Abstract
There is growing interest in non-invasive brain stimulation (NIBS) as a novel treatment option for substance-use disorders (SUDs). Recent momentum stems from a foundation of preclinical neuroscience demonstrating links between neural circuits and drug consuming behavior, as well as recent FDA-approval of NIBS treatments for mental health disorders that share overlapping pathology with SUDs. As with any emerging field, enthusiasm must be tempered by reason; lessons learned from the past should be prudently applied to future therapies. Here, an international ensemble of experts provides an overview of the state of transcranial-electrical (tES) and transcranial-magnetic (TMS) stimulation applied in SUDs. This consensus paper provides a systematic literature review on published data - emphasizing the heterogeneity of methods and outcome measures while suggesting strategies to help bridge knowledge gaps. The goal of this effort is to provide the community with guidelines for best practices in tES/TMS SUD research. We hope this will accelerate the speed at which the community translates basic neuroscience into advanced neuromodulation tools for clinical practice in addiction medicine.
Keywords: Substance use disorder, Addiction, Non-invasive brain stimulation, Transcranial, lectrical stimulation, Transcranial magnetic stimulation, rTMS, tDCS, tES, NIBS, Psychiatry
1. Introduction
Human neuroimaging and preclinical investigations have advanced our knowledge of the neural circuitry that perpetuates the cycle of relapse and recovery in substance use disorders (SUD). The challenge now is to translate this knowledge into evidence-based interventions for patients with SUDs (Ekhtiari and Paulus, 2016). Two tools that demonstrate promise in bridging this gap are transcranial electrical stimulation (tES) and transcranial magnetic stimulation (TMS) (Fig. 1) (Coles et al., 2018; Hone-Blanchet et al., 2015; Yavari et al., 2016). While these non-invasive brain stimulation (NIBS) techniques are still in an early stage of development for SUDs, there is a growing international community of investigators who are attempting to optimize, evaluate, and validate their use as novel treatments for individuals seeking treatment for SUDs. Three meta-analyses show preliminary but promising results with tES/TMS in addiction medicine (Jansen et al., 2013; Maiti et al., 2017; Slotema et al., 2010). While, other non-electromagnetic technologies for NIBS, such as ultrasound and near infrared light, may offer benefits in the future, they are less well developed and have not been studied for SUDs at present, and therefore are not included in this review.
Fig. 1.
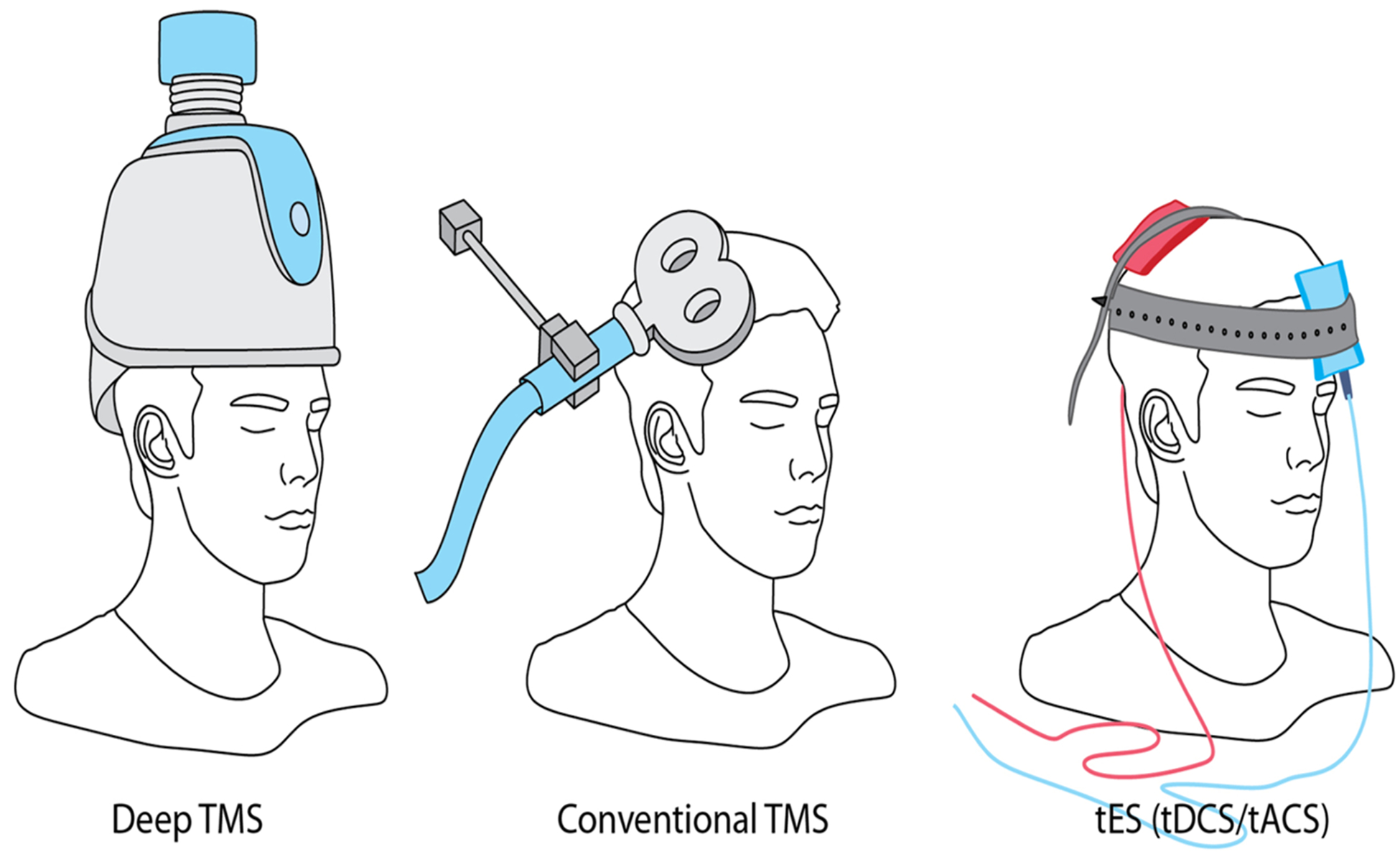
Three major non-invasive brain stimulation technologies. Repeated transcranial magnetic stimulation (rTMS) in its both conventional and deep forms and also transcranial direct current stimulation (tDCS) have been used frequently in addiction medicine trials. Other forms of the transcranial electrical stimulation (tES), like transcranial alternating current stimulation (tACS) have not been used in published addiction medicine trials (till June 1, 2018).
The purpose of this consensus paper is to review the current body of knowledge of the utility of NIBS for SUDs and our current understanding of the biological basis through which these techniques modulate the brain. An important challenge has been the tremendous variability in the methods and outcome measures across tES/TMS trials in SUDs. Additionally, as with most innovative approaches, many (although not all) of the NIBS studies published in the SUD field have small sample sizes, do not contain rigorous control conditions, and are not sufficiently blinded. This makes reproducibility and interpretation difficult.
To address these limitations and to propose a new framework for future research, we have assembled an international collaborative group of investigators with expertise in neuromodulation and addiction research (international network of tES/TMS trials for addiction medicine (INTAM)). We had three webinars (September 2018, January 2019, and April 2019; recorded videos of the webinars are available on YouTube), and two joint meetings in parallel to the New York city neuromodulation meeting (August 2018) and 3rd international brain stimulation conference in Vancouver (February 2019). We review the literature, discuss current gaps in our knowledge, and provide strategies aimed at bridging these gaps. This consensus paper proposes guidelines for best practices in tES/TMS SUD research. Our hope is that this will accelerate the speed with which we can work together as a community to translate basic neuroscience findings into advanced neuromodulation tools for clinical practice.
2. General methodological parameter space in tES/TMS
2.1. TMS technical specifications
Transcranial magnetic stimulation is based on the electromagnetic induction principle where brief focal electromagnetic pulses penetrate the skull to stimulate target brain regions. The magnetic field is usually strong enough to induce firing of neurons beneath the area where the coil is positioned over the scalp. TMS pulses can be applied as single pulses (spTMS), as two paired-pulses (PP-TMS), or as repetitive trains of stimulation that may be either continuous at a specific frequency (repetitive- or rTMS), or patterned with specific inter-train intervals (e.g. either intermittent or continuous theta-burst stimulation, iTBS/cTBS). Both sp- and PP-TMS can be used to measure cortical excitability; rTMS and TBS have been shown to induce changes and after-effects in cortical excitability (section 2c). For all applications of TMS, and indeed for all types of NIBS, stimulation frequency, pattern of stimulation, and the intensity of the stimulation are crucial parameters. These factors affect the magnitude of the delivered electric field and activation of neuronal populations relative to the cortical gyri surface. Equally critical are safety parameters and ensuring stimulation tolerability of the stimulation. For most applications, stimulation intensity may be set as a percentage of an individual’s threshold for inducing an electromyographic motor evoked potential (MEP) of a peripheral muscle (Rossi et al., 2009).
2.2. tES technical specifications
In contrast to TMS, transcranial electrical stimulation (tES) involves delivering low-intensity electric currents through electrodes placed on the scalp and/or upper body. The two most common stimulation paradigms are transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS). Depending on the targeted area(s) of stimulation, carbon rubber or silver chloride electrodes covered in a conductive liquid or gel are placed over the scalp, with lead wires connecting to a battery powered constant-current stimulator that delivers currents in the milli-ampere ranges. Conventional doses, with stimulation intensities up to 2 mA, and durations of up to 30 min, are considered safe as measured by behavioral outcomes and neuroimaging, in both humans and in animals (Bikson et al., 2016). In contrast to TMS, tDCS does not directly induce neuronal firing, but is thought to modulate cortical excitability by a polarity-dependent shift of the neuronal membrane potential (Bindman et al., 1964; Nitsche and Paulus, 2000; Nitsche and Paulus, 2001). The directionality of membrane polarization is determined by the electrical field orientation relative to neuronal orientation (Kabakov et al., 2012; Rawji et al., 2018); (Woods et al., 2016a). In this sense, “anodal” or “cathodal” tDCS should be understood as indicative of placement of the anode or cathode electrodes over a target region (Woods et al., 2016a). “High-definition” tES, using arrays of multiple smaller electrodes, may provide more focal stimulation (Datta et al., 2009; Edwards et al., 2013). On the macroscopic level, placement of the anode over the motor cortex enhances excitability, while placement of the cathode over the motor cortex reduces cortical excitability (Bindman et al., 1964; Nitsche and Paulus, 2000; Nitsche and Paulus, 2001). tACS is based on the delivery of an alternating current with a sinusoidal or other patterned waveform (Antal et al., 2008) to modulate the power and/or phase of endogenous brain oscillations through entrainment (Ali et al., 2013; Reato et al., 2013). Both the frequency and relative phase are critical parameters in tACS experiments (Giordano et al., 2017; Kutchko and Frohlich, 2013; Yavari et al., 2018).
2.3. tES/TMS mechanisms and dependent factors
Considerable insights into the mechanism of tES/TMS have been provided by human neuroimaging and electromyographic (EMG) studies that measure motor excitability after brain stimulation. High-frequency rTMS (> 5 Hz) facilitates, while low-frequency rTMS (< 1 Hz) inhibits motor cortical excitability (Pascual-Leone et al., 1994). Likewise, iTBS and cTBS were shown to facilitate and reduce motor cortical excitability, respectively, but with a shorter stimulation duration compared to rTMS (Cárdenas-Morales et al., 2010; Huang et al., 2005). Anodal tDCS at a 1 mA intensity applied for 13 min over the motor cortex was shown to enhance cortical excitability, while cathodal tDCS applied for 9 min reduced excitability, both effects lasting for up to 1 h after stimulation (Nitsche and Paulus, 2001; Nitsche et al., 2003c). The primary hypothesized mechanism underlying the neuromodulatory effects of these techniques is long-term potentiation (LTP)- or long-term depression (LTD)-like change in the synaptic coupling of neurons (see reviews by Chervyakov et al., 2015; Stagg and Nitsche, 2011). Directionality of LTP or LTD is dependent on the frequency (for TMS), intensity and duration of the stimulation. A rapid post-synaptic increase in calcium ions can induce LTP, which has been noted with high frequency (10 Hz) rTMS, iTBS, or anodal tDCS, whereas a slower and sustained flux of calcium ions induces LTD after 1 Hz rTMS, cTBS, and cathodal tDCS (Cirillo et al., 2017; Huang et al., 2005; Jamil et al., 2016; Liebetanz et al., 2003). Primary sources of evidence in tACS studies suggest that stimulation applied at an endogenous oscillatory frequency for several minutes induces sustained after-effects (Ahn et al., 2019b; Heise et al., 2016; Kasten et al., 2016; Wischnewski et al., 2018; Zaehle et al., 2010), which are associated with NMDA receptor-mediated plasticity (Wischnewski et al., 2018). This after-effect could even be long lasting in the time scale of days to weeks after multiple sessions of tACS (Ahn et al., 2019a). A recent longitudinal study investigated alcohol intake and dopamine transporter (DAT) availability in the striatum of people with alcohol use disorder (AUD) before and after deep rTMS. With respect to sham stimulation, real stimulation significantly reduced both alcohol craving and intake and DAT availability. This study suggested a potential clinical efficacy of rTMS in throughout its modulatory effect on dopaminergic terminals (Addolorato et al., 2017).
In some cases, longer and higher-amplitude stimulation induces stronger and longer effects. For instance, enhanced clinical effects in depressed patients are noted after doubling the number of rTMS pulses (Hadley et al., 2011; Kaster et al., 2018); enhanced after-effects of tDCS on cortical excitability can be induced by increasing the duration of stimulation from a few seconds to several minutes (Nitsche et al., 2003b; Nitsche and Paulus, 2000; Nitsche and Paulus, 2001). However, further extension of stimulation duration and/or intensity does not necessarily increase its efficacy (Esmaeilpour et al., 2018). Extending the duration of anodal tDCS can convert after-effects from excitability enhancement to diminution in some cases (Monte-Silva et al., 2013). Increasing the current intensity of cathodal tDCS from 1 to 2 mA reverses the effects from excitability diminution to enhancement (Batsikadze et al., 2013). Similarly, doubling the duration of iTBS converted its faciliatory after-effects into inhibitory while inhibitory cTBS became faciliatory by doubling stimulation duration (Gamboa et al., 2010). Likewise, increasing the TBS intensity converted its inhibitory effects to faciliatory (Doeltgen and Ridding, 2011). Decreasing the intensity of 140 Hz tACS from 1 mA in 0.2 mA steps, generated excitation at 1 mA, no effect at 0.6 and 0.8 mA, and inhibition at 0.4 mA (Moliadze et al., 2012). Moreover, an increasing number of studies have shown the relevance of a state-dependency of stimulation after-effects. Thus, details of NIBS effects are dependent on a variety of factors and may vary across brain regions in ways yet to be explored.
Based on the principle of homeostatic plasticity, stimulation that is given simultaneously versus preceding or followed by another intervention can change the effect of the primary stimulus. The direction of synaptic plasticity may be due to regulatory mechanisms that aim to prevent a ‘runaway’ effect of synaptic plasticity (Karabanov et al., 2015). Moreover, effects of tES/TMS can also be affected by the ‘baseline’ brain state, in which the excitability profile of the brain area might disrupt the expected mechanism of stimulation. These differential effects were for example observed when opposing effects of rTMS were found in epileptic patients under excitability-inhibiting medication (Fregni et al., 2006). Cue exposure in PTSD patients differentiated response to deep TMS (Isserles et al., 2013) and anodal tDCS resulted in excitability diminution when it was applied to the motor cortex while subjects performed voluntary muscle contraction (Thirugnanasambandam et al., 2011). The above studies should be interpreted with the caveat that “excitability” typically indicates responsiveness to one form of TMS and applied to the motor cortex. Taken together, it should be kept in mind that neuroplastic after-effects of tES/TMS are sensitive to stimulation parameters, but may also vary across regions and brain state.
2.4. Timing: single vs. multiple sessions
In order to establish protocols for clinically relevant long-lasting effects, an ongoing effort of research has been dedicated to exploring the effects of repeating stimulation, either by applying stimulation daily over several days or weeks, or repeating stimulation within a single daily session, separated by a critical time window. In general, repeating stimulation over multiple days has demonstrated efficacy in various clinical applications, such as treatment of bipolar disorder, pain and depression for tDCS (Sampaio-Junior et al., 2018; Kuo et al., 2014; Sampaio-Junior et al., 2018), as well as treatment of depression using rTMS (Rapinesi et al., 2015a; Senova et al., 2019). In fact, the number of repetitions may even play a decisive role, as in one study, it was found that at least 4–6 weeks of daily rTMS over left DLPFC was required to induce significant clinical improvement by active compared with sham TMS (O’Reardon et al., 2007), whereas a parallel study which performed rTMS for 3 weeks found no difference in response between active and sham rTMS (Herwig et al., 2007).
With regard to addiction studies, positive evidence also exists for lasting effects of repeated stimulation, such as reduced craving following a weekly application of tDCS over five weeks for alcohol addiction (da Silva et al., 2013), enhanced abstinence rates for up to three months following four sessions of iTBS for smokers (Dieler et al., 2014) and reduced cigarette consumption and nicotine dependence for up to six months following 13 sessions of rTMS (Dinur-Klein et al., 2014a). However, even with these promising results, systematic or face-to-face studies comparing different repetition intervals are missing, and are crucially needed in order to determine effective repetition rates and durations. The importance of this issue also underlies the need for determining the optimal repetition intervals between sessions. In studies using both tDCS and TMS, the duration of the repetition interval has been found to be critical in modulating plasticity, while also avoiding homeostatic mechanisms that may limit or counter-act plasticity (Goldsworthy et al., 2013; Monte-Silva et al., 2013; Thickbroom, 2007; Tse et al., 2018). For example, in a study on depression, repeating rTMS twice daily with a 15-min interval between stimulation blocks resulted in superior effects compared to a once daily application with the same number of pulses (Modirrousta et al., 2018). For addiction, the number of studies investigating the effect of interval timings remains scarce. In a population of patients with alcohol dependency, five sessions of bilateral tDCS over the DLPFC and administered twice daily for 13 min with a 20-min interval led to positive effects on relapse reduction and improved the quality of life, which lasted for up to six months (Klauss et al., 2014). In summary, although there is promising evidence for persisting and long-lasting effects with repeated stimulation sessions, the relatively large heterogeneity of these studies with regard to stimulation technique, timing, repetition, and montage precludes a clear understanding of how repetition may affect therapeutic outcomes in SUD, warranting a need for systematic research designs (Trojak et al., 2017).
2.5. Sham intervention
Adequate sham stimulation protocols are a critical factor in clinical trials to ensure that effects can be ascribed specifically to tES/TMS. For tES, blinding can be achieved by applying stimulation for a short duration (10–30 s in different studies) with a fade in/out of the current to induce a cutaneous sensation similar to that of the respective effective stimulation protocol. This method does not induce aftereffects, has been shown to produce reliable blinding and is suitable for double-blind experiments (Ambrus et al., 2012). Efforts to enhance sham reliability are ongoing (Fonteneau et al., 2019; Garnett and den Ouden, 2015; Kessler et al., 2012).
Since TMS generates sensory perceptions, such as clicking sounds, and muscle contractions (Conde et al., 2019), sham strategies aim to mimic the sight, sound, and feel of real stimulation, while attempting to avoid any direct neuronal effects on the central nervous system. In order to serve as a reliable control, for both, placebo and sensory effects of TMS, sham TMS is either performed by tilting the coil away from the scalp (Sandrini et al., 2011), using sham-coils that include tangential elements to the scalp surface similar to active coils without electromagnetic discharges (e.g. Levkovitz et al., 2015), and using electrical stimulation under the coil to mimic muscle contractions. Specifically, some sham coils use an electrical stimulator to apply a weak electrical pulse time-locked to a TMS pulse to simulate the cutaneous sensation associated with activating scalp muscles. These techniques have shown the best similarity regarding the look, sound, and feeling of active stimulation (Duecker and Sack, 2015; Mennemeier et al., 2009). Nevertheless, experienced participants may be able to discriminate between active and sham TMS (Duecker and Sack, 2015; Rossi et al., 2007). Sham TMS approaches require further development but may be sufficient in clinical settings in which patients are generally naïve to TMS (Duecker and Sack, 2015; Levkovitz et al., 2015; Sheffer et al., 2013a; 2013b).
Ultimately, the appropriateness of a specific sham protocol depends also on the respective experimental design (e.g. it might be easier for participants to discern between real and sham stimulation in crossover, as compared to parallel group trials). There are ongoing efforts by the TMS community to evaluate and revise sham protocols in order to increase rigor across the field (Bikson et al., 2018a; Opitz et al., 2014).
2.6. Study Design: crossover vs. parallel vs. open label
When designing a brain stimulation study, several factors should be considered in order to achieve reliable findings. These factors include the choice of study population, the possibility of carry-over effects, costs, subject compliance, and statistical power. Generally, studies that do not expect to find high levels of inter-individual variability may opt for parallel group designs. In these studies, two independent groups of subjects receive either active or sham stimulation and are directly compared. Consequently, one may need to recruit a relatively large number of participants in order to find meaningful statistical effects. When inter-individual variability is high, a crossover design may be more beneficial. However, care must be taken to avoid carry-over effects, and unblinding may occur in patients that experience both active and sham stimulation. Investigators may also consider parsimoniously factorial designs where two features of a tES/rTMS intervention are varied (e.g., intensity and duration) including a sham.
In open-label studies, both the researcher and the participant know what type of stimulation/intervention the participant is receiving. Though this may introduce bias in the experimental findings, an open-label design might be used in spite of these weaknesses for pilot studies, when the main question asked is if it is promising to conduct larger, but more demanding sham-controlled trials. It should, however be kept in mind that the interpretability of the results of those studies is seriously limited. To explore the parameter space of stimulation to find the most efficient parameter set, blinded pilot studies with sham arms are mandatory.
3. Current status of evidence
A comprehensive literature search was conducted on PubMed with articles from January 1, 2000 to July 1, 2018. Search terms are shown in Supplementary Fig. 1 and included both TMS and tDCS studies in SUD. For other tES modalities such as tACS/tPCS/tRNS (transcranial alternating current stimulation, transcranial pulsed current stimulation, or transcranial random noise stimulation), we ran a separate search process, which did not return any articles with our inclusion/exclusion criteria in that time frame. A combination of several key terms was used to explore relevant TMS and tDCS articles. The exact combination used is shown in Supplementary Fig. 1.
This search resulted in 218 articles for TMS and 632 articles for tDCS. During the early stage of screening, two independent reviewers (HT and NM) excluded 77 TMS and 558 tDCS records based on their titles and abstracts. Exclusion criteria included: (1) non-peer reviewed book chapters, (2) commentaries, (3) author corrections, and (4) disorders other than substance use disorders (SUDs).
The yield, which included 141 articles for TMS and SUDs and 74 for tDCS and SUDs, were evaluated to identify eligible articles. After an indepth full-text review, 50 TMS articles and 34 tDCS articles were included in the final evaluation. The exclusion criteria at this stage included: (1) review articles, (2) studies with healthy subjects only, (3) case reports, (4) studies with subjects other than humans, (5) basic physiology studies, (6) study protocols, or (7) letter to the editors. One TMS study was duplicated with another title. In addition, single or paired-pulse TMS studies were removed; only repetitive TMS (rTMS) studies were included as we were interested in studies with therapeutic goals, not basic neurophysiology. The PRISMA charts for the inclusion/exclusion procedures for rTMS and tDCS studies are shown in Fig. 2 panel A and B, respectively.
Fig. 2.

PRISMA flow diagrams for published rTMS (panel A) and tDCS (panel B) trials in drug addiction.
3.1. Contribution of countries and drug classes
Among the yield of 84 total articles, 19 articles had corresponding authors from the United States of America (USA), followed by Brazil (n = 13), Italy (n = 11), China (n = 10) and Germany (n = 8). Fourteen countries were represented in the extant published literature. Countries reported different proportions of rTMS vs. tDCS studies (Fig. 3).
Fig. 3.

Contribution by country of tDCS/rTMS studies (n = 84) for addiction medicine, color coded for type of stimulation (Y axis represents number of studies).
Countries targeted different substances: cocaine studies were reported from the USA, Italy, and Brazil; methamphetamine studies from China, Iran, and USA; and heroin/opioid studies only from China (Fig. 4). It is notable that there were 3 articles with 2 drugs as targets. Two out of these 3 articles reported a single trial including patients with two drug use disorders (cocaine or alcohol) (Hanlon et al., 2017; Nakamura-Palacios et al., 2016) and one article including 2 independent trials for cocaine and alcohol (Kearney-Ramos et al., 2018). As these two trials were identical in their design, they were counted as a single study. As a result, the number of articles and studies (trials) in this manuscript are the same (n = 84).
Fig. 4.

The number of tDCS/rTMS studies from each country color coded according to substance type (n = 84 but 3 studies included 2 drugs).
3.2. Number of subjects and sex proportion
Forty-seven studies were reported as parallel design, 33 crossover, and 4 single arm trials. Seventy-five (87%) studies had 30 or fewer subjects in each arm; 10 studies had 30 or more subjects in each arm. From all 84 studies, 11 studies did not report the number of female vs. male participants; 26 studies included only males. In 21 studies the percentage of female participants was 40% or less. Twenty studies had 40–70% female participants; in 6 studies the proportion was greater than 70%. In one study, all participants were female (Hoppner et al., 2011).
3.3. Targeted brain areas
The left DLPFC was the most frequent anatomical target followed by the right DLPFC. In addition, there were seven other brain areas targeted: frontal pole (FP), temporoparietal (TP), inferior frontal gyrus (IFG), superior frontal gyrus (SFG), and motor cortex. There were seven studies that used TMS intended to target deeper and wider regions including anterior cingulate cortex (ACC) and insula (Table 1, Fig. 5). For TMS it is relativity well established that the primary outcome is the activation of the cortex under the TMS coil (Opitz et al., 2011), with secondary activation of networks through synaptic connectivity (Opitz et al., 2016).
Table 1.
Protocols used to stimulate specific areas with tDCS/rTMS (n (number of studies) = 84).
| Target | Type (n) | Protocol Outline (n) | |
|---|---|---|---|
| Right DLPFC | TMS (n=15) | rTMS | 10 Hz (n=6) [1], [2], [3], [4], [5], [6] |
| 20 Hz (n=6) [7], [8], [9], [10], [11], [12] | |||
| iTBS | 50 Hz bursts in 5 Hz (n=1) [13] | ||
| rTMS | 1Hz (n=1) [14] | ||
| rTMS | 10 Hz and 1 Hz (n=1) [15] | ||
| tDCS (n=17) | Anodal tDCS | Cathode on left DLPFC (n=15) [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30] | |
| Cathode on supraorbital (n=1) [31] | |||
| Cathode on occipital region (n=1) [32] | |||
| Left DLPFC | TMS (n=29) | rTMS | 10 Hz (n=16) [4], [6], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46] |
| 15Hz (n=3) [47], [48], [49] | |||
| 20Hz (n=5) [12], [50], [51], [52] | |||
| 10 Hz and 20 Hz (n=1) [53] | |||
| Deep TMS | 15 Hz (n=1) [54] | ||
| rTMS | 1 HZ (n=1) [55] | ||
| rTMS | 10 Hz and 1 Hz (n=2) [15], [56] | ||
| tDCS (n=19) | Anodal tDCS | Cathode on right DLPFC (n=10) [21], [25], [27], [28], [29], [30], [57], [58], [59], [60] | |
| Cathode on supraorbital (n=9) [61], [62], [63], [64], [65], [66], [67], [68], [69] | |||
| Bilateral DLPFC | TMS (n=5) | Deep TMS | 10 Hz (n=2) [70], [71] |
| 20Hz (n=3) [72], [73], [74] | |||
| Left FP | TMS (n=3) | cTBS | 50 Hz bursts in 5 Hz(n=2) [75], [76] |
| rTMS | 5 Hz (n=1) [77] | ||
| SFG (FpZ) | TMS (n=1) | rTMS | 10 Hz and 1 Hz (n=1) [78] |
| Bilateral DLPFC and ACC | TMS (n=1) | Deep TMS | 10 Hz and 1 Hz (n=1) [79] |
| Bilateral DLPFC and Insula | TMS (n=1) | Deep TMS | 10 Hz and 1 Hz (n=1) [80] |
| Bilateral TP | tDCS (n=2) | Cathodal tDCS | Anode on occipital region (n=2) [81], [82] |
| Right IFG | tDCS (n=1) | Anodal tDCS | Cathode on supraorbital (n=1) [65] |
| Left TP | tDCS (n=1) | Cathodal tDCS | Anode on right TP (n=1) [81] |
| Motor cortex | tDCS (n=2) | Anodal tDCS | Cathode on supraorbital (n=2) [83], [84] |
DLPFC, dorsolateral prefrontal cortex: IFG, inferior frontal gyrus; FP, frontal pole; TP, temporoparietal; SFG, superior frontal gyrus; ACC, anterior cingulate cortex. [1], (Mishra et al., 2016); [2], (Qiao et al., 2016); [3], (Jansen et al., 2015); [4], (Mishra et al., 2015); [5], (Mishra et al., 2010); [6], (Camprodon et al., 2007); [7], (Kozak et al., 2018); [8], (Herremans et al., 2016); [9], (Herremans et al., 2015); [10], (Herremans et al., 2013); [11], (Herremans et al., 2012); [12], (Wing et al., 2012); [13], (Dieler et al., 2014); [14], (Trojak et al., 2015); [15], (Liu et al., 2017); [16], (Klauss et al., 2018a); [17], (Shahbabaie et al., 2018a); [18], (Nakamura-Palacios et al., 2016); [19], (Wietschorke et al., 2016); [20],(Batista et al., 2015); [21], (Pripfl and Lamm, 2015); [22], (Conti et al., 2014); [23], (Conti and Nakamura-Palacios, 2014); [24], (Klauss et al., 2014); [25], (Gorini et al., 2014); [26], (Fecteau et al., 2014); [27], (Pripfl et al., 2013); [28], (Boggio et al., 2010); [29], (Boggio et al., 2008); [30], (Fregni et al., 2008); [31], (Shahbabaie et al., 2014); [32], (Mondino et al., 2018); [33], (Kamp et al., 2018); [34], (Liang et al., 2018a); [35], (Zhang et al., 2018); [36], (Li et al., 2017a); [37], (Li et al., 2017b); [38], (Sahlem et al., 2017); [39], (Su et al., 2017); [40], (Del Felice et al., 2016); [41], (Huang et al., 2016); [42], (Shen et al., 2016); [43], (Prikryl et al., 2014); [44], (Pripfl et al., 2014); [45], (Li et al., 2013a); [46], (Amiaz et al., 2009); [47], (Pettorruso et al., 2018); [48], (Terraneo et al., 2016); [49], (Politi et al., 2008); [50], (Sheffer et al., 2018); [51], (Hoppner et al., 2011); [52], (Eichhammer et al., 2003); [53], (Sheffer et al., 2013a; 2013b); [54], (Rapinesi et al., 2016); [55], (Li et al., 2013b); [56], (Baker et al., 2017); [57], (Yang et al., 2017); [58], (den Uyl et al., 2017); [59], (de Almeida Ramos et al., 2016); [60], (Boggio et al., 2009); [61], (Vitor de Souza Brangioni et al., 2018); [62], (den Uyl et al., 2016); [63], (Falcone et al., 2016); [64], (Kroczek et al., 2016); [65], (den Uyl et al., 2015); [66], (Smith et al., 2015); [67], (da Silva et al., 2013); [68], (Xu et al., 2013); [69], (Nakamura-Palacios et al., 2012); [70],(Iran Supreme Leader, 2006); [71], (Bolloni et al., 2016); [72], (Girardi et al., 2014); [73], (Rapinesi et al., 2015b); [74], (Ceccanti et al., 2015); [75], (Hanlon et al., 2017); [76], (Kearney-Ramos et al., 2018); [77], (Hanlon et al., 2016a; 2016b);[78], (Rose et al., 2011); [79], (Martinez et al., 2018); [80], (Dinur-Klein et al., 2014b); [81], (Meng et al., 2014); [82], (Wang et al., 2016); [83] (Batsikadze et al., 2017); [84], (Grundey et al., 2012).
Fig. 5.

Brain targets for TMS/tDCS trials in addiction medicine. Right panel: Seventy seven out of 84 published TMS/tDCS studies (till June 1, 2018) selected DLPFC as the target of stimulation including right, left, or bilateral DLPFC. Each trial with bilateral stimulation counted in both right and left categories. In 13 tDCS studies, one of the electrodes was placed on right supraorbital area (counted as frontal pole). All deep TMS (dTMS) studies are bilateral with both high and low frequency (HF and LF) TMS. Left panel: The number of TMS and tDCS studies in each target. DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; FP, frontal pole; SFG, superior frontal gyrus; IFG, inferior frontal gyrus; TP, temporoparietal and M1, motor cortex.
For conventional tES (tDCS/tACS), using large electrodes, computational models (DaSilva et al., 2015; Datta et al., 2009; Santos et al., 2016) supported by intra-cranial recordings (Huang et al., 2017; Opitz et al., 2013) and imaging studies (Krishnamurthy et al., 2015; Lang et al., 2005) indicate diffuse current flow under and between electrodes representing a large area of potentially stimulated cortex.
3.4. Dosage, number of sessions and follow up
Among 50 TMS studies, nearly half of them (49%) applied 10 Hz pulses to the subjects, 24% used 20 Hz, and 12% performed 1 Hz stimulation. The remained applied 5 and 15 Hz, and in 1 study subjects received 50 Hz pulses in the form of iTBS. The numbers of pulses in a session of TMS are 2000 or less for 82% of TMS studies. In 34 tES trials, 2 mA is the most frequent intensity (21 trials), and second place goes to 1 mA with 10 trials. 0.45 mA and 1.5 mA each have 2 trials. In term of duration, 88% of tES trials have 20 min stimulation or less.
Thirty-seven (44%) tES/TMS trials included only one session of stimulation. Eighteen (21%) had 2–7 sessions of the intervention and 20 (24%) had 8–14 sessions. Nine studies delivered more than 14 sessions (11%) all using TMS; three administered 15 sessions and six administered 20 sessions. Sixty (71%) studies did not include any follow up beyond the day of the intervention. There were only two studies with one-year follow-up, six studies with six months follow-up, and four studies with three months follow-up. Twelve studies had fewer than three months follow-up.
3.5. When to stimulate
There were four distinct time intervals at which rTMS/tDCS interventions were administered: (1) before the participant sought standard treatment (2) while the subject was treatment seeking but before undergoing standard treatment, (3) within the first month of standard treatment (mainly detoxification and stabilization) and (4) after the initial recovery period (more than one month).
All studies in tobacco dependent participants were conducted before starting standard treatment, but strategies addressing other drugs of use were varied (Fig. 6). Seven studies explicitly added rTMS/tDCS to a pharmacotherapy program that included the following: diazepam (n = 3), trazodone (n = 1), nicotine (n = 2), and zolpidem (n = 1). Six studies were combined with psychological interventions. Thirty-four (40%) studies used cue exposure before or during stimulation with the goal of increasing efficacy. There is a growing body of evidence on using tES/TMS to reduce opioid use in healthy people for pain management (e.g., post-surgery) before dependence can start (Borckardt et al., 2017, 2013). This is an interesting field but it is beyond the scope of this review.
Fig. 6.

Phases of recovery during which tDCS/rTMS was administered, divided amongst SUD groups.
3.6. Outcome measures
Sixty-four (76%) studies used craving as their primary outcome measure. Self-report on a visual analogue scale (VAS) was the most frequently used craving measure (30 studies). Eighteen different questionnaires were used to measure drug craving for different drugs with variations in items and structure. Forty-four (52%) articles used change in drug use frequency/amount as an outcome measure. However, only 14 used objective measures such as urine drug tests or breath analyzers. Thirty-six studies (81%) used self-report measures for drug use or addiction severity. Other outcomes such as positive valence (e.g., motivation, willingness, and hedonic tone) (n = 6), negative valence (e.g., depression, anxiety, and withdrawal) (n = 22), cognition (e.g., memory, attention, and inhibition) (n = 20), general mental or physical health (e. g., daily functioning, quality of life and sleep) (n = 10), neurophysiologic measures (e.g., ERP, fMRI, and fNIRS) (n = 27) were also included in the reviewed studies (Fig. 7 and Table 2).
Fig. 7.

Major groups of outcome measures in 84 tDCS/rTMS studies.
Table 2.
Outcome measures in tDCS/rTMS studies.
| Outcome (rTMS/tDCS) | Types of Outcome (rTMS/tDCS) |
|---|---|
| Craving (38/26) | General craving (28/17) |
| Cue-induced craving (11/10) | |
| Implicit craving (0/5) | |
| Positive Valence (5/1) | Hedonic tone (1/0) |
| Willingness (1/0) | |
| Motivation (2/1) | |
| Intensity of enjoyment in chest sensations (1/0) | |
| Negative Valence (13/9) | Depression (8/3) |
| Anxiety/Stress (4/4) | |
| Mood (0/5) | |
| Withdrawal (3/0) | |
| Mental/General health assessment (6/4) | General health/Functioning (2/3) |
| Psychiatric symptoms (4/1) | |
| Drug use/Relapse/Abstinence (27/17) | Self-report (22/14) |
| Objective measure (10/4) | |
| Cognitive Functions (11/9) | General cognitive performance (2/4) |
| Attention/Working memory (5/2) | |
| Memory and learning (2/0) | |
| Executive function/Inhibition (2/0) | |
| Decision making (1/3) | |
| Neurophysiology (14/13) | Brain activity (fMRI, ERP, fNIRS) (7/9) |
| Brain connectivity (fMRI, DTI) (7/3) | |
| Neurophysiological effects (EEG, Heart rate) (2/2) | |
| Dopamine transporter (PET) (1/0) | |
| Cerebral hemodynamic indices (Transcranial | |
| Doppler sonography) (1/0) | |
| Metabolites (MRS) (2/0) | |
| Motor cortex excitability (0/2) | |
| Others (2/0) | Adherence (1/0) |
| Tolerability (1/0) |
4. Clinical targets and outcomes in tES/TMS trials for addiction medicine
Selecting suitable outcome measures for tES/TMS trials in SUDs is always a challenging process. Like any other field, outcome measures need to have high validity (face validity, content validity, and construct validity), reliability (test-retest and inter-rater), variability (broad distribution and range of values), responsiveness (ability to detect change in an individual over time) and feasibility in the clinical context of the trial. As previous sections have mentioned, there is significant variability in the experimental designs employed and the patient populations investigated to date. This variability also extends to the outcome measures that have been evaluated in these trials. In this section, we will introduce standard behavioral and biologic measurement assays that are recommended as robust endpoints for clinical trials in addiction medicine. Some questions include: “What outcome measures should be used in tES/TMS clinical trials of participants with SUDs?”, “What are governmental standards (e.g. FDA) on outcome measures?”, “Are there ‘gold standard’ assessment tools and biomarkers that should be used for evaluating NIBS efficacy in various domains of SUD?” In this section, we provide the reader with some tangible suggestions for dependent measures to consider in neuromodulation studies of SUD.
4.1. Primary endpoint: consumption
To date, the majority of therapeutic agents being used to treat SUDs have received FDA-approval based on their ability to decrease consumption. The gold standard for evaluating efficacy of a therapeutic agent for SUDs is its ability to stop consumption of the substance being used or to reduce consumption to less harmful levels (harm reduction). For example, there are currently four FDA-approved pharmacotherapies for alcohol use disorder - disulfiram, oral naltrexone, extended release injectable naltrexone, and acamprosate. Disulfiram, acamprosate, and oral naltrexone were approved for increasing abstinence more than placebo, whereas extended release injectable naltrexone was approved for increasing the proportion of subjects with no heavy drinking days. Within alcohol treatment research alcohol consumption is frequently measured by patient’s self-report (e.g. the time line follow back method (Sobell et al., 1996)) but can also include biological measures, such as urine ethyl glucuronide (ETG; a measure of recent drinking) and percent carbohydrate deficient transferrin (%CDT; a measure of drinking over several days). Unfortunately, available biomarkers for alcohol use tend to have poor sensitivity and specificity (Tavakoli et al., 2011).
There are also three FDA approved medications for smoking cessation: nicotine replacement therapies (NRTs), bupropion, and varenicline -all of which promote abstinence. As with the alcohol use literature, consumption is frequently measured by self-report, but can also include biological verification such as exhaled carbon monoxide (a measurement of recent use of a combustible agent), or urine cotinine (a metabolite of nicotine). As the methods of delivery for nicotine are evolving and there is a rise of smoking non-tobacco products (e.g. marijuana), a positive carbon monoxide (CO) level may not be sufficient to conclude that someone is smoking tobacco. Combining CO with a urine drug screen and/or urine cotinine may provide a more robust assessment.
Treatment for opioid use disorder typically requires acute detoxification and/or opioid maintenance treatment (i.e., medication assisted treatment). The two primary treatments for opioid use disorder (methadone, buprenorphine) are designed for long term opioid maintenance therapy. Methadone is a mu-opioid receptor agonist whereas buprenorphine is a partial mu-opioid receptor agonist (mu agonist-K antagonist). As with the other classes of substances both self-report and urine drug screens are used for biological verification of consumption. The challenge with opioids is that a general opioid screen will detect morphine and many of its metabolites (e.g. Demerol) but not oxycodone. Oxycodone and buprenorphine often have to be assessed with a specific assay. Additionally, as worldwide production and export of synthetic compounds is evolving, many of these newer compounds are not detected by typical hospital screening tools.
Although there are currently no FDA-approved pharmacotherapies for cocaine use disorder or methamphetamine use disorder, consumption of these drugs can (and should) also be assessed with biological metrics as well as self-report whenever possible.
*Suggestions for measuring consumption:
Whenever possible biomarkers (e.g. ETG, CDT, CO, cotinine, opioid metabolites, and cocaine metabolites) of drug consumption should be assessed repeatedly according to the dissipation rate of the biomarker (for example, CO vanishes within hours while cotinine can be detected over a week after smoking). Additionally, the timeline follow back (TLFB) interview is the gold standard method for assessing days of use (e.g., 30 days) with the caveat that it relies completely on self-report. For longitudinal studies at least a 3 months follow-up period is recommended. The TLFB can be used to create summary measures, including the percentage of days abstinent from substances, number of days used in last month, and number of days used at a high level in last month (i.e. heavy drinking days). See (Donovan et al., 2012) for a thorough review of primary outcome measures in SUD treatment research.
4.2. Surrogate endpoints
Although a reduction or elimination of the consumption of the drug is the ultimate endpoint for clinical trials research, there are also many other behavioral and biologic variables that have been studied extensively and are considered meaningful surrogate endpoints for patients seeking treatment for SUDs. Many of these behavioral domains are described below, appear to be present across multiple types of drug using populations, and have also been observed in preclinical models of drug use (e.g. heightened reactivity to predictive drug cues, perseverative responding, delayed discounting for the drug, response to stress, narrowing of the behavioral repertoire) (Beveridge et al., 2008; Garavan and Hester, 2007; Stalnaker et al., 2009).
4.2.1. Cue-reactivity and self-reported craving
One domain that pharmacotherapy, behavioral treatment, and brain stimulation intervention researchers have embraced is “craving” and its closely related construct “cue-reactivity.” Of the published studies using brain stimulation as a treatment for SUDs thus far, the most common dependent measure has been self-reported craving. While craving is now included in the DSM-5 as a core symptom of SUD, its inclusion has been controversial, due in part to challenges in defining ‘craving’ (Sayette et al., 2000). Support for craving as a key treatment target for SUD comes from multiple behavioral, imaging, pharmacology, and genetics studies (Ekhtiari et al., 2016; Garrison and Potenza, 2014; Haass-Koffler et al., 2014; Sinha, 2009; Tiffany and Wray, 2012). Self-reported craving, however, is often very subjective and is not as closely related to relapse as biological markers of cue-sensitivity (for review see: Hanlon et al., 2016a; 2016b)). This may be because craving as a construct requires that participants have insight into something that is a fairly complex psychological phenomenon. It may also be related to the relatively imprecise ways in which it is sometimes measured (e.g. often on a 1–5 numerical rating scale; (Kavanagh et al., 2013)).
Cue-reactivity is another common dependent measure and can be measured in a more objective manner using behavioral paradigms (e.g. attentional bias) or biological metrics (e.g. EEG, fMRI, pupil dilation, heart rate). Elevated drug-cue elicited brain activity is one of the most widely cited, transdiagnostically relevant traits of current SUD populations. Several retrospective meta-analyses have described that the medial prefrontal cortex and cingulate cortex are reliably activated to drug cues (Schacht et al., 2013). Other meta-analyses have shown that activity in these brain regions may predict relapse across multiple substances (Courtney et al., 2016; Killen and Fortmann, 1997).
As with the suggestion that any clinical research study measuring consumption include a biologic metric (as well as self-report), we highly recommend that studies measuring craving/cue reactivity include a biologic metric, as well as self-report.
*Suggestions for measuring craving/cue-reactivity:
Biologic verification of cue-reactivity (functional MRI, DA and Glutamate PET, eye-tracking/pupilometry, startle response, EEG, physiologic measurements of heart rate, and blood pressure) and self-report measures such as Tiffany Cocaine Craving Inventory (Tiffany et al., 1993), Brief Questionnaire of Smoking Urges (QSU) (Cox et al., 2001), Obsessive Compulsive Drinking Scale (OCDS) (Anton et al., 1995), Alcohol Urge Questionnaire (AUQ) (Bohn et al., 1995), Alcohol Craving Questionnaire (ACU) (Raabe et al., 2005), Penn Alcohol Craving Scale (PACS) (Flannery et al., 1999) and Desire for Drug Questionnaire (DDQ) (Franken et al., 2002). Since craving is a potentially transdiagnostic outcome across different SUDs, there are attempts in developing standardized versions of the above measures/questionnaires that can be used across substances (Franken et al., 2002). There is an ongoing debate that simple scales like single item visual analogue scales (VAS) have face validity but lack content or predictive validity (limited to measure complex multifaceted aspects of craving). Meanwhile, there are hopes for simple craving or drug use self-reports using ecological momentary assessment (EMA) (Jones et al., 2019).
4.2.2. Cognitive and behavioral markers of SUD
While consumption (e.g. smoking cessation, reductions in alcohol use) is the dependent measure currently deemed most important for FDA approval, there are a number of phenotypes that are associated with SUDs and vulnerability to relapse. These include indices of executive control (i.e., inhibitory control, working memory), stress reactivity/distress tolerance (Kwako and Koob, 2017), reward processing (Volkow and Morales, 2015), and assigning value to future rewards (e.g. delayed discounting). tES/TMS may be able to modulate neural systems that orchestrate these cognitive processes and improve performance in executive function and decision-making measures. Some of these measures (i.e. risk-taking and uncertainty-based decision-making) are also meaningfully related to clinical outcomes in addiction medicine (e.g. treatment retention, drug relapse) (Dominguez-Salas et al., 2016). tES/TMS trials in addiction medicine can benefit from adopting a “gradients of transference” approach by testing the link between stimulation/inhibition and (1) related cognitive performance, (2) cognition-related clinical outcomes. However, there are serious concerns regarding the validity and reliability of currently available behavioral tasks in decision making and self-control (Enkavi et al., 2019).
*Suggestions for measuring cognitive and behavioral markers of SUD before and after NIBS treatment:
Inhibitory control (Go/No Go; Kaufman et al., 2003), working memory (N-Back; Watter et al., 2001), temporal processing (Delay Discounting Bickel, 2015), stress reactivity/distress tolerance (TSST; Kirschbaum et al., 1993); personalized guided imagery (Sinha, 2013); risky decision making (Iowa Gambling Task (Bechara et al., 1997) and reward processing (MID; Knutson et al., 2001) could be some of the domains and measures.
4.2.3. Comorbidity and psychosocial correlates of substance use
SUDs are often associated with psychiatric comorbidities (e.g. depression, anxiety), social and environmental deficits (e.g. negative consequences, environmental reward, drug free social network), and poor quality of life with each contributing to a lower likelihood of sustained recovery (Kiluk et al., 2019; Tiffany et al., 2012; Witkiewitz et al., 2019). While an improvement in any of these factors has not yet been the focus of FDA approval, from a clinical perspective it is clear that if a brain stimulation intervention could impact any of these associated factors, there would be a large reduction in social costs associated with SUD.
*Suggestions for measuring functional and health correlates:
Short Inventory of Problems from Alcohol and Drug Use (SIP-AD) or broader quality of life measures like WHOQOL-BREF (Skevington et al., 2004) or 36-item short-form health survey (SF-36) (Ware and Sherbourne, 1992).
5. tES/TMS interactions with contextual treatments and comorbidities
5.1. Contextual treatments (treatment as usual)
The literature to date has been mixed with respect to the efficacy of tES/TMS on SUDs (Spagnolo and Goldman, 2017). Among the various neuromodulation modalities used to treat SUDs, short-term treatment with rTMS and tDCS have shown beneficial effects on both drug consumption and craving (Coles et al., 2018). Nevertheless, optimal stimulation parameters (i.e., duration, number of stimulation treatments, stimulation frequency, intensity, brain region of target and proximity between treatments) have yet to be established (Shahbabaie et al., 2018b). Thus, parsing the different contextual parameters among these trials might lead to more efficient outcomes for targeted treatment of SUDs with or without comorbidities.
Time of Intervention may be a critical component to successful treatment. Treatments for SUDs are typically administered within three stages: 1) pretreatment (i.e., aiding individuals who are considering entering treatment/changing their behavior), 2) detoxification (i.e., helping individuals reduce/eventually quit a substance), or 3) in a long-term recovery phase (i.e., administered post-abstinence as a relapse prevention strategy).
5.1.1. Pretreatment intervention
Individuals recruited for participation in a study with a neuromodulation intervention before starting their SUD treatment (treatment seeker or non-treatment seeker) should be assessed for motivation, insight, and concurrent risky behavior (Bari et al., 2018). Frequently, individuals with SUD are incentivized towards study participation for financial reasons, or other social, economic, and psychological stressors (Luigjes et al., 2015). These contextual factors should be considered carefully as well.
5.1.2. Interventions during detoxification
Following multiple failed detoxification attempts individuals with SUDs may resort to novel interventions such as neuromodulatory techniques to aid in quitting (Bari et al., 2018). Given that the critical period for relapse is within the first few days of abstinence, therapeutic approaches that target both acute cravings and withdrawal symptoms are warranted (Trojak et al., 2015). Several studies have shown promising anti-craving/withdrawal effects of TMS, including reducing drug-seeking and risk-taking behaviors (Jansen et al., 2013; Liang et al., 2018b).
5.1.3. Interventions during long-term recovery
Although the efficacy of neuromodulatory treatments has been promising during detoxification, a major issue facing treatment providers is the high risk of relapse following successful treatment. Non-invasive neuromodulation treatments notably produce temporary effects (i.e., tDCS and TMS) compared to long-term benefits found with more directly-targeted neuromodulatory therapies (i.e., DBS) (Bari et al., 2018). Moreover, studies have shown that there are lower relapse rates after treatment discontinuation when behavioral therapies are combined with medications or neuromodulatory treatments (Jansen et al., 2013). Although the research in this area is limited, there is currently a large momentum to design clinical trials that combine a behavioral prime or a behavioral intervention with the neuromodulation strategy (Section 5.3). This may be particularly valuable in tES literature given the portability of the technology and the ease with which it can be combined with evidence-based behavioral interventions for SUD such as cognitive behavioral therapy, exposure therapy, or contingency management. Thus, given the multifaceted nature of addiction and its treatment, including psychotherapeutic processes like motivational enhancement and cognitive behavioral treatment may be useful as add-on long-term therapies with tES/TMS (Bari et al., 2018).
5.2. Comorbidities with SUDs
Neuromodulatory treatments have also been used for comorbidities with SUDs (Coles et al., 2018). One group studying smokers with schizophrenia demonstrated that rTMS reduced cigarette cravings compared to sham (Wing et al., 2012). In contrast, another study of tDCS in comorbid individuals with schizophrenia and tobacco dependence found no effect on craving or consumption (Smith et al., 2015). Another group using rTMS for comorbid dysthymia and alcohol use disorder, showed decreased alcohol consumption with rTMS (Ceccanti et al., 2015). Finally, a case report of using DBS to treat a woman with refractory OCD resulted in reduced craving and consumption of cigarettes (Mantione et al., 2015). Perhaps a dual benefit of brain stimulation treatments targeting underlying neurobiological factors in SUDs may also extend to deficiencies found in other psychiatric disorders (i.e., nicotinic acetylcholine receptor deficits found in schizophrenia patients, associated with both higher smoking rates and cognitive dysfunction) (Lucatch et al., 2018).
5.3. Combination interventions with tES/TMS
While neuromodulatory techniques are a promising interventional approach in treatment of SUDs but most responses are partial, and even well-documented anti-craving effects of rTMS, do not necessarily translate into reduction in drug use or abstinence (Wing et al., 2013). Combining neuromodulation with behavioral and pharmacotherapeutic interventions may ultimately mitigate these shortcomings.
5.3.1. Cognitive and behavioral interventions (CBT, cognitive training/retraining)
In the treatment of SUDs, behavioral interventions (i.e., motivational interviewing (MI); cognitive behavioral therapy (CBT); contingency management (CM)) have all been used with varying effectiveness to support abstinence and prevent relapse. Accordingly, neuromodulation treatment, like pharmacotherapies, should be seen as an adjunct to behavioral treatment of addiction to ensure prolonged recovery (Spagnolo and Goldman, 2017). For instance, plateaued response from DBS was further improved when structured CBT was added (Widge et al., 2016). Another study delivered rTMS as an adjunctive treatment to transdermal nicotine and group therapy, resulting in decreased craving. However, no differences in smoking were found between active and sham conditions (Wing et al., 2012). Given the level of motivation needed to change a behavior, ensuring that a solid behavioral modification framework of recovery is in place is critical for effective outcomes in neuromodulation trials. Cognitive deficits associated with decreased prefrontal functioning have been related to SUDs (Kozak et al., 2017) and increased risk of relapse (Dominguez-Salas et al., 2016). Given that neuromodulation can improve cognitive control/functioning, it may (in part) diminish the risk for relapse by strengthening cognitive control (Jansen et al., 2013; Schluter et al., 2018).
5.3.2. Pharmacotherapies
Though limited, combining pharmacological treatments with brain stimulation methods has an advantage of reversing plasticity induced by drugs of abuse by targeting the neurocircuits that maintains addictive behaviors (Salling and Martinez, 2016). For instance, nearly 50% of patients become abstinent from cigarettes after treatment with rTMS concurrent with nicotine replacement therapy (Trojak et al., 2015). One study found that adjunctive treatment of rTMS with pharmacotherapy showed improvements in depressive symptoms and craving for alcohol (Rapinesi et al., 2015a).
6. Perspectives on tES/TMS for non-substance-related addictive behaviors
Non-substance-related addictive disorders are frequently comorbid with and share some neurobiological substrates and behavioral manifestations of substance-related addictive disorders. This is particularly true for gambling disorder. It is thus an important question of whether neuromodulation could change these neurobiological vulnerabilities, and thereby have clinical value for non-substance addictive behaviors as well.
Gambling disorder was recognized as the first behavioral addiction, and as such was reclassified within the category of “Substance-related and Addictive Disorders”, in the Diagnostic and Statistical Manual of psychiatric disorders (DSM-5) in 2013. In the ICD-11, gambling disorder was classified within the same super-category of disorders due to substance use or addictive behaviors. In the DSM-5, gaming disorder was placed in the Appendix as a condition requiring more research. There is abundant evidence on similarities between gambling disorder and SUDs regarding genetics, neurobiology, psychological processes, and effectiveness of psychological treatment (Goudriaan et al., 2014). In gambling disorder, a neurocognitive profile showing diminished executive functioning compared to healthy controls (e.g. diminished response inhibition, cognitive flexibility) was related to differential functioning of the DLPFC and anterior cingulate cortex (ACC), both part of the cognitive control circuitry (van Holst et al., 2010). Moreover, increased neural cue reactivity and associated self-reported craving are present in the striatum, orbitofrontal cortex and insular cortex in gambling disorder compared to healthy controls. Abnormal reward and loss processing is evidenced by differential orbitofrontal cortex (OFC) functioning in gamblers and is thought to underlie processes like persistence in gambling despite losses (Clark et al., 2018).
These abnormalities in cognitive-motivational behavioral and frontostriatal functioning in gambling disorder warrant the question of whether tES/TMS may be a promising add-on treatment for gambling disorder and other non-substance-related addictive behaviors. tES/TMS may normalize some of these endophenotypic markers, for instance by enhancing cognitive control through DLPFC stimulation. Neuromodulation like tDCS and rTMS have been shown to reduce craving in substance related disorders (Jansen et al., 2013), and changes in cognitive functioning, mostly indicating improvement of cognitive functioning in addictive disorders following tES/TMS (Schluter et al., 2018). Currently, a very limited number of tES/TMS pilot studies in gambling disorder are present. For instance, in a single session pilot study in nine problem gamblers, high frequency rTMS reduced desire to gamble, whereas cTBS reduced blood pressure, but had no effects on desire to play (Zack et al., 2016). In this same study no effects on impulsive behavior (delay discounting) and Stroop interference were evident. In a sham-controlled cross-over high-frequency rTMS study (left DLPFC), active rTMS diminished craving compared to sham rTMS (Gay et al., 2017). Yet in another trial, low-frequency rTMS over the right DLPFC had similar effects as sham stimulation on craving, though with a large placebo effect (Sauvaget et al., 2018). In a neuroimaging study, active tDCS changed GABA levels in the prefrontal cortex in problem gamblers, but glutamate levels were unaffected, as indicated by magnetic resonance spectroscopy (Dickler et al., 2018). Thus, from these few studies it appears that tES/rTMS has the ability to alter at least some of the working mechanisms that underlie pathological gambling.
As DLPFC targeted TMS has been shown to not only change DLPFC functioning, but also to lead to changes in frontostriatal connectivity in immediate single-session studies in addiction (Jansen et al., 2015), future studies could target the DLPFC in gambling disorder to target the relevant underlying neural circuitry. Rigorously conducted clinical trials are needed to investigate whether tES/TMS and what type of stimulation (e.g. DLPFC stimulation; insular stimulation; cTBS) has the potential to improve cognitive functioning, diminish craving, and/or reduce gambling behavior in disordered gambling.
7. Laterality of stimulation in the treatment of addictive disorders: left or right stimulation?
There is very little information available from empirical studies to help guide the selection of left or right-sided targets for neuromodulation approaches in SUD. Most studies with rTMS have applied excitability enhancing rTMS to the left DLPFC (following the pathway that was forged by depression researchers). In alcohol research, however, there has been a unique emphasis on stimulating the right DLPFC. In a previous meta-analysis, no laterality effect could be found for either right or left DLPFC stimulation, although there was a trend favoring right-sided DLPFC rTMS (Enokibara et al., 2016; Jansen et al., 2013). Recent reviews on the cognitive effects of tES/rTMS in addiction indicate positive effects of both right-sided and left-sided DLPFC stimulation (Naish et al., 2018; Schluter et al., 2018). Both left- and right-sided stimulation have been shown to enable positive effects on cognition and on craving in addictive disorders, these effects may be due to non-focal effects of rTMS. Indeed, lateralized rTMS has been shown to change bilateral brain activation patterns, for instance by activation of monosynaptic afferents in the contralateral hemisphere (Hanlon et al., 2013) and influencing bilateral resting-state functional connectivity of frontostriatal circuits (Schluter et al., 2017). Thus, the question on laterality in the treatment of addictive disorders should be put in a wider perspective, and be approached from a network perspective, where not only laterality, but also the target location is relevant.
8. tES/TMS dosage in the treatment of SUDs
Stimulation parameters, such as duration, number of stimulation sessions, stimulation frequency, intensity, target brain region, and interval between treatments, should be investigated to define the dose response of tES/TMS techniques. Few of these parameters have been systematically investigated for addiction treatment. The majority of brain stimulation studies have adopted protocols that modulate cortical excitability of key brain areas for addiction, such as DLPFC, demonstrating the potential efficacy in reducing drug craving and addictiverelated behaviors (Li et al., 2013a; Politi et al., 2008; Rapinesi et al., 2016; Terraneo et al., 2016). Acute multiple sessions of tDCS stimulation protocols, typically related to facilitative effects on cortical excitability (Nitsche and Paulus, 2000; Paulus, 2003), have been associated with a reduction of spontaneous (Batista et al., 2015; Klauss et al., 2018b), and cue-induced craving (Boggio et al., 2009; Fregni et al., 2008; Gorelick et al., 2014) when applied over the prefrontal cortex. However, there was no effect on spontaneous craving when stimulation sessions were extended (Klauss et al., 2018a). Similarly, rTMS protocols at high frequency (5–25 Hz) have typically been used to excite cortical neurons and elicit LTP-like effects. These methods have been used to reduce spontaneous and cue-induced craving. There are limited available studies with direct comparisons to date. Evidence from depression rTMS studies suggests that longer treatment duration and/or higher number of rTMS sessions could contribute to faster clinical improvement and better outcomes (Schulze et al., 2018). Future studies should focus on two main areas: (1) the personalization of the tES/TMS treatment, and (2) optimization of stimulation parameters, electrodes/coil size and shape, duration, and number of stimulations.
9. Preclinical and Pharmacologic insight into the mechanism of tES/TMS as a tool to decrease drug consumption
Preclinical models have certainly disentangled some of the cellular and molecular mechanisms by which tES/TMS exert their neurophysiological effects, as well as effects of multiple stimulation sessions on drug-related behaviors (Levy et al., 2007). As noted above (e.g. Chen et al., 2013; Levy et al., 2007), tES/TMS induce effects at a cellular level through different mechanisms including the modulation of glutamatergic receptors (Gersner et al., 2011) and neuronal excitability eliciting prolonged, offline after-effects similar to LTP and LTD (Cirillo et al., 2017; Diana et al., 2017; Nitsche and Paulus, 2000; Paulus, 2003). Seminal preclinical studies have extensively demonstrated that tES/TMS induced-LTP/LTD are strictly dependent on NMDA and AMPA receptor signalling (Cirillo et al., 2017; Diana et al., 2017) within glutamatergic synapses within addiction related brain regions (Argilli et al., 2008; Diana et al., 2017; Good et al., 2011).
The dependence on glutamatergic activity is supported by the suppression of tDCS induced effects on the primary motor cortex after NMDA-receptor blockade in humans (Liebetanz et al., 2002; Nitsche et al., 2003a). In addition to glutamatergic signalling, dopaminergic transmission is also likely to play a significant role in shaping some of the TMS-induced effects (Diana, 2011; Strafella et al., 2001). tES/TMS techniques could also exert their effects modulating the expression of neurotrophic factors, such as BDNF, an active regulator of synaptic plasticity, within cortical and subcortical areas (Cirillo et al., 2017; Custodio et al., 2018). More recently, non-synaptic events have been suggested as mediators of tES/TMS long-term effects, including plasticity-related gene expression, and neurogenesis (Spagnolo and Goldman, 2017; Strube et al., 2015; Zhang et al., 2007). Whether these mechanisms are involved in tES/TMS mediated effects in SUDs remains to be explored.
Animal models provide a powerful tool to map brain activation patterns after brain stimulation. The few neuroimaging studies available have suggested that tES/TMS induce neuronal activation both in cortical and subcortical areas (Hanlon et al., 2013; Moore et al., 2016; Uhlirova et al., 2016). Nevertheless, it is clear that understanding the effects of tES/TMS by using animal models is problematic due to technical limitations and different gyral patterns. Coils, electrode sizes, and stimulation power represent major issues preventing a direct comparison of specificity, accuracy, current density and electromagnetic field between human and animal studies (Gorelick et al., 2014; Jackson et al., 2016). Similarly, interspecies differences in anatomy, receptor, and neurotransmitters distributions should be taken into account when assessing tES/TMS neurobiological effects. Technical advances in preclinical studies for tES/TMS are needed in order to increase the focality of stimulation in specific brain areas. The large protocol variability and lack of standardization at present are detrimental and could contribute to the conflicting results reported in the literature. Therefore, future research should focus on the optimization of stimulation targets and stimulation parameters such as electrodes/coil size and shape, duration, and number of stimulation sessions. Interactions between brain states (e.g. anesthesia in animals) and stimulation parameters should be further studied in the context of addiction (e.g., being abstinent or using cocaine will also likely impact the effects of tES/TMS).
10. Biomarkers for treatment selection and monitoring
As with other neuropsychiatric disorders, there are currently no clinically useful biomarkers (a measurable indicator of some biological state or condition) for SUD. Absent such markers, it is impossible to predict an individual’s vulnerability to addiction, the severity of an individual’s current level of dependence, treatment effectiveness, or risk of relapse. A poor understanding of the addicted human brain and the complex actions of a drug on, and neuroplastic consequences to, various neural circuits and neurobiological mechanisms, contribute to the failure in developing more efficacious treatments as the field mostly relies on a symptom checklists and peripheral markers of exposure (e.g., urine drug screen). To move forward in this quest, many believe that a more proximal, brain-based measure of dependence is required. Furthermore, the delineation of potential subtypes of addiction, analogous to what was recently described in treating depression (Drysdale et al., 2017), may result in specialized treatments for unique endophenotypic subtypes.
Emerging evidence suggests that persistent drug use leads to a dysregulation of multiple cognitive constructs subserved by multiple neural circuits, networks, and neurotransmitter systems. As such, to effectively diagnose and treat individuals suffering from SUDs, rather than concentrating on any given brain region or transmitter as currently pursued in most medication discovery, a better understanding of how abused drugs affect the topological organization of brain connectivity networks may have greater strategic importance (Steele et al., 2019). A large-scale network measure of connectivity such as Participation Coefficient or Degree, measured noninvasively with fMRI and/or EEG from either (or both) resting-state and task-based connectivity measures reflects the overall functional properties of the brain and may provide a useful heuristic to explore the efficacy of SUD diagnosis and treatment interventions.
The trajectory of SUD development from impulsive to compulsive use is thought to be reflected in changes in various cognitive constructs and their underlying networks, including reward processing (Haber and Knutson, 2010), salience detection (SN; Seeley et al., 2007), executive control (ECN; Seeley et al., 2007), and internal ruminations (default mode network; DMN; Raichle, 2015). Moreover, SUD is not a static disease but rather is manifest by cycling between phases, including binge/intoxication (i.e. reward seeking), abstinence induced withdrawal (negative affect) and drug craving brain circuits and networks (For review, see; Koob and Volkow, 2016; Spronk et al., 2013; Steele et al., 2018a). The hypothesis of an imbalance between drive state and reward processing (so called ‘go-circuits’) and executive control (‘stop-circuits’) processes (Bechara, 2005; Bickel et al., 2007; Childress et al., 1999; Goldstein and Volkow, 2011; Hu et al., 2015; Volkow et al., 2016) is a manifestation of such dysregulation and has shown promise as a predictor of drug-related treatment outcomes (Adinoff et al., 2015; McHugh et al., 2017; Steele et al., 2014; 2018b).
Most measures of large-scale networks implement MRI based measures, but EEG measures of large-scale networks via phase synchrony holds tremendous potential due to its exquisite temporal resolution (e.g., Aviyente et al., 2017; Watts et al., 2018). Event-related potentials (ERPs) assessing cognitive functions have also successfully predicted SUD treatment outcomes (Fink et al., 2016; Marhe et al., 2013; Nakamura-Palacios et al., 2016; Steele et al., 2014). Biomarker development incorporating multimodal measures, rather than a single modality, and taking advantage of both the high spatial resolution of fMRI and high temporal resolution of EEG is likely to provide a more complete picture of both the cognitive functions and the underlying mechanisms dysregulated in SUD. Identification of biomarkers for both risk and protective factors and targets for treatment will likely uncover a proximal, brain-based measure of dependence essential for individualized medicine and curbing the significant societal impact of SUDs (Yavari et al., 2017).
Non-invasive brain stimulation has shown promise by targeting networks known to be dysregulated in SUDs (For review: Diana et al., 2017). In the quest toward individualized medicine, rich datasets that include several imaging modalities may help fractionate the SUD phenotype and identify the most appropriate treatment type, ‘dosage’ and duration for each individual (c.f., Drysdale et al., 2017). Neuromodulation interventions with focal manipulation of specific dysregulated networks associated with an individual’s SUD subtype (and potential comorbidity with other neuropsychiatric disorders) may provide a unique tool to target those networks most compromised in the individual.
11. Quality standards for designing and reporting clinical research using tES/TMS
11.1. Pre-registration of clinical trials and responsible reporting of “Big Data” projects
Good clinical practice states that all clinical trials should be pre-registered before a study is initiated (Moher et al., 2001). In fact, funding agencies such as the NIH require that a trial must be previously registered when submitting a grant request; the International Committee of Medical Journal Editors (ICMJE) (“ICMJE recommendations,” Accessed 27 Nov 2018) also requires, by policy, that the trial should only be considered for publication if it was registered at or before the time of the first subject enrollment. The ICMJE defines a clinical trial as any research project that prospectively assigns people or a group of people to an intervention, with or without concurrent comparison or control groups, to study the relationship between a health-related intervention and a health outcome. Health-related interventions are those used to modify a biomedical or health-related outcome; examples include drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, and process-of-care changes. Health outcomes are any biomedical or health-related measures obtained in patients or participants, including pharmacokinetic measures and adverse events. According to the NIH, a clinical trial is defined as studies (1) involving human participants; (2) in which participants are assigned prospectively to an intervention and the effects of this intervention are being assessed; (3) the effects are being assessed by a health-related biomedical or behavioral outcome. ClinicalTrials.gov or the WHO International Clinical Trials Registry Platform (ICTRP) are available for this registration process.
While we encourage all researchers with a traditional clinical trial to pre-register their studies, we simultaneously acknowledge that the NIH’s momentum towards data sharing and posting of raw data on biorepositories will provide a wealth of information that may benefit brain stimulation target identification studies in the future. In the field of depression, for example, Drysdale and colleagues recently published a highly influential article in which they aggregated over 1000 behavioral and resting state fMRI datasets that had been collected from patients with major depressive disorder. Through machine learning algorithms they demonstrated that there were 4 prominent “biotypes” (each containing a set of behavioral and brain imaging features) of individuals with depression and that individuals with disrupted activity in the dorsal medial prefrontal cortex happened to be the ones that responded best to rTMS at the dorsal medial prefrontal cortex (Drysdale et al., 2017). While a prospective study is still warranted, this investigation is an elegant demonstration of the manner in which big, shared data can be a common resource to the field. While this is not explicitly a clinical trial it has unique value. It is undeniable that similar studies will likely be attempted in SUD research. In the spirit of transparency, however, we encourage researchers doing this type of datadriven research to report their findings in a traditional publication or on a public database so that the research community is made aware of the results. By increasing the transparency of research and encouraging individuals to publish outcomes which are both consistent and inconsistent with their original hypotheses, we will hopefully help the field move forward in a more efficient manner.
11.2. Outcome measures/Phases of clinical trials
Outcome measures should be defined a priori, as well as the inferential statistical methods that will be used for the analysis of this data. Most recent clinical trials on tES/TMS for SUDs are Phase-II trials (or feasibility/preliminary trials). It is worth discussing that most of them evaluate clinical endpoints, such as craving assessment, substance consumption, and relapse rates (Coles et al., 2018). These outcomes are likely highly clinically relevant, but are likely not as illuminating regarding the biological mechanism of observed effects compared to studies which are able to measure changes in systemic physiology or neurobiology. Studies that are designed with electrophysiological techniques or functional imaging as primary outcomes (Li et al., 2013b; Pripfl et al., 2014) shed light on how stimulation techniques may modulate brain activity to improve symptoms such as craving assessment, substance consumption, and relapse rates. While we realize that these techniques are often very expensive and not available to laboratories in developing nations (in which SUDs may be just as prevalent as in nations with ready access to these techniques), we encourage investigators to collaborate across borders and strive to include a biomarker of treatment efficacy in their experimental design. These studies can also provide important data on mechanistic effects of stimulation on addiction and withdrawal to test and improve existing protocols, as well as to design novel interventions. In this context, the aims of the investigation need to be well defined and surrogate or clinical outcomes need to be pre-specified.
11.3. Sample size calculation and power/effect size - target engagement studies versus clinical efficacy studies
In 2015 the National Institute of Mental Health introduced a new experimental medicine initiative that provided a framework for innovative treatment development. It is now the backbone for many NIH-sponsored neuromodulation treatment grants (Insel, 2015; Krystal et al., 2018). Briefly, the initiative introduced a concept of early “target engagement” studies, which are designed to demonstrate that an intervention can modulate a pre-specified biological or behavioral target. These studies are then followed by a longer period of “clinical efficacy” evaluation. Although this language and framework is not yet required at all NIH institutes, we encourage individuals in the SUD field to use this framework for their studies.
With this framework in mind, careful planning should be under-taken when calculating sample size as this population can be challenging when considering recruitment and adherence. In particular, clinical efficacy studies with multiple-session stimulation protocols and long-term follow-up periods can be problematic, with drop-out rates as high as 89% following 6 months of follow-up (Ceccanti et al., 2015). The researcher must also consider that single-session target engagement studies usually do not have problems with adherence, but effect sizes are often smaller and less meaningful when assessing clinical impact in longer-term studies. A recent review of brain stimulation methods to treat SUDs (Coles et al., 2018) showed that the effect-size of tDCS can vary from Cohen’s d 0.1 to 4.45, depending on the number of stimulation sessions and the substance of abuse, while the effect-size of rTMS varies from Cohen’s d 0.05 to 4.42. Therefore, the researcher must find the balance between two extremes: a study with a large sample size and multiple sessions can become unfeasible due to drop-outs, while a small sample study and insufficient sessions can easily be underpowered. The investigator should generate realistic estimates of the effect size and run different scenarios for power calculation (sensitivity analysis).
11.4. Randomization and blinding
Randomization and allocation concealment are important in the development and design of clinical trials, as blinding protects the internal validity of the study. Although balancing of covariates is possible using different techniques, such as several types of regression analyses, it is necessary to first know these covariates and then correctly collect this data. Randomization, on the other hand, allows not only for balancing of known covariates, but also for unknown ones if the sample size is large enough. In addition, “block randomization,” can help with avoiding imbalance of known participant characteristics (e.g. sex) across conditions.
Blinding has been an important element of debate in the field of tES/TMS, as it depends not only on the technique used (e.g., TMS vs. tDCS) but also on the parameters of stimulation. For instance, a longitudinal trial with parallel design and multiple sessions of tDCS is capable of providing blinding in the same way as a placebo drug (Brunoni et al., 2013). Cross-over designs should be avoided especially when stimulation intensity is equal to or greater than 2 mA due to the risk of unblinding (O’Connell et al., 2012). Papers should not only report blinding procedures, but also blinding-success rates (see Section 2.5) (Gorelick et al., 2014). Importantly, success of blinding procedures should be measured and reported both for participants and study personnel in double-blind studies.
Strategies to mimic the skin sensation of tES/TMS should be pursued. Skin-redness has been more often reported for active than sham-tDCS, but that observation was not correlated with prediction of the treatment effects (Brunoni et al., 2014). Furthermore, pre-treating the scalp where the tDCS electrodes will be placed with topical anesthetic solutions has been attempted. Non-steroidal anti-inflammatory drugs such as ketoprofen can reduce stimulation-induced erythema (Guarienti et al., 2015) and topical lidocaine can reduce the overall skin sensation related to tDCS stimulation. However, these agents are not advisable since there are concerns about systemic absorption from the skin. Objective outcomes (e.g., biomarkers, neurobiologic indicators) that are not as vulnerable to placebo effects are an option. In addition, a run-in phase to exclude high placebo responders can be used to reduce placebo effect (Fregni et al., 2010). A recent systematic review and meta-analysis found that sham tES (a-tDCS, c-tDCS, tACS, tRNS and tPCS) does not result in statistically significant differences in corticospinal excitability (Dissanayaka et al., 2018), meaning that control groups involving sham procedures are reliable from a neurophysiological standpoint in tES studies.
11.5. Reporting clinical context
Clinical characteristics of the sample can affect results of studies and therefore should be considered and reported carefully. As populations with SUDs are highly variable, it is important to report enough details of the clinical characteristics in order to determine generalizability. Substance use history as well as inclusion and exclusion criteria, should be clearly defined. One future research modification is to enroll subjects based on the specific behavior rather than signs and symptoms defining SUD. An effort towards this end is to use the Research Domain Criteria (RDoC) (Insel et al., 2010) for recruitment. For instance, a study may include subjects with “drug craving disorder” that includes alcohol craving, smoking craving, and methamphetamine craving, in order to target a specific neural circuit associated with craving behavior rather than a specific drug. The RDoC initiative, launched by the National Institute of Mental Health can play an important role in this process, changing how researchers currently design mental disorders trials, including addiction.
It is also important to have a good characterization of the study population for comparison purposes and meta-analyses. Clinical trials are usually designed based on relevant preliminary data and often cite related systematic reviews to justify the new study. Reliable interpretation of results of clinical trials requires a discussion in the context of findings from similar research such as up-to-date systematic reviews. Therefore, studies should reference the preliminary data in order to build knowledge based on tES/TMS (Clarke et al., 2007).
11.6. Reporting tES specifications
tES specifications must be based on biological reasoning with a predefined hypothesis on how these interventions will affect addiction. Reproducibility of the results is paramount and there must be a clear description of the protocol used for the interventions (Brunoni et al., 2012). Montages with anode and cathode positions (including number of respective electrodes) (Nasseri et al., 2015), how this montage was determined (measurements, neuro-navigation), size and shape of electrodes (rectangular, circular or EEG type), current intensity, duration, number of sessions and interval between sessions (Thibaut et al., 2017) are extremely important parameters that need to be made explicit (Pinto et al., 2018). For tACS, a particularly vexing problem for the field has been the confusion between peak-to-peak and true (baseline-to-peak) amplitude. Detailed definitions of waveform parameters, ideally combined with a plot of the waveform, are recommended. Allowed and prohibited concomitant interventions should be clearly defined (Paulus, 2011). Similarly, the duration of active stimulation and the process for ramping up and down the stimulation used in sham studies should be clearly defined.
11.7. Reporting TMS specifications
TMS can be used for stimulation or neurophysiological assessment. For stimulation, specifications must also be based on a predefined hypothesis, with clear definition of location; coil shape (H-coil, figure-8 coil) (Coles et al., 2018); frequency (high vs low-frequency stimulation and/or patterned stimulation, if present); number of pulses (and train of pulses in case of high-frequency); duration of the stimulation session; intensity (if defined as a percentage of the motor threshold or a percentage of the output of the machine); and number of sessions (Schluter et al., 2018). It should be clearly defined how the target location was found and whether or not neuronavigation was used. Training of TMS administrators (staff administering interventions) should be specified (either as referenced to a protocol or using the supplementary data). Researchers carrying out TMS protocols should be rigorously trained in the technique to ensure the quality of the technical procedures, guaranteeing subject safety and care, and maximizing the possible benefits for patients (Lefaucheur et al., 2014). For TMS assessments, cortical excitability can be measured by single or paired-pulsed techniques. Specifications must include a detailed description of how measurements were done, such as: motor evoked potential (MEP); resting motor threshold (MT); short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF). Blinding procedures and methods should be clearly defined for TMS studies (sham coil, coil tilted, metal plate under coil) (Gorelick et al., 2014).
12. Safety
The safety of tES and TMS has been exhaustively reviewed, including some consideration of use in the treatment of addiction. TMS safety guidelines are largely based around minimizing risk of seizures (Rossi et al., 2009). There is currently no direct evidence for increased risk of serious or non-serious adverse events from TMS or tES in the treatment of addiction that are casually linked to stimulation. Further, there is no evidence that standard TMS safety guidelines need to be specifically adjusted in addiction treatment. Nonetheless, increased vigilance is always warranted when theoretical concerns exist or in specific patient subgroups with limited prior data. Any factor that independently increases the risk of a seizure (e.g. cocaine use, alcohol withdrawal, benzodiazepine/barbiturate use/withdrawal in opioid use, tramadol use, bupropion in nicotine treatment) can, in theory, increase brain sensitivity to TMS induced seizures.
Similarly, there is no evidence that best practices for tES research (Antal et al., 2017; Bikson et al., 2016, 2018b; Woods et al., 2016b) need to be adjusted in addiction treatment. tES is not associated with severe adverse events, however, skin irritation can be produced when standard protocols are not followed (Bikson et al., 2016). Any risk factors for tES/TMS that applies to the general population (e.g. presence of implants, skull defects) would similarly apply in addiction treatment.
For investigational treatments, risk/benefit analysis is always specific to each human trial along with any associated regulatory restrictions. In order to evaluate if there is eventually a need to propose
specific safety TMS or tES guidelines for addiction treatment, studies should carefully report their methods, safety monitoring measures, and side effects assessments. For instance, patient’s selection criteria (e.g., use status such as if they are recently abstinent and for how long or current user) will provide information on potential status or sub-groups that may be more hazardous to deliver TMS or tES. Similarly, assessment of adverse events specific to addiction treatment may be included (e.g., craving level) which will inform on whether these should become part of safety TMS or tES guidelines. In regards to safety monitoring, if there is increased risk, one should consider in adding safety monitoring measures (e.g. urinary samples, blood alcohol levels) to identify if a patient is currently using a substance and potentially not reporting the substance use. Finally, there is great interest in treating patients with addiction as early as possible in their disorders. Thus, safety guidelines in regards to teenagers or children should be carefully respected when delivering TMS or tES in younger populations with addiction.
13. Advancements in tES/TMS technologies
Non-invasive brain stimulation modulates neuronal activity through synergistic interaction with endogenous activity and thus the response to stimulation is state-dependent (Alagapan et al., 2016; Schmidt et al., 2014). The response to stimulation varies from person to person and from moment to moment. Likely, this explains the substantial heterogeneity of findings in this field. One promising way to address this challenge is to refine, adapt, and individualize stimulation such that the applied perturbation is modulated (ideally in real-time) to maximize the desired effect on neuronal network dynamics (Thut et al., 2017). Examples of this feedback approach includes stimulation at specific phases of an ongoing oscillation, stimulation in response to the detection of a specific waveform in the EEG, and stimulation as a function of a non-neuronal physiological variable.
These new approaches bring a new set of challenges for research studies. First, feedback control requires measurement of brain activity combined with application of stimulation. However, since both recording and stimulation are based on electromagnetic fields, tracking brain activity during stimulation is difficult due to interference leading to stimulation artifacts that are orders of magnitude larger than the recorded brain signals. New algorithms for stimulation artifact removal and stimulation approaches that do not suffer from this cross-contamination are under active development. In addition, there are unresolved questions about how to choose the appropriate control conditions and how to analyze responses when a research participant has received a unique stimulation pattern that depended on ongoing activity fluctuations. In addition, development of the next generation non-invasive stimulation technology includes efforts to deliver higher field strength with higher spatial precision to deeper brain structures (Grossman et al., 2017; Vöröslakos et al., 2018). This can lead to automated closed loop stimulation, on the condition that neural correlates are developed that represent the craving or other symptoms one attempts to treat.
14. Multisite RCTs for addiction medicine: hopes and challenges
A comprehensive literature search was conducted on PubMed (without time limits). We used similar terms to those presented in Section 2, but with the addition of ‘multisite’ and its derivates (e.g., ‘multicenter, ‘multi-site’, “multi-site”, etc.). This search did not return any articles, and to the best of our knowledge there are no currently published multisite RCTs that used neuromodulation for the treatment of addiction. However, several such studies are registered at ClinicalTrials.gov, all conducted in adults (18–22 to 65–70 years old participants) from both sexes.
The most advanced one (NCT02126124; estimated to complete recruitment on February 2019) is evaluating deep TMS (dTMS) as an aid to smoking cessation based on a previous double blind sham-controlled study (Dinur-Klein et al., 2014b). In this study, a total of 224 participants in 18 sites received either sham or real stimulation (10 Hz, 120% RMT, 1800 pulses per daily session) over the prefrontal and insular cortices for 3 weeks, with maintenance once a week for an additional 3 weeks and a follow-up after 4 months. The primary objective of the study is to compare the four-week continuous quit rate between the active and sham treatment groups. Brain imaging data and behavioral assessments are collected before TMS, and after 10 days of TMS, 1 month follow up, and 2 months follow up.
In another study (NCT03576781; estimated to end on December 2019), 78 opioid dependent individuals and healthy controls are randomized to receive either one placebo-like TMS treatment, or one of two real TMS treatments aimed to increase activity in the DLPFC with iTBS (600 pulses, 110% RMT) or decrease activity in the MPFC with cTBS (600 pulses, 110% RMT). The study measures changes in pain (using MRI-based thermal pain paradigm) and craving, and the results will be used to design a larger clinical trial of rTMS as an innovative, new treatment option for pain in opioid dependent individuals.
Finally, another study (NCT03333460; estimated to end on December 2020) evaluates whether rTMS can reduce cocaine craving and use, and affect mood, behavioral, and cognitive alterations associated with prolonged cocaine use. In this study, a total of 80 participants receive either sham or real rTMS (15 Hz, 100% RMT) over the left DLPFC for two weeks (twice a day), with a maintenance intervention (twice a week for 3 months) and a follow-up of additional 3 months. The primary objective of the study is to compare changes to cocaine craving and consumption, as indicated by self-reports and urine drug screen, and it also includes Pre- and Post-treatment fMRI scans while drug-related images are presented.
The challenges of designing and conducting multisite neuromodulation studies for addiction treatment are not fundamentally different from those conducted for depression (e.g., Levkovitz et al., 2015; O’Reardon et al., 2007) or of addiction treatment studies in which other interventions (e.g. pharmacotherapies) have been used (e.g., Falk et al., 2018). The challenge of bringing in the participants every day for treatment (rather than taking a medication at home and coming only for evaluations and follow-ups) is greater, and therefore only subjects highly motivated to adhere to the study should be recruited. Home use tES devices might be a solution for this limitation (Shaw et al., 2017). Clearly, large controlled multicenter studies that allow validation and generalization of positive preliminary studies are still lacking in this field to establish neuromodulation as a validated tool in the treatment of addiction.
Conclusion
The overarching goal of INTAM is to determine the extent to which tES/TMS will improve the degree and extent of recovery from SUDs. Research to date has tested the effect of tES/TMS on clinical outcomes (e.g., substance use) as well as established mechanisms of change (e.g., craving). Despite these efforts, consensus has been difficult to determine due to the variability in methodology across tES/TMS studies.
Moving forward, the critical mass of expertise through INTAM and utilization of a shared matrix of research questions and protocols will maximize the likelihood of recruiting large clinical cohorts that reflect the heterogeneity of addiction, further facilitating fully powered statistical tests of the effects of tES/TMS on clinical outcomes and mechanisms of change. A shared matrix of research questions and outcome measures for the biomarkers, mechanisms and clinical outcomes of addiction will form the basis of INTAM. In this context, it will also be critical to define the clinical population (e.g., substance, disorder severity, age, comorbidities) and treatment setting (e.g., detoxification, relapse prevention).
Pre-registration of study protocols and primary and secondary outcomes is encouraged to promote transparency. Shared protocols will enable higher rigor and reproducibility. Protocols should ideally contain (1) a detailed description of materials and equipment, (2) step-by-step administration instructions with accompanying video examples, where appropriate, (3) information on troubleshooting strategies, and (4) guides for data processing, analysis, and interpretation. These protocols could then be used to establish multicenter trials. Finally, the use of an online data and registration platform will facilitate comparative and integrative analysis.
The establishment of shared research questions, protocols and data repository does not come without its challenges. Both within and across cultures, there exist different norms and ideas about what constitutes a clinically significant change. For instance, not all cultures emphasize ‘abstinence only’ in treatment, necessitating the ongoing awareness and open discussion of these types of assumptions. Further, a risk in prioritizing well-established procedures and findings is the reduction in acknowledgment of innovative approaches and newly formed theories. This is particularly critical for a field in its early stages, such as tES/TMS for addiction.
Therefore, it will be important for INTAM to balance standardization with the embrace of a flexible approach and concentrated effort to regularly integrate and update novel findings into the shared protocols and a data repository. Although the role of tES/TMS in future daily clinical practice in addiction medicine is unclear, we hope this collaborative effort will lead the research community to high quality empirical research on these techniques such that clinical efficacy can be established. Through larger, sham-controlled studies with more uniform reporting standards in tES/TMS research, we will be better prepared to deliver something meaningful to our patients. Finally, we acknowledge that SUD is a uniquely intransigent condition which will likely not be solved by a single brain stimulation intervention. By performing rigorous studies in tES and TMS however, we will be able to confidently approach the next frontier of experimental medicine in SUD - combining targeted brain stimulation with pharmacotherapy and behavioral management in order to optimize treatment efficacy for a given individual.
Supplementary Material
Funding
Xavier Noël is supported by a grant of the Belgian Fund for Scientific Research (grant PDR/OL T.0146.18). Anna E. Goudriaan and Renée S. Schluter are funded by an innovative VIDI grant awarded to AG (grant number 91713354) by the Netherlands Health Research Organization (ZonMW). Elliot Stein and Vaughn Steele are supported by the Intramural Research Program of the National Institute on Drug Abuse (NIDA), National Institutes of Health, Baltimore, Maryland and The Center on Compulsive Behavior. Antonio Verdejo-García is supported by Australian Medical Research Future Fund (MRF1141214). Laurie Zawertailo received funding from Canadian Institutes of Health Research, Canadian Cancer Society, Ontario Ministry of Health, and Global Research Awards in Nicotine Dependence. Flavio Frohlich received funding from NIH, Bran and Behavior Research Foundation, Human Frontiers Science Program, Tal Medical, and the Neurocare Group. Shirley Fecteau is supported by the Canada Research Chair in Cognitive Neuroplasticity. Stacey Daughters is supported by the NIH/NIDA (R01DA026424). Michael A. Nitsche receives funding from the German Research Foundation (DFG; SFB 1280 Project A6), and the German Federal Ministry of Education and Research (BMBF, GCBS grant 01EE1403C). Coleen A. Hanlon received funding from the NIDA (R01DA04447) and NIAAA (P50AA010761). Vincent Van Waes is supported by the Fondation pour la Recherche en Alcoologie (FRA) and the Région Bourgogne Franche-Comté. Ganesan Venkatasubramanian is supported by the Swarnajayanti Fellowship Grant (DST/SJF/LSA-02/2014–15) by the Department of Science and Technology, Government of India. Michael J. Wesley receives funding from the NIDA (K01DA043652; R01DA045023 and R01DA047368).
Footnotes
Declaration of Competing Interest
Flavio Frohlich is the founder, majority owner, and Chief Scientific Officer of Pulvinar Neuro LLC. Michael Nitsche is a member of the Scientific Advisory Board of Neuroelectrics. Colleen A. Hanlon has served as a consultant for Brain Research and Development Services (Brainsway). Other authors reported no conflict of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.neubiorev.2019.06.007.
References
- Addolorato G, Antonelli M, Cocciolillo F, Vassallo GA, Tarli C, Sestito L, … Di Giuda D, 2017. Deep transcranial magnetic stimulation of the dorsolateral prefrontal cortex in alcohol use disorder patients: effects on dopamine transporter availability and alcohol intake. Eur. Neuropsychopharmacol 27 (5), 450–461. 10.1016/j.euroneuro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Gu H, Merrick C, McHugh M, Jeon-Slaughter H, Lu H, … Stein EA, 2015. Basal hippocampal activity and its functional connectivity predicts cocaine relapse. Biol. Psychiatry 78 (7), 496–504. 10.1016/j.biopsych.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Mellin JM, Alagapan S, Alexander ML, Gilmore JH, Jarskog LF, Frohlich F, 2019a. Targeting reduced neural oscillations in patients with schizophrenia by transcranial alternating current stimulation. Neuroimage 186, 126–136. 10.1016/j.neuroimage.2018.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Prim JH, Alexander ML, McCulloch KL, Frohlich F, 2019b. Identifying and engaging neuronal oscillations by transcranial alternating current stimulation in patients with chronic low back pain: a randomized, crossover, double-blind, sham-controlled pilot study. J. Pain 20 (3). 10.1016/j.jpain.2018.09.004.277.e271-277.e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagapan S, Schmidt SL, Lefebvre J, Hadar E, Shin HW, Frӧhlich F, 2016. Modulation of cortical oscillations by low-frequency direct cortical stimulation is state-dependent. PLoS Biol. 14 (3), e1002424 10.1371/journal.pbio.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MM, Sellers KK, Frohlich F, 2013. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J. Neurosci 33 (27), 11262–11275. 10.1523/jneurosci.5867-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrus GG, Al-Moyed H, Chaieb L, Sarp L, Antal A, Paulus W, 2012. The fade-in - Short stimulation - Fade out approach to sham tDCS - Reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimul. 5 (4), 499–504. 10.1016/j.brs.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A, 2009. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 104 (4), 653–660. 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- Antal A, Alekseichuk I, Bikson M, Brockmoller J, Brunoni AR, Chen R, … Paulus W, 2017. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol 128 (9), 1774–1809. 10.1016/j.clinph.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W, 2008. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 1 (2), 97–105. 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P, 1995. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol. Clin. Exp. Res 19 (1), 92–99. [DOI] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A, 2008. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J. Neurosci 28 (37), 9092–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviyente S, Tootell A, Bernat EM, 2017. Time-frequency phase-synchrony approaches with ERPs. Int. J. Psychophysiol 111, 88–97. 10.1016/j.ijpsycho.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Baker TE, Lesperance P, Tucholka A, Potvin S, Larcher K, Zhang Y, … Conrod P, 2017. Reversing the atypical valuation of drug and nondrug rewards in smokers using multimodal neuroimaging. Biol. Psychiatry 82 (11), 819–827. 10.1016/j.biopsych.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Bari A, DiCesare J, Babayan D, Runcie M, Sparks H, Wilson B, 2018. Neuromodulation for substance addiction in human subjects: a review. Neurosci. Biobehav. Rev 95, 33–43. 10.1016/j.neubiorev.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM, 2015. A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int. J. Neuropsychopharmacol 18 (12) pyv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA, 2013. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J. Physiol 591 (7), 1987–2000. 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsikadze G, Paulus W, Hasan A, Grundey J, Kuo MF, Nitsche MA, 2017. Compromised neuroplasticity in cigarette smokers under nicotine withdrawal is restituted by the nicotinic alpha4beta2-receptor partial agonist varenicline. Sci. Rep 7 (1), 1387 10.1038/s41598-017-01428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, 2005. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci 8, 1458 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR, 1997. Deciding advantageously before knowing the advantageous strategy. Science 275 (5304), 1293–1295. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ, 2008. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos. Trans. Biol. Sci 363 (1507), 3257–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, 2015. Discounting of delayed rewards as an endophenotype. Biol. Psychiatry 77 (10), 846–847. 10.1016/j.biopsych.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA, 2007. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 90, S85–S91. 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Brunoni AR, Charvet LE, Clark VP, Cohen LG, Deng ZD, … Lisanby SH, 2018a. Rigor and reproducibility in research with transcranial electrical stimulation: an NIMH-sponsored workshop. Brain Stimul. 11 (3), 465–480. 10.1016/j.brs.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, … Woods AJ, 2016. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 9 (5), 641–661. 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Paneri B, Mourdoukoutas A, Esmaeilpour Z, Badran BW, Azzam R, … Tyler WJ, 2018b. Limited output transcranial electrical stimulation (LOTES-2017): engineering principles, regulatory statutes, and industry standards for wellness, over-the-counter, or prescription devices with low risk. Brain Stimul. 11 (1), 134–157. 10.1016/j.brs.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ, Redfearn JWT, 1964. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol 172 (3), 369–382. 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F, 2009. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci. Lett 463 (1), 82–86. 10.1016/j.neulet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, … Fregni F, 2008. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend. 92 (1–3), 55–60. 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F, 2010. Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend. 112 (3), 220–225. 10.1016/j.drugalcdep.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA, 1995. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol. Clin. Exp. Res 19 (3), 600–606. [DOI] [PubMed] [Google Scholar]
- Bolloni C, Panella R, Pedetti M, Frascella AG, Gambelunghe C, Piccoli T, … Diana M, 2016. ). Bilateral transcranial magnetic stimulation of the prefrontal cortex reduces cocaine intake: a pilot study. Front. Psychiatry 7 10.3389/fpsyt.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borckardt JJ, Reeves ST, Milliken C, Carter B, Epperson TI, Gunselman RJ, … George MS, 2017. Prefrontal versus motor cortex transcranial direct current stimulation (tDCS) effects on post-surgical opioid use. Brain Stimul. 10 (6), 1096–1101. 10.1016/j.brs.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borckardt JJ, Reeves ST, Robinson SM, May JT, Epperson TI, Gunselman RJ, … George MS, 2013. Transcranial direct current stimulation (tDCS) reduces postsurgical opioid consumption in total knee arthroplasty (TKA). Clin. J. Pain 29 (11), 925–928. 10.1097/AJP.0b013e31827e32be. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, … Fregni F, 2012. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 5 (3), 175–195. 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Schestatsky P, Lotufo PA, Bensenor IM, Fregni F, 2014. Comparison of blinding effectiveness between sham tDCS and placebo sertraline in a 6-week major depression randomized clinical trial. Clin. Neurophysiol 125 (2), 298–305. 10.1016/j.clinph.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, … Fregni F, 2013. The sertraline vs. Electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 70 (4), 383–391. 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martínez-Raga J, Alonso-Alonso M, Shih M-C, Pascual-Leone A, 2007. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 86 (1), 91–94. 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cárdenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schönfeldt-Lecuona C, 2010. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 22 (4), 294–306. 10.1007/s10548-009-0084-7. [DOI] [PubMed] [Google Scholar]
- Ceccanti M, Inghilleri M, Attilia ML, Raccah R, Fiore M, Zangen A, 2015. Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation. A pilot study. Can. J. Physiol. Pharmacol 93 (4), 283–290. 10.1139/cjpp-2014-0188. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou C, Wu B, Wang Y, Li Q, Wei Y, … Zou D, 2013. Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res. 210 (3), 1260–1264. [DOI] [PubMed] [Google Scholar]
- Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA, 2015. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front. Hum. Neurosci 9 (303). 10.3389/fnhum.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’brien CP, 1999. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry 156 (1), 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, … Di Lazzaro V, 2017. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 10 (1), 1–18. [DOI] [PubMed] [Google Scholar]
- Clark L, Boileau I, Zack M, 2018. Neuroimaging of reward mechanisms in Gambling disorder: an integrative review. Mol. Psychiatry 10.1038/s41380-018-0230-2. [DOI] [PubMed] [Google Scholar]
- Clarke M, Hopewell S, Chalmers I, 2007. Reports of clinical trials should begin and end with up-to-date systematic reviews of other relevant evidence: a status report. J. R. Soc. Med 100 (4), 187–190. 10.1177/014107680710011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles AS, Kozak K, George TP, 2018. A review of brain stimulation methods to treat substance use disorders. Am. J. Addict 27 (2), 71–91. 10.1111/ajad.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde V, Tomasevic L, Akopian I, Stanek K, Saturnino GB, Thielscher A, … Siebner HR, 2019. The non-transcranial TMS-evoked potential is an inherent source of ambiguity in TMS-EEG studies. Neuroimage 185, 300–312. 10.1016/j.neuroimage.2018.10.052. [DOI] [PubMed] [Google Scholar]
- Conti CL, Moscon JA, Fregni F, Nitsche MA, Nakamura-Palacios EM, 2014. Cognitive related electrophysiological changes induced by non-invasive cortical electrical stimulation in crack-cocaine addiction. Int. J. Neuropsychopharmacol 17 (9), 1465–1475. 10.1017/S1461145714000522. [DOI] [PubMed] [Google Scholar]
- Conti CL, Nakamura-Palacios EM, 2014. Bilateral transcranial direct current stimulation over dorsolateral prefrontal cortex changes the drug-cued reactivity in the anterior cingulate cortex of crack-cocaine addicts. Brain Stimul. 7 (1), 130–132. 10.1016/j.brs.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJO, Ray LA, 2016. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict. Biol 21 (1), 3–22. 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG, 2001. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res 3 (1), 7–16. 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Custodio JCDS, Martins CW, Lugon MDMV, Rodrigues LM, Figueiredo SG, Nakamura-Palacios EM, 2018. Prefrontal BDNF levels after anodal epidural direct current stimulation in rats. Front. Pharmacol 9, 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F, … Nakamura-Palacios EM, 2013. Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J. Physiol. Paris 107 (6), 493–502. 10.1016/j.jphysparis.2013.07.003. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Truong DQ, DosSantos MF, Toback RL, Datta A, Bikson M, 2015. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front. Neuroanat 9, 89 10.3389/fnana.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M, 2009. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2 (4), 201–207. 10.1016/j.brs.2009.03.005.207.e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Ramos R, Taiar I, Trevizol AP, Shiozawa P, Cordeiro Q, 2016. Effect of a ten-day prefrontal transcranial direct current stimulation protocol for crack craving: a proof-of-concept trial. J. ECT 32 (3), e8–e9. [DOI] [PubMed] [Google Scholar]
- Del Felice A, Bellamoli E, Formaggio E, Manganotti P, Masiero S, Cuoghi G, … Serpelloni G, 2016. Neurophysiological, psychological and behavioural correlates of rTMS treatment in alcohol dependence. Drug Alcohol Depend. 158, 147–153. 10.1016/j.drugalcdep.2015.11.018. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Rinck M, Lindenmeyer J, Wiers RW, 2017. A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict. Biol 22 (6), 1632–1640. 10.1111/adb.12463. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Wiers RW, 2015. Transcranial direct current stimulation, implicit alcohol associations and craving. Biol. Psychol 105, 37–42. 10.1016/j.biopsycho.2014.12.004. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Wiers RW, 2016. Electrophysiological and behavioral effects of combined transcranial direct current stimulation and alcohol approach Bias retraining in hazardous drinkers. Alcohol. Clin. Exp. Res 40 (10), 2124–2133. 10.1111/acer.13171. [DOI] [PubMed] [Google Scholar]
- Diana M, 2011. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry 2, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A, 2017. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat. Rev. Neurosci 18 (11), 685. [DOI] [PubMed] [Google Scholar]
- Dickler M, Lenglos C, Renauld E, Ferland F, Edden RA, Leblond J, Fecteau S, 2018. Online effects of transcranial direct current stimulation on prefrontal metabolites in gambling disorder. Neuropharmacology 131, 51–57. 10.1016/j.neuropharm.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Dieler AC, Dresler T, Joachim K, Deckert J, Herrmann MJ, Fallgatter AJ, 2014. Can intermittent theta burst stimulation as add-on to psychotherapy improve nicotine abstinence? Results from a pilot study. Eur. Addict. Res 20 (5), 248–253. 10.1159/000357941. [DOI] [PubMed] [Google Scholar]
- Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A, 2014a. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76 (9), 742–749. 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A, 2014b. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76 (9), 742–749. 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Dissanayaka TD, Zoghi M, Farrell M, Egan GF, Jaberzadeh S, 2018. Sham transcranial electrical stimulation and its effects on corticospinal excitability: a systematic review and meta-analysis. Rev. Neurosci 29 (2), 223–232. 10.1515/revneuro-2017-0026. [DOI] [PubMed] [Google Scholar]
- Doeltgen SH, Ridding MC, 2011. Low-intensity, short-interval theta burst stimulation modulates excitatory but not inhibitory motor networks. Clin. Neurophysiol 122 (7), 1411–1416. 10.1016/j.clinph.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Dominguez-Salas S, Diaz-Batanero C, Lozano-Rojas OM, Verdejo-Garcia A, 2016. Impact of general cognition and executive function deficits on addiction treatment outcomes: systematic review and discussion of neurocognitive pathways. Neurosci. Biobehav. Rev 71, 772–801. 10.1016/j.neubiorev.2016.09.030. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, … Wells EA, 2012. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction 107 (4), 694–708. 10.1111/j.1360-0443.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, … Liston C, 2017. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med 23 (1), 28–38. 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duecker F, Sack AT, 2015. Rethinking the role of sham TMS. Front. Psychol 6 (210). 10.3389/fpsyg.2015.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M, 2013. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage 74, 266–275. 10.1016/j.neuroimage.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, Hajak G, 2003. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J. Clin. Psychiatry 64 (8), 951–953. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H, Nasseri P, Yavari F, Mokri A, Monterosso J, 2016. Neuroscience of drug craving for addiction medicine: from circuits to therapies. Prog. Brain Res 223, 115–141. 10.1016/bs.pbr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Ekhtiari H, Paulus M, 2016. Preface: neuroscience for addiction medicine: from prevention to rehabilitation. Prog. Brain Res 224, xxv–xxvi. 10.1016/s0079-6123(16)00030-3. [DOI] [PubMed] [Google Scholar]
- Enkavi AZ, Eisenberg IW, Bissett PG, Mazza GL, MacKinnon DP, Marsch LA, Poldrack RA, 2019. Large-scale analysis of test-retest reliabilities of self-regulation measures. Proc. Natl. Acad. Sci. U. S. A 10.1073/pnas.1818430116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokibara M, Trevizol A, Shiozawa P, Cordeiro Q, 2016. Establishing an effective TMS protocol for craving in substance addiction: Is it possible? Am. J. Addict 25 (1), 28–30. 10.1111/ajad.12309. [DOI] [PubMed] [Google Scholar]
- Esmaeilpour Z, Marangolo P, Hampstead BM, Bestmann S, Galletta E, Knotkova H, Bikson M, 2018. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul. 11 (2), 310–321. 10.1016/j.brs.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Bernardo L, Ashare RL, Hamilton R, Faseyitan O, McKee SA, … Lerman C, 2016. Transcranial direct current brain stimulation increases ability to resist smoking. Brain Stimul. 9 (2), 191–196. 10.1016/j.brs.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Ryan ML, Fertig JB, Devine EG, Cruz R, Brown ES, Mendelson J, 2018. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol. Clin. Exp. Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D, Pascual-Leone A, 2014. Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend. 140, 78–84. 10.1016/j.drugalcdep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink BC, Steele VR, Maurer MJ, Fede SJ, Calhoun VD, Kiehl KA, 2016. Brain potentials predict substance abuse treatment completion in a prison sample. Brain Behav. 6 (8), e00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM, 1999. Psychometric properties of the penn alcohol craving scale. Alcohol. Clin. Exp. Res 23 (8), 1289–1295. [PubMed] [Google Scholar]
- Fonteneau C, Mondino M, Arns M, Baeken C, Bikson M, Brunoni AR, … Brunelin J, 2019. Sham tDCS: a hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul. 10.1016/j.brs.2018.12.977. [DOI] [PubMed] [Google Scholar]
- Franken IH, Hendriksa VM, van den Brink W, 2002. Initial validation of two opiate craving questionnaires the obsessive compulsive drug use scale and the desires for drug questionnaire. Addict. Behav 27 (5), 675–685. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Otachi P, Thut G, Rigonatti SP, … Valente K, 2006. Homeostatic effects of plasma valproate levels on corticospinal excitability changes induced by 1Hz rTMS in patients with juvenile myoclonic epilepsy. Clin. Neurophysiol 117 (6), 1217–1227. 10.1016/j.clinph.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Fregni F, Imamura M, Chien HF, Lew HL, Boggio P, Kaptchuk TJ, … group, Ipsw., 2010. Challenges and recommendations for placebo controls in randomized trials in physical and rehabilitation medicine: a report of the international placebo symposium working group. Am. J. Phys. Med. Rehabil 89 (2), 160–172. 10.1097/PHM.0b013e3181bc0bbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS, 2008. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J. Clin. Psychiatry 69 (1), 32–40. [DOI] [PubMed] [Google Scholar]
- Gamboa OL, Antal A, Moliadze V, Paulus W, 2010. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp. Brain Res 204 (2), 181–187. 10.1007/s00221-010-2293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R, 2007. The role of cognitive control in cocaine dependence. Neuropsychol. Rev 17 (3), 337–345. [DOI] [PubMed] [Google Scholar]
- Garnett EO, den Ouden DB, 2015. Validating a sham condition for use in high definition transcranial direct current stimulation. Brain Stimul. 8 (3), 551–554. 10.1016/j.brs.2015.01.399. [DOI] [PubMed] [Google Scholar]
- Garrison KA, Potenza MN, 2014. Neuroimaging and biomarkers in addiction treatment. Curr. Psychiatry Rep 16 (12), 513 10.1007/s11920-014-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay A, Boutet C, Sigaud T, Kamgoue A, Sevos J, Brunelin J, Massoubre C, 2017. A single session of repetitive transcranial magnetic stimulation of the prefrontal cortex reduces cue-induced craving in patients with gambling disorder. Eur. Psychiatry 41, 68–74. 10.1016/j.eurpsy.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Gersner R, Kravetz E, Feil J, Pell G, Zangen A, 2011. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J. Neurosci 31 (20), 7521–7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano J, Bikson M, Kappenman ES, Clark VP, Coslett HB, Hamblin MR, … Calabrese E, 2017. Mechanisms and effects of transcranial direct current stimulation. DoseResponse 15 (1), 1559325816685467 10.1177/1559325816685467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi P, Rapinesi C, Chiarotti F, Kotzalidis GD, Piacentino D, Serata D, … Angeletti G, 2014. Add-on deep transcranial magnetic stimulation (dTMS) in patients with dysthymic disorder comorbid with alcohol use disorder: a comparison with standard treatment. World J. Biol. Psychiatry 16 (1), 66–73. 10.3109/15622975.2014.925583. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12 (11), 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy MR, Pitcher JB, Ridding MC, 2013. Neuroplastic modulation of inhibitory motor cortical networks by spaced theta burst stimulation protocols. Brain Stimul. 6 (3), 340–345. 10.1016/j.brs.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Good CH, Hoffman AF, Hoffer BJ, Chefer VI, Shippenberg TS, Bäckman CM, … Galter D, 2011. Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson’s disease. Faseb J. 25 (4), 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS, 2014. Transcranial magnetic stimulation in the treatment of substance addiction. Ann. N. Y. Acad. Sci 1327 (1), 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini A, Lucchiari C, Russell-Edu W, Pravettoni G, 2014. Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front. Hum. Neurosci 8, 661 10.3389/fnhum.2014.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Yucel M, van Holst RJ, 2014. Getting a grip on problem gambling: what can neuroscience tell us? Front. Behav. Neurosci 8, 141 10.3389/fnbeh.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk H-J, … Boyden ES, 2017. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell 169 (6), 1029–1041. 10.1016/j.cell.2017.05.024. e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundey J, Thirugnanasambandam N, Kaminsky K, Drees A, Skwirba AC, Lang N, … Nitsche MA, 2012. Neuroplasticity in cigarette smokers is altered under withdrawal and partially restituted by nicotine exposition. J. Neurosci 32 (12), 4156–4162. 10.1523/JNEUROSCI.3660-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarienti F, Caumo W, Shiozawa P, Cordeiro Q, Boggio PS, Benseñor IM, … Brunoni AR, 2015. Reducing transcranial direct current stimulation-induced erythema with skin pretreatment: considerations for sham-controlled clinical trials. Neuromodulation 18 (4), 261–265. 10.1111/ner.12230. [DOI] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L, Kenna GA, 2014. Pharmacological approaches to reducing craving in patients with alcohol use disorders. CNS Drugs 28 (4), 343–360. 10.1007/s40263-014-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley D, Anderson BS, Borckardt JJ, Arana A, Li X, Nahas Z, George MS, 2011. Safety, tolerability, and effectiveness of high doses of adjunctive daily left prefrontal repetitive transcranial magnetic stimulation for treatment-resistant depression in a clinical setting. J. ECT 27 (1), 18–25. 10.1097/YCT.0b013e3181ce1a8c. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Canterberry M, Taylor JJ, DeVries W, Li X, Brown TR, George MS, 2013. Probing the frontostriatal loops involved in executive and limbic processing via interleaved TMS and functional MRI at two prefrontal locations: a pilot study. PLoS One 8 (7), e67917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Correia B, Mithoefer O, Kearney-Ramos T, Lench D, … George MS, 2017. Left frontal pole theta burst stimulation decreases orbitofrontal and insula activity in cocaine users and alcohol users. Drug Alcohol Depend. 178, 310–317. 10.1016/j.drugalcdep.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Jones JL, 2016a. Biomarkers for success: using neuroimaging to predict relapse and develop brain stimulation treatments for cocaine-dependent individuals. Int. Rev. Neurobiol 129, 125–156. 10.1016/bs.irn.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Moss H, Canterberry M, George MS, 2016b. Mobilization of medial and lateral frontal-striatal circuits in cocaine users and controls: an interleaved TMS/BOLD functional connectivity study. Neuropsychopharmacology 41 (13), 3032–3041. 10.1038/npp.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise K-F, Kortzorg N, Saturnino GB, Fujiyama H, Cuypers K, Thielscher A, Swinnen SP, 2016. Evaluation of a modified high-definition electrode montage for transcranial alternating current stimulation (tACS) of pre-central areas. Brain Stimul. 9 (5), 700–704. 10.1016/j.brs.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Baeken C, Vanderbruggen N, Vanderhasselt MA, Zeeuws D, Santermans L, De Raedt R, 2012. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend. 120 (1–3), 209–213. 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Herremans SC, De Raedt R, Van Schuerbeek P, Marinazzo D, Matthys F, De Mey J, Baeken C, 2016. Accelerated HF-rTMS protocol has a rate-dependent effect on dACC activation in alcohol-dependent patients: an open-label feasibility study. Alcohol. Clin. Exp. Res 40 (1), 196–205. 10.1111/acer.12937. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Van Schuerbeek P, De Raedt R, Matthys F, Buyl R, De Mey J, Baeken C, 2015. The impact of accelerated right prefrontal high-frequency repetitive transcranial magnetic stimulation (rTMS) on cue-reactivity: an fMRI study on craving in recently detoxified alcohol-dependent patients. PLoS One 10 (8), e0136182 10.1371/journal.pone.0136182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans SC, Vanderhasselt MA, De Raedt R, Baeken C, 2013. Reduced intra-individual reaction time variability during a go-nogo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol. 48 (5), 552–557. 10.1093/alcalc/agt054. [DOI] [PubMed] [Google Scholar]
- Herwig U, Fallgatter AJ, Hoppner J, Eschweiler GW, Kron M, Hajak G, … Schonfeldt-Lecuona C, 2007. Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. Br. J. Psychiatry 191, 441–448. 10.1192/bjp.bp.106.034371. [DOI] [PubMed] [Google Scholar]
- Hone-Blanchet A, Ciraulo DA, Pascual-Leone A, Fecteau S, 2015. Noninvasive brain stimulation to suppress craving in substance use disorders: review of human evidence and methodological considerations for future work. Neurosci. Biobehav. Rev 59, 184–200. 10.1016/j.neubiorev.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppner J, Broese T, Wendler L, Berger C, Thome J, 2011. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J. Biol. Psychiatry 12 (Suppl. 1), 57–62. 10.3109/15622975.2011.598383. [DOI] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y, 2015. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry 72 (6), 584–592. [DOI] [PubMed] [Google Scholar]
- Huang W, Shen F, Zhang J, Xing B, 2016. Effect of repetitive transcranial magnetic stimulation on cigarette smoking in patients with schizophrenia. Shanghai Arch. Psychiatry 28 (6), 309–317. 10.11919/j.issn.1002-0829.216044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X, Parra LC, 2017. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife 6 10.7554/eLife.18834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC, 2005. Theta burst stimulation of the human motor cortex. Neuron 45 (2), 201–206. 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- ICMJE recommendations. (Accessed 27 November 2018). Retrieved from http://www.icmje.org/recommendations/.
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P, 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167 (7), 748–751. 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2015. The NIMH experimental medicine initiative. World Psychiatry 14 (2), 151–153. 10.1002/wps.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iran Supreme Leader. (2006). Macropolicies of Drug Control. Communicated by Iran Supreme Leader on 1385.7.10 [Google Scholar]
- Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, Zangen A, 2013. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder-a pilot study. Brain Stimul. 6 (3), 377–383. 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC, Bikson M, 2016. Animal models of transcranial direct current stimulation: methods and mechanisms. Clin. Neurophysiol 127 (11), 3425–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil A, Batsikadze G, Kuo H-I, Labruna L, Hasan A, Paulus W, Nitsche MA, 2016. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J. Physiol 595 (4), 1273–1288. 10.1113/JP272738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE, 2013. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci. Biobehav. Rev 37 (10 Pt 2), 2472–2480. 10.1016/j.neubiorev.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Jansen JM, van Wingen G, van den Brink W, Goudriaan AE, 2015. Resting state connectivity in alcohol dependent patients and the effect of repetitive transcranial magnetic stimulation. Eur. Neuropsychopharmacol 25 (12), 2230–2239. 10.1016/j.euroneuro.2015.09.019. [DOI] [PubMed] [Google Scholar]
- Jones A, Remmerswaal D, Verveer I, Robinson E, Franken IHA, Wen CKF, Field M, 2019. Compliance with ecological momentary assessment protocols in substance users: a meta-analysis. Addiction 114 (4), 609–619. 10.1111/add.14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabakov AY, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A, 2012. Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J. Neurophysiol 107 (7), 1881–1889. 10.1152/jn.00715.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp D, Engelke C, Wobrock T, Kunze B, Wölwer W, Winterer G, … Landgrebe M, 2018. Letter to the Editor: influence of rTMS on smoking in patients with schizophrenia. Schizophr. Res 192, 481. [DOI] [PubMed] [Google Scholar]
- Karabanov A, Ziemann U, Hamada M, George MS, Quartarone A, Classen J, … Siebner HR, 2015. Consensus paper: probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul. 8 (5), 993–1006. 10.1016/j.brs.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Kasten FH, Dowsett J, Herrmann CS, 2016. Sustained Aftereffect of α-tACS lasts up to 70 min after Stimulation. Front. Hum. Neurosci 10, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaster TS, Daskalakis ZJ, Noda Y, Knyahnytska Y, Downar J, Rajji TK, … Blumberger DM, 2018. Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: a prospective randomized controlled trial. Neuropsychopharmacology 43 (11), 2231–2238. 10.1038/s41386-018-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H, 2003. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J. Neurosci 23 (21), 7839–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney-Ramos TE, Dowdle LT, Lench DH, Mithoefer OJ, Devries WH, George MS, … Hanlon CA, 2018. Transdiagnostic effects of ventromedial prefrontal cortex transcranial magnetic stimulation on cue reactivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 (7), 599–609. 10.1016/j.bpsc.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH, 2012. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 5 (2), 155–162. 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, 1997. Craving is associated with smoking relapse: findings from three prospective studies. Exp. Clin. Psychopharmacol 1064–1297 (Print). [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Fitzmaurice GM, Strain EC, Weiss RD, 2019. What defines a clinically meaningful outcome in the treatment of substance use disorders: reductions in direct consequences of drug use or improvement in overall functioning? Addiction 114 (1), 9–15. 10.1111/add.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH, 1993. The ‘Trier Social Stress Test’-a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28 (1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Klauss J, Anders QS, Felippe LV, Ferreira LVB, Cruz MA, Nitsche MA, Nakamura-Palacios EM, 2018a. Lack of additional effects of extended sessions of transcranial Direct Current Stimulation (tDCS) over dorsolateral prefrontal cortex on craving and relapses in crack-cocaine users. Front. Pharmacol 9, 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J, Anders QS, Felippe LV, Nitsche MA, Nakamura-Palacios EM, 2018b. Multiple sessions of transcranial direct current stimulation (tDCS) reduced craving and relapses for alcohol use: a randomized placebo-controlled trial in alcohol use disorder. Front. Pharmacol 9, 716 10.3389/fphar.2018.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J, Penido Pinheiro LC, Silva Merlo BL, Correia Santos G.d.A., Fregni F, Nitsche MA, Miyuki Nakamura-Palacios E, 2014. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int. J. Neuropsychopharmacol 17 (11), 1793–1803. 10.1017/S1461145714000984. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D, 2001. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12 (17), 3683–3687. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3 (8), 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak K, Barr MS, George TP, 2017. Traits and biomarkers for addiction risk in schizophrenia. Curr. Addict. Rep 4 (1), 14–24. [Google Scholar]
- Kozak K, Sharif-Razi M, Morozova M, Gaudette EV, Barr MS, Daskalakis ZJ, … George TP, 2018. Effects of short-term, high-frequency repetitive transcranial magnetic stimulation to bilateral dorsolateral prefrontal cortex on smoking behavior and cognition in patients with schizophrenia and non-psychiatric controls. Schizophr. Res 10.1016/j.schres.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy V, Gopinath K, Brown GS, Hampstead BM, 2015. Resting-state fMRI reveals enhanced functional connectivity in spatial navigation networks after transcranial direct current stimulation. Neurosci. Lett 604, 80–85. 10.1016/j.neulet.2015.07.042. [DOI] [PubMed] [Google Scholar]
- Kroczek AM, Haussinger FB, Rohe T, Schneider S, Plewnia C, Batra A, Ehlis AC, 2016. Effects of transcranial direct current stimulation on craving, heart-rate variability and prefrontal hemodynamics during smoking cue exposure. Drug Alcohol Depend. 168, 123–127. 10.1016/j.drugalcdep.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Pizzagalli DA, Mathew SJ, Sanacora G, Keefe R, Song A, … Potter W, 2018. The first implementation of the NIMH FAST-FAIL approach to psychiatric drug development. Nat. Rev. Drug Discov 18 (1), 82–84. 10.1038/nrd.2018.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M-F, Paulus W, Nitsche MA, 2014. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage 85, 948–960. 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- Kutchko KM, Frohlich F, 2013. Emergence of metastable state dynamics in interconnected cortical networks with propagation delays. PLoS Comput. Biol 9 (10), e1003304 10.1371/journal.pcbi.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Koob GF, 2017. Neuroclinical framework for the role of stress in addiction. Chronic Stress 1, 2470547017698140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, … Frackowiak RS, 2005. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur. J. Neurosci 22 (2), 495–504. 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, … Garcia-Larrea L, 2014. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol 125 (11), 2150–2206. 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, … Zangen A, 2015. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 14 (1), 64–73. 10.1002/wps.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A, 2007. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J. Neurosci 27 (51), 14179–14189. 10.1523/jneurosci.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Du L, Sahlem GL, Badran BW, Henderson S, George MS, 2017a. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend. 174, 98–105. 10.1016/j.drugalcdep.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, … George MS, 2013a. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol. Psychiatry 73 (8), 714–720. 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Malcolm RJ, Huebner K, Hanlon CA, Taylor JJ, Brady KT, … See RE, 2013b. Low frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex transiently increases cue-induced craving for methamphetamine: a preliminary study. Drug Alcohol Depend. 133 (2), 641–646. 10.1016/j.drugalcdep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sahlem GL, Badran BW, McTeague LM, Hanlon CA, Hartwell KJ, … George MS, 2017b. Transcranial magnetic stimulation of the dorsal lateral prefrontal cortex inhibits medial orbitofrontal activity in smokers. Am. J. Addict 26 (8), 788–794. 10.1111/ajad.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Lin J, Yang J, Li X, Chen Y, Meng X, Yuan J, 2018a. Intervention Effect of Repetitive TMS on Behavioral Adjustment After Error Commission in Long-Term Methamphetamine Addicts: Evidence From a Two-Choice Oddball Task. Neurosci. Bull 34 (3), 449–456. 10.1007/s12264-018-0205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Wang L, Yuan TF, 2018b. Targeting withdrawal symptoms in men addicted to methamphetamine with transcranial magnetic stimulation: a randomized clinical trial. JAMA Psychiatry 75 (11), 1199–1201. 10.1001/jamapsychiatry.2018.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Paulus W, 2003. Pharmacology of transcranial direct current stimulation: missing effect of riluzole Chapter 29 In: In: Paulus W, Tergau F, Nitsche MA, Rothwell JG, Ziemann U, Hallett M (Eds.), Supplements to Clinical Neurophysiology 56 Elsevier, pp. 282–287. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W, 2002. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125 (10), 2238–2247. [DOI] [PubMed] [Google Scholar]
- Liu Q, Shen Y, Cao X, Li Y, Chen Y, Yang W, Yuan T-F, 2017. Either at left or right, both high and low frequency rTMS of dorsolateral prefrontal cortex decreases cue induced craving for methamphetamine. Am. J. Addict 26 (8), 776–779. 10.1111/ajad.12638. [DOI] [PubMed] [Google Scholar]
- Lucatch AM, Lowe DJE, Clark RC, Kozak K, George TP, 2018. Neurobiological determinants of tobacco smoking in schizophrenia. Front. Psychiatry 9, 672 10.3389/fpsyt.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigjes J, van den Brink W, Schuurman PR, Kuhn J, Denys D, 2015. Is deep brain stimulation a treatment option for addiction? Addiction 110 (4), 547–548. 10.1111/add.12773. [DOI] [PubMed] [Google Scholar]
- Maiti R, Mishra BR, Hota D, 2017. Effect of high-frequency transcranial magnetic stimulation on craving in substance use disorder: a meta-analysis. J. Neuropsychiatry Clin. Neurosci 29 (2), 160–171. 10.1176/appi.neuropsych.16040065. [DOI] [PubMed] [Google Scholar]
- Mantione M, Nieman D, Figee M, van den Munckhof P, Schuurman R, Denys D, 2015. Cognitive effects of deep brain stimulation in patients with obsessive-compulsive disorder. J. Psychiatry Neurosci 40 (6), 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhe R, van de Wetering BJ, Franken IH, 2013. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. Biol. Psychiatry 73 (8), 782–788. [DOI] [PubMed] [Google Scholar]
- Martinez D, Urban N, Grassetti A, Chang D, Hu M-C, Zangen A, … Nunes EV, 2018. Transcranial magnetic stimulation of medial prefrontal and cingulate cortices reduces cocaine self-administration: a pilot study. Front. Psychiatry 9 10.3389/fpsyt.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Gu H, Yang Y, Adinoff B, Stein EA, 2017. Executive control network connectivity strength protects against relapse to cocaine use. Addict. Biol 22 (6), 1790–1801. [DOI] [PubMed] [Google Scholar]
- Meng Z, Liu C, Yu C, Ma Y, 2014. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates smoking behavior. J. Psychiatr. Res 54, 19–25. 10.1016/j.jpsychires.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Mennemeier MS, Triggs WJ, Chelette KC, Woods AJ, Kimbrell TA, Dornhoffer JL, 2009. Sham transcranial magnetic stimulation using electrical stimulation of the scalp. Brain Stimul. 2 (3), 168–173. 10.1016/j.brs.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BR, Maiti R, Nizamie SH, 2016. Cerebral Hemodynamics With rTMS in Alcohol Dependence: A Randomized, Sham-Controlled Study. J. Neuropsychiatry Clin. Neurosci 10.1176/appi.neuropsych.15110381.appineuropsych15110381. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK, 2010. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 105 (1), 49–55. 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Praharaj SK, Katshu MZUH, Sarkar S, Nizamie SH, 2015. Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: a randomized double-blind study. J. Neuropsychiatry Clin. Neurosci 27 (1), e54–e59. 10.1176/appi.neuropsych.13010013. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Meek BP, Wikstrom SL, 2018. Efficacy of twice-daily vs once-daily sessions of repetitive transcranial magnetic stimulation in the treatment of major depressive disorder: a retrospective study. Neuropsychiatr. Dis. Treat 14, 309–316. 10.2147/ndt.S151841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG, 2001. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 357 (9263), 1191–1194. [PubMed] [Google Scholar]
- Moliadze V, Atalay D, Antal A, Paulus W, 2012. Close to threshold transcranial electrical stimulation preferentially activates inhibitory networks before switching to excitation with higher intensities. Brain Stimul. 5 (4), 505–511. 10.1016/j.brs.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Mondino M, Luck D, Grot S, Januel D, Suaud-Chagny MF, Poulet E, Brunelin J, 2018. Effects of repeated transcranial direct current stimulation on smoking, craving and brain reactivity to smoking cues. Sci. Rep 8 (1), 8724 10.1038/s41598-018-27057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA, 2013. Induction of late LTP-Like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 6 (3), 424–432. 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Moore K, Madularu D, Iriah S, Yee JR, Kulkarni P, Darcq E, Ferris CF, 2016. BOLD imaging in awake wild-type and mu-opioid receptor knock-out mice reveals on-target activation maps in response to oxycodone. Front. Neurosci 10, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naish KR, Vedelago L, MacKillop J, Amlung M, 2018. Effects of neuromodulation on cognitive performance in individuals exhibiting addictive behaviors: a systematic review. Drug Alcohol Depend. 192, 338–351. 10.1016/j.drugalcdep.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, de Almeida Benevides MC, da Penha Zago-Gomes M, de Oliveira RW, de Vasconcellos VF, de Castro LN, … Fregni F, 2012. Auditory event-related potentials (P3) and cognitive changes induced by frontal direct current stimulation in alcoholics according to Lesch alcoholism typology. Int. J. Neuropsychopharmacol 15 (5), 601–616. 10.1017/S1461145711001040. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Lopes IB, Souza RA, Klauss J, Batista EK, Conti CL, … de Souza RS, 2016. Ventral medial prefrontal cortex (vmPFC) as a target of the dorsolateral prefrontal modulation by transcranial direct current stimulation (tDCS) in drug addiction. J. Neural Transm. (Vienna) 123 (10), 1179–1194. 10.1007/s00702-016-1559-9. [DOI] [PubMed] [Google Scholar]
- Nasseri P, Nitsche MA, Ekhtiari H, 2015. A framework for categorizing electrode montages in transcranial direct current stimulation. Front. Hum. Neurosci 9, 54 10.3389/fnhum.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, … Paulus W, 2003a. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol 553 (Pt 1), 293–301. 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W, 2003b. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin. Neurophysiol 114 (4), 600–604. 10.1016/S1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2000. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol 527 (3), 633–639. 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2001. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57 (10), 1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F, 2003c. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci 15 (4), 619–626. 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- O’Connell NE, Cossar J, Marston L, Wand BM, Bunce D, Moseley GL, De Souza LH, 2012. Rethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2mA. PLoS One 7 (10), e47514 10.1371/journal.pone.0047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, … Sackeim HA, 2007. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 62 (11), 1208–1216. 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Opitz A, Fox MD, Craddock RC, Colcombe S, Milham MP, 2016. An integrated framework for targeting functional networks via transcranial magnetic stimulation. Neuroimage 127, 86–96. 10.1016/j.neuroimage.2015.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz A, Legon W, Mueller J, Barbour A, Paulus W, Tyler WJ, 2014. Is sham cTBS real cTBS? The effect on EEG dynamics. Front. Hum. Neurosci 8, 1043 10.3389/fnhum.2014.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz A, Legon W, Rowlands A, Bickel WK, Paulus W, Tyler WJ, 2013. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage 81, 253–264. 10.1016/j.neuroimage.2013.04.067. [DOI] [PubMed] [Google Scholar]
- Opitz A, Windhoff M, Heidemann RM, Turner R, Thielscher A, 2011. How the brain tissue shapes the electric field induced by transcranial magnetic stimulation. Neuroimage 58 (3), 849–859. 10.1016/j.neuroimage.2011.06.069. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M, 1994. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117 (4), 847–858. 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Paulus W, 2003. Transcranial direct current stimulation (tDCS) Supplements to Clinical Neurophysiology 56 Elsevier, pp. 249–254. [DOI] [PubMed] [Google Scholar]
- Paulus W, 2011. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil 21 (5), 602–617. 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- Pettorruso M, Spagnolo PA, Leggio L, Janiri L, Di Giannantonio M, Gallimberti L, … Martinotti G, 2018. Repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex may improve symptoms of anhedonia in individuals with cocaine use disorder: a pilot study. Brain Stimul 10.1016/j.brs.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Pinto CB, Teixeira Costa B, Duarte D, Fregni F, 2018. Transcranial direct current stimulation as a therapeutic tool for chronic pain. J. ECT 10.1097/YCT.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E, 2008. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am. J. Addict 17 (4), 345–346. 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- Prikryl R, Ustohal L, Kucerova HP, Kasparek T, Jarkovsky J, Hublova V, … Ceskova E, 2014. Repetitive transcranial magnetic stimulation reduces cigarette consumption in schizophrenia patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 49, 30–35. 10.1016/j.pnpbp.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Lamm C, 2015. Focused transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex modulates specific domains of self-regulation. Neurosci. Res 91, 41–47. 10.1016/j.neures.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Neumann R, Kohler U, Lamm C, 2013. Effects of transcranial direct current stimulation on risky decision making are mediated by’ hot’ and’ cold’ decisions, personality, and hemisphere. Eur. J. Neurosci 38 (12), 3778–3785. 10.1111/ejn.12375. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I, Lamm C, 2014. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 7 (2), 226–233. 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Qiao J, Jin G, Lei L, Wang L, Du Y, Wang X, 2016. The positive effects of high-frequency right dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation on memory, correlated with increases in brain metabolites detected by proton magnetic resonance spectroscopy in recently detoxified alcohol-dependent patients. Neuropsychiatr. Dis. Treat 12, 2273–2278. 10.2147/ndt.s106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe A, Grusser SM, Wessa M, Podschus J, Flor H, 2005. The assessment of craving: psychometric properties, factor structure and a revised version of the Alcohol Craving Questionnaire (ACQ). Addiction 100 (2), 227–234. 10.1111/j.1360-0443.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, 2015. The brain’s default mode network. Annu. Rev. Neurosci 38, 433–447. [DOI] [PubMed] [Google Scholar]
- Rapinesi C, Bersani FS, Kotzalidis GD, Imperatori C, Del Casale A, Di Pietro S, … Girardi P, 2015a. Maintenance deep transcranial magnetic stimulation sessions are associated with reduced depressive relapses in patients with unipolar or bipolar depression. Front. Neurol 6, 16 10.3389/fneur.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapinesi C, Curto M, Kotzalidis GD, Del Casale A, Serata D, Ferri VR, … Girardi P, 2015b. Antidepressant effectiveness of deep transcranial magnetic stimulation (dTMS) in patients with major depressive disorder (MDD) with or without alcohol use disorders (AUDs): a 6-month, open label, follow-up study. J. Affect. Disord 174, 57–63. 10.1016/j.jad.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Rapinesi C, Del Casale A, Di Pietro S, Ferri VR, Piacentino D, Sani G, … Girardi P, 2016. Add-on high frequency deep transcranial magnetic stimulation (dTMS) to bilateral prefrontal cortex reduces cocaine craving in patients with cocaine use disorder. Neurosci. Lett 629, 43–47. 10.1016/j.neulet.2016.06.049. [DOI] [PubMed] [Google Scholar]
- Rawji V, Ciocca M, Zacharia A, Soares D, Truong D, Bikson M, … Bestmann S, 2018. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul. 11 (2), 289–298. 10.1016/j.brs.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D, Rahman A, Bikson M, Parra LC, 2013. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front. Hum. Neurosci 7, 687 10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud’homme X, Krystal AD, 2011. Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol. Psychiatry 70 (8), 794–799. 10.1016/j.biopsych.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Rossi S, Ferro M, Cincotta M, Ulivelli M, Bartalini S, Miniussi C, … Passero S, 2007. A real electro-magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS). Clin. Neurophysiol 118 (3), 709–716. 10.1016/j.clinph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol 120 (12), 2008–2039. 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlem GL, Baker NL, George MS, Malcolm RJ, McRae-Clark AL, 2017. Repetitive transcranial magnetic stimulation (rTMS) administration to heavy cannabis users. Am. J. Drug Alcohol Abuse 44 (1), 47–55. 10.1080/00952990.2017.1355920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Martinez D, 2016. Brain stimulation in addiction. Neuropsychopharmacology 41 (12), 2798–2809. 10.1038/npp.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Junior B, Tortella G, Borrione L, Moffa AH, Machado-Vieira R, Cretaz E, … Brunoni AR, 2018. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: a randomized clinical trial. JAMA Psychiatry 75 (2), 158–166. 10.1001/jamapsychiatry.2017.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrini M, Umiltà C, Rusconi E, 2011. The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci. Biobehav. Rev 35 (3), 516–536. 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Santos L, Martinho M, Salvador R, Wenger C, Fernandes SR, Ripolles O, … Miranda PC, 2016. Evaluation of the electric field in the brain during transcranial direct current stimulation: a sensitivity analysis. Conf. Proc. IEEE Eng. Med. Biol. Soc 2016, 1778–1781. 10.1109/embc.2016.7591062. [DOI] [PubMed] [Google Scholar]
- Sauvaget A, Bulteau S, Guilleux A, Leboucher J, Pichot A, Valriviere P, … Grall-Bronnec M, 2018. Both active and sham low-frequency rTMS single sessions over the right DLPFC decrease cue-induced cravings among pathological gamblers seeking treatment: a randomized, double-blind, sham-controlled crossover trial. J. Behav. Addict 7 (1), 126–136. 10.1556/2006.7.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG, 2000. The measurement of drug craving. Addiction 95 (Suppl. 2), S189–S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter RS, Daams JG, van Holst RJ, Goudriaan AE, 2018. Effects of non-invasive neuromodulation on executive and other cognitive functions in addictive disorders: a systematic review. Front. Neurosci 12, 642 10.3389/fnins.2018.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter RS, Jansen JM, van Holst RJ, van den Brink W, Goudriaan AE, 2017. Differential effects of left and right prefrontal high frequency rTMS on resting state fMRI in healthy individuals. Brain Connect. 10.1089/brain.2017.0542. [DOI] [PubMed] [Google Scholar]
- Schmidt SL, Iyengar AK, Foulser AA, Boyle MR, Frohlich F, 2014. Endogenous cortical oscillations constrain neuromodulation by weak electric fields. Brain Stimul. 7 (6), 878–889. 10.1016/j.brs.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Feffer K, Lozano C, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J, 2018. Number of pulses or number of sessions? An open-label study of trajectories of improvement for once-vs. twice-daily dorsomedial prefrontal rTMS in major depression. Brain Stimul. 11 (2), 327–336. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27 (9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senova S, Cotovio G, Pascual-Leone A, Oliveira-Maia AJ, 2019. Durability of antidepressant response to repetitive transcranial magnetic stimulation: systematic review and meta-analysis. Brain Stimul. 12 (1), 119–128. 10.1016/j.brs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Shahbabaie A, Ebrahimpoor M, Hariri A, Nitsche MA, Hatami J, Fatemizadeh E, … Ekhtiari H, 2018a. Transcranial DC stimulation modifies functional connectivity of large-scale brain networks in abstinent methamphetamine users. Brain Behav. 8 (3), e00922 10.1002/brb3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbabaie A, Golesorkhi M, Zamanian B, Ebrahimpoor M, Keshvari F, Nejati V, … Ekhtiari H, 2014. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int. J. Neuropsychopharmacol 17 (10), 1591–1598. 10.1017/S1461145714000686. [DOI] [PubMed] [Google Scholar]
- Shahbabaie A, Hatami J, Farhoudian A, Ekhtiari H, Khatibi A, Nitsche MA, 2018b. Optimizing electrode montages of transcranial direct current stimulation for attentional bias modification in early abstinent methamphetamine users. Front. Pharmacol 9, 907 10.3389/fphar.2018.00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MT, Kasschau M, Dobbs B, Pawlak N, Pau W, Sherman K, … Charvet LE, 2017. Remotely supervised transcranial direct current stimulation: an update on safety and tolerability. J. Vis. Exp 128 10.3791/56211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer CE, Bickel WK, Brandon TH, Franck CT, Deen D, Panissidi L, … Mantovani A, 2018. Preventing relapse to smoking with transcranial magnetic stimulation: feasibility and potential efficacy. Drug Alcohol Depend. 182, 8–18. 10.1016/j.drugalcdep.2017.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer CE, Mennemeier M, Landes RD, Bickel WK, Brackman S, Dornhoffer J, … Brown G, 2013a. Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. J. Subst. Abuse Treat 45 (2), 206–214. 10.1016/j.jsat.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer CE, Mennemeier MS, Landes RD, Dornhoffer J, Kimbrell T, Bickel WK, … Vuong M, 2013b. Focal electrical stimulation as an effective sham control for active rTMS and biofeedback treatments. Appl. Psychophysiol. Biofeedback 38 (3), 171–176. 10.1007/s10484-013-9221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J, Yuan T-F, 2016. 10-hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex reduces heroin cue craving in long-term addicts. Biol. Psychiatry 80 (3), e13–e14. 10.1016/j.biopsych.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Sinha R, 2009. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict. Biol 14 (1), 84–98. 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2013. The clinical neurobiology of drug craving. Curr. Opin. Neurobiol 23 (4), 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skevington SM, Lotfy M, O’Connell KA, 2004. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual. Life Res 13 (2), 299–310. 10.1023/b:Qure.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, Hoek HW, Sommer IE, 2010. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J. Clin. Psychiatry 71 (7), 873–884. 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Smith RC, Boules S, Mattiuz S, Youssef M, Tobe RH, Sershen H, … Davis JM, 2015. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr. Res 168 (1–2), 260–266. 10.1016/j.schres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB, 1996. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 42 (1), 49–54. [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Goldman D, 2017. Neuromodulation interventions for addictive disorders: challenges, promise, and roadmap for future research. Brain 140 (5), 1183–1203. 10.1093/brain/aww284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ, 2013. Characterizing the cognitive effects of cocaine: a comprehensive review. Neurosci. Biobehav. Rev 37 (8), 1838–1859. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA, 2011. Physiological basis of transcranial direct current stimulation. Neuroscientist 17 (1), 37–53. 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G, 2009. Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology 56, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele V, Pariyadath V, Goldstein R, Stein E, 2018a. Reward Circuitry and Drug Addiction: Neurobiology of Mental Illness, 5th ed. Oxford University Press, Oxford, UK. [Google Scholar]
- Steele VR, Ding X, Ross TJ, 2019. Addiction: informing drug abuse interventions with brain networks Connectomics. Elsevier, pp. 101–122. [Google Scholar]
- Steele VR, Fink BC, Maurer JM, Arbabshirani MR, Wilber CH, Jaffe AJ, … Clark VP, 2014. Brain potentials measured during a Go/NoGo task predict completion of substance abuse treatment. Biol. Psychiatry 76 (1), 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Maurer JM, Arbabshirani MR, Claus ED, Fink BC, Rao V, … Kiehl KA, 2018b. Machine learning of functional magnetic resonance imaging network connectivity predicts substance abuse treatment completion. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3 (2), 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A, 2001. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci 21 (15), RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strube W, Nitsche MA, Wobrock T, Bunse T, Rein B, Herrmann M, … Rietschel M, 2015. BDNF-Val66Met-polymorphism impact on cortical plasticity in schizophrenia patients: a proof-of-concept study. Int. J. Neuropsychopharmacol 18 (4) pyu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Zhong N, Gan H, Wang J, Han H, Chen T, … Zhao M, 2017. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: a randomised clinical trial. Drug Alcohol Depend. 175, 84–91. 10.1016/j.drugalcdep.2017.01.037. [DOI] [PubMed] [Google Scholar]
- Tavakoli HR, Hull M, Michael Okasinski L, 2011. Review of current clinical biomarkers for the detection of alcohol dependence. Innov. Clin. Neurosci 8 (3), 26–33. [PMC free article] [PubMed] [Google Scholar]
- Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L, 2016. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: a pilot study. Eur. Neuropsychopharmacol 26 (1), 37–44. 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut A, Russo C, Hurtado-Puerto AM, Morales-Quezada JL, Deitos A, Petrozza JC, … Fregni F, 2017. Effects of transcranial direct current stimulation, transcranial pulsed current stimulation, and their combination on brain oscillations in patients with chronic visceral pain: a pilot crossover randomized controlled study. Front. Neurol 8, 576 10.3389/fneur.2017.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW, 2007. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp. Brain Res 180 (4), 583–593. 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Sparing R, Dafotakis M, Meister IG, Paulus W, Nitsche MA, Fink GR, 2011. Isometric contraction interferes with transcranial direct current stimulation (tDCS) induced plasticity-evidence of state-dependent neuromodulation in human motor cortex. Restor. Neurol. Neurosci 29 (5), 311–320. [DOI] [PubMed] [Google Scholar]
- Thut G, Bergmann TO, Frohlich F, Soekadar SR, Brittain JS, Valero-Cabre A, … Herrmann CS, 2017. Guiding transcranial brain stimulation by EEG/MEG to interact with ongoing brain activity and associated functions: a position paper. Clin. Neurophysiol 128 (5), 843–857. 10.1016/j.clinph.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Friedman L, Greenfield SF, Hasin DS, Jackson R, 2012. Beyond drug use: a systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction 107 (4), 709–718. 10.1111/j.1360-0443.2011.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE, 1993. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 34 (1), 19–28. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM, 2012. The clinical significance of drug craving. Ann. N. Y. Acad. Sci 1248, 1–17. 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojak B, Sauvaget Anne, Fecteau Shirley, Lalanne Laurence, Chauvet-Gelinier Jean-Christophe, Koch Sonja, … Achab Sophia, 2017. Outcome of non-invasive brain stimulation in substance use disorders: a review of randomized sham-controlled clinical trials. J. Neuropsychiatry Clin. Neurosci 29 (2), 105–118. 10.1176/appi.neuropsych.16080147. [DOI] [PubMed] [Google Scholar]
- Trojak B, Meille V, Achab S, Lalanne L, Poquet H, Ponavoy E, … Chauvet-Gelinier JC, 2015. Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: a randomized controlled trial. Brain Stimul. 8 (6), 1168–1174. 10.1016/j.brs.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Tse NY, Goldsworthy MR, Ridding MC, Coxon JP, Fitzgerald PB, Fornito A, Rogasch NC, 2018. The effect of stimulation interval on plasticity following repeated blocks of intermittent theta burst stimulation. Sci. Rep 8 (1), 8526 10.1038/s41598-018-26791-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova H, Klç K, Tian P, Sakadžić S, Gagnon L, Thunemann M, … Yaseen MA, 2016. The roadmap for estimation of cell-type-specific neuronal activity from non-invasive measurements. Philos. Trans. Biol. Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, van den Brink W, Veltman DJ, Goudriaan AE, 2010. Why gamblers fail to win: a review of cognitive and neuroimaging findings in pathological gambling. Neurosci. Biobehav. Rev 34 (1), 87–107 S0149–7634(09)00105–5 [pii]; 10.1016/j.neubiorev.2009.07.007 [doi]. [DOI] [PubMed] [Google Scholar]
- Vitor de Souza Brangioni MC, Pereira DA, Thibaut, Fregni F, Brasil-Neto JP, Boechat-Barros R, 2018. Effects of prefrontal transcranial direct current stimulation and motivation to quit in tobacco smokers: a randomized, sham controlled, double-blind trial. Front. Pharmacol 9, 14 10.3389/fphar.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Morales M, 2015. The brain on drugs: from reward to addiction. Cell 162 (4), 712–725. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT, 2016. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med 374 (4), 363–371. 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vöröslakos M, Takeuchi Y, Brinyiczki K, Zombori T, Oliva A, Fernández-Ruiz A, … Berényi A, 2018. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat. Commun 9 (1), 483 10.1038/s41467-018-02928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shen Y, Cao X, Shan C, Pan J, He H, … Yuan TF, 2016. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates cue-induced craving for heroin. J. Psychiatr. Res 79, 1–3. 10.1016/j.jpsychires.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Ware JE Jr, Sherbourne CD, 1992. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30 (6), 473–483. [PubMed] [Google Scholar]
- Watter S, Geffen GM, Geffen LB, 2001. The n-back as a dual-task: P300 morphology under divided attention. Psychophysiology 38 (6), 998–1003. [DOI] [PubMed] [Google Scholar]
- Watts AT, Tootell AV, Fix ST, Aviyente S, Bernat EM, 2018. Utilizing time-frequency amplitude and phase synchrony measure to assess feedback processing in a gambling task. Int. J. Psychophysiol 132, 203–212. [DOI] [PubMed] [Google Scholar]
- Widge AS, Deckersbach T, Eskandar EN, Dougherty DD, 2016. Deep Brain Stimulation for Treatment-Resistant Psychiatric Illnesses: What Has Gone Wrong and What Should We Do Next? Biol. Psychiatry 79 (4), e9–10. 10.1016/j.biopsych.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Wietschorke K, Lippold J, Jacob C, Polak T, Herrmann MJ, 2016. Transcranial direct current stimulation of the prefrontal cortex reduces cue-reactivity in alcohol-dependent patients. J. Neural Transm. (Vienna) 123 (10), 1173–1178. 10.1007/s00702-016-1541-6. [DOI] [PubMed] [Google Scholar]
- Wing VC, Bacher I, Wu BS, Daskalakis ZJ, George TP, 2012. High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr. Res 139 (1–3), 264–266. 10.1016/j.schres.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, George TP, 2013. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 6 (3), 221–230. 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Engelhardt M, Salehinejad MA, Schutter DJLG, Kuo MF, Nitsche MA, 2018. NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cereb. Cortex 10.1093/cercor/bhy160.bhy160-bhy160. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Wilson AD, Pearson MR, Montes KS, Kirouac M, Roos CR, … Maisto SA, 2019. Profiles of recovery from alcohol use disorder at three years following treatment: can the definition of recovery be extended to include high functioning heavy drinkers? Addiction 114 (1), 69–80. 10.1111/add.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, … Nitsche MA, 2016a. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol 127 (2), 1031–1048. 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, … Nitsche MA, 2016b. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol 127 (2), 1031–1048. 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Fregni F, Brody AL, Rahman AS, 2013. Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front. Psychiatry 4, 112 10.3389/fpsyt.2013.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LZ, Shi B, Li H, Zhang W, Liu Y, Wang H, … Zhang X, 2017. Electrical stimulation reduces smokers’ craving by modulating the coupling between dorsal lateral prefrontal cortex and parahippocampal gyrus. Soc. Cogn. Affect. Neurosci 12 (8), 1296–1302. 10.1093/scan/nsx055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari F, Jamil A, Mosayebi Samani M, Vidor LP, Nitsche MA, 2018. Basic and functional effects of transcranial electrical stimulation (tES)—an introduction. Neurosci. Biobehav. Rev 85, 81–92. 10.1016/j.neubiorev.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Yavari F, Nitsche MA, Ekhtiari H, 2017. Transcranial electric stimulation for precision medicine: a spatiomechanistic framework. Front. Hum. Neurosci 11, 159 10.3389/fnhum.2017.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavari F, Shahbabaie A, Leite J, Carvalho S, Ekhtiari H, Fregni F, 2016. Noninvasive brain stimulation for addiction medicine: from monitoring to modulation. Prog. Brain Res 224, 371–399. 10.1016/bs.pbr.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Zack M, Cho SS, Parlee J, Jacobs M, Li C, Boileau I, Strafella A, 2016. Effects of high frequency repeated transcranial magnetic stimulation and continuous theta burst stimulation on gambling reinforcement, delay discounting, and stroop interference in men with pathological gambling. Brain Stimul. 9 (6), 867–875. 10.1016/j.brs.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Rach S, Herrmann CS, 2010. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One 5 (11), e13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cao X, Liang Q, Li X, Yang J, Yuan J, 2018. High-frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex restores attention bias to negative information in methamphetamine addicts. Psychiatry Res. 265, 151–160. 10.1016/j.psychres.2018.04.039. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mei Y, Liu C, Yu S, 2007. Effect of transcranial magnetic stimulation on the expression of c-Fos and brain-derived neurotrophic factor of the cerebral cortex in rats with cerebral infarct. J. Huazhong Univ. Sci. Technol 27 (4), 415–418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


