Abstract
Humans possess some unique social-cognitive skills and motivations, involving such things as joint attention, cooperative communication, dual-level collaboration and cultural learning. These are almost certainly adaptations for humans' especially complex sociocultural lives. The common assumption has been that these unique skills and motivations emerge in human infancy and early childhood as preparations for the challenges of adult life, for example, in collaborative foraging. In the current paper, I propose that the curiously early emergence of these skills in infancy––well before they are needed in adulthood––along with other pieces of evidence (such as almost exclusive use with adults not peers) suggests that aspects of the evolution of these skills represent ontogenetic adaptations to the unique socio-ecological challenges human infants face in the context of a regime of cooperative breeding and childcare.
This article is part of the theme issue ‘Life history and learning: how childhood, caregiving and old age shape cognition and culture in humans and other animals’.
Keywords: human ontogeny, human life history, sociality, cooperative breeding
1. Introduction
Life-history theory appears in modern developmental psychology mostly in the form of one basic fact: human ontogeny is very slow (and requires much parental investment) relative to that of many other species, including humans' nearest primate relatives. But life-history analyses have the potential to contribute to developmental psychology in other very rich ways as well, based on another key fact: for all species, each step or stage in ontogeny is adapted to the socio-ecological challenges that face the developing individual at that step or stage (or else it will not survive long enough to pass on its genes). Recognition of this basic fact can in many cases contribute to a better understanding of the nature of a particular psychological process by determining more accurately what it is ‘for’, what problems it evolved to solve.
In human ontogeny, the main issue is this. Many complex skills and motivations begin to emerge early in ontogeny because they need much time to develop, and this is especially true of those requiring extensive learning. These are often called ‘deferred adaptations' because they emerge in nascent form before they are really needed. But there are also some skills and motivations that begin to emerge early in ontogeny at least partly because they are needed for the individual to survive and thrive at that particular age. Such ‘ontogenetic adaptations’ emerge when they are needed for the developing individual's well-being, and indeed some of them (e.g. some infant reflexes) disappear after their function has been served. Of course, there is no reason why both processes may not be at work: a skill or motivation emerges that both serves an immediate function at that ontogenetic stage but also is a preparation for adulthood.
In this paper, I would like to use this general framework to focus on a set of uniquely human psychological processes that emerge in human infancy and early childhood. These are the processes that I have previously referred to under the rubric of ‘the 9-month revolution’, but now refer to more generally under the rubric of ‘skills and motivations of shared intentionality’. These include such things as joint attention, cooperative communication, dual-level collaboration and cultural learning, and I have argued in numerous places––based on experiments comparing humans with their nearest primate relatives––that these are all uniquely human and contribute to humans' dominance of the large mammal niche. These skills and motivations have mostly been taken by developmental psychologists (including myself) to be way stations on a trip to an adult endpoint. More recently, however, following Hrdy [1,2]; see also [3], I have come to believe––and will argue here––that these skills and motivations are also ontogenetic adaptations that enable infants and young children to navigate successfully their sociocultural worlds as structured by a cooperative regime of childcare (so-called cooperative breeding), which differs starkly from that of other great ape infants who are taken care of almost exclusively by mothers. Understanding the functions that these uniquely human psychological processes serve in early childhood will help us to better understand both what they are and how they work.
2. The emergence of uniquely human sociality
The individuals of all great ape species go through relatively protracted ontogenies, spending a large portion of their lives in immature form. The human version of this ‘extended immaturity’ life-history pattern is exaggerated and has some special characteristics. Humans' especially long period of immaturity may be most clearly seen by comparing the timing of brain development in humans and their nearest primate relatives. The adult human brain is roughly three times larger than that of other apes. But even neutralizing across this enormous size difference at maturity, the relative rates at which chimpanzee and human brains reach their respective adult sizes are very different. As can be seen in figure 1, already at birth the brains of chimpanzees are about half of their adult size, and then they reach 90% of their adult size by 2 years of age. By stark contrast, the brains of humans are only 20% of their adult size at birth and do not reach 90% of their adult size until 8 years of age. If we assume that chimpanzees are more or less representative of the last common ancestor between humans and other apes in this way (and that brain growth is a proxy for cognitive development), then this huge difference of timing indicates that over the last 6 million years of evolution, human cognitive ontogeny has slowed down dramatically.
Figure 1.
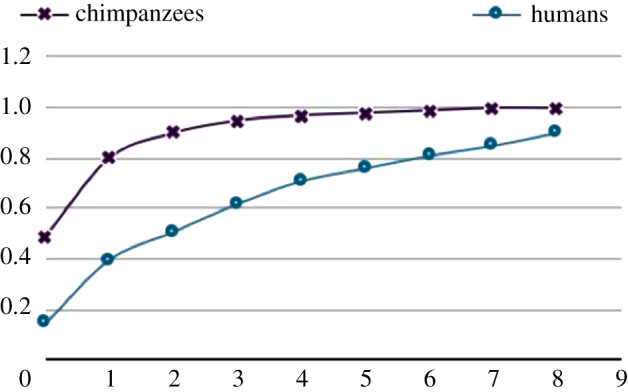
Percentage of adult brain size as a function of age in years in humans and chimpanzees (from [4], based on data from [5]).
Relatedly, human ontogeny, but not the ontogeny of other apes, occurs within the context of a highly cooperative social group, a.k.a. culture, whose members collaborate and help one another in myriad ways, including in foraging and in raising young. In this ultra-cooperative ontogenetic niche, children depend on many more adults in many more ways and for a much longer period of time than do other apes. The most basic way is in obtaining food. Great ape mothers wean their youngsters at around 4 to 5 years of age, and from then on they are on their own. By sharp contrast, after human children wean at around 3 years of age, they are provisioned with food by mothers and other adults until well into adolescence. In a study of hunter-gatherers, Hill & Hurtado [6] found that children typically do not provide their own food at a level sufficient for survival until mid-adolescence, and this same pattern would seem to hold, perhaps even more strongly, for children in modern industrialized societies.
In a similar fashion, great ape youngsters are pretty much on their own for gathering information about the world around them. They may learn things from others, but adults do not actively provision them with needed information via teaching or instruction [7]. Once again in sharp contrast, human children gain much information from intentional adult instruction, and this is true in societies of all types (including in hunter-gatherer groups in which adults instruct children in many non-verbal ways [8,9]). Indeed, for human children to acquire the local cultural skills on which their survival depends––and to develop normally in all kinds of other ways cognitively and socially––adult instruction is absolutely essential.
The key point for current purposes is that the ultra-cooperative ontogenetic niche within which human children develop is what makes possible their especially protracted ontogenies. Adults provisioning youngsters with food and information frees them from the costs and risks of doing so for themselves, and so they may take their time developing their cognitive and social skills.
(a). Uniquely human psychological ontogeny
Human infants possess some unique social-cognitive abilities, and so the question arises whether these special social-cognitive abilities are related in some way to the especially cooperative ontogenetic niche within which they develop and to their especially slow brain growth.
Herrmann et al. [10] administered a comprehensive battery of cognitive tests to large numbers of chimpanzees, orangutans and 2.5-year-old human children. The test battery consisted of 16 different non-verbal tasks assessing all kinds of cognitive abilities involving both physical problems and social problems relevant to primates in their natural environments. The tests relating to the physical world consisted of problems concerning space, quantities, and tools and causality. The tests relating to the social world consisted of problems requiring subjects to imitate another's solution to a problem, communicate non-verbally with others and read the intentions of others from their behaviour. If the difference between human and ape cognition is a difference in something like ‘general intelligence’, then the children should have differed from the apes uniformly across all of the different tasks. But this was not the case. The finding was that the children and apes had similar cognitive skills for dealing with the physical world; but the children––old enough to use some language but still years away from reading, counting or going to school––already had more sophisticated cognitive skills than either ape species for dealing with the social world.
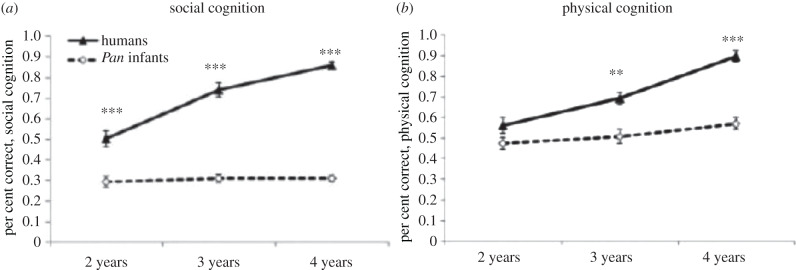
But one criticism of this study is that the apes were adults; perhaps ape youngsters have the same skills as human youngsters (and then lose them). In the most comprehensive study to date of great ape cognitive ontogeny, Wobber et al. [11] administered the same basic test battery as Herrmann et al. [10], but to chimpanzee and bonobo juveniles, at 2, 3 and 4 years of age (some data were collected longitudinally and some were collected cross-sectionally). The overall pattern of results (combining the two species of nonhuman great ape into Pan) is presented in figure 2. The results replicate––using immature apes––the most basic finding of Herrmann et al.: young humans and youngsters of the two species of Pan are indistinguishable in their skills with space, quantities and causality (i.e. physical cognition), but the children are already advanced in their skills with imitation, communication and intention reading (i.e. social cognition). In the period from 2 to 3 years of age, children's skills of social cognition continue increasing in dramatic fashion whereas those of apes do not develop further at all. Consistent with the idea that these rapidly developing social skills are leading the way to human adults' more sophisticated general cognitive skills, in the physical domain children go from being indistinguishable from apes at age 2 years to having significantly better skills at ages 3 and 4. Interestingly, this pattern of behavioural findings parallels rather closely the patterns of brain growth depicted in figure 1: chimpanzees’ skills of both social and physical cognition are basically mature already at 2 years of age (by which point their brains are 90% of adult size), whereas young children's skills in these domains are increasing significantly from age 2 to 4 as their brains go from roughly 50% to 75% of adult size.
Figure 2.
Overall results from Wobber et al. [11] (asterisks indicate a significant species difference).
Human infants’ unique social-cognitive skills are almost certainly adaptations for life in a cultural group, that is, life in which individuals must coordinate, communicate and learn from one another in myriad ways in order to develop normally. On the one hand, these unique skills put infants in a position to learn from adults over many years of interaction all the myriad things they need to learn to survive and thrive effectively in a cooperative cultural group (see [12]). On the other hand, however, it is also possible that these early-emerging skills and motivations are ontogenetic adaptations aimed at the special challenges of the human infancy period itself.
(b). The ontogenetic adaptations hypothesis
As noted above, human children are able to develop slowly because they are provisioned by adults with food and information for a long time. Importantly, and in contrast with other apes, this provisioning of food and information is done not just by mothers but by a variety of other adults. For all four nonhuman ape species, basically 100% of the care of offspring is provided by the mother, and youngsters stay in close proximity to their mothers for some time, often in bodily contact. By contrast, human adults form pair bonds with mates and live in cooperative cultural groups, and so children are raised not only by mothers but also by fathers, other relatives and friends. The outcome is that in human societies of all kinds, from hunter-gatherer groups to modern industrialized nations, after early infancy only about 50% of the care of offspring is provided by mothers, with the other half provided by fathers, grandmothers and friends [1]. This pattern of so-called cooperative breeding enables mothers to forage and engage in a variety of other tasks without distraction, and so to have offspring at more closely spaced intervals than other apes. For infants, this creates more competition for the care and attention of adults.
The need to socially interact with a wide array of different adults, in competition with more siblings and other children, presents unique cognitive and social challenges for human infants. Hrdy [2] argues that it was just these unique socio-ecological challenges that were the primary selective context for the emergence of human children's unique social-cognitive skills and motivations. She proposes that human infants' remarkable skills of social cognition, communication, collaboration and social learning––a.k.a., shared intentionality––are ontogenetic adaptations for navigating effectively the regime of cooperative childcare characteristic of the human, but not other ape, ontogenetic niche. In the context of both more adult carers and more child ‘competitors’, infant strategies to promote mother-infant attachment as characteristic of all primates (e.g. screaming when in danger) are no longer sufficient. Instead, for humans, those infants do best who can discern the thoughts and moods of carers in this new more complex social environment and so more skilfully solicit and recruit from them the help and care that they need.
Tomasello & Cabrera [13] offer a modified version of this hypothesis, emphasizing that such things as infants' emotion sharing, attention sharing (a.k.a. joint attention) and attitude sharing in cooperative communication with adults all tend to align the psychological states of infant and adult, and many studies in social psychology show that aligning psychological states with others––and even aligning behaviour in imitation and collaboration––promotes social bonding (e.g. [14]). As compared with classical forms of attachment and social bonding based on complementary actions (e.g. infants scream and mothers rescue), the sharing of emotions, attention, actions and attitudes may lead to an evolutionarily novel phenomenon: individuals form more positive social relationships with those with whom they share experience. And sharing experience is a key component in virtually all forms of uniquely human cooperation and shared intentionality.
3. Evidence for the ontogenetic adaptations hypothesis
There are three sets of data that support the hypothesis that human children's early-emerging, species-unique skills of shared intentionality are, at least in part, adaptations to the socio-ecological challenges facing them in the context of a cooperative breeding regime of childcare. I treat these each briefly in turn.
(a). Shared intentionality emerges earlier than is needed and promotes social closeness
The basics of humans’ unique skills and motivations of shared intentionality all emerge before 3 years of age, that is, before the traditional age of weaning. Then, there is more than another decade to go before adulthood. If these unique skills and motivations were not needed until adulthood, one might think that they would first emerge only during middle childhood and adolescence. So one very general argument for the ontogenetic adaptations hypothesis is simply that the emergence of children's unique skills and motivations of shared intentionality occurs much earlier than is needed, seemingly, if they are merely preparations for adulthood.
So what might their function be in infancy? As noted above, it is a well-established finding in social psychology that adults feel socially closer to one another when they share experience by, for example, engaging in synchronous movements, sharing political attitudes or even simply watching a film together [14]. Recently, Wolf & Tomasello [15] found that the same holds true between adults and young children. In their study, a 2-year-old child either watched a film with an unfamiliar adult sitting next to them or else the child watched the same film alone with an unfamiliar adult sitting next to her but reading a book (and not watching the film). Later, when the child was given the opportunity to approach their adult partner to play with a toy, children who had previously watched a film with her approached more quickly than did children who had watched the film alone while the adult read a book. This suggests that even something as simple as watching a film together serves to align the psychological states of toddler and adult, which, as between two adults, tends to strengthen their social relationship.
Tomasello & Cabrera [13] assumed that all of this was unique to the human species. However, if one thinks about how things might have got started evolutionarily, infants must have been tapping into something that was already operative in adults. That is, infants' new skills for aligning psychological states with others could only be effective in forming more positive social relationships with adults if adults already possessed some tendencies in this direction also. Interestingly, and counter to their original hypothesis, Wolf & Tomasello [16] thus found that adult chimpanzees who watched a film together subsequently spent more time in proximity to one another than they did if one of them had watched the film alone (with the other similarly close by, but not watching owing to the positioning of the monitor). Although the evolutionary contexts in which such a social process evolved are unknown, it is possible that chimpanzees and other apes developed a tendency to form positive social relationships with those whom they travelled or fought side-by-side, which in both cases involve a visual co-orientation in a common direction.
It may be that the psychological processes involved in the human and ape cases are different (see [17]), but the key point for current purposes is that this finding suggests that early human adults already formed positive social relationships with others by aligning psychological states with them. This set the stage for children to exploit this tendency, that is, to elicit care and attention from adults not just by attachment behaviours (in a relatively inflexible way), but rather using shared intentional activities to form more positive social relationships with them. This stronger positive relationship would make adults prefer to interact with children who engaged them in this way more than ‘competitor’ children.
(b). Infants’ shared intentionality activities are almost exclusively with adults
Another piece of evidence that argues for the ontogenetic adaptation hypothesis is that young children––that is, before the age of weaning at age 3 years––use their species-unique skills of shared intentionality almost exclusively with adults. Of course this pattern might reflect a kind of ‘training wheels’ approach in which children practice their skills with adults and then later use them with more challenging peers, simply as a way of developing those skills effectively. However, this pattern might also reflect something more direct: infants use their species-unique skills with adults because this promotes their development during the infancy period itself.
In a classic study, Bakeman & Adamson [18] documented patterns of joint attention between human infants and mothers, with substantial developments occurring during the 9–18 month age period. Interestingly, Bakeman & Adamson also looked at infant peers interacting together. They found little coordinated joint engagement between them; they mostly played in parallel. This finding is consistent with the proposal that at this early developmental period joint attention is a mode of interaction that adapts infants especially for interactions with their adult carers.
Relatedly, human infants and young children are able to communicate with adults in unique ways, as compared with infant peers. That is, infants communicate with adults using their species-unique forms of cooperative communication––earliest in the form of the pointing gesture but then later via other gestures and language––in ways that they do not do with other infants. Kachel et al. [19] found that young 2-year-old children used their pointing gesture more often for adult partners than for peer partners, even if adult and peer behaved identically. Moreover, as recipients of a pointing gesture, children used an adult's pointing gesture to find a hidden toy more often than they used a peer's. In terms of language, Ninio [20] observed that 2- and 3-year-old children only rarely used language to get peers to jointly attend with them to external entities, whereas the same children did so much more often with adults. These findings are consistent with the idea that children's early skills and motivations of communication are specifically aimed at, and possibly adapted for, adult recipients.
Finally, infants collaborate reasonably well with an adult from before their second birthdays (e.g. [21]). However, infants of this same age are not very skilful with a peer. Brownell & Carriger [22] observed pairs of 18-month-old infants working together in a fairly simple task in which one individual had to move the lever to put a reward in front of a hole, and then the other had to retrieve it. The infants were sometimes successful, but then on the very next trial they could not reproduce their success; they did not seem to know what they were doing. Brownell et al. [23, p. 283] extended these findings using a different task, again focusing on 1- and 2-year-old children and found that ‘one-year-olds' coordinated actions appeared more coincidental than cooperative whereas older children appeared to be more actively cooperating toward a shared goal’. Young children collaborate first and best with adults, not peers.
Overall, then, it would seem that children's earliest skills of shared intentionality––joint attention, cooperative communication and collaboration––are adapted for adult partners who can interact in the requisite ways. Only sometime after that are they able to use these skills equally well with peers. But even then we are talking about peers still in early childhood and before school age, and there are still many years to go before they will be using these skills as adults with adult peers.
(c). Common social-cognitive skills emerge earlier in humans than in apes
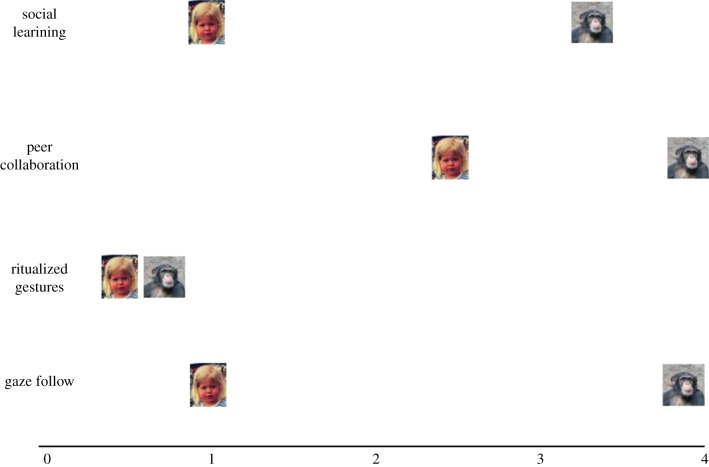
A third piece of evidence for the ontogenetic adaptations hypothesis is the developmental timing of the social skills that human children have in common with other apes. Tomasello [4] compared the developmental timing in humans and great apes in three skills (for which there was sufficient data): gaze following (as a precursor to joint attention), ritualized gestures (as a precursor to cooperative communication), and social learning (as a precursor to cultural learning).
Human infants begin following the gaze direction of others to close-in targets, within peripheral vision, at around six months of age. Following gaze direction to more distant targets emerges at closer to 12 to 13 months of age, and this includes when the gazer looks up to the ceiling as in several ape studies. When an adult looks behind a barrier, infants as young as 12 months of age will locomote some distance so that they can get the right angle to look behind it too, and 14-month-olds know when a demonstrator's gaze is blocked by an opaque barrier (see [4] for a review of the primary studies). In all of these ways, the gaze following of human infants is similar to that of great apes, but it appears much earlier in human than in ape ontogeny. If one compares humans and chimpanzees in each of the experimental paradigms in which they have both been tested ontogenetically, the ages for humans are in all cases at least 1 year earlier, and in many cases more like 2 to 3 years (figure 3). From soon after the middle of the first year, before the nine-month revolution, infants often produce ritualized body movements that are similar to great apes' intentional gestures. For example, having tried to climb up an adult, and having been picked up as a result, they might then raise their arms toward the adult (and perhaps whine) as a request to be picked up. Such gestures are very similar to great apes' ‘intention-movement’ gestures. Infants also at this age do something similar to great apes' ‘attention-getter’ gestures, for example, by making movements or sounds that draw attention to themselves in acts of ‘showing off’. Also, analogous to apes' ritualized reaching cum pointing, infants at around 9 or 10 months of age begin producing a ‘whole hand pointing’ gesture, a kind of ritualized reaching in which they expect an adult to retrieve an object for them. Although human infants' ritualized gestures emerge at more or less the same developmental period as great ape ritualized gestures, they tend to come in a few months earlier (figure 3).
Figure 3.
Summary of results from Tomasello [4], comparing age of emergence of common social-cognitive skills in human and great ape infants and youngsters.
In terms of collaboration, the problem is that great ape infants have been given very little opportunity to collaborate on their own without their mothers, who are normally always present. Some ‘orphan’ juveniles have learned to collaborate with peers, but only after some training from humans. We do not have the needed data, but from what data we do have it is unlikely that great ape infants could collaborate with peers at the tender age of 2 to 3 years, as is typical with human children (figure 3).
Finally, in the months around their first birthdays, human infants begin to socially learn and reproduce the actions of others, some of which apes do as well. Tomasello & Carpenter [24] gave a systematic battery of imitation tasks to three human-raised chimpanzees longitudinally over the first few years of life. Wobber et al. [11] gave some similar but different imitation tasks to chimpanzee and bonobo youngsters. The common tests across species include straight imitation of actions on objects; the imitation of intentional over accidental actions; the reproduction of intended actions in a failed attempts paradigm; the reproduction of the ‘style’ of an action even when it was causally irrelevant and rational imitation (again see [4] for a review of the primary studies). Human children are successful in all of these at around 12 to 16 months of age. Great apes, by contrast, show no evidence of any of these kinds of imitative learning until 2.5 to 3 years of age. Importantly, because of the developmental focus of the relevant ape studies, we have in these data a number of well-documented data points where great apes failed to imitate at younger ages, which are crucial for setting the lower limit in ontogeny. So once again we have a clear developmental shift of skills to a period more than 1 year earlier in human infants (figure 3).
The theoretical question is thus why gaze following, communicative gestures, collaboration and social learning skills should have shifted in great ape ontogeny to a younger age of emergence in humans. There certainly could be other reasons, but this shift is at least consistent with the ontogenetic adaptations hypothesis that human infants at some point began evolving skills for dealing with the special socio-ecological challenges of human infancy in its cooperative breeding context.
4. A hybrid model
A central insight of evolutionary developmental biology (evo-devo; e.g. [25]) is that many, if not most, evolutionary changes result from changes in ontogenetic timing. That is, if across generations an adaptation moves to earlier or later in ontogeny, or takes shorter or longer to develop, the result can be huge changes in the adult phenotype owing to the interaction of this change with existing developmental pathways. For example, when over evolutionary time juvenile characteristics come to be extended into adulthood in a pattern of neoteny, the effects may extend well beyond that characteristic itself (e.g. childhood curiosity combines with adult cognitive and social skills to result in scientific experimentation). Or if it were the case that running was originally an adaptation for adults stalking prey, if it extended to an earlier period in ontogeny, it might now be adaptive for children outrunning predators, which might prompt adults to give children more autonomy at an earlier age, which might then lead to all kinds of other social and cognitive changes.
The way such changes happen are strictly Darwinian, but they must be viewed in a developmental context. Thus, many changes in developmental timing are a simple result of the fact that owing to random variation an adaptation emerges in some individuals a bit earlier or a bit later than the norm, or the adaptation takes shorter or longer to develop than is typical. If there is a fitness benefit for individuals at the extreme––e.g. those in whom the adaptation emerges earliest or lasts longest––then a change in timing for the species should occur. The fitness benefit is first and foremost about the particular developmental periods involved, but it may extend to later developmental periods if, for example, skills developed at one period are especially good preparation for challenges to be faced at some later period. Importantly, any time there is a potential change in developmental timing, there are ‘developmental constraints' that must be overcome, in the sense that the change in timing of one system cannot disrupt already existing systems inordinately; changes in timing must work not just locally, but for the organism as a whole.
In this context, our hybrid model can be re-cast as follows: adaptations for shared intentionality that evolved to facilitate adult activities (i.e. collaborative foraging) ‘migrated down’ in ontogeny because they benefitted immature individuals in their collaborative activities as well. This migration process, in turn, also benefitted later adults, as earlier developing skills enabled extra preparation and practice for collaborative activities during adulthood. At the same time, ontogenetic adaptations for shared intentionality that were beneficial to infants and young children in a cooperative breeding context ‘migrated up’ in ontogeny because they benefitted older children in their collaborative and communicative activities with peers. These individuals were then better adapted for adult activities relying on collaboration and communication. Our proposal is that both of these processes in fact occurred; that is, human cooperative breeding and collaborative forms of subsistence co-evolved, as mothers who received help rearing their young could be much more productive in collaborative foraging (and the helpers would share in the fruits of her labour). This combined hypothesis would explain both why skills of shared intentionality first emerge in contemporary human ontogeny in infants and young children––that is, as ontogenetic adaptations for infancy in a cooperative breeding context––and why contemporary humans have the complex cognitive skills for social and cultural coordination that they do––that is, as these ontogenetic adaptations extended to older children and adults and interacted with other systems for solving adult problems of social and cultural coordination (and perhaps set the stage for new adaptations for these older individuals as well) in ways that increased adult fitness.
Our hybrid model thus comprises both bottom-up and top-down processes of natural selection operating within ontogeny. Bottom-up processes are those in which an ontogenetic adaptation migrates up and increases the fitness of older individuals, and top-down process works in the reverse order. Whichever process is at work, if there is a fitness benefit for adults who possess the competency, then an earlier age of emergence also creates a kind of ontogenetic top-down selective pressure for younger individuals to develop the competency as a deferred adaptation preparing them better for adulthood, assuming that a longer ontogeny, beginning earlier, is advantageous for developing the competency.
5. Conclusion
The current analysis thus stresses that human adults' species-unique skills and motivations of shared intentionality may have two evolutionary sources. One is adults’ need to coordinate and communicate and collaborate with others, because this is what was needed in early humans to succeed in collaborative foraging for resources. But, in addition, as this cooperative lifestyle emerged, a cooperative breeding pattern of childcare also emerged. In this context, infants and young children evolved the ability to exploit pre-existing adult tendencies––in particular, the tendency to form positive social relationships with those with whom one travelled or fought side-by-side––so as to elicit from them greater care and attention.
With regard to this latter function, one can easily see––as already noted extensively by Hrdy [1]––the parallels with the classic functions of attachment. We may thus discern the following pattern over different taxa in the animal world. The infant geese and ducks studied by Konrad Lorenz ‘imprint’ on their mothers in fairly rigid and stereotypical ways (enabling Lorenz to manipulate the process in sometimes humorous ways, for example, getting chicks to imprint on a moving balloon). Bowlby [26] went beyond the imprinting model and focused mainly on primates and other mammals. They ‘attach’ to their mothers based less on rigid and stereotypical innate behaviours, and much more on more flexibly deployed emotions. Although Bowlby and other clinically oriented developmentalists sought to establish long-lasting effects of different styles of attachment on human adult functioning, the original theory posits essentially an ontogenetic adaptation: human infants are adapted to the time-limited socio-ecological challenge that their mothers quite often, relative to other primates, put them down or allow others to hold their babies. Evolutionarily, infants had to find a way to make sure that this practice was not lethal for them.
In this context, we would emphasize that it is perhaps not accurate to say that human infants ‘imprint on’ or ‘attach to’ their mothers or any other adults. Rather, we should say that by aligning psychological states with adults––emotions, attention and actions––infants are forming deeper and more positive social relationships with them. This is because, unlike the case with other primates, infants are not trying to elicit provisioning of food and protection from predation from dedicated carers, with the only competition being other things the carers might be doing. Rather, in the context of cooperative breeding, human infants and young children are attempting to get various adults to prefer interacting with them over other children: they want the adults to have a better relationship with them. Also, because adults already form deeper and closer relationships with those with whom they share psychological states, infants evolved the ability to do this––through such interactive behaviours as joint attention, cooperative communication and collaboration––at a very young age.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Hrdy S. 2009. Mothers and others. The evolutionary origins of mutual understanding. Cambridge, MA: The Belknap Press of Harvard University Press. [Google Scholar]
- 2.Hrdy SB. 2016. Development plus social selection in the emergence of ‘emotionally modern’ humans. In Childhood: origins, evolution, and implications (eds Meehan CL, Crittenden AN), pp. 11–44. Albuquerque, NM: University of New Mexico Press. [Google Scholar]
- 3.Hawkes K. 2014. Primate sociality to human cooperation. Why us and not them? Human Nature 25, 28–48. ( 10.1007/s12110-013-9184-x) [DOI] [PubMed] [Google Scholar]
- 4.Tomasello M. 2019. Becoming human: a theory of ontogeny. Cambridge, MA: Harvard University Press. [Google Scholar]
- 5.Coqueugniot I, Hublin J-J, Veillon F, Houet F, Jacob T. 2004. Early brain growth in Homo erectus and implications for cognitive ability. Nature 231, 299–302. [DOI] [PubMed] [Google Scholar]
- 6.Hill K, Hurtado K. 2009. Cooperative breeding in South American hunter-gatherers. Proc. R. Soc. B 276, 3863–3870. ( 10.1098/rspb.2009.1061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornton A, Raihani NJ. 2008. The evolution of teaching. Anim. Behav. 75, 1823–1836. ( 10.1016/j.anbehav.2007.12.014) [DOI] [Google Scholar]
- 8.Kruger A, Tomasello M. 1996. Cultural learning and learning culture. In Handbook of education and human development: new models of teaching, learning, and schooling (eds Olson D, Torrance N), pp. 353–372. London, UK: Blackwell. [Google Scholar]
- 9.Hewlett BS, Roulette CJ. 2016. Teaching in hunter– gatherer infancy. R. Soc. Open Sci. 3, 150403 ( 10.1098/rsos.150403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann E, Call J, Hernández-Lloreda MV, Hare B, Tomasello M. 2007. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366. ( 10.1126/science.1146282) [DOI] [PubMed] [Google Scholar]
- 11.Wobber V, Herrmann E, Hare B, Wrangham R, Tomasello M. 2013. Differences in the early cognitive development of children and great apes. Dev. Psychobiol 56, 547–573. ( 10.1002/dev.21125) [DOI] [PubMed] [Google Scholar]
- 12.Gopnik A. 2020. Childhood as a solution to explore–exploit tensions. Phil. Trans. R. Soc. B 375, 20190502 ( 10.1098/rstb.2019.0502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomasello M, Gonzalez-Cabrera I. 2017. The role of ontogeny in the evolution of human cooperation. Human Nature 28, 274–288. ( 10.1007/s12110-017-9291-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf W, et al. 2016. Joint attention, shared goals, and social bonding. Br. J. Psychol. 107, 322–337. ( 10.1111/bjop.12144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf W, Tomasello M. 2019. Watching a video together creates social closeness. J. Exp. Child Psychol. 189, 1–12 (104712). [DOI] [PubMed] [Google Scholar]
- 16.Wolf W, Tomasello M. 2019. Visually attending to a video together facilitates great ape social closeness. Proc. R. Soc. B 286, 20190488 ( 10.1098/rspb.2019.0488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf W, Tomasello M. Submitted. Human children, but not great apes become socially closer by sharing an experience in common ground. [DOI] [PubMed]
- 18.Bakeman R, Adamson L. 1984. Coordinating attention to people and objects in mother-infant and peer-infant interactions. Child Dev. 55, 1278–1289. ( 10.2307/1129997) [DOI] [PubMed] [Google Scholar]
- 19.Kachel G, Moore R, Tomasello M. 2018. Two-year-olds use adults' but not peers’ points. Dev. Sci. 21, e12660 ( 10.1111/desc.12660) [DOI] [PubMed] [Google Scholar]
- 20.Ninio A. 2016. Bids for joint attention by parent–child dyads and by dyads of young peers in interaction . J. Child Lang. 43, 135–156. ( 10.1017/S0305000915000082) [DOI] [PubMed] [Google Scholar]
- 21.Warneken F, Chen F, Tomasello M. 2006. Cooperative activities in young children and chimpanzees. Child Dev. 77, 640–663. ( 10.1111/j.1467-8624.2006.00895.x) [DOI] [PubMed] [Google Scholar]
- 22.Brownell CA, Carriger MS. 1990. Changes in cooperation and self-other differentiation during the second year. Child Dev. 61, 1164–1174. ( 10.2307/1130884) [DOI] [PubMed] [Google Scholar]
- 23.Brownell CA, Ramani GB, Zerwas S. 2006. Becoming a social partner with peers: cooperation and social understanding in one- and two-year-olds. Child Dev. 77, 803–821. ( 10.1111/j.1467-8624.2006.00904.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomasello M, Carpenter M. 2005. The emergence of social cognition in three young chimpanzees. Monogr. Soc. Res. Child Dev. 70. [DOI] [PubMed] [Google Scholar]
- 25.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Bowlby J. 1969. Attachment and loss. New York, NY: Basic Books. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.