Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 is the agent responsible for the coronavirus disease 2019 (COVID-19) global pandemic. SARS-CoV-2 is closely related to SARS-CoV, which caused the 2003 SARS outbreak. Although numerous reagents were developed to study SARS-CoV infections, few have been applicable to evaluating SARS-CoV-2 infection and immunity. Current limitations in studying SARS-CoV-2 include few validated assays with fully replication-competent wild-type virus. We have developed protocols to propagate, quantify, and work with infectious SARS-CoV-2. Here, we describe: (1) virus stock generation, (2) RT-qPCR quantification of SARS-CoV-2 RNA; (3) detection of SARS-CoV-2 antigen by flow cytometry, (4) quantification of infectious SARS-CoV-2 by focus-forming and plaque assays; and (5) validated protocols for virus inactivation. Collectively, these methods can be adapted to a variety of experimental designs, which should accelerate our understanding of SARS-CoV-2 biology and the development of effective countermeasures against COVID-19.
Keywords: Coronavirus, Titration, Plaque assay, Focus-forming assay, Flow cytometry, Virus inactivation, SARS-CoV-2
1. Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV)-2 is an enveloped virus with a single-stranded positive-sense RNA genome. Zoonotic transmission of SARS-CoV-2 from an as yet unidentified animal reservoir occurred in late 2019. Subsequent human-to-human transmission by respiratory droplets has resulted in the ongoing Coronavirus disease 2019 (COVID-19) pandemic that has infected millions of people worldwide (Wu et al., 2020b; Zhou et al., 2020; Zhu et al., 2020). The rapid spread and relatively high case fatality rate of COVID-19 has led to an urgent need to develop diagnostics, therapeutics, and vaccines.
The SARS-CoV-2 genome is comprised of approximately 30,000 nucleotides. The first two-thirds of the genome encodes for nonstructural proteins in open reading frames 1a and 1b that principally facilitate genome replication and viral RNA synthesis. The remaining one-third is comprised of genes encoding structural proteins such as spike (S), envelope (E), membrane (M), and nucleocapsid (N), which form the virion, and accessory proteins that regulate host cellular responses. Whole-genome phylogenetic analysis identified the SARS-like bat CoV (GenBank MG772933) as the closest known relative of SARS-CoV-2. Bats also are the reservoir host for SARS-CoV (Wu et al., 2020a). Alignment of SARS-CoV-2 to the consensus sequence of SARS-like CoV revealed 380 amino acid differences including 27 amino acid differences in the S protein and six substitutions in the receptor binding domain (RBD) (Wu et al., 2020a).
SARS-CoV entry is mediated by initial engagement of the RBD of the S protein with the human ACE2 receptor (Li et al., 2003, 2005), and recent studies have established that SARS-CoV-2 utilizes the same receptor for entry (Letko et al., 2020). The S protein also is a key target for neutralizing antibodies and vaccine strategies (Rockx et al., 2008; Sui et al., 2005; Zhu et al., 2007). Although the S protein of SARS-CoV and SARS-CoV-2 are structurally similar (Li et al., 2005; Walls et al., 2020; Wrapp et al., 2020), genetically similar (Walls et al., 2020), and use the same receptor (Lei et al., 2020; Li et al., 2003), neutralizing anti-SARS-CoV RBD antibodies (Abs) generally lack cross-reactivity to SARS-CoV-2 (Wrapp et al., 2020). However, polyclonal sera from mice immunized with recombinant SARS-CoV RBD protein inhibits SARS-CoV-2 infection (Walls et al., 2020). Recent studies have identified cross-reactive, non-neutralizing monoclonal Abs (mAbs) against SARS-CoV and SARS-CoV-2, which were isolated previously using phage display or hybridoma fusion screens (Joyce et al., 2020; ter Meulen et al., 2006; Tian et al., 2020; Tripp et al., 2005; Yuan et al., 2020). Competition binding studies show that two of these mAbs, CR3022 and 240CD, both recognize the SARS-CoV-2 RBD. A co-crystal structure revealed that CR3022 binds an epitope on the RBD distal to the binding site of ACE2 and SARS-CoV neutralizing antibodies (Yuan et al., 2020).
SARS-CoV-2 research must be performed in a biosafety level 3 laboratory by personnel equipped with a powered air-purifying respirator (PAPR). This limitation has compelled the development of many in vitro assays that utilize heterologous pseudotyped viruses expressing the SARS-CoV-2 S protein (Lei et al., 2020; Letko et al., 2020). However, this approach only can be used to study cellular and antibody interactions involving the S protein that principally affect attachment and entry. Here, we developed or adapted multiple methodologies to quantify SARS-CoV-2 infection in vitro using a patient isolate of SARS-CoV-2: 1) RT-qPCR quantification of viral RNA; 2) detection of viral antigen by flow cytometry; 3) focus-forming assay through immunostaining of the S protein and 4) plaque assay. We also have identified and validated chemical and heat treatment methods to inactivate replication-competent virions, which are compatible with downstream quantification assays. Together, the methodologies can be used to examine SARS-CoV-2 pathogenesis and antibody responses, and to screen for potential inhibitors of infection.
2. Results and discussion
Propagation of SARS-CoV-2 in vitro. Isolates of SARS-CoV-2 from patients or animals often need to be propagated to generate high-titer virus stocks. We have tested several cell types and found African Green Monkey cell lines and derivatives thereof to be most permissive to SARS-CoV-2 infection. These include Vero-CCL81 (ATCC-CCL81), Vero-furin (Mukherjee et al., 2016), Vero E6 (ATCC-CRL1586), Vero-TMPRSS2 (Matsuyama et al., 2020), and MA104 (ATCC-CRL-2378.1) cells. Each cell type is sufficient to propagate SARS-CoV-2 using the protocol detailed below. All procedures should be completed only after appropriate safety training is obtained and using aseptic technique within a certified biosafety cabinet under BSL-3 containment.
2.1. Materials needed
Chosen cell type (Vero-CCL81, Vero-furin, Vero E6, Vero-TMPRSS2, and MA104 cells)
Standard media for chosen cell type (see Recipes)
Infection media (see Recipes)
SARS-CoV-2 seed stock
150 cm2 (T150) tissue culture flasks
15 mL disposable polystyrene conical tubes with screw caps (e.g., Falcon)
50 mL disposable polystyrene conical tubes with screw caps (e.g., Falcon)
1.5 mL or 0.5-mL O-ring tubes
-
1.)
In a standard BSL2 laboratory, plate cells for infection one day prior into two T150 flasks in standard media for the chosen cell type. One flask serves as a mock-infected control and the other for infection. Plate cells so they will be ~80–90% confluent the following day. *For instance, plate 1 x 10 7 Vero CCL81 cells per T150 flask. Place flasks in a humidified 37 °C incubator with 5% CO2 overnight.
-
2.)
Transfer flasks into BSL3 facility the following day. Rapidly thaw a SARS-CoV-2 stock at 37 °C. Calculate the volume of virus needed to infect at the desired multiplicity of infection (MOI) using the following formula:
-
3.)
Add the volume of virus calculated above to 20 mL of infection medium.
-
4.)
Remove medium from T150 flasks. Replenish with 20 mL of fresh infection medium for mock-infected flask. Add 20 mL of infection medium containing virus from step #3 to flask for infection.
-
5.)
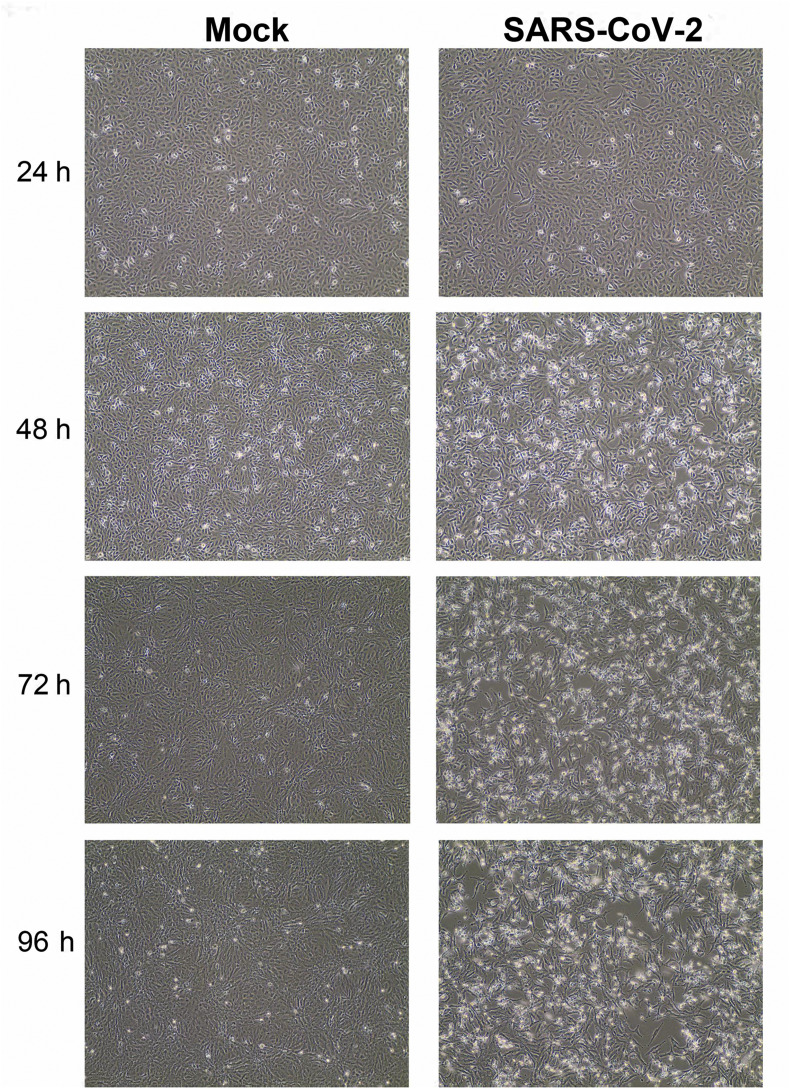
Incubate for 48–72 h at 37 °C monitoring daily for evidence of cytopathic effect (CPE) (Fig. 1 ). Use the mock-infected flask as a control for subtle CPE.
Fig. 1.
SARS-CoV-2 causes cytopathic effect on Vero E6 cell monolayers. Vero E6 cells were inoculated with SARS-CoV-2 at an multiplicity of infection (MOI) of 0.01 plaque forming unit (PFU)/cell and monitored for cytopathic effect at the indicated timepoints. Images were collected using an EVOS XL Core Imaging System. Magnification is 10X for all images.
*Harvesting at 48–72 h post-inoculation has yielded the best titers in our hands; although, titers remain roughly the same when incubated for longer periods (4–5 days). CPE should be apparent by day 3 in Vero or MA104 cells.
-
6.)
To harvest virus, collect the cell culture supernatant by pipetting the media into two 15 mL conical tubes. Centrifuge at 450×g for 5 min at 4 °C to clarify supernatants and pellet cell debris. Combine the supernatant from all tubes into a single vessel and gently mix using a serological pipette to ensure homogeneity across aliquots of the stock. Pipette the supernatant into small aliquots (200–500 μL) in O-ring tubes. Store at −80 °C.
Real-time PCR assay for SARS-CoV-2 detection. Detection of viral RNA by reverse-transcription quantitative polymerase chain reaction (RT-qPCR) using a TaqMan probe is a highly-sensitive and specific method for measuring viral burden in a variety of specimens. Because CoVs generate subgenomic RNAs as a template for translation, the abundance of viral RNA varies for each gene and depends upon the gene position within the genome. Genes located closer to the 3′ end of the (+) sense genome will have a greater abundance of transcripts than those located at the 5′ end of the (+) sense genome. This should be considered when designing primer/probe combinations, as “N gene” transcripts will be more abundant than genomic RNA copies, which can be quantified by targeting sequences within the ORF1a gene. Many primer/probe combinations have been designed and validated, several of which are used in clinical diagnosis (CDC, 2020; Corman et al., 2020). In the clinical setting, precise copy-number quantitation of viral RNA is not necessary and instead sensitivity is paramount. However, quantitative assays are desirable for research applications, and may have utility in longitudinal studies of infected human subjects. RT-qPCR cycle threshold (Ct) values can be converted to transcript or genome copy number equivalents by generating an RNA standard curve, the design and production of which is described below.
2.2. Design of the primer/probe combination
The CoV replication strategy should be considered when designing a RT-qPCR assay. Primer/probe combinations targeting the N gene are most sensitive; those targeting the spike gene can also be used to titer spike-containing pseudoviruses; those targeting the ORF1a gene provide genome equivalents; and those targeting the leader sequence can give an estimation of the total number of viral transcripts (Table 1 ). For a given viral gene target, a template (~500–1000 bp) for in vitro transcription can be generated by RT-PCR using primers that flank the intended target, with the forward (F) primer also including a 5′ T7 promoter sequence (Vogels et al., 2020). If multiple targets are desired, a single dsDNA fragment can be synthesized to include concatenated gene fragments, each of which spans the entirety of the target amplicons. This strategy also can be used to quantify host genes of interest (e.g., ACE2).
Table 1.
Primer/probe combinations for detection of SARS-CoV-2 RNA.
| Assay name | Target | F primer sequence | R primer sequence | Probe Sequence | Designer |
|---|---|---|---|---|---|
| 5′UTR | 5′UTR | ACTGTCGTTGACAGGACACG | AACACGGACGAAACCGTAAG | CGTCTATCTTCTGCAGGCTG | ALB |
| ORF1a | ORF1a | TTCAGTTGACTTCGCAGTGG | GGACGGGTTTGAGTTTTTCA | AACTAACATCTTTGGCACTGTTT | ALB |
| nCoV_ALB | N gene | ATGCTGCAATCGTGCTACAA | GACTGCCGCCTCTGCTC | TCAAGGAACAACATTGCCAA | ALB |
| N1 | N gene | GACCCCAAAATCAGCGAAAT | TCTGGTTACTGCCAGTTGAATCTG | ACCCCGCATTACGTTTGGTGGACC | CDC |
| N2 | N gene | TTACAAACATTGGCCGCAAA | GCGCGACATTCCGAAGAA | ACAATTTGCCCCCAGCGCTTCAG | CDC |
| N3 | N gene | GGGAGCCTTGAATACACCAAAA | TGTAGCACGATTGCAGCATTG | AYCACATTGGCACCCGCAATCCTG | CDC |
ALB = Adam L. Bailey.
CDC = Centers for Disease Control and Prevention.
2.3. Construction of the RNA standard
-
1.
(Day 1) The DNA fragment/amplicon containing the primer/probe targets to be used in the RT-qPCR assay should be introduced into a vector containing a T7 (or other DNA-dependent RNA-polymerase) promoter sequence using Gibson Assembly, restriction enzyme cloning, blunt-end ligation, or gene synthesis. These vectors should be transformed into competent E. coli (e.g., DH5α) for antibiotic selection.
-
2.
(Day 2) Pick clones and amplify to miniprep scale. We normally pick 6 to 12 clones to ensure proper cloning.
-
3.
(Day 3) Purify plasmid from clones, and identify a clone with the proper insert using restriction enzyme digestion and/or Sanger sequencing.
-
4.
(Day 4) Linearize ~2–4 μg of the DNA in preparation for in vitro transcription by performing an overnight restriction digest using a high-fidelity restriction enzyme that cuts each plasmid only once in a position 3′ to the insert. The distance between the T7 transcriptional start-site and the 3′ end restriction site should be ~500–1500 nucleotides.
-
5.
(Day 5) Run the linearized product on a 1% agarose gel. A shift in fragment size should be apparent relative to the non-linearized plasmid. Extract and cleanup the linearized product with a commercially-available gel-extraction (e.g., Qiagen) kit.
-
6.
Perform in vitro transcription using a commercially available kit (e.g., MEGAscript T7). Note: to prevent contamination of PCR workstations with transcribed RNA, all steps hereafter should be performed in a contained hood/workspace that is separate from the area where PCR reaction setup is performed.
-
7.
Digest DNA with DNase, then perform RNA cleanup using a commercially available kit (e.g., MEGApure).
-
8.
Quantify the RNA using a spectrophotometer (e.g., Nanodrop or Qubit) by diluting the RNA with RNase-free water until the concentration is within the analytical measurement range of the spectrophotometer.
-
9.
Calculate the copies of RNA transcript within each μL:
Note: the molecular weight can be calculated online (e.g., OligoCalc website).
-
10.
Dilute the transcript with RNase-free water containing 1% of added ribonuclease inhibitor (e.g., RNaseOUT) to obtain 1–2 mL of standard at a 1 × 1010 copies/μL. Mix by pipette.
-
11.
Aliquot the diluted RNA transcript into PCR strip tubes (with individual caps) in aliquots of 6–12 μL/aliquot.
-
12.
Freeze at −80 °C. The remaining concentrated RNA can be frozen and re-quantified later as needed.
2.4. Validation and use of the RNA standard
The RNA standard is concentrated and poses a risk for contamination of reagents and specimens. Follow best-practices for PCR preparation (Standards Unit, 2010) and only handle RNA standards after all reagents and specimens have been stored. Appropriate no-template controls must be used to eliminate and track possible contamination. Wipe down work areas and pipettes with 10% bleach followed by 70% ethanol. Bleach pipette tips.
-
1.
Create a 20x stock of primer/probe mix by diluting primers to a concentration of 10 μM and probe to a concentration of 2 μM.
-
2.
For “n” number of reactions, create a master-mix for n+1 by combining one-step RT-qPCR reaction buffer, primer/probe mix, and reverse-transcriptase enzyme at the appropriate concentration/volumes. Aliquot master-mix into wells of a RT-qPCR-compatible plate.
-
3.
Separate a single tube containing the RNA standard from the stock. Work quickly to avoid thawing other aliquots in the adjacent strip tubes.
-
4.
Thaw the aliquot and briefly centrifuge to collect contents at the bottom of the tube.
-
5.
Dilute the standard into a volume of RNase-free water to obtain 1.0 × 109 RNA copies per reaction. Mix gently but thoroughly by pipette. Change gloves.
-
6.
Make 10-fold serial dilutions in a PCR strip-tube by transferring 10 μL into 90 μL of RNase-free water. Mix each dilution thoroughly with a p100 pipette set to 70 μL. Discard tips between each dilution.
Note: When testing a new RNA standard, perform serial dilutions several-fold below 1 copy per reaction. Reactions containing less that 1–10 copies/well should fail to amplify.
-
7.
Transfer the appropriate volume of RNA standard from each dilution into the reaction plate using a multichannel pipette.
-
8.Perform real-time PCR using the following thermocycling parameters:
-
1.48 °C for 15 min
-
2.95 °C for 10 min
-
3.95 °C for 15 s
-
4.60 °C for 1 min – Acquire Signal
-
5.Go to "step 3″ 49x (i.e., 50 cycles)
-
1.
Note: these parameters may vary depending on the specific RT-qPCR kit used; our parameters have been tested using the TaqMan RNA-to-CT 1-step kit (Applied Biosystems) on the QuantStudio 6 flex Real-time PCR system (Applied Biosystems).
-
9.
Upon completion of the run, examine your standard curve. Approximately 3.3 Ct should separate each dilution, which corresponds to a change of one log10 copies for a reaction that is >90% efficient.
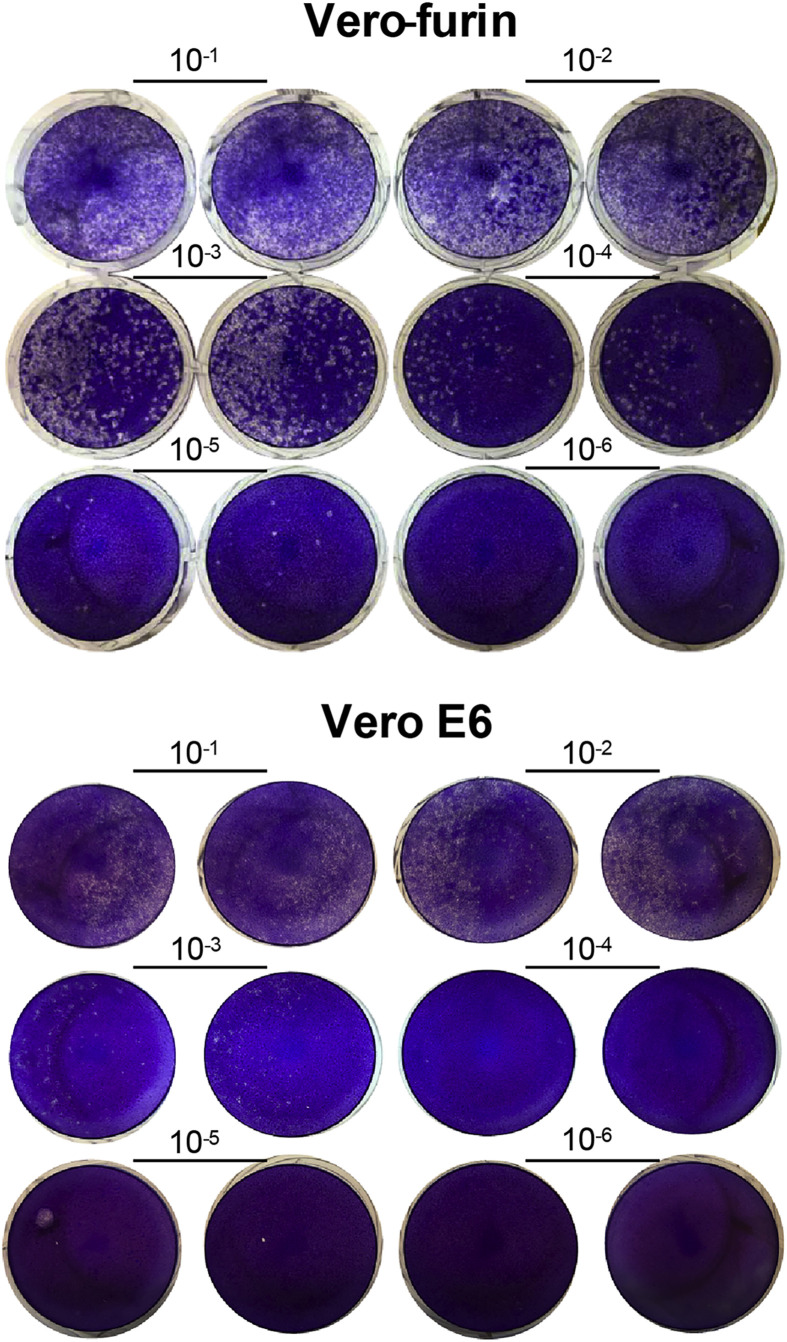
Quantification of SARS-CoV-2 by plaque assay. The plaque assay is the gold standard test for quantifying infectious virus in a sample. The plaque assay measures “plaques,” which describe the zone of cellular death that occurs after one infectious unit has entered a cell and spread to adjacent cells over the time period of incubation (Fig. 2 ). The assay does not rely on the use of any virus-specific reagents, which is beneficial when reagents are unavailable. As this cell-based assay typically is performed in 6-well plates, it is relatively low-throughput, labor-intensive, and may not be reliable when the samples themselves are cytotoxic (e.g., homogenate from certain tissues) or when the virus is poorly cytopathic in a given cell type. Thus, it is important to choose a highly permissive cell type (e.g., Vero E6 cells) for which SARS-CoV-2 causes substantive cell death.
Fig. 2.
Crystal violet stained plaque assay plates. Vero-furin or Vero E6 cells were inoculated with 10-fold serial dilutions of a SARS-CoV-2 stock. Plates were fixed three days post-infection and stained with crystal violet. Wells with individual plaques were used to determine the virus titer (Vero-furin 10−4, Vero E6 10−3). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.5. Materials needed
Vero E6 or Vero-furin cells
Vero cell culture medium (see Recipes)
Infection media (see Recipes)
Virus to be titered
96-well U-bottom plates
6-well or 12-well tissue culture plates
2X MEM + 4% FBS (see Recipes)
2% methylcellulose (see Recipes)
4% paraformaldehyde solution (in PBS)
Crystal violet staining solution (see Recipes)
-
1.
Plate approximately 7.5 × 105 Vero E6 or Vero-furin cells/well into 6-well plates. Plate enough wells to test each dilution in duplicate (starting from 10−1 to 10−6; 10-fold dilutions). Incubate cells overnight (12–18 h) at 37 °C.
*12-well tissue culture plates also will work. Plate approximately 2.5 × 10 5 cells/well.
-
2.
Dilute samples to be titered in infection media in 96-well U-bottom plates. Make a 10-fold dilution series, providing enough volume to add 200 μL per 6-well plate.
-
3.
Remove existing cell culture media from 6-well plates. Add 200 μL of each dilution to one well of a 6-well plate (200uL to 12-well plate) starting with most diluted so the same pipette tip can be used up the dilution series.
-
4.
Incubate 6-well plates at 37 °C in 5% CO2 for 1 h, rocking plates every 15 min to prevent cells from drying out.
-
5.
Meanwhile, mix 2X MEM + 4% FBS with 2% methylcellulose in a 1:1 ratio. Place in 37 °C incubator while plates are incubating to decrease viscosity of the solution.
-
6.
After 1 h incubation, add 2 mL of MEM:methylcellulose mixture to each well of the 6-well plates (1 mL–12-well plate).
-
7.
Incubate plates at 37 °C in 5% CO2 for 3 days.
*After 3 days, plaques should be visible by eye when carefully held up to the light. If plaques are too small to discern, plates can be incubated for an additional day.
-
8.
On day 3, gently remove methylcellulose overlays with a pipette and fix cells by adding 3 mL of 4% paraformaldehyde (PFA) in PBS to each well. Incubate at room temperature for 20 min.
-
9.
Remove 4% PFA into an appropriate hazardous waste container. If this concentration of PFA has been approved as a method of SARS-CoV-2 inactivation by the Institutional Biosafety Committee, plates can be removed from the BSL3.
-
10.
Add 1 mL of 0.05% (w/v) crystal violet in 20% methanol to each well. Incubate for 20–30 min. Remove crystal violet with a pipette and wash twice with dH2O or until excess crystal violet is removed, and plaques are easily visualized.
-
11.
Count the plaques at the dilution in which there are 10–100 plaques. Calculate titer in PFU/mL using the following formula: Titer (PFU/mL) = number of plaques counted × 10^dilution counted × 5 (to get to mL because we added 200 μL of diluted sample)
Quantification of SARS-CoV-2 by focus-forming assay. A focus-forming assay is similar to a plaque assay in that it detects infectious virus in a sample. A “foci” describes the zone of cells that have become infected from a single infectious unit. These foci of cells express high amounts of viral antigen, which can be detected using a virus-specific antibody that is directly conjugated to a colorimetric readout (e.g. peroxidase) or through use of secondary antibodies (Fig. 3 ). This approach adds specificity to the assay, but also increases the number of processing steps post-infection. However, because the focus-forming assay captures infected foci before the cells die and develop into plaques, this assay typically requires shorter incubation times than the plaque assay. It also can be performed in 96-well plate format, which can increase throughput.
Fig. 3.
SARS-CoV-2 focus-forming assay. CCL81, Vero-furin, Vero E6, and MA104 cells were inoculated with 10-fold serial dilutions of a SARS-CoV-2 stock. Plates were fixed 30 h post-infection and stained with CR3022 anti-SARS-CoV-2 antibody (1 μg/mL) overnight followed by anti-human IgG-HRP (1:500) for 2 h. Foci were visualized using TrueBlue substrate and wells with discrete foci were used to determine virus titer (10−3 - 10−4).
2.6. Materials needed
Vero E6 or Vero-furin cells
Vero cell culture medium (see Recipes)
Infection media (see Recipes)
Viral sample to be titered
96-well U-bottom plates
96-well flat-bottom plates
2X MEM + 4% FBS (see Recipes)
2% methylcellulose (see Recipes)
4% PFA
Permeabilization (Perm) wash buffer (see Recipes)
Anti-SARS-CoV-2 antibody (and secondary antibody if primary is not directly conjugated)
Automated cell counter (e.g. CTL BioSpot)
-
1.
Plate Vero E6 or Vero-furin cells in 96-well flat bottom plates at a cell density of 2.5 × 104 cells/well in a total volume of 100 μL/well.
-
2.
Incubate cells overnight (12–18 h) in humidified incubator at 37 °C in 5% CO2.
-
3.
The next day, make 10-fold serial dilutions of viral sample to be titered in infection media in 96-well U-bottom plates. Change tips between dilutions and mix each row well before transferring to the next.
-
4.
Remove existing cell culture media from cells. Transfer 100 μL of the dilutions generated in step #3 to corresponding wells on cell plate starting with the most diluted so the same pipette tip can be used up the dilution series.
-
5.
Incubate at 37 °C for 1 h.
-
6.
Meanwhile, mix 2X MEM + 4% FBS with 2% methylcellulose in a 1:1 ratio. Warm in 37 °C incubator while plates are incubating to decrease viscosity of the solution.
-
7.
Add 100 μL/well MEM:methylcelluose overlay to each well.
-
8.
Incubate at 37 °C for 30 h.
-
9.
Remove methylcellulose:MEM overlays from each well.
-
10.
Add 300 μL of 4% PFA in PBS (see Recipes) to each well.
-
11.
Incubate at room temperature for 20 min.
-
12.
Remove 4% PFA into appropriate waste container.
-
13.
Wash cells with 300 μL of Perm wash (see Recipes) six times to remove any remaining overlay and 4% PFA.
*Plates can now be removed safely from the BSL3 after obtaining Institutional Biosafety Committee approval.
-
14.
Add 50 μL primary antibody/well in Perm wash. Incubate at 4 °C overnight with no rocking or 2 h at room temperature with rocking.
*These conditions are optimized for using CR3022 ( Yuan et al., 2020 ) at 1 μg/mL.
-
15.
Wash three times with 300 μL/well of PBS + Tween wash (see Recipes).
-
16.
Add 50 μL of secondary antibody/well in Perm wash.
*We use goat anti-human IgG-HRP at 1:500 (Sigma).
-
17.
Incubate plates for 2 h at room temperature with rocking or gentle agitation.
-
18.
Wash 3x with 300 μL/well of PBS + Tween wash.
-
19.
Add 50 μL of KPL TrueBlue substrate (from Seracare Life Sciences Inc).
-
20.
Incubate plates at room temperature with rocking until foci are fully developed and visible by eye (~15–30 min).
-
21.
Wash 3x with 300 μL/well of dH2O.
-
22.
Tap plate dry on a paper towel and image with CTL Immunospot plate reader.
Detection of SARS-CoV-2 antigen by flow cytometry. Detection of viral antigen in infected cells using flow-cytometry has a range of applications. In particular, multiplexing of viral staining with live/dead dyes and additional antigen stains can be used to screen infected cells for antibody binding or interrogate a range of biological variables.
2.7. Materials needed
Infected cells
Cell culture medium
96-well V-bottom plates
Dissociation reagent (e.g. Trypsin-EDTA or TrypLE)
FACS wash (see Recipes)
Anti-SARS-CoV-2 primary antibody
Secondary antibody (if primary is not directly conjugated)
-
1.
If cells are adherent, dissociate SARS-CoV-2-infected cells into single-cell suspension using trypsin or EDTA-based dissociation agent.
-
2.
Add cellular growth media and centrifuge in a swinging bucket rotor for 5 min at 500×g.
Note: this must be performed in an aerosol-tight bucket with gasketed lid.
-
3.
Open the gasketed centrifuge lid in the biosafety cabinet, remove samples, and pipette off supernatant. Resuspend cells in 4% PFA (final concentration) diluted in PBS and incubate for 10 min at room temperature.
-
4.
Centrifuge at 600×g for 3 min at 4 °C, remove supernatant, and resuspend cells in FACS wash.
*Cells can now be removed safely from the BSL3 after obtaining Institutional Biosafety Committee approval.
-
5.
Count cells and add 3 × 104–1 × 106 cells/well in a 96-well U-bottom plate for each sample to be tested.
*If desired, cells can be permeabilized using Perm wash (see Recipe below) and stained for intracellular antigen.
-
6.
Centrifuge at 600×g for 3 min at 4 °C, remove supernatant, and resuspend cells with 50μL/well FACS wash containing primary antibody (e.g. containing 2 μg/mL of CR3022). Incubate at 4 °C for 45 min–1 h.
-
7.
Wash twice with FACS wash by repeated centrifugation at 600×g for 3 min at 4 °C, removal of supernatant, and resuspension of pellet in FACS wash.
-
8.
Upon completing the second spin, resuspend cells with FACS wash containing fluorophore-conjugated secondary antibody that recognizes the primary antibody (e.g., goat anti-human IgG Alexa 647 at 1:1000 dilution) for 1 h at 4 °C.
-
9.
Wash twice with FACS wash by repeated centrifugation at 600×g for 3 min at 4 °C, removal of supernatant, and resuspension of pellet in FACS wash. Resuspend after final spin in an appropriate volume for the flow cytometer you will use.
-
10.
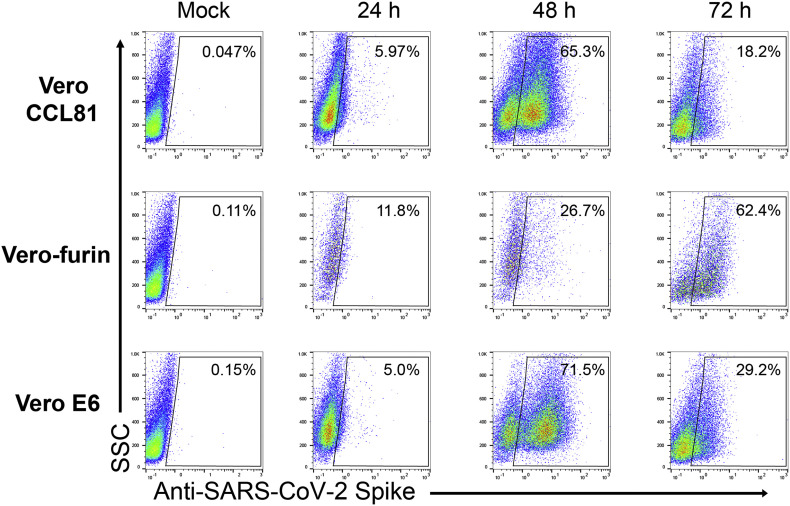
Analyze cells on a flow cytometer (Fig. 4 ).
Fig. 4.
SARS-CoV-2 infected cell flow cytometry plots. Indicated cell types were inoculated with SARS-CoV-2 at an MOI of 0.01 PFU/cell. At each indicated timepoint post-infection, cells were collected and prepared for flow cytometry using CR3022 anti-S as the primary antibody followed by goat-anti-human IgG Alexa 647 as the secondary antibody.
SARS-CoV-2 outgrowth assay for validation of inactivation methods. To evaluate many aspects of COVID-19 biology, methods for inactivating SARS-CoV-2 infectivity are needed so that samples can be worked with safely outside of the BSL3. To test whether a specific method or treatment completely inactivates SARS-CoV-2, a virus outgrowth assay should be used. This type of assay is highly sensitive in that it allows for the outgrowth of as little as a single infectious unit. However, it is not quantitative and must be adapted to the application in question. Alternate agents and methods are benchmarked against “gold-standard” methods of inactivation of SARS-CoV-2 (Table 2 ). We describe methods that we have tested and validated, although before use, individual Institutional Biosafety Committees likely will need to review data before providing clearance.
Table 2.
Methods for inactivation of infectious SARS-CoV-2.
| Method | Specimen type(s) | Specific reagent(s)/method(s) used | Incubation time |
|---|---|---|---|
| Trizol* | Tissue homogenate Cells Biological fluids |
Per manufacturer instructions | |
| Paraformaldehyde* | Cells Biological fluids |
4% final concentration | 10 min |
| Formalin* | Tissues | 10% at a ratio of 1:10 (tissue:formalin) | 7 days |
| Triton-X-100 | Serum Tissue culture media** Cell homogenate*** |
1% triton-X (final conc.) (Millipore-Sigma cat #11332481001) |
20 min |
| Triton-X-100 | Lung homogenate | 60 min | |
| MagMAX | Serum Tissue culture media** |
MagMAX viral RNA kit (ABI cat # AM1939) | 5 min |
| MagMAX | Tissue homogenate Cell homogenate*** |
MagMAX mirVana kit (ABI cat # A27828) | 5 min |
| Heat | Urine Tissue culture media |
50 °C for 5 min followed by 95 °C for 5 min | 10 min |
| Paraformaldehyde | Lung homogenate | 1% (final concentration) | 60 min |
*Gold standard methods.
**Note: tissue culture media is less complex than serum and in some cases is inferred from data showing 100% inactivation in serum.
**Note: cells from tissue culture are less complex than tissues from an infected animal, and this data is inferred from data showing 100% inactivation in homogenized lung tissue.
2.8. Materials needed
Inactivation agent/method of choice
Vero E6 cells
Vero cell culture medium
2.9. Selecting an inactivation agent/method
Selecting the appropriate inactivation agent/method requires a detailed understanding of the project in question: specifically, the properties of the measurand (e.g., DNA, RNA, protein, and cells); the effect of the agent/method on the integrity of the measurand (e.g., fragmentation of DNA by formaldehyde or lysis of RNA by boiling); and the types of specimens that will be treated for inactivation (e.g., whole blood, plasma, cell culture media, cells, and tissues). No one reagent or method works for all applications, and ideally, the activity of each inactivation agent should be tested against each specimen type that will be used in the project. Validation of reagents/methods with a diverse array of applications may result in fewer hours spent performing validation of inactivation reagents.
2.10. Determination of the inactivation agent's cytotoxicity
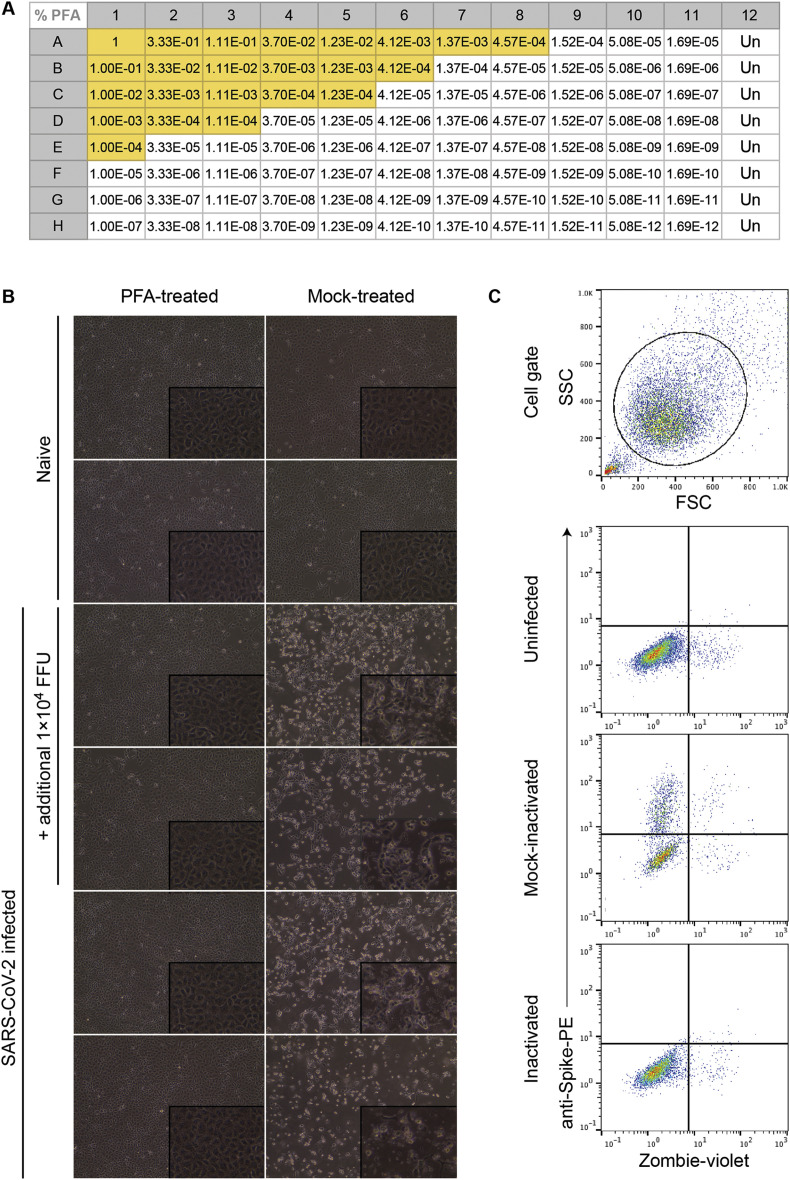
Many commonly used chemical inactivation agents (e.g., chaotropic salts, detergents, and formaldehyde-based solutions) are toxic to cells (Fig. 5 A). Because viruses require infection of a cell to replicate, this toxic effect of the inactivation agent must be diluted sufficiently after the sample has been treated to enable virus outgrowth.
-
1.
(Day −1) Add 2 × 104 cells per well in a flat-bottom tissue-culture 96 well plate and incubate at 37 °C overnight (12–18 h).
-
2.
(Day 0) In a separate U-bottom plate, add 200 μL of the fully-reconstituted inactivation agent to well A1. Make serial 10-fold dilutions down the first column by transferring 18 μL into 162 μL. Make serial 3-fold dilutions across the plate using a multichannel pipette by transferring 60 μL into 120 μL; add only media to column 12.
Fig. 5.
Virus outgrowth assay. (A) Titration of paraformaldehyde toxicity on Vero E6 cells plated in 96-well format as described in the protocol. Yellow shading indicates wells in which cytopathic effect was observed. (B) Cytopathic effect observed in Vero E6 cells following inoculation with SARS-CoV-2 infected lung homogenate, treated with or without 1% PFA for 60 min and diluted 1:15,000. Photographs show cells under phase-contrast at 20X (and 40X, inset) magnification. (C) Flow cytometric analysis of Vero E6 cells inoculated with SARS-CoV-2 following treatment with an inactivation agent or PBS (mock). Cells were dissociated to single-cell suspension once the mock-treated culture displayed CPE consistent with SARS-CoV-2 infection. Viability staining with Zombie violet was performed prior to fixation. Antibody staining was performed on 4% paraformaldehyde-fixed and permeabilized cells using the CR3022 anti-SARS-CoV-2 spike antibody followed by anti-human IgG-BV421 labelled secondary antibody. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
This setup will create a dilution scheme in which the dilution that renders the inactivation agent non-toxic can be assessed in multiple rows.
-
3.
Remove the supernatant from the plate containing Vero cells, and add 100 μL from each corresponding well in the plate containing diluted inactivation agent. Incubate cells at 37 °C overnight.
-
4.
(Day 1) Observe the cells under the microscope, noting wells with obvious cell death. Longer incubation times may be necessary. Mark wells in which toxicity is no longer obvious, and test the cells in these wells for viability and total cell number using trypan blue staining (or a variety of other live/dead counting methods). Use the media-only wells in column 12 for comparison. Wells that have the same viability and number of cells (±10%) should be used to calculate the required dilution factor.
2.11. Sample inactivation and virus outgrowth assay
To determine the ability of the agent/method to fully inactivate infectious virus, several high-titer specimens should be identified. If these are not available, then these specimens can be created by spiking specimens with SARS-CoV-2 virus stock (1:10) or infected cells. Ideally, specimens with the range of characteristics that will be encountered during the project (e.g., icteric, hemolyzed, and lipemic serum specimens) should be tested.
-
1.
Prepare Vero E6 cells in a volume that enables the dilution of inactivation agent to a non-toxic level when >1 μL of treated sample is added. The number of cells also should be such that they will reach approximately 50% confluency upon adhering.
For example, for diluting an agent 1:1000, plate 3-4 × 10 4 Vero E6 cells in 4 mL and add 4 μL of the inactivated sample.
-
2.
For the specimen(s) to be tested, split into two equal aliquots. Subject one to inactivation and the other to mock-inactivation (e.g., with addition of saline or medium instead of inactivation reagent). This should be performed at the temperature and for the duration of time that will be used for inactivation of experimental specimens.
-
3.
Add the appropriate volume of inactivated (and mock-inactivated) sample to the Vero cells.
-
4.
Incubate at 37 °C and observe daily (Fig. 5B). Once obvious signs of CPE are observed, examine the cells and/or supernatant for infection using the flow cytometry (Figure.5C) and/or focus forming assay described above to confirm viral infection.
Conclusions. The emergence of SARS-CoV-2 and the resulting COVID-19 pandemic has strained biomedical resources throughout the world. Necessarily, pressure has been placed on the scientific community to deliver countermeasures for this continually evolving threat. Although new technologies are being applied to address this problem, classical virological methods, such as those presented here, remain important. Within a remarkably short period of time, the scientific community has built an infrastructure for studying SARS-CoV-2, especially given the biosafety concerns surrounding SARS-CoV-2 research. However, given the complex nature of COVID-19 pathophysiology, a critical need remains for developing new modalities for studying and combating this novel disease. Further optimization of assays will be required, and these will include a need to amplify high-titer virus stocks from low-passage patient isolates and develop new culture models to evaluate infectivity, host responses, and outcomes. New methods for SARS-CoV-2 inactivation will be developed, and these will require rigorous validation before wide-scale implementation. Issues regarding SARS-CoV-2 biosafety and biocontainment will continue to evolve as the pandemic progresses, and methods for safely working with and titrating SARS-CoV-2 will require further evaluation.
3. Recipes
3.1. Vero cell culture medium
*For 1 L:
Dulbecco's Modified Eagle Medium (high glucose) supplemented to contain:
10% heat-inactivated fetal bovine serum
1% Glutamax
10 mM HEPES
100 U/mL penicillin/100 U/mL streptomycin
Sterile filter and store at 4 °C.
*Vero-furin media is as stated above with the addition of 5 μg/mL blasticidin.
3.2. MA104 culture medium
*For 1 L:
M199 medium with Earle's salts supplemented to contain:
5% fetal bovine serum
10 mM HEPES
100 U/mL penicillin/100 U/mL streptomycin
2.5 μg/mL amphotericin B
3.3. Infection medium
*For 1 L:
Dulbecco's Modified Eagle Medium (high glucose) supplemented to contain:
2% heat-inactivated fetal bovine serum
10 mM HEPES
100U/mL penicillin/100U/mL streptomycin
3.4. 2X minimal essential medium + 4% FBS
*For 1 L:
200 mL 10X MEM (Sigma #M0275)
20 mL of 1 M l-glutamine
20 mL 1 M HEPES
40 mL heat-inactivated fetal bovine serum
4.2 g sodium bicarbonate
20 mL of 10,000 IU/ml penicillin +10,000 μg/ml streptomycin
To volume with deionized distilled (Milli-Q) water
Sterile filter and store at 4 °C.
3.5. 2% methylcellulose
Autoclave a 250 mL glass bottle containing 2 g carboxymethylcellulose powder (Sigma #M0512) and a stir bar.
Autoclave 100 mL deionized distilled (Milli-Q) water.
When water is cool enough to handle, add to methylcellulose containing bottle.
Stir mixture overnight at 4 °C and then store at 4 °C until ready for use.
3.6. Crystal violet staining solution
*For 100 mL:
50 mg of crystal violet powder (Fisher #C581-100)
20 mL of 100% methanol
80 mL of deionized distilled (Milli-Q) water
Store at room temperature and mix well before each use.
3.7. Perm wash
*For 1L:
1 g of saponin (Sigma, Cat. No: S7900)
1 g of bovine serum albumin (Fraction V)
To volume with 1 L phosphate buffered saline (without Ca or Mg)
Filter sterilize and store at 4 °C until ready for use.
3.8. FACS wash
*For 1 L:
100 mL 10X phosphate-buffered saline
5 mL 1 M EDTA solution
50 mL 5% heat-inactivated fetal bovine serum
10 mL 5% sodium azide solution
835 mL deionized distilled (Milli-Q) water
Filter sterilize and store at 4 °C until ready for use.
3.9. PBS + Tween wash
1 L 10X PBS
9 L ddH2O
50 mL 10% Tween-20
Author contributions
J.B.C. developed the protocols and performed the infection studies with SARS-CoV-2. A.L.B. designed and tested the SARS-CoV-2 primer/probes and developed the inactivation protocols. J.B.C. and A.S.K. performed flow cytometry assays. R.E.C. provided experimental assistance with focus-forming and plaque assays. A.L.B., J.B.C., A.S.K., and M.S.D. wrote the initial manuscript draft.
Declaration of competing interest
M.S.D. is a consultant for Inbios, Vir Biotechnology, NGM Biopharmaceuticals, and on the Scientific Advisory Board of Moderna.
Acknowledgements
This study was supported by NIH contracts and grants (75N93019C00062 and R01 AI127828) and the Defense Advanced Research Project Agency (HR001117S0019). J.B.C. is supported by a Helen Hay Whitney Foundation postdoctoral fellowship.
References
- (CDC), C.f.D.C.a.P . 2020. 2019-Novel Coronavirus (2019-nCoV) Real-Time rRT-PCR Panel Primers and Probes. [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brunink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce M.G., Sankhala R.S., Chen W.-H., Choe M., Bai H., Hajduczki A., Yan L., Sterling S.L., Peterson C.E., Green E.C., Smith C., de Val N., Amare M., Scott P., Laing E.D., Broder C.C., Rolland M., Michael N.L., Modjarrad K. 2020. A Cryptic Site of Vulnerability on the Receptor Binding Domain of the SARS-CoV-2 Spike Glycoprotein. bioRxiv. [Google Scholar]
- Lei C., Fu W., Qian K., Li T., Zhang S., Ding M., Hu S. 2020. Potent Neutralization of 2019 Novel Coronavirus by Recombinant ACE2-Ig. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M. Proceedings of the National Academy of Sciences of the United States of America. 2020. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Sirohi D., Dowd K.A., Chen Z., Diamond M.S., Kuhn R.J., Pierson T.C. Enhancing dengue virus maturation using a stable furin over-expressing cell line. Virology. 2016;497:33–40. doi: 10.1016/j.virol.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Corti D., Donaldson E., Sheahan T., Stadler K., Lanzavecchia A., Baric R. Structural basis for potent cross-neutralizing human monoclonal antibody protection against lethal human and zoonotic severe acute respiratory syndrome coronavirus challenge. J. Virol. 2008;82:3220–3235. doi: 10.1128/JVI.02377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standards Unit, D.f.E. Standards and Training . 2010. Good Laboratory Practice when Performing Molecular Amplification Assays. [Google Scholar]
- Sui J., Li W., Roberts A., Matthews L.J., Murakami A., Vogel L., Wong S.K., Subbarao K., Farzan M., Marasco W.A. Evaluation of human monoclonal antibody 80R for immunoprophylaxis of severe acute respiratory syndrome by an animal study, epitope mapping, and analysis of spike variants. J. Virol. 2005;79:5900–5906. doi: 10.1128/JVI.79.10.5900-5906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen J., van den Brink E.N., Poon L.L., Marissen W.E., Leung C.S., Cox F., Cheung C.Y., Bakker A.Q., Bogaards J.A., van Deventer E., Preiser W., Doerr H.W., Chow V.T., de Kruif J., Peiris J.S., Goudsmit J. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. 2020. Potent Binding of 2019 Novel Coronavirus Spike Protein by a SARS Coronavirus-specific Human Monoclonal Antibody. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp R.A., Haynes L.M., Moore D., Anderson B., Tamin A., Harcourt B.H., Jones L.P., Yilla M., Babcock G.J., Greenough T., Ambrosino D.M., Alvarez R., Callaway J., Cavitt S., Kamrud K., Alterson H., Smith J., Harcourt J.L., Miao C., Razdan R., Comer J.A., Rollin P.E., Ksiazek T.G., Sanchez A., Rota P.A., Bellini W.J., Anderson L.J. Monoclonal antibodies to SARS-associated coronavirus (SARS-CoV): identification of neutralizing and antibodies reactive to S, N, M and E viral proteins. J. Virol. Methods. 2005;128:21–28. doi: 10.1016/j.jviromet.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels C., Fauver J., Ott I., Grubaugh N. 2020. Generation of SARS-COV-2 RNA Transcript Standards for qRT-PCR Detection Assays. protocols.io. [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. New York, N.Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., Sheng J., Quan L., Xia Z., Tan W., Cheng G., Jiang T. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Wu N.C., Zhu X., Lee C.D., So R.T.Y., Lv H., Mok C.K.P., Wilson I.A. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020 doi: 10.1126/science.abb7269. New York, N.Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Chakraborti S., He Y., Roberts A., Sheahan T., Xiao X., Hensley L.E., Prabakaran P., Rockx B., Sidorov I.A., Corti D., Vogel L., Feng Y., Kim J.O., Wang L.F., Baric R., Lanzavecchia A., Curtis K.M., Nabel G.J., Subbarao K., Jiang S., Dimitrov D.S. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]