Abstract
Background: The incidence of cervical cancer in young women is rising, and squamous cell carcinoma makes up a great percentage of the histological types. The presence of aggressive pathologic risk factors following patients’ primary surgery may warrant the use of adjuvant radiotherapy. It is important to weigh up the risks and benefits of using adjuvant radiotherapy for each young patient so as to maximize their prognosis while minimizing the treatment-related morbidity. Methods: A retrospective study was performed. It consisted of 97 patients under 35 years old who were diagnosed with cervical squamous cell carcinoma and underwent treatment at West China Second University Hospital between December 2009 and January 2014. Five-year follow-up, prognostic risks, long-term radiation toxicity, female sexual function, and quality of life were investigated. Results: Adjuvant radiotherapy did improve the prognosis of young patients with lymph node metastases. However, there were few significant differences in progress-free survival and overall survival for the young patients without lymph node metastases following adjuvant radiotherapy. Besides, young patients who took radiotherapy exhibited greater intestinal dysfunction, more severe lower extremities edema, greater sexual dysfunction, and worse long-term quality of life. Conclusion: Young patients with early-stage cervical squamous cell carcinoma without lymph node metastases who have undergone the primary surgery should be counseled in detail before the decision to use adjuvant radiotherapy can be made. The counseling should emphasize not only the benefit that local recurrence rates can be reduced, but also the risks that treatment-related side effects could increase and lower QoL could occur.
Subject terms: Quality of life, Gynaecological cancer
Introduction
Thanks to widespread screening and advanced medical treatment, the incidence and the mortality of cervical cancer have been reduced in developed countries1. However, in the rural developing regions of China, the morbidity of cervical cancer is still high due to the suboptimal medical conditions, making the cervical cancer a major health problem for women2,3. Recent studies showed that the incidence of cervical cancer in young women is rising and squamous cell carcinoma (SCC) still makes up a great percentage of the histological types4,5. To our best knowledge, the persistent infection of high-risk human papillomavirus (HPV) is identified as the most critical factor for the development of cervical cancer6,7. For young women, the squamous-columnar junction of cervix is more vulnerable to HPV infection if she has an early age of active intercourse.
Considering the quality of life (QoL), a primary surgery with radical hysterectomy or trachelectomy without bilateral salpingo-oophorectomy is usually performed for young women with early-stage cervical SCC. According to the National Comprehensive Cancer Network clinical practice guidelines of cervical cancer, if one or more aggressive pathologic risk factors are discoverd after the primary surgery, such as bulky tumor size, deep stromal invasion (DSI), lymph-vascular space invasion (LVSI), pelvic lymph nodes (LN) metastases, parametrial and surgical margin involvement, the use of adjuvant radiotherapy (RT) is warranted. However, it has been reported that using more than one treatments for cervical cancer leads to a potenital increase of related complications and side effects, such as diarrhea, bloody stool, urinary frequency, lower extremities edema, and sexual dysfuction8,9.
Taking into account long-term side effects, sexual function and QoL, adjuvant RT may be declined by young patients with cervical cancer. It is important to weigh up the risks and benefits of using adjuvant RT for each patient so as to maximize their prognosis while minimizing the treatment-related morbidity. Therefore, this study aims to make clear the impact of adjuvant RT on progress-free survival (PFS), overall survival (OS), treatment-related side effects, sexual function, and QoL for patients under 35 years old with early-stage cervical SCC following their primary surgery, compared with that of non-RT (NRT)..
Patients and Methods
This study was performed at the department of gynecology and obstetrics of West China Second University Hospital, Chengdu, China. The follow-up and consent procedures were approved by the Sichuan University Medical Ethical Committee. We confirm that all research was performed in accordance with relevant regulations and informed consent was obtained from all participants.
A total of 191 patients under 35 years old had been diagnosed with cervical SCC and underwent treatment at our department between December 2009 and January 2014. 107 patients (10 patients lost to follow-up) were confirmed to present one or more pathologic risk factors after the primary surgery and were informed to take adjuvant RT. 23 patients out of them declined the following therapy. Consequently, patients were divided into two study groups: the RT and NRT groups. In the RT group, pelvic external beam radiotherapy (EBRT) with intensity-modulated radiation therapy (IMRT) technology was indicated. At the minimum, the radiation volume included upper 3 to 4 cm of the vaginal cuff, the parametria, and immediately adjacent nodal basins. For documented nodal metastasis, the common lilacs should be covered as well. For common iliac or para-aortic nodal involvement, the superior border of the radiation field was increased up to the level of the renal vessels. Vaginal brachytherapy was added as a useful boost for patients with positive pelvic nodes. Patients were treated with definitive EBRT to a dose of 45 to 50 Gy in standard fractionation. The fractionation schemes of brachytherapy included 5.5 Gy × 2 fractions dosed at 5 mm.
The clinical and pathologic characteristics of both study groups were examined. The follow-up included interval history, gynecological examination, and cervical smear test every 3 months for 1 year, every 6 months for another 2 years, and annually afterwards. Long-term radiation toxicity was documented based on the criteria of radiation morbidity scoring of the RT Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC)10. The female sexual function index (FSFI) was used to assess sexual function in women, including desire, arousal, lubrication, orgasm, satisfaction, and pain. Higher scores indicate better sexual function11. The EORTC QLQ-C30 questionnaire was employed to assess the QoL. The standard score was calculated.
Statistical analysis was performed using SPSS software package. Pearson’s chi-square or Fisher exact’s test was implemented to compare the difference in proportions. The PFS and OS curves were constructed using the Kaplan-Meier method and were compared using the log-rank test. Univariate and multivariate analysis were performed using Cox’s multivariate regression model to identify meaningful prognostic factors. Differences in standard scores of female sexual function and QoL between two groups were evaluated using two-tailed Student’s t-test. P < 0.05 was considered to be statistically significant.
Results
The clinical and pathologic characteristics of the two study groups are shown in Table 1. The proportion of FIGO stage II in the RT group was higher than that in the NRT group (45.9% vs 17.4%, P = 0.027). No significant differences in tumor differentiation, size, and pathologic risk factors were found between the two groups.
Table 1.
Characteristics of 97 young patients with SCC in two study groups.
| n (%) | NRT | RT | P | |
|---|---|---|---|---|
| n = 23 (24.8%) | n = 74 (75.2%) | |||
| Age, year | ||||
| Median | 30 | 33 | ||
| Range | 21–35 | 22–35 | ||
| Stage | 0.027* | |||
| I | 59 (60.8) | 19 (82.6) | 40 (54.1) | |
| II | 38 (39.2) | 4 (17.4) | 34 (45.9) | |
| Differentiation | 0.683 | |||
| G1/G2 | 13 (13.4) | 2 (8.7) | 11 (14.9) | |
| G3 | 84 (86.6) | 21 (91.3) | 63 (85.1) | |
| Tumor size | 0.911 | |||
| <4 cm | 60 (61.9) | 14 (60.9) | 46 (62.2) | |
| ≥4 cm | 37 (38.1) | 9 (39.1) | 28 (37.8) | |
| Stromal invasion | 0.288 | |||
| <1/2 | 18 (18.6) | 6 (26.1) | 12 (16.2) | |
| ≥1/2 | 79 (81.4) | 17 (73.9) | 62 (83.8) | |
| LVSI | 0.473 | |||
| Negative | 32 (33.0) | 9 (39.1) | 23 (31.1) | |
| Positive | 65 (67.0) | 14 (60.9) | 51 (68.9) | |
| LN metastases | 0.126 | |||
| Negative | 63 (64.9) | 18 (78.3) | 45 (60.8) | |
| Positive | 34 (35.1) | 5 (21.7)) | 29 (39.2) | |
*P < 0.05.
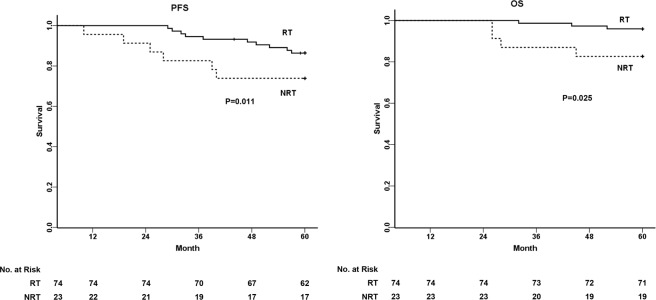
The five-year PFS and OS of all 97 patients were 83.4% and 92.8%, respectively. Adjuvant RT was the significant prognostic factor of the poor PFS (NRT vs. RT = 74.0% vs. 86.4%, P = 0.011) and OS (NRT vs. RT = 82.6% vs. 95.9%, P = 0.025) in young patients with SCC (Fig. 1). Among prognostic factors, LN metastases and adjuvant RT were identified as independent prognostic factors for predicting PFS and OS using Cox’s multivariate regression analysis (Table 2). For patients with LN metastases (Fig. 2A), the five-year PFS and OS were 72.4% and 93.1% in the RT group, respectively, in contrast to 40.0% and 60.0% in the NRT group (P < 0.05). However, for patients with non-LN metastases (Fig. 2B), the five-year PFS and OS had few significant differences between both groups (P > 0.05).
Figure 1.
Survival analysis of young patients with SCC who were informed to take adjuvant radiotherapy. PFS and OS of 97 young patients are shown between two study groups: NRT and RT. The five-year PFS in NRT and RT groups were 74.0% vs. 86.4% (P = 0.011), and the five-year OS in NRT and RT groups were 82.6% vs. 95.9% (P = 0.025).
Table 2.
Prognostic factors of PFS and OS analyzed by Cox proportional hazard models for young patients with SCC.
| Prognostic factors | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95%CI | P | Hazard ratio | 95%CI | P | |
| Bulky tumor | 1.198 | 0.426–3.375 | 0.733 | 3.286 | 0.703–15.348 | 0.130 |
| DSI | 2.300 | 0.527–10.041 | 0.268 | 0.920 | 0.138–6.155 | 0.932 |
| LVSI | 1..451 | 0.464–4.539 | 0.522 | 4.264 | 0.505–35.962 | 0.183 |
| LN metastases | 11.029 | 2.742–44.358 | 0.001** | 9.151 | 1.165–71.857 | 0.035* |
| Adjuvant RT | 6.356 | 1.689–23.917 | 0.006** | 12.260 | 1.646–91.329 | 0.014* |
*P < 0.05.
**P < 0.01.
Figure 2.
Survival analysis of young patients with SCC related to LN metastases between two study groups: NRT and RT. (A) For patients with LN metastases, the five-year PFS in NRT and RT groups were 40.0% vs. 72.4% (P = 0.027), and the five-year OS in NRT and RT groups were 60.0% vs. 93.1% (P = 0.015). (B) For patients with non-LN metastases, the five-year PFS in NRT and RT groups were 83.3% vs. 95.5% (P = 0.100), and the five-year OS in NRT and RT groups were 88.9% vs. 97.8% (P = 0.135).
The incidences of side effects associated with the two adjuvant treatment modalities are shown in Table 3. Compared to the NRT group, patients who had received adjuvant RT exhibited a significantly higher incidence of diarrhea (P = 0.018), bloody stool (P = 0.007), lower extremities edema (P = 0.033), and vaginal dryness (P = 0.000). As shown in Fig. 3, the most common long-term radiation toxicity included radioproctitis, radiocystitis, radiosteitis, lower extremities edema in grade 1, and ureteral obstruction in grade 2. Grade 4 toxicity of lower extremities edema in 2 of the 52 (3.8%) patients were documented.
Table 3.
Comparison of long-term side effects between two study groups.
| n | NRT | RT | P | |
|---|---|---|---|---|
| n = 23 (24.8%) | n = 74 (75.2%) | |||
| Fatigue | 48 | 8 (34.8) | 40 (54.1) | 0.152 |
| Abdominal pain | 79 | 17 (73.9) | 62 (83.8) | 0.358 |
| Diarrhea | 51 | 7 (30.4) | 44 (59.5) | 0.018* |
| Constipation | 65 | 16 (69.6) | 49 (66.2) | 1.000 |
| Bloody stool | 38 | 3 (13.0) | 35 (47.3) | 0.007** |
| Dysuria | 23 | 3 (13.0) | 20 (27.0) | 0.273 |
| Urinary incontinence | 26 | 9 (39.1) | 17 (23.0) | 0.127 |
| Urinary frequency | 24 | 8 (34.8) | 16 (21.6) | 0.201 |
| Lower extremities edema | 49 | 7 (30.4) | 42 (56.8) | 0.033* |
| Vaginal discharge increasing | 21 | 8 (34.8) | 13 (17.6) | 0.090 |
| Vaginal dryness | 52 | 4 (17.4) | 48 (64.9) | 0.000** |
| Lower back pain | 32 | 4 (17.4) | 28 (37.8) | 0.117 |
| Dermal flushing | 27 | 3 (13.0) | 24 (32.4) | 0.122 |
*P < 0.05.
**P < 0.01.
Figure 3.
Long-term radiation toxicity of young patients with cervical SCC. The most common long-term radiation toxicity included radioproctitis, radiocystitis, radiosteitis, lower extremities edema in grade 1, and ureteral obstruction in grade 2.
Table 4 revealed that adjuvant RT-treated subjects exhibited significantly greater sexual dysfunction (21.63 ± 1.64 vs. 22.58 ± 1.34, P = 0.013). Comparing to the NRT group, patients undergoing adjuvant RT often presented difficulty in lubrication (3.34 ± 0.64 vs. 3.69 ± 0.39, P = 0.016), decrease in sexual satisfaction (3.50 ± 0.57 vs. 3.79 ± 0.47, P = 0.030), and increase in dyspareunia (3.43 ± 0.52 vs. 3.68 ± 0.40, P = 0.038).
Table 4.
Standard mean scores of sexual dysfunctions based on FSFI between two study groups.
| NRT (n = 23,) | RT (n = 74) | P | |
|---|---|---|---|
| Desire | 3.86 ± 0.67 | 3.90 ± 0.69 | 0.812 |
| Arousal | 3.64 ± 0.65 | 3.59 ± 0.71 | 0.758 |
| Lubrication | 3.69 ± 0.39 | 3.34 ± 0.64 | 0.016* |
| Orgasm | 3.91 ± 0.61 | 3.85 ± 0.73 | 0.752 |
| Satisfaction | 3.79 ± 0.47 | 3.50 ± 0.57 | 0.030* |
| Pain | 3.68 ± 0.40 | 3.43 ± 0.52 | 0.038* |
| Full scale | 22.58 ± 1.34 | 21.63 ± 1.64 | 0.013* |
*P < 0.05.
There were significant statistical differences in the QoL issues, including physical function, emotional function, constipation, financial difficulties, and global health status between the two modalities (Table 5). The NRT group scored higher on the scale of global health status than the RT group (72.10 ± 14.78 vs. 64.30 ± 16.35, P = 0.044). In functional scales, physical function (NRT vs. RT = 71.30 ± 18.90 vs. 61.80 ± 19.28, P = 0.043) and emotional function (NRT vs. RT = 68.12 ± 16.98 vs. 59.12 ± 16.15, P = 0.023) had minimal differences. Meanwhile, in symptom scales/items, diarrhea (NRT vs. RT = 72.46 ± 23.89 vs. 52.70 ± 33.11, P = 0.009) and financial difficulties (NRT vs. RT = 85.51 ± 16.89 vs. 62.16 ± 34.16, P = 0.002) were significantly different.
Table 5.
Standard mean scores of quality of life (QoL) between two study groups.
| NRT (n = 23,) | RT (n = 74) | P | |
|---|---|---|---|
| Global health status/QoL | 72.10 ± 14.78 | 64.30 ± 16.35 | 0.044* |
| Function scales | |||
| Physical function | 71.30 ± 18.90 | 61.80 ± 19.28 | 0.043* |
| Role function | 60.87 ± 22.25 | 55.86 ± 23.32 | 0.365 |
| Emotional function | 68.12 ± 16.98 | 59.12 ± 16.15 | 0.023* |
| Cognitive function | 86.96 ± 12.26 | 87.61 ± 12.66 | 0.827 |
| Social function | 39.85 ± 16.47 | 37.16 ± 14.48 | 0.453 |
| Symptom scales/items | |||
| Fatigue | 71.98 ± 14.55 | 70.42 ± 19.05 | 0.719 |
| Nausea and vomiting | 79.35 ± 24.60 | 73.65 ± 24.44 | 0.332 |
| Pain | 70.29 ± 27.96 | 73.42 ± 20.99 | 0.566 |
| Dyspnea | 85.51 ± 19.66 | 78.83 ± 23.78 | 0.225 |
| Sleep disturbance | 76.81 ± 27.40 | 83.33 ± 26.60 | 0.310 |
| Appetite loss | 82.61 ± 22.18 | 68.92 ± 30.88 | 0.052 |
| Diarrhea | 72.46 ± 23.89 | 52.70 ± 33.11 | 0.009** |
| Constipation | 89.86 ± 15.68 | 82.88 ± 23.57 | 0.187 |
| Financial difficulties | 85.51 ± 16.89 | 62.16 ± 34.16 | 0.002** |
*P < 0.05.
**P < 0.01.
Discussion
The existing studies on the prognosis of cervical cancer have shown that positive postoperative pathological factors, such as DSI, LVSI, LN metastases, and parametrial and surgical margin involvement, could evidently worsen the prognosis12–15. Adjuvant RT should be considered after the primary surgery if pathologic risk factors are discovered16–18. In this study, LN metastases and adjuvant RT were found to be the most significant independent prognostic factors for young patients with cervical SCC in southwestern China. Interestingly, adjuvant RT did improve the prognosis of young patients with LN metastases. However, there were few significant differences in PFS and OS for young patients without LN metastases who underwent adjuvant RT. Our results demonstrated that adjuvant RT decreased the risk of disease progression and improved overall survival, compared to the scenario when no further treatment was conducted. However, little evidence was found that adjuvant RT might impact the prognosis for young patients without LN metastases. With the gradual improvement of surgical techniques and methods, complete radical surgery was conducted in standard scales without positive surgical margin, parametrium, and residue. This is likely to increase the PFS and OS for young cervical SCC patients without LN metastases, even no adjuvant RT was performed following the primary surgery. In addition, it remains unclear whether the benefits of conducting RT outweigh the risks19. This result prompted us to re-evaluate the risks and benefits of adjuvant RT for young patients with cervical SCC who had undergone the primary surgery.
Previous publications noted that severe side effects and life-threatening toxicity were observed in 6% of irradiated patients, compared to 2% in randomly selected patients who took no further treatment. In addition, 4% patients treated with radiation after radical surgery had grade 4 toxicity17,20. We examined the side effects and toxicity issues for young patients who were or were not treated with adjuvant RT following the primary surgery. In this study, different morbidities and severities of treatment-related side effects occurred in two treatment modalities. Patients in the RT group exhibited greater intestinal dysfunction, such as diarrhea,bloody stool, and Grade 1 to grade 3 toxicity of radioproctitis. The most critical factor contributing to intestinal dysfunction is that RT to pelvic cavity can easily cause telangiectasis of the rectum and damages to the blood vessels of rectal tissues, causing mucous membrane to be pale and fragile, and finally necrotic21. It is also reported that RT to pelvic cavity could result in other parameters associated with intestinal dysfunction, such as an imbalance of intestinal bacteria, bile salt and lactose dysabsorption, and altered intestinal peristalsis22–25. Additionally, higher morbidity of severe lower extremities edema, even in grade 4 toxicity, was found in the RT group. Previous studies have revealed that pelvic lymphadenectomy and nodal irradiation are two leading factors contributing to the development of the lower extremities edema in patients with gynecological malignancies26,27. Lymphatic fluid flow is obstructed primarily by radical surgery, and irradiation of adjacent nodal basins can damage the unhealed lymphatic channels and promote fibrosis of the lymph node and its surrounding tissues28,29. Therefore, adjuvant RT exacerbated the lower extremities edema following pelvic lymphadenectomy.
Sexual dysfunction has been found in almost half the patients with early-stage cervical cancer treated with surgery and RT30. Our results of the FSFI, which measured the sexual function for both study groups, were as expected. We did find differences in terms of lubrication, satisfaction, and dyspareunia between the two groups based on the postoperative therapeutic type. The direct injury during radical surgery causes a shortened vagina and pelvic neural dysfunction, possibly resulting in sexual dysfunction31–33. However, if the young patient did not receive adjuvant RT, the impairment of sexal function could be reversed since the bilateral salpingo-oophorectomy was not performed and the ovarian function remained intact. According to the literature, vaginal stenosis, premature ovarian failure, and descreased libido caused by pelvic RT often result in irreversible sexual dysfunction33,34.
The prolonged survival of cervical cancer by RT has drawed researchers’ attention to its impact on the patient’s QoL. A remarkable result in our study was that different postoperative adjuvant therapies caused significant differences in the long-term QoL. Some treatment-related symptoms did develop, as revealed by the QLQ-C30. Consistent with published studies, diarrhea worsened because of the toxicity of adjuvant RT35,36. Meanwhile, financial difficulties have been found to be a significant factor influencing young SCC patients’ QoL following adjuvant RT. According to our investigation and the literature, financial difficulties arose not only from work interruption, loss of employment and family income during the primary therapy, but also from the high medical expenses of RT and the lack of health insurance coverage in the developing areas of China37,38. Besides, patients in the RT group scored lower on function scales, including their physical and emotional functions. They did not recover or improve as time went by—this finding was largely inconsistent with those in previous studies39,40. Meanwhile, it is noteworthy that young patients who took adjuvant RT had worse long-term global QoL after five years. These results suggested that young patients with early-stage SCC who have pathologic risk factors following the primary surgery should be counseled in detail before the decision to use adjuvant RT can be made. The counseling should emphasize not only the benefit that local recurrence rates can be reduced, but also the risks that treatment-related side effects could increase and lower QoL could occur.
In addition, there were some limitations in this retrospective study. The clinician treatment modalities were uncontrolled, and non-random sampling may cause potential sampling bias. In addition, this retrospective study may underestimate both the frequency and severity of sequelae. These issues can be mitigated using random sampling or randomized experimental designs in the future.
Acknowledgements
This study was partially supported by the National Science Foundation of China (No. 81572573). We are sincerely grateful for the clinical and statistical assistance provided by Dr. Xiaorong Qi, Dr. Xi Tan, and Dr. Ce Bian from West China Second University Hospital.
Author contributions
L.Y.Y., M.R.X., and H.J.W. conceived and designed the study. L.Y.Y. and J.L.Y. performed the study. L.Y.Y. and X.Z. analyzed the data. X.Z. and J.L.Y. contributed materials and analysis tools. L.Y.Y., M.R.X., and H.J.W. prepared all figures and tables, and composed the main manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Cao, Z. China Obstetrics and Gynecology. (ed. 3) 1730–1731 (People’s Medical Publishing House, 2010).
- 4.Torre LA, et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, et al. Comprehensive clinic-pathological characteristics of cervical cancer in southwestern China and the clinical significance of histological type and lymph node metastases in young patients. PloS one. 2013;8:e75849. doi: 10.1371/journal.pone.0075849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masumoto N, et al. Dominant human papillomavirus 16 infection in cervical neoplasia in young Japanese women; study of 881 outpatients. Gynecologic oncology. 2004;94:509–514. doi: 10.1016/j.ygyno.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Moscicki AB. Human papillomavirus disease and vaccines in adolescents. Adolescent medicine: state of the art reviews. 2010;21(347–363):x–xi. [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu WC, et al. Comparison of surgery or radiotherapy on complications and quality of life in patients with the stage IB and IIA uterine cervical cancer. Gynecologic oncology. 2009;115:41–45. doi: 10.1016/j.ygyno.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Carter J, et al. A 2-year prospective study assessing the emotional, sexual, and quality of life concerns of women undergoing radical trachelectomy versus radical hysterectomy for treatment of early-stage cervical cancer. Gynecologic oncology. 2010;119:358–365. doi: 10.1016/j.ygyno.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) International journal of radiation oncology, biology, physics. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 11.Rosen R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. Journal of sex & marital therapy. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 12.Ho CM, et al. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecologic oncology. 2004;93:458–464. doi: 10.1016/j.ygyno.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Vinh-Hung V, et al. Prognostic value of histopathology and trends in cervical cancer: a SEER population study. BMC Cancer. 2007;7:164. doi: 10.1186/1471-2407-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Touboul C, et al. Prognostic factors and morbidities after completion surgery in patients undergoing initial chemoradiation therapy for locally advanced cervical cancer. The oncologist. 2010;15:405–415. doi: 10.1634/theoncologist.2009-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchiole P, et al. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgico-pathological study. Gynecologic oncology. 2005;97:727–732. doi: 10.1016/j.ygyno.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Rotman M, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. International journal of radiation oncology, biology, physics. 2006;65:169–176. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Sedlis A, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecologic oncology. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 18.Monk BJ, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecologic oncology. 2005;96:721–728. doi: 10.1016/j.ygyno.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Rogers, L., Siu, S. S., Luesley, D., Bryant, A. & Dickinson, H. O. Radiotherapy and chemoradiation after surgery for early cervical cancer. Cochrane Database Syst Rev, CD007583, 10.1002/14651858.CD007583.pub3 (2012). [DOI] [PMC free article] [PubMed]
- 20.Peters WA, III, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 21.Haboubi NY, Schofield PF, Rowland PL. The light and electron microscopic features of early and late phase radiation-induced proctitis. Am J Gastroenterol. 1988;83:1140–1144. [PubMed] [Google Scholar]
- 22.Classen J, et al. Radiation-induced gastrointestinal toxicity. Pathophysiology, approaches to treatment and prophylaxis. Strahlenther Onkol. 1998;174(Suppl 3):82–84. [PubMed] [Google Scholar]
- 23.Hauer-Jensen M, Wang J, Boerma M, Fu Q, Denham JW. Radiation damage to the gastrointestinal tract: mechanisms, diagnosis, and management. Curr Opin Support Palliat Care. 2007;1:23–29. doi: 10.1097/SPC.0b013e3281108014. [DOI] [PubMed] [Google Scholar]
- 24.Danielsson A, et al. Chronic diarrhoea after radiotherapy for gynaecological cancer: occurrence and aetiology. Gut. 1991;32:1180–1187. doi: 10.1136/gut.32.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lantz B, Einhorn N. Intestinal damage and malabsorption after treatment for cervical carcinoma. Acta Radiol Oncol. 1984;23:33–36. doi: 10.3109/02841868409135982. [DOI] [PubMed] [Google Scholar]
- 26.Biglia N, et al. Lower limb lymphedema and neurological complications after lymphadenectomy for gynecological cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2015;25:521–525. doi: 10.1097/IGC.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, et al. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2012;22:686–691. doi: 10.1097/IGC.0b013e3182466950. [DOI] [PubMed] [Google Scholar]
- 28.Lawenda BD, Mondry TE, Johnstone PA. Lymphedema: a primer on the identification and management of a chronic condition in oncologic treatment. CA: a cancer journal for clinicians. 2009;59:8–24. doi: 10.3322/caac.20001. [DOI] [PubMed] [Google Scholar]
- 29.Ohara K, et al. Use of the small pelvic field instead of the classic whole pelvic field in postoperative radiotherapy for cervical cancer: reduction of adverse events. International journal of radiation oncology, biology, physics. 2004;60:258–264. doi: 10.1016/j.ijrobp.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Virtanen H, et al. Effects of abdominal hysterectomy on urinary and sexual symptoms. Br J Urol. 1993;72:868–872. doi: 10.1111/j.1464-410x.1993.tb16288.x. [DOI] [PubMed] [Google Scholar]
- 31.Jensen PT, et al. Early-stage cervical carcinoma, radical hysterectomy, and sexual function. A longitudinal study. Cancer. 2004;100:97–106. doi: 10.1002/cncr.11877. [DOI] [PubMed] [Google Scholar]
- 32.Raspagliesi F, et al. Nerve-sparing radical hysterectomy in cervical cancer: evolution of concepts. Gynecologic oncology. 2007;107:S119–121. doi: 10.1016/j.ygyno.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Herzog TJ, Wright JD. The impact of cervical cancer on quality of life–the components and means for management. Gynecologic oncology. 2007;107:572–577. doi: 10.1016/j.ygyno.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Bukovic D, et al. Sexual life after cervical carcinoma. Coll Antropol. 2003;27:173–180. [PubMed] [Google Scholar]
- 35.Ferrandina G, et al. Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: a prospective, longitudinal study. Gynecologic oncology. 2012;124:389–394. doi: 10.1016/j.ygyno.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, et al. PrediQt-Cx: post treatment health related quality of life prediction model for cervical cancer patients. PloS one. 2014;9:e89851. doi: 10.1371/journal.pone.0089851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, et al. Health-related quality of life in locally advanced cervical cancer patients treated with neoadjuvant therapy followed by radical surgery: A single-institutional retrospective study from a prospective database. Gynecologic oncology. 2019;154:583–589. doi: 10.1016/j.ygyno.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Thapa N, et al. Impact of cervical cancer on quality of life of women in Hubei, China. Sci Rep. 2018;8:11993. doi: 10.1038/s41598-018-30506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirchheiner K, et al. Health related quality of life and patient reported symptoms before and during definitive radio(chemo)therapy using image-guided adaptive brachytherapy for locally advanced cervical cancer and early recovery - a mono-institutional prospective study. Gynecologic oncology. 2015;136:415–423. doi: 10.1016/j.ygyno.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Heijkoop ST, et al. Dynamics of patient reported quality of life and symptoms in the acute phase of online adaptive external beam radiation therapy for locally advanced cervical cancer. Gynecologic oncology. 2017;147:439–449. doi: 10.1016/j.ygyno.2017.08.009. [DOI] [PubMed] [Google Scholar]