Summary:
The resting sensory discomfort transiently relieved upon movement of the affected area in restless legs syndrome suggests that sensorimotor integration mechanisms, specifically gating, may be altered in the disease. The authors sought to determine the effects of prepulse auditory and tactile stimulation applied to lower limbs on the blink reflex of patients with restless legs syndrome and healthy subjects. Seventeen patients with restless legs syndrome and 17 age- and sex-matched healthy controls were investigated. Auditory stimuli and tactile lower limb stimulation were applied as prepulses. The R2 response of the blink reflex induced by electrical stimulation applied to the right supraorbital nerve was selected as the test stimulus. Time intervals between prepulses and response-eliciting stimuli were 40, 70, 90, 110, and 200 milliseconds. There were no differences in either the auditory or tactile prepulse conditions between patients and controls and no differences between these measures within subject groups. We concluded that the tactile lower limb and the auditory prepulse effects on the brainstem interneurons mediating the blink reflex share common neural pathways. Because forebrain interneurons mediate these prepulse effects, they are likely not involved in the disordered sensorimotor interaction of restless legs syndrome.
Keywords: Restless legs syndrome, Prepulse inhibition, Startle reflex, Blink reflex
Restless legs syndrome (RLS) is a chronic condition characterized by abnormal sensations causing restlessness (Nagandla and De, 2013; Lugaresi et al., 1986). This condition can be idiopathic or secondary to a number of disorders (Sieminski et al., 2012; Talarico et al., 2013). Discomfort in RLS can lead to a significant alteration in quality of life (Abetz et al., 2004; Nagandla and De, 2013). The resting sensory discomfort is transiently relieved upon movement of the affected area. Involuntary lower limb movements while asleep and sometimes while awake are often associated with RLS (Lugaresi et al., 1986). The pathophysiology of this condition is not fully understood (Ekbom and Ulfberg, 2009; Ferreri and Rossini, 2004; Freeman and Rye, 2013; Nagandla and De, 2013).
Studies have shown that a malfunction of neural pathways located above the spinal cord seems to alter spinal cord activity in RLS (Freeman et al., 2012; Scalise et al., 2010). A likely origin above the spinal cord is supported by the fact that dysregulation of a number of brainstem structures involved in the sleep–wake cycle have been associated with disruption of the sensorimotor homeostasis within the spinal cord (Nagandla and De, 2013; Scaglione et al., 2008). Motor hyperactivity of animal limbs is observed when some of these neural networks do not work properly (Lynch, 1971). Investigations in humans with RLS have suggested that brainstem interneurons are key structures involved in the pathogenesis of this disorder downregulating spinal cord function (Bara-Jimenez et al., 2000, 2007; Frauscher et al., 2007). Therefore, the neurobiological evaluation of these interneurons and associated structures in humans might help in delineating the pathophysiology of RLS.
The functional assessment of sensorimotor behavior-mediated brainstem interneurons can be investigated using the auditory startle reflex (ASR), the blink reflex (BR) and prepulse inhibition (PPI). The ASR is modulated by lower pons interneurons, the orbicularis oculi reflex, or BR induced by electrical supraorbital nerve stimulation is mediated by caudal brainstem lateral reticular interneurons, and the effects of a preceding stimulus on the startle reflex, also known as PPI, is modulated by forebrain circuitry and brainstem circuits that are incompletely known (Bhidayasiri and Truong, 2011; Sanes et al., 1982; Valls-Solé et al., 1999). In individuals with RLS, studies of the BR and the ASR have suggested that caudal brainstem regions downregulate the neural systems believed to be involved in the pathophysiology of RLS (Akyol et al., 2003; Bucher et al., 1996). However, the influence of forebrain circuitry (Castellanos et al., 1996; Swerdlow et al., 1995) on brainstem interneurons in RLS is unknown.
The physiological behavior of forebrain structures and its connections can be investigated by measuring the effects that a weak conditioning stimulus (prepulse, S1) produces on a response caused by sudden and above-threshold startle stimulation (test pulse, S2). The effect of conditioning auditory stimulation (S1) on the ASR (S2) is the most widely and extensively investigated PPI domain (Valls-Sole, 2012). The effects of mechanical and somatosensory prepulses elicited by electrical stimulation of upper limbs on the ASR and BR have also been investigated in humans (Brown et al., 1991; Sanes et al., 1982; Valls-Solé et al., 1994, 1999). These investigations have shown that somatosensory stimulation of upper limbs and auditory prepulse effects on the startle reflex may share common gating mechanisms (Valls-Solé et al., 1994, 1999), some of which are abnormal in a number of neural disorders, such as Huntington’s disease, Tourette’s syndrome, and parkinsonism (Castellanos et al., 1996; Swerdlow et al., 1995; Valls-Solé et al., 2004). Time-locked mechanical stimulation and prepulse electrical stimulation of lower limbs are also responsible for gating orbicularis oculi reflex responses in healthy subjects (Rossi and Scarpini, 1992; Valls-Solé et al., 1994). The behavior of tactile prepulses applied to lower limbs, its relationship with auditory prepulses, and the modulatory action of these stimuli on the orbicularis oculi reflex is unknown in healthy subjects and individuals with neural disorders.
Here, we investigated the behavior of the interneurons involved in the corticosubcortical circuitries believed to mediate PPI (Mazzone et al., 2011; Swerdlow et al., 1995) in patients with RLS and in normal controls. We hypothesized that these behavioral measures would differ between these two groups. If PPI is altered in patients with RLS, it might favor the involvement of forebrain pathways in this disorder. Furthermore, it would demonstrate that the abnormal interneuronal activity in RLS is more widespread than considered to date. However, if this operational measure of sensorimotor activity is normal in RLS, it would imply that the involved circuits are spared and functionally different from those generating the abnormal brainstem responses obtained elsewhere by other neurobiological evaluations (Bucher et al., 1996; Frauscher et al., 2007).
MATERIALS AND METHODS
Patients and Healthy Controls
Seventeen patients (5 male, 12 female; age: 48.8 ± 11.5 years old) with RLS and 17 age- and sex-matched healthy controls (5 male, 12 female; age: 48.7 ± 11.2 years old) were investigated. All patients were screened with a complete medical and neurological history and clinical examination. Patients with RLS fulfilled the international validated criteria (The International Restless Legs Syndrome Study Group, 2003). Patients and controls were screened with blood and urine tests including a complete blood count, levels of electrolytes, creatinine, glucose, iron, transferrin, ferritin, and a urine drug screen. Subjects were excluded if they had any biochemical abnormalities. In addition, patients and controls were excluded if they were taking and unable to discontinue any of the following medications for five half-lives: carbidopa–levodopa, dopamine agonists, selective serotonin receptor agonists, benzodiazepines, neuroleptics, amantadine, opiates, anxiolytics, sedatives, clonidine, N-methyl-D-aspartate receptor antagonists, monoamine oxidase inhibitors, anticholinergics, anticonvulsants, antihistamines, or lithium.
Test Protocol
Healthy subjects and patients were placed in a supine position, and the skin over the forehead and around the right eye was cleaned using alcohol wipes. Adhesive disposable surface recording electrodes were placed on the lateral canthus of the eye and below the lower eyelid in line with the pupil over the orbicularis oculi muscle. A ground electrode was placed on the right hand. Impedances were kept below 20 kΩ.
The test stimulus was electric stimulation of the right supraorbital nerve through surface silver cup electrodes. The cathode was placed over the supraorbital notch. The anode was placed 2 cm superior and lateral to the cathode. The test stimulus consisted of an electrical square wave pulse lasting 0.2 milliseconds, which was set a level of 3 times the sensory threshold. The response R2 of the BR was chosen (Kimura, 2001; Leon-S et al., 1997; Leon-Sarmiento et al., 2005; Neumann, 2004) as the control response. This response has shown the most reliable conservative and convincing demonstration of the prepulse effects on the BR (Ludewig et al., 2003). This reflex response appears at around 20 ~ 25 milliseconds after applying an electrical stimulation on the supraorbital nerves and has a duration of 30 ~ 40 milliseconds (Kimura, 2001; Leon-Sarmiento et al., 2010). The R2 area was calculated in µVms2. It was measured in a window 21 to 80 milliseconds after the onset of the eliciting stimulus (Blumenthal et al., 2005).
Auditory Prepulse Inhibition Testing
The methodology described by Swerdlow et al. (1995) and Schmolesky et al. (1996) was followed. Headphones were placed over the ears, and they were given the instruction to gently close their eyes. The conditioning stimulus was an acoustic stimulation of 16 dB above a 70 dB background of 20 milliseconds duration (Ludewig et al., 2003).
Tactile Prepulse Inhibition Testing
The reported methodology from Rossi and Scarpini (1992) and Valls-Solé et al. (1994) was followed. The electrical conditioning stimulus consisted of 0.2 milliseconds duration, square-wave electric pulse delivered through surface electrodes to the tibial nerve of the right leg, with the anode located 3 cm distal to the cathode. The tactile prepulse stimulation was kept constant during the experiment, and it was set to two times the subject’s perceptual threshold obtained by following the ascending and descending methods of limits (Sanes et al., 1982). At these threshold values, the conditioning stimulus did not elicit reflex responses in the right orbicularis oculi muscle (Valls-Solé et al., 1994).
Conditioning Test Protocol
The lead intervals were counted from prepulse onset and eliciting stimulus onset. The time intervals between the prepulse lead stimulus and the eliciting stimulus were 40, 70, 90, 110, and 200 milliseconds. The prepulse/pulse combinations were intermixed with four pulse-only stimuli. The computer randomly generated the order of both the prepulse/pulse combinations and the pulse-only combination. Each set of prepulse/pulse stimuli or pulse-only stimuli were performed 60 seconds apart to prevent habituation. Participants were instructed to open their eyes between each trial. The sequence was repeated 4 times for a total of 36 separate trials. For a given interstimulus period, each individual’s response was the subject’s R2-measured average response associated with the prepulse stimulation divided by their average R2-measured response associated with the pulse-only stimulation. Conditions were identical for the auditory and lower limb testing condition. Data were collected in the electromyographic machine and analyzed offline. Band-pass filters were set between 20 and 2,000 Hz.
Prepulse Inhibition Analysis
All results were analyzed using the National Instruments Labview 6.1 program. Responses with voluntary muscle activity were rejected. For analysis, the waveforms were rectified, and the ipsilateral R2 component of the BR was calculated in milliseconds × millivolts. Each interstimulus interval (ISI) group and pulse-only group was then averaged. For analysis, each ISI was divided by the pulse-only signal to represent the percent change of the R2 area. The results were expressed as mean ± SEM.
Statistical Analysis
Because the BR responses particularly at high ISI tend to converge at zero, the data were log-transformed before analysis. Statistical analyses were performed using the R statistical package (Baier et al., 2011). A linear mixed-model approach was used to examine the relationships between stimulation interval and patient/healthy volunteer status about both auditory and tactile stimulation. This model easily accommodates covariates (in this case, age and gender used for matching patients and controls), does not require balanced data, and allows for more flexible error structures. Time interval was treated as a 5-level factor (qualitatively similar results were obtained when treated as a continuous covariate), and interest focused upon the interactions and main effects of time interval, patient/control status, and stimulation type. We did separate analyses that examined differences between patients and controls for a given stimulation type and differences between stimulation type within each group. Of primary interest in the analysis of auditory or tactile response is the main effect of patient/control status and whether there is an interaction between patient/control status and stimulation interval (Davis, 2002). P value was set at < 0.05.
Ethics
All participants gave written informed consent. The study was approved by the Institutional Review Board of the NINDS (NIH) and has been performed in accordance with the Ethical Standards of the Declaration of Helsinki and later amendments.
RESULTS
All individuals completed the experiments and tolerated the procedures with no concerns. No patient reported any pain or discomfort during the experiment, except for the mild pinprick sensation from the electrical stimulation. None of the subjects fell asleep during the study.
Auditory Prepulse Inhibition
No significant group effects (patient/control) on PPI (P = 0.16, F: 1, 30) nor a significant interaction between stimulation interval and group status (P = 0.35, F: 4, 128) were found. The PPI was also unaffected by gender (P = 0.79) or age (P = 0.24) (Fig. 1). Although there was a trend for PPI differences at the intermediate interstimuli periods with less PPI inhibition in patients, there was a considerable overlap between PPI from patients and controls at different ISIs.
FIG. 1.
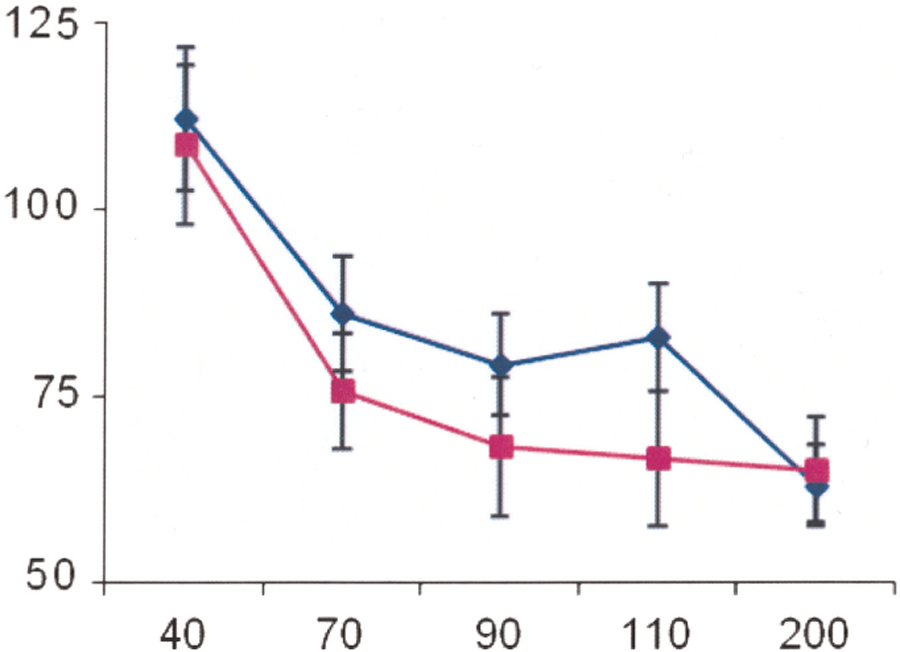
Time course of the effects of an auditory prepulse stimulus on R2 test reflex responses elicited by a blink reflex. The size of the conditioned R2 responses is expressed as a percentage of their unconditioned values. Blue diamond: patients with restless legs syndrome; purple square: healthy controls. Y-axis: size of the conditioned reflex responses in percentage; X-axis: conditioning test interval in milliseconds.
Tactile Prepulse Inhibition
The findings for tactile PPI were similar to those for auditory PPI (Fig. 2). There was neither a significant group effect (P = 0.31, F: 1, 30) nor a significant interaction between interval and status (P = 0.31, F: 4, 128) on the PPI responses. Borderline significance was found for age (P = 0.07) and gender (0.05) with older individuals and females having less PPI.
FIG. 2.
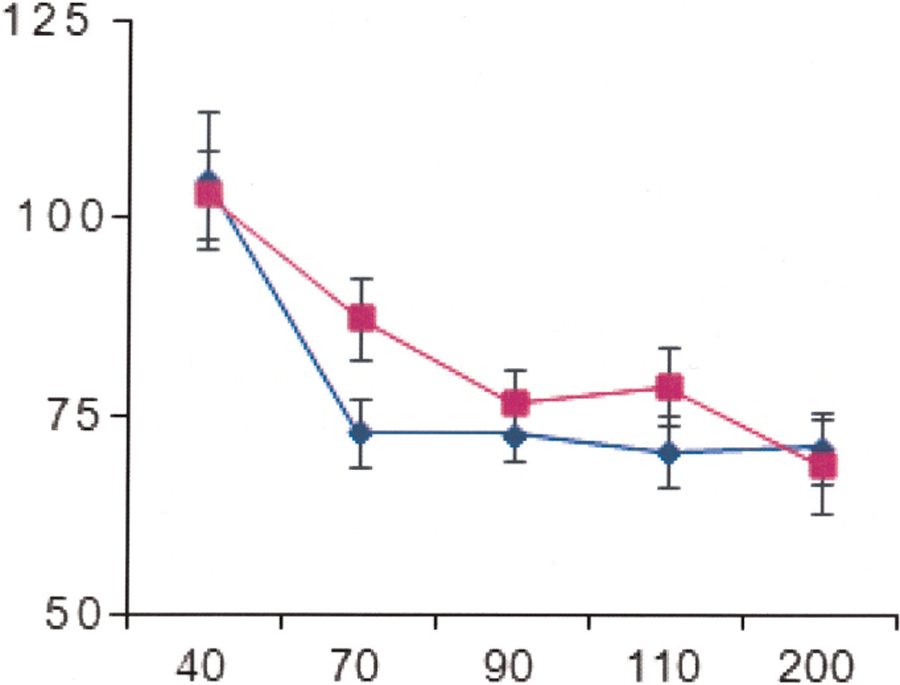
Time course of the effects of a tactile prepulse stimulus applied to the tibial nerve on R2 test reflex responses elicited by a blink reflex. The size of the conditioned R2 responses is expressed as a percentage of their unconditioned values. Blue diamond: patients with restless legs syndrome; purple square: healthy control. Y-axis: size of the conditioned reflex responses in percentage; X-axis: conditioning test interval in milliseconds.
Patients With Restless Legs Syndrome and Prepulse Inhibition Patterns
Although PPI patterns from patients did not differ from controls for either type of stimulation pattern, we wanted to look for differences between stimulation patterns for each group. Modeling indicated no significant interaction between stimulation type and stimulus interval (P = 0.39, F: 4, 144) on PPI. However, the main effect for stimulus type on PPI was significant (P = 0.0024, F: 1, 148) when the interaction was removed. The main effect corresponded to a larger response for tactile stimulation among the patients (0.16 units higher on the log-transformed scale). Age and gender did not influence these results (P > 0.1 for both).
Healthy Controls and Prepulse Inhibition Patterns
No interaction was found in the PPI responses between stimulation type and interval (P = 0.33, F: 4, 144). After removing the interval interaction, the effect of stimulation type on the PPI remained nonsignificant (P = 0.37, F: 1, 148). Neither age nor gender showed significant interaction with the PPI responses.
DISCUSSION
We have demonstrated for the first time that the inhibitory effects exerted by auditory stimuli and tactile stimuli applied to lower limbs on the R2 response of the BR are comparable in normal subjects. The auditory effects we found in this investigation were similar to those reported by Schmolesky et al. (1996). The neural inhibition found in this study by applying a prepulse tactile stimulation to the tibial nerve resembled the effects of sural nerve PPI electrical stimulation on the BR of healthy individuals investigated by Rossi and Scarpini (1992). One difference is that the inhibition of the R2 response found by these authors started at shorter ISI (e.g., 40 milliseconds) than that found by us. The most likely explanation for the differences in the changes of the magnitude of the R2 response of the BR obtained in our group of healthy subjects in comparison with findings of Rossi and Scarpini (1992) are the ISIs we used. We used longer intervals than any previous study on PPI using tactile stimulation (60 seconds) as this avoids the habituation of the R2 response of the BR (Ison et al., 1990; Leon-S et al., 1995).
The similarities of the effects of the two different stimulus modalities on the R2 response of the orbiculari oculi reflex are difficult to understand on anatomical grounds. The best explanation for our findings is that both modalities converge on a common neural tollgate within the brainstem interneurons (Rimpel et al., 1982; Steidl et al., 2004; Valls-Solé et al., 1994). Such a key relay station would synchronize in a nonspecific way for the surrounding environment to ensure an appropriate PPI within the central nervous system. In accordance with these statements, Groves et al. (1973) found that a number of neurons within the bulbopontine reticular formation of rats responded similarly to both tactile and auditory stimuli. Although this explanation would support the similarities found in the BR data obtained by following auditory and tactile prepulse stimuli for the ISIs investigated here, more work on this is needed.
Another novel finding of this investigation is that by using the R2 response of the orbicularis oculi reflex as probe, the PPI exerted by auditory and tactile lower limb stimulation in individuals with RLS was found to be within normal limits. These unexpected results contrast with the abnormal brainstem interneuron activity found previously in the BR and ASR of RLS (Bucher et al., 1996; Frauscher et al., 2007). These findings should not be considered paradoxical but rather complementary because the PPI, BR, and ASR reflect the function of different pathways (Brown et al., 1991; Valls-Sole, 2012). Together, they can give a more complete picture of the pathophysiology of RLS (Fig. 3).
FIG. 3.
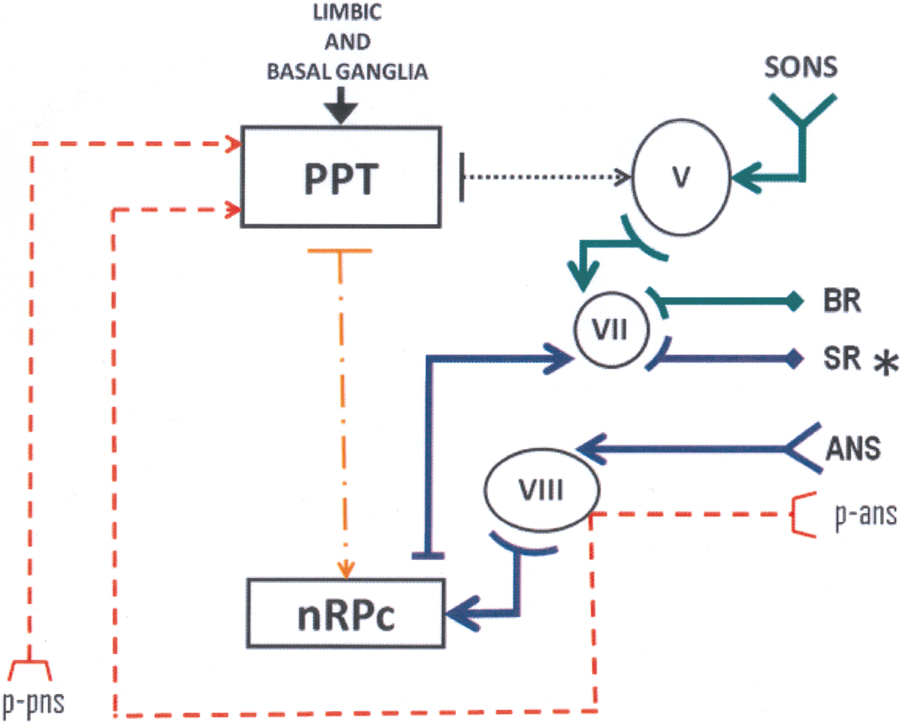
Simplified model of the interaction of afferent and efferent pathways involved in the prepulse inhibition, tactile blink reflex (BR) and auditory startle reflex (ASR) in humans. Red broken lines: Prepulses originated in the peripheral nerves (p-pns) and in the auditory system (p-ans); green lines: circuit for the electrically elicited BR; blue lines: circuit for the ASR. Black dotted line: inhibitory action exerted by the pedunculopontine nucleus (PPN) on the trigeminal nucleus (V). Yellow dotted and broken line: inhibitory action exerted by the PPN on the nucleus reticularis pontis caudalis. VII: Brainstem nucleus of the facial nerve; VIII: brainstem nucleus of the auditory nerve. SR: startle reflex, which is abnormal (asterisk) in restless legs syndrome.
The R2 response of the BR induced by single electrical stimulation of the supraorbital nerve is normal in RLS (Bucher et al., 1996). This indicates that the neural pathways involved with this motor response and their associated trigeminal nuclei are normal in RLS (Cohen et al., 1989; Kimura, 2001; Pellegrini et al., 1995; Sohn et al., 2004). However, the excitability of the ASR was increased in patients with RLS (Frauscher et al., 2007). Of note, the latency calculated from responses recorded from leg muscles of patients with RLS was shorter than in controls; further, the area under the curve of the motor responses was greater in this group of patients than the values measured in the controls (Frauscher et al., 2007). From the pictures presented by these authors, it could be inferred that the duration of the R2 response, and very likely the one from R3, was prolonged as well (Frauscher et al., 2007). Of note, the ASR of rats with an iron deficiency (Unger et al., 2006), a biochemical marker associated with RLS (Rizzo et al., 2013), was also found abnormal. These findings indicate that subcortical pathways that pass to the lower pons and medulla mediated by the nucleus reticularis pontis caudalis (Bhidayasiri and Troung, 2011; Yeomans and Frankland, 1995) are abnormal in RLS.
Forebrain circuitries and the pedunculopontine tegmental nucleus and its associated neurotransmitters modulate the effects exerted by auditory and tactile prepulses on the R2 responses induced by electrical stimulation of the supraorbital nerve. These are spared in RLS (Eisensehr et al., 2001; Michaud et al., 2002; Stiasny-Kolster et al., 2004; Tribl et al., 2004). The influence of forebrain circuitries on brainstem interneurons investigated by the PPI in iron-deficient rats (Burhans et al., 2006) is normal. These investigations support our findings, which clearly indicate that the neural circuitry that inhibits the effects of the prepulses on the R2 motor response elicited by supraorbital nerve electrical stimulation, mediated by the main sensory nuclei of the trigeminal nerve, is normal in patients with RLS. These results contrast with the abnormalities found in the brainstem interneurons of a number of movement disorders (Fig. 4). Moreover, they indicate that the lower pons plays a central role in the pathophysiology of RLS.
FIG. 4.
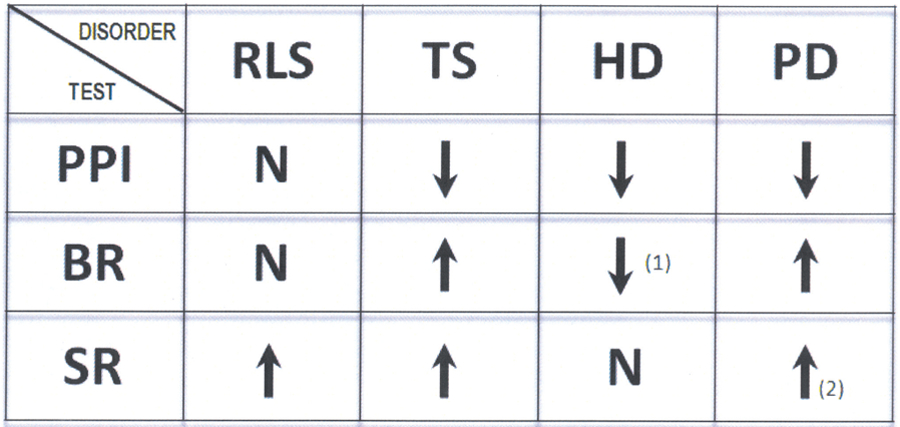
Summary of the prepulse inhibition, blink reflex, and startle reflex data obtained from individuals with restless legs syndrome, Tourette syndrome, Huntington’s disease, and Parkinson’s disease. N: normal; arrow up: increased excitability; arrow down: decreased excitability. (1): Some patients may show normal or increased excitability, (2): some patients may show normal excitability. Adapted from Bucher et al., 1996; Castellanos et al., 1996; Frauscher et al., 2007; Kofler et al., 2001; Swerdlow et al., 1995; Valls-Sole et al., 2004; Williams et al., 2008. Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
ACKNOWLEDGMENTS
This study was supported by the NINDS Intramural program. F. E. Leon-Sarmiento was also supported by a grant from the Department of Defense of the United States (USAMRAAW81XWH-09–1-0467). The authors would like to thank Dr. Neil Jeffries for his help with the statistical analysis.
Footnotes
Disclosure of Commercial Interest: M. Hallett serves as Chair of the Medical Advisory Board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the Editorial Board of 20 journals and received royalties from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer, and Elsevier. He has received honoraria for lecturing from Columbia University. M. Hallett’s research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds came from the Kinetics Foundation, for studies of instrumental methods to monitor Parkinson’s disease, and BCN Peptides, S.A., for treatment studies of blepharospasm, Merz, for a study of hand dystonia, and Allergan through Mt. Sinai, for a study of the use of ultrasound to treat dystonia and spasticity.
REFERENCES
- Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther 2004;26:925–935. [DOI] [PubMed] [Google Scholar]
- Akyol A, Kiylioglu N, Kadikoylu G, et al. Iron deficiency anemia and restless legs syndrome: is there an electrophysiological abnormality? Clin Neurol Neurosurg 2003;106:23–27. [DOI] [PubMed] [Google Scholar]
- Baier T, Neuwirth E, De Meo M. Creating and deploying an application with (R) Excel and R. The R Journal 2011;3:5–11. [Google Scholar]
- Bara-Jimenez W, Aksu M, Graham B, et al. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology 2000;54:1609–1615. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Aksu M, Leon-Sarmiento FE, et al. Effects of dopamine agonist treatment on spinal cord excitability in patients with restless legs syndrome. Mov Dis 2007;22:4. [Google Scholar]
- Bhidayasiri R, Truong DD. Startle syndromes. Handb Clin Neurol 2011;100:421–430. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, et al. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology 2005;42:1–15. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, et al. New observations on the normal auditory startle reflex in man. Brain 1991;114:1891–1902. [DOI] [PubMed] [Google Scholar]
- Bucher SF, Trenkwalder C, Oertel WH. Reflex studies and MRI in the restless legs syndrome. Acta Neurol Scand 1996;94:145–150. [DOI] [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Wiesinger J, et al. Iron deficiency affects acoustic startle response and latency, but not prepulse inhibition in young adult rats. Physiol Behav 2006;87:917–924. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Fine EJ, Kaysen D, et al. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol Psychiatry 1996;39:33–41. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Ludlow CL, Warden M, et al. Blink reflex excitability recovery curves in patients with spasmodic dysphonia. Neurology 1989;39:572–577. [DOI] [PubMed] [Google Scholar]
- Davis CS. Statistical methods for the analysis of repeated measurements New York: Springer, 2002. [Google Scholar]
- Eisensehr I, Wetter TC, Linke R, et al. IBZM SPECT in drug-naïve and levodopa-treated idiopathic restless legs syndrome. Neurology 2001;57:1307–1309. [DOI] [PubMed] [Google Scholar]
- Ekbom K, Ulfberg J. Restless legs syndrome. J Intern Med 2009;266:419–431. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Rossini PM. Neurophysiological investigations in restless legs syndrome and other disorders of movement during sleep. Sleep Med 2004;5:397–399. [DOI] [PubMed] [Google Scholar]
- Frauscher B, Löscher W, Högl B, et al. Auditory startle reaction is disinhibited in idiopathic restless legs syndrome. Sleep 2007;30:489–493. [DOI] [PubMed] [Google Scholar]
- Freeman A, Pranski E, Miller RD, et al. Sleep fragmentation and motor restlessness in a Drosophila model of Restless Legs Syndrome. Curr Biol 2012;22:1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AA, Rye DB. The molecular basis of restless legs syndrome. Curr Opin Neurobiol 2013;23:895–900. [DOI] [PubMed] [Google Scholar]
- Groves PM, Miller SW, Parker MV, Rebec GV. Organization by sensory modality in the reticular formation of the rat. Brain Res 1973;54:207–224. [DOI] [PubMed] [Google Scholar]
- Ison JR, Sanes JN, Foss JA, Pinckney LA. Facilitation and inhibition of the human startle blink reflexes by stimulus anticipation. Behav Neurosci 1990;104:418–429. [DOI] [PubMed] [Google Scholar]
- Kimura J Electrodiagnosis in disease of nerve and muscles 3rd ed. Oxford: Oxford University Press, 2001. [Google Scholar]
- Kofler M, Muller J, Wenning GK, et al. The auditory startle reaction in parkinsonian disorders. Mov Disord 2001;16:62–71. [DOI] [PubMed] [Google Scholar]
- Leon-S FE, Arimura K, Arimura Y, Sonoda Y, Osame M. Contralateral early blink reflex in patients with HTLV-I associated myelopathy/tropical spastic para-paresis. J Neurol Sci 1995;128:51–57. [DOI] [PubMed] [Google Scholar]
- Leon-S FE, Arimura K, Suwazono S, Arimura Y, Osame M. The effects of shounousui on the three responses of the blink reflex in man. Muscle Nerve 1997;20:110–112. [DOI] [PubMed] [Google Scholar]
- Leon-Sarmiento FE, Bayona-Prieto J, Gomez J. Neurophysiology of blepharospasm and multiple system atrophy: clues to its pathophysiology. Parkinsonism Relat Disord 2005;11:199–201. [DOI] [PubMed] [Google Scholar]
- Leon-Sarmiento FE, Bayona-Prieto J, Moscoso F. Incomplete facial nerve palsy: new lessons from activated orbicularis oculi muscles. South Med J 2010;103:581–584. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Ludewig S, Seitz A, et al. The acoustic startle ref lex and its modulation: effects of age and gender in humans. Biol Psychol 2003;63:311–323. [DOI] [PubMed] [Google Scholar]
- Lugaresi E, Cirignotta F, Coccagna G, Montagna P. Nocturnal myoclonus and restless legs syndrome. Adv Neurol 1986;43:295–307. [PubMed] [Google Scholar]
- Lynch G Behavioral excitability and the giant-celled pontine reticular formation (nucleus reticularis pontis oralis). Brain Res 1971;32:449–453. [DOI] [PubMed] [Google Scholar]
- Mazzone P, Sposato S, Insola A, Scarnat E. The deep brain stimulation of the pedunculopontine tegmental nucleus: towards a new stereotactic neurosurgery. J Neural Transm 2011;118:1431–1451. [DOI] [PubMed] [Google Scholar]
- Michaud M, Soucy JP, Chabli A, et al. SPECT imaging of striatal pre and post-synaptic dopaminergic status in restless legs syndrome with periodic limb movements of sleep. J Neurol 2002;249:164–170. [DOI] [PubMed] [Google Scholar]
- Nagandla K, De S. Restless legs syndrome: pathophysiology and modern management. Postgrad Med J 2013;89:402–410. [DOI] [PubMed] [Google Scholar]
- Neumann DL. The effects of lead stimulus and reflex stimulus modality on modulation of the blink reflex at very short, short, and long lead intervals. Percept Psychophys 2004;66:141–151. [DOI] [PubMed] [Google Scholar]
- Pellegrini JJ, Horn AK, Evinger C. The trigeminally evoked blink reflex. I. Neuronal circuits. Exp Brain Res 1995;107:166–180. [DOI] [PubMed] [Google Scholar]
- Rimpel J, Geyer D, Hopf HC. Changes in the blink responses to combined trigeminal, acoustic and visual repetitive stimulation, studied in the human subject. Electroencephalogr Clin Neurophysiol 1982;54:552–560. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Manners D, Testa C, et al. Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov Dis 2013;28:1886–1890. [DOI] [PubMed] [Google Scholar]
- Rossi A, Scarpini C. Gating of trigemino-facial reflex from low-threshold trigeminal and extratrigeminal cutaneous fibres in humans. J Neurol Neurosurg Psychiatry 1992;55:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Foss JA, Ison JR. Conditions that affect the thresholds of the components of the eyeblink reflex in humans. J Neurol Neurosurg Psychiatry 1982;45:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaglione C, Vetrugno R, Plazzi G, et al. Nonreciprocal inhibition in primary restless legs syndrome. Mov Dis 2008;23:96–100. [DOI] [PubMed] [Google Scholar]
- Scalise A, Pittaro-Cadore I, Janes F, et al. Changes of cortical excitability after dopaminergic treatment in restless legs syndrome. Sleep Med 2010;11:75–81. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Boelhouwer AJ, Blumenthal TD. The effect of acoustic pulse intensity upon the electrically elicited blink reflex at positive and negative stimulus onset asynchronies. Biol Psychol 1996;44:69–84. [DOI] [PubMed] [Google Scholar]
- Sieminski M, Bilińska M, Nyka WM. Increased frequency of restless legs syndrome in myasthenia gravis. Eur Neurol 2012;68:166–170. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Voller B, Dimyan M, et al. Cortical control of voluntary blinking: a transcranial magnetic stimulation study. Clin Neurophysiol 2004;115:341–347. [DOI] [PubMed] [Google Scholar]
- Steidl S, Faerman P, Li L, Yeomans JS. Kynurenate in the pontine reticular formation inhibits acoustic and trigeminal nucleus-evoked startle, but not vestibular nucleus-evoked startle. Neuroscience 2004;126:127–136. [DOI] [PubMed] [Google Scholar]
- Stiasny-Kolster K, Möller JC, Zschocke J, et al. Normal dopaminergic and serotonergic metabolites in cerebrospinal fluid and blood of restless legs syndrome patients. Mov Dis 2004;19:192–196. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Paulsen J, Braff DL, et al. Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry 1995;58:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico G, Canevelli M, Tosto G, et al. Restless legs syndrome in a group of patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2013;28:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Restless Legs Syndrome Study Group. Validation of the international syndome study group rating scale for restless legs syndrome. Sleep Med 2003;4:21–132. [DOI] [PubMed] [Google Scholar]
- Tribl GG, Asenbaum S, Happe S, et al. Normal striatal D2 receptor binding in idiopathic restless legs syndrome with periodic leg movements in sleep. Nucl Med Commun 2004;25:55–60. [DOI] [PubMed] [Google Scholar]
- Unger EL, Bianco LE, Burhans MS, et al. Acoustic startle response is disrupted in iron-deficient rats. Pharmacol Biochem Behav 2006;84:378–384. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Cammarota A, Alvarez R, Hallett M. Orbicularis oculi responses to stimulation of nerve afferents from upper and lower limbs in normal humans. Brain Res 1994;650:313–316. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Valldeoriola F, Molinuevo JL, et al. Prepulse modulation of the startle reaction and the blink reflex in normal human subjects. Exp Brain Res 1999;129:49–56. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Muñoz JE, Valldeoriola F. Abnormalities of prepulse inhibition do not depend on blink reflex excitability: a study in Parkinson’s disease and Huntington’s disease. Clin Neurophysiol 2004;115:1527–1536. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J Assessment of excitability in brainstem circuits mediating the blink reflex and the startle reaction. Clin Neurophysiol 2012;123:13–20. [DOI] [PubMed] [Google Scholar]
- Williams DR, Doyle LM, Lees AJ, Brown P. The auditory startle response in parkinsonism may reveal the extent but not type of pathology. J Neurol 2008;255:628–632. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Brain Res Rev 1995;21:301–314. [DOI] [PubMed] [Google Scholar]


