Abstract
Pulmonary hypertension (PH) is a frequent and important complication of chronic obstructive pulmonary disease (COPD). It is associated with worse clinical courses with more frequent exacerbation episodes, shorter survival, and greater need of health resources. PH is usually of moderate severity and progresses slowly, without altering right ventricular function in the majority of cases. Nevertheless, a reduced subgroup of patients may present disproportionate PH, with pulmonary artery pressure (PAP) largely exceeding the severity of respiratory impairment. These patients may represent a group with an exaggerated vascular impairment (pulmonary vascular phenotype) to factors that induce PH in COPD or be patients in whom idiopathic pulmonary arterial hypertension (PAH) coexist. The present review addresses the current definition and classification of PH in COPD, the distinction among the different phenotypes of pulmonary vascular disease that might present in COPD patients, and the therapeutic approach to PH in COPD based on the available scientific evidence.
Keywords: chronic lung disease, pulmonary circulation, vascular remodeling, pulmonary arterial pressure
Epidemiology of Pulmonary Hypertension in COPD
Pulmonary hypertension (PH) is a functional disorder that can occur clinically as an isolated disease (idiopathic pulmonary arterial hypertension), which etiology is unknown, or as a complication associated with other processes. The current classification of pulmonary hypertension (PH) reflects this concept and subdivides it into five broad categories that include processes with common pathogenic mechanisms.1 Pulmonary hypertension due to lung disease and/or hypoxia (group 3 of the classification)1 (Table 1) is one of the most common forms of PH, being chronic obstructive pulmonary disease (COPD) the most frequent cause.
Table 1.
Pulmonary Hypertension Due to Lung Diseases and/or Hypoxiaa
| Obstructive lung disease |
| Restrictive lung disease |
| Other lung disease with mixed restrictive/obstructive pattern |
| Hypoxia without lung disease |
| Developmental lung disorders |
Note: aFrom the Pulmonary Hypertension Classification updated at the 6th World Symposium on Pulmonary Hypertension, Nice, France, 2018.
Pulmonary hypertension is defined in hemodynamic terms by an abnormal increase of pulmonary arterial pressure (PAP). In the last Word Symposium on Pulmonary Hypertension, held in Nice in February–March 2018, it was proposed to lower the previous cutoff value of mean PAP (mPAP) to define PH, adapting it to the normal value of 14.0±3.3 mmHg, shown by Kovacs et al after reviewing more than 1000 right heart catheterizations in healthy subjects.2 Accordingly, the new proposed value to define PH is a mPAP greater than 20 mmHg, which is the upper normal limit shown in healthy subjects,2 with a pulmonary vascular resistance (PVR) greater than 3 Wood Units.
The prevalence of PH in patients with COPD (COPD-PH) is not negligible, and, in general, it is dependent on the severity of the disease. Specific genetic signatures are also linked with the development of PH in COPD.3 Studies in patients with spirometric Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 4 have shown that up to 90% of patients might have abnormal mPAP (>20 mmHg), with most of them ranging between 20 and 35 mmHg. Approximately, only 1% to 5% of patients with COPD have mPAP greater than 35–40 mmHg at rest.4 Interestingly, even under moderate exercise conditions, most COPD patients show a rapid rise in mPAP, indicating loss of lung vasculature, vascular distensibility and/or vessel recruitment capability.5 It should be noted that in COPD, PH at rest or during exercise may be also due to comorbid left heart disease (more frequently), sleep disordered breathing or chronic pulmonary thrombosis.
In COPD, PH is usually of moderate severity, without altering right ventricular function in most cases.6,7 Nevertheless, a small subgroup of patients may present severe PH, with PAP values exceeding those expected according to the severity of respiratory impairment. These patients may depict a clinical picture similar to more severe forms of PH and have greater mortality.
Pathobiology of Pulmonary Hypertension in COPD
Remodeling of pulmonary vessels is the major cause of PH in COPD. It affects small and precapillary arteries and has been identified at different degrees of disease severity.8,9 The most prominent feature of pulmonary vascular remodeling in COPD is the enlargement of the intimal layer in muscular arteries. Intimal hyperplasia is produced by the proliferation of smooth muscle cells (SMC), which in advanced stages conform bundles of longitudinal muscle that differs from the normal circumferential disposition, and deposition of elastic and collagen fibers.8,9 In the arterioles, there is development of a medial coat of circular smooth muscle bounded by a new elastic lamina.
Intimal hyperplasia of pulmonary muscular arteries is apparent at all disease stages. In mild-to-moderate COPD the majority of SMC proliferating in hyperplasic intimas have a poorly differentiated phenotype, as shown by the lack of expression of contractile filaments that are characteristic of mature cells.8
Changes in the tunica media are less conspicuous and the majority of morphometric studies have failed to demonstrate differences in the thickness of the muscular layer when comparing COPD patients with control subjects.10–12
The pathophysiologic mechanisms underlying the development of PH in COPD are still poorly understood. It is believed that the sequence of changes that lead to PH begins at early disease stages by an impairment of endothelial function in pulmonary vessels.13 Our group has previously suggested that in COPD, such endothelial impairment could be caused by cigarette smoke products, which represents an early triggering factor for pulmonary vascular alterations.14 Products contained in cigarette smoke play a critical role in the initiation of pulmonary endothelial cell alterations.14 However, these changes are not sufficient to explain the development of PH, and even less to explain why some patients with COPD develop severe PH.
Other mechanisms such as a decrease in pulmonary vascular surface, parenchymal loss, air-trapping, abnormal pulmonary mechanics, and alveolar hypoxia could concur and promote the development of PH in COPD.15–17 Hypoxemia has been suggested to be a PH trigger, but the relationship between PAP and arterial PO2 (PaO2) has great variability,18 suggesting that there must be additional factors explaining the development of PH in COPD.
Endothelial dysfunction in COPD-PH is associated with an impaired release of endothelium-derived vasodilating agents (nitric oxide, prostacyclin) and increased expression of growth factors.14 Smoking induces remodeling of pulmonary arteries, which could be further enhanced by chronic hypoxia.19 Hypoxia induces endothelial cell damage, causing the release of molecules such as endothelin that leads to neighboring smooth muscle cell vasospasm and proliferation.19 Although initial stages of vascular remodeling due to cigarette smoke exposure or early hypoxia exposure could still be a reversible process, persistent inflammation and chronic hypoxia seem to be largely irreversible.19 Once the pulmonary artery wall has been remodeled, the intima layer thickens and the neointima shaped, remodeling reversion of the pulmonary vascular vessels is not possible.
Vascular stiffness is increased due to a higher deposition of elastic and collagen fibers. This results in an increase of PVR with the subsequent increase in PAP. Small reductions in the diameter of remodeled small pulmonary arteries can lead to significant increases in PVR.19
Clinical Implications and Diagnostic Tools
The diagnosis of COPD-PH is a difficult task, especially in its mildest form. Symptoms due to PH, such as dyspnea or fatigue, are difficult to differentiate from the clinical picture of COPD itself. On the other hand, the identification of some clinical signs can be masked by the existence of lung hyperinflation, the large oscillations in intrathoracic pressure, or superimposed respiratory sounds (rhonchi or crackles). Thus, typical findings such as ejection click increased pulmonary component of the second tone or pansystolic murmur of tricuspid regurgitation may not be recognized. Frequently, the suspicion of PH in COPD is based on the presence of peripheral edema.20 Complementary examinations such as chest x-ray or EKG have low sensitivity in the detection of PH.21 Pulmonary function tests are necessary for the diagnosis of COPD, although there are no specific patterns of impairment of pulmonary function associated with the development of PH. In situations where the lung parenchyma is preserved, the existence of PH may be associated with a decrease in carbon monoxide diffusing capacity (DLCO).
Other non-invasive modalities that might raise suspicion for the presence of PH in COPD include circulating biomarkers, abnormalities in exercise testing, altered blood gases, and changes at the echocardiography and other imaging tests. Plasma levels of BNP or NT-pro-BNP are elevated in severe COPD-PH but have less sensitivity and specificity for moderate PH and may be confounded by left heart abnormalities.22,23 In COPD, PH is generally associated with diminished exercise capacity and greater impairment of gas exchange at rest or during exercise than that expected based on ventilatory limitation.
Echocardiography is a non-invasive and easily accessible tool and is critical in the diagnosis of any patient with suspected PH. It allows the evaluation of hypertrophy or dilatation of the right ventricle and the estimation of systolic PAP. However, echocardiography presents technical difficulties in COPD patients due to the hyperinflation of the chest.24 Echocardiography should be performed when it is considered that PH may have a significant contribution to the patient’s clinical status, such as dissociation between the intensity of symptoms and the degree of functional impairment; existence of manifest disparity between the decrease in DLCO and the degree of spirometric impairment; or the suspicion of disproportionate PH based on physical examination and complementary examinations (chest x-ray, EKG).25,26
The ratio of the main pulmonary artery to the ascending aorta diameter (PA/Ao) on the computed tomography (CT) scan may predict PH in COPD, with a ratio >1 (range 0.9–1.1) suggested as a threshold.27–29 Combining PA/Ao and other non-invasive measures (including echocardiographic and other physiologic variables) improves the accuracy for predicting PH. Additionally, other CT markers such measurements of small vessels surface, as well as assessments of bronchial wall and emphysematous changes, are emerging and might be used for predicting and phenotyping PH in COPD.30,31
Recent studies with cardiac magnetic resonance (CMR) have demonstrated that parameters of pulmonary artery stiffness such as pulse wave velocity have high sensitivity and specificity for identifying PH.32 In addition, other parameters assessed by CMR such as right ventricular mass index and pulmonary artery relative area change are useful in assessing the prognosis of COPD-PH.33 Thus, CMR appears to have a promising role in the early non-invasive assessment of PH in COPD.
Pulmonary hemodynamics assessed by right cardiac catheterization is the gold standard tool to confirm the diagnosis of PH. However, due to its invasive nature, despite being a safe procedure in expert hands, it is not routinely recommended in the evaluation of patients with COPD. However, in certain circumstances, such as frequent episodes of right ventricular failure, or candidates for lung transplantation or lung volume reduction, right heart catheterization might be indicated, after a comprehensive assessment using noninvasive tools. The procedure should be done while the patient is in a stable condition and taking into account the appropriate setting of the zero-reference level and the effects of respiratory swings on pressure readings.34,35
According to the results of right heart catheterization, the 6th World Symposium on Pulmonary Hypertension proposed a hemodynamic classification of COPD-PH into three categories:36 without PH, with PH and with severe PH (Table 2).
Table 2.
Hemodynamic Classification of Pulmonary Hypertension in COPD
| COPD without PH | mPAP <21 mmHg mPAP 21–24 mmHg with PVR <3 WU |
| COPD with PH | mPAP 21–24 mmHg with PVR ≥3 WU mPAP 25–34 mmHg |
| COPD with severe PH | PAP ≥35 mmHg PAP ≥25 mmHg with CI <2.0 L/min/m2 |
Abbreviations: PH, pulmonary hypertension; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; WU, Wood units (mmHg/L/min); CI, cardiac index.
Pulmonary Vascular Disease Phenotypes in COPD
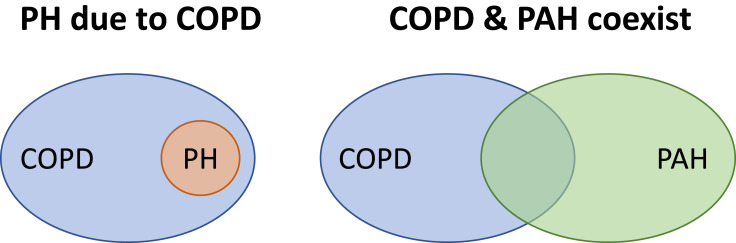
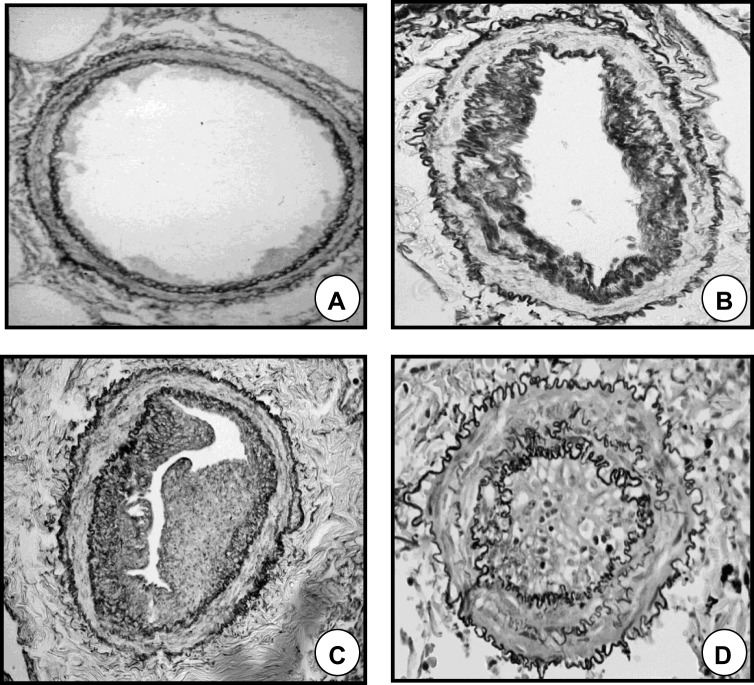
The group of COPD patients with severe PH is an intriguing one. The reason why a small cluster of COPD patients (3–5%) present with severe hemodynamic impairment has not been elucidated. Hypothetically, it might represent a subgroup with a particularly aggressive form of PH, or patients in which pulmonary arterial hypertension (PAH) (group 1 of the PH classification) coexists with COPD by chance (Figure 1). In the past, the term “out-of-proportion” PH was used to define this subgroup of patients, but this term has been abandoned because it is unclear whether the disproportion to the PAP value should be considered based on the severity of the airflow obstruction, the extent of parenchymal derangement or other parameters. In a recent perspective article, Kovacs et al34 proposed the term “pulmonary vascular phenotype” to define this subgroup of patients. The pulmonary vascular phenotype in COPD is characterized by severe PH,36 less severe airflow limitation (mostly moderate obstruction), hypoxemia, very low DLCO, normo- or hypocapnia, and exercise limitation of cardiovascular origin.34,37 Interestingly, the vascular lesions in COPD with severe PH are morphologically similar to those seen in idiopathic PAH11 and differ from those observed in patients with COPD and moderate PH12 (Figure 2). The identification of COPD patients with the pulmonary vascular phenotype and the distinction of those in which COPD and idiopathic PAH coexist is based on clinical judgement, taking into account a set of parameters (Table 3). These parameters include clinical features, pulmonary function testing, chest imaging, hemodynamic profile and the pattern of the response to cardiopulmonary exercise testing. It would be interesting to integrate these variables in a composite score that would assist the clinician to establish a pre-test probability of the pulmonary vascular phenotype or co-existing PAH before undertaking the invasive confirmation of PH with right heart catheterization (Figure 3).
Figure 1.
Potential explanations for severe pulmonary hypertension in COPD. Hypothetically, in patients with COPD, severe pulmonary hypertension (PH) may occur as a consequence of an aggressive form of pulmonary vascular disease (PH due to COPD), or because there is a co-existing pulmonary arterial hypertension.
Figure 2.
Remodeling of pulmonary muscular arteries in pulmonary hypertension. (A) Pulmonary muscular artery of a control subject without COPD. (B) Pulmonary muscular artery with a moderately remodeled intima in a patient with COPD and mild pulmonary hypertension. (C) Pulmonary muscular artery with a highly enlarged intima from a patient with COPD and severe pulmonary hypertension. (D) Pulmonary artery showing a prominent muscular hypertrophy in a patient with pulmonary arterial hypertension.
Table 3.
Assessment of the Pulmonary Vascular Phenotype in COPD
| Clinical features |
| Exclusion of other causes of PH |
| ● Chronic thromboembolism: angio-CT, V-Q scintigraphy |
| ● Left heart disease: echocardiography, right heart catheterization |
| ● Sleep breathing disorders |
| Risk factors for PAH |
| ● HIV infection |
| ● BMPR2 mutations |
| ● Portal hypertension |
| ● Connective tissue disease |
| Pulmonary function testing |
| ● Forced spirometry |
| ● CO diffusing capacity (DLCO) |
| ● Arterial blood gases: PaO2 and PaCO2 |
| Imaging techniques |
| ● High-resolution CT scan: assessment of extent and severity of emphysema |
| Right heart catheterization |
| ● Mean PAP |
| ● Cardiac index |
| Cardiopulmonary exercise testing |
| ● Cardiovascular limitation pattern |
| ● Preserved ventilatory response |
| ● Gas exchange inefficiency: low end-tidal CO2, high VE/VCO2 slope |
| Circulating biomarkers |
| ● BNP or NT-proBNP |
Abbreviations: PH, pulmonary hypertension; angio-CT, computed tomography angiogram; V-Q, ventilation and perfusion; PAH, pulmonary arterial hypertension; HIV, human immunodeficiency virus; BMPR2, bone morphogenetic protein receptor type 2; PAP, pulmonary artery pressure; VE, minute ventilation; VCO2, carbon dioxide output; BNP, brain natriuretic peptide; NT-proBNP, N-terminal prohormone of brain natriuretic peptide.
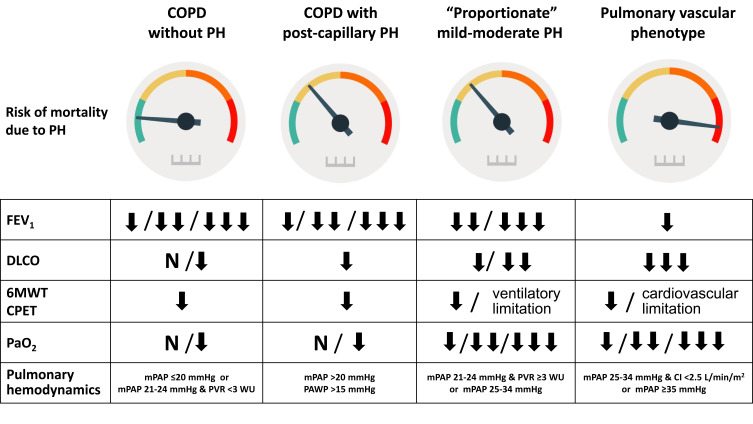
Figure 3.
Pulmonary vascular disease phenotypes in COPD. Risk of death due to pulmonary hypertension and features in different pulmonary vascular disease phenotypes in COPD.
Abbreviations: PH, pulmonary hypertension; FEV1, forced expiratory volume in the first second; DLCO, diffusing capacity for carbon monoxide; 6MWT, six-minute walking test; CPET, cardiopulmonary exercise test; PaO2, partial pressure of arterial oxygen; mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; CI, cardiac index; PAWP, pulmonary artery wedge pressure.
It has been previously established that the presence of PH has a stronger association with mortality in COPD than the forced expiratory volume in the first second (FEV1) or gas exchange variables.38 In addition, an enlarged pulmonary artery diameter, as detected by CT scan, predicts hospitalization due to acute COPD exacerbation.27,39
The majority of patients with COPD-PH present with severe or very severe airflow obstruction (GOLD spirometric stages 3 or 4, FEV1 <50% of predicted) or severe emphysema in the CT scan, and mild-to-moderate precapillary PH (mPAP 21–34 mmHg, cardiac index >2.5 L/min/m2). These cases could be considered as having “proportionate PH” (Figure 3).
Another phenotype to consider in COPD is the presence of postcapillary PH,34 due to the frequent association with left heart disease. Sometimes the presence of heart failure with preserved ejection fraction is difficult to detect. The combination of clinical features and results of non-invasive exams may help to establish the probability of this condition40 (Figure 3).
Treatment of PH in COPD
According to what we know so far, PH should not be seen as a simple complication of hypoxemia that occurs in the most advanced stages of the disease. Furthermore, it should be borne in mind that pulmonary circulation disorders can also be observed in patients with mild disease without hypoxemia, and even in smokers without airflow obstruction. These considerations are of interest when addressing the treatment of pulmonary vascular disorders in COPD.
First of all, COPD should be optimally treated according to current guidelines. In patients with hypoxemia, the correction of alveolar hypoxia with long-term oxygen treatment (LTOT) appears to be the most logical intervention to reduce PH. In stabilized hypoxemic COPD patients, LTOT for 15 h/day prevents the progressive increase of PAP and, when used >18 h/day, produces a slight decrease of PAP.39,41 However, in COPD patients with moderate resting (SpO2 89–93%) or exercise-induced (<90% for ≥10 seconds) oxygen desaturation, LTOT does not provide benefit in terms of survival or hospitalizations.41 Therefore, in COPD, LTOT is the most appropriate treatment for PH associated with respiratory failure, although it has little impact on pulmonary hemodynamics and does not reverse the pathological lesions of the pulmonary circulation.
The safety and efficacy of PAH-targeted therapy in COPD-PH has been evaluated in recent years. Seven randomized controlled trials (RCTs) have evaluated the safety and efficacy of PAH-targeted therapy in COPD-PH, see Nathan et al36 and Gredic et al42 for a detailed review of the trials. Most of these studies were conducted in a limited number of subjects, in some of them PH was diagnosed on the basis of echocardiographic findings, and all but one evaluated COPD patients with mild-to-moderate PH.36,42 Only Vitulo et al43 evaluated COPD patients with severe PH, which was defined by mPAP >35mmHg if FEV1 was <30% of predicted, or by mPAP ≥30mmHg if FEV1 was ≥30% of predicted.
Recent meta-analyses44–46 have analyzed the pooled results of these RCTs. According to these meta-analyses, targeted PAH therapy definitely improves pulmonary hemodynamics, assessed by right heart catheterization.44,45 Such beneficial hemodynamic effect has been demonstrated with both bosentan, an endothelin-1 receptor antagonist, and sildenafil, a phosphodiesterase-5 (PDE5) inhibitor.44,45
The effect of PAH-targeted therapy on exercise capacity in patients with COPD-PH is less apparent. Individual studies show mixed results.36,42 One of the meta-analysis showed improvement in the distance covered at the 6 minutes walking test (6MWD), whereas other two meta-analyses failed to show any improvement in 6MWD.44–46 The lack of consistent improvement in exercise capacity with PAH-targeted therapy could be explained, at least in part, by the design of the studies. The only two studies that showed an improvement in 6MWD47,48 have some methodological limitations, since both included a reduced number of patients, in one of them the diagnosis of PH was made by echocardiography47 and the other had an open-label design.48 Differences in the effects on 6MWD are not due to the type of drug, since inconsistent effects have been shown using bosentan or PDE5 inhibitors.36,42 In addition to the methodological differences among RCTs, it must be considered that COPD itself limits exercise tolerance and hence differences in COPD severity might influence too the results of the 6MWD and explain, in part, discrepancies among studies.
The efficacy of PAH-targeted therapy on quality of life or dyspnea in patients with COPD-PH is also limited and, in general, with disappointing results.44,45 Most of the studies did not show any improvement in quality of life. However, Vitulo et al43 showed an improvement in the BODE (body mass index, lung obstruction, dyspnea and exercise capacity) score, the modified Medical Research Council scale, and the SF-36 general health domain, after 16 weeks of treatment with sildenafil 20 mg t.i.d in COPD patients who had severe PH.43
In summary, current evidence does not support that in patients with COPD-PH, the improvement in pulmonary hemodynamics shown with targeted PAH therapy results in greater exercise capacity and/or improvement in symptoms.
The effect of targeted PAH therapy on survival in COPD-PH has been assessed retrospectively in studies with a mixed population of patients with different respiratory diseases (not only COPD), and usually with severe PH. Having these limitations in mind, two studies showed longer survival in patients that had been treated with targeted PAH therapy, which in most cases consisted of sildenafil or tadalafil, a PDE5 inhibitor, as compared with untreated patients.22,23 Of note, in one of these studies, survival benefit with targeted PAH therapy was only apparent in patients who had severe PH, whereas no benefit was observed in patients with mild-to-moderate PH.23
In terms of safety, the vasodilator effect of targeted PAH therapy may worsen gas exchange in COPD due to the inhibition of hypoxic pulmonary vasoconstriction, which increases ventilation-perfusion (VA/Q) mismatching.13,49 Evidence for the long-term effect of targeted PAH therapy on gas exchange in COPD-PH is heterogeneous.44 While deterioration of gas exchange was shown in some studies with the long-term use of bosentan or sildenafil, no change was observed in others using sildenafil or tadalafil43,50-52 and rarely resulted in treatment withdrawal.53
Rehabilitation with exercise training is an established therapy for patients with COPD. In patients with PAH, supervised exercise rehabilitation has also been shown to improve exercise tolerance.54 There is very few information on the effects of pulmonary rehabilitation in patients with COPD-PH. In a RCT performed by our group, addressed to evaluate the effects of sildenafil added to a program of pulmonary rehabilitation in patients with COPD-PH, we showed that all patients improved significantly exercise endurance with pulmonary rehabilitation, irrespective if they received sildenafil or placebo.52 Indeed, sildenafil did not provide additional benefit to that obtained with pulmonary rehabilitation alone.52 Therefore, exercise training should be considered in patients with COPH-PH as a therapeutic option addressed to improve exercise tolerance.36
In summary, unfortunately, to date, we do not have appropriate therapeutic alternatives for the treatment of PH in patients with COPD, and available drugs are limited to supportive care.19 Most RCTs conducted with targeted PAH therapy have failed to demonstrate symptomatic improvement or improvement in exercise tolerance.15,52 Nevertheless, it should be considered that most of RCTs conducted in COPD-PH involved patients with mild-to-moderate PH. There is also a concern that in COPD the administration of targeted PAH therapy may worsen gas exchange.49 Therefore, further studies are needed to identify novel targets directed to prevent or reduce the uncontrolled cellular hyperproliferation in pulmonary arteries, as well as the determinants of the heterogeneity in its presentation, in order to find suitable therapeutic alternatives, particularly in COPD patients with severe PH or the “pulmonary vascular phenotype”. We consider that future RCTs in COPD-PH should target specifically this subgroup of patients and consider new trial endpoints, as it has been already done in PAH, given the lack of sensitivity of 6MWD to reflect hemodynamic changes in COPD.
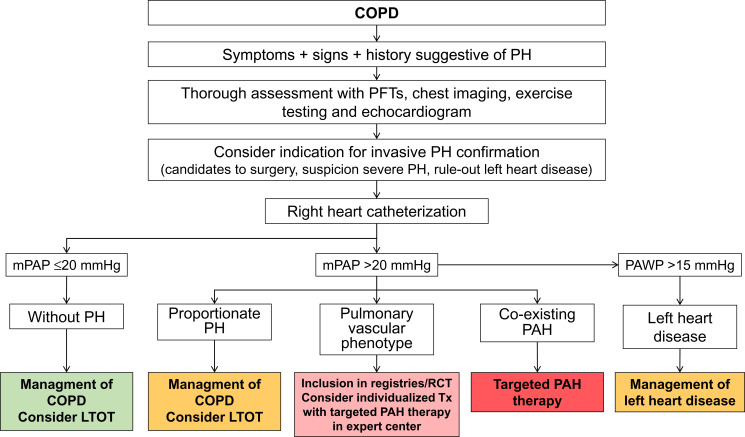
We can conclude that the treatment of choice for patients with COPD-PH and associated hypoxemia is long-term oxygen therapy. Regarding the use of targeted PAH therapy, it should be restricted to those patients with PAH and co-existing COPD. The use of targeted PAH in the “pulmonary vascular phenotype” should be considered on individual basis, circumscribed to centers expert in both PH and COPD, and employed in the context of RCTs and/or clinical registries, in order to acquire firm evidence about its safety and efficacy (Figure 4). With the currently available information, the use of targeted PAH therapy in COPD patients with mild-to-moderate or “proportionate” PH is discouraged.
Figure 4.
Diagnosis and management of pulmonary hypertension in COPD.
Abbreviations: COPD, chronic obstructive pulmonary disease; PH, pulmonary hypertension; mPAP, mean pulmonary arterial pressure; PAWP, pulmonary arterial wedge pressure; LTOT, long-term oxygen treatment; RCT, randomized controlled trials; Tx, treatment; PAH, pulmonary arterial hypertension.
Conclusion
Pulmonary hypertension is a serious complication in the natural history of COPD. Its presence is associated with reduced survival and has been identified as a predictive factor of greater use of health resources. Pulmonary vascular disease covers a wide spectrum of COPD. Moderate degrees of PH are relatively frequent in COPD, usually associated with severe airflow obstruction or parenchymal destruction. There is a subgroup of patients in whom PH reaches a severe, disproportionately high value with mild airflow obstruction, which is similar to those who present idiopathic PAH. In these patients, clinical judgement based on a multidimensional assessment must discern whether they have a “pulmonary vascular phenotype” or co-existing PAH.
There are indications that the sequence of alterations that lead to PH begins in the early stages of the disease from pulmonary endothelial dysfunction. As a consequence of this dysfunction, there is an imbalance in the substances of endothelial synthesis, in favor of those that produce vasoconstriction and promote cell proliferation and the deposition of extracellular matrix in the arterial wall.
Regarding treatment, PAH-targeted therapy should be restricted to those patients with co-existing PAH. It could also be considered in individual cases with the “pulmonary vascular phenotype” in expert PH and COPD centers, in the context of clinical trials or registries.
Disclosure
Dr Joan Albert Barberà reports grants, personal fees from Actelion, grants from Ferrer, grants from GlaxoSmithKline, grants, personal fees from Merck Sharp & Dohme, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–894. doi: 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann J, Wilhelm J, Olschewski A, Kwapiszewska G. Microarray analysis in pulmonary hypertension. Eur Respir J. 2016;48(1):229–241. doi: 10.1183/13993003.02030-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaouat A, Bugnet A-S, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189–194. doi: 10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 5.Weitzenblum E, Sautegeau A, Ehrhart M, Mammosser M, Hirth C, Roegel E. Long-term course of pulmonary arterial pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1984;130(6):993–998. doi: 10.1164/arrd.1984.130.6.993 [DOI] [PubMed] [Google Scholar]
- 6.Portillo K, Torralba Y, Blanco I, et al. Pulmonary hemodynamic profile in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1313–1320. doi: 10.2147/COPD.S78180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilde JM, Skjørten I, Hansteen V, et al. Haemodynamic responses to exercise in patients with COPD. Eur Respir J. 2013;41(5):1031–1041. doi: 10.1183/09031936.00085612 [DOI] [PubMed] [Google Scholar]
- 8.Santos S, Peinado VI, Ramírez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19(4):632–638. doi: 10.1183/09031936.02.00245902 [DOI] [PubMed] [Google Scholar]
- 9.Peinado VI, Gómez FP, Barberà JA, et al. Pulmonary vascular abnormalities in chronic obstructive pulmonary disease undergoing lung transplant. J Hear Lung Transpl. 2013;32(12):1262–1269. doi: 10.1016/j.healun.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 10.Santos S, Peinado VI, Ramírez J, et al. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(9):1250–1256. doi: 10.1164/rccm.200210-1233OC [DOI] [PubMed] [Google Scholar]
- 11.Carlsen J, Hasseriis Andersen K, Boesgaard S, Iversen M, Steinbrüchel D, Bøgelund Andersen C. Pulmonary arterial lesions in explanted lungs after transplantation correlate with severity of pulmonary hypertension in chronic obstructive pulmonary disease. J Hear Lung Transpl. 2013;32(3):347–354. doi: 10.1016/j.healun.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 12.Bunel V, Guyard A, Dauriat G, et al. Pulmonary arterial histologic lesions in patients with COPD with severe pulmonary hypertension. Chest. 2019;156(1):33–44. doi: 10.1016/j.chest.2019.02.333 [DOI] [PubMed] [Google Scholar]
- 13.Barberà JA, Blanco I. Pulmonary hypertension in patients with chronic obstructive pulmonary disease: advances in pathophysiology and management. Drugs. 2009;69(9):1153–1171. doi: 10.2165/00003495-200969090-00002 [DOI] [PubMed] [Google Scholar]
- 14.Blanco I, Piccari L, Barberà JA. Pulmonary vasculature in COPD: the silent component. Respirology. 2016;21(6):984–994. doi: 10.1111/resp.12772 [DOI] [PubMed] [Google Scholar]
- 15.Opitz I, Ulrich S. Pulmonary hypertension in chronic obstructive pulmonary disease and emphysema patients: prevalence, therapeutic options and pulmonary circulatory effects of lung volume reduction surgery. J Thorac Dis. 2018;10(Suppl 23):S2763–S2774. doi: 10.21037/jtd.2018.07.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Washko GR, Nardelli P, Ash SY, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease a longitudinal observational study. Am J Respir Crit Care Med. 2019;200(4):454–461. doi: 10.1164/rccm.201811-2063OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahaghi FN, Argemí G, Nardelli P, et al. Pulmonary vascular density: comparison of findings on computed tomography imaging with histology. Eur Respir J. 2019;54(2):1900370. doi: 10.1183/13993003.00370-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166(3):314–322. doi: 10.1164/rccm.2107027 [DOI] [PubMed] [Google Scholar]
- 19.Sysol JR, Machado RF. Classification and pathophysiology of pulmonary hypertension. Contin Cardiol Educ. 2018;4(1):2–12. doi: 10.1002/cce2.71 [DOI] [Google Scholar]
- 20.Weitzenblum E, Apprill M, Oswald M, Chaouat A, Imbs JL. Pulmonary hemodynamics in patients with chronic obstructive pulmonary disease before and during an episode of peripheral edema. Chest. 1994;105(5):1377–1382. doi: 10.1378/chest.105.5.1377 [DOI] [PubMed] [Google Scholar]
- 21.Oswald-Mammosser M, Oswald T, Nyankiye E, Dickele MC, Grange D, Weitzenblum E. Non-invasive diagnosis of pulmonary hypertension in chronic obstructive pulmonary disease. Comparison of ECG, radiological measurements, echocardiography and myocardial scintigraphy. Eur J Respir Dis. 1987;71(5):419–429. [PubMed] [Google Scholar]
- 22.Tanabe N, Taniguchi H, Tsujino I, et al. Multi-institutional retrospective cohort study of patients with severe pulmonary hypertension associated with respiratory diseases. Respirology. 2015;20(5):805–812. doi: 10.1111/resp.12530 [DOI] [PubMed] [Google Scholar]
- 23.Lange TJ, Baron M, Seiler I, Arzt M, Pfeifer M. Outcome of patients with severe ph due to lung disease with and without targeted therapy. Cardiovasc Ther. 2014;32(5):202–208. doi: 10.1111/1755-5922.12084 [DOI] [PubMed] [Google Scholar]
- 24.Greiner S, Jud A, Aurich M, et al. Reliability of noninvasive assessment of systolic pulmonary artery pressure by doppler echocardiography compared to right heart catheterization: analysis in a large patient population. J Am Heart Assoc. 2014;3(4). doi: 10.1161/JAHA.114.001103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowak J, Hudzik B, Jastrzȩbski D, et al. Pulmonary hypertension in advanced lung diseases: echocardiography as an important part of patient evaluation for lung transplantation. Clin Respir J. 2018;12(3):930–938. doi: 10.1111/crj.12608 [DOI] [PubMed] [Google Scholar]
- 26.D’Andrea A, Stanziola A, Di Palma E, et al. Right ventricular structure and function in idiopathic pulmonary fibrosis with or without pulmonary hypertension. Echocardiography. 2016;33(1):57–65. doi: 10.1111/echo.12992 [DOI] [PubMed] [Google Scholar]
- 27.Wells JM, Washko GR, Han MLK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yagi M, Taniguchi H, Kondoh Y, et al. CT-determined pulmonary artery to aorta ratio as a predictor of elevated pulmonary artery pressure and survival in idiopathic pulmonary fibrosis. Respirology. 2017;22(7):1393–1399. doi: 10.1111/resp.13066 [DOI] [PubMed] [Google Scholar]
- 29.Alkukhun L, Wang XF, Ahmed MK, et al. Non-invasive screening for pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2016;117:65–72. doi: 10.1016/j.rmed.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coste F, Benlala I, Dournes G, Girodet PO, Laurent F, Berger P. Assessing pulmonary hypertension in COPD. Is there a role for computed tomography? Int J COPD. 2019;14:2065–2079. doi: 10.2147/COPD.S207363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahaghi FN, Vegas-Sanchez-Ferrero G, Minhas JK, et al. Ventricular geometry from non-contrast non-ECG-gated CT scans: an imaging marker of cardiopulmonary disease in smokers. Acad Radiol. 2017;24(5):594–602. doi: 10.1016/j.acra.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agoston-Coldea L, Lupu S, Mocan T. Pulmonary artery stiffness by cardiac magnetic resonance imaging predicts major adverse cardiovascular events in patients with chronic obstructive pulmonary disease. Sci Rep. 2018;8(1). doi: 10.1038/s41598-018-32784-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johns CS, Rajaram S, Capener DA, et al. Non-invasive methods for estimating mPAP in COPD using cardiovascular magnetic resonance imaging. Eur Radiol. 2018;28(4):1438–1448. doi: 10.1007/s00330-017-5143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovacs G, Agusti A, Barberá JA, et al. Pulmonary vascular involvement in chronic obstructive pulmonary disease is there a pulmonary vascular phenotype? Am J Respir Crit Care Med. 2018;198(8):1000–1011. doi: 10.1164/rccm.201801-0095PP [DOI] [PubMed] [Google Scholar]
- 35.Weitzenblum E, Mammosser M, Ehrhart M. Evolution and prognosis of pulmonary hypertension in chronic obstructive pulmonary diseases. Herz. 1986;11(3):147–154. [PubMed] [Google Scholar]
- 36.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53(1):1801914. doi: 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boerrigter BG, Bogaard HJ, Trip P, et al. Ventilatory and cardiocirculatory exercise profiles in COPD: the role of pulmonary hypertension. Chest. 2012;142(5):1166–1174. doi: 10.1378/chest.11-2798 [DOI] [PubMed] [Google Scholar]
- 38.Ehlken N, Lichtblau M, Klose H, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J. 2016;37(1):35–44. doi: 10.1093/eurheartj/ehv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medrek SK, Sharafkhaneh A, Spiegelman AM, Kak A, Pandit LM. Admission for COPD exacerbation is associated with the clinical diagnosis of pulmonary hypertension: results from a retrospective longitudinal study of a veteran population. COPD J Chronic Obstr Pulm Dis. 2017;14(5):484–489. doi: 10.1080/15412555.2017.1336209 [DOI] [PubMed] [Google Scholar]
- 40.Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1):1801897. doi: 10.1183/13993003.01897-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albert RK, Au DH, Blackford AL, et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617–1627. doi: 10.1056/NEJMoa1604344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gredic M, Blanco I, Kovacs G, et al. Pulmonary hypertension in chronic obstructive pulmonary disease. Br J Pharmacol. 2020:bph.14979. doi: 10.1111/bph.14979. [DOI] [PubMed] [Google Scholar]
- 43.Vitulo P, Stanziola A, Confalonieri M, et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: a randomized controlled multicenter clinical trial. J Hear Lung Transpl. 2017;36(2):166–174. doi: 10.1016/j.healun.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Tang S, Liu K, et al. Therapy in stable chronic obstructive pulmonary disease patients with pulmonary hypertension: a systematic review and meta-analysis. J Thorac Dis. 2015;7(3):309–319. doi: 10.3978/j.issn.2072-1439.2015.02.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prins KW, Duval S, Markowitz J, Pritzker M, Thenappan T. Chronic use of PAH-specific therapy in world health organization group III pulmonary hypertension: a systematic review and meta-analysis. Pulm Circ. 2017;7(1):145–155. doi: 10.1086/690017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J, Song JH, Park DA, Lee JS, Do LS, Oh YM. Systematic review and meta-analysis of pulmonary hypertension specific therapy for exercise capacity in chronic obstructive pulmonary disease. J Korean Med Sci. 2013;28(8):1200–1206. doi: 10.3346/jkms.2013.28.8.1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao RS, Singh S, Sharma BB, Agarwal VV, Singh V. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci. 2011;53(2):81–85. [PubMed] [Google Scholar]
- 48.Valerio G, Bracciale P, Grazia D’agostino A. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2009;3(1):15–21. doi: 10.1177/1753465808103499 [DOI] [PubMed] [Google Scholar]
- 49.Blanco I, Gimeno E, Munoz PA, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med. 2010;181(3):270–278. doi: 10.1164/rccm.200907-0988OC [DOI] [PubMed] [Google Scholar]
- 50.Lederer DJ, Bartels MN, Schluger NW, et al. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD J Chronic Obstruct Pulm Dis. 2012;9(3):268–275. doi: 10.3109/15412555.2011.651180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stolz D, Rasch H, Linka A, et al. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J. 2008;32(3):619–628. doi: 10.1183/09031936.00011308 [DOI] [PubMed] [Google Scholar]
- 52.Blanco I, Santos S, Gea J, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J. 2013;42(4):982–992. doi: 10.1183/09031936.00176312 [DOI] [PubMed] [Google Scholar]
- 53.Calcaianu G, Canuet M, Schuller A, Enache I, Chaouat A, Kessler R. Pulmonary arterial hypertension-specific drug therapy in COPD patients with severe pulmonary hypertension and mild-to-moderate airflow limitation. Respiration. 2016;91(1):9–17. doi: 10.1159/000441304 [DOI] [PubMed] [Google Scholar]
- 54.Grünig E, Eichstaedt C, Barberà J-A, et al. ERS statement on exercise training and rehabilitation in patients with severe chronic pulmonary hypertension. Eur Respir J. 2019;53(2):1800332. doi: 10.1183/13993003.00332-2018 [DOI] [PubMed] [Google Scholar]