Abstract
Objective
Qualitative research exploring patient preferences regarding the mode of treatment administration for psoriatic arthritis (PsA) is limited. We report patient preferences and their reasons across PsA treatment modes.
Methods
In this global, cross-sectional, qualitative study, interviews were conducted with adult patients with PsA in Brazil, France, Germany, Italy, Spain, the UK, and the US. Patients were currently taking a disease-modifying antirheumatic drug (DMARD). Patients indicated the order and strength of preference (0–100; 100 = strongest) across four modes of treatment administration: oral (once daily), self-injection (weekly), clinic injection (weekly), and infusion (monthly); reasons for preferences were qualitatively assessed. Descriptive statistics were reported. Fisher’s exact tests and t-tests were conducted for treatment mode outcomes.
Results
Overall, 85 patients were interviewed (female, 60.0%; mean age, 49.8 years). First-choice ranking (%) and mean [standard deviation] preference points were: oral (49.4%; 43.9 [31.9]); self-injection (34.1%; 32.4 [24.8]); infusion (15.3%; 14.5 [20.0]); clinic injection (1.2%; 9.2 [10.0]). Of 48 (56.5%) patients with a strong first-choice preference (ie point allocation ≥60), 66.7% chose oral administration. Self-injection was most often selected as second choice (51.8%), clinic injection as third (49.4%), and infusion as fourth (47.1%). Oral administration was the first-choice preference in the US (88.0% vs 38.0% in Europe). The most commonly reported reason for oral administration as the first choice was speed and ease of administration (76.2%); for self-injection, this was convenience (75.9%). The most commonly reported reason for avoiding oral administration was concern about possible drug interactions (63.6%); for self-injection, this was a dislike of needles or the injection process (66.7%).
Conclusion
Patients with PsA preferred oral treatment administration, followed by self-injection; convenience factors were common reasons for these preferences. Overall, 43.5% of patients did not feel strongly about their first-choice preference and may benefit from discussions with healthcare professionals about PsA treatment administration options.
Keywords: psoriatic arthritis, patient preference, qualitative research, treatment administration
Plain Language Summary
Treatments for psoriatic arthritis (PsA) can be administered by different modes: by mouth (oral administration, eg pills), injection, or drip (infusion). Previous research has shown that patients with PsA usually prefer oral administration, but little is known about the reasons for such preferences.
In this study, patients with PsA were interviewed about their preferences for treating their PsA with the following modes of administration: oral administration once daily, injection weekly (self-injection or injection at a clinic), and infusion monthly.
Oral administration was the first choice for almost half of the patients, followed by self-injection, infusion, and then clinic injection. Patients mostly liked how fast and easy it was to take/swallow oral treatments; the main reason for avoiding oral treatments was possible interactions with other pills.
Patients mostly liked self-injection because it could be done at home; the most common reason for disliking self-injection was to avoid needles and pain.
The most common reason for liking infusion was that it was not administered very often, but patients who disliked infusion wanted to avoid visiting the doctor.
The few patients who liked clinic injection felt more comfortable with experts giving their treatment, felt safe, and liked the fast results. However, almost all patients preferred not to have clinic injections because they wanted to avoid visiting the doctor.
These results give specific explanations for patient preferences regarding PsA treatment modes of administration. Considering these results may support the shared decision-making process between healthcare professionals and patients when choosing the most suitable treatment option.
Introduction
Psoriatic arthritis (PsA) is a progressive, debilitating, immune-mediated inflammatory disease with multiple disease manifestations and comorbidities,1–4 which can substantially impact patients’ health-related quality of life.3
According to the 2015 evidence-based treatment recommendations by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the European League Against Rheumatism, non-steroidal anti-inflammatory drugs and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) should be used in the early treatment of PsA.5,6 For patients in whom these are unsuccessful, biologic DMARDs (bDMARDs), such as tumor necrosis factor inhibitors (TNFi), and targeted synthetic DMARDs (tsDMARDs), such as apremilast (a phosphodiesterase inhibitor), are recommended.5,6 More recent guidelines, jointly published in 2018 by the American College of Rheumatology and the National Psoriasis Foundation, recommend starting a TNFi (bDMARD) over csDMARDs or apremilast (tsDMARD) in treatment-naïve patients with active PsA, and switching to a TNFi over csDMARDs, apremilast, or the Janus kinase inhibitor, tofacitinib, in patients with active PsA despite csDMARD treatment; however, the guidelines also acknowledge that treatment with oral medications can be considered if patients prefer these to injectable therapy.7 csDMARDs and tsDMARDs are mostly administered orally, whereas bDMARDs require administration by intravenous infusion or subcutaneous injection.8
Patient-physician shared decision-making is key to optimizing the management of patients with PsA and improving outcomes.5,6,9 Patients may be more likely to adhere to a treatment or disease management plan if they have been involved in the decision.10,11 With studies reporting non-adherence to TNFi treatment in patients with PsA ranging from 24.5% to 55.0%,12,13 improving treatment choice is vital. Previous research has shown that patients with PsA consider the route of administration an important medication attribute and tend to prefer oral administration.14 However, an in-depth analysis of the reasons behind patients’ preference for certain modes of treatment administration is lacking. Qualitative research could improve understanding of patients’ motivations and attitudes underlying certain preferences, which in turn might help physicians consider patient preferences and characteristics when making treatment decisions.
This qualitative study aimed to provide evidence-based data on the preferences for modes of treatment administration and reasons for these preferences among patients with PsA. Specific objectives of this survey were to explore the perceptions, beliefs, and attitudes associated with preferences for modes of treatment administration, identify patient characteristics that may be associated with specific preferences, and better understand the experience of patients with PsA.
Patients and Methods
In this global, cross-sectional, qualitative study, in-depth interviews were conducted with patients from Brazil, Europe (France, Germany, Italy, Spain, and the UK), and the US, who reported a previous physician diagnosis of PsA. The RTI International Institutional Review Board reviewed the study on ethical grounds in the US and deemed it exempt. In Brazil and Europe, the study was exempt from ethics committee review, in accordance with national criteria, as it was considered to meet the criteria for market research. All patients provided written informed consent, and received an honorarium in appreciation of their time.
In each country, patients from the community were recruited via telephone by qualitative research recruiters managing databases of individuals who were local to the area and who agreed to be contacted for participation in research studies. Potential participants were identified based on their current or past report of health conditions and/or treatment experience and then further screened for the specific criteria for this study. Eligible patients were aged ≥18 years, self-reported having a physician/clinical diagnosis of PsA, and were taking a csDMARD, tsDMARD, or bDMARD. The recruiters confirmed potential participants’ eligibility using a recruitment screening questionnaire. Participants’ demographic characteristics and PsA treatment information were collected at screening.
The total sample size and sample plan for this qualitative study were determined to allow generalizability as well as to permit select subgroup and country comparisons. Recruitment quotas by country were 10 patients each from Brazil, France, Germany, Italy, Spain, and the UK (total quota for Europe: 50 patients), and 25–30 patients from the US. More patients were recruited from the US to allow comparison between the US and Europe. The following subgroup quotas for each country were established to obtain a sample representative of the PsA population and to facilitate analysis by country and/or sociodemographic factors: females (50–60%); age <50 years (40–60%); current or recent (≤2 years) use of bDMARDs for PsA (45–55%), and bDMARD-naïve patients (ie had never used a bDMARD at all: 40–60%).
All interviews were conducted in person by interviewers from RTI Health Solutions or subcontractors using the native language of the country and following a semi-structured guide to ensure consistency. In the US, interviews were conducted by experienced interviewers from RTI. In Europe and Brazil, interviews were conducted by experienced, approved RTI subcontractor, AplusA. RTI provided training and ensured standardization of the interview process. The interviewers had no previous/personal knowledge of the patients. Each patient was interviewed once for up to 1 hour and the interviews were audio-recorded. The topics discussed included: patients’ preferences for modes of treatment administration and their reasons for these preferences; their general experiences with PsA; current and past treatments and modes of treatment administration; current PsA symptoms, including the presence of psoriasis (PsO), pain, fatigue (0–10 scales, where 0 = none and 10 = the worst possible PsO, pain, or fatigue in the past 7 days), and the severity of PsA (mild, moderate, severe, or very severe) and how their symptoms had changed since diagnosis (improvement, no change, worsening); and current satisfaction with treatment (0–10 scale, where 10 = greatest satisfaction).
To evaluate PsA treatment mode preference, patients assessed four modes of treatment administration: oral (once daily), self-injection (weekly), clinic injection (weekly), and infusion (monthly) (Figure S1). Patients were asked for their preferred mode of treatment administration, then rated each mode (with 100 points allocated across modes; where more points represented stronger preferences, and the sum of points across the four modes was 100), from which a strength of preference was determined. “Strong” preferences were indicated by a point allocation at or above the median (50th percentile) number of points allocated across the first-ranked mode (≥60 points), “moderate” preferences were indicated by a point allocation between the 25th and 50th percentiles (50–59 points), and “weak” preferences were indicated by a point allocation less than the 25th percentile (≤49 points). Lastly, patients discussed their reasons for preference and point allocation.
All interview data, including de-identified transcripts, were translated into English as needed. Qualitative analysis of the transcripts and the interviewers’ field notes was facilitated using ATLAS.ti software (v7.5). Patterns in the interview data were identified, summarized, and characterized using qualitative content analysis methods, and thematic codes were derived from the transcripts to identify dominant concepts across the interviews.15 To facilitate the identification of concepts and comparison across interviews, patient input on treatment mode preferences, experiences, beliefs, likes, and dislikes were coded by two coders in accordance with a codebook, which was updated as new concepts were identified during the transcript review. Example coding trees for administration mode likes and dislikes are presented in Figure S2. The first 10 transcripts were double-coded (ie coded by both coders) and discrepancies resolved. A random selection of approximately 10% of the remaining transcripts were double-coded for additional quality control. The sociodemographic, lifestyle, and clinical data provided by patients at screening and during the interview were summarized using descriptive analysis methods. Quantitative analyses of the ranking outcome and preference point variables were conducted using SAS® software, Version 9.4 for Windows.16
To assess associations between significant differences in treatment mode preferences and patient and disease characteristics, the following variables were dichotomized into subgroups: sex, age, time since PsA diagnosis, current PsA pain severity, current fatigue severity, current PsO severity (dermatological and nail symptoms), impact, current PsA overall severity, health conditions in addition to PsA, daily number of medications in addition to those for PsA, smoking status, weekly alcohol consumption, weekly exercise, education level, employment status, relationship status, life stage (ie children at home), health care coverage (insurance type), disposable household income, current and any experience with modes of treatment administration, current and any experience with bDMARDs, current and any experience with methotrexate, and satisfaction with PsA treatment. Thresholds used to dichotomize these variables were based on meaning, distribution, and sample size, as applicable.
Although the design of this primarily qualitative study was not intended for statistical comparisons, for the treatment mode outcome variables, independent two-group t-tests (preference point means) and Fisher’s exact tests (first-choice proportions of patients) were used.17 A P value <0.05 was used for all comparisons.
Results
Patient Characteristics
Overall, 85 interviews were conducted; 25 patients were from the US, and 10 patients each were from Brazil, France, Germany, Italy, Spain, and the UK. Patient-reported demographics and clinical characteristics are presented in Table 1; country-specific data are presented in Table S1. Slightly more than half of the 85 patients were female (60.0%), and most were White (89.3%). The mean age was 49.8 years; mean (standard deviation [SD]) age at diagnosis of PsA was 39.6 (13.0) years. Most patients were educated to secondary school (36.8%) or college/university (51.3%) level. Table S2 presents additional data on the patients’ lifestyles and general health characteristics, as well as the specialties of the physicians managing their disease. The majority of patients were diagnosed and managed by rheumatologists (82.4% and 77.6%, respectively) and dermatologists (11.8% and 15.3%, respectively).
Table 1.
Participant Characteristics, by Region and Overalla
| US (N = 25) | Europeb (N = 50) | Brazil (N = 10) | Total (N = 85) | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Sex, n (%) | ||||
| Female | 18 (72.0) | 27 (54.0) | 6 (60.0) | 51 (60.0) |
| Current age (years), mean (SD) | 48.1 (12.4) | 51.1 (12.9) | 47.3 (10.0) | 49.8 (12.5) |
| Education,cn (%) | ||||
| Primary school | 1 (4.0) | 6 (14.6) | 2 (20.0) | 9 (11.8) |
| Secondary school | 6 (24.0) | 19 (46.3) | 3 (30.0) | 28 (36.8) |
| College/university degree | 18 (72.0) | 16 (39.0) | 5 (50.0) | 39 (51.3) |
| Prefer not to answer | 0 (0) | 9 (18.0) | 0 (0) | 9 (10.6) |
| Relationship status,cn (%) | ||||
| Single | 7 (28.0) | 5 (12.2) | 2 (20.0) | 14 (18.4) |
| Cohabiting, married, or civil partnership | 13 (52.0) | 33 (80.5) | 7 (70.0) | 53 (69.7) |
| Otherd | 5 (20.0) | 3 (7.3) | 1 (10.0) | 9 (11.8) |
| Prefer not to answer | 0 (0) | 9 (18.0) | 0 (0) | 9 (10.6) |
| Race/ethnicity,cn (%) | ||||
| White | 19 (76.0) | 21 (100.0) | 10 (100.0) | 50 (89.3) |
| Black | 2 (8.0) | 0 (0) | 0 (0) | 2 (3.6) |
| Asian | 1 (4.0) | 0 (0) | 0 (0) | 1 (1.8) |
| Hispanic | 3 (12.0) | 0 (0) | 0 (0) | 3 (5.4) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Prefer not to answer | 0 (0) | 29 (58.0) | 0 (0) | 29 (34.1) |
| Healthcare coverage,cn (%) | ||||
| Private | 18 (72.0) | 8 (19.5) | 4 (40.0) | 30 (39.5) |
| Medicare, Medicaid, or public assistance | 7 (28.0) | 32 (78.0) | 6 (60.0) | 45 (59.2) |
| Military | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| None | 0 (0) | 1 (2.4) | 0 (0) | 1 (1.3) |
| Prefer not to answer | 0 (0) | 9 (18.0) | 0 (0) | 9 (10.6) |
| Clinical characteristics | ||||
| Age (years) at PsA diagnosis, mean (SD) | 37.6 (13.3) | 41.1 (12.9) | 37.1 (12.5) | 39.6 (13.0) |
| PsO, NRS (0–10),e mean (SD) | 4.0 (2.8) | 2.7 (3.0) | 5.4 (3.1) | 3.4 (3.0) |
| Joint/ligament pain, NRS (0–10),e mean (SD) | 4.7 (2.5) | 4.6 (2.6) | 5.6 (2.0) | 4.7 (2.5) |
| Fatigue, NRS (0–10),e mean (SD) | 5.3 (2.8) | 4.9 (2.9) | 6.5 (2.5) | 5.2 (2.8) |
| Impact, NRS (0–10),e,f mean (SD) | 5.7 (3.0) | 5.4 (3.1) | 9.1 (1.4) | 5.9 (3.1) |
| Severity of PsA, n (%) | ||||
| Mild | 9 (36.0) | 15 (30.0) | 1 (10.0) | 25 (29.4) |
| Moderate | 11 (44.0) | 23 (46.0) | 8 (80.0) | 42 (49.4) |
| Severe | 5 (20.0) | 9 (18.0) | 0 (0) | 14 (16.5) |
| Very severe | 0 (0) | 3 (6.0) | 1 (10.0) | 4 (4.7) |
| Current PsA and PsO signs and symptoms,gn (%) | ||||
| Joint pain | 22 (88.0) | 44 (88.0) | 8 (80.0) | 74 (87.1) |
| Skin patches or plaques (flaking, redness) | 20 (80.0) | 29 (58.0) | 10 (100.0) | 59 (69.4) |
| Stiffness | 17 (68.0) | 25 (50.0) | 3 (30.0) | 45 (52.9) |
| Joint swelling | 15 (60.0) | 24 (48.0) | 2 (20.0) | 41 (48.2) |
| Skin discomfort (eg itching, painful, bleeding, etc.) | 15 (60.0) | 17 (34.0) | 5 (50.0) | 37 (43.5) |
| Unusual fatigue | 9 (36.0) | 18 (36.0) | 2 (20.0) | 29 (34.1) |
| Joint damage | 5 (20.0) | 17 (34.0) | 2 (20.0) | 24 (28.2) |
| Joint tenderness | 5 (20.0) | 16 (32.0) | 2 (20.0) | 23 (27.1) |
| Swollen or inflamed (“sausage”) fingers or toes (dactylitis) | 4 (16.0) | 13 (26.0) | 2 (20.0) | 19 (22.4) |
| Inflammatory back pain (back pain/stiffness) | 6 (24.0) | 12 (24.0) | 0 (0) | 18 (21.2) |
| Sleep problems | 5 (20.0) | 10 (20.0) | 1 (10.0) | 16 (18.8) |
| Nail changes (ie pitting or small dents, separation from nail bed) | 6 (24.0) | 7 (14.0) | 1 (10.0) | 14 (16.5) |
| Tenderness or swelling of ligament/tendon that connects to the bone, commonly the heel or elbow (enthesitis) | 3 (12.0) | 6 (12.0) | 1 (10.0) | 10 (11.8) |
| Eye irritation, redness, disturbed vision | 1 (4.0) | 5 (10.0) | 0 (0) | 6 (7.1) |
Notes: aAll variables were patient-reported. bIncludes France, Germany, Italy, Spain, and the UK. cPercentages for responses other than “Prefer not to answer” do not include any patients who selected “Prefer not to answer”. dSeparated, divorced, or widowed. e0 indicated “none” and 10 indicated the “worst possible” of the variable. fBased on the question “How, if at all, have your PsA symptoms (including skin and musculoskeletal [joint, spine, swelling, tenderness] symptoms) impacted your life?”. gPatients could report >1 response; percentages total >100%.
Abbreviations: NRS, numerical rating scale; PsA, psoriatic arthritis; PsO, psoriasis; SD, standard deviation.
Patient clinical characteristics are also presented in Table 1 and Table S1. Using a scale from 0 (none) to 10 (worst possible), patients, on average, rated their PsA-related pain at 4.7, their PsA-related fatigue at 5.2, and the overall impact of their PsA at 5.9. When asked to categorize the severity of their PsA as mild, moderate, severe, or very severe, nearly one-half of patients said moderate (49.4%; n = 42), approximately one-third selected mild (29.4%; n = 25), and just over one-fifth said severe (16.5%; n = 14) or very severe (4.7%; n = 4). Most patients reported currently experiencing joint pain (87.1%; n = 74) and skin patches/plaques (69.4%; n = 59). Overall, approximately two-thirds of patients reported some aspect of improvement (67.1%; n = 57), one-quarter reported no change (24.7%; n = 21), and one-half reported an element of worsening (49.4%; n = 42) (Table S1).
All 85 patients were currently taking some form of DMARD, with 57 patients (67.1%) taking a csDMARD and 48 (56.5%) taking a bDMARD. Nearly one-quarter of patients (23.5%; n = 20) were taking a combination of csDMARDs and bDMARDs, and 11.8% (n = 10) were taking a steroid (Table 2; Table S3). Over half of the patients were currently receiving oral (62.4%; n = 53) and injection (63.5%; n = 54) modes of administration, while only five (5.9%) patients were receiving infusions (Table 2; Table S3).
Table 2.
Medication Use by Region and Overall
| US (N = 25) | Europea (N = 50) | Brazil (N = 10) | Total (N = 85) | |
|---|---|---|---|---|
| Current PsA medication typeb | ||||
| DMARD type, n (%) | ||||
| Any DMARD | 25 (100.0) | 50 (100.0) | 10 (100.0) | 85 (100.0) |
| csDMARD monotherapy (no bDMARD) | 5 (20.0) | 25 (50.0) | 5 (50.0) | 35 (41.2) |
| MTX (included in csDMARD monotherapy) | 5 (100.0) | 19 (76.0) | 2 (40.0) | 26 (74.3) |
| bDMARD monotherapy (no csDMARD) | 9 (36.0) | 15 (30.0) | 4 (40.0) | 28 (32.9) |
| csDMARD + bDMARD (combination therapy) | 9 (36.0) | 10 (20.0) | 1 (10.0) | 20 (23.5) |
| MTX (included in csDMARD + bDMARD) | 7 (77.8) | 8 (80.0) | 1 (100.0) | 16 (80.0) |
| tsDMARD (apremilast) | 2 (8.0) | 0 (0) | 0 (0) | 2 (2.4) |
| Steroid, n (%) | 0 (0) | 10 (20.0) | 0 (0) | 10 (11.8) |
| csDMARDs,c n (%) | 16 (64.0) | 35 (70.0) | 6 (60.0) | 57 (67.1) |
| MTX | 12 (75.0) | 27 (77.1) | 3 (50.0) | 42 (73.7) |
| Oral | 9 (75.0) | 17 (63.0) | 3 (100.0) | 29 (69.0) |
| Injection | 3 (25.0) | 9 (33.3) | 0 (0) | 12 (28.6) |
| Oral and injection | 0 (0) | 1 (3.7) | 0 (0) | 1 (2.4) |
| Otherd | 3 (18.8) | 12 (34.3) | 3 (50.0) | 18 (31.6) |
| bDMARDs, n (%) | 18 (72.0) | 25 (50.0) | 5 (50.0) | 48 (56.5) |
| Adalimumab | 6 (33.3) | 9 (36.0) | 2 (40.0) | 17 (35.4) |
| Etanercept | 6 (33.3) | 8 (32.0) | 1 (20.0) | 15 (31.3) |
| Secukinumab | 4 (22.2) | 1 (4.0) | 1 (20.0) | 6 (12.5) |
| Infliximab | 2 (11.1) | 2 (8.0) | 1 (20.0) | 5 (10.4) |
| Golimumab | 0 (0) | 4 (16.0) | 0 (0) | 4 (8.3) |
| Ustekinumab | 0 (0) | 1 (4.0) | 0 (0) | 1 (2.1) |
| Current administration mode,c,e n (%) | ||||
| Oral f | 14 (56.0) | 34 (68.0) | 5 (50.0) | 53 (62.4) |
| Injectionsg | 17 (68.0) | 32 (64.0) | 5 (50.0) | 54 (63.5) |
| Infusion h | 2 (8.0) | 2 (4.0) | 1 (10.0) | 5 (5.9) |
| Previous experience,i n (%) | ||||
| bDMARD experience | 19 (76.0) | 26 (52.0) | 6 (60.0) | 51 (60.0) |
| MTX experience | 16 (64.0) | 45 (90.0) | 5 (50.0) | 66 (77.6) |
| Any injection experience | 21 (84.0) | 40 (80.0) | 7 (70.0) | 68 (80.0) |
| Any infusion experience | 4 (16.0) | 18 (36.0) | 3 (30.0) | 25 (29.4) |
Notes: aIncludes France, Germany, Italy, Spain, and the UK. bAt screening, patients were asked to specify any current prescription medications for PsA other than pain medication or topical medication for plaque psoriasis. This information was reviewed during the subsequent study interview. cPatients could report >1 response; percentages total >100%. dLeflunomide, sulfasalazine, azathioprine, hydroxychloroquine, and others. ePatients were asked whether current PsA medications were taken orally (tablet or pill), by injection (self-injected at home or at doctor’s office or hospital clinic), or by intravenous infusion. fRegardless of mode, the 53 patients receiving an oral mode of treatment for PsA were taking the following types of PsA treatments: steroids (n = 10); csDMARDs (n = 50); MTX (n = 36); bDMARDs (n = 21). gRegardless of mode, the 54 patients receiving an injection for PsA were taking the following types of PsA treatments: steroids (n = 8); csDMARDs (n = 27); MTX (n = 23); bDMARDs (n = 43). hRegardless of mode, the five patients receiving an infusion for PsA were taking the following types of PsA treatments: steroids (n = 0); csDMARDs (n = 4); MTX (n = 3); bDMARDs (n = 5). iPatients were asked "Have you ever taken a biologic to treat your PsA? Have you ever taken any medicine on a regular basis, for any condition, that required injection? Have you ever taken methotrexate to treat your PsA?"
Abbreviations: bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease-modifying antirheumatic drug; MTX, methotrexate; PsA, psoriatic arthritis; tsDMARD, targeted synthetic DMARD.
Treatment Mode Preferences
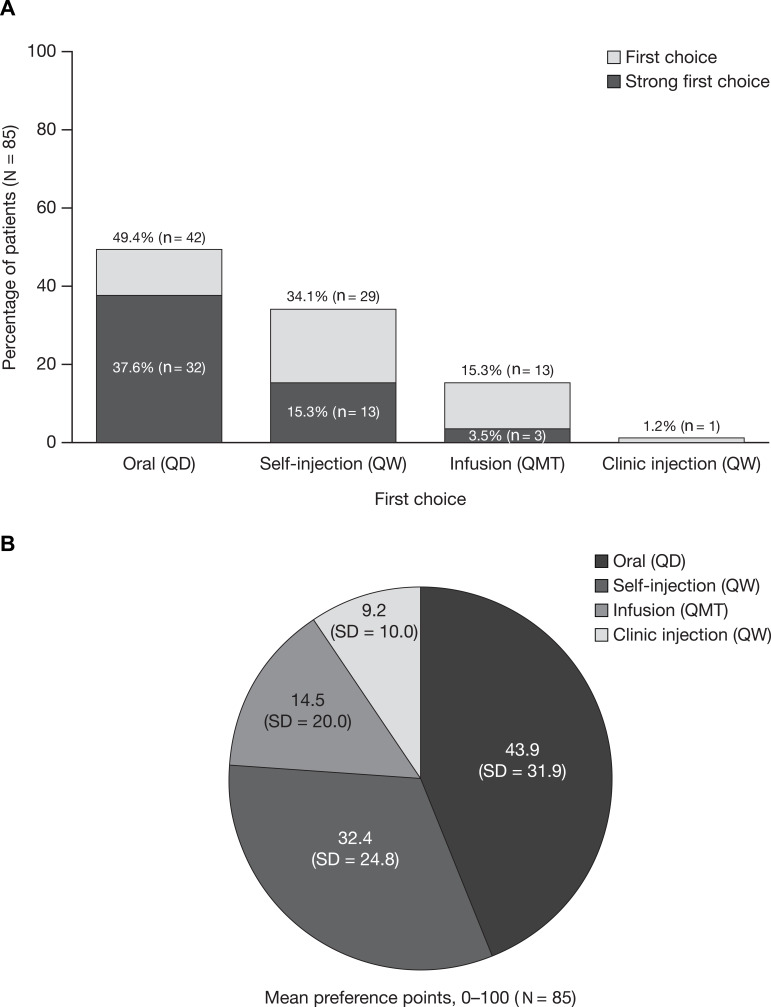
Oral administration (chosen by 49.4%; n = 42) and self-injection (chosen by 34.1%; n = 29) were most commonly chosen as patients’ first-choice mode of treatment administration (Figure 1A). These modes of treatment administration also received the most points in the points allocation task, with mean (SD) point allocations of 43.9 (31.9) and 32.4 (24.8), respectively, followed by infusion (14.5 [20.0]) and clinic injection (9.2 [10.0]) (Figure 1B). Overall, 56.5% of patients (n = 48) had a “strong” first-choice preference for treatment mode, 29.4% (n = 25) had a “moderate” first-choice preference, and 14.1% (n = 12) had a “weak” first-choice preference. Among patients with a “strong” preference, most (66.7%; n = 32) had a preference for oral administration. No patients had a strong preference for clinic injection (Figure 1A). When the threshold for a “strong” first-choice preference was adjusted from ≥60 to ≥50 or ≥70 points, 85.9% (n = 73) and 41.2% (n = 35) of patients, respectively, had a “strong” preference (Figure S3). In both cases, oral administration remained the most common “strong” first-choice preference.
Figure 1.
Mode of treatment administration ranking and point allocationa. (A) First-choiceb mode of treatment administration (ranking) and (B) mode of treatment administration 100-point allocation means (N = 85). aPatients were asked ‘assuming equal effectiveness, safety, and cost, if you had 100 points to assign across these four modes of administration to reflect your preferences, how would you allocate these points? The more points you give to a mode means the more you prefer that mode of administration. You can assign as many or as few points, even zero points, as you want to each of the four modes, so long as the points across all four modes sum to 100.’ bA patient’s first-choice mode was the mode with the most points allocated; a “strong” first-choice preference was defined as a mode with point allocation ≥60.
Abbreviations: QD, once per day; QMT, once per month; QW, once per week; SD, standard deviation.
Self-injection was most commonly selected as patients’ second-choice mode of treatment administration (51.8%; n = 44). Clinic injection (chosen by 49.4%; n = 42) and infusion (chosen by 47.1%; n = 40) were most commonly selected as patients’ third- and fourth-choice modes of treatment, respectively.
Reasons for Treatment Mode Preferences
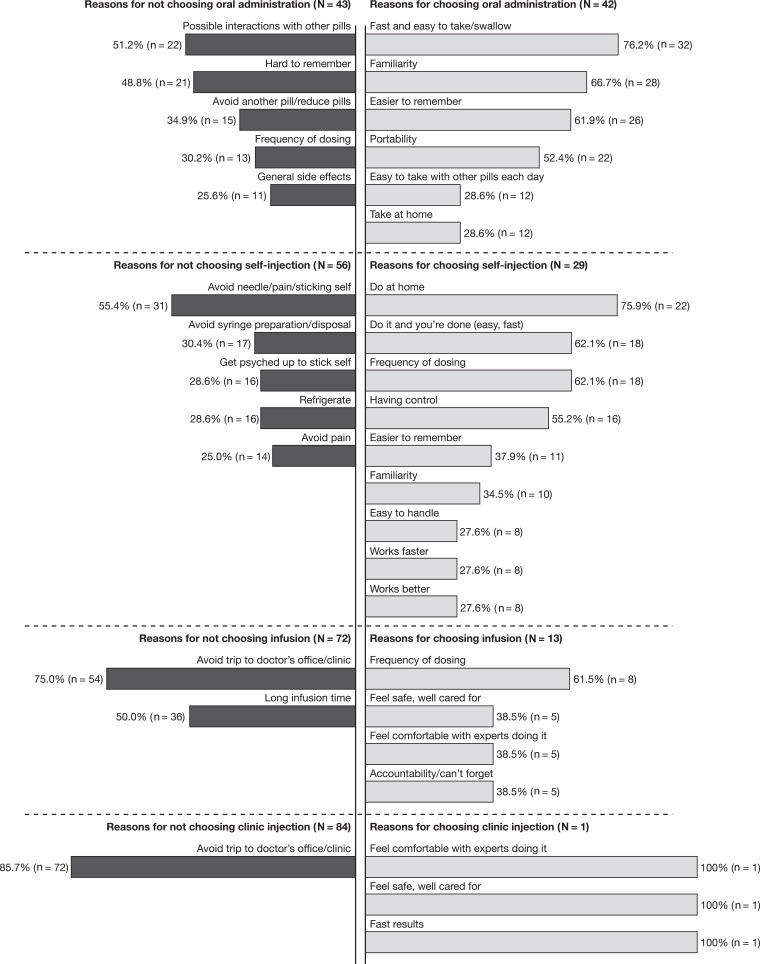
The most common (≥25%) reasons provided by patients in support of their first-choice preference, and reasons for why they did not prefer or did not give more preference points to modes of treatment administration other than their first choice, are shown in Figure 2. Examples of quotations from patients regarding the reasons for their preferences are provided in Tables S4 (oral administration), S5 (self-injection), S6 (infusion), and S7 (clinic injection).
Figure 2.
Most commona reasonsb for choosing and not choosing the most preferred mode of administration. aReported by ≥25% of patients. bPatients were asked about how they had assigned their 100 points to the modes of treatment administration: “Why is your first-choice mode your first choice? Why is that important to you? What else makes it your first choice? Why is your second/third/fourth choice so far/close in preference to your first/second/third choice? What do you like about your second/third/fourth-choice mode? What do you dislike about your second/third/fourth-choice mode? What else, if anything, is related to your first-choice mode being your most preferred way to take your PsA treatment? How, if at all, do you think that your past experiences with treatments for PsA or treatments for any other conditions affect your preference for your first-choice mode? Like what?”.
Abbreviation: PsA, psoriatic arthritis.
The most common reasons provided by patients who selected oral administration as their preferred mode of treatment administration, included speed and ease of administration (76.2%; n = 32), familiarity (66.7%; n = 28), ease of remembering (61.9%; n = 26), and portability (52.4%; n = 22) (Figure 2). Patients who chose self-injection as their preferred mode cited at-home administration (75.9%; n = 22), speed and ease of administration (62.1%; n = 18), less-frequent dosing (62.1%; n = 18), and having a feeling of control (55.2%; n = 16). Those who chose infusion did so due to less-frequent dosing intervals (61.5%; n = 8), feelings of safety and being well cared for, comfort with having experts administer treatment, and accountability (38.5%; n = 5 for each). The one patient who chose clinic injection preferred this mode also due to the comfort of having experts administer treatment and feelings of safety and being well cared for, as well as the rapid onset of action of the treatment.
Patients who did not prefer oral administration were concerned about interactions with other medications (51.2%; n = 22), found it difficult to remember taking a pill (48.8%; n = 21), and were reluctant to take another pill (34.9%; n = 15) (Figure 2). Patients who did not prefer the self-injection mode cited a desire to avoid needles (55.4%; n = 31), to avoid syringe preparation and disposal (30.4%; n = 17), or to avoid having to mentally prepare or “psych” themselves up for an injection (28.6%; n = 16). Those who did not prefer the infusion mode found it inconvenient to go to the doctor’s office or clinic (75.0%; n = 54) and found the infusion times too long (50.0%; n = 36). Of the 85 patients included in this analysis, 84 did not prefer the clinic injection mode, mainly due to the inconvenience of clinic visits (85.7%; n = 72) (Figure 2).
Patients who provided fewer than 10 preference points to either oral administration (n = 11) or self-injection (n = 15) modes of treatment administration were considered mode-avoidant. Patients who avoided oral administration were often concerned about possible drug interactions (63.6%; n = 7) or just did not want to take another pill (54.5%; n = 6) (Table S8). Among the reasons cited for the avoidance of self-injection, nearly all were to do with the dislike of needles (66.7%; n = 10) or other steps involved in the process (Table S9).
Association Between Treatment Mode Preferences and Patient and Disease Characteristics
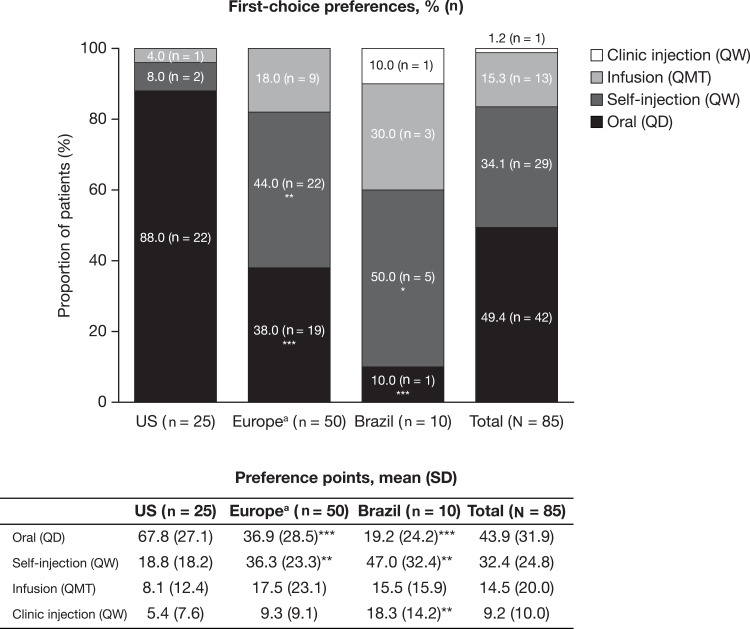
More patients in the US than in Europe preferred oral administration as their first-choice mode of treatment administration (88.0% [n = 22] and 38.0% [n = 19], respectively; P < 0.001). First-choice preferences for self-injection and, to a lesser extent, infusion (which was not statistically significant), were higher in Europe than in the US (P < 0.01 for self-injection) (Figure 3).
Figure 3.
First-choice mode of treatment administration preference and preference points, by region. *P < 0.05; **P < 0.01; ***P < 0.001 vs the US. aIncludes France, Germany, Italy, Spain and the UK.
Abbreviations: QD, once per day; QMT, once per month; QW, once per week; SD, standard deviation.
Discussion
This international qualitative study examined 85 patients’ individual beliefs and preferences related to PsA modes of treatment administration. Overall, oral administration was the most preferred mode of treatment administration, with approximately half of the patients (49.4%) ranking this as their first choice; self-injection, infusion, and clinic injection were named first-choice by 34.1%, 15.3%, and 1.2% of patients, respectively.
These results were generally consistent with a choice-based conjoint survey study among US patients with PsA. Among the attributes evaluated, this analysis found the route of administration to be the first- and second-most important treatment attribute to patients with Medicare and commercial insurance coverage, respectively; among the routes assessed, oral administration was preferred.14 In prior studies of the mode of treatment administration preferences among patients with rheumatoid arthritis (RA), oral administration was also preferred to self-injection, infusion, and clinic injection.18,19
Patients’ most frequently reported reasons for selecting oral administration as the first-choice mode of treatment administration were speed and ease of administration, and familiarity. Reasons for not selecting the oral mode of treatment administration included concerns about possible drug–drug interactions and difficulty with remembering. The reasons provided by the patients for their preferences in this study are similar to those reported in a study of the mode of TNFi administration preferences among TNFi-naïve Italian patients with RA,20 and to medication attributes that were considered most important in a survey of patients with RA.18
More than half of patients (56.5%) indicated a strong first-choice mode of treatment administration. Among these patients, most chose oral administration (66.7%), followed by self-injection (27.1%) and infusion (6.3%); no patients had a strong preference for clinic injection. Interestingly, 43.5% of patients did not state a strong first-choice preference at all, suggesting that these patients may be receptive to, and benefit from, discussions with their healthcare professionals and/or patient support groups about PsA mode of treatment administration options.
Subgroup analyses linked differences in preferences to certain patient characteristics (Table 3). Specifically, more patients in the US than in Europe chose oral administration as their first choice, whereas more patients in Europe preferred self-injection and, to a lesser extent, infusion. This may be due to several differences in the sample demographics and disease characteristics observed between the US and Europe; for instance, more patients in the US had a college degree or higher, were non-smokers, had private healthcare coverage, and reported no health conditions in addition to PsA. As seen in the subgroup analyses, patients with a college degree or higher were also more likely to prefer oral administration, and less likely to prefer infusion, than those without a college degree. Similarly, non-smokers were more likely to prefer oral administration, and less likely to prefer infusion, than current smokers (Table 3).
Table 3.
Statistically Significant Subgroup Comparisonsa
| Subgroup Comparisons (Dichotomy, n) | Higher Preference (Mean or Proportion) for … | ||
|---|---|---|---|
| Oral Mode of Administration Among … | Self-injection Mode of Administration Among … | Infusion Mode of Administration Among … | |
| Education level (<college degree, college degree or higher) | College degree or higher** | — | <College degree** |
| Current smoker (yes/no) | No* | — | Yes*** |
| Current drinker (yes/no) | Yes* | — | — |
| Daily medicines (0–2/≥3) | ≥3* | — | — |
| Current injections (yes/no) | No* | Yes** | — |
| Current bDMARDs (yes/no) | — | Yes* | — |
| Previous injections (yes/no) | — | Yes* | — |
| Previous bDMARDs (yes/no) | — | Yes* | — |
| Healthcare coverage (private/public) | Private*** | Public* | — |
Notes: *P < 0.05; **P < 0.01; ***P < 0.001 for mean and/or proportion comparison. aThe subgroup (eg “college degree or higher”, “yes”, “private”) with the statistically significantly higher preference for each mode of treatment administration (within each subgroup comparison) is shown; “–” indicates that there was no significant difference between the preferences of the subgroups (eg there is a higher preference for oral administration among patients with a college degree or higher compared with those with less than a college degree).
Abbreviations: bDMARDs, biologic disease-modifying antirheumatic drugs.
Other differences were observed that are consistent with the impact of familiarity with a mode of treatment and the desire to stay on the same route of administration: bDMARD-experienced patients and those currently receiving bDMARDs were more likely to prefer self-injection than bDMARD-naïve patients and those not currently receiving bDMARDs, and patients who were administering self-injections were less likely to prefer oral administration and more likely to prefer self-injections than those not currently administering self-injections (Table 3). No other statistically significant associations were observed between preferred and current modes of treatment. The current and previous rates of injection experience were comparable between the US and Europe. However, while the rates of current and previous bDMARD experience were higher among patients in the US than in Europe, more patients in Europe than the US preferred self-injections. These results suggest a potential true preference difference between patients in the US and Europe that may partially be explained by differences in access to or cost of medications, or other reasons that are not directly related to the mode of treatment administration.
Limitations of this study must be noted. The qualitative design, the purposeful sampling method that may facilitate selection bias, and the relatively small sample size, may limit the ability to generate meaningful inferential statistics. The generalizability of the results may also be limited as the study was only conducted across seven countries, in three regions, and there were high proportions of female patients in the US and white patients across all regions. In addition, the cross-sectional sample did not allow the study of change over time, and future research could explore such changes using a longitudinal study design. Furthermore, any differences between individual countries or between additional subgroups beyond those reported could not be assessed due to small sample sizes.
Finally, not all available modes of treatment were evaluated in the analyses, and the four modes of treatment administration analyzed here are not reflective of all currently approved PsA treatment frequencies. Given the importance of dosing frequency, the modes of treatment administration explored in this study were specifically assigned to a certain dosing frequency (selected during the design of the study), in order to obtain clear preferences and rationales from patients (as opposed to a conjoint study, for instance, which can explore the trade-offs among dosing frequencies and mode of treatment administration). Patients were also asked to assume equal efficacy and safety profiles across the treatment modes. It is possible that the assigned dosing frequency may have impacted the patients’ decisions in choosing a mode of treatment administration; dosing frequency and mode of treatment administration were not independent of each other.
Conclusions
In conclusion, this qualitative, descriptive study presented concrete explanations for patient preferences regarding specific modes of treatment administration in PsA. These findings were identified in a sample of patients selected to represent the PsA population and could be used to generate specific hypotheses for future studies. Understanding the reasons for these preferences may support the shared decision-making process when choosing appropriate treatments, and may increase patient satisfaction with, and adherence to, treatments.
Acknowledgments
The authors would like to thank the patients who have participated in this study. The authors would also like to thank Dr Suzanne Grieb, PhD, MSPH, for her input on the design of the study and interpretation of the data, and Rebecca Rushton and Jennifer Dine for their support in coding the interview transcripts. Medical writing support, under the guidance of the authors, was provided by Kate Lothman, BA, of RTI Health Solutions, Research Triangle Park, NC, USA, and Christina Viegelmann, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163[6]:461–464).
Funding Statement
This study was supported by Pfizer Inc and was performed under a research contract between RTI Health Solutions and Pfizer Inc.
Data Sharing Statement
Data are available from Pfizer Inc on reasonable request.
Author Contributions
DA, MEH, JFM, PMY, TMB, CE, M-AH, and LF contributed to the conception or design of the study. CE participated in the acquisition of the data. DA, RL, PMY, JCC, TMB, M-AH, and LF contributed to the analysis of the data. TMB was involved in coding the interview transcripts. All authors contributed to the interpretation of the data, drafted the article or revised it critically for important intellectual content, gave final approval of the version of the article to be published, and agree to be accountable for all aspects of the work.
Disclosure
DA has received grant/research support from AbbVie, Bristol-Myers Squibb, and MSD; is a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Medac, Merck, MSD, Pfizer Inc, Roche, and UCB; and is a member of speakers’ bureaus for AbbVie, Bristol-Myers Squibb, Eli Lilly, Janssen, Medac, Merck, MSD, Pfizer Inc, Roche, and UCB. MEH is a consultant for and reports personal fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB. JFM is a consultant and/or investigator for and reports personal fees from AbbVie, Aclaris, Almirall, Arena, Avotres, Biogen, Celgene, Dermavant, Eli Lilly, GSK, Incyte, Janssen, Leo Pharma, Merck, Novartis, Pfizer Inc, EMD Serono, Samumed, Sanofi Regeneron, Sun Pharma, and UCB. RR is a consultant for AbbVie, Janssen, Novartis, and Pfizer Inc; and is a member of speakers’ bureaus for AbbVie, Janssen, Novartis, and Pfizer Inc. HB is a consultant for Pfizer Inc. RL was an employee and shareholder of Pfizer Inc at the time of analysis. RL reports personal fees from Pfizer Pharma GmbH, during the conduct of the study; personal fees from AbbVie Inc and Pfizer Pharma GmbH, outside the submitted work. TMB and CE are employees of RTI Health Solutions (non-profit independent research institute), which receives funding from pharmaceutical companies for research consulting services. TMB and CE receive no compensation from the pharmaceutical companies, and their RTI salary is not connected to the projects they work on or the clients for whom research is conducted. PMY, JCC, M-AH, and LF are employees of, and hold stock/stock options in, Pfizer Inc. The authors report no other conflicts of interest in this work.
References
- 1.Helliwell P, Coates L, Chandran V, et al. Qualifying unmet needs and improving standards of care in psoriatic arthritis. Arthritis Care Res. 2014;66(12):1759–1766. doi: 10.1002/acr.22404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med. 2017;17(1):65–70. doi: 10.7861/clinmedicine.17-1-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. Pharm Ther. 2010;35(12):680–689. [PMC free article] [PubMed] [Google Scholar]
- 4.Kaine J, Song X, Kim G, Hur P, Palmer JB. Higher incidence rates of comorbidities in patients with psoriatic arthritis compared with the general population using U.S. administrative claims data. J Manage Care Spec Pharm. 2019;25(1):122–132. doi: 10.18553/jmcp.2018.17421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573 [DOI] [PubMed] [Google Scholar]
- 6.Gossec L, Smolen JS, Ramiro S, et al. European league against rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337 [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken). 2019;71(1):2–29. doi: 10.1002/acr.23789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–970. doi: 10.1056/NEJMra1505557 [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Braun J, Dougados M, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann Rheum Dis. 2014;73(1):6–16. doi: 10.1136/annrheumdis-2013-203419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189–199. [PMC free article] [PubMed] [Google Scholar]
- 11.Betteridge N, Boehncke WH, Bundy C, Gossec L, Gratacós J, Augustin M. Promoting patient-centred care in psoriatic arthritis: a multidisciplinary European perspective on improving the patient experience. J Eur Acad Dermatol Venereol. 2016;30(4):576–585. doi: 10.1111/jdv.13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chastek B, Fox KM, Watson C, Gandra SR. Etanercept and adalimumab treatment patterns in psoriatic arthritis patients enrolled in a commercial health plan. Adv Ther. 2012;29(8):691–697. doi: 10.1007/s12325-012-0039-3 [DOI] [PubMed] [Google Scholar]
- 13.Saad AA, Ashcroft DM, Watson KD, Hyrich KL, Noyce PR, Symmons DP. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British society of rheumatology biologics register. Arthritis Res Ther. 2009;11(2):R52. doi: 10.1186/ar2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11(8):408–417. [PMC free article] [PubMed] [Google Scholar]
- 15.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi: 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 16.SAS Proprietary Software, Version 9.4 [Computer Program]. Cary NC:SAS Institute Inc. [Google Scholar]
- 17.Rosner B. Fundamentals of Biostatistics. 8th ed. Boston, MA: Cengage Learning; 2015. [Google Scholar]
- 18.Louder AM, Singh A, Saverno K, et al. Patient preferences regarding rheumatoid arthritis therapies: a conjoint analysis. Am Health Drug Benefits. 2016;9(2):84–93. [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor PC, Betteridge N, Brown TM, et al. Treatment mode preferences in rheumatoid arthritis: moving toward shared decision-making. Patient Prefer Adherence. 2020;14:119–131. doi: 10.2147/PPA.S220714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpato S, Antivalle M, Favalli EG, et al. Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology (Oxford). 2010;49(2):289–294. doi: 10.1093/rheumatology/kep354 [DOI] [PubMed] [Google Scholar]