Abstract
Purpose
To survey healthcare workers (HCW) on availability and use of personal protective equipment (PPE) caring for COVID-19 patients in the intensive care unit (ICU).
Materials and method
A web-based survey distributed worldwide in April 2020.
Results
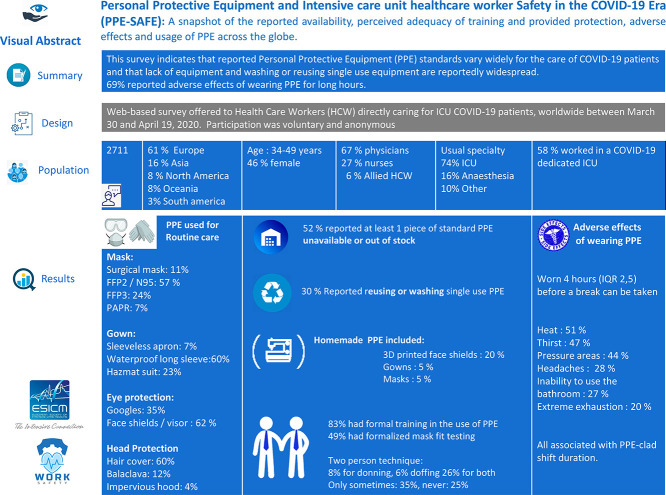
We received 2711 responses from 1797 (67%) physicians, 744 (27%) nurses, and 170 (6%) Allied HCW. For routine care, most (1557, 58%) reportedly used FFP2/N95 masks, waterproof long sleeve gowns (1623; 67%), and face shields/visors (1574; 62%). Powered Air-Purifying Respirators were used routinely and for intubation only by 184 (7%) and 254 (13%) respondents, respectively. Surgical masks were used for routine care by 289 (15%) and 47 (2%) for intubations. At least one piece of standard PPE was unavailable for 1402 (52%), and 817 (30%) reported reusing single-use PPE. PPE was worn for a median of 4 h (IQR 2, 5). Adverse effects of PPE were associated with longer shift durations and included heat (1266, 51%), thirst (1174, 47%), pressure areas (1088, 44%), headaches (696, 28%), Inability to use the bathroom (661, 27%) and extreme exhaustion (492, 20%).
Conclusions
HCWs reported widespread shortages, frequent reuse of, and adverse effects related to PPE. Urgent action by healthcare administrators, policymakers, governments and industry is warranted.
Keywords: COVID-19, Personal protective equipment, Safety, Health care workers, Intensive care
Graphical abstract
1. Introduction
The SARS-CoV-2 virus and the disease it causes (Coronavirus Disease 2019; COVID-19) has created a global public health emergency following its first appearance in December 2019 [1]. As of early june 2020 there had been more than 6.4 million confirmed cases and 385,000 deaths reported worldwide [2].
This highly contagious virus poses a significant but largely preventable risk to healthcare workers (HCW) [3]. In some areas, HCW have comprised up to 11% of all confirmed COVID-19 cases with an increasing number of occupationally attributed deaths being reported [4,5]. Use of personal protective equipment (PPE) can markedly reduce the infection risk associated with caring for COVID-19 patients [6,7]. While there is little evidence to which PPE offers the best protection, training in donning and doffing, simulation and face to face instructions are likely beneficial [8]. As a result of adequacy of instruction, availability of fit-testing, and supply limitations [9], HCW may not be utilizing PPE as per recommended guidelines [6,10,11].
Reports of PPE scarcity and unavailability are emerging worldwide. HCWs report on social media and the general press resorting to reusing PPE or using household and self-made items in place of PPE. While limited evidence exists on the effectiveness of these practices, it has sometimes been done on the advice of their employers or health organisations [12,13]. Pictures of HCWs' faces bruised by wearing masks for extended periods have been used to illustrate the extreme work conditions when caring for such patients. While pain, heat stress and fluid loss with using Powered Air-Purifying Respirators (PAPR) were predicted by experimental data [14], there are no real-life reports of this issue when using PPE that is available to HCWs.
The objective of this study was to describe the current reported practices, availability, training, confidence in the use and adverse effects due to extended use of PPE by HCWs from around the world caring for COVID-19 patients who require ICU management.
2. Methods
A web-based survey was conducted in order to elicit HCW reports surrounding PPE related to the COVID-19 pandemic. Participation was voluntary and anonymous. This study was approved and granted a waiver of signed individual informed consent by the Royal Brisbane and Women's Hospital Human Research Ethics Committee (LNR/2020/QRBW/63041), Brisbane, Australia.
2.1. Survey instrument
The survey target population was all HCW of any discipline or training background or level who are directly involved in the management of COVID-19 patients in a critical care setting. A 2-part study-specific survey was designed (see electronic supplement). In the first part, questions surrounding basic demographic, training experience, and institutional work characteristics were elicited. No specific identifying data (i.e. name, date of birth) was requested The second part comprised of a series of questions regarding the usual practices and availability of PPE, along with perceptions of its adequacy in terms of supply and training in the workplace as well as adverse effects of wearing PPE on the HCW.
Questions were developed and the survey pre-tested for ease of administration, flow, and content by management committee members and by experienced clinician volunteers. Following iterative revisions, the final survey was developed. An English language version was prepared then translated in the French, Spanish and Italian languages.
The survey started with a binary question: if the respondent declared directly caring for COVID-19 patients in the ICU setting the survey was continued and the response categorized as valid. In the opposite case the survey was terminated, and the response categorized as invalid.
2.2. Survey administration
The final survey was prepared using the Surveymonkey® online platform (SVMK Inc., San Mateo, USA) and posted at https://www.surveymonkey.com/r/PPE-SAFE. The survey was planned to be open for 2 weeks starting March 30. Only the English language version was initially available with the others implemented as of April 7, 2020. Duration of the survey was subsequently extended and we report data collected between March 30 and April 20, 2020.
Subjects were invited to participate through several venues including email invitations using mailing lists of the European Society of Intensive Care Medicine, Australia and New Zealand Intensive Care Society, Australian College of Critical Care Nurses, and the European Society of Clinical Microbiology and Infectious Diseases. In addition, ad hoc emails and advertisements were made via personal networks and social media accounts of management committee members.
2.3. Data management and analysis
Survey results were exported to and analysed using Stata 15.1 (Stata Corp, College Station, USA). Means with standard deviations (SD) and medians with interquartile ranges (IQR) were used to describe normally and non-normally distributed continuous variables, respectively. Differences in grouped means and medians were tested using the t-test and Wilcoxon rank-sum test, respectively. Categorical data were compared using the Chi-square or Fisher Exact Tests. A p-value less than 0.05 was deemed to represent significance for all comparisons. We performed univariate logistic regression to test the effect of PPE-clad shift duration, modelled as a continuous variable, on adverse effects. We used a separate univariate model for each adverse effect, and for any adverse effect.
3. Results
3.1. Description of the respondents
Valid responses were received from 2711 of 4879 (56%) individuals who accessed the survey. Of which 1797 (67%) were physicians, 744 (27%) were nurses, and 170 (6%) were allied HCW (Table 1 and figure e-sup 1). The median age was 41 (IQR, 34–49), 1254 (46%) were female. As detailed in the electronic supplement, respondents worked in 90 different countries, mostly from Europe (1666; 61%) followed by Asia (437; 16%), and North America (224; 8%). Most (1585; 58%) respondents worked in a COVID-19 dedicated ICU, including 281 (10%) in another area re-purposed as a COVID-19 ICU. One third (924; 34%) of subjects reported working in an ICU that contained patients with and without COVID-19, and 201 (7%) worked in other areas. As shown in Table 1, several characteristics were different among those working in COVID-19 dedicated or repurposed ICUs as compared to mixed or other ICUs.
Table 1.
Comparison of demographic and workplace attributes among respondents working in COVID-19 dedicated or repurposed ICUs as compared to mixed or other critical care areas.
| Factor | Total |
Mixed ICU, COVID-19 ICU or other |
COVID-19 dedicated or re-purposed ICU |
|---|---|---|---|
| n = 2711 | n = 1126 | n = 1585 | |
| Age | 41 (34–49) | 42 (35–50) | 41 (34–48) |
| Female gender | 1254 (46%) | 481 (43%) | 773 (49%) |
| ICU experience (Years) | 10 (4–18) | 10 (5–20) | 10 (4–17) |
| PPE shift duration (hours) | 4 (2–6) | 4 (2–6) | 4 (2–6) |
| Position | |||
| Nurse | 744 (27%) | 240 (31%) | 504 (32%) |
| Physician | 1797 (67%) | 808 (72%) | 989 (62%) |
| Allied Health | 170 (6%) | 78 (7%) | 92 (6%) |
| Usual specialty | |||
| Anaesthesia | 430 (16%) | 171 (15%) | 259 (16%) |
| Intensive Care | 2019 (74%) | 833 (74%) | 1186 (75%) |
| Emergency | 72 (3%) | 40 (4%) | 32 (2%) |
| Other | 190 (7%) | 82 (7%) | 108 (7%) |
| Continent | |||
| Africa | 66 (2%) | 44 (4%) | 22 (1%) |
| Asia | 437 (16%) | 263 (23%) | 174 (11%) |
| Europe | 1666 (61%) | 470 (42%) | 1196 (75%) |
| North America | 224 (8%) | 105 (9%) | 119 (8%) |
| Oceania | 229 (8%) | 194 (17%) | 35 (2%) |
| South America | 89 (3%) | 50 (4%) | 39 (2%) |
| Hospital type | |||
| Community/urban | 741 (27%) | 268 (24%) | 472 (30%) |
| Tertiary | 1548 (57%) | 657 (58%) | 891 (56%) |
| Private | 237 (9%) | 123 (11%) | 114 (7%) |
| Remote/regional | 186 (7%) | 78 (7%) | 108 (7%) |
| Running capacity | |||
| Well above | 690 (26%) | 141 (13%) | 549 (35%) |
| Above | 586 (22%) | 169 (15%) | 417 (26%) |
| Below | 663 (25%) | 400 (36%) | 263 (17%) |
| Unsure | 57 (2%) | 29 (3%) | 28 (2%) |
| Usual | 699 (26%) | 375 (34%) | 324 (20%) |
Data in n (%) for categorical variables and medians with interquartile ranges (IQR) for continuous variables. Type of ICU denotes today's place of work, Mixed ICU includes any ICU that treats patients with or without COVID-19, as opposed to COVID-19 dedicated or repurposed ICU that only treats patients with COVID-19. PPE shift duration denotes the duration the HCW remains dressed in PPE before being able to take a break.
3.2. PPE usage
In the routine care of patients with COVID-19 most respondents reported use of FFP2/N95 masks (1557; 58%), Surgical masks were reportedly used for routine care in 289 (15%) cases but infrequently (47, 2%) for intubations. Waterproof long sleeve gowns (1623; 67%), and face shields/visor (1574; 62%). Use of PAPR was infrequent with routine care (184; 7%) or intubation (254, 13%). Their use was more frequent in Asian and North American countries compared with Oceania and Europe but was not associated with the type of ICU, it's capacity or current workload. Variations between countries were wide and shown in the electronic supplementary Tables 3.
A comparison of PPE usage between professions is shown in the electronic supplementary Table 2. Comparisons should be interpreted with caution as due to the nature of the survey it is unknown if differences between respondents may is due to their institution or profession.
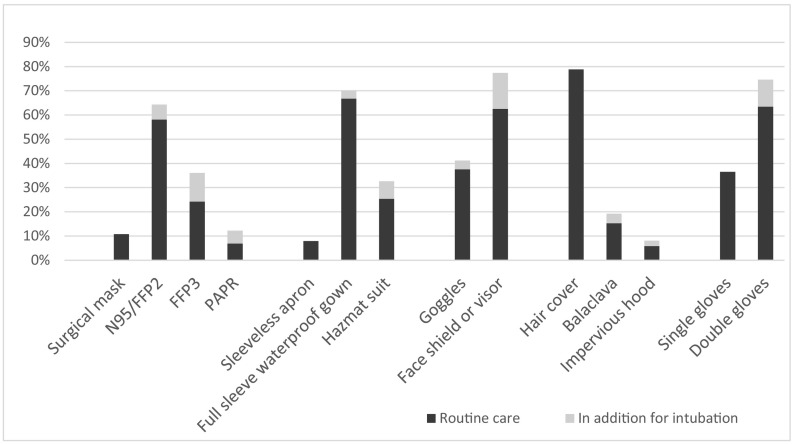
A comparison of the PPE used in routine care and for intubation among the respondents is shown in Fig. 1 . Six hundred and twenty-eight (23%) subjects reported use of different mask for intubation compared to routine care. The corresponding numbers for gown and eye protection are 284 (12%) and 495 (20%). (Table 2 ).
Fig. 1.
PPE used for routine care and intubation.
PPE used by HCWs for routine care (black bars), and if anything, what additional PPE is used for intubation of COVID-19 patients in an ICU.
Table 2.
Shortages and Reuse of single use PPE.
| Used for routine care | Reported as Missing | Washed or reused | |
|---|---|---|---|
| Mask (n = 2679/2711)* | |||
| Surgical Mask | 289, (11%) | 11, (4%) | 13, (4%) |
| N95/FFP2 maks | 1557, (57%) | 127, (8%) | 267, (17%) |
| FFP3 mask | 649, (24%) | 78, (12%) | 107, (16%) |
| PAPR | 184, (7%) | 16, (9%) | n/a |
| None reported | 32 (1%) | ||
| Gown (n = 2432/2711)* | |||
| Sleeveless apron | 193, (7%) | 3, (2%) | 5, (3%) |
| Full sleeve waterproof gown | 1623, (60%) | 115, (7%) | 183, (11%) |
| Hazmat suit | 616, (23%) | 73, (12%) | 66, (11%) |
| None reported | 279 (10%) | ||
| Eye Protection (n = 2519/2711)* | |||
| Goggles | 945, (35%) | 28, (3%) | 326, (34%) |
| Face shield or visor | 1574, (58%) | 131, (8%) | 820, (52%) |
| None reported | 192, (7%) | ||
| Head protection (n = 2075/2711)* | |||
| Hair cover | 1636, (60%) | 43, (3%) | 41, (3%) |
| Balaclava | 317, (12%) | 26, (8%) | 8, (3%) |
| Impervious hood | 122, (4%) | 5, (4%) | 11, (9%) |
| PAPR | 184 (7%) | ||
| None reported | 452, (17%) | ||
Data are expressed in n(%). Denotes number of valid responses for PPE used for routine care. Reported as missing denoted PPE that would normally be used but is not available. None reported denotes respondents that did not report using any equipment in that category of PPE. Washed or reused denotes single use PPE that is washed or reused due to stock or availability issues. PAPR shown as Mask and Head protection as includes a hood and shown as n/a for reuse as they are reusable by design.
* Respondents who reported using a piece of equipment in that category of PPE
3.3. PPE availability
More than half of respondents (1402, 52%) reported at least one piece of the standard PPE as not available, and 817 (30%) reported that at least a piece of single-use PPE was being reused or washed as a result of shortages (Table 2). The distribution of PPE that was reportedly not available or being reused is shown in Table 2. Overall few respondents indicated that no additional PPE should be provided. Among the 1184 (44%) respondents that detailed additional need, this was most commonly Hazmat suits and PAPRs. Homemade solutions to PPE shortages included 3D printed face shields (529, 20%), homemade gowns (163, 5%), and homemade masks (145, 5%). There were wide variations between countries, with some reporting up to 19% of some items missing and others up to 39% being reused (tables electronic supplement 2).
3.4. Knowledge and training
Most of the respondents (2245, 83%) reported that they had formal training in the use of PPE. That included training at commencement in the institution (336, 13%) and within the last 2 months due to the COVID-19 pandemic (1509, 60%). Most reported they would benefit from additional training, this included simulation (1224, 49%) or demonstration by infection control specialists (478, 19%), and didactic teaching (220, 9%). Less than half reported having formalized mask fit testing at any time (1243, 49%). A two-person technique was reportedly used for donning (193, 8%), doffing (159, 6%), or both (643, 26%), sometimes (881, 35%) but never in almost one-quarter (639, 25%) of respondents. There was a strong association between reporting never use of a 2 persons technique and never receiving PPE training, fit testing, and low confidence in using recommended PPE (p < .001 for all comparisons).
3.5. Confidence
Almost half (1211, 45%) reported being very or confident with their technique in using the available PPE and 138 (5%) were not confident at all. Confidence in the adequacy of protection was reported by 1187 (44%), while 376 (14%) were not confident at all. This was similar for doctors, nurses and allied health (p = .93). There was a strong association between confidence in protection and the absence of PPE shortage and confidence in technique (p < .001 for both comparisons).
3.6. Adverse effects
The median duration of a shift while wearing PPE without the ability to take a break (PPE-Shift) as 4 h (IQR 2, 5 h). This was similar for nurses (median 4, IQR 2, 6 h) and doctors (median 4, IQR 3,5 h).
Adverse effects were reported by 80%, including heat (1266, 51%), thirst (1174, 47%), pressure areas (1088, 44%), headaches (696, 28%), inability to use the bathroom (661, 27%) and extreme exhaustion (4924, 20%) (Table 3 ). They were all associated with longer duration of shifts wearing PPE (Table 4 ).
Table 3.
Adverse effects according to PPE-Shift duration.
| PPE-Shift duration: | <3 h n = 727 |
3–5.9 h n = 1097 |
6–8.9 h n = 524 |
>9 h n = 128 |
|---|---|---|---|---|
| Any adverse effects | 445, (69%) | 815, (86%) | 369, (87%) | 86, (83%) |
| Extreme exhaustion | 77, (12%) | 187, (20%) | 116, (27%) | 32, (31%) |
| Inability to use the bathroom | 61, (9%) | 261, (28%) | 176, (41%) | 47, (45%) |
| Headaches | 118, (18%) | 297, (31%) | 137, (32%) | 36, (35%) |
| Thirst | 216, (33%) | 525, (55%) | 213, (50%) | 63, (61%) |
| Heat | 290, (45%) | 524, (55%) | 230, (54%) | 56, (54%) |
| Pressure areas | 237, (37%) | 495, (52%) | 193, (45%) | 42, (40%) |
| Other | 17, (3%) | 14, (1%) | 11, (3%) | 2, (2%) |
PPE-Shift duration denotes the amount of time in hours that the HCW is wearing PPE without the ability to take a break. Data expressed in n(%).
Table 4.
Effect of PPE-clad shift duration on adverse effects experienced by HCW.
| Adverse effect | OR (per 1-h shift duration) | Lower 95% CI | Upper 95% CI | p |
|---|---|---|---|---|
| Any | 1.24 | 1.18 | 1.30 | <0.001 |
| Extreme exhaustion | 1.15 | 1.11 | 1.20 | <0.001 |
| Inability to use bathroom | 1.27 | 1.22 | 1.31 | <0.001 |
| Headaches | 1.13 | 1.09 | 1.17 | <0.001 |
| Thirst | 1.16 | 1.12 | 1.20 | <0.001 |
| Heat | 1.07 | 1.04 | 1.10 | <0.001 |
| Pressure areas | 1.06 | 1.02 | 1.09 | 0.001 |
Univariate logistic regression of duration of PPE-clad shift on adverse effects experienced by HCWs. The odds ratio represents the change in odds of having the adverse effect with every 1-h increase in PPE-clad shift duration.
4. Discussion
This survey provides a snapshot of the reported availability, perceived adequacy of training and provided protection, adverse effects and usage of PPE among HCW managing COVID-19 patients in critical care environments from across the globe. It is important to note that these responses are likely influenced by how burdened HCW are, the safety culture, and the baseline resources in their institutions. While these data do not prove adequacy or inadequacy of PPE per se, they do lend important insights into what HCW are experiencing in this novel pandemic situation.
It is important to recognize that information on human-to-human COVID-19 transmission is still emerging. While respiratory droplets are considered as the main route of transmission, airborne transmission resulting from aerosol-generating procedures likely is a mode [15]. Surface contamination with transmission using contact means is another route of infection transfer [16].
Recommendations for PPE vary significantly both between and within countries. As an example, airborne precautions are recommended only for high-risk procedures in some countries whereas this is routinely in others [6,10,11,17]. Furthermore, shortages of PPE equipment has led to practices to reduce, reuse, or substitute lesser or non-approved products in an attempt to address inadequate supply of PPE [18].
Variability in knowledge, training and technique, such as the formal fit testing of respirators or the use of a 2 persons technique for donning and doffing PPE are correlated with confidence and likely impact safety of HCWs managing ICU patients infected with COVID-19. These factors contribute to a sense of uncertainty and lack of confidence in a safe workplace among HCW [9,19].
Access to appropriate PPE was the first of 8 sources of anxiety in a group of HCWs interviewing during the first week of the pandemic [20]. This is likely further exacerbated by frequent changes in guidelines and public health messages. Those may be secondary to epidemiological changes, the rapidly accumulating knowledge but also by the scarcity of the resource, further increasing anxiety and distrust from HCWs.
The shortages and concerns surrounding provision of adequate PPE represents a major issue from a supply chain perspective. This further raises serious concerns about equity and justice related to provision for those most in need. At local levels, reports of PPE being stolen from healthcare institutions, misappropriated, or hoarded have occurred such that this equipment may not be available to those at highest risk [18]. At subnational and national levels this has also become a concern as bidding wars and re-direction of orders has occurred. Recent examples of countries threatening to block export shipments of PPE to other countries has further exacerbated concerns by HCW around access to appropriate PPE. While it is likely that innovative approaches and ramp-up of domestic manufacturing processes may help to meet demand, it is a serious risk for low income countries who may ultimately suffer the greatest adverse effects of lack of PPE.
Confirming social media and widely distributed photos of HCWs bruised faces, most respondents have reported adverse effects from PPE. This question the safety of currently available PPE when it is worn for an extended duration. Most of the available PPE was designed and manufactured for single-use and brief duration of use. These findings call for urgent design and manufacture of PPE that can be safely worn and remains effective for extended durations. It also reinforces the need for recruitment of an increased health care workforce. This would allow for surge capacity whilst minimizing harm to frontline staff.
There are some limitations of this study that must be noted. First, it is a voluntary survey and responses reflect opinions and perceptions alone. They may not necessarily reflect actual practices as these are not confirmed through audit. Second, we did not use a systematic sampling strategy but rather made the survey broadly available and accordingly there is no denominator to establish a response rate. Therefore, our results may reflect a small portion and potentially biased reflection of the true opinions of all HCW. By using scientific society mailing lists we may have skewed the sampling towards the geographical location of their members. However, we elected to pursue this study approach in order to obtain a contemporary view. Given the time frame and rapid changes related to this pandemic, we therefore elected to pursue this study without subsequent formalized sampling strategy. This allowed the identification of trends in reported use of PPE rather than real time data. Third, the study has an over-representation by physicians which may underestimate the burden of adverse effects caused by PPE. Fourth, there is an underrepresentation of low- and middle-income countries, which may have skewed the results. Finally, we only offered the survey in English, French, Spanish and Italian. This may have been a barrier for some HCW to participate and may have resulted in a selection of respondents that may be different had we included options for other languages.
5. Conclusion
In summary this survey study provides a snapshot of reported PPE practices availability, and confidence in adequacy to provide protection among HCWs at the frontlines of the COVID-19 pandemic. Respondents report widespread shortages and reuse of single-use PPE items. Half of the respondents had never had fit-testing of masks. Adverse effects from PPE usage frequently reported and mostly associated with PPE-clad shift duration. Urgent action by healthcare administrators, policymakers, governments and industry is warranted to address these issues.
Funding acknowledgements
This project has been realised by the authors without specific funding. Survey web platform and manpower has been provided in kind by ESICM.
Andrew Conway Morris is supported by a Clinical Research Career Development Fellowship from the Wellcome Trust (WT 2055214/Z/16/Z). Niccolò Buetti is currently receiving a Post.doc Mobility grant from the Swiss National Science Foundation (grant number: P400PM_183865) and a grant from the Bangerter-Rhyner Foundation.
Other acknowledgements
We thank Luca Buetti and Luisa Nobile for their assistance with translating the survey in the Italian language and Alba Llorens for her assistance with the Spanish language. We thank the respondents for completing the survey while working in ICUs during the pandemic.
Declaration of Competing Interest
Dr. Tabah has nothing to disclose, Dr. Ramanan has nothing to disclose, Prof. Laupland has nothing to disclose, Dr. Buetti has nothing to disclose, Dr. Cortegiani has nothing to disclose, Mr. Mellinghoff has nothing to disclose, Dr. Conway Morris reports grants from Wellcome Trust, during the conduct of the study; Dr. Camporota has nothing to disclose, Dr. Zappella has nothing to disclose, Dr. Vidal has nothing to disclose, Dr. Elhadi has nothing to disclose, Dr. Povoa reports personal fess from Orion, personal fees from Pfizer and personal fees from Technofage, Dr. Amrein reports grants, personal fees and other from Fresenius Kabi, personal fees from Vifor Pharma, personal fees from Shire now part of Takeda, outside the submitted work, Dr. Derde reports grants from European Union, grants from ZonMw, outside the submitted work, Guy Francoishas nothing to disclose, Dr. Bassetti reports grants and personal fees from Pfizer, grants and personal fees from MSD, grants and personal fees from Menarini, grants and personal fees from Angelini, personal fees from Astellas, personal fees from Nabriva, grants and personal fees from Paratek, personal fees from Gilead, personal fees from Basilea, personal fees from Cidara, personal fees from Molteni, outside the submitted work; Dr. Ssi Yan Kai has nothing to disclose, Dr. De Waelereports grants from Research Foundation Flanders, during the conduct of the study; other from Bayer, other from Pfizer, other from MSD, other from Grifols, other from Accelerate, outside the submitted work;.
Acknowledgements
This study was endorsed by, and communications were sent to the members of:
-
•
European Society of Intensive Care Medicine (ESICM)
-
•
European Society of Clinical Microbiology and Infectious Diseases Study Group for Infections in Critically Ill Patients – ESCMID ESGCIP
-
•
Società Italiana di Anestesia Analgesia Rianimazione e Terapia Intensiva (SIAARTI)
-
•
Associazione Nazionale Infermieri di Area Critica (ANIARTI).
-
•
Società Italiana di Terapaia Antinfettiva (SITA)
Without mention of endorsement communications were sent to the members of:
-
•
Société Française d'Anesthésie et de Réanimation (SFAR)
-
•
The Australian and New Zealand Intensive Care Society (ANZICS)
-
•
College Of Intensive Care Medicine of Australia and New Zealand (CICM ANZ)
-
•
Sociedad Argentina de Terapia Intensiva (SATI)
-
•
The Eurobact II study group.
-
•
The DIANA study group
-
•
Society for Healthcare Epidemiology of America (SHEA)
-
•
Sociedade Portuguesa de Cuidados Intensivos (SPCI)
-
•
Departments and networks of the PPE-SAFE contributors
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2020.06.005.
Appendix A. Supplementary data
Supplementary tables and figures
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. New England J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.University JH Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map
- 3.Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q., et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323(19):1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH . 2020. Coronavirus disease 2019 (COVID-19): situation report, 82. [Google Scholar]
- 5.Guardian T. Doctors, nurses, porters, volunteers: the UK health workers who have died from Covid-19 2020. https://www.theguardian.com/world/2020/apr/16/doctors-nurses-porters-volunteers-the-uk-health-workers-who-have-died-from-covid-19 Available from.
- 6.Alhazzani W, Moller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Dsease 2019 (COVID-19). Intensive Care Med 2020. [DOI] [PMC free article] [PubMed]
- 7.Cook TM. Personal protective equipment during the COVID-19 pandemic - a narrative review. Anaesthesia. 2020. [DOI] [PMC free article] [PubMed]
- 8.Verbeek JH, Rajamaki B, Ijaz S, Sauni R, Toomey E, Blackwood B, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev 2020(4). [DOI] [PMC free article] [PubMed]
- 9.Kamerow D. Covid-19: the crisis of personal protective equipment in the US. BMJ. 2020;369:m1367. doi: 10.1136/bmj.m1367. [DOI] [PubMed] [Google Scholar]
- 10.Organization WH . World Health Organization; 2020. Infection prevention and control during health care when COVID-19 is suspected: Interim guidance, 19 March 2020. [Google Scholar]
- 11.England P.H. 2020. COVID-19 personal protective equipment (PPE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England P.H. Considerations for acute personal protective equipment (PPE) shortages 2020. 2020. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/managing-shortages-in-personal-protective-equipment-ppe Available from.
- 13.Center for Disease Control and Prevention C Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html Available from.
- 14.Loibner M., Hagauer S., Schwantzer G., Berghold A., Zatloukal K. Limiting factors for wearing personal protective equipment (PPE) in a health care environment evaluated in a randomised study. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0210775. [e0210775-e] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhen-Dong G., Zhong-Yi W., Shou-Feng Z., Xiao L., Lin L., Chao L., et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging Infect. Dis. J. 2020;26:7. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. New England J. Med. 2020. [DOI] [PMC free article] [PubMed]
- 17.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568–576. doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages — the need for ventilators and personal protective equipment during the Covid-19 pandemic. New England J. Med. 2020;328:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 19.Godlee F. Protect our healthcare workers. BMJ. 2020;369:m1324. [Google Scholar]
- 20.Shanafelt T., Ripp J., Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133–2134. doi: 10.1001/jama.2020.5893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables and figures