Abstract
Vaccine solutions rarely reach the public until after an outbreak abates; an Ebola vaccine was approved 5 years after peak outbreak and SARS, MERS, and Zika vaccines are still in clinical development. Despite massive leaps forward in rapid science, other regulatory bottlenecks are hamstringing the global effort for pandemic vaccines.
Keywords: COVID-19, vaccine development, regulation, innovation speed
When an international team of researchers led by Jeremy Farrar from the Wellcome Trust published an article last November in Nature calling for theories and practices underpinning infectious disease epidemiology to keep pace with the translational nature of epidemics in the 21st century [1], few thought the next pandemic would come so soon. Yet, when the COVID-19 outbreak was confirmed by Wuhan Municipal Health Commission on 31 December 2019, the scientific community acted swiftly, collaborating intensely across disciplines and sharing new knowledge in a truly open innovation approach to advancing scientific research [2., 3., 4., 5.].
The combined efforts of the scientific community, mobilization of public health resources, increased public and private investment, and a ‘completely new culture of doing research’ [6] accelerated the first novel vaccine candidate through discovery, lead-candidate optimization, preclinical development, and into clinical trials within 2 months of the outbreak. Four additional candidates entered the clinic within the next month and 18 more are expected to begin clinical trials by late 2020 [7].
Rapid Development
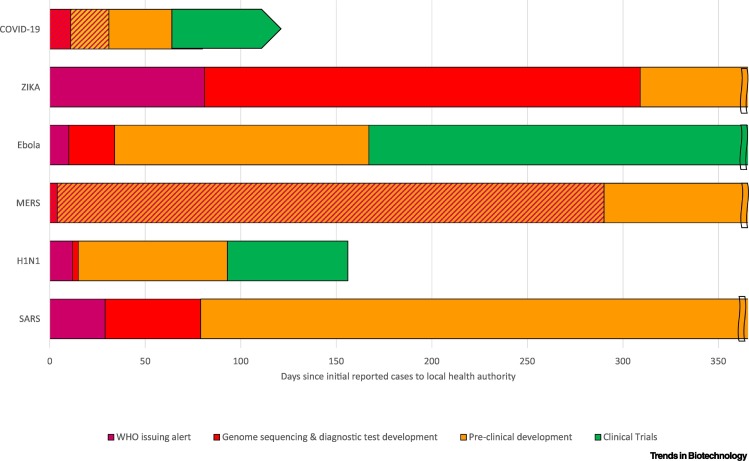
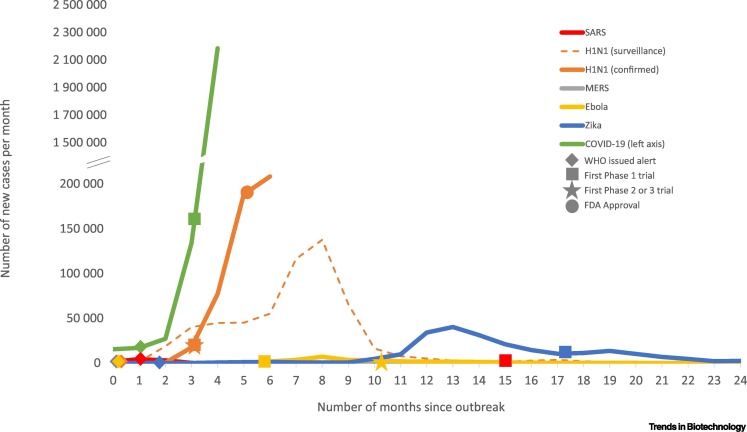
The rapid spread of COVID-19 helps us understand the volume, diversity, and quality of resources mobilized to realize these unprecedented vaccine development speeds, especially when we compare them with the response to other global outbreaks over the last two decades (Figure 1). The first novel vaccine candidate for COVID-19 began clinical trials 1 month faster than the first candidate for the H1N1 swine flu epidemic in 2009 (93 days) and 3 months faster than the first Ebola vaccine candidate in 2014 (167 days) (Table S1 in the supplemental information online). These vaccine candidates also started clinical trials ahead of their respective outbreak peaks (Figure 2).
Figure 1.
Timing and Duration of Key Development Milestones towards a Vaccine in First 365 Days of Recent Major Global Outbreaks.
Double lines at 365 days represent ongoing activities. Slanted lines indicate overlap in development milestones; the genomes of Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2; COVID-19) were sequenced and diagnostic tests developed before the World Health Organization (WHO) issued their alert. See Table S1 in the supplemental information online for dates of featured events.
Double lines at 365 days represent ongoing activities. Slanted lines indicate overlap in development milestones; the genomes of Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2; COVID-19) were sequenced and diagnostic tests developed before the World Health Organization (WHO) issued their alert. See Table S1 in the supplemental information online for dates of featured events.
Figure 2.
Number of Reported Cases per Month and Key Events in the Development of a Novel Vaccine for Global Epidemics and Pandemics over the Last Two Decades.
See Table S1 in the supplemental information online for key events. Data sources: severe acute respiratory syndrome (SARS): World Health Organization (WHO)i; H1N1: WHOii, FluNet/WHO Global Influenza Surveillance and Response System (GISRS)iii (H1N1 data comes from surveillance of global circulation of influenza viruses; number of specimens positive for H1N1 reported to GISRS); Middle East respiratory syndrome (MERS): European Centre for Disease Prevention and Control (ECDC)iv; Ebola: Centers for Disease Control and Prevention (CDC)v; Zika: Pan American Health Organization (PAHO)/WHOvi; COVID19: Johns Hopkins Universityvii (COVID-19 cases for month 4 are from 1 April 2020 to 27 April 2020).
See Table S1 in the supplemental information online for key events. Data sources: severe acute respiratory syndrome (SARS): World Health Organization (WHO)i; H1N1: WHOii, FluNet/WHO Global Influenza Surveillance and Response System (GISRS)iii (H1N1 data comes from surveillance of global circulation of influenza viruses; number of specimens positive for H1N1 reported to GISRS); Middle East respiratory syndrome (MERS): European Centre for Disease Prevention and Control (ECDC)iv; Ebola: Centers for Disease Control and Prevention (CDC)v; Zika: Pan American Health Organization (PAHO)/WHOvi; COVID19: Johns Hopkins Universityvii (COVID-19 cases for month 4 are from 1 April 2020 to 27 April 2020).
Yet, only the H1N1 vaccine was approved in time to deliver a preventative solution to the then ongoing public health crisis. Novel vaccines against the viruses responsible for the 2003 severe acute respiratory syndrome (SARS), 2012 Middle East respiratory syndrome (MERS), and 2015 Zika virus outbreaks are still in clinical testing, long after their respective outbreaks peaked and the World Health Organization (WHO) declared the outbreaks over. While there is now an Ebola vaccine, it took 5 years to complete 12 supporting and one pivotal clinical trial, then be reviewed and approved (in 2019) by the European Medicines Agency (EMA) and the FDA Centre for Biological Evaluation and Research (CBER).
The reality is that a 5 year development program for a novel vaccine is fast. The only other novel vaccines approved by CBER in 2019, one for dengue fever (Dengvaxia, Sanofi) and one for smallpox and monkeypox (Jynneos, Bavarian Nordic), were both in clinical trials for approximately 15 years. Five-year clinical development is fast, even for drugs, and generally limited to ground-breaking precision medicines for indications with unmet medical need where the FDA’s Centre for Drug Evaluation and Research works intensively with drug developers to impart their knowledge and expertise, built over decades and across thousands of new drug development programs [8,9].
To achieve the Ebola vaccines’ 5-year clinical program, global regulators from developed countries combined and leveraged their collective expertise to assist the African Vaccine Regulatory Forum and worked closely with vaccine developer Merck to conduct supporting and pivotal trials when and where the outbreak was underway [10]. This significantly improved the regulatory and ethics timelines in key areas of presubmission discussions with sponsors and manufacturers, authorization of clinical trials and emergency use of investigational vaccines, and implementation of joint reviews for ethical and regulatory approvals [10,11]. The leadership of WHO was the cornerstone of this coordinated regulatory response.
Regulatory Bottlenecks
While there are ongoing collaborative efforts around the harmonization of regulatory frameworks in pharmaceuticals and medical devices, the ability to coordinate cross-national regulatory responses in times of crises remains a sticking point. For example, while Merck submitted the same evidence dossier for the Ebola vaccine to both the EMA and FDA at the same time, there were different meetings, review and response timelines, and questions from each, and ultimately the vaccine was approved 1 month earlier in Europe than the United Statesviii. Further, regulatory and ethical issues could make it challenging for SARS, MERS, and Zika candidates to even complete clinical testing now that their respective outbreaks are over. For instance, the development of a Zika vaccine still presents unresolved questions around the identification of immune markers likely to predict clinical benefit, the role of flavivirus immunity in vaccine safety, and ethical concerns related to the vaccination of pregnant women [12].
There is ongoing debate about how long a novel COVID-19 vaccine will really take to advance through trials, achieve regulatory approval across multiple jurisdictions, and be manufactured and distributed at the required scale to impact global immunity and prevent further transmission. Vaccines often present commercial viability challenges due to long, complex development timelines and ‘capacity to pay’ issues in developing countries. While some that achieve global population health success, such as the human papillomavirus vaccine, can provide substantial return on investmentix, these trends have meant that, compared with novel therapeutics, far fewer vaccines have made it through clinical testing. As a result, regulators and vaccine developers lack experience in vaccine development comparable with novel therapeutics [13]. This could become increasingly problematic if all current COVID-19 clinical and preclinical vaccine candidates are simultaneously vying for regulators’ attention and resources to expeditiously review clinical protocols and evidence dossiers. Not to mention disruption to other clinical programs, as regulators are redirected to reviewing the clinical protocols of dozens of COVID-19 vaccine candidates and even more therapeutic candidates. Beyond these bottlenecks, the underlying support required within bioanalytical and pathology providers will also be critical to ensure rapid availability and interpretation of clinical data.
The changing dynamics of the disease as it continues to emerge could also affect the development of vaccines when trial designs are informed by the epidemiology of the virus. As governments implement lockdowns and nonessential travel and transmission rates decline, clinical trial recruitment will become challenging across the potentially dozens of independent clinical programs striving for patients and healthy volunteers. In addition, patient screening for COVID-19-negative status and monitoring of potential virus exposure while on study will create additional challenges and opportunities for early trial endpoints.
Potential Solutions
If regulatory bottlenecks prevent solutions being brought to the public in time to reduce the impact of an epidemic, then we need to consider alternatives that deliver solutions at an earlier stage of an epidemic, in the geographical jurisdictions where the outbreak is occurring. Governments can leverage harmonized regulatory standards, mutual recognition, and regulatory reliance mechanisms to enhance existing regulatory pathways and accelerate and coordinate approvals globallyx. This would be particularly beneficial in countries that traditionally suffer submission and approval lags for novel drugs and vaccines.
With the data at hand from previous outbreaks, we can consider predictive epidemiological models that can guide globally coordinated responses ahead of the curve and take advantage of the availability of patients across what are typically northern and southern hemisphere seasonal outbreaks. Public–private partnerships, such as the Accelerating COVID-19 Therapeutic Interventions and Vaccines partnership led by the National Institutes of Health, and programs for expedited responses from leading regulators such as the FDA’s Coronavirus Treatment Acceleration Program and the EMA’s Rapid Response team could be pre-emptively designed and developed for global health emergencies, rather than introduced during an outbreak, and when governments with advanced health systems such as Australia, New Zealand, South Korea, Hong Kong, Taiwan, and Singapore have already flattened their curves. This may point to an expanded leadership role for international organizations like WHO to coordinate regulatory jurisdictions in a more focused and targeted manner. In short, global coordination of regulatory efforts for COVID-19 are still lagging research and clinical development efforts.
We also need to be better prepared in the areas of preclinical science, including animal challenge models, clinical trial pathways from safety to efficacy, and for innovative and adaptive clinical trial designs such as umbrella studies (where multiple candidates are tested under a single protocol) to become the norm in times of epidemics. While some of these trial initiatives have started to emerge, such as the SOLIDARITY trial, they are still few and far between. This is especially problematic considering there are currently over 500 randomized controlled studies for COVID-19 preventatives and treatments, 31 of which have at least two arms and a target sample size of at least 1000 patients (some with recruitment targets of 40 000–55 000) [14]. Large, randomized and placebo-controlled studies have been associated with longer total development times, as opposed to smaller, nonrandomized single-arm or extension-arm studies [8]. Last, guidance documents for pandemic trials should be available in anticipation of pandemics, ready to be triggered as soon as the WHO declares a public health emergency, and remain in effect after the public health crisis has abated.
While it is clear the scientific community’s attention is now shifting towards regulatory solutions and regulators are demonstrating flexible regulatory processes, there is no time for competition and fragmentation among governments and regulators. Regulation requires collaboration, especially as a solution would provide neither prophylactic nor therapeutic benefit to communities, or commercial return to the developer, if marketed after the peak of the outbreak.
Acknowledgments
The authors would like to thank the anonymous reviewer and the Editor for comments and suggestions to improve the manuscript. M. Oyola-Lozada would like to acknowledge Peru's National Council for Science, Technology and Technological Innovation (CONCYTEC) for supporting her doctoral research at The University of Queensland.
Disclaimer Statement
Professor Munro is Project Director of the Coalition for Epidemic Preparedness Innovations (CEPI) funded Vaccine Rapid Response pipeline at the University of Queensland.
Footnotes
Supplemental information associated with this article can be found online at https://doi.org/10.1016/j.tibtech.2020.06.004.
Resources
iwww.who.int/csr/sars/country/en/iiwww.who.int/csr/disease/swineflu/updates/en/iiihttp://apps.who.int/flumart/Default?ReportNo=10ivwww.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-31-december-2017-6-january-2018-week-1vwww.cdc.gov/vhf/ebola/history/2014-2016-outbreak/cumulative-cases-graphs.html#anchor_1515096547931viwww.paho.org/data/index.php/es/?option=com_content&view=article&id=529:zika-subregions-es&Itemid=353viihttps://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6viiihttps://cdn.ymaws.com/www.casss.org/resource/resmgr/wcbp/wcbp_2020/Duffy_Kim_Hassis_Kim_Slides.pdfixwww.mrknewsroom.com/newsroom/news-releases/news-details/2020/Merck-Announces-Fourth-Quarter-and-Full-Year-2019-Financial-Results/default.aspxxwww.ifpma.org/resource-centre/covid-19-biopharmaceutical-industry-regulatory-guiding-principles/Supplemental Information
Featured events in the development of vaccines against modern pandemics
References
- 1.Bedford J. A new twenty-first century science for effective epidemic response. Nature. 2019;575:130–136. doi: 10.1038/s41586-019-1717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu B. Open access epidemiological data from the COVID-19 outbreak. Lancet Infect. Dis. 2020;20:534. doi: 10.1016/S1473-3099(20)30119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu D.K.W. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corman V. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Eurosurveillance. 2012;17:20285. doi: 10.2807/ese.17.39.20285-en. [DOI] [PubMed] [Google Scholar]
- 6.Kupferschmidt K. ‘A completely new culture of doing research.’ Coronavirus outbreak changes how scientists communicate. Science. 2020 doi: 10.1126/science.abb4761. Published online February 26, 2020. [DOI] [Google Scholar]
- 7.Le T. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 8.Pregelj L. Precision medicines have faster approvals based on fewer and smaller trials than other medicines. Health Aff. (Millwood) 2018;37:724–731. doi: 10.1377/hlthaff.2017.1580. [DOI] [PubMed] [Google Scholar]
- 9.Darrow J.J. FDA approval and regulation of pharmaceuticals, 1983-2018. JAMA. 2020;323:164–176. doi: 10.1001/jama.2019.20288. [DOI] [PubMed] [Google Scholar]
- 10.Akanmori B. The African vaccine regulatory forum (AVAREF): a platform for collaboration in a public health emergency. WHO Drug Info. 2015;29:127–132. [Google Scholar]
- 11.Henao-Restrepo A.M. On a path to accelerate access to Ebola vaccines: the WHO's research and development efforts during the 2014–2016 Ebola epidemic in West Africa. Curr. Opin. Virol. 2016;17:138–144. doi: 10.1016/j.coviro.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vannice K.S. Demonstrating vaccine effectiveness during a waning epidemic: a WHO/NIH meeting report on approaches to development and licensure of Zika vaccine candidates. Vaccine. 2019;37:863–868. doi: 10.1016/j.vaccine.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgson J. The pandemic pipeline. Nat. Biotechnol. 2020;38:523–532. doi: 10.1038/d41587-020-00005-z. [DOI] [PubMed] [Google Scholar]
- 14.Davis J.S., Ferreira D., Denholm J.T., Tong S.Y.C. Clinical trials for the prevention and treatment of coronavirus disease 2019 (COVID-19): The current state of play. Med. J. Aust. 2020 doi: 10.5694/mja2.50673. https://www.mja.com.au/journal/2020/clinical-trials-prevention-and-treatment-coronavirus-disease-2019-covid-19-current Published online: 27 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Featured events in the development of vaccines against modern pandemics