Highlights
-
•
Severe COVID-19 illness from infection with SARS-CoV-2 is associated with a high incidence of thromboembolic events.
-
•
Major thromboembolic episodes following previous COVID-19 in ambulant patients without risk factors for thrombophilia are not described in the literature.
-
•
A unique case of acute cor pulmonale from a saddle pulmonary embolism following previous COVID-19, managed successfully by thrombolysis, is reported here.
-
•
The case highlights the importance of planning randomized controlled trials to determine whether prophylactic anticoagulation should be prolonged even in patients with moderately severe COVID-19.
Keywords: COVID-19, Thromboembolism, Prophylactic anticoagulation
Abstract
Severe coronavirus disease 2019 (COVID-19) is known to be associated with a heightened risk of thromboembolism. However, the risk associated with mild and moderate illness from COVID-19 is unknown, and there is no current recommendation for prophylaxis against thromboembolism in patients after hospital treatment, unless there are established thrombophilic risk factors. We report the case of a 52-year-old woman who presented with massive saddle pulmonary embolism 1 week after initial hospital discharge, which was treated successfully with thrombolysis. This case raises the question of whether extended prophylactic anticoagulation should be considered even in low-risk COVID-19 cases.
Introduction
The global pandemic of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected more than 6.8 million people globally, with a death toll exceeding 400 000 at the time of writing; almost all countries across the world have been affected. Although COVID-19 has protean clinical manifestations involving almost every organ system in the human body, pneumonia, acute respiratory distress syndrome (ARDS), diarrhoea, septic shock, acute kidney injury (AKI), disseminated intravascular coagulation (DIC), and rhabdomyolysis are the more commonly described clinical features (Guan et al., 2020). Arterial and/or venous thromboembolic events can also be important manifestations of severe cases of COVID-19, although the actuarial prevalence or incidence of this complication is unknown, especially in those with mild disease. A recent autopsy series of 12 cases revealed that unsuspected venous thromboembolism was present in seven patients (58.3%), and pulmonary embolism (PE) was the direct cause of death in four patients (33.3%) (Wichmann et al., 2020). Acute cor pulmonale and cardiac arrest have also been described in critically ill patients (Creel-Bulos et al., 2020).
We report a case of acute massive PE occurring 1 week after patient discharge following initial hospital treatment for COVID-19 pneumonia, which was managed successfully by thrombolysis. This raises serious concerns about the indications for extended prophylactic anticoagulation in such cases.
Case report
A 52-year-old woman presented with difficulty in breathing and a productive cough for 2 weeks, with a previous history of well-controlled asthma and essential hypertension and without any past or family history of thrombophilia. She had bibasal lung crackles and an oxygen saturation of 93% while breathing 40% oxygen through a Venturi mask. Other initial investigations were as follows (with the reference laboratory range in parenthesis): white cell count 9.2 × 109/l (4.5–11 × 109/l), lymphocytes 0.56 × 109/l (1.5–4 × 109/l), serum creatinine 129 μmol/l (45–84 μmol/l), blood urea 5.3 mmol/l (2.5–7.8 mmol/l), pH 7.43 (7.35–7.45), pO2 7.34 kPa (11–14 kPa), pCO2 5.09 kPa (4.6–6.0 kPa), bicarbonate 25.5 mmol/l (22–28 mmol/l), lactate 0.4 mmol/l (0.5–16 mmol/l), and C-reactive protein (CRP) 112 mg/l, with normal liver function tests. The baseline electrocardiogram showed only sinus tachycardia. Her reverse transcriptase-polymerase chain reaction (RT-PCR) test for SARS-CoV-2 ribonucleic acid was positive, and a chest radiograph showed bilateral lower zone airspace opacities consistent with COVID-19 pneumonia.
The patient was initially managed with intravenous hydration, oral doxycycline, oxygen, and prophylactic enoxaparin. Subsequently, she was weaned off oxygen and was discharged 4 days after initial admission.
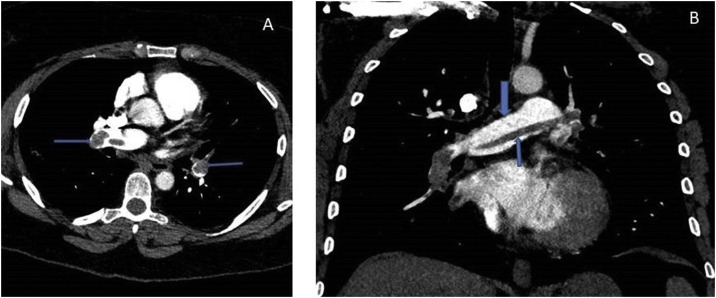
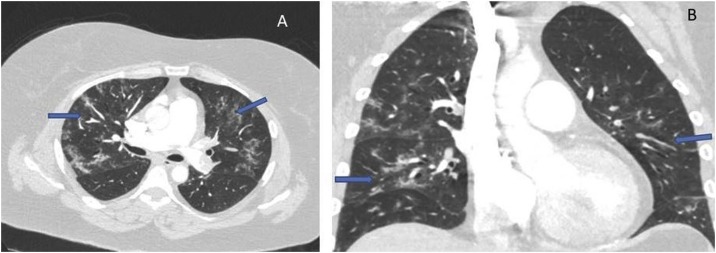
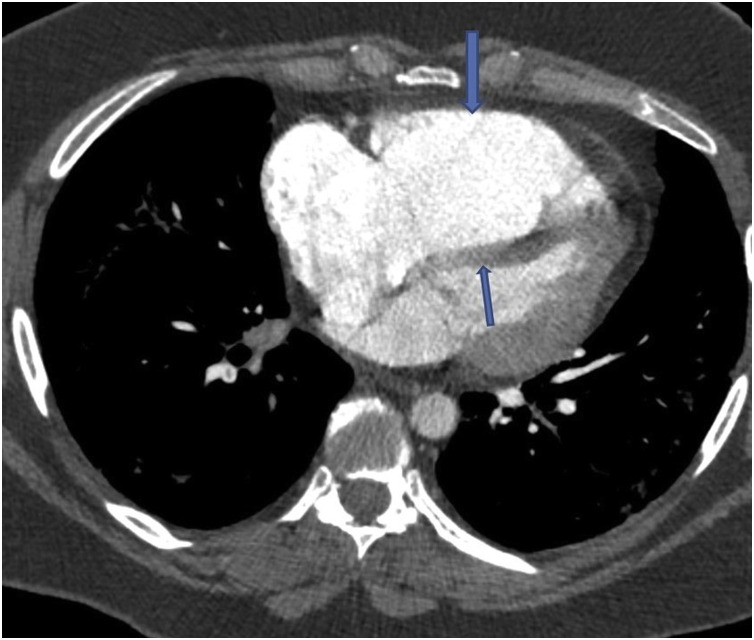
One week later she presented again with a syncopal episode and severe breathlessness, chest tightness, tachycardia, hypoxia, and hypotension. Her blood profile showed serum troponin 205 ng/l (reference range 5–14 ng/l), D-dimer 6.26 μg/ml (reference range 0–0.5 μg/ml), CRP 7 mg/l, and a lymphocyte count of 0.95 × 109/l. A chest radiograph revealed improving bibasilar lung infiltrates in comparison to the radiograph taken 11 days before. An electrocardiogram showed S1Q3T3 pattern suggestive of probable acute PE. An urgent computed tomography pulmonary angiogram (CTPA) revealed bilateral extensive thromboembolism in the pulmonary arterial branches (Figure 1 A) and a saddle PE in the main pulmonary artery bifurcation (Figure 1B). There was right ventricular (RV) dilatation and deviation of the interventricular septum to the left ventricle, suggesting RV strain pattern (Figure 2 ). The lung windows of the CTPA showed resolving COVID-19 pneumonia (Figure 3 A, B).
Figure 1.
A. Axial view of the computed tomographic pulmonary angiography (CTPA) showing thrombi in the pulmonary artery branches (arrows) B. Coronal view of CTPA: thick arrow shows the bifurcation of the pulmonary artery, and thin arrow the saddle embolus.
Figure 2.
Computed tomographic image of the dilated right ventricle (thick arrow) and the bulging interventricular septum into the left ventricle (thin arrow) from right ventricular strain.
Figure 3.
(A) Axial and (B) coronal views of the computed tomographic images (lung windows) showing ground glass opacification and pulmonary infiltrates (arrows) of resolving Covid-19 pneumonia.
The patient was immediately thrombolysed with recombinant tissue plasminogen activator in the intensive treatment unit (ITU), with rapid improvement of the hypoxia and hypotension. Within 16 hours of successful thrombolysis, she was able to maintain oxygen saturation of 98–100% on room air. After the initial management with therapeutic anticoagulation using subcutaneous enoxaparin, she was discharged home on the fourth day of the second hospital admission on oral rivaroxaban 15 mg twice daily for 21 days, followed by a maintenance dose of 20 mg daily for 3 more months.
Discussion
Based on observations from multiple clinical studies, severe COVID-19 disease requiring ITU management is now established as a highly thrombophilic state, with an estimated incidence of varying degrees of thromboembolic episodes ranging from 21% to 58% (Wichmann et al., 2020, Creel-Bulos et al., 2020, Helms et al., 2020, Lodigiani et al., 2020, Klok et al., 2020). A significant proportion of these patients have developed thromboembolic events even while on prophylactic anticoagulation therapy, challenging our conventional concepts about anticoagulation protocols in the ITU management of critically ill patients. Emerging data and clinical experience suggest an increased prevalence of venous thromboembolic events (VTE) in COVID-19, especially in patients with severe disease requiring hospitalization, and even among those who are not critically ill.
Current explanations for the pathogenesis of COVID-19-associated hypercoagulability include hypoxia and systemic inflammation secondary to COVID-19 that may lead to high levels of inflammatory cytokines and activation of the coagulation pathway. However, the exact mechanisms causing thromboembolic episodes remain elusive. Endothelial inflammation with very high levels of von Willebrand factor antigen and factor VIII, hypoxemia-induced vasoconstriction promoting vaso-occlusion, activation of hypoxia-inducible factors (HIFs) resulting in the induction or inhibition of many genes including tissue factor (TF) and plasminogen activator inhibitor 1 (PAI-1), elevated levels of lupus anticoagulant, direct activation coagulation cascades, and endothelial injury by the virus have all been proposed as putative mechanisms (Helms et al., 2020).
All hospitalized patients with COVID-19 should receive pharmacological thromboprophylaxis as per the standard international recommendations (Bikdeli et al., 2020). Some groups even recommend empirical therapeutic anticoagulation in severely ill COVID-19 patients treated in the ITU, based on past experience of a very high risk of thromboembolism for this high-risk patient group (Bikdeli et al., 2020, Song et al., 2020). Fibrinolytic therapy or a catheter-based intervention for the removal of the thrombus is the treatment of choice for haemodynamically unstable patients with massive PE and acute cor pulmonale. Thrombolysis has previously been found to reduce the odds of death (odds ratio 0.57, 95% confidence interval 0.37–0.87; p = 0.01) and recurrence of PE (odds ratio 0.51, 95% confidence interval 0.29–0.89; p = 0.02), but also to increase the risk of bleeding in patients with massive PE (Hao et al., 2018). The risk of recurrence after an episode of unprovoked venous thromboembolism treated with anticoagulation for 3 months was found to be 10% within the first year, 16% at 2 years, 25% at 5 years, and 36% at 10 years (Khan et al., 2019). However, the role and the duration of subsequent oral therapy with warfarin or non-coumarin anticoagulants for the prevention of future thromboembolism among COVID-19 patients is currently unknown.
Clinical management guidelines regarding the prophylaxis and treatment of venous thromboembolism in COVID-19 patients is a rapidly evolving topic, with new information often released based on emerging evidence from multi-centre randomized controlled trials (RCTs). There are a number of ongoing international RCTs to evaluate the risks and benefits of anticoagulation in patients with COVID-19, which are expected to shed more light on this grey area for the development of a best global consensus in the near future (Covid-19 illness, 2020).
The US National Institutes of Health (NIH) has recently updated the guidelines for antithrombotic therapy in patients with COVID-19 who are discharged from the hospital (The National Institute of Health, 2020). The guidelines state that routine post-discharge VTE prophylaxis is not recommended for patients with COVID-19. The benefits of post-discharge prophylaxis for certain high-risk patients without COVID-19 led to the US Food and Drug Administration approval of two current regimens: (1) rivaroxaban 10 mg daily for 31–39 days, and (2) betrixaban 160 mg on day 1, followed by betrixaban 80 mg once daily for 35–42 days (Spyropoulos et al., 2020, Cohen et al., 2016). Inclusion criteria for the RCTs that have studied these regimens included a modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE)-VTE score of ≥4, or a modified IMPROVE-VTE score ≥2 and D-dimer level more than two times the upper limit of normal (Spyropoulos et al., 2020); and age ≥75 years, or age >60 years and a D-dimer level more than two times the upper limit of normal, or age 40–60 years and a D-dimer level more than two times the upper limit of normal plus a previous VTE event or cancer (Cohen et al., 2016). Post-discharge VTE prophylaxis should consider the individual patient’s risk factors, including reduced mobility, bleeding risks, and feasibility before any treatment decision is made.
Bikdeli et al. recently updated a document to provide guidance from the Anticoagulation Forum, a North American organization of anticoagulation providers, regarding the use of anticoagulant therapies in patients with COVID-19 (Bikdeli et al., 2020). This paper outlining issues related to thrombotic disease with implications for prevention and therapy has been endorsed by the International Society for Thrombosis and Haemostasis, the North American Thrombosis Forum, the European Society of Vascular Medicine, and the International Union of Angiology. The paper discusses in-hospital and post-discharge VTE prevention, the treatment of suspected but unconfirmed VTE, laboratory monitoring of COVID-19, associated anticoagulant therapies, and essential elements for optimized transitions of care specific to patients with COVID-19.
The risks of arterial and venous thromboembolism in patients with mild to moderate COVID-19 disease are not well established. In the case presented here, there were no identifiable risk factors for thromboembolism at the time of initial discharge from the hospital and the patient was fully ambulant. However, presentation with a sub-lethal massive PE within a week raises serious concerns about the heightened risk of thromboembolism even in patients with moderate COVID-19 illness. The role of extended prophylactic anticoagulation should be studied in such patients in randomized trials. The duration of therapeutic anticoagulation following an episode of thromboembolism in COVID-19 patients may also need further investigations considering the hyper-exaggerated coagulopathy in these cases, because our current concepts on disease management are largely challenged by this enigmatic disease.
Funding
No funding was received for this work.
Ethical approval
Ethical approval was not needed as this is just a case report; informed signed consent for publication was obtained from the patient
Conflict of interest
There are no competing interests to declare among the authors of this work.
References
- Guan W.J., Ni Z.Y., Hu Y. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(April (18)):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.P., Lütgehetmann M. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;(May) doi: 10.7326/M20-2003. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creel-Bulos C., Hockstein M., Amin N., Melhem S., Truong A., Sharifpour M. Acute Cor Pulmonale in Critically Ill Patients with Covid-19. N Engl J Med. 2020;(May) doi: 10.1056/NEJMc2010459. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Tacquard C., Severac F. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;(May) doi: 10.1007/s00134-020-06062-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191(April):9–14. doi: 10.1016/j.thromres.2020.04.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;(April) doi: 10.1016/j.thromres.2020.04.041. pii: S0049-3848(20)30157-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. J Am Coll Cardiol. 2020;(April) doi: 10.1016/j.jacc.2020.04.031. pii: S0735-1097(20)35008-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.C., Wang G., Zhang W. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res. 2020;7(April (1)):19. doi: 10.1186/s40779-020-00247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q., Dong B.R., Yue J., Wu T., Liu G.J. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev. 2018;12(December) doi: 10.1002/14651858.CD004437.pub5. CD004437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F., Rahman A., Carrier M. MARVELOUS Collaborators. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis. BMJ. 2019;366(July):l4363. doi: 10.1136/bmj.l4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongoing clinical trials on anticoagulation for the patients with Covid-19 illness. Available at: https://www.clinicaltrials.gov/ct2/results?cond=COVID&term=anticoagulation&cntry=&state=&city=&dist= Accessed on June 06, 2020.
- The National Institute of Health. Antithrombotic Therapy in Patients with COVID-19. Available at: https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/ Accessed on June 06, 2020.
- Spyropoulos A.C., Lipardi C., Xu J. Modified IMPROVE VTE Risk score and elevated D-dimer identify a high venous thromboembolism risk in acutely ill medical population for extended thromboprophylaxis. TH Open. 2020;4(1):e59–e65. doi: 10.1055/s-0040-1705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.T., Harrington R.A., Goldhaber S.Z. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375(6):534–544. doi: 10.1056/NEJMoa1601747. [DOI] [PubMed] [Google Scholar]