Abstract
Few studies have focused on the transmission efficiency of asymptomatic carriers of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Our follow-up study was performed on 147 asymptomatic carriers in Anhui Province. Of these, 50.0% were male, 50.3% were older than 40 years, 43.8% were farmers, and 68.7% were from the north of Anhui Province. 16 of the 147 asymptomatic carriers developed symptoms in the following 14 days of isolated observation, and were subsequently diagnosed as confirmed cases. The possible latent infection period was found to range from 1–5 days before onset, with a median time of 2 days. The second attack rate for the 16 confirmed cases who had transferred from being asymptomatic carriers was 9.7% (23/236 close contacts), while for the 131 asymptomatic carriers the rate was 2.6% (24/914 close contacts), showing a significant difference in second attack rate between the two groups (p<0.001). Our study indicated that COVID-19 cases are contagious during the incubation period, and that close contact screening should be extended to include the incubation period. Our results also showed that the transmission efficiency for asymptomatic carriers was lower than that for confirmed case.
Keywords: coronavirus disease 19, SARS-CoV-2, asymptomatic infection, emerging infectious disease
Introduction
COVID-19 is a novel infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This disease mainly affects the lung, but can also cause damage to the intestinal tract, liver, and nervous system, with corresponding symptoms (Lu et al., 2020). The outbreak originated in Wuhan city, Hubei Province in December 2019, and quickly spread to provinces and cities across the country and aboard (Li et al., 2020, WHO, 2020, Zhu et al., 2019). As of 28 August, 2020, there were 85 022 confirmed cases in China, including 4634 deaths and 80 126 recovered cases (China NHCotPsRo, 2020a). Human-to-human transmission of SARS-CoV-2 had been demonstrated mainly through respiratory droplets and contact (Chan et al., 2020). The continuing challenges of the epidemic and research progress in China have led to emergency responses from the Chinese government, including the issuance of regulatory documents (China NHCotPsRo, 2020b, China NHCotPsRo, 2020c).
Clinical symptoms of SARS-CoV-2 infection include fever, cough, and fatigue, and in a few cases stuffy nose, runny nose, and diarrhea (China NHCotPsRo, 2020b). Severe cases result in acute respiratory distress syndrome, septic shock, and coagulopathically caused death (Huang et al., 2020). As a highly contagious infectious disease, its sources of infection include not only confirmed cases, but also asymptomatic carriers (Huang et al., 2020, Cohen, 2020). Asymptomatic carriers of SARS-CoV-2 infection are always without clinical symptoms, but positive for the viral nucleic acid test. Most are found during the screening of close contacts, and because the laboratory tests are performed at an early stage, some asymptomatic cases go on to develop illness after screening.
For this study, we conducted a follow-up study on asymptomatic carriers in Anhui Province, and analyzed the features of these infections. Our findings provide evidence for the need to modify the preventive measures.
Materials and methods
Study design
This was a follow-up study, conducted in Anhui Province, China.
Study cases
147 asymptomatic carriers of SARS-CoV-2 infection were recruited for this study from Anhui Province. All asymptomatic carriers fulfilled the following criteria: (1) without symptoms of fever, cough, and fatigue; (2) no radiographic evidence of pneumonia; (3) with normal white cell count and normal lymphocyte count; and (4) positive nucleic acid test for SARS-CoV-2.
Data collection
A questionnaire was formulated to gather data on: (1) demographic information, such as name, age, gender, and occupation; (2) clinical symptoms, including fever, chill, cough, and fatigue if these developed during the 14-day observation period; (3) laboratory testing related to SARS-CoV-2 infection, including nucleic test result and timing of test; (4) close contact information, such as numbers, confirmed cases, and asymptomatic carriers.
Laboratory testing
Respiratory samples, including sputum and throat swab samples, were collected from all asymptomatic carriers by trained inspection personnel. Viral RNA was extracted using a TANBead nucleic acid kit (Taiwan Advanced Nanotech Inc, Taiwan, China) in a biosafety level-2 laboratory. Tests for the ORF1ab and N genes in respiratory samples were carried out using a 2019-nCoV dual fluorescent PCR kit (Shanghai BioGerm Medical Biotechnology Co., Ltd, Shanghai, China and DAAN Gene Co., Ltd, Sun Yat-sen University, Guangdong, China).
Statistical analysis
Microsoft Office software (version 2019) was employed to input and check the data, and SPSS software (version 11.0) was used to analyze the data. Categorical variables were summarized as frequencies and percentages, and continuous variables were described using medians. The frequencies of categorical variables were compared using the chi-square test, as appropriate. Tests with a p-value less than 0.05 were considered statistically significant.
Ethical statement
Collection of data and samples from patients was part of a routine surveillance and outbreak investigation, and was therefore exempt from assessment by the institutional review board (IRB). The IRB of Anhui Provincial Center for Disease Control and Prevention reviewed the study.
Results
Demographic features
Table 1 shows the demographic features of the 147 asymptomatic carriers. Of these, 15.6% (23/147) were under 20 years old, and 64.6% (95/147) were aged 20–59 years. 51.7% (76/147) of the carriers were male, and 94.6% (139/147) were found by close contact screening. A total of 16 asymptomatic carriers developed symptoms during the following 14-day observation period.
Table 1.
Demographic features and transmission efficiency assessment for asymptomatic carriers of SARS-CoV-2 infection.
| Variables |
n (%) |
p-value | ||
|---|---|---|---|---|
| All carriers (n = 147) | Asymptomatic carriers ( n = 131) |
Confirmed cases transferred from asymptomatic carriers (n = 16) | ||
| Age | 0.195 | |||
| < 20 years old | 23 (15.6) | 23 (17.6) | 0 (0.0) | |
| 20–39 years old | 50 (34.0) | 43 (32.8) | 7 (43.8) | |
| 40–59 years old | 45 (30.6) | 41 (31.3) | 4 (25.0) | |
| ≥ 60 years old | 29 (19.7) | 24 (18.3) | 5 (31.2) | |
| Gender | 0.500 | |||
| mMale | 76 (51.7) | 69 (52.7) | 7 (43.8) | |
| Female | 71 (48.3) | 62 (47.3) | 9 (56.2) | |
| Source of identification | 0.793 | |||
| Close contact screening | 139 (94.6) | 123 (93.9) | 16 (100.0) | |
| Traceability investigation | 2 (1.4) | 2 (1.5) | 0 (0.0) | |
| Extended screening | 6 (4.1) | 6 (4.6) | 0 (0.0) | |
| Close contact | <0.001 | |||
| Second attack rate | 4.1 | 2.6 | 9.7 | |
| Positive close contacts | 47 | 24 | 23 | |
| Total close contacts | 1150 | 914 | 236 | |
Latent infectivity assessment
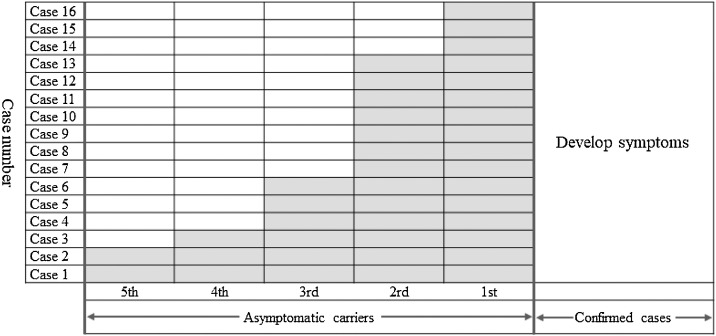
The results of our latent infectivity assessment are shown in Figure 1 . The latent infectivity period was evaluated using the 16 confirmed cases who had transferred from being asymptomatic carriers. Two cases had tested positive 5 days before developing symptoms, one case 4 days before, three cases 3 days before, seven cases 2 days before, and three cases just 1 day prior to becoming ill. The median period was calculated as 2 days (range 1–5).
Figure 1.
Assessment of latent infectivity, based on 16 confirmed cases who had transferred from being asymptomatic carriers.
Transmission efficiency assessment
A comparison of second attack rates for asymptomatic carriers and confirmed cases is shown in Table 1. A total of 1150 close contacts was determined in relation to the 147 asymptomatic carriers. 47 close contacts tested positive for SARS-CoV-2 infection during the 14-day observation period. The 16 confirmed cases who had previously been asymptomatic accounted for 236 close contacts, with a second attack rate of 9.7%, while the remaining 131 asymptomatic carriers accounted for 914 close contacts, with a second attack rate of 2.6%. There was a significant difference between two groups (χ (Li et al., 2020) = 24.257; p < 0.001).
Discussion
Asymptomatic infection, as part of the SARS-CoV-2 infection spectrum, has been reported in a previous study (Chan et al., 2020). An asymptomatic carrier is defined as someone with a positive SARS-CoV-2 test from respiratory samples, including sputum and throat swab, but who displays no symptoms. Some studies have examined asymptomatic carriers and analyzed the incubation period (Pan et al., 2020, Wang et al., 2020). However, none has been conducted on the assessment of transmission efficiency and latent infection period with regards to asymptomatic infection. We performed this follow-up study in Anhui Province to examine these topics. Our findings could provide evidence to support the revision of control measures for SARS-CoV-2 infection.
Asymptomatic carriers can be identified in four ways. First, and most important, is active detection among close contacts. The second is based on epidemiological investigations surrounding suspected or confirmed cases. The third involves looking at the exposed population identified by tracking the SARS-CoV-2 infection source. Finally, asymptomatic carriers can be found through active surveillance in the epidemic areas. Our study confirmed that close contact screening was the main way to locate asymptomatic carriers, with the same result also being reported in a previous study (Pan et al., 2020). This revealed that close contacts identified by screening should be isolated because they are at risk of infection.
According to guidelines aimed at preventing SARS-CoV-2 infection, as potential infectious sources, asymptomatic carriers should be quarantined for 14 days from the first positive laboratory test (China NHCotPsRo, 2020d). 10.9% of the asymptomatic carriers in our study subsequently developed symptoms during the observation period to become confirmed cases. Those cases occurred during the incubation period, a median of 2 days after testing positive (range 1–5 days). Thus, close contact screening should not only focus on patients who have developed illness, but should also be extended to include asymptomatic cases during the incubation period, in order to reduce the infection risk of SARS-CoV-2 (Hu et al., 2020). Our study also assessed the transmission efficiency of asymptomatic carriers. Although the attack rate was lower than that for confirmed cases, it was shown to cause infection in 2.6% of close contacts.
In conclusion, although the transmission efficiency of asymptomatic carriers is lower than that for confirmed cases, the transmission risk from carriers should not be ignored. Second, close contacts of those infected with SARS-CoV-2 should undergo detailed observation and active laboratory screening, with these measures being included in the formulating of guidelines relating to SARS-CoV-2. Finally, close contact screening should be extended to include those present up to 5 days before the onset of illness in confirmed cases.
Funding
This study was supported by grants from Anhui Provincial Department of Science and Technology, Anhui Provincial Health Commission Emergency Research Project of Novel Coronavirus Infection (grant numbers 202004a07020002; 202004a07020004).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
We would like to thank the physicians and staff at the 16 municipal centers for Disease Control and prevention in Anhui Province for collecting the information of asymptomatic carriers of SARS-CoV-2 infection.
References
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases 2020. Geneva. [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. China Novel Coronavirus I, Research T, 2020. A Novel Coronavirus from Patients with Pneumonia in China. N Engl J Med. 2019;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China NHCotPsRo . 2020. Epidemic situation of Corona Virus Disease 19 on February 25. [Google Scholar]
- Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China NHCotPsRo . 2020. Corona Virus Disease 19 is included in the management of legal infectious diseases. [Google Scholar]
- China NHCotPsRo . 2020. Office of the State Administration of Traditional Chinese Medicine. Diagnosis and treatment of Corona Virus Disease 19 (Trial version 7) [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.N.D. 2020. World on alert for potential spread of new SARS-like virus found in China. [Google Scholar]
- Pan X., Chen D., Xia Y., Wu X., Li T., Ou X. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu Y., Liu L., Wang X., Luo N., Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-Coronavirus-2 in Shenzhen, China. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China NHCotPsRo . 2020. The Prevention and Control Plan of Corona Virus Disease 19 (Version 5) [Google Scholar]
- Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in China. Sci. China Life Sci. 2020 doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]