To the Editor:
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has suddenly emerged, resulting in a pandemic. At present, there is no known safe and effective antiviral agent for COVID-19 treatment. Matsuyama et al1 suggested that ciclesonide (Alvesco [(11β, 16α)-16, 17-[[(R)-cyclohexylmethylene]bis(oxy)]-11-hydroxy-21-(2-methyl-1-oxopropoxy)-pregna-1, 4-diene-3, 20-dione]), an inhaled glucocorticoid, could inhibit the replication of SARS-CoV-2 genomic RNA by targeting the viral endonuclease NSP15. However, there is no information concerning the detailed pharmacological and/or molecular mechanisms underlying this response. Here, we present an in silico study that elucidates a mechanism whereby ciclesonide might inhibit SARS-CoV-2 replication.
The full-length nucleotide sequences of NSP15 and RdRp (RNA-dependent RNA polymerase) genes identified in GenBank (accession no. MN908947) were used for homology modeling. The 3-dimensional structures used for the docking simulation analysis were obtained from the PubChem database, including the structural data for nonesterified ciclesonide (compound identification [CID] number 6918155), esterified ciclesonide (desisobutyryl-ciclesonide [des-CIC], CID number 6918281), and fluticasone propionate (CID number 444036). Protein structure models of NSP15 and RdRp proteins (Protein Data Bank identification numbers 6VWW and 6NUR, respectively) were constructed as previously described.2 AutoDock Vina software was used for computationally simulating the molecular recognition process (docking simulation) of the proteins and these drugs.3 Detailed procedures of the docking simulations have been previously reported.4
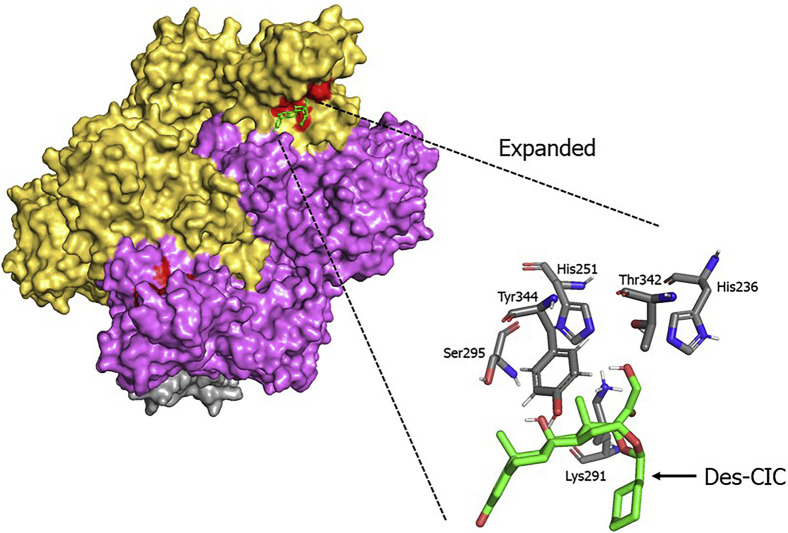
As shown in Fig 1 , our docking simulations revealed that des-CIC could bind to the active site of NSP15 endonuclease with a binding energy of −8.5 kcal/mol. The des-CIC binding sites within NSP15 included His236, His251, Lys291, Ser295, Thr342, and Tyr344 (Fig 1). Most of the interactions between NSP15 and des-CIC were estimated as hydrogen bonds. Similarly, nonesterified ciclesonide could also interact with active-site residues of NSP15 (−7.5 kcal/mol, data not shown). In contrast, neither ciclesonide variant could bind to the active site of SARS-CoV-2 RdRp (data not shown). Moreover, fluticasone propionate, another inhaled glucocorticoid, could not bind to NSP15 or RdRp (data not shown). These results suggested that both esterified and nonesterified derivatives of ciclesonide had the capacity to interact with NSP-15, thereby possessing the capacity to inhibit replication of the SARS-CoV-2 viral genome.
Fig 1.
Detailed interaction between esterified ciclesonide (des-CIC) and active sites (red regions) of SARS-CoV-2 NSP15 endonuclease. Structure mappings of des-CIC and NSP15 endonuclease were constructed using the space-filling or stick model.
Nonesterified ciclesonide is metabolized by tissue esterases, resulting in des-CIC.5 Thus, des-CIC may be the predominant form of ciclesonide in vivo. Interestingly, we found that both nonesterified ciclesonide and des-CIC were capable of interacting with NSP15, and the interaction of des-CIC with NSP15 involved the larger of the 2 predicted binding energies. As such, replication inhibition of the viral genome may relate primarily to the actions of des-CIC. However, it is critical to recognize that there is scarce information available with respect to the RNA replication mechanisms catalyzed by RdRp and NSP15. Ciclesonide is currently approved for the treatment of asthma and allergic rhinitis and has few to no adverse effects.6 , 7 Conclusively, this agent is an important candidate for consideration as potential therapy for COVID-19, and our study results may contribute to the design of other antiviral drugs against SARS-CoV-2.
Footnotes
This work was partly supported by a commissioned project for Research on Emerging and Re-emerging Infectious Diseases from the Japan Agency for Medical Research and Development (AMED, grant no. 20fk0108103h0202).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, et al. The inhaled corticosteroid blocks coronavirus RNA replication by targeting viral NSP15. BioRxiv 987016 [Preprint]. 2020. https://doi.org/10.1101/2020.03.11.987016. [DOI] [PMC free article] [PubMed]
- 2.Aso J, Kimura H, Ishii H, Saraya T, Kurai D, Matsushima Y, et al. Molecular evolution of the fusion protein (F) gene in human respirovirus 3 [published online ahead of print January 15, 2020]. Front Microbiol 2020;10:3054. https://doi.org/10.3389/fmicb.2019.03054. [DOI] [PMC free article] [PubMed]
- 3.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forli S., Huey R., Pique M.E., Sanner M., Goodsell D.S., Olson A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat Protoc. 2016;11:905–919. doi: 10.1038/nprot.2016.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nave R., Meyer W., Fuhst R., Zech K. Formation of fatty acid conjugates of ciclesonide active metabolite in the rat lung after 4-week inhalation of ciclesonide. Pulm Pharmacol Ther. 2005;18:390–396. doi: 10.1016/j.pupt.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Vogelmeier C.F., Hering T., Lewin T., Sander P., Bethke T.D. Efficacy and safety of ciclesonide in the treatment of 24,037 asthmatic patients in routine medical care. Respir Med. 2010;105:186–194. doi: 10.1016/j.rmed.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Ratner P.H., Wingertzahn M.A., van Bavel J.H., Hampel F., Darken P.F., Shah T. Efficacy and safety of ciclesonide nasal spray for the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;118:1142–1148. doi: 10.1016/j.jaci.2006.07.050. [DOI] [PubMed] [Google Scholar]