Abstract
Dr. Thomas Dougherty and his Oncology Foundation of Buffalo were the first to support my (S.O.G.) research into the effects of photodynamic therapy (PDT) on the host immune system. The small grant I was awarded in 2002 launched my career as an independent researcher; at the time there were few studies on the importance of the immune response on the efficacy of PDT and no studies demonstrating the ability of PDT to enhance anti-tumor immunity. Over the last decades the interest in PDT as an enhancer of anti-tumor immunity and our understanding of the mechanisms by which PDT enhances anti-tumor immunity have dramatically increased. In this review article, we look back on the studies that laid the foundation for our understanding and provide an update on current advances and therapies that take advantage of PDT enhancement of immunity.
Graphical Abstract
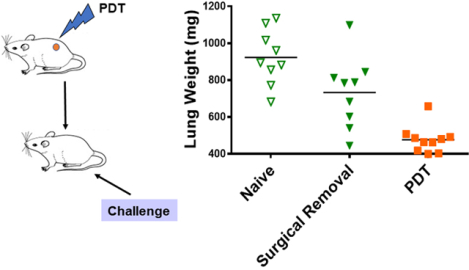
PDT treatment of tumor-bearing patients and animals enhances anti-tumor immunity. Tumor-bearing mice treated with PDT, but not surgery, develop immunity against subsequent tumor growth (as measured here by increased lung weight). In this review article, we look back on the studies that laid the foundation for our understanding and provide an update on current advances and therapies that take advantage of PDT enhancement of immunity.
INTRODUCTION
Photodynamic Therapy (PDT) is approved by both the Food and Drug Administration and by the European Medicine Agency as curative therapy for pre- cancer lesions and solid tumors and as palliative therapy for advanced malignancies. PDT is minimally invasive with high specificity of action on tumor tissue. PDT involves topical or systemic administration of a photosensitive drug (Photosensitizer; PS) followed by illumination of the tumor with light of appropriate wavelength that excites the PS. Energy from excited PS converts molecular oxygen available in tumor tissue to reactive oxygen species (ROS) (1–4). The generation of ROS causes direct cytotoxicity of cells in the tumor microenvironment resulting in tumor cell death and destruction of tumor vasculature. Loss of vasculature depletes the tumor microenvironment of essential survival components: oxygen and nutrition (2, 3, 5–7). Pre-clinical studies in mouse models and clinical studies have shown that PDT efficacy depends on the presence of an intact immune system (1). PDT-induced traumatic insult and oxidative stress to the tumor tissue activates an acute inflammatory process required for removal of tissue-debris and for restoration of homeostasis. In addition, immunogenic cell death (ICD) caused by PDT releases damage associated molecular patterns (DAMPs) that activate innate immunity, which leads to activation of adaptive immunity (8, 9). Henderson et al have shown that PDT regimens can be developed to activate anti-tumor immunity (5). Multiple studies have linked PDT-induced acute inflammation to enhancement of systemic anti-tumor immunity (10, 3, 11, 12).
In this review, we discuss PDT-induction of ICD and the resultant inflammation and subsequent activation of anti-tumor immunity, thus highlighting the potential of PDT to act as adjuvant immunotherapy.
COMPONENTS OF PDT: PHOTOSENSITIZER, LIGHT AND MOLECULAR OXYGEN
PSs typically contain a tetrapyrrole structure as found in porphyrins such as protoporphyrin. The first photosensitizing material used in preclinical studies was hematoporphyrin derivative (HPD), a collection of monomeric and oligomeric porphyrin ethers and esters (13). Dr. Thomas Dougherty and colleagues at Roswell Park Comprehensive Cancer Center developed Photofrin® or Porfimer sodium, a purified version of HPD that lacks the monomers. Photofrin® (absorption peak of 630nm) was the first PS to be approved by FDA for clinical PDT in the Unites States (3, 14). Although Photofrin has a preference for tumor cells, it also has an extended period of retention in normal tissues resulting in photodamage to skin upon exposure to sunlight (15). In addition, 630 nm, the wavelength of light that excites Photofrin, has low tissue penetration capacity making the development of next generation PS necessary for PDT. PSs with increased tumor specificity and stronger absorbance (>650 nm) are currently under various stages of clinical trial with some already receiving approval for clinical use (16).
Photochemical reactions during PDT generate singlet-state oxygen (1O2) which requires energy transfer from PS to molecular oxygen. Hence, tissue oxygenation is critical during PDT efficacy. Several studies have demonstrated that light delivery at low rates (i.e. low fluence rates) results in oxygen conservation (17–20). PDT induction of acute inflammation is regulated by fluence rate (5). Henderson et al showed that low fluence rates result in high levels of inflammation, which are characterized by increases in inflammatory cytokines and neutrophils infiltration into the tumor bed. Subsequent studies have demonstrated that induction of acute inflammation, in particular mobilization of neutrophils, is required for PDT enhancement of anti-tumor immunity.
Immunological consequences of cell death by PDT
The mechanisms leading to cell death upon PDT have been widely studied. Depending on PS localization and extent of photodamage, PDT can induce cell death by necrosis, apoptosis, autophagy or paraptosis (21–24, 3, 25–32). Each of these cell death programs can result in exposure or release of intracellular components known as damage associated molecular patterns (DAMPs) or alarmins. DAMPs are recognized by pattern recognition receptors (PRRs) expressed on immune cells. DAMPs binding to PRRs results in immune cell activation; thus cell death that triggers activation of immune cells is referred to as immunogenic cell death (ICD) (33–36). PDT induced cell death programs that have been associated with ICD are discussed below:
Apoptosis.
Apoptosis is the most widely studied form of ICD in the context of PDT. PDT can cause rapid apoptosis or programmed cell death characterized by chromatin condensation and cellular fragmentation (21). PS localization dictates the mechanism of apoptosis. Localization of PS to mitochondria and subsequent mitochondrial photodamage results in release of cytochrome c to cytosol, thereby initiating apoptosis (23, 31). Additionally, both mitochondrial and ER photodamage results in destruction of the anti-apoptotic proteins Bcl2 and Bcl-xL without affecting pro-apoptotic protein Bax (29, 37, 32); thus further promoting apoptotic cell death. Photosensitizer localization to lysosomes results in release of lysosomal proteases to cytosol, thereby cleaving pro-apoptotic protein Bid to its active form tBid. This truncated form of Bid binds to mitochondrial membrane and initiates apoptosis (38–40). PDT can also induce death-receptor mediated apoptosis when PSs localizing to plasma membrane are used. Photodamage to plasma membrane results in multimerization of death receptors like Fas which belongs to TNF-receptor superfamily. Initiation of Fas signaling results in cleavage of procaspase 8 to the active caspase 8, triggering apoptotic program (7).
Apoptosis is generally a tolerogenic process and functions to maintain homeostatic cellular balance However, apoptotic tumor cells are reported to be highly immunogenic (41–44). Indeed, multiple studies have demonstrated that Photofrin-PDT results in surface expression of HSPs, CRT and release of HMGB1 (45, 46), all of which are characteristic of ICD. Garg et al have demonstrated surface expression of CRT and secretion of ATP upon immunogenic apoptosis induced by Hypericin-based PDT (47). Jalili et al have reported both apoptosis and necrosis of EMT6 murine mammary tumor cell line upon Photofrin-PDT and increased expression of HSPs (48).
Autophagy.
PDT can induce autophagy in which bulk of the cytoplasm is sequestered in double membrane bound vacuoles called autophagosomes. Conditions of cellular starvation and mitochondrial toxicity trigger formation of autophagosomes, which carry cellular components to lysosomes for degradation (49, 50). Generation of ROS during PDT is a powerful trigger for formation of autophagosomes (22, 24, 30). Cancer cells use autophagy in a cytoprotective capacity to remove sources of ROS following PDT. However, cancer cell death by autophagy becomes prominent when apoptotic machinery is impaired or when photodamaged components are incapable of recycling (49, 24, 51, 52, 26).
To determine the effect of PDT-induced autophagy on anti-tumor immune activation, Korbelik et al injected SCCVII tumor bearing mice with in vitro PDT treated SCCVII cells that were incubated with or without lethal autophagy inhibitors (53). Incubation of PDT-treated SCCVII cells with autophagy inhibitor resulted in increased rate of tumor growth. This suggests PDT-induced cell death by autophagy might play role in activating anti-tumor immunity. Chemotherapy-induced autophagy has been demonstrated to release DAMPs such as CRT, HMGB-1 and ATP, thus strengthening the argument for potential of autophagy as a mode of immunogenic cell death during cancer treatment involving cellular toxicity (54).
ICD.
PRRs that bind to DAMPs are either soluble (pentraxins and complement proteins) or membrane bound (Toll-like receptors; TLRs); PRRs are expressed on innate cells and DAMP binding to PRRs initiates activation of these cells. PDT results in accumulation of the pentraxins, such as serum amyloid P component (SAP) and C-reactive protein (CRP) (55) (56). Korbelik et al have demonstrated association of complement receptors and TLRs with PDT-generated DAMPs (55, 45). PDT-induced ICD, along with PDT-induced acute inflammation, is considered to be the initiating step toward PDT enhancement of anti-tumor immunity. This process is discussed in detail below.
Inflammation and activation of innate immunity
Destruction of tumor tissue by PDT elicits an immediate localized inflammatory response aimed at containing and clearing the debris, restoring normal tissue function and homeostasis. Damage to tumor tissue results in release of lipid membrane derived arachidonic acid metabolites (prostaglandin, leukotriene, thromboxanes), rapid upregulation of inflammatory cytokines such as MIP2 (CXCL2), IL6, IL-1β, TNFα, and activation of complement (10, 57–60). Together, these factors facilitate the influx of innate immune cells into the tumor for attack and removal of dying tumor cells (10–12). Multiple studies have revealed the importance of PDT-induced inflammation in enhancing anti-tumor immunity (61, 5, 62). The involvement of innate immune cells in PDT-induced inflammation and subsequent anti-tumor immunity is discussed below.
Involvement of neutrophils in PDT efficacy and activation of anti-tumor immunity.
There are multiple reports of local and systemic neutrophilia upon PDT (61, 10, 11, 5, 62). Photodamage to tumor vasculature causes contraction of endothelial cells allowing neutrophil adhesion to sub-endothelial matrix via the β2 integrin receptors (63, 64). PDT results in increased expression of adhesion molecules. E-selectin and ICAM1, are critical for neutrophil adhesion on tumor microvessels and entry into tumor tissue (11, 65). PDT also induces local expression of the chemokine MIP2 that facilitates neutrophil migration to tumor bed (11). In addition, PDT induces local and systemic activation of complement, which is critical for neutrophil infiltration into the tumor (12). Complement activation releases anaphylatoxins such as C3a and C5a from tumor tissue, which promote vascular permeability (66, 67). Thus, structural changes in vascular endothelial walls and regulatory factors expressed upon PDT causes accumulation of neutrophils at the vascular interface of photodamaged tumor tissue, generates chemotactic gradient across the vascular membrane, and facilitates infiltration of neutrophils to the tumor (10–12). The recruitment of neutrophils to PDT-treated tumor tissue is supported by the accompanying strong acute phase response, which is characterized by increased serum level of acute phase proteins (APPs) such as CRP, mannose binding lectins (MBLs) and SAP (68). APPs facilitate the systemic mobilization of neutrophils from storage pools and increased maturation of neutrophil progenitors in bone marrow and egress from bone marrow (10, 69). Following PDT, neutrophils also enter the tumor draining lymph nodes (TDLNs) via high endothelial venules (HEVs) in an IL-17 and IL-1β driven MIP2 mediated pathway (61). This infiltration of neutrophils to TDLNs is short-lived and resolves within 24 hours of PDT. PDT-induced local and systemic neutrophilia contributes to PDT efficacy by destroying tumor tissue and contributing to the activation of anti-tumor CD8+ T cells. Several studies have demonstrated poor PDT efficacy and reduction in number of activated anti-tumor CD8+ T cells when neutrophil entry into tumors and TDLNs is blocked (61, 5, 62, 65). Although neutrophils are considered to be critical to induction of anti-tumor immunity following PDT, cell markers used to define neutrophils (CD11b and Gr1) are also expressed on myeloid-derived suppressor cells (MDSCs) (70) thus calling into question the nature of the cells induced by PDT. However, Brackett et al performed extensive characterization of the induced cells, concluding that they could be classified as neutrophils based on histology and lack of suppressive activity (61).
Involvement of macrophages in immune activation and effector function after PDT.
Macrophages are phagocytic cells that differentiate from monocytes and express PRRs, including TLRs. PDT induced release of Hsp70, a TLR2/4 binding DAMP, from tumor cells results in TLR2/4 activation of macrophages and release of TNFα (45). TNFα is a cytolytic cytokine which may mediate indirect cytotoxicity of tumor cells following PDT. Macrophages also express complement receptors, which enable them to phagocytose tumor cells opsonized with C3 and MBLs, thus facilitating the clearance of photodamaged tumor tissue (55).
Involvement of NK cells in PDT efficacy.
Studies by Gollnick et al have revealed role of natural killer (NK) cells in PDT. In vitro studies using human and murine colon carcinoma cell lines reveal increased expression of MHC class I-like molecule MICA and NKG2D ligand on PDT treated tumor cells (71). Park et al also reported similar results (72). These molecules are ligands for activation receptors on NK cells, thus indicating a possible role of NK cells in augmentation of anti-tumor immunity following PDT. Gollnick et al reported that control of distant disease by CD8+T cells, following PDT of the primary tumor, is improved in the presence of NK cells (73). Thus, PDT of primary tumors may enhance NK cell-mediated immunity to metastatic tumors. Systemic depletion of NK cells reduced PDT efficacy in EMT6 tumor model. However, splenic NK cells isolated from PDT treated mice were non-cytotoxic to EMT6 cells in vitro (74). This suggests an indirect mechanism of action of NK cells in PDT-induced anti-tumor immunity.
Activation of dendritic cells following PDT.
Dendritic cells (DCs) are professional antigen presenting cells (APCs) that activate adaptive immune cells by presenting antigens on their surface in the context of major histocompatibility complexes. Antigen presentation by immature DCs in the absence of co-stimulatory molecules generates tolerogenic T cell environment. However, activation/ maturation of DCs in an inflammatory setting allows increased expression of MHC class II and co-stimulatory molecules. Co-incubation of DCs with PDT-treated tumor cell lysates induce phenotypic and functional maturation of DCs (75, 76). DCs express PRRs that recognize DAMPS. Since tumor cells release or expose DAMPs during PDT-induced ICD, it is likely that signal transduction through ligand binding of PRRs facilitate DC activation after PDT. Indeed Wang et al reported that incubating DCs with ALA-PDT treated SCCVII cells in presence of blocking agents of DAMPs abrogated phenotypic and functional maturation of the DCs (77).
Activation of adaptive immunity by PDT
The need for an intact adaptive immune system for PDT efficacy is supported by multiple studies. PDT efficacy in scid mice bearing EMT6 tumors was improved by reconstitution with splenocytes derived from PDT-cured immunocompetent mice (74, 78). Transfer of T lymphocytes from immunocompetent naïve mice to EMT6 tumor bearing scid mice improved PDT efficacy in the immunocompromised mice, suggesting specific role of T cells in anti-tumor immunity after PDT. In addition, EMT6 tumor bearing nude mice, that lack only T lymphocytes respond significantly poorer to PDT than BALB/c mice (79, 80). Clinical studies have also shown that an intact immune system supports PDT efficacy (81).
The induction of adaptive immunity or antigen specific immune response upon PDT was first demonstrated in elegant studies performed by Canti et al (82). This group demonstrated that immunocompromised mice (scid) that were cured of MS-2 fibrosarcoma by PDT failed to resist re-challenge with the original tumor cells. On the contrary, immunocompetent tumor-bearing mice cured by PDT were resistant to re-challenge with the original tumor cells but not to challenge with unrelated tumor cells. These results demonstrate that PDT activates adaptive anti-tumor immunity and specific anti-tumor immune memory.
Adaptive immunity is broadly classified into Type1 and Type 2 immunity. Type 1 immunity is facilitated by CD4+ T cells that express cytokines such as IL-12 and IFN-γ and leads to activation of CD8+ T cells which have cytotoxic functions. Type 2 immunity, also considered to be tissue protective immunity, is skewed toward CD4+ T cells of the Th2 phenotype which express cytokines such as IL-4 and facilitate antibody production by B cells (83). Korbelik et al performed studies by depleting specific T cell populations from splenocytes of PDT-cured immunocompetent mice. Engraftment of these specific T cell depleted splenocytes in scid mice and subsequent PDT revealed critical role of CD8+ cytotoxic T lymphocytes (CTLs) in induction of anti-tumor immunity following PDT and a supportive role of CD4+ helper T lymphocytes (78). CD8+ T cell depletion followed by PDT resulted in reduced PDT efficacy, confirming the role of CTLs in PDT-induced anti-tumor immunity (74). Several studies have shown that PDT enhances the activation of tumor specific anti-tumor CD8+ T cells (84, 85, 62). Interestingly, depletion of CD4+ T cells inhibited tumor growth in both untreated and PDT-treated tumor bearing mice, which may be due to elimination of simultaneous depletion of regulatory T (Treg) cells; thus making it difficult to interpret the role of CD4+ T cells in adaptive immune response following PDT. To further dissect the involvement of T cells in anti-tumor immunity following PDT, Kabingu et al used a two-tumor model where BALB/c mice were inoculated with EMT6 cells on both shoulders. PDT of one tumor led to increased infiltration of CD8+ T cells but not CD4+ T cells into the untreated contralateral tumor. Using an experimental model of lung metastasis, Kabingu et al also showed that depletion of CD4+ T cells prior to PDT of the primary tumor had no effect on growth of existing lung tumors while depletion of CD8+ T cells prior to PDT of the primary tumor significantly increased the number of lung tumor nodules (73). These studies confirm the critical role of CD8+ T cells in the anti-tumor immune response following PDT and demonstrate a limited role of CD4+ T cells. In contrast, Preise et al reported delayed or reduced tumor growth in naïve mice when CD4 T cells were adoptively transferred from PDT-cured mice. These CD4+ T cells had increased expression of IFNγ upon restimulation, thereby confirming Th1 phenotype (86). Th17 cells, a subset of CD4+ T cells that produce the cytokine IL-17, have recently being categorized as components of Type 3 immunity; Th17 cells play important role in neutrophil activation (83). Brackett et al identified an increased number of Th17 cells in the TDLN after PDT and a critical role of IL-17 on the efficient recruitment of neutrophils to TDLNs after PDT (61). As already discussed, neutrophils facilitate accumulation of activated CD8+ T cells in the tumor tissue after PDT along with improved PDT efficacy (62). These results indicate PDT-mediated activation of a Type 3 immunity that leads to Type 1 immunity via inflammation characterized by neutrophilia. The mechanism of this switch is unclear but may be related to the inherent plasticity of Th17 cells (87).
Immunosuppressive role of PDT
Several studies have reported that in addition to immune activation, PDT can also promote immune suppression, which was first shown as reduced immune response to application of the hapten dinitroflurobenzene (DFNB) (88, 89). A subsequent study reported a transient increase in immunosuppressive Tregs in spleens and TDLNs of tumor bearing mice following PDT. This transient increase in Tregs might be a component of the homeostatic machinery needed to regulate immune activation following PDT (90).
Recently, Korbelik et al have demonstrated increase in granulocytic myeloid derived suppressor cells (MDSCs) or granulocytic myeloid regulatory cells (Mregs) following vaccination of tumor bearing mice with PDT treated tumor cells (91, 92). Since systemic neutrophilia immediately following PDT plays beneficial role in PDT efficacy, Korbelik et al studied the effect of immediate and delayed inhibition of Gr1+ cells by administering Gr1-depleting antibody immediately following PDT or 1 hour after PDT. Depletion of Gr1+ cells immediately after PDT reduced cure rate of SCCVII tumor bearing mice, in line with the established role of neutrophils in PDT-induced anti-tumor immunity. However, delayed depletion of Gr1+ cells also improved PDT efficacy, suggesting a delayed accumulation of MDSCs or Mregs replacing the initial neutrophilia.
Thus, combination of PDT with inhibitors of immune suppressive cells might improve PDT efficacy. Indeed studies have demonstrated improved cure rate when all trans retinoic acid (ATRA) that facilitates conversion of immune suppressive MDSCs to a non-suppressive mature phenotype is administered in the context of PDT (91). Similar effects were obtained with inhibitors of Tregs such as cyclophosphamide and CD25 depleting antibody (91, 90, 93, 94).
PDT increases expression of the inflammatory mediator Prostaglandin E2 by tumor cells and immune cells along with increased expression of Cycloxygenase 2 (COX2), an enzyme that catalyzes rate-limiting step of PGE2 pathway (95–98). It is well established that COX2/PGE2 expression by tumor cells facilitates tumor progression by affecting angiogenesis, proliferation of tumor cells and suppression of anti-tumor immunity (99–103). PGE2 reduces the effect of necrotic cells upon macrophage production of the anti-tumor cytokines such as TNFα and IFNγ. Thus, PGE2 is considered as an ‘inhibitory DAMP’ (104). Studies have shown improved PDT efficacy upon prolonged blocking of PGE2 synthesis pathway following PDT (97, 105).
Clinical evidence of PDT-enhanced anti-tumor immunity
The first indication for a role of anti-tumor immunity on clinical outcome of PDT was reported by Abdel-Hady et al when they showed that vulval intraepithelial neoplasia (VIN) patients who were non-responsive to ALA-PDT had higher likelihood of having MHC-I negative tumors and reduced CD8+ T cell accumulation in the treated tumor (81). Kabingu et al reported enhancement of anti-tumor immunity when PBMCs of BCC patients treated with PDT displayed increased tumor antigen recognition and cytokine production (106). Thong et al published a case study of 64 year old patient with multifocal angiosarcoma of the head and neck whose tumors regressed upon high dose brachytherapy but recurred within 1 year. Fotolon based PDT of the recurrent tumors resulted in spontaneous remission of the untreated tumors. Biopsy of these untreated tumors exhibited increased infiltration of CD8 T cells (107). In another report of a Phase I clinical trial involving patients with breast cancer progression following mastectomy and electron-beam radiation therapy, treatment with continuous low irradiance PDT (CLIPT) resulted in complete or partial response in 67% of patients (6 out of 9); 2 out of 9 patients demonstrated regression of distant tumors (108). This clinical effect of low-dose PDT on anti-tumor immunity is in line with pre-clinical studies showing that low dose PDT enhanced anti-tumor immunity more effectively than high dose PDT (5, 62). PDT was also shown to reduce immunosuppression, thRough reduction in Tregs, in invasive esophageal squamous cell carcinoma (ESCC) patients (8).
Potential of PDT as adjuvant for checkpoint blockade therapy
Immune checkpoint blockade has established itself as a promising cancer therapy in the recent years. One of the conditions for the success of this therapy is activation of anti-tumor immunity prior to checkpoint blockade. PDT has the potential to be an ideal immune adjuvant to checkpoint blockade therapy for two reasons: firstly, as stated in the previous sections, PDT regimens can be designed to activate anti-tumor immunity; secondly, PDT has limited off target effects due to preferential retention of PS in tumor cells and illumination within the tumor boundary.
Several pre-clinical and clinical studies have reported improved PDT efficacy upon combination with immune checkpoint inhibitors. In an orthotopic model of murine renal carcinoma, O’Shaghnessy et al demonstrated increased regression of primary tumors treated with vascular targeted PDT combined with anti-PD1 and anti-PD-L1 treatment. The combination treatment also prevented lung metastasis. Neither treatment alone was efficacious. Efficacy of the combination therapy was attributed to an increase in the ratio of CD8+ and CD4+ T cells to Tregs (109). Santos et al reported a case study of 62 year old patient with locally advanced SCC of mouth floor that was progressing after surgery, radiation therapy and cisplatin (RT/CT) but regressed following Redaporfin-PDT and anti-PD1 therapy (110). The success of PDT and checkpoint blockade combination therapy depends on the immune enhancement by PDT and not on the tumor ablative capacity of PDT. This is evident from study by Muchowicz et al where blocking of lymphatic regeneration after PDT by administration of inhibitory molecules impaired DC migration to TDLNs and intratumoral accumulation of tumor specific CD8+T cells (111).
Potential for PDT-induced ICD as cancer vaccines
Cancer vaccines involve introduction of lethally damaged cancer cells to tumor bearing hosts with the aim of activating tumor specific host immune response. Traditional methods of cancer vaccine generation relied on radiation-induced lethal damage to cancer cells. However, these vaccines are poorly immunogenic and requires adjuvants for robust immune response. PDT induction of ICD (discussed above) suggests that cancer cells treated by PDT may be excellent cancer vaccines. Gollnick et al generated cancer vaccines from Photofrin-PDT treated lysates of the murine mammary EMT6 and mastocytoma P815 cells. Administration of these lysates to syngeneic mice once a week for four weeks followed by inoculation of the EMT6 or P815 tumor cells revealed protective role of the cancer vaccines as evident by delayed tumor growth or abrogation of tumor establishment in these mice. Vaccines generated from PDT-treated tumor cells also imparted greater protection against tumor challenge than vaccines generated UV or IR treated tumor cells. PDT-generated cancer vaccines activated anti-tumor immunity by facilitating phenotypic and functional maturation of DCs and cytolytic activity of splenocytes (112). Korbelik et al generated SCCVII cancer vaccines by BPD-PDT followed by lethal irradiation. Introduction of these vaccines to SCCVII tumor bearing mice significantly delayed tumor growth and even resulted in cures. Vaccine treated mice had increased DCs, T cells, B cells in TDLNs along with higher numbers of memory T cells. Examination of PDT treated tumor cells isolated after vaccination showed that they were coated with complement protein C3. The importance of complement in effectiveness of the cancer vaccine-induced immune response was demonstrated by reduced efficacy upon complement blocking (55).
Recently Garg et al have described the success of DC vaccine generated upon co-culture of bone marrow derived DCs (BMDCs) with Hypericin-PDT treated murine glioma cell line GL261; hypericin-PDT induced ICD in GL261 cells. Administration of DCs co-cultured with PDT-treated tumor cells provided protective immunity against orthotopic implantation of parental cells in syngeneic mice. This protective immunity conferred by DC vaccine relied on CD8+ T cells, generation of ROS during ICD of the glioma cells and expression of DAMPs upon ICD. Vaccination also improved infiltration of Th1 and Th17 cells and reduction of Tregs in the brain. Th1 and Th17 are associated with improved prognosis in human glioma, thus bringing to light the clinical relevance of PDT-vaccines in glioma treatment (113). Zheng et al reported similar results when BMDCs were co-cultured with Hypericin-PDT treated Lewis Lung Carcinoma cells (LLC). They also demonstrated improved activation of tumor-specific T cells along with a reduction in Tregs (76).
Improving the future of PDT as immune therapy by superior targeting of PS to tumor
In recent years, several strategies for controlled and targeted delivery of PS to tumor have emerged, thus increasing specificity of photodamage to tumor cells. Notable among these are near-infrared photoimmunotherapy (NIR-PIT) (114–117) and nanoparticles (118–120).
NIR-PIT targets the PS IRDye700 to tumor tissue by conjugating it with monoclonal antibody to antigens that are widely expressed on tumor cells. Excitation of the PS is achieved by illumination with NIR light, which has higher tissue penetration capacity than most wavelengths used for PDT. NIR-PIT induces ICD with exposure of HSPs, ATP and HMGB1 that results in maturation of dendritic cells (121). Bao et al targeted IRDye700 to subcutaneous murine 4T1 tumor by utilizing a Fab fragment of antibody that binds to CD276, an antigen preferentially expressed on tumor cells. Although targeted NIR therapy reduced tumor regrowth, it significantly increased PD-L1 expression on tumor cells. Combination of CD276-targeted NIR-PIT and anti-PD-L1 treatment suppressed regrowth of the subcutaneous tumor and prevented lung metastasis by increasing intratumoral accumulation of CD8+T cells (114). Similar results were reported in another study in which IRDye700 was targeted to the integrin αvβ6, which is widely expressed on cancer cells (122). In a combination study with CD44-targeted NIR-PIT and anti-PD-1 administration in subcutaneous model of MC38 murine colon carcinoma, Nagaya et al demonstrated rejection of both treated primary tumor and untreated contralateral tumor, antigen specific T cell response and resistance to tumor establishment upon rechallenge (116).
PS specificity for tumors can also be increased using nanoparticles. Nanoparticles accumulate in tumor tissues due to the enhanced penetration and retention effect (EPR) that results from tumor vessel leakiness (118, 123, 119, 120). Song et al have reported development of nanoparticles containing PS conjugated to immune checkpoint inhibitors. These nanoparticles accumulate in tumors due to the EPR effect. Targeted delivery of PS and checkpoint inhibitor delayed of tumor regrowth, prevented lung metastasis and caused systemic increase of CD8+T cells (124). Hybrid nanoparticles that release PS and glucocorticoid-induced TNF receptor family-related protein/poly(lactic-co-glycolic acid) (GITR-PLGA) take advantage of the immune activating role of PDT and GITR-PLGA mediated inhibition of immunosuppression to enhance the number of anti-tumor CD8+ T cells in the tumor (109).
CONCLUSION
PDT is gaining popularity across US, Europe, Japan, China and other Asian nations due to its selectivity to tumors and multiple mechanisms of action including enhancement of protective anti-tumor immunity. Mechanisms by which PDT augments anti-tumor immunity are becoming increasingly clear as roles of immunogenic cell death, complement, inflammation and adaptive immunity are being delineated (Figure 1). Combination therapies that take advantage of immunostimulant role of PDT will pave way for successful implementation of PDT as curative therapy in clinical setting. Successful targeting of PS and immunostimulatory agents to tumor tissue by employing nanoparticle delivery methods is a great stride forward in improving efficacy of PDT.
Figure 1:
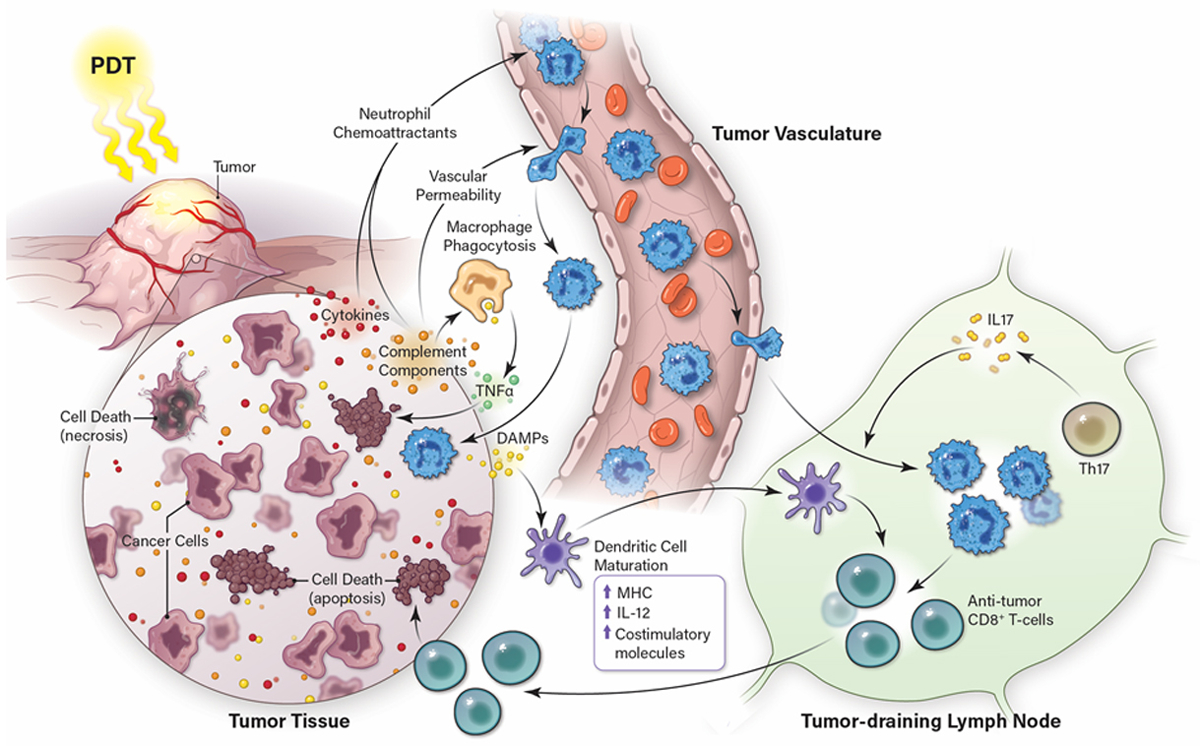
Induction of Anti-tumor Immunity by PDT. PDT of tumors causes immunogenic cell death that is characterized by the release of immune activating DAMPs and is accompanied by induction of acute inflammation that leads to IL-17 dependent neutrophil infiltration into the treated tumor bed and tumor draining lymph node. DAMPs stimulate dendritic cell maturation; mature dendritic cells work in concert with neutrophils to enhanced anti-tumor specific CD8+ T cell activation and increased tumor cell death.
ACKNOWLEDGEMENTS
This publication was supported in part by the National Cancer Institute of the National Institute of Health under Award 5P01CA98156 (S.O.G.) and the Roswell Park Alliance Foundation.
Footnotes
This article is part of a Special Issue dedicated to Dr. Thomas Dougherty.
REFERENCES:
- 1.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC and Golab J (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61, 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolmans DE, Kadambi A, Hill JS, Waters CA, Robinson BC, Walker JP, Fukumura D and Jain RK (2002) Vascular accumulation of a novel photosensitizer, MV6401, causes selective thrombosis in tumor vessels after photodynamic therapy. Cancer research 62, 2151–2156. [PubMed] [Google Scholar]
- 3.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J and Peng Q (1998) Photodynamic therapy. Journal of the National Cancer Institute 90, 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ochsner M (1997) Photophysical and photobiological processes in the photodynamic therapy of tumours. Journal of photochemistry and photobiology. B, Biology 39, 1–18. [DOI] [PubMed] [Google Scholar]
- 5.Henderson BW, Gollnick SO, Snyder JW, Busch TM, Kousis PC, Cheney RT and Morgan J (2004) Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer research 64, 2120–2126. [DOI] [PubMed] [Google Scholar]
- 6.Krammer B (2001) Vascular effects of photodynamic therapy. Anticancer research 21, 4271–4277. [PubMed] [Google Scholar]
- 7.Oleinick NL, Morris RL and Belichenko I (2002) The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 1, 1–21. [DOI] [PubMed] [Google Scholar]
- 8.Reginato E, Lindenmann J, Langner C, Schweintzger N, Bambach I, Smolle-Juttner F and Wolf P (2014) Photodynamic therapy downregulates the function of regulatory T cells in patients with esophageal squamous cell carcinoma. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 13, 1281–1289. [DOI] [PubMed] [Google Scholar]
- 9.Wachowska M, Muchowicz A and Demkow U (2015) Immunological aspects of antitumor photodynamic therapy outcome. Central-European journal of immunology 40, 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecic I and Korbelik M (2002) Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer letters 183, 43–51. [DOI] [PubMed] [Google Scholar]
- 11.Gollnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, Vaughan L, Wang WC, Unger E and Henderson BW (2003) Role of cytokines in photodynamic therapy-induced local and systemic inflammation. British journal of cancer 88, 1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korbelik M, Merchant S, Stott B, Cecic I, Payne P and Sun J (2006) Acute phase response induced following tumor treatment by photodynamic therapy: relevance for the therapy outcome. SPIE. [Google Scholar]
- 13.Lipson RL, Baldes EJ and Gray MJ (1967) Hematoporphyrin derivative for detection and management of cancer. Cancer 20, 2255–2257. [DOI] [PubMed] [Google Scholar]
- 14.McBride G (2002) Studies expand potential uses of photodynamic therapy. Journal of the National Cancer Institute 94, 1740–1742. [DOI] [PubMed] [Google Scholar]
- 15.Nowis D, Makowski M, Stoklosa T, Legat M, Issat T and Golab J (2005) Direct tumor damage mechanisms of photodynamic therapy. Acta biochimica Polonica 52, 339–352. [PubMed] [Google Scholar]
- 16.Gollnick SO and Brackett CM (2010) Enhancement of anti-tumor immunity by photodynamic therapy. Immunologic research 46, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch TM, Hahn SM, Evans SM and Koch CJ (2000) Depletion of tumor oxygenation during photodynamic therapy: detection by the hypoxia marker EF3 [2-(2-nitroimidazol-1[H]-yl)-N-(3,3,3-trifluoropropyl)acetamide]. Cancer research 60, 2636–2642. [PubMed] [Google Scholar]
- 18.Foster TH, Hartley DF, Nichols MG and Hilf R (1993) Fluence rate effects in photodynamic therapy of multicell tumor spheroids. Cancer research 53, 1249–1254. [PubMed] [Google Scholar]
- 19.Henderson BW, Busch TM, Vaughan LA, Frawley NP, Babich D, Sosa TA, Zollo JD, Dee AS, Cooper MT, Bellnier DA, Greco WR and Oseroff AR (2000) Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer research 60, 525–529. [PubMed] [Google Scholar]
- 20.Zilberstein J, Bromberg A, Frantz A, Rosenbach-Belkin V, Kritzmann A, Pfefermann R, Salomon Y and Scherz A (1997) Light-dependent oxygen consumption in bacteriochlorophyll-serine-treated melanoma tumors: on-line determination using a tissue-inserted oxygen microsensor. Photochemistry and photobiology 65, 1012–1019. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal ML, Clay ME, Harvey EJ, Evans HH, Antunez AR and Oleinick NL (1991) Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer research 51, 5993–5996. [PubMed] [Google Scholar]
- 22.Azad MB, Chen Y and Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxidants & redox signaling 11, 777–790. [DOI] [PubMed] [Google Scholar]
- 23.Chiu S, Evans HH, Lam M, Nieminen A and Oleinick NL (2001) Phthalocyanine 4 photodynamic therapy-induced apoptosis of mouse L5178Y-R cells results from a delayed but extensive release of cytochrome c from mitochondria. Cancer letters 165, 51–58. [DOI] [PubMed] [Google Scholar]
- 24.Dewaele M, Martinet W, Rubio N, Verfaillie T, de Witte PA, Piette J and Agostinis P (2011) Autophagy pathways activated in response to PDT contribute to cell resistance against ROS damage. Journal of cellular and molecular medicine 15, 1402–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessel D (2019) Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochemistry and photobiology 95, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessel D and Oleinick NL (2010) Photodynamic therapy and cell death pathways. Methods in molecular biology (Clifton, N.J.) 635, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessel D and Oleinick NL (2018) Cell Death Pathways Associated with Photodynamic Therapy: An Update. Photochemistry and photobiology 94, 213–218. [DOI] [PubMed] [Google Scholar]
- 28.Kessel D and Reiners JJ Jr. (2017) Effects of Combined Lysosomal and Mitochondrial Photodamage in a Non-small-Cell Lung Cancer Cell Line: The Role of Paraptosis. Photochemistry and photobiology 93, 1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HR, Luo Y, Li G and Kessel D (1999) Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer research 59, 3429–3432. [PMC free article] [PubMed] [Google Scholar]
- 30.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L and Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. The EMBO journal 26, 1749–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varnes ME, Chiu SM, Xue LY and Oleinick NL (1999) Photodynamic therapy-induced apoptosis in lymphoma cells: translocation of cytochrome c causes inhibition of respiration as well as caspase activation. Biochemical and biophysical research communications 255, 673–679. [DOI] [PubMed] [Google Scholar]
- 32.Xue LY, Chiu SM and Oleinick NL (2001) Photochemical destruction of the Bcl-2 oncoprotein during photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene 20, 3420–3427. [DOI] [PubMed] [Google Scholar]
- 33.Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology 81, 1–5. [DOI] [PubMed] [Google Scholar]
- 34.Garg AD, Nowis D, Golab J and Agostinis P (2010) Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis : an international journal on programmed cell death 15, 1050–1071. [DOI] [PubMed] [Google Scholar]
- 35.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV and Agostinis P (2010) Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochimica et biophysica acta 1805, 53–71. [DOI] [PubMed] [Google Scholar]
- 36.Kroemer G, Galluzzi L, Kepp O and Zitvogel L (2013) Immunogenic cell death in cancer therapy. Annual review of immunology 31, 51–72. [DOI] [PubMed] [Google Scholar]
- 37.Xue LY, Chiu SM, Fiebig A, Andrews DW and Oleinick NL (2003) Photodamage to multiple Bcl-xL isoforms by photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene 22, 9197–9204. [DOI] [PubMed] [Google Scholar]
- 38.Chiu SM, Xue LY, Lam M, Rodriguez ME, Zhang P, Kenney ME, Nieminen AL and Oleinick NL (2010) A requirement for bid for induction of apoptosis by photodynamic therapy with a lysosome- but not a mitochondrion-targeted photosensitizer. Photochemistry and photobiology 86, 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiners JJ Jr., Caruso JA, Mathieu P, Chelladurai B, Yin XM and Kessel D (2002) Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell death and differentiation 9, 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan Q, Liu L, Xing D and Chen Q (2008) Bid is required in NPe6-PDT-induced apoptosis. Photochemistry and photobiology 84, 250–257. [DOI] [PubMed] [Google Scholar]
- 41.Gamrekelashvili J, Ormandy LA, Heimesaat MM, Kirschning CJ, Manns MP, Korangy F and Greten TF (2012) Primary sterile necrotic cells fail to cross-prime CD8(+) T cells. Oncoimmunology 1, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J and Wainstok R (2003) Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. Journal of immunology (Baltimore, Md. : 1950) 171, 5940–5947. [DOI] [PubMed] [Google Scholar]
- 43.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, Manns MP and Greten TF (2003) Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. International journal of cancer 103, 205–211. [DOI] [PubMed] [Google Scholar]
- 44.Schnurr M, Scholz C, Rothenfusser S, Galambos P, Dauer M, Robe J, Endres S and Eigler A (2002) Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer research 62, 2347–2352. [PubMed] [Google Scholar]
- 45.Korbelik M, Sun J and Cecic I (2005) Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer research 65, 1018–1026. [PubMed] [Google Scholar]
- 46.Korbelik M, Zhang W and Merchant S (2011) Involvement of damage-associated molecular patterns in tumor response to photodynamic therapy: surface expression of calreticulin and high-mobility group box-1 release. Cancer immunology, immunotherapy : CII 60, 1431–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, Annaert W, Golab J, de Witte P, Vandenabeele P and Agostinis P (2012) A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. The EMBO journal 31, 1062–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jalili A, Makowski M, Switaj T, Nowis D, Wilczynski GM, Wilczek E, Chorazy-Massalska M, Radzikowska A, Maslinski W, Bialy L, Sienko J, Sieron A, Adamek M, Basak G, Mroz P, Krasnodebski IW, Jakobisiak M and Golab J (2004) Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells . Clinical cancer research : an official journal of theAmerican Association for Cancer Research 10, 4498–4508. [DOI] [PubMed] [Google Scholar]
- 49.Deretic V, Saitoh T and Akira S (2013) Autophagy in infection, inflammation and immunity. Nature reviews. Immunology 13, 722–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamb CA, Yoshimori T and Tooze SA (2013) The autophagosome: origins unknown, biogenesis complex. Nature reviews. Molecular cell biology 14, 759–774. [DOI] [PubMed] [Google Scholar]
- 51.Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC and Kroemer G (2017) Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nature reviews. Clinical oncology 14, 247–258. [DOI] [PubMed] [Google Scholar]
- 52.Kessel D and Oleinick NL (2009) Initiation of autophagy by photodynamic therapy. Methods in enzymology 453, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korbelik M (2015) Impact of cell death manipulation on the efficacy of photodynamic therapy-generated cancer vaccines. World journal of immunology 5, 95–98. [Google Scholar]
- 54.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L and Kroemer G (2011) Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science (New York, N.Y.) 334, 1573–1577. [DOI] [PubMed] [Google Scholar]
- 55.Korbelik M and Sun J (2006) Photodynamic therapy-generated vaccine for cancer therapy. Cancer immunology, immunotherapy : CII 55, 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merchant S and Korbelik M (2013) Upregulation of genes for C-reactive protein and related pentraxin/complement proteins in photodynamic therapy-treated human tumor cells: enrolment of PI3K/Akt and AP-1. Immunobiology 218, 869–874. [DOI] [PubMed] [Google Scholar]
- 57.Evans S, Matthews W, Perry R, Fraker D, Norton J and Pass HI (1990) Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. Journal of the National Cancer Institute 82, 34–39. [DOI] [PubMed] [Google Scholar]
- 58.Gollnick SO, Liu X, Owczarczak B, Musser DA and Henderson BW (1997) Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer research 57, 3904–3909. [PubMed] [Google Scholar]
- 59.Kick G, Messer G, Goetz A, Plewig G and Kind P (1995) Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-kappa B DNA binding. Cancer research 55, 2373–2379. [PubMed] [Google Scholar]
- 60.Nseyo UO, Whalen RK, Duncan MR, Berman B and Lundahl SL (1990) Urinary cytokines following photodynamic therapy for bladder cancer. A preliminary report. Urology 36, 167–171. [DOI] [PubMed] [Google Scholar]
- 61.Brackett CM, Muhitch JB, Evans SS and Gollnick SO (2013) IL-17 promotes neutrophil entry into tumor-draining lymph nodes following induction of sterile inflammation. Journal of immunology (Baltimore, Md. : 1950) 191, 4348–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kousis PC, Henderson BW, Maier PG and Gollnick SO (2007) Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer research 67, 10501–10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Vree WJ, Essers MC, de Bruijn HS, Star WM, Koster JF and Sluiter W (1996) Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer research 56, 2908–2911. [PubMed] [Google Scholar]
- 64.Sluiter W, de Vree WJ, Pietersma A and Koster JF (1996) Prevention of late lumen loss after coronary angioplasty by photodynamic therapy: role of activated neutrophils. Molecular and cellular biochemistry 157, 233–238. [DOI] [PubMed] [Google Scholar]
- 65.Sun J, Cecic I, Parkins CS and Korbelik M (2002) Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 1, 690–695. [DOI] [PubMed] [Google Scholar]
- 66.Cecic I, Sun J and Korbelik M (2006) Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of host neutrophils and other immune cells. Photochemistry and photobiology 82, 558–562. [DOI] [PubMed] [Google Scholar]
- 67.Gennaro R, Simonic T, Negri A, Mottola C, Secchi C, Ronchi S and Romeo D (1986) C5a fragment of bovine complement. Purification, bioassays, amino-acid sequence and other structural studies. European journal of biochemistry 155, 77–86. [DOI] [PubMed] [Google Scholar]
- 68.Korbelik M, Cecic I, Merchant S and Sun J (2008) Acute phase response induction by cancer treatment with photodynamic therapy. International journal of cancer 122, 1411–1417. [DOI] [PubMed] [Google Scholar]
- 69.Cecic I, Parkins CS and Korbelik M (2001) Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochemistry and photobiology 74, 712–720. [DOI] [PubMed] [Google Scholar]
- 70.Veglia F, Perego M and Gabrilovich D (2018) Myeloid-derived suppressor cells coming of age. Nat Immunol 19, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belicha-Villanueva A, Riddell J, Bangia N and Gollnick SO (2012) The effect of photodynamic therapy on tumor cell expression of major histocompatibility complex (MHC) class I and MHC class I-related molecules. Lasers in surgery and medicine 44, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park MJ, Bae JH, Chung JS, Kim SH and Kang CD (2011) Induction of NKG2D ligands and increased sensitivity of tumor cells to NK cell-mediated cytotoxicity by hematoporphyrin-based photodynamic therapy. Immunological investigations 40, 367–382. [DOI] [PubMed] [Google Scholar]
- 73.Kabingu E, Vaughan L, Owczarczak B, Ramsey KD and Gollnick SO (2007) CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. British journal of cancer 96, 1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hendrzak-Henion JA, Knisely TL, Cincotta L, Cincotta E and Cincotta AH (1999) Role of the immune system in mediating the antitumor effect of benzophenothiazine photodynamic therapy. Photochemistry and photobiology 69, 575–581. [PubMed] [Google Scholar]
- 75.Kushibiki T, Tajiri T, Tomioka Y and Awazu K (2010) Photodynamic therapy induces interleukin secretion from dendritic cells. Int J Clin Exp Med 3, 110–114. [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng Y, Yin G, Le V, Zhang A, Chen S, Liang X and Liu J (2016) Photodynamic-therapy Activates Immune Response by disrupting Immunity Homeostasis of Tumor Cells, which Generates Vaccine for Cancer Therapy. International journal of biological sciences 12, 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang X, Ji J, Zhang H, Fan Z, Zhang L, Shi L, Zhou F, Chen WR, Wang H and Wang X (2015) Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget 6, 44688–44702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Korbelik M and Dougherty GJ (1999) Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer research 59, 1941–1946. [PubMed] [Google Scholar]
- 79.Korbelik M, Krosl G, Krosl J and Dougherty GJ (1996) The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer research 56, 5647–5652. [PubMed] [Google Scholar]
- 80.Rocha LB, Gomes-da-Silva LC, Dabrowski JM and Arnaut LG (2015) Elimination of primary tumours and control of metastasis with rationally designed bacteriochlorin photodynamic therapy regimens. European journal of cancer (Oxford, England : 1990) 51, 1822–1830. [DOI] [PubMed] [Google Scholar]
- 81.Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, Stern PL, Moore JV, Corbitt G, Kitchener HC and Hampson IN (2001) Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer research 61, 192–196. [PubMed] [Google Scholar]
- 82.Canti GL, Lattuada D, Nicolin A, Taroni P, Valentini G and Cubeddu R (1994) Immunopharmacology studies on photosensitizers used in photodynamic therapy. SPIE. [Google Scholar]
- 83.Yamaguchi T, Takizawa F, Fischer U and Dijkstra JM (2015) Along the Axis between Type 1 and Type 2 Immunity; Principles Conserved in Evolution from Fish to Mammals. Biology 4, 814–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mroz P, Szokalska A, Wu MX and Hamblin MR (2010) Photodynamic therapy of tumors can lead to development of systemic antigen-specific immune response. PloS one 5, e15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mroz P, Vatansever F, Muchowicz A and Hamblin MR (2013) Photodynamic therapy of murine mastocytoma induces specific immune responses against the cancer/testis antigen P1A. Cancer research 73, 6462–6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Preise D, Oren R, Glinert I, Kalchenko V, Jung S, Scherz A and Salomon Y (2009) Systemic antitumor protection by vascular-targeted photodynamic therapy involves cellular and humoral immunity. Cancer immunology, immunotherapy : CII 58, 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazzoni A, Maggi L, Liotta F, Cosmi L and Annunziato F (2019) Biological and clinical significance of T helper 17 cell plasticity. Immunology 158, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reddan JC, Anderson CY, Xu H, Hrabovsky S, Freye K, Fairchild R, Tubesing KA and Elmets CA (1999) Immunosuppressive Effects of Silicon Phthalocyanine Photodynamic Therapy. Photochemistry and photobiology 70, 72–77. [PubMed] [Google Scholar]
- 89.Lynch DH, Haddad S, King VJ, Ott MJ, Straight RC and Jolles CJ (1989) SYSTEMIC IMMUNOSUPPRESSION INDUCED BY PHOTODYNAMIC THERAPY (PDT) IS ADOPTIVELY TRANSFERRED BY MACROPHAGES. Photochemistry and photobiology 49, 453–458. [DOI] [PubMed] [Google Scholar]
- 90.Reginato E, Mroz P, Chung H, Kawakubo M, Wolf P and Hamblin MR (2013) Photodynamic therapy plus regulatory T-cell depletion produces immunity against a mouse tumour that expresses a self-antigen. British journal of cancer 109, 2167–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Korbelik M, Banáth J and Saw KM (2015) Immunoregulatory Cell Depletion Improves the Efficacy of Photodynamic Therapy-Generated Cancer Vaccines. Int J Mol Sci 16, 27005–27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Korbelik M, Banath J and Zhang W (2016) Mreg Activity in Tumor Response to Photodynamic Therapy and Photodynamic Therapy-Generated Cancer Vaccines. Cancers 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oh DS, Kim H, Oh JE, Jung HE, Lee YS, Park JH and Lee HK (2017) Intratumoral depletion of regulatory T cells using CD25-targeted photodynamic therapy in a mouse melanoma model induces antitumoral immune responses. Oncotarget 8, 47440–47453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castano AP, Mroz P, Wu MX and Hamblin MR (2008) Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proceedings of the National Academy of Sciences of the United States of America 105, 5495–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henderson BW and Donovan JM (1989) Release of prostaglandin E2 from cells by photodynamic treatment in vitro. Cancer research 49, 6896–6900. [PubMed] [Google Scholar]
- 96.Hendrickx N, Volanti C, Moens U, Seternes OM, de Witte P, Vandenheede JR, Piette J and Agostinis P (2003) Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38 MAPK in hypericin-mediated photodynamic therapy of human cancer cells. The Journal of biological chemistry 278, 52231–52239. [DOI] [PubMed] [Google Scholar]
- 97.Ferrario A, Von Tiehl K, Wong S, Luna M and Gomer CJ (2002) Cyclooxygenase-2 inhibitor treatment enhances photodynamic therapy-mediated tumor response. Cancer research 62, 3956–3961. [PubMed] [Google Scholar]
- 98.Song J, Wei Y, Chen Q and Xing D (2014) Cyclooxygenase 2-mediated apoptotic and inflammatory responses in photodynamic therapy treated breast adenocarcinoma cells and xenografts. Journal of photochemistry and photobiology. B, Biology 134, 27–36. [DOI] [PubMed] [Google Scholar]
- 99.Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E and Reis e Sousa C (2015) Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 162, 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ke J, Yang Y, Che Q, Jiang F, Wang H, Chen Z, Zhu M, Tong H, Zhang H, Yan X, Wang X, Wang F, Liu Y, Dai C and Wan X (2016) Prostaglandin E2 (PGE2) promotes proliferation and invasion by enhancing SUMO-1 activity via EP4 receptor in endometrial cancer. Tumour Biol 37, 12203–12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawamori T, Uchiya N, Sugimura T and Wakabayashi K (2003) Enhancement of colon carcinogenesis by prostaglandin E2 administration. Carcinogenesis 24, 985–990. [DOI] [PubMed] [Google Scholar]
- 102.Kamiyama M, Pozzi A, Yang L, DeBusk LM, Breyer RM and Lin PC (2006) EP2, a receptor for PGE2, regulates tumor angiogenesis through direct effects on endothelial cell motility and survival. Oncogene 25, 7019–7028. [DOI] [PubMed] [Google Scholar]
- 103.Jain S, Chakraborty G, Raja R, Kale S and Kundu GC (2008) Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer research 68, 7750–7759. [DOI] [PubMed] [Google Scholar]
- 104.Hangai S, Ao T, Kimura Y, Matsuki K, Kawamura T, Negishi H, Nishio J, Kodama T, Taniguchi T and Yanai H (2016) PGE2 induced in and released by dying cells functions as an inhibitory DAMP. Proceedings of the National Academy of Sciences of the United States of America 113, 3844–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Makowski M, Grzela T, Niderla J, L. A. M, Mroz P, Kopee M, Legat M, Strusinska K, Koziak K, Nowis D, Mrowka P, Wasik M, Jakobisiak M and Golab J (2003) Inhibition of cyclooxygenase-2 indirectly potentiates antitumor effects of photodynamic therapy in mice. Clinical cancer research : an official journal of the American Association for Cancer Research 9, 5417–5422. [PubMed] [Google Scholar]
- 106.Kabingu E, Oseroff AR, Wilding GE and Gollnick SO (2009) Enhanced systemic immune reactivity to a Basal cell carcinoma associated antigen following photodynamic therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 15, 4460–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thong PS, Ong KW, Goh NS, Kho KW, Manivasager V, Bhuvaneswari R, Olivo M and Soo KC (2007) Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. The Lancet. Oncology 8, 950–952. [DOI] [PubMed] [Google Scholar]
- 108.Morrison SA, Hill SL, Rogers GS and Graham RA (2014) Efficacy and safety of continuous low-irradiance photodynamic therapy in the treatment of chest wall progression of breast cancer. The Journal of surgical research 192, 235–241. [DOI] [PubMed] [Google Scholar]
- 109.O’Shaughnessy MJ, Murray KS, La Rosa SP, Budhu S, Merghoub T, Somma A, Monette S, Kim K, Corradi RB, Scherz A and Coleman JA (2018) Systemic Antitumor Immunity by PD-1/PD-L1 Inhibition Is Potentiated by Vascular-Targeted Photodynamic Therapy of Primary Tumors. Clinical cancer research : an official journal of the American Association for Cancer Research 24, 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santos LL, Oliveira J, Monteiro E, Santos J and Sarmento C (2018) Treatment of Head and Neck Cancer with Photodynamic Therapy with Redaporfin: A Clinical Case Report. Case Rep Oncol 11, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muchowicz A, Wachowska M, Stachura J, Tonecka K, Gabrysiak M, Wolosz D, Pilch Z, Kilarski WW, Boon L, Klaus TJ and Golab J (2017) Inhibition of lymphangiogenesis impairs antitumour effects of photodynamic therapy and checkpoint inhibitors in mice. European journal of cancer (Oxford, England : 1990) 83, 19–27. [DOI] [PubMed] [Google Scholar]
- 112.Gollnick SO, Vaughan L and Henderson BW (2002) Generation of effective antitumor vaccines using photodynamic therapy. Cancer research 62, 1604–1608. [PubMed] [Google Scholar]
- 113.Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW and Agostinis P (2016) Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Science translational medicine 8, 328ra327. [DOI] [PubMed] [Google Scholar]
- 114.Bao R, Wang Y, Lai J, Zhu H, Zhao Y, Li S, Li N, Huang J, Yang Z, Wang F and Liu Z (2019) Enhancing Anti-PD-1/PD-L1 Immune Checkpoint Inhibitory Cancer Therapy by CD276-Targeted Photodynamic Ablation of Tumor Cells and Tumor Vasculature. Molecular pharmaceutics 16, 339–348. [DOI] [PubMed] [Google Scholar]
- 115.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL and Kobayashi H (2011) Cancer cell–selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nature Medicine 17, 1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nagaya T, Friedman J, Maruoka Y, Ogata F, Okuyama S, Clavijo PE, Choyke PL, Allen C and Kobayashi H (2019) Host Immunity Following Near-Infrared Photoimmunotherapy Is Enhanced with PD-1 Checkpoint Blockade to Eradicate Established Antigenic Tumors. Cancer Immunology Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sato K, Nagaya T, Choyke PL and Kobayashi H (2015) Near infrared photoimmunotherapy in the treatment of pleural disseminated NSCLC: preclinical experience. Theranostics 5, 698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grimland JL, Wu C, Ramoutar RR, Brumaghim JL and McNeill J (2011) Photosensitizer-doped conjugated polymer nanoparticles with high cross-sections for one- and two-photon excitation. Nanoscale 3, 1451–1455. [DOI] [PubMed] [Google Scholar]
- 119.Kim H, Mun S and Choi Y (2013) Photosensitizer-conjugated polymeric nanoparticles for redox-responsive fluorescence imaging and photodynamic therapy. Journal of Materials Chemistry B 1, 429–431. [DOI] [PubMed] [Google Scholar]
- 120.Yang Y, Hu Y and Wang H (2016) Targeting Antitumor Immune Response for Enhancing the Efficacy of Photodynamic Therapy of Cancer: Recent Advances and Future Perspectives. Oxidative medicine and cellular longevity 2016, 5274084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ogawa M, Tomita Y, Nakamura Y, Lee M-J, Lee S, Tomita S, Nagaya T, Sato K, Yamauchi T, Iwai H, Kumar A, Haystead T, Shroff H, Choyke PL, Trepel JB and Kobayashi H (2017) Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 8, 10425–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao L, Zhang C, Gao D, Liu H, Yu X, Lai J, Wang F, Lin J and Liu Z (2016) Enhanced Anti-Tumor Efficacy through a Combination of Integrin alphavbeta6-Targeted Photodynamic Therapy and Immune Checkpoint Inhibition. Theranostics 6, 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, Jain RK and McDonald DM (2000) Openings between defective endothelial cells explain tumor vessel leakiness. The American journal of pathology 156, 1363–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song W, Kuang J, Li CX, Zhang M, Zheng D, Zeng X, Liu C and Zhang XZ (2018) Enhanced Immunotherapy Based on Photodynamic Therapy for Both Primary and Lung Metastasis Tumor Eradication. ACS nano 12, 1978–1989. [DOI] [PubMed] [Google Scholar]


