Abstract
Background.
Among women diagnosed with non-endometrioid endometrial carcinoma (EC), we investigated associations between race/ethnicity and receipt of guideline-concordant treatment (GCT), as well as relationships between GCT and survival.
Methods.
We used the National Cancer Database and identified 21,177 non-Hispanic White (NHW), 6657 non-Hispanic Black (NHB), 1689 Hispanic, and 903 Asian/Pacific Islander (AS/PI) women diagnosed with non-endometrioid EC between 2004 and 2014. Year-specific National Comprehensive Cancer Network (NCCN) guidelines were used to classify GCT. We used multivariable logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between race/ethnicity and GCT receipt. Multivariable-adjusted Cox proportional hazards models were used to estimate hazards ratios (HRs) and 95% CIs for relationships between GCT and overall survival in the total study population and stratified by race/ethnicity.
Results.
Overall, 43.8% of women with non-endometrioid EC received GCT. Compared to NHW women, NHB (OR = 1.01, 95% CI = 0.95–1.07), Hispanic (OR = 1.01, 95% CI = 0.91–1.12) and AS/PI women (OR = 1.10, 95% CI = 0.96–1.26) did not have significantly different odds of receiving GCT. GCT was significantly associated with improved survival among NHW (HR = 0.84, 95% CI = 0.80–0.87), NHB (HR = 0.85, 95% CI = 0.80–0.91), and Hispanic women (HR = 0.84, 95% CI = 0.72–0.98) but not among AS/PI women (HR = 0.97, 95% CI = 0.78–1.19).
Conclusions.
While more than half of women with non-endometrioid EC did not receive GCT, no difference in GCT receipt by race/ethnicity was observed. When received, GCT was associated with improved survival in almost all racial groups. Interventions to improve GCT adherence may improve survival for most women with non-endometrioid EC.
Keywords: Uterus neoplasm, Radiation treatment, Chemotherapy, Race, Disparities, Hospital-based cancer registry
1. Introduction
Uterine cancer is the fourth most common cancer in U.S. women and the most common gynecologic malignancy, with increasing age-adjusted incidence rates observed for women of all race/ethnic groups [1]. Approximately 90% of uterine cancers are endometrial carcinoma (EC), which encompass a heterogeneous group of tumors characterized by differences in presentation, etiology, and outcomes [2]. The majority of ECs are endometrioid, with more common presentation among White women, hormonally driven etiology, and generally favorable prognosis [2]. On the other hand, non-endometrioid tumors, which include serous, clear cell, and carcinosarcomas, are more commonly diagnosed among Black women, with an unclear etiology, and unfavorable survival [3–5]. Even within these broad histological classifications, Black women have worse prognosis than White women [1].
Differences in treatment represent a potential mechanism that may underlie racial disparities in EC; however, inconsistencies have been observed. For example, some report that non-Hispanic Black (NHB) women undergo surgery less frequently than non-Hispanic White (NHW) women, while others report no difference in primary surgical management [6–8]. Some studies have observed that Hispanic women with deeply-invasive or high-grade disease may undergo lymphadenectomy less frequently than their NHW counterparts; others have not observed these differences [9–11]. A recent NRG Oncology/GOG analysis reported increased odds of receiving radiation plus chemotherapy among NHB women compared to NHW women, even after stratification according to tumor characteristics [6]. A Surveillance, Epidemiology, End Results (SEER) database analysis found that a larger proportion of Hispanic women underwent no treatment at all, when compared to their NHW counterparts [11]. Among women who did undergo treatment, Hispanic women were more likely to receive chemotherapy and less likely to receive surgery than NHW women [6,11]. Although these disparities persisted after accounting for mediating factors such as income and education, these studies are limited in that they evaluate single components of a treatment plan (i.e. surgery, radiation, etc.) individually, as opposed to the complete course of treatment as a unit, within the context of established guidelines [12].
Guideline-concordant treatment (GCT) for EC can be determined using National Comprehensive Cancer Network Practice Guidelines, which are evidence-based recommendations developed by expert consensus and are recognized as the standard of care. These guidelines specify the total course of treatment (surgery with or without radiation and/or chemotherapy) based on tumor characteristics. In a retrospective cohort of 629 EC survivors enrolled in the Women’s Health Initiative (WHI), no racial disparities in GCT receipt were observed, but the authors recognized this was likely a result of low numbers of non-White EC cases. In addition, neither the WHI study nor any prior study has focused explicitly on women with non-endometrioid EC, which is of particular interest given high mortality associated with this subtype and the disproportionate occurrence among minority women [1]. We hypothesized GCT receipt would be lower among minority as compared with White women with non-endometrioid EC and that GCT would be associated with improved survival, potentially indicating an intervenable pathway that may reduce disparate outcomes for women with this malignancy.
2. Methods
2.1. Data source
Data were obtained from the National Cancer Database (NCDB), a hospital-based cancer registry containing data from over 1400 facilities accredited by the American College of Surgeons’ Commission on Cancer (CoC.) This database includes approximately 70% of all cancer cases diagnosed in the United States. We specifically used the publicly shared subset of the NCDB dataset known as the Participant User Files (PUF) (version 2015). All variables, including information on sociodemographic characteristics, tumor characteristics, hospital attributes, insurance status, and treatment components including surgery, radiation, chemotherapy, and immunotherapy, were captured using standardized codes defined by the Facility Oncology Registry Data Standards (FORDS) [13]. All data are de-identified, and the study was considered exempt by the Ohio State University Institutional Review Board (IRB).
2.2. Study population
Between 2004 and 2014, 443,680 uterine cancers were recorded in the NCDB, of which 57,472 women were diagnosed with invasive non-endometrioid EC [International Classification of Diseases (ICD)-10 morphology codes for carcinosarcoma (8950, 8951, 8980, 8981, 8982), clear cell (8310), and serous (8441, 8460, 8461)]. We excluded women with missing values for stage (n = 12,814), missing vital status (n = 5469), and missing follow-up time (n = 16) or 0 days of follow-up time (n = 14).
To classify GCT, non-missing data on surgical and adjuvant treatment are needed. We therefore excluded women with missing information on surgery (n = 2689), chemotherapy (n = 132), hormone therapy (n = 734), radiation treatment (n = 369), or multiple treatment types (n = 402). An additional 919 women whose GCT status could not be classified due to missing substage (if NCCN treatment guidelines required substage to be known) and 209 women who had a discordant treatment sequence (e.g. radiation preceded surgery) were also excluded, as the database did not collect information regarding the context and clinical indication for these scenarios. Finally, for our main exposure variable, race/ethnicity, we restricted the analysis to NHW (n = 21,177), NHB (n = 6657), Hispanic (n = 1689), or Asian/Pacific Islander (AS/PI, n = 903), for a total of 30,426 women in the analytic cohort.
2.3. Guideline concordant treatment
GCT receipt was defined using the NCCN Clinical Practice Guidelines for EC. The guidelines recommend various regimens of surgery (total hysterectomy with bilateral salpingo-oophorectomy), chemotherapy, and radiation (vaginal brachytherapy and pelvic radiation) as appropriate treatments for non-endometrioid EC. GCT receipt was defined by year, due to periodic revisions of the NCCN guidelines. If a patient’s received treatment did not match the expected treatment options based on clinical characteristics, the patient was coded as non-concordant. For example, the 2014 NCCN Clinical Practice Guidelines recommend that a woman diagnosed with serous EC have primary surgical treatment, with adjuvant systemic chemotherapy and/or radiation depending on pathologic stage [14]. Supplemental Table 1 shows the specific combinations of treatment that qualified as GCT according to year of diagnosis, histology, and stage.
2.4. Vital status
Vital status was collected in 5-year cycles. No information on cause of death is available in the NCDB; therefore, all-cause mortality was computed as the time between diagnosis and date of death from any cause (among women who died) or last date of contact (among women alive at the end of follow-up).
2.5. Covariates
Epidemiological, tumor, and hospital characteristics were categorized as follows: race/ethnicity (NHW, NHB, Hispanic, AS/PI); age at diagnosis (18–44, 45–54, 55–64, 65–74, ≥75 years); stage at diagnosis as defined by the 7th Edition American Joint Commission on Cancer (AJCC) pathologic stage (I, IA, IB, IC, II, IIA, IIB, III, IIIA, IIIB, IIIC, IV, IVA, IVB); histology (carcinosarcoma, clear cell, serous); Charlson/Deyo comorbidity index (0, 1, 2, and ≥3); hospital location (Northeast, South, Midwest, Mountain, Pacific); urban/rural status as defined by the Federal Information Processing System (metro, urban, rural); education quartiles based on the proportion of the population in a particular zip code that did not receive a high school diploma compared to national distributions derived from the 2012 American Community Survey data (≥21%, 13%–20.9%, 7%–12.9%, <7%); household income quartiles based on patients’ zip code residencies compared to national distributions derived from the 2012 American Community Survey data (≤$38,000, $38,000–$47,999, $48,000–$62,999, ≥$63,000); insurance status at time of diagnosis (private, Medicaid, Medicare, other government insurance, uninsured); and hospital facility type (community cancer, comprehensive community cancer, academic/research, integrated network cancer, unknown). Community cancer hospitals treat between 100 and 500 newly diagnosed cancer cases a year and participate in cancer related clinical research but may refer patients for part of their treatment. Comprehensive community cancer programs offer the same range of services as the community cancer centers but treat 500 or more cancer cases annually. Academic research facilities treat N500 newly diagnosed cases each year and have residency programs as well as ongoing research. Integrated network cancer centers are organizations collaborating with various care facilities, at least one being a CoC accredited cancer program.
2.6. Statistical analysis
Frequency distributions of epidemiological, tumor, and hospital characteristics according to race/ethnicity were evaluated using chi-square tests. We computed univariable odds ratios (OR) and 95% confidence intervals (CIs) for relationships between these characteristics and GCT receipt (yes vs. no) using logistic regression. Age, stage, histology, hospital type, and insurance status were associated with GCT in univariable models at p < 0.05 and were therefore included in the multivariable logistic regression model. Although race was not significantly associated with GCT receipt in the univariable model, this variable was included in the final model.
Univariable Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% CIs for the association between GCT and overall survival in the total study population and stratified by race/ethnicity. Potential confounders of the GCT-overall survival relationship were adjusted for in the multivariable models and included race/ethnicity (overall model only) age, stage, histology, hospital type, and insurance status. We evaluated the proportional hazards assumption using plots of the Schoenfeld residuals vs. log follow-up time for GCT and noted no significant deviations. All analyses were performed in SAS version 9.4. All p-values were two-sided.
3. Results
3.1. Study population
In total, 30,426 women met the inclusion criteria for analysis. Table 1 provides characteristics of the total study population and according to race/ethnicity. The majority of women were NHW (N = 21,117, 69.4%) followed by NHB (N = 6657, 21.9%), and in all racial demographics, the largest proportion of women were diagnosed between 65 and 74 years of age. AS/PI women were more likely than NHW, NHB, and Hispanic women to present with stage IA disease (27.0% vs. 25.4%, 25.1%, and 25.0%, respectively, p < 0.0001) and serous histology (56.2% vs. 51.9%, 51.8%, and 50.9%, respectively, p < 0.0001). NHW women were more likely to have no comorbidities as compared with NHB, Hispanic, or AS/PI women (76.5% vs. 66.7%, 71.0%, and 75.8%, respectively, p < 0.0001). NHB women were more likely to receive care at an academic/research hospital compared with NHW, Hispanic, and AS/PI women (53.2% vs. 44.5%, 48.1%, 49.7%, respectively, p < 0.0001) and to live in areas of low median household income (41.8% vs. 29.0%, 12.4%, and 7.0%, respectively, p < 0.0001). Hispanic women were more likely to live in areas with low proportions of high school completion as compared with NHB, AS/PI, and NHW women (50.9% vs. 34.5%, 20.4%, and 11.3%, respectively, p < 0.0001).
Table 1.
Distributions of epidemiological, tumor, and hospital characteristics in the overall study population and according to race/ethnicity, N = 30,426.
| Overall (n = 30,426) | Race/ethnicity, n (Col %) | p | ||||
|---|---|---|---|---|---|---|
| NHW (n = 21,177) | NHB (n = 6657) | Hispanic (n = 1689) | AS/PI (n = 903) | |||
| GCC | 0.46 | |||||
| Yes | 13,337 (43.8%) | 9240 (43.6%) | 2943 (44.2%) | 738 (43.7%) | 416 (46.1%) | |
| No | 17,089 (56.2%) | 11,937 (56.4%) | 3714 (55.8%) | 951 (56.3%) | 487 (53.9%) | |
| Age | <0.0001 | |||||
| 18–44 | 435 (1.4%) | 280 (1.3%) | 61 (0.9%) | 69 (4.1%) | 25 (2.8%) | |
| 45–54 | 1823 (6.0%) | 1243 (5.9%) | 350 (5.3%) | 148 (8.8%) | 82 (9.1%) | |
| 55–64 | 9087 (29.9%) | 5799 (27.4%) | 2424 (36.4%) | 552 (32.7%) | 312 (34.6%) | |
| 65–74 | 11,296 (37.1%) | 7810 (36.9%) | 2545 (38.2%) | 624 (36.9%) | 317 (35.1%) | |
| ≥75 | 7785 (25.6%) | 6045 (28.6%) | 1277 (19.2%) | 296 (17.5%) | 167 (18.5%) | |
| Stage | <0.0001 | |||||
| I | 167 (0.6%) | 126 (0.6%) | 27 (0.4%) | 8 (0.5%) | 6 (0.7%) | |
| IA | 7726 (25.4%) | 5386 (25.4%) | 1673 (25.1%) | 423 (25.0%) | 244 (27.0%) | |
| IB | 4183 (13.8%) | 3092 (14.6%) | 773 (11.6%) | 212 (12.6%) | 106 (11.7%) | |
| IC | 1017 (3.3%) | 806 (3.8%) | 152 (2.3%) | 40 (2.4%) | 19 (2.1%) | |
| II | 1468 (4.8%) | 961 (4.5%) | 374 (5.6%) | 91 (5.4%) | 42 (4.7%) | |
| IIA | 445 (1.5%) | 298 (1.4%) | 99 (1.5%) | 33 (2.0%) | 15 (1.7%) | |
| IIB | 789 (2.6%) | 530 (2.5%) | 197 (3.0%) | 40 (2.4%) | 22 (2.4%) | |
| III | 181 (0.6%) | 127 (0.6%) | 36 (0.5%) | 12 (0.7%) | 6 (0.7%) | |
| IIIA | 2497 (8.2%) | 1830 (8.6%) | 456 (6.9%) | 141 (8.4%) | 70 (7.8%) | |
| IIIB | 604 (2.0%) | 419 (2.0%) | 128 (1.9%) | 39 (2.3%) | 18 (2.0%) | |
| IIIC | 5480 (18.0%) | 3597 (17.0%) | 1411 (21.2%) | 318 (18.8%) | 154 (17.1%) | |
| IV | 513 (1.7%) | 348 (1.6%) | 97 (1.5%) | 50 (3.0%) | 18 (2.0%) | |
| IVA | 569 (1.9%) | 424 (2.0%) | 107 (1.6%) | 22 (1.3%) | 16 (1.8%) | |
| IVB | 4787 (15.7%) | 3233 (15.3%) | 1127 (16.9%) | 260 (15.4%) | 167 (18.5%) | |
| Histology | <0.0001 | |||||
| Carcinosarcoma | 11,207 (36.8%) | 7630 (36.0%) | 2657 (39.9%) | 634 (37.5%) | 286 (31.7%) | |
| Clear cell | 3422 (11.3%) | 2563 (12.1%) | 554 (8.3%) | 195 (11.6%) | 110 (12.2%) | |
| Serous | 15,797 (51.9%) | 10,984 (51.9%) | 3446 (51.8%) | 860 (50.9%) | 507 (56.2%) | |
| Charlson comorbidity score | <0.0001 | |||||
| 0 | 22,518 (74.0%) | 16,194 (76.5%) | 4441 (66.7%) | 1199 (71.0%) | 684 (75.8%) | |
| 1 | 6411 (21.1%) | 4063 (19.2%) | 1760 (26.4%) | 409 (24.2%) | 179 (19.8%) | |
| 2 | 1197 (3.9%) | 742 (3.5%) | 355 (5.3%) | 65 (3.9%) | 35 (3.9%) | |
| ≥3 | 300 (1.0%) | 178 (0.8%) | 101 (1.5%) | 16 (1.0%) | 5 (0.6%) | |
| Hospital type | <0.0001 | |||||
| Community cancer program | 1220 (4.0%) | 897 (4.2%) | 186 (2.8%) | 66 (3.9%) | 71 (7.9%) | |
| Comprehensive community cancer program | 11,046 (36.3%) | 8285 (39.1%) | 1912 (28.7%) | 538 (31.9%) | 311 (34.4%) | |
| Academic/research program | 14,230 (46.8%) | 9427 (44.5%) | 3541 (53.2%) | 813 (48.1%) | 449 (49.7%) | |
| Integrated network cancer program | 3733 (12.3%) | 2443 (11.5%) | 997 (15.0%) | 232 (13.7%) | 61 (6.8%) | |
| Other/unknown | 197 (0.7%) | 125 (0.6%) | 21 (0.3%) | 40 (2.4%) | 11 (1.2%) | |
| Hospital location | <0.0001 | |||||
| Northeast | 7375 (24.2%) | 5380 (25.4%) | 1430 (21.5%) | 418 (24.8%) | 147 (16.3%) | |
| South | 10,796 (35.5%) | 6412 (30.3%) | 3665 (55.1%) | 585 (34.6%) | 134 (14.8%) | |
| Midwest | 7268 (23.9%) | 5873 (27.7%) | 1194 (17.9%) | 117 (6.9%) | 84 (9.3%) | |
| Mountain | 1056 (3.5%) | 899 (4.3%) | 48 (0.7%) | 87 (5.2%) | 22 (2.4%) | |
| Pacific | 3734 (12.3%) | 2488 (11.8%) | 299 (4.5%) | 442 (26.2%) | 505 (55.9%) | |
| Unknown | 197 (0.7%) | 125 (0.6%) | 21 (0.3%) | 40 (2.4%) | 11 (1.2%) | |
| Urban/rural | <0.0001 | |||||
| Metro | 24,997 (82.2%) | 16,758 (79.1%) | 5816 (87.4%) | 1590 (94.1%) | 833 (92.3%) | |
| Urban | 3924 (12.9%) | 3242 (15.3%) | 588 (8.8%) | 58 (3.4%) | 36 (4.0%) | |
| Rural | 547 (1.8%) | 457 (2.2%) | 85 (1.3%) | 4 (0.2%) | 1 (0.1%) | |
| Unknown | 958 (3.2%) | 720 (3.4%) | 168 (2.5%) | 37 (2.2%) | 33 (3.7%) | |
| Incomea | <0.0001 | |||||
| ≤$38,000 | 5970 (19.6%) | 2632 (12.4%) | 2785 (41.8%) | 490 (29.0%) | 63 (7.0%) | |
| $38,000-$47,999 | 6631 (21.8%) | 4694 (22.2%) | 1432 (21.5%) | 401 (23.7%) | 104 (11.5%) | |
| $48,000-$62,999 | 7821 (25.7%) | 5857 (27.7%) | 1277 (19.2%) | 428 (25.3%) | 259 (28.7%) | |
| ≥$63,000 | 9642 (31.7%) | 7746 (36.6%) | 1070 (16.1%) | 361 (21.4%) | 465 (51.5%) | |
| Unknown | 362 (1.2%) | 248 (1.2%) | 93 (1.4%) | 9 (0.5%) | 12 (1.3%) | |
| Education (% without high school diploma)b | <0.0001 | |||||
| ≥21% | 5735 (18.9%) | 2396 (11.3%) | 2295 (34.5%) | 860 (50.9%) | 184 (20.4%) | |
| 13%−20.9% | 7897 (26.0%) | 4907 (23.2%) | 2412 (36.2%) | 372 (22.0%) | 206 (22.8%) | |
| 7%−12.9% | 9412 (30.9%) | 7514 (35.5%) | 1329 (20.0%) | 301 (17.8%) | 268 (29.7%) | |
| <7% | 7033 (23.1%) | 6119 (28.9%) | 533 (8.0%) | 147 (8.7%) | 234 (25.9%) | |
| Unknown | 349 (1.2%) | 241 (1.1%) | 88 (1.3%) | 9 (0.5%) | 11 (1.2%) | |
| Insurance | <0.0001 | |||||
| None | 954 (3.1%) | 418 (2.0%) | 351 (5.3%) | 142 (8.4%) | 43 (4.8%) | |
| Private | 10,497 (34.5%) | 7373 (34.8%) | 2217 (33.3%) | 540 (32.0%) | 367 (40.6%) | |
| Medicaid | 1480 (4.9%) | 620 (2.9%) | 524 (7.9%) | 252 (14.9%) | 84 (9.3%) | |
| Medicare | 16,842 (55.4%) | 12,360 (58.4%) | 3388 (50.9%) | 708 (41.9%) | 386 (42.8%) | |
| Other government | 217 (0.7%) | 146 (0.7%) | 56 (0.8%) | 8 (0.5%) | 7 (0.8%) | |
| Unknown | 436 (1.4%) | 260 (1.2%) | 121 (1.8%) | 39 (2.3%) | 16 (1.8%) | |
p-Value compares distributions among NHW, NHB, Hispanic, and AS/PI women.
Income: quartiles based on equally proportioned income ranges among all U.S. zip codes.
Education: quartiles based on proportion of adults in the patient’s zip code who did not graduate from high school.
3.2. Odds of GCT receipt according to race/ethnicity
In the multivariable analysis, odds of receiving GCT did not differ by race/ethnicity (Table 2). Compared to NHW women, ORs for NHB, Hispanic, and AS/PI women were 1.01 (95% CI = 0.95–1.07), 1.01 (95% CI = 0.91–1.12), and 1.10 (95% CI = 0.96–1.26), respectively. Older age at diagnosis was associated with higher odds of GCT receipt. Advanced stage was significantly associated with lower odds of GCT receipt: compared to women with stage I disease, women with stages IIIVB disease were between 27% and 67% less likely to receive GCT. Furthermore, women with clear cell (OR = 0.78, 95% CI = 0.72–0.84) or serous (OR = 0.78, 95% CI = 0.74–0.82) histology showed lower odds of receiving GCT compared to women with carcinosarcoma. Income, education level, and presence of comorbidities were not associated with GCT.
Table 2.
Univariable and multivariable odds ratios (ORs) and 95% confidence intervals (CIs) for associations between epidemiological, tumor, and hospital characteristics and GCC, N = 30,426.
| Guideline concordant treatment, n (Row %) | Univariable OR (95% CI) | P | Multivariable OR (95% CI)a | P | ||
|---|---|---|---|---|---|---|
| No (n = 17,089) | Yes (n = 13,337) | |||||
| Race/ethnicity | 0.46 | 0.59 | ||||
| NHW | 11,937 (56.4%) | 9240 (43.6%) | 1.00 | 1.00 | ||
| NHB | 3714 (55.8%) | 2943 (44.2%) | 1.02 (0.97–1.08) | 1.01 (0.95–1.07) | ||
| Hispanic | 951 (56.3%) | 738 (43.7%) | 1.00 (0.91–1.11) | 1.01 (0.91–1.12) | ||
| AS/PI | 487 (53.9%) | 416 (46.1%) | 1.10 (0.97–1.26) | 1.10 (0.96–1.26) | ||
| Age | <0.0001 | <0.0001 | ||||
| 18–44 | 261 (60.0%) | 174 (40.0%) | 1.00 | 1.00 | ||
| 45–54 | 1037 (56.9%) | 786 (43.1%) | 1.14 (0.92–1.41) | 1.30 (0.98–1.73) | ||
| 55–64 | 5022 (55.3%) | 4065 (44.7%) | 1.21 (1.00–1.48) | 1.37 (1.04–1.81) | ||
| 65–74 | 6153 (54.5%) | 5143 (45.5%) | 1.25 (1.03–1.52) | 1.40 (1.06–1.84) | ||
| ≥75 | 4616 (59.3%) | 3169 (40.7%) | 1.03 (0.85–1.25) | 1.19 (0.90–1.57) | ||
| Stage | <0.0001 | <0.0001 | ||||
| I | 80 (47.9%) | 87 (52.1%) | 1.00 | 1.00 | ||
| IA | 3016 (39.0%) | 4710 (61.0%) | 1.44 (1.06–1.95) | 1.53 (1.12–2.08) | ||
| IB | 2824 (67.5%) | 1359 (32.5%) | 0.44 (0.32–0.60) | 0.46 (0.33–0.62) | ||
| IC | 547 (53.8%) | 470 (46.2%) | 0.79 (0.57–1.10) | 0.82 (0.59–1.14) | ||
| II | 1087 (74.1%) | 381 (26.0%) | 0.32 (0.23–0.45) | 0.33 (0.24–0.46) | ||
| IIA | 274 (61.6%) | 171 (38.4%) | 0.57 (0.40–0.82) | 0.61 (0.43–0.88) | ||
| IIB | 443 (56.2%) | 346 (43.9%) | 0.72 (0.51–1.00) | 0.76 (0.54–1.06) | ||
| III | 117 (64.6%) | 64 (35.4%) | 0.50 (0.33–0.77) | 0.53 (0.35–0.82) | ||
| IIIA | 1422 (57.0%) | 1075 (43.1%) | 0.70 (0.51–0.95) | 0.73 (0.53–1.00) | ||
| IIIB | 409 (67.7%) | 195 (32.3%) | 0.44 (0.31–0.62) | 0.45 (0.31–0.63) | ||
| IIIC | 3236 (59.1%) | 2244 (41.0%) | 0.64 (0.47–0.87) | 0.67 (0.49–0.91) | ||
| IV | 348 (67.8%) | 165 (32.2%) | 0.44 (0.31–0.62) | 0.47 (0.33–0.67) | ||
| IVA | 365 (64.2%) | 204 (35.9%) | 0.51 (0.36–0.73) | 0.54 (0.38–0.76) | ||
| IVB | 2921 (61.0%) | 1866 (39.0%) | 0.59 (0.43–0.80) | 0.62 (0.45–0.84) | ||
| Histology | <0.0001 | <0.0001 | ||||
| Carcinosarcoma | 6030 (53.8%) | 5177 (46.2%) | 1.00 | 1.00 | ||
| Clear cell | 1971 (57.6%) | 1451 (42.4%) | 0.86 (0.79–0.93) | 0.78 (0.72–0.84) | ||
| Serous | 9088 (57.5%) | 6709 (42.5%) | 0.86 (0.82–0.90) | 0.78 (0.74–0.82) | ||
| Charlson comorbidity score | 0.72 | |||||
| 0 | 12,617 (56.0%) | 9901 (44.0%) | 1.00 | - | ||
| 1 | 3639 (56.8%) | 2772 (43.2%) | 0.97 (0.92–1.03) | - | ||
| 2 | 668 (55.8%) | 529 (44.2%) | 1.01 (0.90–1.13) | - | ||
| ≥3 | 165 (55.0%) | 135 (45.0%) | 1.04 (0.83–1.31) | - | ||
| Hospital type | 0.04 | 0.02 | ||||
| Community cancer program | 679 (55.7%) | 541 (44.3%) | 1.00 | 1.00 | ||
| Comprehensive community cancer program | 6329 (57.3%) | 4717 (42.7%) | 0.94 (0.83–1.05) | 0.90 (0.80–1.02) | ||
| Academic/research program | 7877 (55.4%) | 6353 (44.7%) | 1.01 (0.90–1.14) | 0.97 (0.86–1.10) | ||
| Integrated network cancer program | 2096 (56.2%) | 1637 (43.9%) | 0.98 (0.86–1.12) | 0.93 (0.81–1.06) | ||
| Other/unknown | 108 (54.8%) | 89 (45.2%) | 1.03 (0.76–1.40) | 1.30 (0.86–1.96) | ||
| Hospital location | <0.0001 | |||||
| Northeast | 4244 (57.6%) | 3131 (42.5%) | 1.00 | |||
| South | 5976 (55.4%) | 4820 (44.7%) | 1.09 (1.03–1.16) | - | ||
| Midwest | 3995 (55.0%) | 3273 (45.0%) | 1.11 (1.04–1.19) | - | ||
| Mountain | 657 (62.2%) | 399 (37.8%) | 0.82 (0.72–0.94) | - | ||
| Pacific | 2109 (56.5%) | 1625 (43.5%) | 1.04 (0.97–1.13) | - | ||
| Unknown | 108 (54.8%) | 89 (45.2%) | 1.12 (0.84–1.48) | - | ||
| Urban/rural | 0.99 | |||||
| Metro | 14,035 (56.2%) | 10,962 (43.9%) | 1.00 | - | ||
| Urban | 2203 (56.1%) | 1721 (43.9%) | 1.00 (0.94–1.07) | - | ||
| Rural | 311 (56.9%) | 236 (43.1%) | 0.97 (0.82–1.15) | - | ||
| Unknown | 540 (56.4%) | 418 (43.6%) | 0.99 (0.87–1.13) | - | ||
| Incomeb | 0.20 | |||||
| ≤$38,000 | 3357 (56.2%) | 2613 (43.8%) | 1.00 | - | ||
| $38,000-$47,999 | 3723 (56.2%) | 2908 (43.9%) | 1.00 (0.94–1.08) | - | ||
| $48,000-$62,999 | 4337 (55.5%) | 3484 (44.6%) | 1.03 (0.96–1.10) | - | ||
| ≥$63,000 | 5450 (56.5%) | 4192 (43.5%) | 0.99 (0.93–1.06) | - | ||
| Unknown | 222 (61.3%) | 140 (38.7%) | 0.81 (0.65–1.01) | - | ||
| Education (% without high school diploma)c | 0.27 | |||||
| ≥21% | 3268 (57.0%) | 2467 (43.0%) | 1.00 | - | ||
| 13%−20.9% | 4414 (55.9%) | 3483 (44.1%) | 1.05 (0.98–1.12) | - | ||
| 7%−12.9% | 5257 (55.9%) | 4155 (44.2%) | 1.05 (0.98–1.12) | - | ||
| <7% | 3938 (56.0%) | 3095 (44.0%) | 1.04 (0.97–1.18) | - | ||
| Unknown | 212 (60.7%) | 137 (39.3%) | 0.86 (0.69–1.07) | - | ||
| Insurance | <0.0001 | <0.0001 | ||||
| None | 530 (55.6%) | 424 (44.4%) | 1.00 | 1.00 | ||
| Private | 5842 (55.7%) | 4655 (44.4%) | 1.00 (0.87–1.14) | 0.99 (0.86–1.13) | ||
| Medicaid | 862 (58.2%) | 618 (41.8%) | 0.90 (0.76–1.06) | 0.93 (0.79–1.10) | ||
| Medicare | 9450 (56.1%) | 7392 (43.9%) | 0.98 (0.86–1.12) | 1.04 (0.90–1.21) | ||
| Other government | 114 (52.5%) | 103 (47.5%) | 1.13 (0.84–1.52) | 1.11 (0.82–1.51) | ||
| Unknown | 291 (66.7%) | 145 (33.3%) | 0.62 (0.49–0.79) | 0.65 (0.51–0.83) | ||
Hospital location not included in adjusted model due to collinearity with facility type.
Income: quartiles based on equally proportioned income ranges among all U.S. zip codes.
Education: quartiles based on proportion of adults in the patient’s zip code who did not graduate from high school.
3.3. GCT receipt and overall survival according to race/ethnicity
There was a racial disparity in mortality consistent with the established literature, in which NHB women experienced higher mortality than women in other race/ethnic groups. Overall mortality was 49.6% and proportions by race/ethnicity were 49.1%, 54.6%, 40.2%, and 42.2% among NHW, NHB, Hispanic, and AS/PI women, respectively. Fig. 1 shows multivariable-adjusted associations between GCT receipt and overall survival. In the overall study population, GCT was associated with improved survival (HR = 0.85, 95% CI = 0.82–0.87), which was also observed among NHW (HR = 0.84, 95% CI = 0.80–0.87), NHB (HR = 0.85, 95% CI = 0.80–0.91), and Hispanic (HR = 0.84, 95% CI = 0.72–0.98), but not among AS/PI women (HR = 0.97, 95% CI = 0.78–1.19).
Fig. 1.
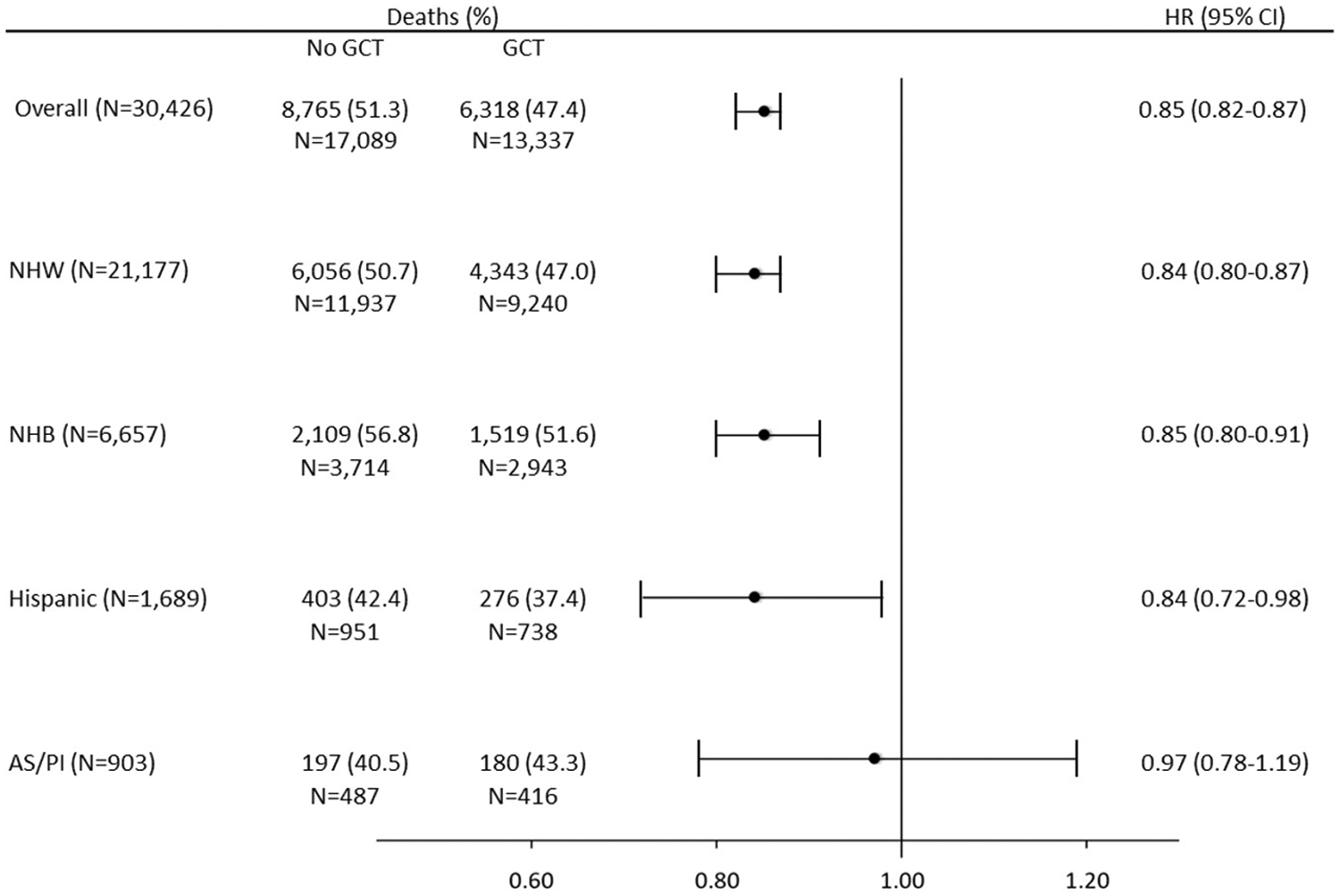
Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between GCT and overall survival in the overall study population and stratified by race/ethnicity.
4. Discussion
In this retrospective cohort study including >30,000 women with non-endometrioid EC, we found that over half of women did not receive GCT. Contrary to our hypothesis, no difference in GCT according to race/ethnicity was observed. In survival models, we noted improved overall survival associated with GCT receipt in the overall study population and according to race, with a similar magnitude of association for NHW, NHB, and Hispanic women. Our findings suggest that adherence to GCT may play a role in improving survival for most women with non-endometrioid EC, which are typically characterized by an aggressive disease course and high mortality.
Although we did not observe racial disparities in receipt of GCT in this study population, investigations among women with ovarian, cervical, and vulvar cancers have revealed that NHB women are less likely to receive GCT than NHW, with an associated negative impact on survival [9,15,16]. Moreover, our group has recently evaluated racial disparities in receipt of GCT among women diagnosed with endometrioid EC and we observed lower odds of GCT receipt among minority women compared with White women [17]. Importantly, the EC literature has focused primarily on individual components of therapy and not the full course of treatment within the context of established guidelines. For example, an analysis of the SEER database for women with non-endometrioid EC found that a significantly lower proportion of Hispanic women received radiation (39.5%) compared to their NHW counterparts (42.3%) [10]. Our investigation is the first to comprehensively assess patient treatment courses for women with non-endometrioid EC with a specific focus on GCT. We demonstrated that GCT is similar for patients of all racial groups, which is encouraging and potentially provides an example of care without a race-dependent health disparity.
While racial disparities in GCT receipt were not observed, we did note an overall low prevalence of GCT receipt. Among all women, only 43.8% received GCT, signifying the need for efforts to consistently provide care per the guideline recommendations. Non-endometrioid EC has been recognized as more aggressive than its endometrioid EC counterpart, with difference in molecular characteristics, etiology, presentation, and survival [2,3,5,18]. Provider decision-making based on patient and tumor characteristics may contribute to the low proportion of women who receive GCT, but these considerations are not captured with the current database variables. Research on the impact of GCT on outcomes for gynecologic malignancies is overall limited. Adherence to GCT in ovarian cancer may be associated with improved quality of care and outcomes [15], but studies in endometrioid EC have demonstrated mixed conclusions [19,20]. To our knowledge, no prior study has specifically evaluated GCT in patients with non-endometrioid EC. As patients with this histology have poorer clinical outcomes, it may be even more important to obtain a clear understanding of their received treatment and optimize their adherence to standards of care.
NCCN guidelines have consistently been recognized as a standard of care in clinical practice, with evidence-based recommendations and frequent updates [16]. Lack of recognition regarding appropriate treatment is therefore unlikely to be the cause of the low prevalence of GCT receipt. However, the quality of the underlying evidence that informs the guidelines may contribute to the low proportion of GCT we observed. Although several randomized clinical trials of women with EC have been conducted, these findings mostly apply to women with endometrioid tumors. Recently completed randomized trials including a larger proportion of women with non-endometrioid EC tumors may lead to important changes in the treatment guidelines for women with these aggressive tumors, which could subsequently increase adoption by clinicians [21,22]. Nonetheless, computational models suggest that closing GCT gaps may improve survival disparities [8]; therefore, studies investigating provider decision-making and identification of barriers to GCT may offer concrete opportunities for intervention. Encouraging increased adherence to standards of care is imperative, as demonstrated by our data that receipt of GCT was linked with improved survival both overall and within most racial demographics.
Strengths of this study include the quality of data analyzed. The NCDB contains a large number of complete records for women with the rarer subtypes of EC, with comprehensive information regarding treatment, tumor characteristics, and demographic factors. This level of detailed data allowed us to consider possible confounders and ensure appropriate comparison of temporally accurate guidelines at the time of each diagnosis. However, several limitations exist. First, specific details regarding individual treatment plans were not available, leading us to approximate GCT receipt in this study. For example, information on specific chemotherapy regimens and radiation courses, including delays, durations of treatment, dose adjustments, and therapeutic agent changes were unavailable. Provider and patient preferences, as well as system factors, that may have influenced treatment plans are also not captured in this database. This study therefore could not assess the clinical decision-making that may have prompted deviations from GCT. Additional limitations include the large number of records we excluded due to missing treatment and pathologic substage information, lack of central pathology review, limited baseline patient data, and no information on recurrence or cause of death. Potential avenues for improvement include expanding national database variables in order to provide insight into possible deviations from GCT, as well as developing medical records tools to better document patient care plans and reasons for deviations within the explicit framework of GCT.
In conclusion, although women with non-endometrioid EC have an overall low rate of GCT, when provided, GCT is associated with improved overall survival for all races except AS/PI women. While no racial disparity in receipt of GCT was observed, all-cause mortality is still highest for NHB women, and continued investigations into the etiology and potential solutions to this disparity are necessary. Currently, adherence to GCT, and the context surrounding non-concordant treatment plans, is difficult to fully characterize, due to limitations in understanding the provider perspective as well as patient and system factors that may in-fluence care. Developing interventions to improve systems process towards greater understanding of clinical management and adherence to NCCN guidelines may contribute to improving patient outcomes.
Supplementary Material
HIGHLIGHTS.
Over half of women with non-endometrioid endometrial cancer do not receive guideline-concordant treatment.
Rates of guideline-concordant treatment in non-endometrioid endometrial cancer do not differ by race.
Guideline-concordant treatment adherence interventions may improve survival.
Research is needed into barriers to adherence to treatment guidelines.
Acknowledgements
This work was supported by the National Cancer Institute, National Institutes of Health, United States (K01CA21845701A1) to ASF.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2020.03.016.
Declaration of competing interest
The authors have no relevant conflicts of interest.
References
- [1].Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R, The growing burden of endometrial cancer: a major racial disparity affecting black women, Cancer Epidemiol. Biomark. Prev 24 (2015) 1407–1415. [DOI] [PubMed] [Google Scholar]
- [2].Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. , Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial, Gynecol. Oncol 129 (2013) 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. , Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers, Br. J. Cancer 94 (2006) 642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Setiawan VW, Pike MC, Kolonel LN, Nomura AM, Goodman MT, Henderson BE, Racial/ethnic differences in endometrial cancer risk: the multiethnic cohort study, Am. J. Epidemiol 165 (2007) 262–270. [DOI] [PubMed] [Google Scholar]
- [5].Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. , Type I and II endometrial cancers: have they different risk factors? J. Clin. Oncol 31 (2013) 2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Felix AS, Cohn DE, Brasky TM, Zaino R, Park K, Mutch DG, et al. , Receipt of adjuvant endometrial cancer treatment according to race: an NRG Oncology/Gynecologic Oncology group 210 study, Am. J. Obstet. Gynecol 219 (2018)(459.e451–459.e411). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sud S, Holmes J, Eblan M, Chen R, Jones E, Clinical characteristics associated with racial disparities in endometrial cancer outcomes: a surveillance, epidemiology and end results analysis, Gynecol. Oncol 148 (2018) 349–356. [DOI] [PubMed] [Google Scholar]
- [8].Doll KM, Winn AN, Goff BA, Untangling the Black-White mortality gap in endometrial cancer: a cohort simulation, Am. J. Obstet. Gynecol 216 (2017) 324–325. [DOI] [PubMed] [Google Scholar]
- [9].Rauh-Hain JA, Melamed A, Schaps D, Bregar AJ, Spencer R, Schorge JO, et al. , Racial and ethnic disparities over time in the treatment and mortality of women with gynecological malignancies, Gynecol. Oncol 149 (2018) 4–11. [DOI] [PubMed] [Google Scholar]
- [10].Mahdi H, Hou H, Kowk LL, Moslemi-Kebria M, Michener C, Type II endometrial cancer in Hispanic women: tumor characteristics, treatment and survival compared to non-Hispanic white women, Gynecol. Oncol 133 (2014) 512–517. [DOI] [PubMed] [Google Scholar]
- [11].Rodriguez AM, Schmeler KM, Kuo YF, Disparities in endometrial cancer outcomes between non-Hispanic White and Hispanic women, Gynecol. Oncol 135 (2014) 525–533. [DOI] [PubMed] [Google Scholar]
- [12].Fader AN, Habermann EB, Hanson KT, Lin JF, Grendys EC, Dowdy SC, Disparities in treatment and survival for women with endometrial cancer: a contemporary national cancer database registry analysis, Gynecol. Oncol 143 (2016) 98–104. [DOI] [PubMed] [Google Scholar]
- [13].Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, et al. , Using the national cancer database for outcomes research: a review, JAMA Oncol 3 (2017) 1722–1728. [DOI] [PubMed] [Google Scholar]
- [14].National Comprehensive Cancer Network, NCCN guidelines, Available from URL https://www.nccn.org/professionals/physician_gls/default.aspx, Accessed date: June 2017.
- [15].Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, et al. , Disparities in ovarian cancer care quality and survival according to race and socioeconomic status, J. Natl. Cancer Inst 105 (2013) 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bristow RE, Chang J, Ziogas A, Anton-Culver H, Adherence to treatment guidelines for ovarian cancer as a measure of quality care, Obstet. Gynecol 121 (2013) 1226–1234. [DOI] [PubMed] [Google Scholar]
- [17].Kaspers M, Llamocca E, Quick A, Dholakia J, Salani R, Felix AS, Black and Hispanic women are less likely than White women to receive guideline-concordant endometrial cancer treatment, Am. J. Obstet. Gynecol (2020) 10.1016/j.ajog.2020.02.041(In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mendivil A, Schuler KM, Gehrig PA, Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes, Cancer Control 16 (2009) 46–52. [DOI] [PubMed] [Google Scholar]
- [19].Boll D, Verhoeven RH, van der Aa MA, Lybeert ML, Coebergh JW, Janssen-Heijnen ML, Adherence to national guidelines for treatment and outcome of endometrial cancer stage I in relation to co-morbidity in southern Netherlands 1995–2008, Eur. J. Cancer 47 (2011) 1504–1510. [DOI] [PubMed] [Google Scholar]
- [20].Eggink FA, Mom CH, Boll D, Ezendam NPM, Kruitwagen R, Pijnenborg JMA, et al. , Compliance with adjuvant treatment guidelines in endometrial cancer: room for improvement in high risk patients, Gynecol. Oncol 146 (2017) 380–385. [DOI] [PubMed] [Google Scholar]
- [21].Randall ME, Filiaci V, McMeekin DS, von Gruenigen V, Huang H, Yashar CM, et al. , Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer, J. Clin. Oncol 37 (21) (2019) 1810–1818 Jco1801575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matei D, Filiaci V, Randall ME, Mutch D, Steinhoff MM, DiSilvestro PA, et al. , Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer, N. Engl. J. Med 380 (2019) 2317–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


