Abstract
Objective
We previously reported an algorithm that identifies women at high risk of postoperative morbidity & mortality (M/M) as a tool to triage between neoadjuvant chemotherapy and primary surgery for epithelial ovarian cancer (EOC). We sought to independently validate its performance using multicenter data.
Methods
Women who underwent surgery for stage IIIC/IV EOC between 1/1/2014 and 12/31/2017 were identified from the National Surgical Quality Improvement Program database and classified as “high risk” or “triage appropriate” using our algorithm. Outcomes were compared between triage appropriate and high-risk women using the chi-square test.
Results
1,777 women met inclusion criteria; the mean age was 62.6 years and 81.9% had stage IIIC disease. Nationally, the surgical complexity scores were low (69.8% low, 25.2% intermediate and 5.0% high). “High risk” women had 2-fold higher rate of severe 30-day complication or death (6.2% vs 3.5%; p =0.01), a 3-fold higher rate of 30-day mortality (1.4% vs 0.5%; p=0.08), and a higher risk of death following a severe complication (11.1% vs. 0%, p=0.11). A sensitivity analysis excluding women with unknown albumin who didn’t meet other high risk criteria showed similar results: severe 30-day complications or death (6.2% vs 3.5%; p=0.02) and 30-day mortality (1.4% vs 0.3%; p=0.04) for “high risk” vs “triage appropriate” women.
Conclusions
Primary cytoreductive surgery to minimal residual disease remains the goal for EOC. We verify that our algorithm can identify women at risk of M/M using national multicenter data, despite a low complexity surgical setting and using 30-day mortality (vs. 90-day). Objective surgical risk assessment for ovarian cancer should be standard of care and can be incorporated into practice using the Mayo triage algorithm.
Keywords: Epithelial ovarian cancer, Primary debulking surgery, Morbidity and mortality, Mayo triage algorithm
Introduction
Cytoreductive surgery with adjuvant chemotherapy has been the cornerstone of treatment for advanced epithelial ovarian cancer (EOC). When disease burden is high, the required surgical effort and resultant surgical morbidity and mortality (M/M) after cytoreductive surgery is increased.1, 2 Given the complexity of surgery needed, we must be able to identify individuals at unacceptable risk of serious complications or death due to patient-related factors (i.e., comorbidities, frailty, malnutrition etc.). Neoadjuvant chemotherapy (NACT) followed by interval cytoreductive surgery is an alternate approach for such patients with lower postoperative morbidity and mortality.3, 4 While observational studies have suggested that primary cytoreductive surgery is superior to NACT in terms of survival, 5, 6, 7, 8 prospective randomized trials suggest that NACT is not inferior to primary cytoreductive surgery.3, 4 Unfortunately the choice between primary cytoreductive surgery and NACT is often based on subjective bias, institutional preference or center experience.
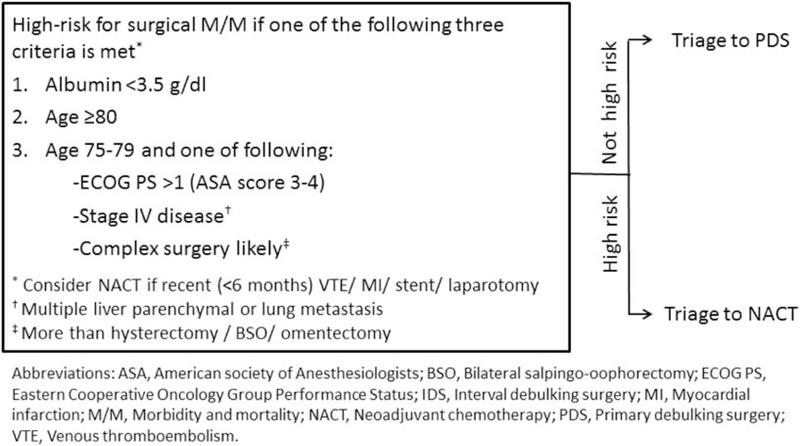
Irrespective of the timing of surgery (primary vs. interval), debulking to minimal residual disease with low surgical M/M would yield the best outcomes in EOC. The risk of short-term M/M associated with cytoreductive surgery should therefore be weighed against its survival benefit. We believe that the treatment approach for each patient should be chosen based on measurable risk factors which predict perioperative M/M.9, 10 We previously reported a triage algorithm to identify women at high risk of poor operative outcomes to successfully improve surgical outcomes after cytoreductive surgery for EOC. 11 (Figure 1) This provides a sensible, evidence-based approach to frame the discussion on patient selection for surgery. This is relevant to both the primary question of triage between NACT and primary cytoreductive surgery, but the bigger question as to surgical eligibility at any time in the spectrum of treatment. Both situations require ascertainment of patient risk factors as well as the anticipated complexity of surgery. Most patients can tolerate a low complexity surgery but if high complexity surgery is anticipated the risks are substantially higher. Preoperative chemotherapy may have a role in reducing surgical complexity for relevant patients.12, 13
Figure 1:
Mayo triage algorithm to predict surgical morbidity and mortality after cytoreductive surgery for advanced epithelial ovarian cancer
Within our institution, our triage algorithm was successful in identifying women at increased risk of surgical M/M after cytoreductive surgery. The initial implementation of the Mayo triage algorithm resulted in a significant decrease in 90-day mortality from 8.9% to 2.6% - these rates reflecting an underlying high complexity surgical setting for most patients.11 As the next step, we sought to externally validate the performance of our triage algorithm in order to test the reproducibility and generalizability of our findings using national multi-institutional data.
Methods
Women who underwent cytoreductive surgery for stage IIIC/IV EOC between 1/1/2014 and 12/31/2017 were identified from the National Surgical Quality Improvement Program (NSQIP) database using hysterectomy targeted participant use files (PUF). When compared to the general PUF, the hysterectomy targeted PUF contains more data points relevant to EOC such as surgical staging and residual disease. Women with a diagnosis of malignant neoplasm of ovary, fallopian tube or peritoneum were identified using ICD-9 and ICD-10 diagnosis codes (183.x, 158.8, 158.9, C48.1, C48.2, C56, C56.1, C56.2, C56.9, C57.0, C57.00, C57.01, C57.02, C57.4). We excluded women with the following characteristics preoperatively: ASA score ≥5, ventilator dependence, open wound, acute renal failure, undergoing dialysis, sepsis within 48h prior to surgery, and emergent surgery. The Mayo Clinic institutional review board waived review of our study because it included de-identified patient data.
Surgical complexity score (SCS) was assigned using a previously published scoring system14 after mapping the CPT codes for the principal operative procedure, other procedures, or concurrent procedures to the 12 procedure categories in the surgical complexity scoring system. We retrospectively applied our triage algorithm and classified women as “high risk” if they met at least one of the three criteria listed, or “triage appropriate” if they had no high-risk factors at the time of cytoreductive surgery. (Figure 1) A priori use of the algorithm would have recommended NACT for the high risk women.
Outcomes studied included severe 30-day complications, 30-day mortality and residual disease after surgery. Severe 30-day complications (Accordion grade 3 postoperative complications) were defined as occurrence of at least one of the following events within 30 days after surgery: unplanned reoperation, septic shock, progressive renal insufficiency, acute renal failure requiring dialysis, or ventilator for > 48 hours. Outcomes were compared between groups using the two-sided Fisher’s exact test or chi-square test. Odds ratios (OR) and corresponding 95% confidence intervals (CI) are reported to summarize the strength of the association. For the primary analysis, women without documented albumin levels were assumed to have normal levels and were classified as “triage appropriate” if they did not meet any other high risk criteria. We performed a sensitivity analysis excluding women without documented albumin levels who did not meet any other high risk criteria. P values <0.05 were considered statistically significant. All statistical analyses were performed using the SAS version 9.4 software package (SAS Institute, Inc.; Cary, NC).
Results
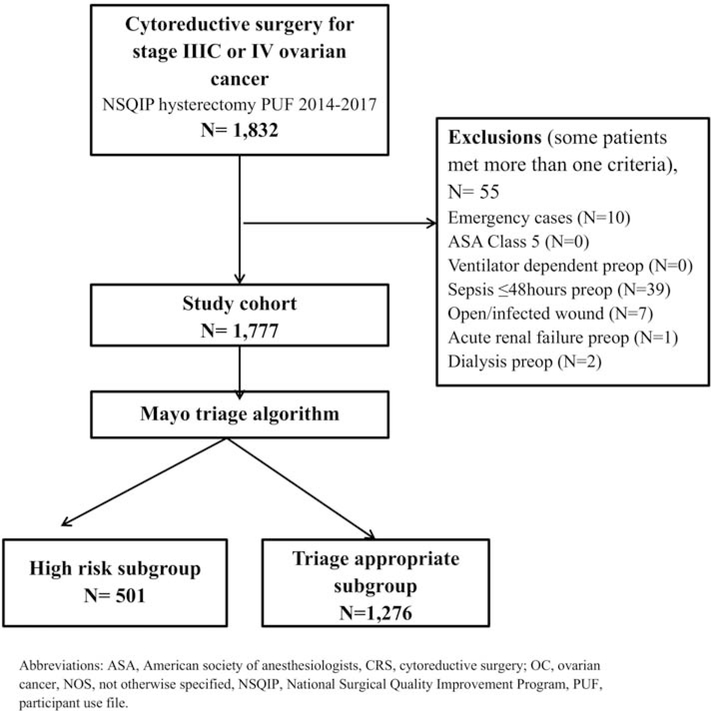
The mean age of 1,777 included women (Figure 2) was 62.6 years and 81.9% (1,455/1,777) had stage IIIC disease. Most women in this cohort (69.8%) underwent low complexity surgery. Only 5% of women underwent high complexity surgery. Preoperative albumin was normal in 60%, low (<3.5 g/dL) in 16.5%, and not recorded in 23.5%. (Table 1) Median operative time was 192 minutes consistent with relatively low complexity operations. Residual disease documentation (a quality measure) was not recorded in 16.8% of women.
Figure 2:
Participant flow diagram
Table 1:
Characteristics of 1,777 women who underwent surgery for stage IIIC or IV ovarian cancer between 1/1/2014 – 12/31/2017
| Characteristic | Total (N=1,777) | High risk subgroup (N=501) | Triage appropriate subgroup (N=1,276) |
|---|---|---|---|
| Age (years), mean (SD) | 62.6 (11.5) | 69.6 (12.2) | 59.9 (9.9) |
| Race/ethnicity | |||
| Non-Hispanic White | 1246 (70.1) | 377 (75.2) | 869 (68.1) |
| Hispanic White | 54 (3.0) | 11 (2.2) | 43 (3.4) |
| Black or African American | 91 (5.1) | 28 (5.6) | 63 (4.9) |
| Asian, Native Hawaiian or Pacific Islander | 65 (3.7) | 10 (2.0) | 55 (4.3) |
| American Indian or Alaska Native | 7 (0.4) | 2 (0.4) | 5 (0.4) |
| Other or Unknown | 314 (17.7) | 73 (14.6) | 241 (18.9) |
| Body Mass Index(kg/m2) | |||
| <25.0 | 656 (36.9) | 191 (38.1) | 465 (36.4) |
| 25.0–39.9 | 1008 (56.7) | 281 (56.1) | 727 (57.0) |
| ≥40.0 | 95 (5.3) | 23 (4.6) | 72 (5.6) |
| Not documented | 18 (1.0) | 6 (1.2) | 12 (0.9) |
| Functional Status | |||
| Independent | 1765 (99.3) | 494 (98.6) | 1271 (99.6) |
| Partially Dependent | 10 (0.6) | 7 (1.4) | 3 (0.2) |
| Not documented | 2 (0.1) | 0 (0.0) | 2 (0.2) |
| American Society of Anesthesiologists (ASA) score | |||
| <3 | 597 (33.6) | 112 (22.4) | 485 (38.0) |
| ≥3 | 1179 (66.3) | 389 (77.6) | 790 (61.9) |
| Not documented | 1 (0.1) | 0 (0.0) | 1 (0.1) |
| Preoperative albumin (g/dL) | |||
| ≥3.5 | 1067 (60.0) | 141 (28.1) | 926 (72.6) |
| <3.5 | 293 (16.5) | 293 (58.5) | 0 (0.0) |
| Not documented | 417 (23.5) | 67 (13.4) | 350 (27.4) |
| FIGO stage | |||
| IIIC | 1455 (81.9) | 412 (82.2) | 1043 (81.7) |
| IV | 322 (18.1) | 89 (17.8) | 233 (18.3) |
| Surgical complexity | |||
| Low | 1240 (69.8) | 347 (69.3) | 893 (70.0) |
| Intermediate | 448 (25.2) | 125 (25.0) | 323 (25.3) |
| High | 89 (5.0) | 29 (5.8) | 60 (4.7) |
| Operative time (minutes), Median (IQR) | 192 (135, 263) | 180 (129, 255) | 195 (140, 266) |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; SD, standard deviation.
Results are reported as N (%) unless otherwise noted.
Upon retrospectively applying our triage algorithm (Figure 1), 28.2% (501/1777) met the high risk criteria for surgical M/M, and the remaining 71.8% (1276/1777) were “triage appropriate”. Characteristics of included women by risk classification are shown in Table 1. “High risk” women were older (mean, 69.6 vs 59.9 years), more likely to have ASA score ≥3 (77.6% vs 61.9%), and have low serum albumin (<3.5 g/dL, 58.5% vs 0%). FIGO staging, surgical complexity, and operative time were similar for the “high risk” women and “triage appropriate” women.
Of the 1777 women, 417 (23.5%) women had no documented preoperative albumin. For the initial analysis, these women (n=350) were considered as “triage appropriate” in the absence of other high-risk factors. If other high-risk factors were present (n=67) they were considered “high risk”. Women who were scored as “high risk” by our algorithm had worse outcomes compared to those identified as appropriate for cytoreductive surgery. (Table 2, Main analysis) Specifically, “high risk” women had 2-fold higher rate of severe 30-day complications or death (6.2% vs 3.5%; OR 1.80, 95% CI 1.13–2.89; p = 0.01) and a 3-fold higher rate of 30-day mortality (1.4% vs 0.5%; OR 2.57, 95% CI 0.90–7.36; p = 0.08). Complete cytoreduction was achieved in 51.1% of “triage appropriate” women as compared to 46.1% of “high risk” women ( p<0.001; 47.4% (208/439) vs. 37.3% 387/1039 when excluding women with RD not recorded, p<0.001).
Table 2:
Outcomes after cytoreductive surgery for ovarian cancer among high risk women as compared to triage appropriate women
| Outcome | Main analysis† | Sensitivity analysis‡ | ||||
|---|---|---|---|---|---|---|
| High risk subgroup (N=501) | Triage appropriate subgroup (N=1,276) | p value^ | High risk subgroup (N=501) | Triage appropriate subgroup (N=926) | p value^ | |
| Severe 30-day complication or death* | 31 (6.2%) | 45 (3.5%) | 0.013 | 31 (6.2%) | 32 (3.5%) | 0.016 |
| 30-day mortality | 7 (1.4%) | 7 (0.5%) | 0.08 | 7 (1.4%) | 3 (0.3%) | 0.039 |
| Residual disease | <0.001 | <0.001 | ||||
| No Gross residual disease | 231 (46.1%) | 652 (51.1%) | 231 (46.1%) | 475 (51.3%) | ||
| Gross residual disease** | 208 (41.5%) | 387 (30.3%) | 208 (41.5%) | 273 (29.5%) | ||
| Not recorded | 62 (12.4%) | 237(18.6%) | 62 (12.4%) | 178 (19.2%) | ||
| Surgical Complexity | 0.64 | 0.97 | ||||
| Low | 347 (69.3%) | 893 (70.0%) | 347 (69.3%) | 642 (69.3%) | ||
| Intermediate | 125 (24.9%) | 323 (25.3%) | 125 (24.9%) | 233 (25.2%) | ||
| High | 29 (5.8%) | 60 (4.7%) | 29 (5.8%) | 51 (5.5%) | ||
Main analysis: Assumes albumin was ≥3.5 g/dL for those without documented albumin levels
Sensitivity analysis: Patients with undocumented albumin who didn’t meet the high risk criteria based on their other characteristics were excluded
Comparisons between the two groups were evaluated based on the two-sided Fisher’s exact test for 30-day mortality and the two-sided chi-square test for all other variables.
Severe 30 day complications (Accordion grade 3+ postoperative complications) were defined as occurrence of at least one of the following events: unplanned reoperation, septic shock, progressive renal insufficiency, acute renal failure requiring dialysis, on ventilator for > 48 hours, or death within 30 days.
Gross residual disease includes those documented as < 1 cm, 1–2 cm, >2 cm, or visible tumor NOS.
We performed a sensitivity analysis excluding the 350 women with unknown preoperative albumin who didn’t meet any other high risk criteria. Of the remaining 1427 women, 501 (35.1%) met the high risk criteria for surgical M/M, and the remaining 926 (64.9%) were “triage appropriate”. The sensitivity analysis showed similar results with increased risk of severe 30-day complications or death (6.2% vs 3.5%; OR 1.84, 95% CI 1.11–3.06: p = 0.02), 30-day mortality (1.4% vs 0.3%; OR=4.36, 95% CI 1.12–16.93; p = 0.04), and lower rates of complete cytoreduction (46.1% vs 51.3%, p < 0.001). (Table 2, sensitivity analysis)
We have previously published that this triage algorithm can identify women who are least able to survive after major complication. To test this in the national cohort, we examined the relationship between mortality following a severe complication and triage risk categorization. We used the sensitivity analysis cohort for this analysis (excluding the 350 women with unknown albumin who didn’t meet any other high risk criteria). Overall, severe postoperative complications occurred in 3.9% (56/1427) and 30-day mortality in 0.7% (10/1427) of women. The 30-day mortality following a severe complication was higher in “high risk” women vs. “triage appropriate” women (11.1% (3/27) vs. 0% (0/29); OR=8.43, 95% CI 0.42–171.20; p=0.11). The difference was significant in magnitude although power was limited by small number of events. For comparison, the 30-day mortality in the absence of a severe postoperative complication was not significantly different in the two groups: 0.8% (4/474) among “high risk” women and 0.3% (3/897) for “triage appropriate” women (p = 0.24). This reinforces the concept of lack of resiliency to tolerate severe complication in the “high risk” women, and that our model is able to identify these women.
Finally we looked at the impact of surgical complexity on M/M, recognizing that the majority of patients had low complexity surgery. The rate of severe 30-day complications or death was higher for women who underwent intermediate or high complexity surgery when compared to those who underwent low complexity surgery (7.3% (39/537) vs. 3.0% (37/1240), p <0.001). We wanted to quantify the increased risk of M/M in “high risk” women based on surgical complexity (Table 3). After intermediate or high complexity surgery, the rate of severe 30-day complications or death was significantly higher for “high risk” women vs. “triage appropriate” women (11.0% (17/154) vs. 5.7% (22/383); p=0.03). After low complexity surgery, this difference was greatly reduced and not statistically significant (4.0% (14/347) vs. 2.6% (23/893); p=0.18). These data support that the increased risk of M/M seen in triage high-risk women, increases with increasing surgical complexity.
Table 3:
Relationship between surgical complexity, triage risk classification, and outcomes
| 30-day outcomes | All women (N=1,777) | High risk Subgroup (N=501) | Triage appropriate subgroup (N=1,276) | p value† |
|---|---|---|---|---|
| Low surgical complexity subgroup (N=1,240) | ||||
| Severe 30-day complication | 31/1240 (2.5%) | 12/347 (3.5%) | 19/893 (2.1%) | 0.18 |
| 30-day mortality | 9/1240 (0.7%) | 4/347 (1.2%) | 5/893 (0.6%) | 0.28 |
| Severe 30-day complication or death | 37/1240 (3.0%) | 14/347 (4.0%) | 23/893 (2.6%) | 0.18 |
| Intermediate/high surgical complexity subgroup (N=537) | ||||
| Severe 30-day complication | 36/537 (6.7%) | 15/154 (9.7%) | 21/383 (5.5%) | 0.07 |
| 30-day mortality | 5/537 (0.9%) | 3/154 (2.0%) | 2/383 (0.5%) | 0.15 |
| Severe 30-day complication or death | 39/537 (7.3%) | 17/154 (11.0%) | 22/383 (5.7%) | 0.03 |
Comparisons between the two groups were evaluated based on the two-sided Fisher’s exact test for 30-day mortality and the two-sided chi-square test for all other variables.
Discussion
In this study, we used an independent multicenter national dataset to test the ability of our triage algorithm to identify women at increased risk of surgical M/M after cytoreductive surgery. Despite studying a cohort where surgical complexity was low in nearly70% of cases, our algorithm identified a subgroup of women who were at a 2 fold increased risk of severe 30-day postoperative complications or death and 3 fold increased risk of 30-day mortality after cytoreductive surgery. “High risk” women were also more likely to die within 30 days following a severe complication (11.1% vs 0%, p=0.11) and less likely to have complete cytoreduction. This is consistent with previously reported findings from our institutional cohort11 and supports the validity and generalizability of our findings.
Following the initial implementation of the triage algorithm within our institution, we observed a significant decrease in 90-day mortality in our institutional cohort from 8.9% among 620 surgical cases during 2003–2011 to 2.6% among 232 surgical cases during 2012-July 2016.11 In the NSQIP cohort, 30-day mortality for “high risk” women was 1.4% compared to 0.5% for “triage appropriate” women. The observed 3 fold increased risk of 30-day mortality for “high risk” women in the NSQIP cohort is similar to what we previously reported in our institutional cohort (70.8% reduction in deaths). While the 3 fold increase in mortality for the “high risk” women is consistent within our institutional dataset as well as the NSQIP dataset, the absolute risk difference depends on the baseline risk within each cohort which depends on surgical complexity and whether 30 or 90 day is used for measuring mortality.
The absolute mortality rates in the NSQIP cohort are lower than in our institutional cohort, and this is likely related to two factors: using 30-day mortality instead of 90-day mortality, and the profound differences in the rates of surgical complexity. With our improving ability to care for critically ill postoperative patients, 30-day mortality rate may not capture the true landscape of postoperative morbidity after surgery for EOC. The 90-day mortality rate (not reported in NSQIP), is a better metric for EOC because it captures women who do not recover enough after complex cytoreductive surgery to proceed to chemotherapy and die between 30 and 90 days after surgery. The 90-day mortality rate is usually double that of the 30-day mortality rate.15 In a National Cancer Database (NCDB) analysis of 24,827 women from 602 hospitals, 30-day mortality was 2.1% (95% CI 2.0–2.3) compared with 90-day mortality of 5.1% (95% CI 4.8–5.4%). We found that 70% of the NSQIP cohort underwent a low complexity surgery, compared to 18.5% in our contemporary institutional cohort from 2012 – July 2016. Similarly the rate of high complexity surgery was only 5% in NSQIP cohort vs. 27.2% in our institutional cohort. This is also reflected in the median operative time of 192 minutes in the NSQIP cohort compared to 315 minutes in our institutional cohort. The operative time reported by aggressive surgical centers range from 240 to 451 minutes for primary cytoreductive surgery, and 275 to 302 minutes for interval cytoreductive surgery.7, 12, 13 The rate of severe 30-day complications or death was higher after intermediate or high complexity surgery vs. low complexity surgery (7.3% (39/537) vs. 3.0%(37/1240), p<0.001) in the NSQIP cohort.
We previously reported that our algorithm identifies women who more likely to succumb to severe postoperative complications. The 90-day mortality following a severe postoperative complication was significantly higher in our institutional cohort prior to implementation of our triage algorithm as compared to after (28.3% vs 2.4%, p <0.001). In the NSQIP cohort, we were able to demonstrate higher mortality following a severe postoperative complication in the “high risk” subgroup (11.1% vs 0%) despite using 30-day mortality, although the difference was not statistically significant. These findings confirm the ability of our algorithm to identify women with decreased reserve who are less likely to recover from severe complications.
Strengths of our study include using data from multiple institutions to validate the performance of our algorithm across surgical practices supporting the generalizability of our findings. The data submitted to NSQIP is collected by a trained and certified Surgical Clinical Reviewer (SCR). Further, Inter-Rater Reliability (IRR) Audits are conducted to ensure that the data are of the highest quality. Additional strengths include large sample size, and inclusion of only advanced EOC. Limitations include a retrospective design and the risk of bias inherent in this study design. Large databases are also prone to misclassification bias; however, we expect this to be similar across groups. As described above, the surgical complexity in this cohort was low, and 90-day mortality was not available. However, we were able to demonstrate a significant difference in outcomes between “high risk” women and “triage appropriate” women despite low SCS, and use of 30-day mortality. Lastly, we were unable to study the impact of triage classification on postoperative chemotherapy delivery since this information is not available in NSQIP.
Cytoreductive surgery with the goal of minimal residual disease remains the standard of care for advanced ovarian cancer. Some patients are poor candidates for surgery due to high risk of postoperative morbidity and mortality. We present a surgical risk stratification tool that is able to identify women at risk of poor short-term outcomes following cytoreductive surgery. This tool, while developed in a setting where most patients underwent moderate or high complexity surgery, is shown to perform well in an independent, multi-center and lower complexity cohort. Given the reproducibility and generalizability of our findings, our triage algorithm can be used to determine the suitability of surgery in varied clinical settings. We currently use our algorithm to help in the decision between primary cytoreductive surgery and NACT at initial presentation; we also use our algorithm to assess eligibility for interval cytoreductive surgery following NACT. Objective surgical risk assessment should be standard of care in the treatment planning for EOC and can be incorporated into practice using our evidence based triage algorithm.
The Mayo triage algorithm identifies women at highest risk of morbidity & mortality (M/M) after cytoreductive surgery
Ability of our algorithm to identify women at risk of M/M after cytoreduction is verified using national multicenter data
Given the reproducible findings, our algorithm can be applied to ovarian cancer management in varied clinical settings.
Acknowledgments
Funding source
This work was supported by grants from the National Cancer Institute (P50CA136393) and the National Center for Advancing Translational Sciences (CTSA Grant Number UL1 TR002377), components of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Conflicts of interest
None of the authors has any conflicts of interest to declare.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benedetti Panici P, Di Donato V, Fischetti M et al. Predictors of postoperative morbidity after cytoreduction for advanced ovarian cancer: Analysis and management of complications in upper abdominal surgery. Gynecol Oncol. 2015. June;137(3):406–11. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Janco JM, Mariani A et al. Risk-prediction model of severe postoperative complications after primary debulking surgery for advanced ovarian cancer. Gynecol Oncol. 2016. January;140(1):15–21. doi: 10.1016/j.ygyno.2015.10.025. Epub 2015 Nov 2. [DOI] [PubMed] [Google Scholar]
- 3.Vergote I, Tropé CG, Amant F et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010. September 2;363(10):943–53. [DOI] [PubMed] [Google Scholar]
- 4.Kehoe S, Hook J, Nankivell M et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015. July 18;386(9990):249–57. [DOI] [PubMed] [Google Scholar]
- 5.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ, Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis, J. Clin. Oncol 20 (5) (2002) 1248–1259. [DOI] [PubMed] [Google Scholar]
- 6.Chi DS, Musa F, Dao F, et al. , An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT), Gynecol. Oncol 124 (1) (2012) 10–14. [DOI] [PubMed] [Google Scholar]
- 7.Chi DS, Eisenhauer EL, Zivanovic O, et al. , Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm, Gynecol. Oncol 114 (1) (2009) 26–31. [DOI] [PubMed] [Google Scholar]
- 8.Harter P, Muallem ZM, Buhrmann C, et al. , Impact of a structured quality management program on surgical outcome in primary advanced ovarian cancer, Gynecol. Oncol 121 (3) (2011) 615–619. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Janco JM, Mariani A, et al. , Risk-predictionmodel of severe postoperative complications after primary debulking surgery for advanced ovarian cancer, Gynecol. Oncol 140 (1) (2016) 15–21. [DOI] [PubMed] [Google Scholar]
- 10.Aletti GD, Eisenhauer EL, Santillan A, et al. , Identification of patient groups at highest risk from traditional approach to ovarian cancer treatment, Gynecol. Oncol 120 (1) (2011. January) 23–28. [DOI] [PubMed] [Google Scholar]
- 11.Narasimhulu DM, Kumar A, Weaver AL, McGree ME, Langstraat CL, Cliby WA. Using an evidence-based triage algorithm to reduce 90-day mortality after primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2019. October;155(1):58–62. [DOI] [PubMed] [Google Scholar]
- 12.Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer. 2016. May;59:22–33. [DOI] [PubMed] [Google Scholar]
- 13.Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016. September;64:22–31. [DOI] [PubMed] [Google Scholar]
- 14.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007. December;197(6):676.e1–7. [DOI] [PubMed] [Google Scholar]
- 15.Spencer RJ, Hacker KE, Griggs JJ, Rice LW, Reynolds RK, Uppal S. Ninety-Day Mortality as a Reporting Parameter for High-Grade Serous Ovarian Cancer Cytoreduction Surgery. Obstet Gynecol. 2017. August;130(2):305–314. [DOI] [PubMed] [Google Scholar]