Abstract
Background:
The proportions of patients with oesophageal adenocarcinoma (OAC) diagnosed by Barrett’s oesophagus surveillance or with preexisting Barrett’s oesophagus are unclear.
Aim:
A systematic review and meta-analysis to estimate the prevalence of prior and concurrent Barrett’s oesophagus diagnosis among patients with OAC or oesophagogastric junction adenocarcinomas (OGJAC).
Methods:
We searched PubMed and Embase to identify studies published 1966–1/8/2020 that examined the prevalence of prior (≥6 months) or concurrent Barrett’s diagnosis (at cancer diagnosis) among OAC and OGJAC patients. Random effects models estimated overall and stratified pooled prevalence rates.
Results:
A total of 69 studies, including 33,002 OAC patients (53 studies) and 2,712 with OGJAC (28 studies) were included. The pooled prevalence of prior Barrett’s oesophagus diagnosis in OAC was 11.8% (95% confidence interval [CI] 8.4–15.6%). The prevalence of prior Barrett’s oesophagus diagnosis was higher in single-center resection studies (16.0%, 95%CI 8.7–24.9%) than population-based cancer registry studies (8.4%, 95%CI 5.5–11.9%). The prevalence of concurrent Barrett’s oesophagus in OAC was 56.6% (95%CI 48.5–64.6%). Studies with 100% early stage OAC had higher prevalence of concurrent Barrett’s oesophagus (91.3%, 95%CI 82.4–97.6%) than studies with <50% early OAC (39.7%, 95%CI 33.7–45.9%). In OGJAC, the prevalence of prior and concurrent Barrett’s oesophagus was 23.2% (95%CI 7.5–44.0%) and 26.3% (95%CI 17.8–35.7%), respectively.
Conclusions:
Most patients with OAC have Barrett’s oesophagus. Our meta-analysis found ~12% of OAC patients had prior Barrett’s diagnosis, but concurrent Barrett’s oesophagus was found in ~57% at the time of OAC diagnosis. This represents a considerable missed opportunity for Barrett’s oesophagus screening.
Keywords: oesophageal adenocarcinoma, Barrett’s oesophagus, oesophagogastric junction adenocarcinoma, prevalence, systematic review, meta-analysis
Introduction
Barrett’s oesophagus is the only known precursor lesion of oesophageal adenocarcinoma (OAC) [1], a rapidly increasing, highly fatal cancer [2, 3]. Much clinical effort has therefore focused on ongoing surveillance after an index Barrett’s oesophagus diagnosis. However, absolute risk of OAC in Barrett’s oesophagus without dysplasia is low (0.1–0.5% per year vs. 6% per year in Barrett’s oesophagus with high-grade dysplasia [1, 4, 5]), with conflicting evidence as to whether Barrett’s oesophagus surveillance reduces OAC-related mortality [6–9].
Furthermore, the vast majority of OAC cases have no prior diagnosis of Barrett’s oesophagus at their cancer diagnosis. The size of this gap is best demonstrated in the last meta-analysis to determine the prevalence of prior Barrett’s oesophagus diagnosis among resected OAC cases that included studies published from 1966–2000 [10]. Among 12 studies involving 1503 patients with resected OAC, only 4.7% had a prior diagnosis of Barrett’s oesophagus. This meta-analysis excluded studies of non-resected OAC cases and therefore did not account for prior diagnosis of Barrett’s oesophagus among patients with all stages of OAC. Additionally, it included studies that combined OAC and oesophagogastric junction adenocarcinomas (OGJAC), therefore independent prevalence estimates for OAC and OGJAC were not reported. Evaluating data from more contemporary studies may be more informative regarding prevalence of Barrett’s oesophagus in OAC as newer cohorts are more likely to have a prior diagnosis of Barrett’s and include early stage OAC patients given increased Barrett’s oesophagus screening and surveillance practices following GI societal recommendations in 1998 [11].
Therefore, we conducted a systematic review and meta-analysis of the published literature to estimate the prevalence of prior as well as concurrent Barrett’s oesophagus diagnosis among OAC and OGJAC patients. In addition, we focused on Barrett’s oesophagus prevalence within studies published in the past 10 years.
Methods
Search Strategy and Selection Criteria:
We conducted and reported this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. Two authors (MCT, NM) independently searched Pubmed and Embase databases for full, original research studies published in print or online in English from 1966 to January 8, 2020 with the following inclusion criteria: 1) reported number of patients with OAC and/or OGJAC; 2) reported number of patients within the cohort with prior and/or concurrent Barrett’s oesophagus diagnosis; and 3) only human studies. We excluded: 1) studies that did not report both numbers of patients with OAC/OGJAC and Barrett’s oesophagus; 2) abstracts only; 3) reviews, editorials, letters to the editor; 4) studies with <10 OAC/OGJAC patients due to imprecise prevalence estimates; or 5) studies with OAC/OGJAC cell lines, tissues, or xenografts. The search strategy was conducted by a medical librarian (A.S.), and the details of Medical Subject Headings (MeSH) search are included in Appendix 1. Our search strategy also included ancestry search of bibliographies of all included studies and any relevant systematic reviews to identify additional studies that may have been missed. All studies included by either reviewer underwent a second review to exclude studies with indistinguishably combined OAC/OGJAC cohorts and studies with potentially overlapping populations. For manuscripts with potentially overlapping study populations, only the one with the largest sample size was included. Consensus was reached by both authors (MCT, NM) for final study inclusion.
OAC/OGJAC and Barrett’s oesophagus identification:
In studies that specified cancer locations based on Siewert classification [13], Siewert I tumors (midpoint 1cm to 5cm proximal to anatomic OGJ) were included with OAC, while Siewert II (midpoint 1cm proximal to 2cm distal to anatomic OGJ) were included with OGJAC. Siewert III and cardia cancers were excluded. Early stage OAC/OGJAC, or those amenable to resection, were defined as T1, T2 or stage 1 or 2 cancers. Prior Barrett’s oesophagus was defined as diagnosed ≥6 months prior to OAC/OGJAC diagnosis, when specified. We defined concurrent Barrett’s oesophagus as Barrett’s found on histopathology at the time of cancer diagnosis. Studies that reported numbers with Barrett’s oesophagus at the time of cancer diagnosis but did not specify how many of these were diagnosed prior to cancer diagnosis were classified as concurrent Barrett’s oesophagus to reduce misclassification of prior Barrett’s oesophagus diagnosis. We included studies that defined Barrett’s oesophagus as “specialized intestinal epithelium”, “intestinal metaplasia”, “columnar epithelium”, or endoscopic appearance of Barrett’s oesophagus.
Data Abstraction and Quality Assessment:
Two authors (MCT, NM) independently abstracted data from included studies including: study characteristics (i.e., study design, location, study period, study site, and study population), patient clinical and sociodemographic characteristics (i.e., method of OAC/OGJAC determination, Barrett’s oesophagus determination, Siewert classification, mean age, percent male, percent White, and percent diagnosed with early stage OAC/OGJAC), number of patients with OAC/OGJAC, number of patients with Barrett’s oesophagus, and assessment of study quality. If a subgroup of the study was included in our review but demographics were only reported for the whole population, we included the reported mean age and percent males.
Assessment of study quality was modified from the “The Joanna Briggs Institute Prevalence Critical Appraisal Tool”, which is a validated critical appraisal tool for systematic reviews addressing questions of prevalence [14]. Two authors independently assessed sample representation, participant recruitment, sample size, cohort descriptions, standardized measurement of Barrett’s oesophagus and OAC/OGJAC, reliable measurement, confounding factors, and subpopulation identification. Studies were determined to have adequately identified sub-populations if they included data on number of early stage cancers and type of study as these were the stratified analysis conducted. Discordance in data abstraction or quality assessment were resolved by consensus agreement by both abstractors.
Statistical Analysis:
We used random effects analysis to estimate pooled prevalence rates of prior and concurrent Barrett’s oesophagus diagnosis among OAC and of OGJAC patients along with their 95% confidence intervals (CI). For studies that did not report prevalence of Barrett’s oesophagus, we calculated the prevalence based on reported numbers of OAC/OGJAC and Barrett’s oesophagus. We used recommended Tukey Freeman arcsine transformed proportion and variance estimates for meta-analytic calculations of prevalence data [15], with results presented as forest plots. Between-study heterogeneity was assessed using the Higgins inconsistency index (I2), and I2>50% was considered substantial heterogeneity [16]. To assess for potential small study or publication bias, we used Egger’s and graphically by evaluating asymmetry in funnel plots of the Tukey Freeman arcsine transformed proportion versus its standard error. We also assessed secular trends in pooled estimate over time meta-analyses.
We also performed a priori specified stratified subgroup and sensitivity analyses to better qualify our findings on overall association between prior and concurrent Barrett’s oesophagus and OAC/OGJAC and to identify factors related to design that contributed to any observed between-study (non-random) variation. These included meta-analyses to obtain pooled prevalence for specified sub-groups based on study site (single-center, multi-center, population-based), location (U.S./Canada, Europe/Australia, Asia, other), method of OAC determination (resection only, biopsy/surgical pathology, database/diagnosis code), sample size (<100, 100–1000, >1000), male proportion (in tertiles), and proportion of early cancers (<50%, 50–99%, 100%). We also assessed if any of these factors was a significant contributor to between-study heterogeneity using univariable meta-regression when there were a minimum 10 studies.
Finally, we performed sensitivity analyses including: 1) restriction to studies published in the last 10 years, and 2) replacing two large, population-based U.S. studies reporting patients from the Surveillance, Epidemiology, and End Results (SEER) [17] and American College of Surgeons (ACS) databases [18] with 11 additional US studies that were initially excluded due to potentially overlapping study populations as the SEER and ACS database studies.
We performed main meta-analyses including pooled estimates, forest plots, I2 and related sub-group analyses using the metafor package implemented in Stata version 14 (StataCorp, College Station, TX) and used OpenMEE (http://www.cebm.brown.edu/openmee/) [19], which stores Tukey Freeman arcsine transformed proportion and variance estimates, for performance of remaining analyses, including meta-regression. All reported p-values are two-sided with p<0.05 indicating statistical significance.
Results
We identified and reviewed 5,057 potentially relevant studies. Of these, 180 studies were included by at least 1 reviewer for a second review. We excluded 117 studies on second review, including 26 that did not meet inclusion criteria, 26 that reported combined OAC/OGJAC numbers, and 63 with potentially overlapping study populations (of which 11 US studies were used in a sensitivity analysis) (Figure 1).
Figure 1.
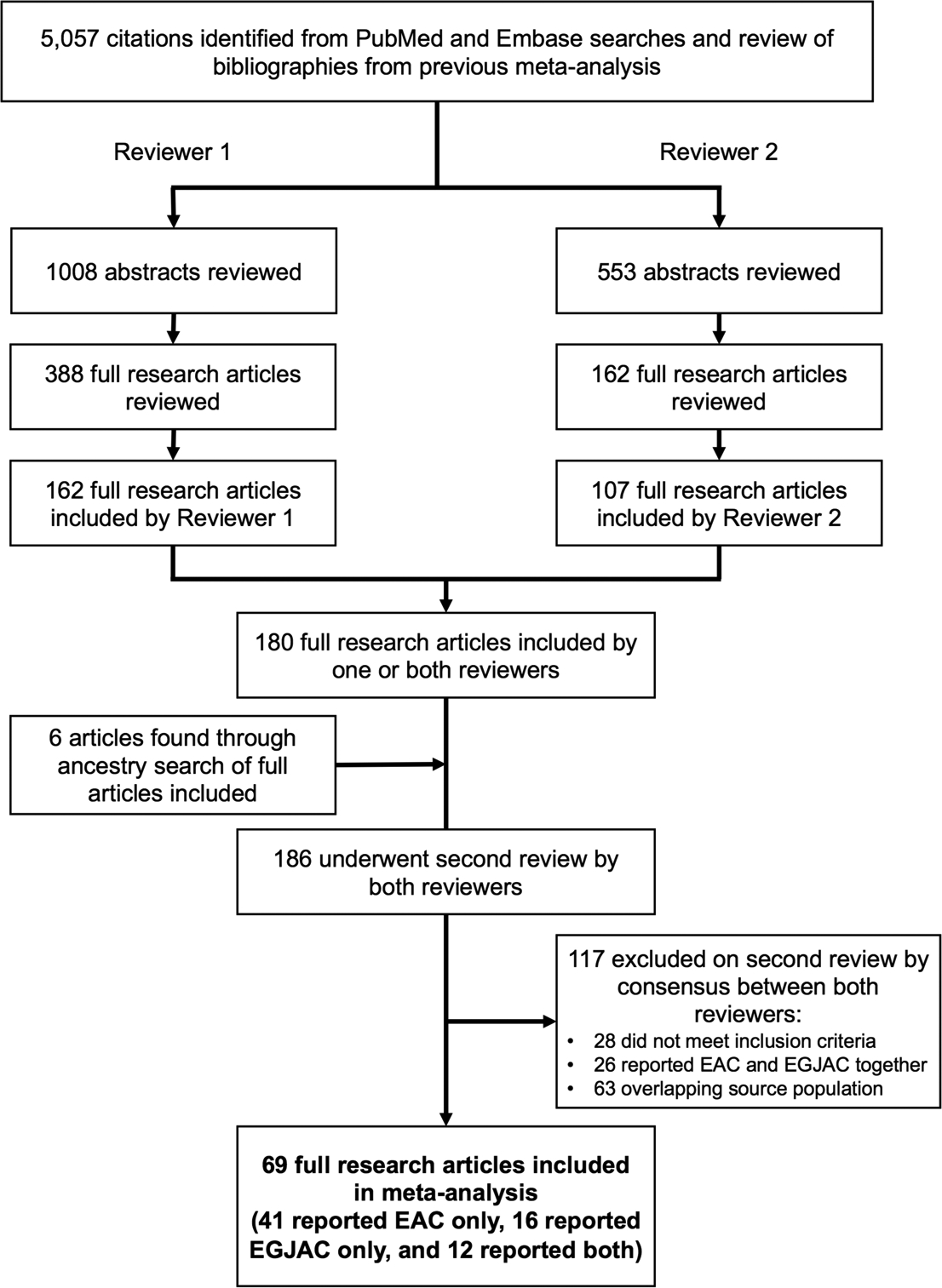
Study flow diagram.
A total of 69 studies met the eligibility criteria and included 33,002 patients with OAC from 53 studies and 2,712 with OGJAC from 28 studies. These studies were published from 1978 to 2019 and were conducted in U.S./Canada (n=25, 36.2%) [17, 18, 20–42], Europe (n=30, 43.5%) [43–72], Asia (n=11, 15.9%) [73–83], Africa (n=1, 1.4%) [84], South America (n=1, 1.4%) [85], or Australia (n=1, 1.4%) [86] and included 55 single-center (79.7%), 7 multi-center (10.1%), and 7 population-based studies (10.1%) (Tables 1, 2). OAC and OGJAC determination was made using surgical or endoscopic specimens (n=59 studies), diagnosis codes (n=3 studies), or cancer registry (n=7 studies). In 15 studies of prior Barrett’s oesophagus diagnosis prevalence, previous Barrett’s oesophagus diagnosis determination was made using histopathology (n=5 studies), Barrett’s oesophagus registry/database/history (n=5 studies), diagnosis codes (n=2 study), a combination of methods (n=1), or using methods that were not reported (n=2 studies). In 59 studies that reported concurrent Barrett’s oesophagus diagnosis prevalence, concurrent Barrett’s oesophagus determination was made using histopathology (n=53 studies), Barrett’s registry/database (n=1 study), survey (n=1 study), a combination of methods (n=1), or was not reported (n=3 studies). Demographic and clinical characteristics of the included studies are shown in Supplementary Table 1.
Table 1.
Study characteristics of 53 studies of 33,002 oesophageal adenocarcinoma (OAC) patients.
| Ref | Author | Year | Study Design | Study Location | Study Period | OAC determination | Barrett’s Oesophagus determination | # Total OAC | # Prior BE Diagnosis | # Concurrent BE |
|---|---|---|---|---|---|---|---|---|---|---|
| Single-center Studies | ||||||||||
| [40] | Amlashi | 2018 | cross-sectional | Houston, Texas | 2002–2015 | hospital cancer registry of patients after chemoradiation therapy | pathology | 129 | 71 | |
| [73] | Bai | 2006 | cross-sectional | Xian, China | 1/1995–12/1999 | pathology reports (biopsy, surgical resection) | pathology (biopsy or surgery) | 29 | 11 | |
| [43] | Bellone | 2007 | case series | Turin, Italy | 1/2002–12/2005 | surgical resection pathology | pathology | 21 | 5 | |
| [20] | Bergeron | 2014 | case series | Ann Arbor, Michigan | 7/2005–7/2011 | surgical resection pathology (only Tis or T1 cancers) | pathology | 89 | 67 | |
| [45] | Cavallin | 2018 | cohort | Padova, Italy | 1/1980–12/2011 | hospital database | hospital database | 1243 | 208 | |
| [41] | Chandrasoma | 2007 | case series | Los Angeles, California | 1997–2000 | surgical resection pathology | pathology | 38 | 25 | |
| [22] | Chen | 1995 | case series | Winston-Salem, North Carolina | 1975–1992 | hospital cancer registry | pathology (biopsy or surgery) | 64 | 25 | |
| [46] | Cijs | 2010 | cohort | Rotterdam, Netherlands | 1/1985–1/2005 | surgical resection pathology | pathology | 596 | 179 | |
| [47] | Collard | 2001 | cohort | Brussels, Belgium | 11/1984–1/2000 | surgical resection pathology | pathology | 183 | 77 | |
| [48] | Curran | 1992 | case series | Galway, Ireland | NR | surgical resection pathology | pathology | 23 | 4 | |
| [49] | Driessen | 2003 | cross-sectional | Leuven, Belgium | 1993–2000 | surgical resection pathology | pathology (biopsy or surgery) | 135 | 127 | |
| [24] | Duhaylongsod | 1991 | cross-sectional | Durham, North Carolina | 1985–1990 | surgical resection pathology | pathology | 57 | 16 | |
| [86] | Epari | 2009 | case series | Melbourne, Australia | 5/1993–5/2006 | surgical resection pathology | pathology (biopsy or surgery) | 93 | 53 | |
| [67] | Grimm | 2010 | case series | Wuerzburg, Germany | 1/2001–6/2004 | surgical resection pathology | pathology | 60 | 41 | |
| [51] | Holscher | 1997 | cohort | Cologne, Germany | 1982–1995 | surgical resection pathology | BE surveillance, pathology (EGD or surgery) | 41 | 8 | 36 |
| [26] | Haggitt | 1978 | case series | Boston, Massachusetts | 1927–1976 | surgical resection pathology | pathology | 14 | 12 | |
| [85] | Henry | 2014 | cross-sectional | Botucatu, Brazil | 2007–2012 | pathology | NR | 50 | 9 | |
| [52] | Khan | 2004 | cohort | Nottingham, UK | 1987–2001 | surgical resection pathology | pathology | 130 | 45 | |
| [54] | Le Page | 2015 | cross-sectional | Edinburgh, UK | 2005–2013 | endoscopic or surgical resection pathology | pathology | 83 | 68 | |
| [38] | Levine | 1984 | case series | Philadelphia, Pennsylvania | 1979–1982 | hospital pathology records | pathology | 17 | 17 | |
| [29] | Li | 2017 | case series | Halifax, Canada | 2005–2013 | endoscopic or surgical resection pathology (only T1 cancers) | pathology | 23 | 23 | |
| [76] | Liu | 2014 | case series | Henan, China | 2002–2011 | pathology; surgical resection pathology | pathology | 217 | 10 | |
| [37] | Melis | 2013 | case series | Tampa, Florida | 6/1994–1/2011 | oesophageal cancer database of surgical resections | history of BE | 540 | 155 | |
| [55] | Moghissi | 2009 | case series | East Yorkshire, UK | 1997–2009 | pathology (only intramucosal cancers) | pathology | 35 | 20 | |
| [30] | Moon | 1992 | cross-sectional | Milwaukee, Wisconsin | 1974–1990 | surgical resection pathology | pathology | 88 | 40 | |
| [31] | Naunheim | 1995 | case series | St. Louis, Missouri | 1986–1993 | pathology (cancers treated with neoadjuvant chemotherapy) | pathology | 28 | 16 | |
| [68] | Nowicki | 2018 | case series | Bydgoszcz, Poland | 2004–2014 | hospital endoscopy database | BE surveillance | 46 | 3 | |
| [32] | Nurkin | 2014 | case series | Buffalo, New York | 2001–2012 | endoscopic resection pathology | pathology | 44 | 44 | |
| [65] | Peracchia | 1999 | case series | Milan, Italy | 11/1992–5/1998 | surgical resection pathology | BE surveillance | 59 | 6 | |
| [34] | Qumseya | 2013 | case series | Jacksonville, Florida | 2003–2010 | endoscopic resection pathology | pathology | 64 | 61 | |
| [35] | Reyes | 1981 | case series | Hines, Illinois | 1953–1979 | surgical resection pathology | pathology | 12 | 6 | |
| [57] | Reynolds | 2011 | case series | Dublin, Ireland | 2004–2008 | pathology from database | BE surveillance | 100 | 23 | |
| [66] | Ribet | 1992 | cross-sectional | Lille Cedex, France | 1970–1988 | surgical resection pathology | pathology | 28 | 2 | 13 |
| [69] | Ruffato | 2016 | case series | Bologna, Italy | 2001–2013 | surgical resection pathology | pathology | 202 | 58 | |
| [39] | Sawas | 2019 | cohort | Rochester, Minnesota | 1996–1997, 2009–2012 | hospital database | endoscopy and/or pathology | 462 | 241 | |
| [59] | Schurr | 2006 | case series | Hamburg, Germany | NR | surgical resection pathology | pathology | 45 | 22 | |
| [60] | Shearer | 2007 | cross-sectional | Glasgow, UK | 1995–2000 | surgical resection pathology | pathology | 15 | 12 | |
| [70] | Siewert | 2006 | cohort | Munich, Germany | 7/1982–12/2005 | surgical resection pathology | pathology | 621 | 494 | |
| [36] | Steiger | 1987 | case series | Allen Park, Michigan | 1975–1982 | surgical resection pathology | pathology | 11 | 2 | |
| [62] | Van Sandick | 2000 | case series | Amsterdam, Netherlands | 1/1993–1/1998 | surgical resection pathology (only pT1 cancers) | pathology | 20 | 20 | |
| [64] | Wijnhoven | 1999 | cross-sectional | Rotterdam, Netherlands | 1987–1997 | surgical resection pathology | pathology | 111 | 60 | |
| Multi-center Studies | ||||||||||
| [72] | Borg | 2016 | case series | Lund & Malmo, Sweden | 1/2006–12/2010 | surgical resection pathology | pathology | 60 | 23 | |
| [81] | Imamura | 2019 | case series | Tokyo, Kumamoto, Fukuoka, Japan | 2006–2013 | surgical resection pathology | pathology | 47 | 35 | |
| [71] | Kunzli | 2018 | case series | Amsterdam & Nieuwegein, Netherlands | 1/2012–8/2016 | endoscopic resection pathology | pathology | 35 | 30 | |
| [84] | Mchembe | 2013 | case series | Bugando, Tanzania | 2008–2013 | pathology | pathology | 13 | 3 | |
| [61] | Sillah | 2009 | cross-sectional | Manchester, UK | 2004–2007 | surgical resection pathology | NR | 248 | 107 | |
| Population-based Studies | ||||||||||
| [44] | Bhat | 2015 | cross-sectional | Northern Ireland | 2003–2008 | Northern Ireland Cancer Registry | Northern Ireland Barrett’s Oesophagus Register >6 months prior to OAC | 716 | 52 | |
| [102] | Cook | 2016 | case-control | USA (SEER-Medicare) | 1994–2009 | SEER-Medicare Cancer Registry (based on ICD9/10 codes) | ICD 9/10 code >6 months prior to OAC diagnosis | 5271 | 662 | |
| [18] | Daly | 2000 | case series | USA (National Cancer Database) | 1/1994–12/1994 | National Cancer Database- American College of Surgeons (based on ICD codes) | survey | 2110 | 777 | |
| [53] | Lagergren | 1999 | case-control | Sweden | 1994–1997 | pathology | pathology | 189 | 118 | |
| [56] | Rantanen | 2016 | case series | Finland | 1980–2007 | EGD/surgical resection pathology | pathology (biopsy or surgery) | 103 | 12 | 31 |
| [63] | Verbeek | 2014 | cross-sectional | Netherlands | 1999–2009 | Netherlands Cancer Registry | Dutch Pathology Registry | 9780 | 791 | |
| [42] | Wenker | 2018 | cross-sectional | USA (National VA Database) | 2002–2016 | VA Cancer Registry (based on ICD codes) | ICD 9 code >6 months prior to OAC diagnosis | 8564 | 419 | |
BE: Barrett’s oesophagus; NR: not reported; EGD: esophagogastroduodenoscopy
Table 2.
Study characteristics of 28 studies of 2,712 oesophagogastric junction adenocarcinomas (OGJAC) patients.
| Ref | Author | Year | Study Design | Study Location | Study Period | OGJAC determination | Barrett’s oesophagus determination | OGJAC definition | # Total OGJAC | # Prior BE Diagnosis | # Concurrent BE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single-center Studies | |||||||||||
| [40] | Amlashi | 2018 | cross-sectional | Houston, Texas | 2002–2015 | hospital cancer registry of patients after chemoradiation therapy | pathology | Siewert 2 | 98 | 26 | |
| [73] | Bai | 2006 | cross-sectional | Xian, China | 1/1995–12/1999 | pathology reports (biopsy, surgical resection) | pathology (biopsy or surgery) | Siewert 2 | 80 | 5 | |
| [21] | Cameron | 2002 | case-control | Rochester, Minnesota | 1/1996–12/1999 | endoscopic or surgical resection pathology (only cancers <2cm) | pathology | within 2 cm of OGJ | 22 | 7 | |
| [23] | Demicco | 2011 | case series | Boston, Massachusetts | 1/2000–5/2008 | surgical resection pathology | pathology (biopsy or surgery) | Siewert 2 | 106 | 19 | 64 |
| [50] | Fein | 1998 | case series | Wuerzburg, Germany | 1992–1997 | surgical resection pathology | pathology | Siewert 2 | 30 | 1 | |
| [25] | Gaca | 2006 | cross-sectional | Durham, North Carolina | 7/1992–2/2001 | surgical resection pathology | NR | within 5cm of OGJ | 96 | 42 | |
| [83] | Gupta | 2001 | case series | Chandigarh, India | 1989–1994 | surgical resection pathology | NR | at or extending 2cm distal to OGJ | 28 | 0 | |
| [74] | Horii | 2011 | case-control | Sendai, Japan | 2000–2009 | pathology (cancers limited to submucosa) | pathology | within 2cm of OGJ and midpoint on oesophageal side | 46 | 23 | |
| [82] | Imai | 2013 | case series | Shizuoka, Japan | 9/2002–3/2009 | endoscopic resection pathology | pathology | Siewert 2 | 49 | 7 | |
| [75] | Kamada | 2012 | cross-sectional | Kurashiki, Japan | 1/2001–12/2008 | endoscopic or surgical resection pathology | pathology | Siewert 2 | 80 | 6 | |
| [27] | Karl | 2000 | cross-sectional | Tampa, Florida | 1989–1999 | surgical resection pathology | pathology | NR | 115 | 56 | |
| [28] | Lada | 2013 | cohort | Rochester, New York | 2000–2011 | surgical resection pathology | medical record review, EGD, pathology | NR | 211 | 73 | |
| [77] | Nagami | 2014 | cross-sectional | Osaka, Japan | 2007–2011 | endoscopic dissection pathology | pathology | Siewert 2 | 43 | 14 | |
| [33] | Pera | 1993 | cross-sectional | Rochester, Minnesota | 1974–1989 | pathology | pathology | extending across OGJ | 14 | 5 | |
| [57] | Reynolds | 2011 | cohort | Dublin, Ireland | 2004–2008 | pathology from database | NR | Siewert 2 | 53 | 1 | |
| [58] | Saha | 2009 | case-control | West Yorkshire, UK | 1/2000–12/2006 | surgical resection pathology (only pT1 cancers) | pathology | Siewert 2 | 44 | 28 | 31 |
| [39] | Sawas | 2019 | cohort | Rochester, Minnesota | 1996–1997, 2009–2012 | hospital database | endoscopy and/or pathology | Siewert 2 | 288 | 140 | |
| [59] | Schurr | 2006 | prospective case series | Hamburg, Germany | NR | surgical resection pathology | pathology | Siewert 2 | 40 | 5 | |
| [60] | Shearer | 2007 | prospective case series | Glasgow, UK | 1995–2000 | surgical resection pathology | pathology | Siewert 2 | 26 | 13 | |
| [70] | Siewert | 2006 | cohort | Munich, Germany | 7/1982–12/2005 | surgical resection pathology | pathology | Siewert 2 | 485 | 27 | |
| [78] | Tsuji | 2004 | cross-sectional | Osaka, Japan | NR | surgical resection pathology | pathology | Siewert 2 | 23 | 2 | |
| [62] | Van Sandick | 2000 | cross-sectional | Amsterdam, Netherlands | 1/1993–1/1998 | surgical resection pathology (only pT1 cancers) | pathology | 12 | 12 | ||
| [64] | Wijnhoven | 1999 | cross-sectional | Rotterdam, Netherlands | 1987–1997 | surgical resection pathology | pathology | Siewert 2 | 141 | 18 | |
| Multi-center Studies | |||||||||||
| [72] | Borg | 2016 | case series | Lund & Malmo, Sweden | 1/2006–12/2010 | surgical resection pathology | pathology | NR | 45 | 11 | |
| [80] | Huang | 2011 | cross-sectional | Nanjing, China and Boston, Massachusetts | 2004–2008, 1991–2008, 1999–2008 | surgical resection pathology | pathology | within 2 cm of OGJ | 70 | 26 | |
| [81] | Imamura | 2019 | case series | Tokyo, Kumamoto, Fukuoka, Japan | 2006–2013 | surgical resection pathology | pathology | Siewert 2 | 273 | 69 | |
| [79] | Yuasa | 2006 | case series | Nagoya, Japan | 1987–2003 | surgical resection pathology | pathology | Siewert 2 | 40 | 2 | |
| Population-based Studies | |||||||||||
| [56] | Rantanen | 2016 | cross-sectional | Finland | 1980–2007 | biopsy/surgical resection pathology | EGD/pathology | NR | 154 | 4 | 24 |
BE: Barrett’s oesophagus; OGJ: oesophagogastric junction; NR: not reported; EGD: esophagogastroduodenoscopy
Evaluation of Studies for Risk of Bias
Risk of bias assessments for the 69 included studies are shown in Supplementary Table 2. Overall, only 10 of 69 studies included a representative sample of the target population, enrolled consecutive patients, had adequate sample size, and reliably measured OAC/OGJAC and Barrett’s oesophagus using objective criteria [18, 23, 24, 46–49, 53, 63, 73]. Few studies fully described the characteristics of OAC/OGJAC patients (n=10), but most studies did not clearly state that consecutive patients were included (n=44) or reliably measure both OAC/OGJAC and Barrett’s oesophagus using histopathology and intestinal metaplasia or goblet cells as standard criteria (n=41). All studies had adequate sample size as we excluded studies with <10 OAC/OGJAC patients. Twenty-nine studies had a representative sample of OAC/OGJAC patients (i.e., included all cancer stages). Most studies included cancer stage data allowing stratified subgroup analysis based on percent early cancers (n=48).
Pooled Prevalence of Barrett’s oesophagus in OAC
Among 11 studies including 25,248 patients with OAC, the pooled prevalence of prior Barrett’s oesophagus diagnosis was 11.8% (95% CI 8.4–15.6%; I2=98%) (Figure 2). Three studies defined prior Barrett’s oesophagus diagnosis as that was diagnosed ≥6 months prior to cancer diagnosis [17, 42, 44] but did not specify the number of Barrett’s oesophagus detected within 12 months; one study defined prior Barrett’s oesophagus as that diagnosed ≥12 months prior to cancer diagnosis [63], and the rest did not specify time interval for defining prior Barrett’s oesophagus. The pooled prevalence of prior Barrett’s oesophagus among 814 OAC patients was higher in the 6 single-center studies, including mostly surgical resections (16.0%, 95% CI 8.7–24.9%; I2=83%) than among the 24,434 OAC patients in the 5 population-based cancer registry studies (8.4%, 95% CI 5.5–11.9%; I2=98%) (heterogeneity between groups p=0.05). Of 5 studies that specify patients who received Barrett’s oesophagus surveillance endoscopy [51, 57, 63, 65, 68], the prevalence of OAC patients that were diagnosed based on Barrett’s oesophagus surveillance endoscopy was 11.6% (95% CI 4.0–22.1%).
Figure 2.
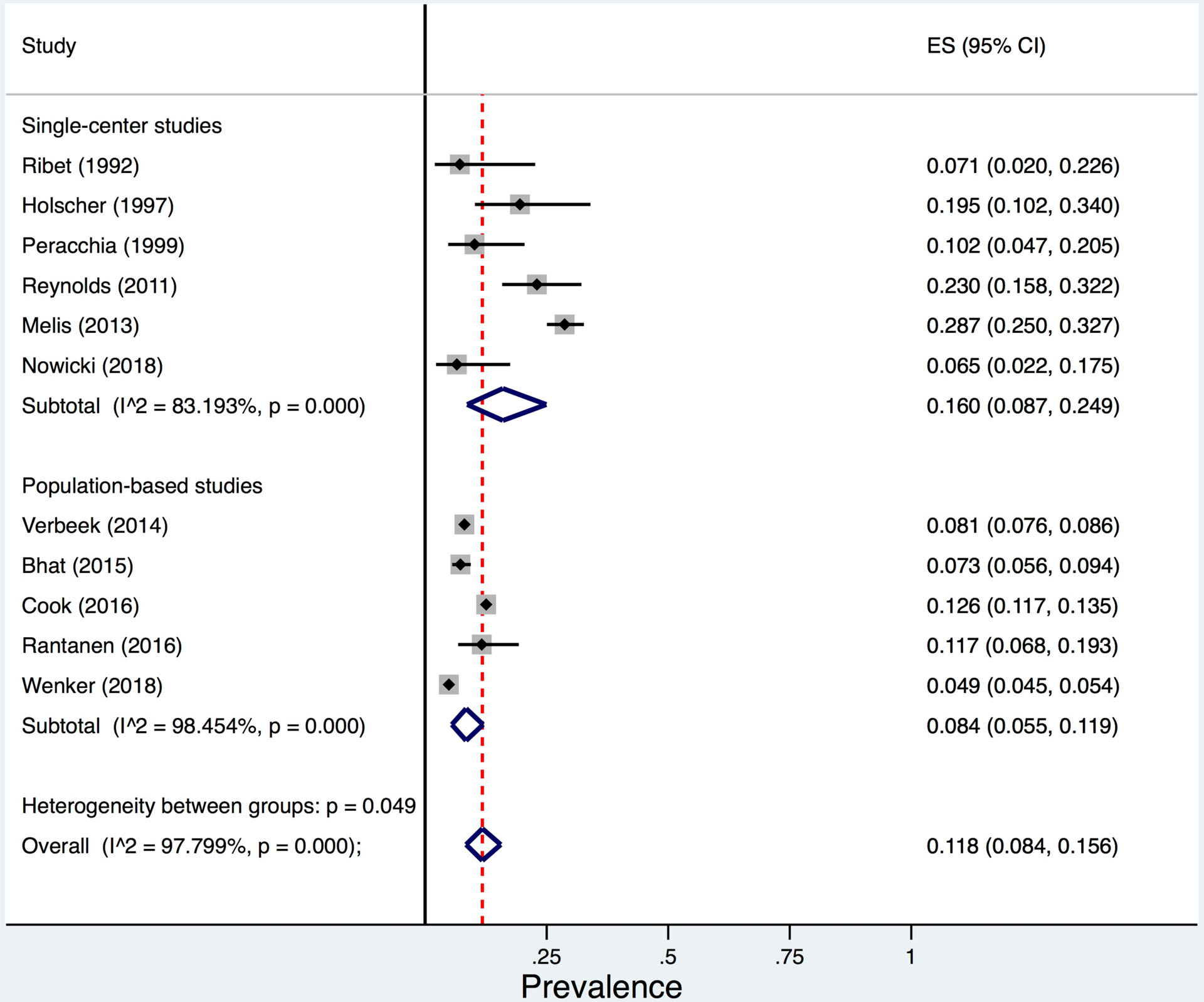
Pooled prevalence of prior Barrett’s oesophagus diagnosis among 25,248 oesophageal adenocarcinoma patients from 11 studies.
In 45 studies of 7,926 OAC patients, the prevalence of concurrent Barrett’s oesophagus was 56.6% (95% CI 48.5–64.6%; I2=98%). Stratified meta-analyses showed numerically lower prevalence of concurrent Barrett’s oesophagus among population based studies (3 studies; 2,402 OAC patients; prevalence=43.0%, 95% CI 26.5–60.4%; I2 not calculated) than single-center studies (39 studies; 5,121 OAC patients; prevalence=58.3%, 95% CI 47.5–68.6%; I2=98%) and multi-center studies (5 studies; 403 OAC patients; prevalence=54.7%, 95% CI 34.8–73.8%; I2=91%); however, the test for heterogeneity between these three sub-groups was not statistically significant (p=0.35) (Figure 3). When evaluating the association between cancer stage and prevalence of concurrent Barrett’s oesophagus, we found that in 10 studies (451 OAC patients) with 100% early stage cancers, the pooled prevalence of concurrent Barrett’s oesophagus was higher (91.3%, 95% CI 82.4–97.6%; I2=86%) than in 7 studies (1,011 OAC patients) with 50–99% of the cohort with early stage cancers (43.8%, 95% CI 12.2–78.4%; I2=99%) and 13 studies (3,616 OAC patients) with <50% of the cohort with early stage cancers (39.7%, 95% CI 33.7–45.9%; I2=89%) (p<0.001) (Figure 4).
Figure 3.

Pooled prevalence of concurrent Barrett’s oesophagus diagnosis among 7,926 oesophageal adenocarcinoma patients from 45 studies.
Figure 4.
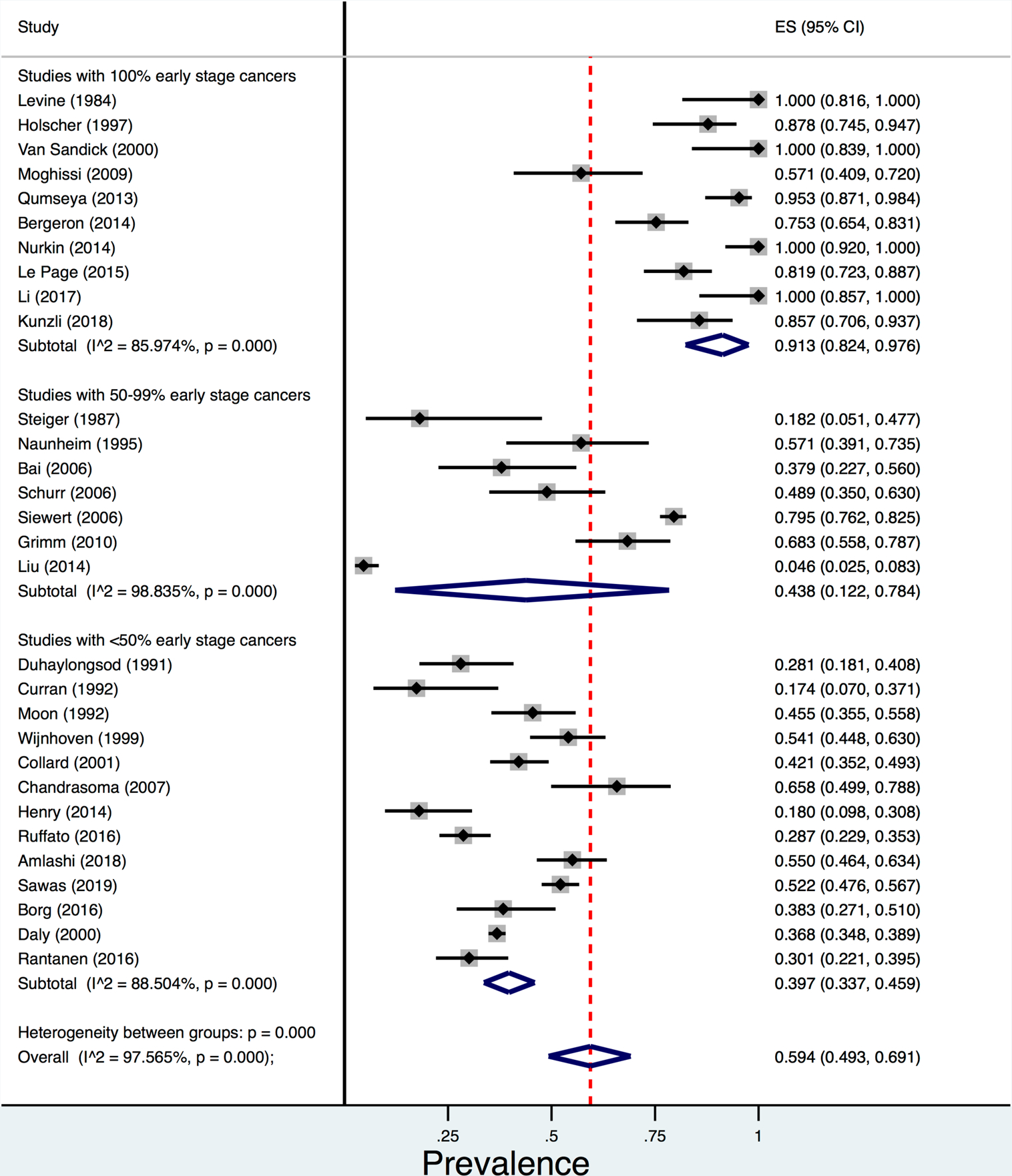
Pooled prevalence of concurrent Barrett’s oesophagus diagnosis among oesophageal adenocarcinoma (OAC) patients comparing 10 studies (451 OAC patients) with 100% early stage cancers to 7 studies (1,011 OAC patients) with 50–99% early stage cancers and 13 studies (3,616 OAC patients) with <50% early stage cancers.
Sensitivity analyses
Meta-analysis restricted to studies published in the last 10 years of the review (2010–2020) showed pooled prevalence of prior Barrett’s oesophagus was 11.8% (95% CI 8.2–16.0, I2=98%) (8 studies; 25,120 OAC patients) and the pooled prevalence of concurrent Barrett’s oesophagus was 56.2% (95% CI 42.0–69.9%, I2=98%) (18 studies; 3,520 OAC patients), which was not different from the overall prevalence (Figure not shown).
We conducted a sensitivity analysis excluding the two large US population-based cohorts [17, 18] and replaced them with 11 US studies with potentially overlapping study populations that were excluded in the primary analysis (Supplementary Table 3) [87–97]. The pooled prevalence of prior Barrett’s oesophagus among 14 studies including 20,891 OAC patients was 11.4% (95% CI 8.3–14.8%; I2=96%) (Supplementary Figure 1). The prevalence of concurrent Barrett’s oesophagus among 6,867 OAC patients in 53 studies was 53.4% (95% CI 45.5–61.1%; I2=97%) (Supplementary Figure 2). Both estimates were similar to the pooled prevalence of prior Barrett’s oesophagus diagnosis in the primary analysis.
Bias and heterogeneity assessments
Graphical funnel plot and Egger’s test demonstrated significant small study bias (p<0.001); although pooled meta-analytic estimates of studies stratified by sample size (<100, 100–1000, >1000 OAC cases) demonstrated only modest differences in pooled prevalence estimates (Figure 5). Among the other variables examined as potential factors explaining observed between-study heterogeneity in univariable meta-regression, method of OAC determination (resection only, biopsy/surgical pathology, database/diagnosis code) and proportion of early cancers (categorically <50%, 50–99%, 100%) were significant predictors (p=0.03 and <0.001, respectively) (Supplementary Table 4).
Figure 5.
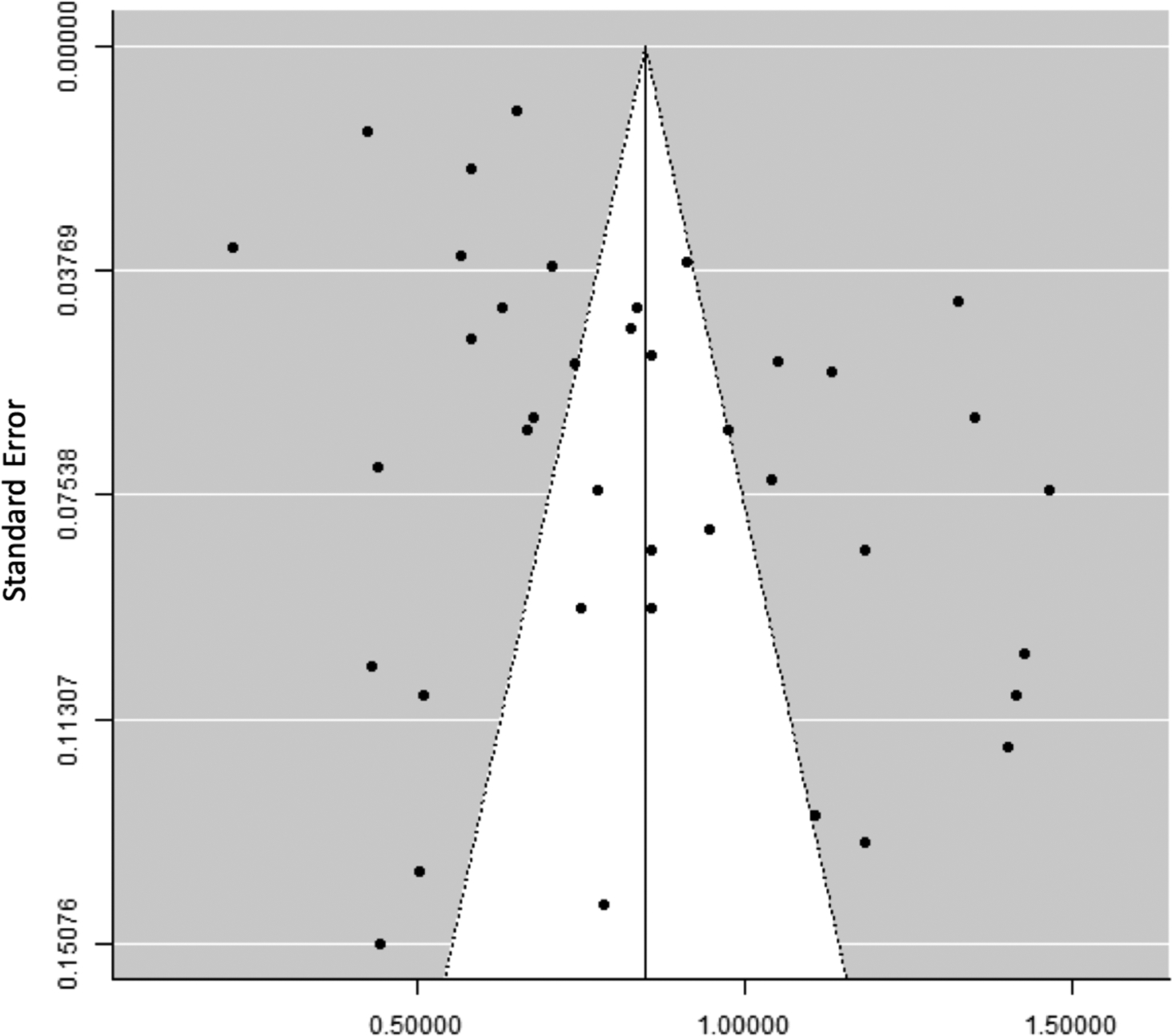
Funnel plot showing significant small study bias among 45 studies evaluating prevalence of concurrent Barrett’s oesophagus diagnosis among 7,926 oesophageal adenocarcinoma patients.
In the cumulative meta-analysis where we examined how the observed pooled estimate changed with studies subsequently added over time, the cumulative pooled prevalence was initially much higher based on studies published prior to 1990, then attenuated with addition of studies published in the 1990s, and largely stabilized by 2000 onwards (Supplementary Figure 3).
Pooled Prevalence of Barrett’s oesophagus in OGJAC
Our primary analysis included 6 studies with 664 patients with OGJAC and found a pooled prevalence of prior Barrett’s oesophagus diagnosis of 23.2% (95% CI 7.5–44.0%; I2=97%) (Supplementary Figure 4). The pooled prevalence of prior Barrett’s oesophagus was higher in the 5 single-center OGJAC studies (29.3%, 95% CI: 13.1–48.8%) compared with the 1 population-based study (2.6%, 95% CI: 1.0–6.5%) (p<0.001). In 25 studies of 2,352 OGJAC patients, the pooled prevalence of concurrent Barrett’s oesophagus was 26.3% (95% CI 17.8–35.7%; I2=95%) (Supplementary Figure 5). The pooled prevalence of concurrent Barrett’s oesophagus among OGJAC patients was no different in the 20 single-center studies (28.1%, 95% CI: 17.1–40.5%) as the 4 multi-center (22.3%, 95% CI: 12.0–34.7%) and 1 population-based study (15.6%, 95% CI: 10.7–22.1%) (p=0.11). Given limited number of studies, we did not perform the Egger’s test or meta-regression for OGJAC studies.
Discussion
Among patients with OAC, prior Barrett’s oesophagus diagnosis was present in 11.8%, while 56.6% had concurrent Barrett’s oesophagus diagnosed at the time of OAC. Studies that included all early stage OAC patients had a higher prevalence of concurrent Barrett’s oesophagus (91.3%) than studies that included all stages of OAC (39.7%). Among those with OGJAC, the pooled prevalence of prior Barrett’s oesophagus diagnosis was 23.2%, and concurrent Barrett’s oesophagus was 26.3%.
Our study reported a higher prevalence of prior Barrett’s oesophagus diagnosis than the one previous meta-analysis (4.7% ± 2.9%) of studies published through 2000 [10]. The previous meta-analysis included 12 studies comprising 1503 cases of OAC/OGJAC but included studies that combined OAC with high-grade dysplasia, gastric cardia and OGJAC (5 studies), which may account for the smaller Barrett’s oesophagus prevalence compared to our meta-analysis. We excluded 6 of these studies from our analysis (5 indistinguishably combined OAC and OGJAC, 1 with overlapping population as another included study). We found a similar prevalence of prior Barrett’s oesophagus diagnosis (11.8%) when pooling contemporary studies published in the last 10 years, although these studies included cohorts as far back as 1980, so likely did not reflect the most contemporary practice patterns. We were unable to restrict to solely contemporary cohorts as none of the studies reporting prior Barrett’s oesophagus diagnosis were limited to cohorts from the last 10 years.
The low prevalence of previously known Barrett’s oesophagus diagnosis among OAC patients likely reflects missed screening opportunities in patients who did in fact have underlying Barrett’s oesophagus. It is possible but less likely that these patients did not have any of the known demographic or clinical Barrett’s oesophagus risk factors (reflux symptoms, obesity, age >50, male, Caucasians, family history of esophageal cancer). Detailed data on Barrett’s oesophagus risk factors were not reported in the studies, and 15.9% of studies reported racial breakdown. We did not estimate prevalence of prior endoscopy in this meta-analysis but previous studies suggest Barrett’s oesophagus is under-diagnosed prior to OAC due to lack of endoscopic screening. Our previous study of 182 patients with OAC reported only 24.7% underwent any pre-OAC diagnosis endoscopy, and of those who did not undergo previous endoscopy, most had risk factors for Barrett’s oesophagus or OAC (63.5%) [98]. When we pooled studies that confirmed the concurrent presence of Barrett’s oesophagus on histopathology at the time of cancer diagnosis, we found a much higher prevalence of Barrett’s oesophagus, indicating that the majority of OAC patients have underlying Barrett’s oesophagus that was not previously diagnosed. We further examined studies that included only early stage OAC and found the pooled prevalence of concurrent Barrett’s oesophagus was much higher (91.3%) compared to studies with all stages of OAC (39.7%). This finding strongly supports that advanced OAC likely overgrows the underlying Barrett’s oesophagus making Barrett’s detection easier in earlier stages [99]. A second explanation to account for the considerable proportion of OAC patients without Barrett’s oesophagus at the time of OAC diagnosis (60.3% in studies with all OAC stages) may be a mechanism of OAC development that excludes Barrett’s oesophagus. A recent study found improved survival in OAC patients with synchronous Barrett’s oesophagus compared to those without Barrett’s oesophagus after adjusting for cancer stage, proposing the possibility of a different phenotype of OAC development [8].
We found 23.2% of those with OGJAC had a prior Barrett’s oesophagus diagnosis and 26.3% had Barrett’s oesophagus confirmed on histopathology at the time of cancer diagnosis; this estimate has not previously been reported. OGJAC shares several risk factors with OAC (e.g., reflux, obesity) [100]. OGJAC are classified by location using the Siewert classification [13]. Siewert type I and II are treated similarly to OAC, while Siewert III and cardia cancers are treated following gastric cancer protocols [50, 101]. We included Siewert I OGJAC with OAC, and Siewert II cancers with OGJAC [13]. Our findings confirm that Siewert II OGJAC occurs sometimes in the setting of Barrett’s oesophagus, and some OGJAC may follow the same carcinoma sequence and share the same risk factors as Barrett’s oesophagus. However, Barrett’s oesophagus was found at the time of cancer diagnosis less commonly in OGJAC than in OAC, which may point to a different mechanism of OGJAC pathogenesis apart from Barrett’s oesophagus in some cases of OGJAC.
Our meta-analysis has multiple strengths including structured comprehensive search strategy resulting in >5000 reviewed studies by 2 reviewers, use of ancestry/bibliography searches to identify any missed studies, contacting study authors for unpublished data, and defining prior and concurrent Barrett’s oesophagus diagnosis, OAC, and OGJAC a priori. We employed a methodologically rigorous approach including sub-group analysis by study site and proportion of early cancers and sensitivity analyses restricted to contemporary studies from the last 10 years and replacing 2 large US population-based studies with 11 excluded US single-center studies. Additionally, we used multiple methods including meta-regression and cumulative meta-analysis to identify potential sources of between-study heterogeneity and bias.
Our meta-analysis has several limitations. There were no studies that were prospectively conducted with the aim of evaluating Barrett’s oesophagus prevalence among OAC/OGJAC patients, thus systematic evaluation for Barrett’s oesophagus was lacking across studies. Especially among studies that define Barrett’s oesophagus using registry or databases, the prevalence of Barrett’s oesophagus may have been under-estimated as it was not systematically captured at the time of cancer diagnosis. The definitions of Barrett’s oesophagus, OAC, OGJAC varied across studies in completeness. Only 3 studies specified prior Barrett’s oesophagus diagnosis as >6 months [17, 42, 44] and 1 additional study as >12 months [63] prior to cancer diagnosis. In order to reduce misclassification of prior Barrett’s oesophagus diagnosis cases, we classified cases as concurrent Barrett’s oesophagus diagnosis when studies did not specifically state that there was a prior diagnosis or that cancer was found on Barrett’s oesophagus surveillance endoscopy; therefore, in the studies without mention of timing of Barrett’s oesophagus diagnosis, cases of prior Barrett’s oesophagus and concurrent Barrett’s oesophagus may have been misclassified. There was also poor reporting on race/ethnicity and consecutiveness of study population. The overall sample size was relatively small, including those with prior Barrett’s oesophagus diagnosis in OAC and OGJAC. Additionally, significant heterogeneity was seen among the reported pooled prevalence. Meta-regression analyses demonstrated that variable proportion of early OAC accounted for some of this heterogeneity, and stratified meta-analysis by proportion of early OAC decreased the heterogeneity seen in the overall pooled prevalence. The significant small study bias is likely substantially explained by the relation of sample size to method of OAC determination, as smaller studies tended to determine OAC on resection specimens and larger studies on registry/diagnosis codes.
Overall, although up to 91% of all newly diagnosed early stage OAC patients had Barrett’s oesophagus seen on histopathology at the time of cancer diagnosis, the prevalence of known Barrett’s oesophagus prior to OAC diagnosis remains low among these patients. These findings call for increased use of Barrett’s oesophagus screening protocols.
Supplementary Material
Acknowledgements
Financial support: The work was supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338) and in part by the resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), at the Michael E. DeBakey VA Medical Center, Houston, TX. Dr. White receives research support from the U.S. Department of Veterans Affairs (CX001430).
Footnotes
Competing interests: None.
DISCLAIMER
The opinions expressed reflect those of the authors and not necessarily those of the Department of Veterans Affairs, the US government or Baylor College of Medicine.
References
- 1.Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365(15):1375–83. [DOI] [PubMed] [Google Scholar]
- 2.Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer epidemiology. 2016;41:88–95. [DOI] [PubMed] [Google Scholar]
- 3.Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Annals of oncology : official journal of the European Society for Medical Oncology. 2012;23(12):3155–62. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi A, Puli S, El-Serag HB, et al. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: a meta-analysis. Gastrointestinal endoscopy. 2008;67(3):394–8. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2014;79(6):897–909.e4; quiz 83.e1, 83.e3. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Naik AD, Duan Z, et al. Surveillance endoscopy is associated with improved outcomes of oesophageal adenocarcinoma detected in patients with Barrett’s oesophagus. Gut. 2016;65(8):1252–60. [DOI] [PubMed] [Google Scholar]
- 7.Wenker TN, Tan MC, Liu Y, et al. Prior Diagnosis of Barrett’s Esophagus Is Infrequent, but Associated with Improved Esophageal Adenocarcinoma Survival. Digestive diseases and sciences. 2018;63(11):3112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawas T, Killcoyne S, Iyer PG, et al. Identification of Prognostic Phenotypes of Esophageal Adenocarcinoma in 2 Independent Cohorts. Gastroenterology. 2018;155(6):1720–8.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corley DA, Mehtani K, Quesenberry C, et al. Impact of endoscopic surveillance on mortality from Barrett’s esophagus-associated esophageal adenocarcinomas. Gastroenterology. 2013;145(2):312–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulai GS, Guha S, Kahn KL, et al. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122(1):26–33. [DOI] [PubMed] [Google Scholar]
- 11.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. The American journal of gastroenterology. 1998;93(7):1028–32. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. The British journal of surgery. 1998;85(11):1457–9. [DOI] [PubMed] [Google Scholar]
- 14.Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. International journal of health policy and management. 2014;3(3):123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of public health = Archives belges de sante publique. 2014;72(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook MB, Drahos J, Wood S, et al. Pathogenesis and progression of oesophageal adenocarcinoma varies by prior diagnosis of Barrett’s oesophagus. British journal of cancer. 2016;115(11):1383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly JMF W;Little AG;Winchester DP;McKee RF;Stewart AK;Fremgen AM. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. Journal of the American College of Surgeons. 2000;190:562–72; discussion 72–3. [DOI] [PubMed] [Google Scholar]
- 19.Wallace BC, Lajeunesse MJ, Dietz G, et al. OpenMEE: Intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods in Ecology and Evolution. 2017;8(8):941–7. [Google Scholar]
- 20.Bergeron EJ Jules L,;Chang Andrew C;Orringer Mark B;Reddy Rishindra M. Endoscopic ultrasound is inadequate to determine which T1/T2 esophageal tumors are candidates for endoluminal therapies. The Journal of thoracic and cardiovascular surgery. 2014;147:765–71: Discussion 71–3. [DOI] [PubMed] [Google Scholar]
- 21.Cameron AJ, Souto EO, Smyrk TC. Small adenocarcinomas of the esophagogastric junction: association with intestinal metaplasia and dysplasia. The American journal of gastroenterology. 2002;97(6):1375–80. [DOI] [PubMed] [Google Scholar]
- 22.Chen MYMO, David J;Gelfand David W. More Evidence for the Increasing Prevalence of Adenocarcinoma of the Esophagus over an 18-Year Period. Journal of Clinical Gastroenterology. 1995;21:254–5. [DOI] [PubMed] [Google Scholar]
- 23.Demicco EGF, Alton B;Baba Yoshifumi;Agbor-Etang Brian;Bergethon Kristin;Mandal Rajni;Daives Diane;Fukuoka Junya;Shimizu Michio;Dias-Santagata Dora;Ogino Shuji;Iafrate A John;Gaissert Henning A;Mino-Kenudson Mari. The dichotomy in carcinogenesis of the distal esophagus and esophagogastric junction: intestinal-type vs cardiac-type mucosa-associated adenocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:1177–90. [DOI] [PubMed] [Google Scholar]
- 24.Duhaylongsod FGW WG. Barrett’s esophagus and adenocarcinoma of the esophagus and gastroesophageal junction. The Journal of thoracic and cardiovascular surgery. 1991;102:36–41; discussion −2. [PubMed] [Google Scholar]
- 25.Gaca JGP, Rebecca P;Peterson Bercedis L;Harpole David H;D’Amico Thomas A;Pappas Theodore N;Seigler Hilliard F;Wolfe Walter G;Tyler Douglas S. Pathologic nodal status predicts disease-free survival after neoadjuvant chemoradiation for gastroesophageal junction carcinoma. Annals of surgical oncology. 2006;13:340–6. [DOI] [PubMed] [Google Scholar]
- 26.Haggitt RC, Tryzelaar J, Ellis FH, et al. Adenocarcinoma complicating columnar epithelium-lined (Barrett’s) esophagus. American journal of clinical pathology. 1978;70(1):1–5. [DOI] [PubMed] [Google Scholar]
- 27.Karl RCS R;Boulware D;Baker S;Coppola D Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Annals of surgery. 2000;231:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lada MJN, Dylan R;Han Michelle;Timratana Poochong;Alsalahi Omran;Peyre Christian G;Jones Carolyn E;Watson Thomas J;Peters Jeffrey H. Gastroesophageal reflux disease, proton-pump inhibitor use and Barrett’s esophagus in esophageal adenocarcinoma: Trends revisited. Surgery. 2013;154:856–64; discussion 64–6. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Yamashita DT, Hawel JD, et al. Endoscopic mucosal resection versus esophagectomy for intramucosal adenocarcinoma in the setting of barrett’s esophagus. Surgical endoscopy. 2017;31(10):4211–6. [DOI] [PubMed] [Google Scholar]
- 30.Moon MRS WJ;Haasler GB;Condon RE. Transhiatal and transthoracic esophagectomy for adenocarcinoma of the esophagus. Archives of surgery (Chicago, Ill : 1960). 1992;127:951–5. [DOI] [PubMed] [Google Scholar]
- 31.Naunheim KSP PJ; Roy TS;Schlueter JM;Kim H;Baue AE. Multimodality therapy for adenocarcinoma of the esophagus. The Annals of thoracic surgery. 1995;59:1085–90; discussion 90–1. [DOI] [PubMed] [Google Scholar]
- 32.Nurkin SJN, Hector R;Yendamuri Sai;LeVea Charles M;Nwogu Chumy E;Groman Adrienne;Wilding Gregory;Bain Andrew J;Hochwald Steven N;Khushalani Nikhil I. Outcomes of endoscopic resection for high-grade dysplasia and esophageal cancer. Surgical endoscopy. 2014;28:1090–5. [DOI] [PubMed] [Google Scholar]
- 33.Pera MC A;Trastek VF;Carpenter HA;Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510–3. [DOI] [PubMed] [Google Scholar]
- 34.Qumseya BJP, Abraham;Rizk Cynthia;Cangemi David J;Wolfsen Christianne;Raimondo Massimo;Woodward Timothy A;Wallace Michael B;Wolfsen Herbert C. Survival in esophageal high-grade dysplasia/adenocarcinoma post endoscopic resection. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2013;45:1028–33. [DOI] [PubMed] [Google Scholar]
- 35.Reyes CVW T Primary adenocarcinoma of the esophagus: a review of 12 cases. Journal of surgical oncology. 1981;18:153–8. [DOI] [PubMed] [Google Scholar]
- 36.Steiger ZW RF;Leichman L;Busuito MJ;Rosenberg JC. Primary adenocarcinoma of the esophagus. Journal of surgical oncology. 1987;36:68–70. [DOI] [PubMed] [Google Scholar]
- 37.Melis M, Weber J, Shridhar R, et al. Body mass index and perioperative complications after oesophagectomy for adenocarcinoma: a systematic database review. BMJ Open. 2013;3(5):02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine MS, Caroline D, Thompson JJ, et al. Adenocarcinoma of the esophagus: relationship to Barrett mucosa. Radiology. 1984;150(2):305–9. [DOI] [PubMed] [Google Scholar]
- 39.Sawas T, Azad N, Killcoyne S, et al. Comparison of Phenotypes and Risk Factors for Esophageal Adenocarcinoma at Present vs Prior Decades. Clin Gastroenterol Hepatol. 2019;08:08. [DOI] [PubMed] [Google Scholar]
- 40.Amlashi FG, Wang X, Davila RE, et al. Barrett’s Esophagus after Bimodality Therapy in Patients with Esophageal Adenocarcinoma. Oncology. 2018;95(2):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandrasoma P, Wickramasinghe K, Ma Y, et al. Adenocarcinomas of the distal esophagus and “gastric cardia” are predominantly esophageal carcinomas. Am J Surg Pathol. 2007;31(4):569–75. [DOI] [PubMed] [Google Scholar]
- 42.Wenker TN, Tan MC, Liu Y, et al. Prior Diagnosis of Barrett’s Esophagus Is Infrequent, but Associated with Improved Esophageal Adenocarcinoma Survival. Dig Dis Sci. 2018;63(11):3112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bellone GS, Dino;Chiusa Luigi;Brondino Gabriele;Carbone Anna;Prati Adriana;Scirelli Tiziana;Camandona Michele;Palestro Giorgio;Dei Poli Marcello. Transforming growth factor-beta binding receptor endoglin (CD105) expression in esophageal cancer and in adjacent nontumorous esophagus as prognostic predictor of recurrence. Annals of surgical oncology. 2007;14:3232–42. [DOI] [PubMed] [Google Scholar]
- 44.Bhat SKM, Damian T;Coleman Helen G;Johnston Brian T;Cardwell Christopher R;McMenamin Una;Bannon Finian;Hicks Blanaid;Kennedy Grace;Gavin Anna T;Murray Liam J. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut. 2015;64:20–5. [DOI] [PubMed] [Google Scholar]
- 45.Cavallin F, Scarpa M, Cagol M, et al. Esophageal Cancer Clinical Presentation: Trends in the Last 3 Decades in a Large Italian Series. Annals of surgery. 2018;267(1):99–104. [DOI] [PubMed] [Google Scholar]
- 46.Cijs TM, Verhoef C, Steyerberg EW, et al. Outcome of esophagectomy for cancer in elderly patients. The Annals of thoracic surgery. 2010;90(3):900–7. [DOI] [PubMed] [Google Scholar]
- 47.Collard JM. Exclusive radical surgery for esophageal adenocarcinoma. Cancer. 2001;91:1098–104. [PubMed] [Google Scholar]
- 48.Curran AJG DB;O’Muircheartaigh I;Keeling P. Transhiatal oesophagectomy in the management of advanced oesophageal carcinoma. Journal of the Royal College of Surgeons of Edinburgh. 1992;37:225–8. [PubMed] [Google Scholar]
- 49.Driessen AVR D;De Leyn P;Filez L;Peeters M;Winnepenninckx V;Penninckx F;Lerut T;Ectors N;Belgian Contact Group HP. Are carcinomas of the cardia oesophageal or gastric adenocarcinomas? European journal of cancer (Oxford, England : 1990). 2003;39:2487–94. [DOI] [PubMed] [Google Scholar]
- 50.Fein M, Fuchs KH, Ritter MP, et al. Application of the new classification for cancer of the cardia. Surgery. 1998;124(4):707–13; discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 51.Hölscher AHB E;Schneider PM;Siewert JR. Early adenocarcinoma in Barrett’s oesophagus. The British journal of surgery. 1997;84:1470–3. [PubMed] [Google Scholar]
- 52.Khan OAA C;Soomro I;Duffy JP;Morgan WE;Beggs FD. Pathological determinants of survival in node-negative oesophageal cancer. The British journal of surgery. 2004;91:1586–91. [DOI] [PubMed] [Google Scholar]
- 53.Lagergren JB R;Lindgren A;Nyrén O Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. The New England journal of medicine. 1999;340:825–31. [DOI] [PubMed] [Google Scholar]
- 54.Le Page PAV Pras P;Penman Ian D;Couper Graeme W;Paterson-Brown Simon;Lamb Peter J. Surgical and endoscopic management of high grade dysplasia and early oesophageal adenocarcinoma. The surgeon : journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2015. [DOI] [PubMed] [Google Scholar]
- 55.Moghissi KD, Kate;Stringer Mark;Thorpe JAC. Photofrin PDT for early stage oesophageal cancer: long term results in 40 patients and literature review. Photodiagnosis and photodynamic therapy. 2009;6:159–66. [DOI] [PubMed] [Google Scholar]
- 56.Rantanen T, Oksala N, Sand J. Adenocarcinoma of the oesophagus and oesophagogastric junction: Analysis Of incidence and risk factors. Anticancer Research. 2016;36(5):2323–9. [PubMed] [Google Scholar]
- 57.Reynolds JVM E;Ravi N;Donohoe C;O’Toole D;O’Byrne K;Hollywood D Modern oncologic and operative outcomes for oesophageal cancer treated with curative intent. Irish medical journal. 2011;104:235–8. [PubMed] [Google Scholar]
- 58.Saha AKS, Christopher D.;Sue-Ling Henry;Dexter Simon P. L.;Sarela Abeezar I. Comparison of oncological outcomes after laparoscopic transhiatal and open esophagectomy for T1 esophageal adenocarcinoma. Surgical endoscopy. 2009;23:119–24. [DOI] [PubMed] [Google Scholar]
- 59.Schurr PGY, Emre F;Kaifi Jussuf T;Lasch Steffi;Strate Tim;Kutup Asad;Cataldegirmen Guel;Bubenheim Michael;Pantel Klaus;Izbicki Jakob R. Lymphatic spread and microinvolvement in adenocarcinoma of the esophago-gastric junction. Journal of surgical oncology. 2006;94:307–15. [DOI] [PubMed] [Google Scholar]
- 60.Shearer CJG, James J;Neilson Lisa J;Stuart Robert C. Modified classification for adenocarcinoma of the gastro-oesophageal junction. ANZ journal of surgery. 2007;77:544–9. [DOI] [PubMed] [Google Scholar]
- 61.Sillah KP, Susan A.;Watkins Gillian R.;McShane James;West Catharine M.;Page Richard;Welch Ian M. The degree of circumferential tumour involvement as a prognostic factor in oesophageal cancer☆☆☆. European Journal of Cardio-Thoracic Surgery. 2009;36:368–73. [DOI] [PubMed] [Google Scholar]
- 62.van Sandick JW, van Lanschot JJ, ten Kate FJ, et al. Pathology of early invasive adenocarcinoma of the esophagus or esophagogastric junction: implications for therapeutic decision making. Cancer. 2000;88(11):2429–37. [DOI] [PubMed] [Google Scholar]
- 63.Verbeek REL Max;Ten Kate Fiebo J W;van Hillegersberg Richard;Vleggaar Frank P;van Baal Jantine W P M;van Oijen Martijn G H;Siersema Peter D. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: a population-based cohort study. The American journal of gastroenterology. 2014;109:1215–22. [DOI] [PubMed] [Google Scholar]
- 64.Wijnhoven BPS PD;Hop WC;van Dekken H;Tilanus HW. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. The British journal of surgery. 1999;86:529–35. [DOI] [PubMed] [Google Scholar]
- 65.Peracchia AB L;Via A;Incarbone R Current trends in the surgical treatment of esophageal and cardia adenocarcinoma. Journal of experimental & clinical cancer research : CR. 1999;18:289–94. [PubMed] [Google Scholar]
- 66.Ribet MEM EA. Reflux esophagitis and carcinoma. Surgery, gynecology & obstetrics. 1992;175:121–5. [PubMed] [Google Scholar]
- 67.Grimm M, Lazariotou M, Kircher S, et al. MMP-1 is a (pre-)invasive factor in Barrett-associated esophageal adenocarcinomas and is associated with positive lymph node status. J. 2010;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nowicki A, Kula Z, Swierszczynska A. Barrett's esophagus and gland cancer - the experience of one center. Pol Przegl Chir. 2018;90(3):19–24. [DOI] [PubMed] [Google Scholar]
- 69.Ruffato A, Lugaresi M, Mattioli B, et al. Total Lymphadenectomy and Nodes-Based Prognostic Factors in Surgical Intervention for Esophageal Adenocarcinoma. Ann Thorac Surg. 2016;101(5):1915–20. [DOI] [PubMed] [Google Scholar]
- 70.Siewert JR, Stein HJ, Feith M. Adenocarcinoma of the esophago-gastric junction. Scand J Surg. 2006;95(4):260–9. [DOI] [PubMed] [Google Scholar]
- 71.Künzli HT, Belghazi K, Pouw RE, et al. Endoscopic management and follow-up of patients with a submucosal esophageal adenocarcinoma. United European Gastroenterology Journal. 2018;6(5):669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Borg D, Hedner C, Gaber A, et al. Expression of IFITM1 as a prognostic biomarker in resected gastric and esophageal adenocarcinoma. Biomark Res. 2016;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yi Bai J-GL;Dang Cheng-Xue. Adenocarcinoma of the Esophagogastric Junction in China according to Siewert’s classification. Japanese journal of clinical oncology. 2006;36:364–7. [DOI] [PubMed] [Google Scholar]
- 74.Tomoyuki Horii TK;Abe Yasuhiko;Kikuchi Ryosuke;Unakami Hiroyuki;Iijima Katsunori;Imatani Akira;Ohara Shuichi;Shimosegawa Tooru. Two distinct types of cancer of different origin may be mixed in gastroesophageal junction adenocarcinomas in Japan: evidence from direct evaluation of gastric acid secretion. Scandinavian journal of gastroenterology. 2011;46:710–9. [DOI] [PubMed] [Google Scholar]
- 75.Hiromichi Kamada TK,;Yamanaka Yoshiyuki;Manabe Noriaki;Kusunoki Hiroaki;Shiotani Akiko;Inoue Kazuhiko;Hata Jiro;Matsumoto Hideo;Akiyama Takashi;Hirai Toshihiro;Sadahira Yoshito;Haruma Ken. Relationship between gastroesophageal junction adenocarcinoma and Helicobacter pylori infection in Japan. Digestion. 2012;85:256–60. [DOI] [PubMed] [Google Scholar]
- 76.Liu SD James Y;Yao Lena;Li Xiaohong;Reid Brian;Self Steve;Ma Jie;Chang Yuxi;Feng Shixian;Tapsoba Jean de Dieu;de Dieu Tapsoba Jean;Sun Xibin Xin;Sun Xibin Xin. Esophageal Adenocarcinoma and Its Rare Association with Barrett’s Esophagus in Henan, China. PloS one. 2014;9:e110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirohisa Nagami YM;Shiba Masatsugu;Obayashi Tomoko;Omissnami Masaki;Fukunaga Shusei;Sugimori Satoshi;Yamagami Hirokazu;Tanigawa Tetsuya;Watanabe Kenji;Watanabe Toshio;Tominaga Kazunari;Fujiwara Yasuhiro;Arakawa Tetsuo. Clinical Efficacy of Endoscopic Submucosal Dissection for Adenocarcinomas of the Esophagogastric Junction. Endoscopy international open. 2014;2:E15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shingo Tsuji NI;Tsukamoto Yoshitane;Mano Masayuki;Kasugai Tsutomu;Miyashiro Isao;Doki Yuichiro;Iishi Hiroyasu;Kudo Masatoshi. Mucin phenotypic expression and background mucosa of esophagogastric junctional adenocarcinoma. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2004;7:97–103. [DOI] [PubMed] [Google Scholar]
- 79.Hideo Yuasa NM;Yamada Tatsuharu;Ebata Tomoki;Nimura Yuji;Hattori Tatsuo. Clinicopathologic comparison of Siewert type II and III adenocarcinomas of the gastroesophageal junction. World journal of surgery. 2006;30:364–71. [DOI] [PubMed] [Google Scholar]
- 80.Huang QF Xiangshan;Agoston Agoston T;Feng Anning;Yu Huiping;Lauwers Gregory;Zhang Lihua;Odze Robert D. Comparison of gastro-oesophageal junction carcinomas in Chinese versus American patients. Histopathology. 2011;59:188–97. [DOI] [PubMed] [Google Scholar]
- 81.Imamura Y, Watanabe M, Toihata T, et al. Recent Incidence Trend of Surgically Resected Esophagogastric Junction Adenocarcinoma and Microsatellite Instability Status in Japanese Patients. Digestion. 2019;99(1):6–13. [DOI] [PubMed] [Google Scholar]
- 82.Imai K, Kakushima N, Tanaka M, et al. Validation of the application of the Japanese curative criteria for superficial adenocarcinoma at the esophagogastric junction treated by endoscopic submucosal dissection: A long-term analysis. Surgical Endoscopy. 2013;27(7):2436–45. [DOI] [PubMed] [Google Scholar]
- 83.Gupta NM, Jindal R, Prakash O, et al. Comparison of the clinical profile and outcome for squamous cell carcinoma and adenocarcinoma of the distal esophagus and cardia in India. Surg. 2001;31(5):400–4. [DOI] [PubMed] [Google Scholar]
- 84.Mchembe MDR Peter F;Chalya Phillipo L;Jaka Hyasinta;Koy Mheta;Mahalu William. Endoscopic and clinicopathological patterns of esophageal cancer in Tanzania: experiences from two tertiary health institutions. World Journal of Surgical Oncology. 2013;11:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henry MACdAL Mauro Masson;Ribeiro Priscila Watson;Rodrigues Maria Aparecida Marchesan. Epidemiological features of esophageal cancer. Squamous cell carcinoma versus adenocarcinoma. Acta cirúrgica brasileira / Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia. 2014;29:389–93. [DOI] [PubMed] [Google Scholar]
- 86.Epari KC Richard. Oesophagectomy for tumours and dysplasia of the oesophagus and gastro-oesophageal junction. ANZ journal of surgery. 2009;79:251–7. [DOI] [PubMed] [Google Scholar]
- 87.Girvin GWM GH; Bates DM;Garscia JM;Clyde;Lin PH. Treating esophageal cancer with a combination of chemotherapy, radiation, and excision. American journal of surgery. 1995;169:557–9. [DOI] [PubMed] [Google Scholar]
- 88.Hoff SJ, Sawyers JL, Blanke CD, et al. Prognosis of adenocarcinoma arising in Barrett’s esophagus. The Annals of thoracic surgery. 1998;65:176–80; discussion 80–1. [DOI] [PubMed] [Google Scholar]
- 89.Birgisson SR TW;Easley KA;Richter JE. The lack of association between adenocarcinoma of the esophagus and gastric surgery: a retrospective study. The American journal of gastroenterology. 1997;92:216–21. [PubMed] [Google Scholar]
- 90.Firoozi B, Vega KJ, Holland BK, et al. Epidemiologic pattern of esophageal cancer at an inner-city university hospital. Journal of the Association for Academic Minority Physicians : the official publication of the Association for Academic Minority Physicians. 1999;10:44–7. [PubMed] [Google Scholar]
- 91.Sabel MSP K;Toon H;Smith JL. Adenocarcinoma of the esophagus with and without Barrett mucosa. Archives of surgery (Chicago, Ill : 1960). 2000;135:831–5; discussion 6. [DOI] [PubMed] [Google Scholar]
- 92.Scott Bolton JW TT;Yeo CJ;Cameron JL;Heitmiller RF. Esophagectomy for adenocarcinoma in patients 45 years of age and younger. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2001;5:620–5. [DOI] [PubMed] [Google Scholar]
- 93.Swanson SJB HF;Bueno R;Jaklitsch MT;Lukanich JM;Allred E;Mentzer SJ;Sugarbaker DJ. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection and cervical esophagogastrostomy for esophageal carcinoma. The Annals of thoracic surgery. 2001;72:1918–24; discussion 24–5. [DOI] [PubMed] [Google Scholar]
- 94.Corley DAL, Theodore R;Habel Laurel A;Weiss Noel S;Buffler Patricia A. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–40. [DOI] [PubMed] [Google Scholar]
- 95.Hashemi NL David;DiMarino Anthony J;Cohen Sidney. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Digestive diseases and sciences. 2009;54:1708–12. [DOI] [PubMed] [Google Scholar]
- 96.Leers JMD, Steven R;Chan Nadia;Ayazi Shahin;Oezcelik Arzu;Abate Emmanuele;Banki Farzaneh;Lipham John C;Hagen Jeffrey A;DeMeester Tom R. Clinical characteristics, biologic behavior, and survival after esophagectomy are similar for adenocarcinoma of the gastroesophageal junction and the distal esophagus. The Journal of thoracic and cardiovascular surgery. 2009;138:594–602; discussion 1–2. [DOI] [PubMed] [Google Scholar]
- 97.Singhal S, Kapoor H, Subramanian S, et al. Polymorphisms of Genes Related to Function and Metabolism of Vitamin D in Esophageal Adenocarcinoma. J Gastrointest Cancer. 2019;50(4):867–78. [DOI] [PubMed] [Google Scholar]
- 98.Hammad TA, Thrift AP, El-Serag HB, et al. Missed Opportunities for Screening and Surveillance of Barrett’s Esophagus in Veterans with Esophageal Adenocarcinoma. Digestive diseases and sciences. 2019;64(2):367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cameron AJ, Lomboy CT, Pera M, et al. Adenocarcinoma of the esophagogastric junction and Barrett’s esophagus. Gastroenterology. 1995;109(5):1541–6. [DOI] [PubMed] [Google Scholar]
- 100.Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Seminars in radiation oncology. 2013;23(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers (Version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed June 24, 2018. [updated May 22, 2018. Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
- 102.Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


