Abstract
The success of organ transplantation is limited by the complications of immunosuppression, by chronic rejection and by the insufficient organ supply and thousands of patients die every year while waiting for a transplant. With recent progress in xenotransplantation permitting porcine organ graft survival of months or even years in non-human primates, there is renewed interest in its potential to alleviate the organ shortage. Many of these advances are the result of our heightened capacity to modify pigs genetically, particularly with the development of CRISPR/Cas9-based gene editing methodologies. While this approach allows the engineering of pig organs that are less prone to rejection, the clinical application of xenotransplantation will require the ability to avoid the ravages of a multi-faceted attack on the immune system, while preserving the capacity to protect both the recipient and the graft from infectious microorganisms. In this review, we will discuss the potential and limitations of these modifications and how the engineering of the graft can be leveraged to alter the host immune response so that all types of immune attack are avoided.
One sentence summary.
Are genetically engineered pigs the answer to the organ shortage?
Introduction
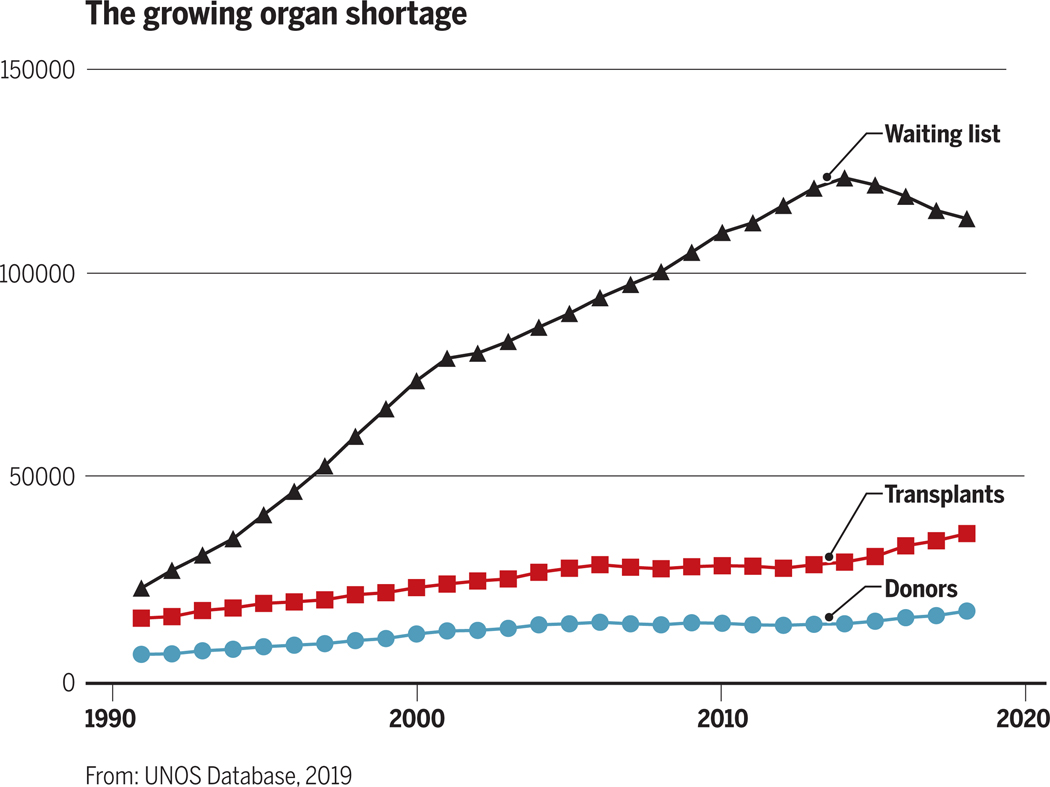
In 2017 there were 114,000 patients in the United States on waiting lists for organ transplantation (http://optn.transplant.hrsa.gov). On average, 20 of these patients die every day because of failure to obtain a suitable organ. Disturbingly, the disparity between the number of patients on transplant waiting lists versus the number of available organs continues to increase (Figure 1).
Figure 1:
The growing allogeneic organ supply/demand imbalance has resulted in an expanding transplant waiting list.
Three possible approaches to solving the organ shortage have emerged: 1) Increasing deceased donor donation. Since only a very small fraction of deaths result in transplantable organs (i.e. brain deaths and donations after cardiac death, which account for less than 1% of mortalities), the potential success of this approach is limited. Even in countries with “presumed consent” laws for removal of organs from brain-dead individuals who have not specified their wishes regarding organ donation, the disparity between organ need and availability continues to increase1; 2) “Bioengineering” of transplantable organs. This approach often uses stem or progenitor cells to populate organ scaffolds produced either by 3-D printing or by decellularization of human or animal organs. This approach has not yet been successful in large animal models and is hampered by significant hurdles that will need to be overcome to produce an organ that can actually function as a replacement organ. Additionally, unless stem cells are obtained from the intended recipient, there will be antigenic differences requiring immunosuppression and the scaffolds themselves may be immunogenic; 3) The use of organs from animals, or xenotransplantation, the subject of this review. It is conceivable to have an essentially limitless supply of tissues and organs of lower mammalian species such as pigs. If successful, therefore, xenotransplantation could provide a near-term solution for the human organ shortage.
The field of xenotransplantation was jumpstarted by a series of chimpanzee to human renal transplants by Reemtsma et al. in the early 1960s2. One patient survived for nine months following transplantation of a chimpanzee kidney. However, it was clear even then that chimpanzees, as an endangered species, could not meet the growing need for organ transplants. A flurry of attempts to utilize much more readily available baboons as sources of kidneys and hearts met with dismal failure3,4. The use of lower mammalian species was not attempted because such transplants into nonhuman primates were uniformly rejected in minutes to hours, due to the high level of natural antibodies (NAbs) present across such discordant species barriers5.
For the next 20 years, clinical needs were largely met by deceased donation. The enormous success of allogeneic transplantation following the introduction of much-improved immunosuppressive drugs in the late 1980s paradoxically led to a resurgence of interest in xenotransplantation. By this time, the use of chimpanzees as organ sources had been outlawed and the use of other nonhuman primates was plagued by problems of size, availability, viral pathogens and ethics. Nevertheless, several attempts to utilize baboon organs in humans were made with patient survivals ranging from just 20 to 70 days6. Collectively, these issues led the field of transplantation to seek another, less problematic xenograft source animal.
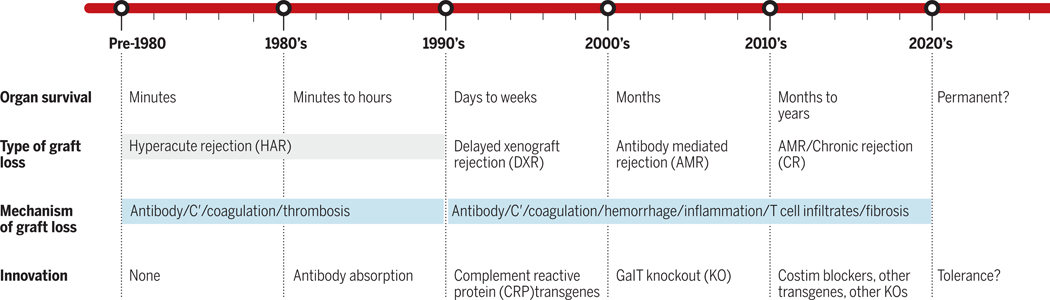
By the mid-1990s, most investigators agreed that the pig would be the most appropriate xenograft source animal for a number of reasons, including size, availability, breeding characteristics and physiologic similarities to human beings. The main impediment to use of the pig was the presence of a high level of NAbs to porcine antigens in old world primates and humans that leads to what has been termed hyperacute rejection (HAR) of xenotransplants5 (Figure 2). However, with advances in genome editing and our understanding of immune rejection of porcine organs, pig to human organ transplantation might be a realistic option in the near future.
Figure 2:
Schematic timeline showing advances in overcoming the immunologic challenges to xenograft survival and the impact on pig organ survival times in primates.
Successful xenotransplantation would have many advantages over allogeneic transplantation, including essentially unlimited availability and far better quality control for both function and absence of infectious organisms in transplanted organs. At the moment elective transplants are only possible for living donation, but if xenotransplanation become a reality, transplants would become elective operations that could be offered before severe complications of organ failure arise. In this review, we summarize what is now known about the immunologic barriers to xenotransplantation and describe progress to date in overcoming these barriers, especially through genetic engineering of pigs.
1. Immune barriers to Xenotransplantation
Transplants from pigs to primates are subject to vigorous immunologic rejection involving both innate and adaptive immune responses. The innate barriers include monocytes and macrophages (reviewed in 7), natural killer (NK) cells (reviewed in 8); and complement and coagulation pathways (reviewed in 8). In addition, natural antibodies (Nab), which are often classified as innate immune components, as they arise without exposure to their known ligands, present another formidable barrier. The most important of these Nabs recognize α-galactose-1,3-galactose (Gal). Gal is a carbohydrate moiety displayed on numerous cell surface glycoproteins and glycolipids of pigs and most other mammalian species, excepting humans and Old World monkeys, due to a frame shift mutation in α−1,3-galactosyltransferase (GalT) in our ancestors9. The high levels of NAbs to Gal in human serum likely reflect exposure to it on numerous microorganisms. Anti-Gal NAbs constitute about 1–4% of circulating human immunoglobulin (Ig)10,11 and include both IgM and IgG. As is discussed below, anti-Gal NAbs were a major early obstacle to xenotransplantation. Antibodies to Gal are responsible for HAR, which occurs within hours following transplant. Attempts to absorb or neutralize these antibodies led to delayed rejection, but without long-term success12 When HAR was successfully prevented by the removal of the Gal epitope from pigs using nuclear transfer technology in the early 2000’s13–15, it became evident that natural antibodies against other pig specificities16 less prominent than Gal, can mediate a more delayed type of vascular xenograft rejection, termed “acute vascular rejection” or “delayed xenograft rejection (DXR)”, which occurs over days to weeks, rather than minutes to hours, as is the case with HAR. Non-Gal NAbs showed binding to multiple cell surface antigens, and many recognize an SDa red blood cell antigen-like terminal carbohydrate produced by a β−1,4 N-acetylgalactosaminyl transferase 2 (B4GalNT2)17 or a N-glycolylneuraminic acid (NeuGc) ligand that is expressed by glycoproteins and gangliosides. NeuGc is highly expressed on endothelial cells of all mammals except humans18 and anti-NeuGc NAbs are present in the majority of human sera19, reflecting a point mutation in the human gene for the CMP-NeuAc hydroxylase (CMAH) that produces the NeuGc epitope20.
B and T cells are the adaptive immune barriers in xenotransplantation.
B cell responses can be dampened by adequate T cell immunosuppression or tolerance induction, although some responses may be T cell-independent 21. T cells can directly attack the graft and can also promote B and NK cell responses. T cell responses, including cytotoxicity, cytokine production, and recruitment and activation of innate cytotoxic cells, are not fully overcome by immunosuppressive therapy in pig-to-primate xenotransplantation22,23. While early studies of mouse anti-pig immune responses revealed very weak direct T cell xenoreactivity, this was due largely to the failure of multiple accessory receptor/ligand interactions between species24 and did not necessarily implicate poor TCR-ligand interactions in xenogeneic combinations. Indeed, the ability of highly disparate porcine MHC molecules to effectively mediate positive selection of murine T cells25 (reviewed in 26) and to select a diverse human T cell repertoire27 suggests that these xenogeneic interactions are highly effective. Direct activation of human CD8+ and CD4+ T cells by porcine SLA class I and class II molecules, respectively, has been described, and are similar in magnitude to direct alloresponses28, reflecting in part the demonstrated efficacy of a number of adhesive and costimulatory interactions between human T cells and porcine ligands. The inability of pig cytokine receptors to respond to human IFN-γ29 may limit the upregulation of MHC and costimulatory molecules by pig cells in the presence of human T cells.
Indirect recognition, in which recipient APCs process and present donor antigens on recipient MHC molecules, is also potent in the human anti-pig direction28,30 and is stronger than the indirect response to alloantigens28, in keeping with the prediction of a greater number of protein, and hence peptide, polymorphisms between than within a species. Indirect activation of IFN-γ-producing recipient T cells was implicated in the rejection of porcine islet xenografts in monkeys, which was not prevented by a strong immunosuppressive regimen that prevented allograft rejection, highlighting the potency of xenogeneic responses31. Indirect memory T cell responses to porcine antigens in non-human primates (NHPs) have been reported to be more resistant to immunosuppression than direct xenoresponses32. Since induced antibody responses to transplanted organs largely reflect presentation of donor antigens by recipient B cells to peptide-reactive T helper cells, IgG responses in NHPs receiving porcine heart or kidney transplants16,33,34 suggest a potent indirect T cell xenoresponse.
2. Role of Genetic Engineering in Overcoming Immune Barriers to Xenotransplantation
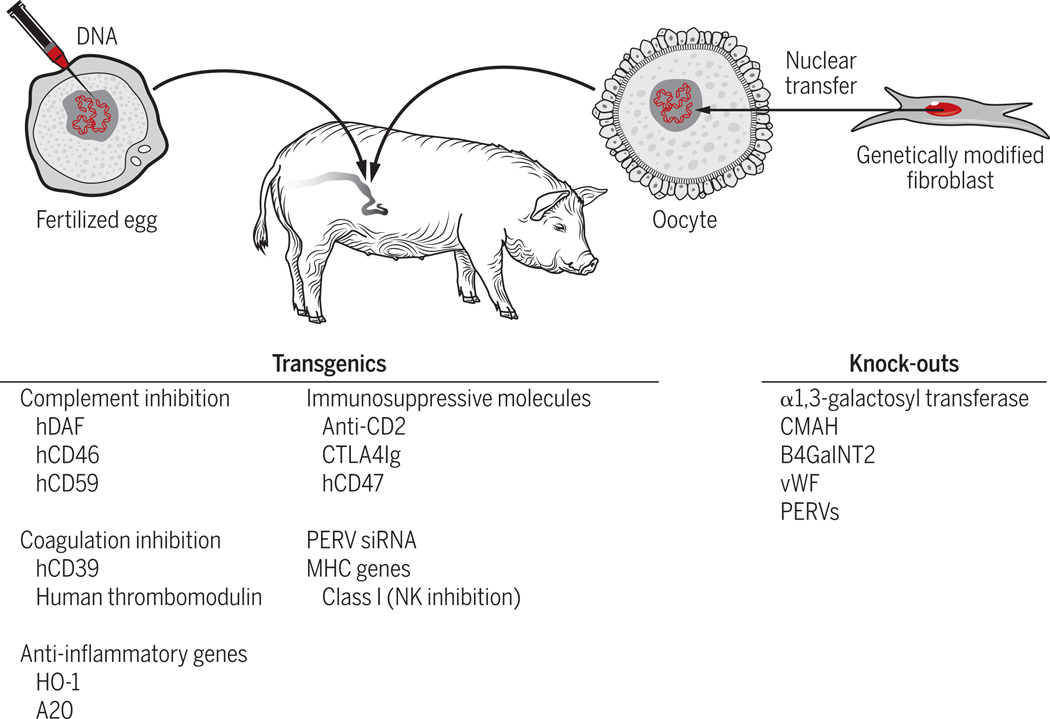
Given the great evolutionary distance between pigs and primates (estimated to be 70 to 80 million years), it is remarkable how similar these two species have remained, both physiologically and immunologically37. Nevertheless, genetic disparities that occurred during evolution resulted in incompatibilities that need to be overcome in order for xenotransplantation to be successful. Fortunately, largely due to their favorable breeding characteristics, pigs are particularly amenable to engineering of their genome. Enormous progress has been made over the past two decades toward overcoming relevant inter-species incompatibilities through such genetic engineering (GE). Genetic modifications that have been introduced into pigs are summarized in Figure 3.
Figure 3:
Genetic modifications that have been made in pigs to facilitate pig to human organ transplantation. See text for details.
A. Hyperacute Rejection:
As mentioned above, NAbs in primates reactive with porcine cell surface antigens led to HAR within minutes to hours following the completion of vascular anastomoses. Since the initiation of HAR required complement activation, the first genetic modifications (GMs) of pigs involved introduction of transgenic human complement regulatory proteins (CRPs) into the pig genome. CRPs are expressed on normal endothelial cells to prevent collateral damage to host cells and modulate inflammation when complement is activated in the destruction of microorganisms. To enhance these protective mechanisms in pigs whose organs would be exposed to human antibodies and complement, DNA constructs for three such human CRPs (CD55, CD46 and CD59) were introduced into porcine fertilized ova via pronuclear injection38. Expression of human CRPs in pig endothelial cells mitigated, but did not eliminate HAR, prolonging organ graft survival for days to weeks when NAbs were removed by absorption procedures39. Unfortunately, NAbs returned after the transplants, often at even higher titers, causing DXR12.
In the early 2000’s, following the demonstration of cloning through nuclear transfer in mammalian species (i.e. the Dolly experiment40), several laboratories knocked out the GalT gene by homologous recombination in cultured fetal fibroblasts, followed by nuclear transfer into enucleated ova and implantation of the resulting modified embryos into receptive sows13. The process was repeated, to knock out the second allele for this enzyme, and the resulting offspring no longer expressed Gal. Pig organs were no longer affected by high titers of anti-Gal in primate recipients, thus largely eliminating the problem of HAR and markedly extending xenograft survival33,41.
Further advances in techniques for genome editing, including the use of Zinc Finger nucleases, Talens (transcription activator-like effector nucleases) and most recently, CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats and their associated RNA-guided DNA endonuclease #9)42, have made it increasingly feasible to change the pig genome in ways intended to improve the success of xenotransplantation. All of these techniques have been used to modify the genome through nuclear transfer of nuclei from gene-edited somatic cells.
B. Non-Gal-mediated DXR:
As discussed above, while the problem of HAR was overcome by genetic engineering, DXR mediated by non-Gal NAbs became the next major hurdle for xenotransplantation. The discovery of the B4Gal and NeuGc specificities led to the generation of pigs with the genes responsible for their production knocked out. Paradoxically, these developments highlighted a limitation of non-human primates as a model to study xenotransplanation of pig organs. While humans lack a functional gene for CMAH that is required for NeuGc synthesis20 and often have Nabs against NeuGc in their sera19,43,44, NHPs have a functional CMAH enzyme45. Given this difference between humans and NHPs, effectively modeling rejection due to NeuGc specificity in NHP recipients is impossible. Indeed, deletion of CMAH in addition to GalT in porcine cells increased the binding of baboon sera, suggesting that removal of NeuGc revealed another antibody target recognized by baboon sera46. A KO of the B4GalNT2 (1,4-N-acetylgalactosaminyltransferase) enzyme has also been produced by several groups. In fact “triple KO” pigs that lack GalT, B4GalNT2, CMAH have been generated and human sera show reduced levels of NAbs binding to these pig cells compared to cells lacking only Gal and NeuGc. The binding of sera from transplant waitlisted humans to triple KO (GalT KO, CMAH KO, B4GalNT2 KO) pig target cells is reportedly similar to their binding to allogeneic targets47.
A variety of additional GE approaches to overcoming the early antibody-mediated rejection of xenografts have been developed, including: 1) transgenic expression of human (h) hemoxygenase-1 (hHO-1), an enzyme that converts heme to bilirubin, carbon monoxide, and free iron. HO-1 helps cells to protect themselves from oxidative injury through anti-inflammatory, anti-apoptotic, and anti-proliferative effects through mechanisms that likely involve, at least in part, the production of CO48. Although this gene has now been included in several multi-gene edited swine donors, its effect on xenograft survival remains unclear; 2) transgenic expression of hA20, a human TNF-induced zinc finger protein enzyme that inhibits NF-kappa B activation and TNF-mediated apoptosis. Expression of this transgene in pigs was limited to the heart, where it appeared to partially inhibit ischemia-perfusion injury, although there was no demonstrable increase in organ survival49; 3) transgenic expression of human thrombomodulin (hTM) (CD141), an anticoagulant protein expressed on the surface of ECs. It was included among the transgenic modifications of pig hearts that was thought to be instrumental in allowing long-term survival of heterotopic heart xenografts to baboons36,50; 4) KO of porcine von Willebrand Factor (vWF): vWF is a glycoprotein that induces platelet adhesion at sites of vascular damage by binding to and stabilizing Factor VIII. It is hoped that the KO pig will mitigate induction of clotting at the interface of primate blood with porcine ECs. This protective function has been demonstrated ex vivo but its effect on xenograft survival has not yet been established49; 5) transgenic expression of hCD39 (ectonucleoside triphosphate diphosphohydrolase-1; ENTPD-1), an enzyme that hydrolyzes ATP and ADP to AMP, which is subsequently hydrolyzed to adenosine, providing anti-thrombotic and cardiovascular protective effects. The hCD39 transgene has been included in several pig-to-primate xenograft models51 but without clear evidence for benefit.
Using serial nuclear transfer, pigs have been produced that have both GalT and CMAH knocked out, express multiple human CRPs and express transgenes encoding the anti-inflammatory/anti-apoptotic molecules HO-1 and A2052.
C. T cell-mediated rejection:
The use of genetic engineering to subvert T cell immune responses and even make xenogeneic organs invisible to immune attack is appealing. Approaches that have been explored include the introduction of FasL53, CTLA4Ig54 and anti-CD2 monoclonal antibodies55 into the source pig, aiming to achieve local immunosuppression following transplantation. Pigs expressing transgenic CTLA4Ig ubiquitously have been shown to be capable of reproduction despite global immunosuppression54 and corneal transplants from them showed improved survival in NHPs56. Transgenic CTLA4Ig expression under control of the insulin promoter improved porcine islet survival in humanized mice57. However, immune evasion by a xenograft may impair immune protection from infection or viral reactivation. The absence of class I MHC could also make xenografts more susceptible to natural killer (NK) cell-mediated rejection, although this might be overcome by expression of transgenic HLA-E molecules58–60. Invisibility to direct immune attack would not obviate indirect recognition of xenoantigens with consequential antibody- and cytokine-mediated effector mechanisms.
Other transgenes such as hCD47 are discussed in more detail elsewhere in this review and additional KOs and transgenic modifications are undoubtedly in progress. However, it is important to note, as also described above for the CMAH KO, that unless a genetic modification can be demonstrated to be of value for the survival of xenografts in primates, it may be counterproductive to include it in a multi-gene construct, since every genetic modification carries with it the potential to affect pig health or produce neoantigens that may be immunogenic.
3. Recent advances in overcoming rejection in NHPs
A. Combined immunosuppression and genetic engineering:
The GE approaches discussed above, combined with advances in immunosuppressive therapies, especially costimulatory blockers, have permitted long-term organ and islet graft survival in NHPs, resulting in a resurgence in optimism about the potential for successful clinical xenotransplantation. Blockade of the B7-CD2835 and the CD40/CD154 pathways36 has played a significant role in these successes. The major advances in islet, kidney and heart transplantation are discussed here. Liver xenotransplantation has been much more challenging, as these grafts have not yet persisted beyond about one month in NHPs61–63.
Islet xenotransplantation:
Islet xenotransplantation is relatively non-invasive and is a non-life-supporting organ xenograft, leading to the notion that it may be quite readily applied in the clinic. Advances in porcine islet xenograft survival in NHPs have resulted from the use of immunosuppression that includes costimulatory blockade22,64,65, but the grafts were ultimately rejected despite heavy immunosuppression. While prolonged survival of porcine islets expressing multiple human CRPs and CTLA4Ig has been reported, the role of GE in promoting islet xenograft survival in primates remains unclear66. The GalT knockout modification may be unnecessary for adult pig islet transplantation, as these do not express Gal67. However, human CRPs and anti-coagulation proteins may alleviate the immediate innate immune response that destroys islets immediately when they are injected into the portal circulation68. Given that diabetes can usually be controlled with insulin therapy, islet allotransplantation, which also requires chronic immunosuppression and usually only achieves a transient insulin-free period69, is reserved for the small subset of patients with life-threatening diabetes complications like hypoglycemic unawareness. Islet xenotransplantation on a large scale could best be justified if the need for immunosuppression could be avoided, for example by islet encapsulation or tolerance induction.
Kidney xenotransplantation:
Recently, with the use of powerful T cell-depleting and costimulatory blocking immunosuppression and selection of recipients with low non-Gal natural antibody levels, GalT knockout kidney graft survivals of >400 days have been reported in a few animals70–73 . Immunosuppression included T and B cell depletion, steroids and costimulatory blockade, with rapamycin in one model and MMF in the other, and the grafts were eventually rejected. On the basis of these results, it has been suggested that kidney xenotransplantation could be offered in the near future to patients who are unlikely to receive an allograft due to high levels of alloantibodies, the existence of underlying renal disease that is likely to recur rapidly in an allograft, or a lack of vascular access for dialysis74. The inability to predict outcomes in humans of GalT/B4/CMAH triple KO kidney transplants on the basis of NHP studies has led some to argue that clinical trials of porcine kidney transplantation are currently justified75. However, size incompatibility between humans and pigs could be another a significant problem if conventional-size pigs were used76, as is discussed in more detail below.
It is likely that xenograft tolerance would broaden the potential applicability of porcine kidney xenotransplantation and accelerate progress. As is discussed below, a tolerance approach has led to graft acceptance >6 months in NHPs76 without any rejection. Limitations to animal survival in this model have been consequences of nephrotic syndrome that may reflect physiologic differences between pigs and baboons. While this problem can be controlled by treatment with rituximab and CTLA4Ig77–79, it is unknown whether this will be an issue in humans.
Heart transplantation:
Large animal models for orthotopic, life-sustaining heart transplantation are technically challenging and most heart xenotransplantation has been heterotopic, such that the graft is connected to the recipient great vessels in the abdominal cavity and serves as an accessory rather than a functioning heart. Heterotopic porcine GalT KO, human CRP and thrombomodulin transgenic heart grafts have survived in baboons for periods of several years with long-term immunosuppression36. Maintenance immunosuppression included CD40 blockade, upon which graft survival was critical, as dose reductions led to antibody-mediated rejection. More recently, advances have been made in orthotopic cardiac xenotransplantation, with life-sustaining graft survival >6 months of GalT KO, human CD46 and human thrombomodulin pig hearts transplanted into baboons treated with rituximab, ATG, anti-CD40/CD40L, mycophenolate mofetil and steroids80. Success was dependent on non-ischemic preservation of the heart prior to transplantation. Myocardial hypertrophy was a significant initial limitation to long-term survival, and this was controlled by maintaining low blood pressure in the recipient and using rapamycin. Therefore, miniature pigs may be needed as source animals for clinical cardiac xenotransplantation. Candidates for cardiac xenotransplantation who will otherwise die waiting for an allograft could include patients experiencing failure of and alloantibody formation while on left ventricular assist devices, failure of primary cardiac allografts, and complications of or contraindications to total artificial hearts74.
B. Tolerance:
While the advances in GE and immunosuppression discussed above have greatly improved xenograft survival in primates, the eventual rejections seen in the presence of immunosuppressive therapy raise the concern that long-term rejection-free survival may not be reliably achieved with the use of clinically tolerable levels of immunosuppression. Induction of tolerance, defined here as the absence of a destructive response to the graft in the absence of global immunosuppression, is therefore an important goal for the long-term success of clinical xenotransplantation. Two approaches to tolerance induction that have shown promise in large animal models are mixed hematopoietic chimerism and porcine thymic transplantation.
Mixed hematopoietic chimerism:
Mixed chimerism denotes a state in which hematopoietic elements of the donor and recipient co-exist. It can be achieved by transferring donor hematopoietic cells (e.g. bone marrow) to a recipient that has been immunosuppressed and conditioned in a way that does not eliminate host hematopoiesis (i.e. non-myeloablative) but is sufficient to make “space” for engraftment of the donor marrow. Mixed chimerism is associated with induction of tolerance to organ grafts from the same donor. However, the use of HC transplantation (HCT) for the purpose of tolerance induction is only acceptable if the conditioning regimen used to permit engraftment of donor hematopoietic cells does not ablate recipient hematopoiesis and has relatively low toxicity and if there is essentially no risk of graft-vs-host disease (GVHD). These criteria have been met in rodent models but have been more challenging to achieve in non-human primates and humans. Transient mixed allogeneic chimerism has recently led to successful renal allograft tolerance across HLA barriers in clinical studies (reviewed in81). Preclinical studies in rodents and humanized mice (reviewed in82) have indicated that non-myeloablative mixed chimerism induction can achieve xenograft tolerance, but the innate and adaptive immune barriers to xenogeneic HC engraftment are much stronger than those to allogeneic HCs (reviewed in 8,82). Studies in the rat-to-mouse and pig-to-humanized mouse xenograft models have shown that this approach has the advantage of inducing xenogeneic tolerance not only in the T cell compartment, but also among Nab-producing B cells and NK cells83 (reviewed in 8). Furthermore, recipient HCs have a competitive advantage over xenogeneic HCs85, likely due to homologous species preference for interactions with hematopoietic cytokines and other molecules such as adhesion molecules86,87,88,89. Genetic engineering approaches, such as expressing human hematopoietic cytokine receptors in pigs, are likely to enhance the establishment of porcine hematopoietic chimerism, as shown in pig cytokine transgenic (Tg) mice86,90.
Once engraftment of xenogeneic HCs is achieved, pre-existing natural IgM antibodies against the donor disappear in mixed xenogeneic chimeras (reviewed in 91). Using GalT-deficient mice, the loss of anti-donor Nabs in mixed chimeras was shown to be due to specific B cell tolerance induction, initially via anergy and later by a deletional mechanism (reviewed in 92). Mixed chimerism prevented all types of rejection of primarily vascularized xenografts, including hyperacute, acute vascular, T cell-mediated and chronic rejection (reviewed in 92). Thus, mixed chimerism has the combined ability to tolerize donor-reactive T cells robustly, also preventing induced antibody responses, as well as T cell-independent Nab-producing B cells.
Another innate immune barrier to xenogeneic and not to allogeneic HC engraftment is posed by macrophages (reviewed in7). While pig CD47 binds well to the human macrophage inhibitory receptor SIRPα, a signal leading to SIRPα phosphorylation fails to be transmitted, resulting in failure of the “don’t eat me” signal and rapid destruction of the xenogeneic HCs. Introduction of human CD47 into porcine HCs reduces their phagocytosis by human macrophages93. Importantly, a human CD47 transgene on porcine HCs enhanced porcine chimerism in baboons receiving non-myeloablative conditioning, resulting in markedly prolonged survival of donor pig skin grafts compared to that in animals receiving non-CD47-expressing pig HCs, in the complete absence of immunosuppression94. Chimerism and tolerance induction in baboons may be further enhanced by injecting the hCD47-Tg transgenic HCs directly into the bones, an approach that enhances porcine HC survival95.
NK cells pose a greater barrier to xenogeneic than to allogeneic HC engraftment. Once xenogeneic chimerism is achieved, however, NK cells demonstrate tolerance to the donor (reviewed in 82). While the role of NK cells in the allogenic setting has been studied extensively, studies on NK cells in the context of pig-NHP transplants is still a relatively new area and much remains to be learned. It is likely that these studies will also influence genetic engineering of pigs for transplanation.
Thymic transplantation:
An alternative to mixed chimerism as a means of inducing deletional T cell tolerance is transplantation of the donor thymus. In the thymus, potentially autoreactive T-cells are deleted or anergized by exposure to the appropriate self antigens presented by either bone marrow-derived cells or thymic stromal cells (i.e. negative selection), playing a key role in tolerance to self antigens. If one transplants an allogenic donor thymus into a recipient depleted of mature T cells but still having bone marrow stem cells that can migrate to the donor thymus, newly developing T cells are similarly exposed to negative selection by both host and donor elements (likely dendritic cells and thymic epithelial cells), leading to loss of reactivity to both host and donor96,97. Intrathymic self tolerance induction also involves the presentation of tissue-restricted antigens (TRAs) by medullary epithelial cells. These TRAs play a role in deleting thymocytes that recognize them and in generating Tregs with specificity for them.
Pig to mouse and humanized mouse models
Early studies in a pig-to-mouse model demonstrated the capacity of grafted porcine fetal thymic tissue to reconstitute functional host T-cells in thymectomized, T cell-depleted immunocompetent mice96,97. Murine thymocyte populations in the porcine thymic grafts were phenotypically indistinguishable from normal host thymocytes and peripheral CD4+ T-cells recovered, demonstrating tolerance to the donor and the recipient in vivo and in vitro that extended to acceptance of donor pig skin grafts without immunosuppression96,97. Intrathymic clonal deletion was shown to be a major mechanism of tolerance to the xenogeneic donor and the recipient. Tregs developing in the porcine thymus graft were also implicated in the suppression of residual mouse anti-pig responses. Despite the exclusive role of porcine MHC in thymic positive selection, these T cells responded to protein antigens presented by murine MHC molecules and protected the mice from an opportunistic pathogen. These and other results in the model are consistent with the generation in the pig thymus of a sufficiently diverse TCR repertoire to permit recognition of foreign antigens on recipient MHC despite positive selection by porcine MHC (reviewed in82).
Proof that the porcine thymic transplantation approach could achieve human T cell tolerance has come from humanized mouse models in which human T cells develop normally from hematopoietic progenitors and are centrally tolerized to porcine xenoantigens as well as human “self” antigens (due to the presence of human APCs in the porcine thymic grafts) in pig thymic grafts in immunodeficient mice98,99.
Pig to primate transplants
Fetal pig thymic tissue transplanted to baboons resulted in donor-specific hyporesponsiveness in vitro and prolongation of porcine skin graft survival. However, only a small amount of thymic epithelium remained at the implantation site by day 60102. We reasoned that the lack of permanent tolerance in grafted baboons might reflect incomplete T-cell depletion, which needed to be exhaustive in mice in order to prevent eventual rejection of the thymus graft103.
Given earlier successes of vascularized thymus grafts in the form of composite thymokidney (TK) and vascularized thymic lobe (VTL) transplantation for induction of tolerance across allogeneic barriers in pigs (reviewed in100), this approach was tested across the xenogeneic pig-to-primate barrier. It was first applied using hDAF transgenic kidney donors, immunoabsorption of natural anti-Gal antibodies on Gal columns and immunosuppression including thymectomy, cobra venom factor (CVF) and T-cell depletion104. The vascularized thymic grafts were found to support reconstitution of recipient-type T-cells at early time points, as demonstrated by recovery of CD45RA high/CD4+ cells in the peripheral blood. In addition, the thymic grafts induced in vitro donor-specific unresponsiveness that was maintained for approximately 2 months. However, all grafts were lost thereafter to humoral rejection following the inexorable return of anti-Gal antibodies.
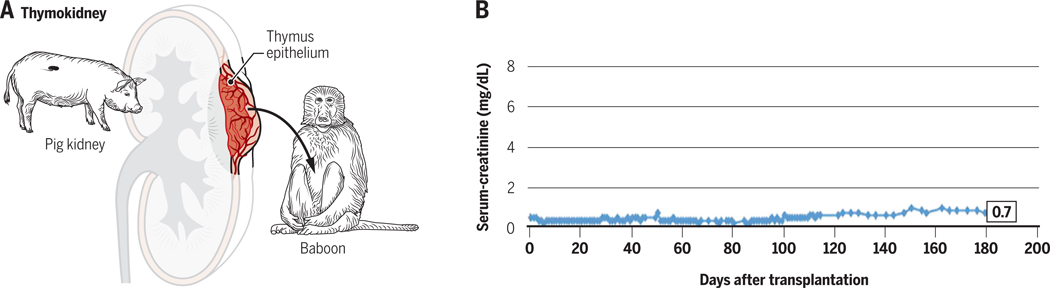
These TK and VTL experiments were subsequently repeated using GalT-KO swine donors, for which the presence or return of anti-Gal antibodies would no longer pose a threat. The improvement of results was remarkable, with renal xenograft survivals increasing to over 80 days and most animals expiring due to causes other than rejection33,105. While immunosuppression was not fully withdrawn before the demise of these animals, these results suggested that in the absence of rejection caused by returning anti-Gal antibodies, the TK and VTL tolerance-inducing regimens could prevent T cell-mediated responses as well as new, T-cell-dependent antibody responses, thereby permitting much longer survival of the xenografted organs. Recently, further modification of the induction regimen has facilitated survival of life-supporting thymokidneys for over 6 months, with loss due to cortical necrosis likely reflecting graft growth, without evidence of rejection, despite tapering of immunosuppression prior to sacrifice (Figure 4).
Figure 4:
A. Schematic of a thymokidney transplant from a pig to a baboon. B. Long-term survival of a GalT KO miniature swine thymokidney graft in a baboon with tapering immunosuppression. Serum creatinine was normal for >6 months and the graft grew markedly and was lost to cortical necrosis with no evidence of rejection at Day 193 post-transplant. Reprinted with permission from American Journal of Transplantation, 2017, 17:1778–1790.
Several limitations associated with the generation of a human T cell repertoire in a xenogeneic porcine thymus deserve consideration. Human T cells positively selected by porcine MHC would preferentially recognize microbial antigens presented by porcine MHC, effectively protecting the graft from infection but perhaps less effectively protecting the host against microbial pathogens. Indeed, in humanized mice, human T cells that developed in a pig thymus demonstrated reduced responses to peptides presented by human APCs compared to those developing in a human thymus graft99. Additionally, porcine thymic epithelium might fail to completely negatively select conventional T cells or optimally positively select Tregs recognizing human TRAs. Therefore, we are exploring the idea of constructing a “hybrid thymus”, in which recipient thymic epithelial cells obtained from human thymectomy specimens are injected into the porcine thymic tissue 106.
4. Requirements for a successful source animal:
As described above (see Introduction), the pig is generally considered to be the most suitable xenograft source animal, because of its size, favorable breeding characteristics and the similarity of many of its organ systems to those of humans37. In this section, we will consider these and other source animal characteristics that will likely be required for the success of xenotransplantation. We will also discuss several more subtle characteristics that may be of particular importance for transplantation of certain organs.
A). Availability:
Unlike the NHPs used as donors for the early clinical xenotransplantation efforts, swine are not an endangered species. Over 100 million pigs are slaughtered for food each year in the United States alone. Although there are animal rights groups that oppose the use of animals for any purpose, it seems unlikely that society would wish to limit the number of swine whose organs might be used to save the lives of human beings. However, not all swine would be appropriate for this purpose, since special breeding and maintenance would be needed to satisfy scientific and regulatory criteria.
B). Safety:
As in the case of allogeneic transplantation, the two most important safety concerns for xenotransplantation are the risk of failure due to rejection and the risk of side effects due to the immunosuppressive medications required to prevent rejection, most importantly infections, to which these medications lower resistance. During the late1990’s, discussions around xenotransplantation became focused on the risk of infectious transmission from pigs to humans. This resulted in large part from the observation that a porcine kidney cell line was capable of transmitting c-type porcine endogenous retroviruses (PERVs) to human cell lines in vitro107. There was a theoretical concern that these porcine PERVs could infect human xenograft recipients and cause disease due to insertional effects, causing disruption of normal gene function or oncogenesis. Considerable research on the potential for human infection by PERV ensued, resulting in the realization that primary human cells are very difficult to infect due to the presence of APOBEC restriction factors and that it was primarily PERC A-C recombinants that infected cell lines in vitro (reviewed in 108). An initial report that PERV infected human islets implanted in immunodeficient mice109 was later found to reflect pseudotyping of PERV virions by murine endogenous retrovirus envelope proteins, that permitted infection of human cells living in the same mice as the porcine islet110 or thymic111 grafts. Most importantly, despite the development of sensitive assays for its detection112, PERV infection has not been detected in any of over 200 patients who were exposed to porcine cells or xenografts in recent decades(reviewed in108). Hundreds of non-human primates receiving porcine xenografts have also failed to show evidence of PERV infection, but their cells are less readily infectable than human cells (reviewed in108). These developments have led to acceptance in the xenotransplantation community that the theoretical risk of PERV infection can be managed with appropriate surveillance and oversight from national health regulatory agencies108,113. Additionally, several groups have developed GE methodologies to further reduce this risk, by introducing small inhibitory RNA into the genome114 or by using CRISPR-Cas9 technology to knockout PERVs115,116. A concern with the latter approach is the potential for off-target genetic modifications that could have deleterious effects117, especially when so many loci are targeted simultaneously.
While so much attention has been focused on the theoretical risk of PERV infection, it may have been underappreciated that porcine xenografts, when performed appropriately, can carry less infectious risk than human allografts108,118. Human deceased allograft donors are screened for a variety of known human viruses, but the time-urgency of utilizing organs from brain-dead human donors often makes it difficult to carry out sufficient safety testing. Currently, approximately 0.2% of transplant recipients experience unexpected infections transmitted from the allograft119. In contrast, raising of source pigs in a carefully controlled and monitored environment can virtually eliminate this concern. US regulatory agencies 120,121 , the World Health Organization (WHO) and the International Xenotransplantation Association (IXA) have developed guidelines and recommendations for xenotransplantation clinical trials122,123. The recommendations include use of a “designated pathogen-free” pig that is housed in a closed facility where the risk of introducing new pathogens is minimized108.
Besides the risk of transmission of porcine microbial infections to the immunocompromised host, porcine viruses that do not possess the ability to infect the human host may still pose a risk to the graft, particularly because the recipients typically will not have T cell immunity to porcine viruses that recognize these antigens in the context of porcine MHC (swine leukocyte antigens, SLA).
C). Size:
One of the attractive features of swine as potential xenograft sources is the fact that their organs could potentially be matched to the size of any potential human recipient. Which strain of swine will be most appropriate on this basis remains undecided. One criterion might be the age at which an animal might be anticipated to reach the size required for a particular recipient. Domestic swine, which are being used by most groups developing genetically engineered swine as potential xenograft donors, attain mature adult weights of over 1000 pounds, so that their organs would be too large for use in humans after the age of about one year. In contrast, miniature swine, that have been developed extensively for this purpose in the laboratory of the authors, achieve maximum adult weights of 200 to 300 pounds, similar to those of humans, so that their organs could be of potential use at any age. While it is not yet clear how much of the growth potential of a transplanted organ is intrinsic to the organ itself versus extrinsically controlled by the size of the recipient, transplantation of kidneys from conventional swine to miniature swine with a tolerance induction regimen was associated with persistent graft growth in the miniature swine, reaching volumes 3.7 times their initial volume over 3 months, versus 1.2 times their initial volume for miniature swine kidneys over the same time period124. Similarly increased growth ratios were seen for lung allografts, in this case leading to impaired function of the organs124.Pig-to-baboon kidney xenografts have also been associated with rapid growth of the organs leading to cortical necrosis124.
D). Breeding Characteristics:
In addition to making it possible to raise large numbers of animals quickly and relatively inexpensively, their breeding characteristics make swine one of the few large animal species in which it is possible to carry out a selective genetic breeding program. They have large litter sizes (5 – 10 offspring), early sexual maturity (5 months), short gestation time (114 days), and frequent estrus cycles (every three weeks). Thus, it has been possible to produce miniature swine homozygous for the MHC in a relatively short time 125, as well as one inbred line which has reached greater than 94% coefficient of inbreeding, and has been demonstrated to be histocompatible, allowing transplants within this line to be accepted without exogenous immunosuppression 126. GE of appropriate xenograft donors is enhanced by the availability of such inbred animals. Thus, if one starts with a highly inbred animal, it is possible to combine any number of independently-introduced transgenes into the same genetic background by simple crossbreeding and selection for the desired transgenes among the offspring, which retain the same genetic background. Having inbred donor animals is of particular importance for approaches to xenotransplantation tolerance for two reasons: 1) cells (+/− gene modifications) capable of inducing tolerance can be used from one animal followed by an organ from another, perhaps with different gene modifications tailored for that organ’s function; and 2) if, for any reason, the transplanted organ needs to be replaced, the recipient would remain tolerant to a second organ from an animal of the same line.
E. Structural and Physiologic Similarity to Humans:
The pig has been widely agreed upon as the optimal source animal for human transplantation in large part due to its physiologic similarities to humans37. Most tissues and organs of swine bear a remarkable resemblance to those of humans both in structure and physiology. This includes the heart and circulatory system, the kidney, the pancreas, the liver, the lungs and even the skin127, which is almost indistinguishable histologically from skin of humans. The major difference that may have to be taken into account in choosing the optimal source animal is likely to be size, as discussed in detail above. This factor may be of more importance for thoracic organs, that are confined to a closed cavity, then for abdominal organs, for which additional room for growth might be possible. Nevertheless, excessive growth could lead to organs that would outgrow their blood supply, causing the kind of cortical necrosis already seen in the case of porcine kidneys transplanted between animals of markedly different eventual size76.
However, organ-specific physiologic differences are to be expected, especially if the organ produces a species-specific product, such as erythropoietin produced in the kidney, or coagulation factors produced by the liver. As long as the number of such differences is limited for each organ, the use of selective genetic modifications or of biological replacement therapies may be able to overcome the incompatibility. As more prolonged pig organ xenograft survival is achieved in NHPs, previously-unknown physiologic incompatibilities may emerge that will require appropriate therapy or additional pig genetic modification. As an example, pig liver xenograft survival up to 28 days was recently achieved in baboons when it was recognized that liver-derived porcine coagulation factor VII was insufficient and needed augmentation from human prothrombin concentrate to prevent coagulopathy and thrombocytopenia62,63. Likewise, advances in pig-to-primate islet xenotransplantation have revealed differing metabolic requirements between the species128. Recently, it has been suggested that differences in the intrinsic blood pressures of pigs vs baboons may contribute to myocardial hypertrophy in orthotopic heart transplantation80. While a detailed discussion of potential physiologic differences between pigs and humans is beyond the scope of this article, it is worth pointing out, in the context of the tolerance approach discussed above, that suboptimal xenogeneic hematopoietic cell engraftment partly reflects physiologic incompatibilities in the hematopoietic microenvironment in xenogeneic recipients129.
5). Conclusions:
The remarkable clinical success of transplantation over the past several decades has paradoxically led to an increasing shortage of transplantable human organs, by increasing the waiting list of patients aware that transplants could save their lives. Although there are other, competing technologies also under development, xenotransplantation is likely to the best near-term solution for this organ shortage. Many of the obstacles that have previously inhibited progress in xenotransplantation have now been overcome through GE of pigs to make their organs more compatible with human immune systems and physiology. Even with these modifications, however, it is not yet clear that the use of clinically-acceptable levels of immunosuppression could permit xenograft survivals similar to those of allografts. To achieve this goal, tolerance may be required. Additional GE of pigs to improve the likelihood of tolerance induction to pigs is also underway. Judicious combinations of modifying host immunity and genetically engineering swine donors is likely to lead to clinical success of xenotransplantation in the near future.
Abbreviations
- Gal
α1,3Gal
- αGalT
α1,3Galactosyl transferase
- APC
antigen-presenting cell
- CMV
cytomegalovirus
- ECs
endothelial cells
- GVHD
graft-versus-host disease
- HAR
hyperacute rejection
- HCT
hematopoietic cell transplantation
- HLA
human leukocyte antigen
- HSC
hematopoietic stem cell
- IL-15
interleukin 15
- MHC
major histocompatibility complex
- NHP
non-human primate
- NK cell
natural killer cells
- TRA
tissue-restricted antigen
References
- 1.Levitt M. Could the organ shortage ever be met? Life Sci Soc Policy. 2015;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reemtsma K, McCracken BH, Schlegel JU. Renal heterotransplantation in man. AnnSurg. 1964;160:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy JD, Timmis HH, Chavez CM, Lehan PH, Hellems HK, Fabian LW. Heart transplantation. JMissState MedAssoc. 1968;9(3):105–110. [PubMed] [Google Scholar]
- 4.Starzl TE, Marchioro TL, Peters GN. Renal heterotransplantation from baboon to man: experience with six cases. Transplantation. 1964;2:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt JL, Fischel RJ, Matas AJ, Reif SA, Bolman RM, Bach FH. Immunopathology of hyperacute xenograft rejection in a swine-to-primate model. Transplantation. 1991;52:214–220. [DOI] [PubMed] [Google Scholar]
- 6.Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, Doyle H, Zeevi A, Warty V, Michaels M, Kusne S, Rudert WA, Trucco M. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Yang YG. Innate cellular immunity and xenotransplantation. Curr Opin Organ Transplant. 2012;17(2):162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Imm Revs. 2014;258(1):241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263(33):17755–17762. [PubMed] [Google Scholar]
- 10.Parker W, Bruno D, Holzknecht ZE, Platt JL. Characterization and affinity isolation of xenoreactive human natural antibodies. J Immunol. 1994;153(8):3791–3803. [PubMed] [Google Scholar]
- 11.McMorrow IM, Comrack CA, Sachs DH, DerSimonian H. Heterogeneity of human anti-pig natural antibodies cross-reactive with the Gal(alpha1,3)Galactose epitope. Transplantation. 1997;64(3):501–510. [DOI] [PubMed] [Google Scholar]
- 12.Kozlowski T, Fuchimoto Y, Monroy R, Bailin M, Martinez-Ruiz R, Foley A, Xu Y, Awwad M, Fishman J, Andrews D, Ritzenthaler J, Sablinski T, Ierino FL, Sachs DH. Apheresis and column absorption for specific removal of Gal-alpha-1,3 Gal natural antibodies in a pig-to-baboon model. Transplant Proc. 1997;29(1–2):961. [DOI] [PubMed] [Google Scholar]
- 13.Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of {alpha}−1,3-Galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295:1089–1092. [DOI] [PubMed] [Google Scholar]
- 14.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, Im GS, Liu Z, Mell GD, Murphy CN, Park KW, Rieke A, Ryan DJ, Sachs DH, Forsberg EJ, Prather RS, Hawley RJ. Production of {alpha}−1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004;101:7335–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, Wise Y, Liu Y, Xiang Y, Copeman L, Liu W, Jevnikar A, Wall W, Cooper DK, Murase N, Dai Y, Wang W, Xiong Y, White DJ, Zhong R. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. NatMed. 2005;11(12):1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91(3):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miwa Y, Kobayashi T, Nagasaka T, Liu D, Yu M, Yokoyama I, Suzuki A, Nakao A. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation. 2004;11(3):247–253. [DOI] [PubMed] [Google Scholar]
- 19.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9(6):376–381. [DOI] [PubMed] [Google Scholar]
- 20.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273(25):15866–15871. [DOI] [PubMed] [Google Scholar]
- 21.Chong AS-F, Shen J, Xiao F, Blinder L, Wei L, Sankary H, Foster P, Williams J. Delayed xenograft rejection in the concordant hamster heart into Lewis rat model. Transplantation. 1996;62:90–96. [DOI] [PubMed] [Google Scholar]
- 22.Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala P, Murtaugh MP, Kirchhof N, Schuurman HJ. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. [DOI] [PubMed] [Google Scholar]
- 23.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, Weber CJ, Pearson TC, Rajotte RV, Larsen CP. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. [DOI] [PubMed] [Google Scholar]
- 24.Moses RD, Winn HJ, Auchincloss H. Evidence that multiple defects in cell-surface molecule interactions across species differences are responsible for diminished xenogeneic T cell responses. Transplantation. 1992;53:203–209. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Swenson K, Sergio JJ, Sykes M. Pig MHC mediates positive selection of mouse CD4+ T cells with a mouse MHC-restricted TCR in pig thymus grafts. JImmunol. 1998;161:1320–1326. [PubMed] [Google Scholar]
- 26.Li HY YG; Sykes M. Thymic education of human T cells and regulatory T cell development in humanized mice In: Poluektova LYG-M JV; Manz MG; Tager AM, ed. Humanized mice for HIV research. Vol. 10 New York: Springer; 2014. [Google Scholar]
- 27.Shimizu I, Fudaba Y, Shimizu A, Yang YG, Sykes M. Comparison of human T cell repertoire generated in xenogeneic porcine and human thymus grafts. Transplantation. 2008;86(4):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorling A, Lombardi G, Binns R, Lechler RI. Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur J Immunol. 1996;26(6):1378–1387. [DOI] [PubMed] [Google Scholar]
- 29.Sultan P, Murray AG, McNiff JM, Lorber MI, Askenase PW, Bothwell AL, Pober JS. Pig but not human interferon-gamma initiates human cell-mediated rejection of pig tissue in vivo. Proc Natl Acad Sci U S A. 1997;94(16):8767–8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155(11):5249–5256. [PubMed] [Google Scholar]
- 31.Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transpl Immunol. 2009;21(2):81–86. [DOI] [PubMed] [Google Scholar]
- 32.Buhler L, Illigens BM, Nadazdin O, Tena A, Lee S, Sachs DH, Cooper DK, Benichou G. Persistence of Indirect but Not Direct T Cell Xenoresponses in Baboon Recipients of Pig Cell and Organ Transplants. Am J Transplant. 2016;16(6):1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O’malley P, Nobori S, Vagefi PA, Patience C, Fishman J, Cooper DK, Hawley RJ, Greenstein J, Schuurman HJ, Awwad M, Sykes M, Sachs DH. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. [DOI] [PubMed] [Google Scholar]
- 34.Davila E, Byrne GW, LaBreche PT, McGregor HC, Schwab AK, Davies WR, Rao VP, Oi K, Tazelaar HD, Logan JS, McGregor CG. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation. 2006;13(1):31–40. [DOI] [PubMed] [Google Scholar]
- 35.Wu G, Pfeiffer S, Schroder C, Zhang T, Nguyen BN, Lea W, Kelishadi S, Atkinson JB, Schuurman HJ, White DJ, Azimzadeh AM, Pierson RN, III. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation. 2005;12(3):197–208. [DOI] [PubMed] [Google Scholar]
- 36.Mohiuddin MM, Singh AK, Corcoran PC, Thomas Iii ML, Clark T, Lewis BG, Hoyt RF, Eckhaus M, Pierson Iii RN, Belli AJ, Wolf E, Klymiuk N, Phelps C, Reimann KA, Ayares D, Horvath KA. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachs DH. The pig as a potential xenograft donor. VetImmunolImmunopathol. 1994;43:185–191. [DOI] [PubMed] [Google Scholar]
- 38.Niemann H, Rath D. Progress in reproductive biotechnology in swine. Theriogenology. 2001;56(8):1291–1304. [DOI] [PubMed] [Google Scholar]
- 39.Buhler L, Friedman T, Iacomini J, Cooper DK. Xenotransplantation--state of the art--update 1999. Front Biosci. 1999;4:D416–432. [DOI] [PubMed] [Google Scholar]
- 40.Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380(6569):64–66. [DOI] [PubMed] [Google Scholar]
- 41.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DK. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11(1):29–31. [DOI] [PubMed] [Google Scholar]
- 42.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, Burlak C, Wang ZY, Reyes LM, Ivary B, Yin F, Blankenship RL, Paris LL, Tector AJ. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20(1):27–35. [DOI] [PubMed] [Google Scholar]
- 44.Burlak C, Paris LL, Lutz AJ, Sidner RA, Estrada J, Li P, Tector M, Tector AJ. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant. 2014;14(8):1895–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irie A, Suzuki A. CMP-N-Acetylneuraminic acid hydroxylase is exclusively inactive in humans. Biochem Biophys Res Commun. 1998;248(2):330–333. [DOI] [PubMed] [Google Scholar]
- 46.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, Butler JR, Sidner R, Tector M, Tector J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22(3):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martens GR, Reyes LM, Butler JR, Ladowski JM, Estrada JL, Sidner RA, Eckhoff DE, Tector M, Tector AJ. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs. Transplantation. 2017;101(4):e86–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lundvig DM, Immenschuh S, Wagener FA. Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly? Front Pharmacol. 2012;3:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niemann H, Petersen B. The production of multi-transgenic pigs: update and perspectives for xenotransplantation. Transgenic Res. 2016;25(3):361–374. [DOI] [PubMed] [Google Scholar]
- 50.Singh AK, Chan JL, DiChiacchio L, Hardy NL, Corcoran PC, Lewis BGT, Thomas ML, Burke AP, Ayares D, Horvath KA, Mohiuddin MM. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation. 2018:e12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SJ, Kim JS, Chee HK, Yun IJ, Park KS, Yang HS, Park JH. Seven Years of Experiences of Preclinical Experiments of Xeno-Heart Transplantation of Pig to Non-Human Primate (Cynomolgus Monkey). Transplant Proc. 2018;50(4):1167–1171. [DOI] [PubMed] [Google Scholar]
- 52.Fischer K, Kraner-Scheiber S, Petersen B, Rieblinger B, Buermann A, Flisikowska T, Flisikowski K, Christan S, Edlinger M, Baars W, Kurome M, Zakhartchenko V, Kessler B, Plotzki E, Szczerbal I, Switonski M, Denner J, Wolf E, Schwinzer R, Niemann H, Kind A, Schnieke A. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci Rep. 2016;6:29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsuyuki S, Kono M, Bloom ET. Cloning and potential utility of porcine Fas ligand: overexpression in porcine endothelial cells protects them from attack by human cytolytic cells. Xenotransplantation. 2002;9(6):410–421. [DOI] [PubMed] [Google Scholar]
- 54.Bahr A, Kaser T, Kemter E, Gerner W, Kurome M, Baars W, Herbach N, Witter K, Wunsch A, Talker SC, Kessler B, Nagashima H, Saalmuller A, Schwinzer R, Wolf E, Klymiuk N. Ubiquitous LEA29Y Expression Blocks T Cell Co-Stimulation but Permits Sexual Reproduction in Genetically Modified Pigs. PLoS One. 2016;11(5):e0155676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nottle MB, Salvaris EJ, Fisicaro N, McIlfatrick S, Vassiliev I, Hawthorne WJ, O’Connell PJ, Brady JL, Lew AM, Cowan PJ. Targeted insertion of an anti-CD2 monoclonal antibody transgene into the GGTA1 locus in pigs using FokI-dCas9. Sci Rep. 2017;7(1):8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vabres B, Le Bas-Bernardet S, Riochet D, Cherel Y, Minault D, Hervouet J, Ducournau Y, Moreau A, Daguin V, Coulon F, Pallier A, Brouard S, Robson SC, Nottle MB, Cowan PJ, Venturi E, Mermillod P, Brachet P, Galli C, Lagutina I, Duchi R, Bach JM, Blancho G, Soulillou JP, Vanhove B. hCTLA4-Ig transgene expression in keratocytes modulates rejection of corneal xenografts in a pig to non-human primate anterior lamellar keratoplasty model. Xenotransplantation. 2014;21(5):431–443. [DOI] [PubMed] [Google Scholar]
- 57.Klymiuk N, van BL, Bahr A, Offers M, Kessler B, Wuensch A, Kurome M, Thormann M, Lochner K, Nagashima H, Herbach N, Wanke R, Seissler J, Wolf E. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes. 2012;61(6):1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weiss EH, Lilienfeld BG, Muller S, Muller E, Herbach N, Kessler B, Wanke R, Schwinzer R, Seebach JD, Wolf E, Brem G. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87(1):35–43. [DOI] [PubMed] [Google Scholar]
- 59.Lilienfeld BG, Crew MD, Forte P, Baumann BC, Seebach JD. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation. 2007;14(2):126–134. [DOI] [PubMed] [Google Scholar]
- 60.Puga Yung G, Bongoni AK, Pradier A, Madelon N, Papaserafeim M, Sfriso R, Ayares DL, Wolf E, Klymiuk N, Bahr A, Constantinescu MA, Voegelin E, Kiermeir D, Jenni H, Rieben R, Seebach JD. Release of pig leukocytes and reduced human NK cell recruitment during ex vivo perfusion of HLA-E/human CD46 double-transgenic pig limbs with human blood. Xenotransplantation. 2018;25(1). [DOI] [PubMed] [Google Scholar]
- 61.Shah JA, Navarro-Alvarez N, DeFazio M, Rosales IA, Elias N, Yeh H, Colvin RB, Cosimi AB, Markmann JF, Hertl M, Sachs DH, Vagefi PA. A Bridge to Somewhere: 25-day Survival After Pig-to-Baboon Liver Xenotransplantation. Ann Surg. 2016;263(6):1069–1071. [DOI] [PubMed] [Google Scholar]
- 62.Navarro-Alvarez N, Shah JA, Zhu A, Ligocka J, Yeh H, Elias N, Rosales I, Colvin R, Cosimi AB, Markmann JF, Hertl M, Sachs DH, Vagefi PA. The Effects of Exogenous Administration of Human Coagulation Factors Following Pig-to-Baboon Liver Xenotransplantation. Am J Transplant. 2016;16(6):1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah JA, Patel MS, Elias N, Navarro-Alvarez N, Rosales I, Wilkinson RA, Louras NJ, Hertl M, Fishman JA, Colvin RB, Cosimi AB, Markmann JF, Sachs DH, Vagefi PA. Prolonged Survival Following Pig-to-Primate Liver Xenotransplantation Utilizing Exogenous Coagulation Factors and Costimulation Blockade. Am J Transplant. 2017;17(8):2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markmann JF, Bartlett ST, Johnson P, Korsgren O, Hering BJ, Scharp D, Kay TW, Bromberg J, Odorico JS, Weir GC, Bridges N, Kandaswamy R, Stock P, Friend P, Gotoh M, Cooper DK, Park CG, O’Connell PJ, Stabler C, Matsumoto S, Ludwig B, Choudhary P, Khovatchev B, Rickels MR, Sykes M, Wood K, Kraemer K, Hwa A, Stanley E, Ricordi C, Zimmerman M, Greenstein J, Montanya E, Otonkoski T. Executive Summary of IPITA-TTS Opinion Leaders Report on the Future of beta-Cell Replacement. Transplantation. 2016;100(7):e25–31. [DOI] [PubMed] [Google Scholar]
- 65.Cardona K, Milas Z, Strobert E, Cano J, Jiang W, Safley SA, Gangappa S, Hering BJ, Weber CJ, Pearson TC, Larsen CP. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant. 2007;7(10):2260–2268. [DOI] [PubMed] [Google Scholar]
- 66.Bottino R, Wijkstrom M, van der Windt DJ, Hara H, Ezzelarab M, Murase N, Bertera S, He J, Phelps C, Ayares D, Cooper DK, Trucco M. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14(10):2275–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dor FJ, Cheng J, Alt A, Cooper DK, Schuurman HJ. Galalpha1,3Gal expression on porcine pancreatic islets, testis, spleen, and thymus. Xenotransplantation. 2004;11(1):101–106. [DOI] [PubMed] [Google Scholar]
- 68.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779–1784. [DOI] [PubMed] [Google Scholar]
- 69.Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, O’Connell PJ, Greenbaum C, Kudva YC, Brayman KL, Aull MJ, Larsen C, Kay TW, Fernandez LA, Vantyghem MC, Bellin M, Shapiro AM. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care. 2012;35(7):1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, Ezzelarab M, Long C, Klein E, Wagner R, Phelps C, Ayares D, Shapiro R, Humar A, Cooper DK. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22(4):302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB, Larsen CP, Ford ML, Lutz AJ, Tector M, Newell KA, Tector AJ, Adams AB. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22(3):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim SC, Wakwe W, Higginbotham LB, Mathews DV, Breeden CP, Stephenson AC, Jenkins J, Strobert E, Price K, Price L, Kuhn R, Wang H, Yamniuk A, Suchard S, Farris AB 3rd, Pearson TC, Larsen CP, Ford ML, Suri A, Nadler S, Adams AB. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am J Transplant. 2017;17(5):1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adams AB, Kim SC, Martens GR, Ladowski JM, Estrada JL, Reyes LM, Breeden C, Stephenson A, Eckhoff DE, Tector M, Tector AJ. Xenoantigen Deletion and Chemical Immunosuppression Can Prolong Renal Xenograft Survival. Ann Surg. 2018;268(4):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooper DKC, Wijkstrom M, Hariharan S, Chan JL, Singh A, Horvath K, Mohiuddin M, Cimeno A, Barth RN, LaMattina JC, Pierson RN 3rd, Selection of Patients for Initial Clinical Trials of Solid Organ Xenotransplantation. Transplantation. 2017;101(7):1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper DKC, Gaston R, Eckhoff D, Ladowski J, Yamamoto T, Wang L, Iwase H, Hara H, Tector M, Tector AJ. Xenotransplantation-the current status and prospects. Br Med Bull. 2018;125(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanabe T, Watanabe H, Shah JA, Sahara H, Shimizu A, Nomura S, Asfour A, Danton M, Boyd L, Dardenne Meyers A, Ekanayake-Alper DK, Sachs DH, Yamada K. Role of Intrinsic (Graft) Versus Extrinsic (Host) Factors in the Growth of Transplanted Organs Following Allogeneic and Xenogeneic Transplantation. Am J Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamada K, Shah JA, Tanabe T, Lanaspa MA, Johnson RJ. Xenotransplantation: Where Are We with Potential Kidney Recipients? Recent Progress and Potential Future Clinical Trials. Curr Transplant Rep. 2017;4(2):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamada KT T; Lanaspa M; Watanabe H; Rivard C; Sekijima M; Shah J; Tasaki M; Sahara H,; Shimizu A; Sachs D; Johnson R. The roles of CD80 upregulation and human CD47 expression on glomerular posdocytes in development of proteinuria following pig-to-baboon renal xenotransplantation. Xenotransplantation. 2017;24(5):31. [Google Scholar]
- 79.Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J Am Soc Nephrol. 2014;25(4):737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, Dashkevich A, Baehr A, Egerer S, Bauer A, Mihalj M, Panelli A, Issl L, Ying J, Fresch AK, Buttgereit I, Mokelke M, Radan J, Werner F, Lutzmann I, Steen S, Sjoberg T, Paskevicius A, Qiuming L, Sfriso R, Rieben R, Dahlhoff M, Kessler B, Kemter E, Klett K, Hinkel R, Kupatt C, Falkenau A, Reu S, Ellgass R, Herzog R, Binder U, Wich G, Skerra A, Ayares D, Kind A, Schonmann U, Kaup FJ, Hagl C, Wolf E, Klymiuk N, Brenner P, Abicht JM. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564(7736):430–433. [DOI] [PubMed] [Google Scholar]
- 81.Sykes M. Hematopoietic cell transplantation for tolerance induction: animal models to clinical trials. Transplantation. 2009;87(3):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sykes M. IXA Honorary Member Lecture, 2017: The long and winding road to tolerance. Xenotransplantation. 2018;25(3):e12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li HW, Vishwasrao P, Holzl MA, Chen S, Choi G, Zhao G, Sykes M. Impact of Mixed Xenogeneic Porcine Hematopoietic Chimerism on Human NK Cell Recognition in a Humanized Mouse Model. Am J Transplant. 2017;17(2):353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sharabi Y, Aksentijevich I, Sundt III TM, Sachs DH, Sykes M. Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. JExpMed. 1990;172:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gritsch HA, Sykes M. Host marrow has a competitive advantage which limits donor hematopietic repopulation in mixed xenogeneic chimeras. Xenotransplant. 1996;3:312–320. [Google Scholar]
- 86.Chen AM, Zhou Y, Swenson K, Sachs DH, Sykes M, Yang Y-G. Porcine stem cell engraftment and seeding of murine thymus with class II+ cells in mice expressing porcine cytokines: Toward tolerance induction across discordant xenogeneic barriers. Transplantation. 2000;69:2484–2490. [DOI] [PubMed] [Google Scholar]
- 87.Simon AR, Warrens AN, Yazzie NP, Seebach JD, Sachs DH, Sykes M. Cross-species interaction of porcine and human integrins with their respective ligands. Implications for xenogeneic tolerance induction. Transplantation. 1998;66:385–394. [DOI] [PubMed] [Google Scholar]
- 88.Warrens AN, Simon AR, Theodore PR, Sykes M. Human-porcine receptor-ligand compatibility within the immune system: relevance for xenotransplantation. Xenotransplant. 1999;6:75–78. [DOI] [PubMed] [Google Scholar]
- 89.Emery DW, Sachs DH, LeGuern C. Culture and characterization of hematopoietic progenitor cells from miniature swine. ExpHematol. 1996;24:927–935. [PubMed] [Google Scholar]
- 90.Yang Y-G, Chen AM, Garrett LJ, Sergio JJ, Theodore P, Awwad M, Verhalen J, Bodine DM, Sachs DH, Sykes M. Development and analysis of transgenic mice expressing porcine hematopoietic cytokines: a model for achieving durable porcine hematopoietic chimerism across an extensive xenogeneic barrier. Xenotransplant. 2000;7:58–64. [DOI] [PubMed] [Google Scholar]
- 91.Bardwell PD, Ohdan H, Sykes M. B cell tolerance and xenotransplantation. Curr Op Organ Transplantation. 2005;10(3):252–258. [Google Scholar]
- 92.Ohdan H, Sykes M. B cell tolerance to xenoantigens. Xenotransplantation. 2003;10(2):98–106. [DOI] [PubMed] [Google Scholar]
- 93.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, Sykes M, Yang YG, Ohdan H. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104(12):5062–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tena AA, Sachs DH, Mallard C, Yang YG, Tasaki M, Farkash E, Rosales IA, Colvin RB, Leonard DA, Hawley RJ. Prolonged Survival of Pig Skin on Baboons After Administration of Pig Cells Expressing Human CD47. Transplantation. 2017;101(2):316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tasaki M, Wamala I, Tena A, Villani V, Sekijima M, Pathiraja V, Wilkinson RA, Pratts S, Cormack T, Clayman E, Arn JS, Shimizu A, Fishman JA, Sachs DH, Yamada K. High Incidence of Xenogenic Bone Marrow Engraftment in Pig-to-Baboon Intra-Bone Bone Marrow Transplantation. Am J Transplant. 2015;15(4):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee LA, Gritsch HA, Sergio JJ, Arn JS, Glaser RM, Sablinski T, Sachs DH, Sykes M. Specific tolerance across a discordant xenogeneic transplantation barrier. ProcNatlAcadSciUSA. 1994;91:10864–10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M. Skin graft tolerance across a discordant xenogeneic barrier. Nature Med. 1996;2:1211–1216. [DOI] [PubMed] [Google Scholar]
- 98.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. JImmunol. 1999;162:3402–3407. [PubMed] [Google Scholar]
- 99.Kalscheuer HO T; Dahmani A; Li H; Holzl M; Yamada K; Sykes M. Xenograft tolerance and immune function of human T cell developing in pig thymus xenografts. Journal of Immunology. 2014;192(7):3442–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sachs DH. Immune Tolerance, Xenografts, and Large-Animal Studies in Transplantation. Ann Am Thorac Soc. 2017;14(Supplement_3):S220–S225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Auchincloss H Jr., Mayer T, Ghobrial R, Winn HJ. T-cell subsets, bm mutants, and the mechanisms of allogeneic skin graft rejection. ImmunolRes. 1989;8:149–164. [DOI] [PubMed] [Google Scholar]
- 102.Wu A, Yamada K, Neville DM, Awwad M, Wain JC, Shimizu A, Gojo S, Kitamura H, Colvin RB, Cooper DK, Sykes M, Sachs DH. Xenogeneic thymus transplantation in a pig-to-baboon model. Transplantation. 2003;75(3):282–291. [DOI] [PubMed] [Google Scholar]
- 103.Zhao Y, Swenson K, Wekerle T, Rodriguez-Barbosa JI, Arn JS, Sykes M. The critical role of mouse CD4+ cells in the rejection of highly disparate xenogeneic pig thymus grafts. Xenotransplant. 2000;7:129–137. [DOI] [PubMed] [Google Scholar]
- 104.Barth RN, Yamamoto S, LaMattina JC, Kumagai N, Kitamura H, Vagefi PA, Awwad M, COLVIN RB, Cooper DK, Sykes M, Sachs DH, Yamada K. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation. 2003;75(10):1615–1624. [DOI] [PubMed] [Google Scholar]
- 105.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, Ishikawa Y, Schule P, Arn JS, Robson SC, Fishman JA, Sykes M, Sachs DH, Yamada K. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9(12):2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khosravi Maharlooei MH M; Li H; Vecchione A; Misra A; Madley R; Zhao G; Sykes M. Generation of human/pig hybrid thymus to achieve immune tolerance to pig antigens with optimal immune function. Xenotransplantation. 2017;24(5):15. [Google Scholar]
- 107.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nature Med. 1997;3:282–286. [DOI] [PubMed] [Google Scholar]
- 108.Fishman JA. Infectious disease risks in xenotransplantation. Am J Transplant. 2018;18(8):1857–1864. [DOI] [PubMed] [Google Scholar]
- 109.Clemenceau B, Jegou D, Martignat L, Sai P. Microchimerism and transmission of porcine endogenous retrovirus from a pig cell line or specific pathogen-free pig islets to mouse tissues and human cells during xenografts in nude mice. Diabetologia. 2002;45(6):914–923. [DOI] [PubMed] [Google Scholar]
- 110.van der Laan LJ, Lockey C, Griffeth BC, Frasier FS, Wilson CA, Onions DE, Hering BJ, Long Z, Otto E, Torbett BE, Salomon DR. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice [In Process Citation]. Nature. 2000;407(6800):90–94. [DOI] [PubMed] [Google Scholar]
- 111.Yang YG, Wood JC, Lan P, Wilkinson RA, Sykes M, Fishman JA, Patience C. Mouse retrovirus mediates porcine endogenous retrovirus transmission into human cells in long-term human-porcine chimeric mice. J Clin Invest. 2004;114(5):695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wynyard S, Garkavenko O, Elliot R. Multiplex high resolution melting assay for estimation of Porcine Endogenous Retrovirus (PERV) relative gene dosage in pigs and detection of PERV infection in xenograft recipients. J VirolMethods. 2011;175(1):95–100. [DOI] [PubMed] [Google Scholar]
- 113.Cooper DKC, Pierson RN 3rd, Hering BJ, Mohiuddin MM, Fishman JA, Denner J, Ahn C, Azimzadeh AM, Buhler LH, Cowan PJ, Hawthorne WJ, Kobayashi T, Sachs DH. Regulation of Clinical Xenotransplantation-Time for a Reappraisal. Transplantation. 2017;101(8):1766–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dieckhoff B, Petersen B, Kues WA, Kurth R, Niemann H, Denner J. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation. 2008;15(1):36–45. [DOI] [PubMed] [Google Scholar]
- 115.Yang L, Guell M, Niu D, George H, Lesha E, Grishin D, Aach J, Shrock E, Xu W, Poci J, Cortazio R, Wilkinson RA, Fishman JA, Church G. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015;350(6264):1101–1104. [DOI] [PubMed] [Google Scholar]
- 116.Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, Zhao HY, Wang Y, Kan Y, Shrock E, Lesha E, Wang G, Luo Y, Qing Y, Jiao D, Zhao H, Zhou X, Wang S, Wei H, Guell M, Church GM, Yang L. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357(6357):1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schaefer KA, Wu WH, Colgan DF, Tsang SH, Bassuk AG, Mahajan VB. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat Methods. 2017;14(6):547–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nellore A, Fishman JA. Donor-derived infections and infectious risk in xenotransplantation and allotransplantation. Xenotransplantation. 2018;25(4):e12423. [DOI] [PubMed] [Google Scholar]
- 119.Ison MG. The epidemiology and prevention of donor-derived infections. Adv Chronic Kidney Dis. 2009;16(4):234–241. [DOI] [PubMed] [Google Scholar]
- 120.Food and Drug Administration C UD. Source animal product, preclincial, and clinical issues regarding the use of xenotransplantation products in humans: guidance for industry. 2016. [Google Scholar]
- 121.Bloom ET. National policies for xenotransplantation in the USA. Xenotransplantation. 2007;14(4):345–346. [DOI] [PubMed] [Google Scholar]
- 122.WHO. Second WHO global consultation on regulatory requirement for xenotrasnplantation clinical trialsx. 2001. [Google Scholar]
- 123.Hering BJ, Cozzi E, Spizzo T, Cowan PJ, Rayat GR, Cooper DK, Denner J. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--Executive summary. Xenotransplantation. 2016;23(1):3–13. [DOI] [PubMed] [Google Scholar]
- 124.Tanabe T, Watanabe H, Shah JA, Sahara H, Shimizu A, Nomura S, Asfour A, Danton M, Boyd L, Dardenne Meyers A, Ekanayake-Alper DK, Sachs DH, Yamada K. Role of Intrinsic (Graft) Versus Extrinsic (Host) Factors in the Growth of Transplanted Organs Following Allogeneic and Xenogeneic Transplantation. Am J Transplant. 2017;17(7):1778–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sachs DH. MHC-Homozygous miniature swine In: Swindle MM, Moody DC, Phillips LD, eds. Swine as models in biomedical research. Ames,IA: Iowa State University Press; 1992:3–15. [Google Scholar]
- 126.Mezrich JD, Haller GW, Arn JS, Houser SL, Madsen JC, Sachs DH. Histocompatible miniature swine: an inbred large-animal model. Transplantation. 2003;75(6):904–907. [DOI] [PubMed] [Google Scholar]
- 127.Holzer PW, Leonard DA, Shanmugarajah K, Moulton KN, Ng ZY, Cetrulo CL Jr., Sachs DH. A Comparative Examination of the Clinical Outcome and Histological Appearance of Cryopreserved and Fresh Split-Thickness Skin Grafts. J Burn Care Res. 2017;38(1):e55–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Graham ML, Bellin MD, Papas KK, Hering BJ, Schuurman HJ. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation. 2011;18(6):328–342. [DOI] [PubMed] [Google Scholar]
- 129.Gritsch HA, Glaser RM, Emery DW, Lee LA, Smith CV, Sablinski T, Arn JS, Sachs DH, Sykes M. The importance of non-immune factors in reconstitution by discordant xenogeneic hematopoietic cells. Transplantation. 1994;57:906–917. [DOI] [PubMed] [Google Scholar]