Significance
Transport of IgG antibodies from the maternal to the fetal circulation is a key process for neonatal immunity, as neonates cannot sufficiently generate IgG antibodies to reach protective levels during the first months after birth. In humans and other primates, maternal to fetal transport of IgG antibodies is largely mediated through the placental tissue. FcRn has been previously identified as the major driver of IgG transplacental transport. Here we examine whether other receptors, such as FcγRs, also contribute to the maternal-fetal IgG transfer. By characterizing the Fc domain structure of paired maternal-fetal IgG samples and modeling transplacental IgG transport in genetically engineered mouse strains, we determined that FcRn, but not FcγRs, is the major receptor that mediates transplacental IgG transport.
Keywords: immunoglobulin, IgG, placental transfer, Fc domain, FcRn
Abstract
The IgG Fc domain has the capacity to interact with diverse types of receptors, including the neonatal Fc receptor (FcRn) and Fcγ receptors (FcγRs), which confer pleiotropic biological activities. Whereas FcRn regulates IgG epithelial transport and recycling, Fc effector activities, such as antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis, are mediated by FcγRs, which upon cross-linking transduce signals that modulate the function of effector leukocytes. Despite the well-defined and nonoverlapping functional properties of FcRn and FcγRs, recent studies have suggested that FcγRs mediate transplacental IgG transport, as certain Fc glycoforms were reported to be enriched in fetal circulation. To determine the contribution of FcγRs and FcRn to the maternal-fetal transport of IgG, we characterized the IgG Fc glycosylation in paired maternal-fetal samples from patient cohorts from Uganda and Nicaragua. No differences in IgG1 Fc glycan profiles and minimal differences in IgG2 Fc glycans were noted, whereas the presence or absence of galactose on the Fc glycan of IgG1 did not alter FcγRIIIa or FcRn binding, half-life, or their ability to deplete target cells in FcγR/FcRn humanized mice. Modeling maternal-fetal transport in FcγR/FcRn humanized mice confirmed that only FcRn contributed to transplacental transport of IgG; IgG selectively enhanced for FcRn binding resulted in enhanced accumulation of maternal antibody in the fetus. In contrast, enhancing FcγRIIIa binding did not result in enhanced maternal-fetal transport. These results argue against a role for FcγRs in IgG transplacental transport, suggesting Fc engineering of maternally administered antibody to enhance only FcRn binding as a means to improve maternal-fetal transport of IgG.
Maternal to fetal transfer of IgG antibodies is central to neonatal immunity. This is because IgGs are not produced by the neonate at mature levels for nearly a year after birth, making maternal antibodies a principal mechanism of immunity during this vulnerable period of development. In species such as primates, this transfer is largely the result of transplacental transport of IgG. The neonatal Fc receptor (FcRn) is recognized as the primary transporter of IgGs across epithelial cells of the placenta. Indeed, seminal studies in mice as well as in ex vivo human placental assay systems have established that the transport of IgG molecules across the placental tissue is exclusively mediated by FcRn (1, 2). In addition to transport across placental cell layers, FcRn extends the half-life of IgGs through a mechanism of protective recycling that rescues IgGs from catabolic pathways. These functions of FcRn are possible because of high-affinity interactions between FcRn and monomeric IgGs, triggered by acidification of endosomes after passive uptake of IgGs that are present in the extracellular space. The high affinity of FcRn-IgG interactions enables transport of IgGs within endosomes from the apical cell surface to the basolateral cell surface (in transplacental IgG transport) or back to the apical surface (for recycling of IgGs), where a return to neutral pH causes release of IgGs from FcRn back into circulation (3, 4).
FcRn interacts with the IgG heavy chain at the interface between the CH2 and CH3 domains of the IgG Fc (5, 6). This is in contrast to interactions with Fc gamma receptors (FcγRs), which bind the hinge proximal region of the CH2 domain of IgG. Interactions between FcRn with IgGs are generally not thought to be impacted by IgG subclass or Fc glycosylation, while specific IgG allotypes (e.g., Gm3[b], Gm3[g]) may impact FcRn binding (7–10). This is in stark contrast to FcγR-IgG interactions, which are profoundly impacted by both IgG subclass and Fc glycosylation (11, 12). While several prior studies have determined that FcRn is the major transporter of IgGs from maternal to fetal circulation (1, 2, 13), important questions remain regarding the biology of transplacental IgG transport. These include the mechanism(s) governing preferential transfer of the IgG1 subclass and the variation of transfer efficiency that can occur based on IgG Fab specificity. Furthermore, whether FcγRs have any role in transplacental IgG transport or whether specific posttranslational modifications of IgGs can impact transfer through any mechanism are questions that have not been resolved (14–18).
An intriguing prospect for enhancing neonatal immunity has focused on the dynamics of transplacental transport and postulates that increasing transport of protective IgGs to the fetus during gestation could profoundly boost and extend neonatal immunity. This could be done by maternal administration of recombinant, antipathogen antibodies that are specifically engineered for increased placental transport. Through enhancing transfer of protective monoclonal antibodies, this strategy could reduce infant mortality while avoiding direct delivery of IgGs to newborns. Feasibility of this approach, however, requires that there is absolute clarity about mechanisms regulating the transport of IgGs across the placenta.
Although earlier studies that defined the dependence of IgG transplacental transport on FcRn have excluded a role for FcγRs in this process (1, 2), recent studies have suggested that posttranslational modifications of IgGs and a specific low-affinity FcγR, FcγRIIIa, have a role in transplacental IgG transfer (19, 20). These studies characterized paired maternal and cord blood IgG, reporting a significant increase in digalactosylation on total cord IgGs (but not on antigen-specific IgGs) and suggesting that this modification may direct transplacental transfer of maternal IgGs in a process they term “placental sieving.” Through a series of binding and in vitro cellular assays, the authors conclude that digalactosylation of IgG increases the affinity of IgGs for both FcRn and FcγRIIIa and propose that FcγRIIIa contributes to transplacental transfer of galactosylated IgGs. Furthermore, they report that digalactosylation enhances FcγRIIIa-mediated cellular activation.
In view of the importance of this topic and a prior study of IgG Fc glycosylation from paired maternal and cord IgGs that used rigorous methods for relative quantitation of Fc glycans and found no significant differences in IgGs transferred across the placenta (14), we have re-examined key conclusions from these studies. We characterized paired maternal and fetal IgGs from two separate clinical cohorts and have used a combination of in vitro approaches and in vivo functional studies to test the hypothesis that FcγRIIIa or other FcγRs may have a role in transplacental IgG transfer. In two clinical cohorts, we observed no consistent difference between digalactosylation of maternal and cord IgG1, the most abundant IgG subclass. Some small, but statistically significant differences were observed in IgG2 galactosylation between maternal and cord IgGs. IgG2 is the second most abundant IgG subclass and does not engage FcγRIIIa. Biochemical and in vivo functional studies designed to investigate whether galactosylation of IgG (either IgG1 or IgG2) impacts interactions with FcRn or FcγRIIIa found no evidence of modulation of IgG-FcγR or IgG-FcRn interactions through Fc galactosylation, in vitro or in vivo. To confirm the results, we developed an in vivo murine model in which human FcγRs and FcRn are expressed in place of their murine counterparts and found no evidence that enhancing IgG Fc binding to FcγRIIIa (or to other FcγRs) results in increased maternal to fetal IgG transport. In contrast, enhancing IgG-FcRn binding resulted in significantly increased transplacental transport of maternal IgGs. Our results thus support the conclusion that Fc glycosylation is unlikely to be a significant modifier of maternal-to-fetal IgG transport and that engineering IgGs to enhance FcγRIIIa binding will not result in enhanced transplacental transport.
Results
IgG Fc Glycans Do Not Predict the Efficiency of Transplacental IgG Transfer.
To investigate whether differences in posttranslational modifications, such as glycosylation, of IgGs are found in paired maternal-fetal samples and thus suggest a role in transplacental IgG transfer, we characterized the glycosylation of IgG Fc domains from paired maternal and venous cord (fetal) bloods from two geographically distinct clinical cohorts (SI Appendix, Table S1). One cohort of mothers and infants from which samples were studied is located in Tororo, a region of Uganda where malaria is endemic (21, 22). Some mothers in the cohort had a malaria infection during pregnancy while others remained free from malaria infection (SI Appendix, Table S1A). From these Ugandan samples, we profiled both total and malaria specific, anticircumsporozoite protein (CSP) IgGs. Samples from a second Nicaraguan cohort were drawn during a period when Zika virus infections were endemic in the region. Mothers in the study had a PCR-confirmed Zika virus (ZIKV) infection during pregnancy or had confirmed infection via the NS1 Blockade-of-Binding (BOB) enzyme-linked immunosorbent assay (ELISA) assay (23) and had Zika envelope protein (E)-reactive IgGs in serum (SI Appendix, Table S1B). From the Nicaraguan study, we profiled total IgGs and anti-Zika E IgGs.
To characterize Fc glycoforms, we employed a previously validated and sensitive method (24), in which trypsin-digested, purified IgGs are subjected to nanoscale liquid chromatography coupled to tandem mass spectrometry (nano LC-MS/MS), enabling relative quantification of IgG subclass-specific Fc glycoforms. Subclass-specific analysis of Fc glycans is critical for hypothesis generation around the potential function(s) associated with specific Fc modifications as the role of various Fc glycoforms on non-IgG1 subclasses is not yet defined. In samples from both cohorts, we observed no statistically significant differences between maternal and cord IgGs for the majority of Fc glycoforms (Figs. 1 and 2 and SI Appendix, Table S2). In IgGs from the Ugandan cohort (Fig. 1), there were no significant differences between maternal and cord Fc glycoforms of total or of anti-CSP IgG1. Analysis of IgG2 showed some small, but statistically significant, differences in galactosylation of total IgG with decreased monogalactosylation in cord blood and increased digalactosylation and total galactosylation in cord, compared with maternal, blood. Although statistically significant, each of these modifications varied by only ∼2% between maternal and cord IgGs (SI Appendix, Table S2). In contrast, no differences were observed in anti-CSP IgG2. Total IgG (all subclasses) monogalactosylation was significantly different, with the mean abundance of monogalactosylation being ∼1.4% lower in cord, relative to maternal, blood (Fig. 1 and SI Appendix, Table S2). No differences in Fc glycosylation of anti-CSP IgGs (all subclasses) were observed (Fig. 1). The largest mean difference observed in Fc glycans between cord and maternal blood from the Ugandan cohort was in monogalactosylation of bulk IgG2, with cord blood having ∼2.3% lower levels than maternal blood (Fig. 1 and SI Appendix, Table S2).
Fig. 1.
Relative abundance of IgG Fc glycans in paired maternal and cord bloods in the Ugandan cohort. Total or antimalaria CSP IgGs were purified from maternal or venous cord blood, and the relative abundance of the following Fc glycoforms were characterized for each IgG category: G0, G1, G2, G0F, G1F, G2F, total galactosylation (G), total fucosylation (F), total bisection (N), and total sialylation (S). Significance was assessed by two-way ANOVA with Bonferroni’s multiple comparisons test where P < 0.0125 was considered significant, *P < 0.0125, **P < 0.0025, and ***P < 0.00025. Figure is related to SI Appendix, Tables S1 and S2.
Fig. 2.
Relative abundance of IgG Fc glycans in paired maternal and cord blood in the Nicaraguan cohort. Total or anti-Zika virus envelope (E) IgGs were purified from maternal or venous cord blood, and the relative abundance of the following Fc glycoforms were characterized for each IgG category: G0, G1, G2, G0F, G1F, G2F, total galactosylation (G), total fucosylation (F), total bisection (N), and total sialylation (S). Significance was assessed by two-way ANOVA with Bonferroni’s multiple comparisons test where P < 0.0125 was considered significant, *P < 0.0125, **P < 0.0025, and ***P < 0.00025. Figure is related to SI Appendix, Tables S1 and S2.
In IgGs from the Nicaraguan study (Fig. 2), total cord IgG1 levels of Fc monogalactosylation were reduced and increased in sialylation relative to total maternal IgG1, while anti-E IgG1 total galactosylation was significantly elevated in cord blood (Fig. 2). Total cord IgG2 was significantly elevated in total galactosylation and cord anti-E IgG2 digalactosylation and total galactosylation were elevated over maternal blood IgG (Fig. 2). Total cord IgG (all subclasses) Fc monogalactosylation was significantly reduced, but was elevated in digalactosylated IgGs while cord anti-E IgG (all subclasses) was elevated in total galactosylation. The largest mean difference observed in Fc glycans between cord and maternal blood from the Nicaraguan cohort was in total galactosylation of bulk IgG2, with cord blood having ∼5.6% higher levels than maternal blood (Fig. 2 and SI Appendix, Table S2).
Overall, we found that there were no consistent trends in Fc glycosylation between cord and maternal IgGs which would indicate a role for IgG glycosylation in transplacental transport of IgGs. Transplacental IgG transfer in these two clinical cohorts was not a function of Fc galactosylation (Figs. 1 and 2 and SI Appendix, Table S2). Furthermore, no difference between maternal and cord IgG fucosylation was observed, which could support a potential role for FcγRIIIa in transplacental IgG transport. With respect to IgG subclasses, results from the Ugandan and Nicaraguan cohorts were consistent with one another and with prior studies demonstrating a clear bias in transfer of IgG1 from maternal to cord blood and reduced transfer of the IgG2 subclass (Fig. 3 and SI Appendix, Table S2) (25–27).
Fig. 3.
IgG subclass distribution in paired maternal and cord bloods. (A and B) Total or antimalaria CSP IgG from the Ugandan cohort. (C and D) Total or anti-Zika virus envelope (E) IgG from the Nicaraguan cohort. Data from Fig. 3 are quantified in SI Appendix, Table S2. The abundance of specific IgG subclasses is shown. Significance was assessed by paired t test with Bonferroni’s correction. ****P < 0.0001. Figure related to SI Appendix, Tables S1 and S2.
Galactosylation of IgG Fc Does Not Significantly Impact Binding to FcRn or FcγRs In Vitro.
Previous studies have established that core fucosylation of the Fc reduces affinity of IgG1 for FcγRIIIa, while galactosylation alone has no impact on FcγRIIIa binding and/or antibody-dependent cell-mediated cytotoxicity (28, 29). Galactosylation has been observed to increase Fc-FcγRIIIa binding, but only in the absence of fucosylation (30). Recently, it has been reported that IgG galactosylation, in the presence of fucosylation, impacts IgG binding to FcγRIIIa and FcRn (19). Given these contrasting reports on the role of Fc galactosylation in modulating FcγR and FcRn affinity, we performed a comprehensive analysis of the FcγR and FcRn affinity of IgG Fc glycoforms with defined, homogenous glycan structures. Using a chemoenzymatic glycosylation remodeling method (31, 32), we generated homogenously agalactosylated (G0) or digalactosylated (G2) human IgG1 variants of the monoclonal antibody (mAb) 6A6 that specifically recognizes the murine platelet glycoprotein IIb (33). Production of G0 or G2 of anti-gpIIb mAb was further stratified by core fucosylation (G0F and G2F, respectively), and the homogeneity of the IgG glycoforms was assessed by LC-MS analysis (SI Appendix, Fig. S1).
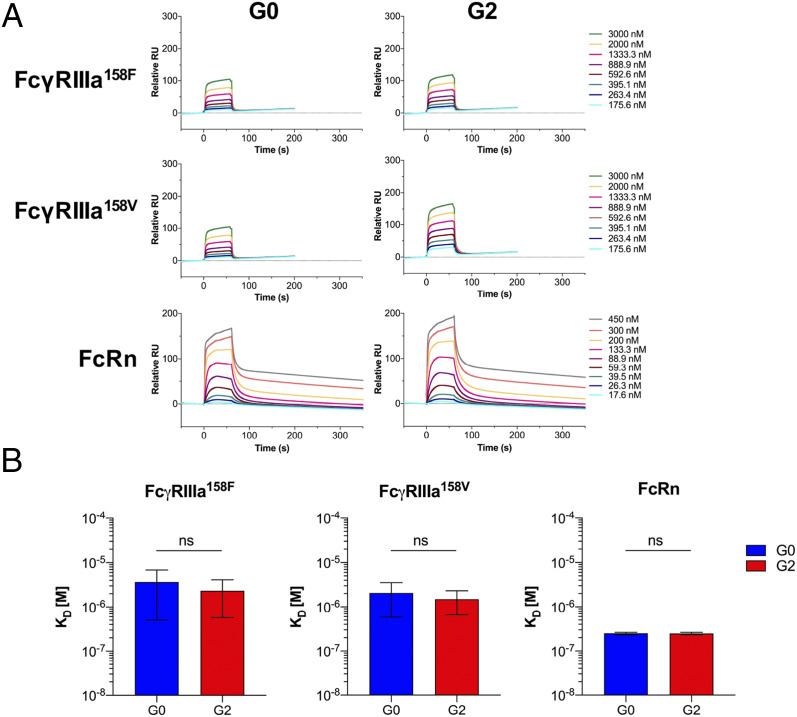
Human IgG1 variants of 6A6 mAb were tested by surface plasmon resonance (SPR) for binding to all human FcγRs and to human FcRn. As expected, Fc fucosylation of mAb 6A6 significantly reduced binding to both FcγRIIIa158F and FcγRIIIa158V alleles (Fig. 4 A and B). In contrast, fucosylation did not significantly impact interactions with FcRn. The galactosylation status of the mAb 6A6 had no impact on FcγRIIIa (or other FcγR) or FcRn binding (Fig. 4 A and B and SI Appendix, Fig. S2A). To exclude mAb clone-specific effects, the results of these SPR binding studies were confirmed using homogenous Fc glycoforms (G0 or G2) of the antiinfluenza hemagglutinin (HA) mAb FI6 (SI Appendix, Fig. S3A).
Fig. 4.
Effect of IgG1 core fucosylation and galactosylation on FcγR engagement. (A) The affinity of glycoengineered mouse-human chimeric IgG1 of anti-gpIIb (6A6) mAb for human FcγRIIIa158F, FcγRIIIa158V, and FcRn ectodomains was determined by SPR analysis, and representative SPR sensorgrams are presented. Data correspond to one experiment per interaction tested, which is representative of three independent experiments that gave similar results. (B) The equilibrium dissociation constant (KD, M) and the SD are indicated for each tested interaction. Significance was assessed by one-way ANOVA followed by Bonferroni multiple comparison test. ns: not significant; ***P < 0.005; ****P < 0.0001. Figure is related to SI Appendix, Figs. S1–S3.
Although analysis of the two clinical cohorts revealed no differences in Fc glycosylation between cord and maternal IgG1, a minor, yet statistically significant, difference was evident in the levels of IgG2 Fc galactosylation between cord and maternal IgG (Figs. 1 and 2 and SI Appendix, Table S2). To address whether Fc galactosylation of IgG2 impacts FcγR and/or FcRn affinity, we generated homogenously agalactosylated (G0) or digalactosylated (G2) human IgG2 variants of both anti-gpIIb 6A6 and anti-HA FI6 mAbs (SI Appendix, Fig. S1) and assessed their affinities for human FcγRs and FcRn by SPR. As observed for IgG1 (Fig. 4), the galactosylation status of IgG2 had no impact on either FcγR or FcRn binding affinity (Fig. 5 A and B and SI Appendix, Fig. S2B). Thus, using homogeneously glycosylated IgGs revealed no significant differences in FcγR or FcRn binding affinities for galactosylated or agalactosylated Fc glycoforms for either the IgG1 or IgG2 subclasses.
Fig. 5.
Effect of IgG2 core fucosylation and galactosylation on FcγR engagement. (A) The affinity of glycoengineered mouse-human chimeric IgG2 of anti-gpIIb (6A6) mAb for human FcγRIIIa158F, FcγRIIIa158V, and FcRn ectodomains was determined by SPR analysis, and representative SPR sensorgrams are presented. Data correspond to one experiment per interaction tested, which is representative of two or three independent experiments that gave similar results. (B) The equilibrium dissociation constant (KD [M]) and the SD are indicated for each tested interaction. Significance was assessed by unpaired t test; ns: not significant. Figure is related to SI Appendix, Figs. S1–S3.
Galactosylation of IgG Fc Does Not Significantly Impact In Vivo Activity.
To validate the physiological significance of these in vitro binding results, we assessed the in vivo effector function of Fc glycoforms of cytotoxic antibodies in FcγR humanized mice, which recapitulate the unique features of human FcγR physiology (34). To characterize the Fc effector activity conferred by Fc galactosylation, we took advantage of the well-defined in vivo platelet depleting activity of anti-gpIIb mAb 6A6. In mice that are fully humanized for FcγRs, administration of the chimeric mAb 6A6/hIgG Fc causes platelet counts to drop through the engagement of the activating FcγR, FcγRIIIa, on myeloid cells. Platelet depletion is quantitatively increased as the binding affinity of the 6A6 human IgG Fc for FcγRIIIa is increased (34, 35). Thus, this system can be used to dissect how Fc modifications directly impact binding to FcγRIIIa and thereby its function. Using the homogenous glycovariants of human IgG1 and IgG2 mAb 6A6 previously characterized for their in vitro FcγR binding affinity (Figs. 4 and 5), we observed that the absence of Fc fucosylation, regardless of galactosylation status, resulted in enhanced platelet depletion by mAb 6A6 (Fig. 6). This was consistent with the increased affinity of afucosylated 6A6 glycoforms for FcγRIIIa, when compared with fucosylated 6A6 variants (Figs. 4 A and B and 5). In contrast, there was no difference in the activity of mAbs based on G0 or G2 glycoforms (Fig. 6 A, Upper). Similarly, no difference was observed in the activity of G0 or G2 glycoforms in IgG2 subclass-mediated depletion (Fig. 6 A, Lower).
Fig. 6.
Evaluation of the in vivo Fc effector function and half-life of human IgG antibodies in FcγR and FcγR/FcRn humanized mice. (A) The cytotoxic activity of glycoengineered mAbs was evaluated in FcγR humanized mice. Time-course analysis of platelet counts following administration of glycoengineered anti-gpIIb (6A6) IgG1 (Upper) and IgG2 (Lower) mAb (2 mg/kg) is presented as the mean ± SEM from three to eight mice/group. The Fc domain variant N297A, lacking Fc glycosylation and therefore the FcγR binding ability, is used as negative control. Significance was assessed by two-way ANOVA followed by Bonferroni multiple comparison test; *P < 0.05 G0 vs. G0F and G2 vs. G2F. (B and C) The in vivo half-life of glycoengineered antihemagglutinin (FI6) IgG1 (Upper) and IgG2 (Lower) mAb (50 µg/mouse) was evaluated in FcRn/FcγR humanized mice. Results are presented as the mean ± SD from four to five mice/group. Significance was assessed by unpaired t test. ns: not significant. Figure is related to SI Appendix, Figs. S4 and S5.
In addition to evaluating the Fc effector function of Fc glycovariants, we determined the pharmacokinetics profile of these glycovariants in vivo. Given the substantial interspecies differences between humans and mice in the affinity for human IgG antibodies for FcRn, we generated a mouse strain engineered to express all classes of human FcγRs and FcRn in lieu of the mouse orthologs of these receptors. Importantly, FcγR expression on effector leukocytes in the FcγR/FcRn humanized mice was consistent with expression on human leukocyte populations (SI Appendix, Fig. S4). To characterize the in vivo activity of human IgG antibodies in this mouse strain, we performed a series of studies to evaluate human FcRn- and FcγR-mediated functions. Engineering IgG for higher affinity toward human FcRn using M428L/N434S (LS) mutations enhanced IgG half-life in the FcγR/FcRn humanized mice (SI Appendix, Fig. S5A) (36). Cytotoxic antibody effector function was characterized using models of CD4+ T cell depletion and the mAb 6A6-mediated model of platelet depletion. As expected, chimeric anti-CD4 or 6A6 human IgG1 mAbs, Fc-engineered for higher affinity for activating FcγRIIa and FcγRIIIa and reduced for FcγRIIb, using the G236A/A330L/I332E (GAALIE) Fc mutations, were significantly more efficient at mediating depletion of CD4+T cells and platelets, respectively, compared with wild-type human IgG1 mAb variants or non-FcγR–binding mAb variants (G236R/L328R [GRLR] Fc mutations) (SI Appendix, Fig. S5 B–D) (37). Thus, human antibody functions mediated by human FcRn and FcγRs in this mouse model were intact.
To evaluate the role of IgG galactosylation status in the modulation of the FcRn-mediated IgG recycling, we compared the in vivo half-life of agalactosylated (G0) and digalactosylated (G2) Fc glycoforms of the anti-HA mAb FI6. Consistent with the SPR affinity data (Figs. 4 and 5), the presence or absence of galactose residues had no significant impact on the in vivo half-life of either IgG1 (Fig. 6 B and C, Upper) or IgG2 (Fig. 6 B and C, Lower) variants of mAb FI6. Overall, our studies in FcγR/FcRn humanized mouse strains revealed that the galactosylation status of IgG1 and IgG2 antibodies had no impact on their capacity to interact with FcγRs or FcRn, modulating either IgG effector functions or half-life, respectively.
Engineering IgG Fcs for Enhanced Binding to FcγRs Does Not Impact Transplacental IgG Transfer In Vivo.
While our data do not support the contention that substantive changes in glycan profiles between maternal and fetal compartments are seen in matched samples from different patient cohorts, we nevertheless sought to directly test whether engineering IgGs for enhanced binding to any FcγR(s) might impact transplacental IgG transfer in vivo. To experimentally evaluate this possibility, we developed a mouse model using the FcγR/FcRn humanized mice described above. Human IgG1 was transferred to pregnant mice 1 d prior to delivery, and the levels of human IgG1 were characterized in the neonates. Human IgG1 levels in neonatal mice remained relatively unchanged in the 8 d following delivery when mice were housed with their biological mothers (Fig. 7A). Next, we sought to determine whether this antibody was acquired by pups via transplacental transfer or via GI tract-mediated transfer after nursing (milk-acquired IgGs). To study this, we compared transfer of human IgG to pups that were housed after delivery with their birth mothers, that had been administered human IgG1 1 d prior to delivery, or with surrogate mothers that were not administered any antibody prior to delivery. This comparison revealed that pups that did not receive human IgG via GI tract-mediated transfer had a rapid decline in serum IgG levels (Fig. 7 B and C). Thus, we determined that the level of transplacentally transferred IgG could be ascertained in the pups on the day of delivery, prior to feeding, a time point comparable to obtaining cord blood upon delivery in our human cohorts. To validate the GI tract-mediated IgG transfer, we studied IgG levels in naive pups that were housed with mothers that were administered human IgG prior to delivery. Indeed, serum IgG levels rose rapidly in the pups, demonstrating substantial GI tract-mediated IgG transfer (Fig. 7D). Thus, we concluded that the day of delivery was ideal for studying transplacentally transferred IgG in this model system.
Fig. 7.
Evaluation of transplacental transfer of human IgG antibodies in FcRn/FcγR humanized mice. (A) Pregnant FcRn/FcγR humanized mice were injected i.v. with human IgG1 mAb (3BNC117; 400 μg) 1 d prior to delivery, and the serum levels of human IgG1 were evaluated in the pups at different time points postpartum. (B) Experimental overview to differentiate placental- from GI-tract–mediated maternal-to-fetal transfer of human IgG in FcRn/FcγR humanized mice involved the use of surrogate mothers that were injected or not with human IgG antibodies. (C) To assess the contribution of placental-mediated IgG transfer, pups born to mothers that were injected with human mAbs (3BNC117 hIgG1; 100 μg i.v.) 1 d prior to delivery remained either with their biological mother (nonsurrogate) or transferred to naive surrogate mothers. Human IgG levels in the serum of pups were quantified at different time points following birth. n = 4/group; ****P < 0.0001; **P = 0.006 surrogate vs. nonsurrogate. (D) Comparison of placental- (red boxes) and GI-tract–mediated (gray boxes) transfer of human IgG antibodies from maternal to fetal circulation. n = 4–5 mice/group; **P = 0.001, ****P < 0.0001. (E) FcγR and FcRn binding profile of Fc domain mutants with differential receptor affinity that were generated to study the contribution of human FcRn and FcγRs to the transplacental transfer of human IgG antibodies. (F) Experimental strategy to evaluate the maternal-to-fetal transport efficacy of human IgG antibodies in FcRn/FcγR humanized mice. (G) Comparison of the human IgG levels (expressed as pg/mg of fetal tissue) in fetuses born to mothers previously injected (1 d prior to delivery) with Fc domain variants of a human IgG1 mAb (3BNC117; 100 μg i.v.) n = 3–9/group; ns: not significant vs. WT; *P < 0.02 vs. WT or GAALIE; **P = 0.003 vs. GRLR. Figure is related to SI Appendix, Figs. S4 and S5.
To determine whether FcγRs may have a role in transplacental IgG transfer, we developed variants of a human IgG1 mAb that were engineered for enhanced or reduced binding to specific human FcγRs or to human FcRn (Fig. 7E). Human mAb variants were administered to pregnant mice (on day 19 of pregnancy) 1 d prior to induced delivery, and antibody levels were quantified in pups upon delivery (Fig. 7F). Engineering of mAbs for enhanced binding to activating FcγRs (FcγRIIa and FcγRIIIa), using the GAALIE mutations, had no impact on transplacental IgG transfer (Fig. 7G). Additionally, abrogating FcγR binding using GRLR mutations had no impact on transplacental IgG transfer. In contrast, engineering the Fc for enhanced affinity to human FcRn using LS mutations significantly increased the efficiency of transplacental IgG transfer (Fig. 7G).
In summary, studies using this model of transplacental transfer of IgG demonstrated that, while FcRn could be harnessed to increase transplacental IgG transfer, modulating antibodies for enhanced binding to FcγRs did not impact the transplacental transfer efficacy of human IgGs.
Discussion
Here, using a variety of in vitro and in vivo approaches, we addressed questions also asked by recent studies as to whether FcγRs may be involved in transplacental transfer of human IgGs (14, 19, 20). Unlike some studies (19, 20), but consistent with others (1, 2, 14), we found that there is no enrichment for specific Fc glycoforms in cord blood over paired maternal blood and demonstrated that maternal-fetal transfer of IgG antibodies is primarily mediated through FcRn, while FcγRs have a limited contribution to this process. A significant difference in methodologies exists between these reports in that the Fc glycan analysis was not done in an IgG subclass-specific manner in these studies (19, 20); rather, these studies characterized total IgG Fc glycans (antigen-specific IgG or bulk IgG). This methodology does not account for the different activities that can be conferred by modification of different IgG subclasses by the same N-glycan. In contrast, the present study reports relative quantification of Fc glycoforms on specific IgG subclasses. Together, our study shows no consistent trends among cohorts, indicating that Fc glycosylation is likely not a significant modifier of maternal-to-fetal IgG transport.
Our studies also find that Fc galactosylation had no statistically significant impact on FcγR binding, including FcγRIIIa, or on human FcRn binding for either the IgG1 or IgG2 subclasses. In vivo analysis of different IgG glycoforms in mice expressing human FcγRs and FcRn and lacking in their murine homologs confirmed that galactosylation had no statistically significant impact on FcγRIIIA-mediated effector function or FcRn-mediated half-life. Finally, direct analysis of transplacental transport of human IgG in a murine model demonstrated that modulating binding of maternal antibodies to FcγRs, including FcγRIIIA, had no impact on neonatal antibody concentration. In contrast, modulating maternal antibody FcRn affinity resulted in statistically significant increases in neonatal antibody concentration. While Jennewein et al. (19) propose that the enhanced FcγRIIIa-mediated effector function of cord blood IgG relative to maternal IgG is a function of enriched galactosylation of IgG in cord blood, we suggest that this activity is likely a consequence of the well-documented bias in transplacental transfer of the IgG1 subclass. IgG1 antibodies have enhanced affinity for activating FcγRs, including FcγRIIIa; thus, this straightforward hypothesis could explain the difference in functional activity between maternal and fetal IgGs that was observed in their study (19).
We conclude that engineering antibodies for enhanced FcγRIIIa binding (or for binding to any FcγR) will not result in increased transplacental IgG transfer and is not a viable strategy to pursue for extending neonatal immunity. In contrast, our studies support the engineering of mAbs for enhanced FcRn affinity to increase the efficiency of IgG transfer from mother to fetus.
Materials and Methods
Clinical Cohorts and Samples.
Paired maternal and cord blood samples were obtained from women and infants enrolled in PROMOTE (NCT02163447), a randomized clinical trial of novel antimalarial chemoprevention regimens in eastern Uganda (21, 22). The study was approved by the Institutional Review Boards of the Makerere University School of Biomedical Sciences, the Uganda National Council for Science and Technology, and the University of California San Francisco. Written informed consent was obtained from all study participants. Paired maternal and cord blood samples were obtained from the Nicaraguan Zika Positives (NZP) study approved by the University of California, Berkeley, institutional review board (IRB) protocol # 2016–10-9265 and by the Nicaraguan (Sustainable Sciences Institute, Nicaragua) IRB protocol # NIC-MINSA/CNDR CIRE-19/12/16–078). All samples were de-identified prior to use in the present study. The NZP study enrolled pregnant women confirmed as rRT-PCR ZIKV+ by the Ministry of Health or with a history of Zika-like symptoms during their pregnancy. For those who displayed Zika-like symptoms but did not have a ZIKV+ rRT-PCR result, recent ZIKV infection was confirmed via the NS1 BOB ELISA assay (23).
Mice.
All in vivo experiments were performed in compliance with federal laws and institutional guidelines and have been approved by the Rockefeller University Institutional Animal Care and Use Committee. Mice were bred and maintained at the Comparative Bioscience Center at the Rockefeller University. FcγR humanized mice (FcγRαnull, hFcγRI+, FcγRIIaR131+, FcγRIIb+, FcγRIIIaF158+, and FcγRIIIb+) were generated in the C57BL/6 background and have been extensively characterized in previous studies (34). FcRn humanized mice (B6.Cg-Fcgrttm1Dcr Tg[FCGRT)32Dcr/DcrJ] were purchased from The Jackson Laboratory; these mice were deficient in mouse FcRn and expressed human FcRn as a transgene (38, 39). FcγR/FcRn humanized mice were generated by crossing the FcγR humanized strain to the FcRn humanized mice.
IgG Fc Glycan and IgG Subclass Analysis.
Methods for relative quantification of Fc glycoforms and IgG subclasses have been described (24, 40). Briefly, IgGs were purified from serum by protein G purification. Antigen-specific IgGs were isolated on N-hydroxysuccinimide (NHS) agarose resin (ThermoFisher; 26196) coupled to the relevant protein. Following tryptic digestion of purified IgGs, nano LC-MS/MS analysis was performed on tryptic peptides containing the N279 glycan using an UltiMate3000 nanoLC (Dionex) coupled with a hybrid triple quadrupole linear ion trap mass spectrometer, the 4000 Q Trap (SCIEX). Data were acquired using Analyst 1.6.1 software (SCIEX) for precursor ion scan triggered information dependent acquisition analysis for initial discovery-based identification. For quantitative analysis of the glycoforms at the N297 site across the three IgG subclasses (IgG1, IgG2, and IgG3/G4), multiple-reaction monitoring (MRM) analysis for selected target glycopeptides was applied to the samples using the nanoLC-4000 Q Trap platform after trypsin digestion. The m/z of 4-charged ions for all different glycoforms of the core peptides from three different subclasses as Q1 and the fragment ion at m/z 366.1 as Q3 for each of the transition pairs were used for MRM assays. A native IgG tryptic peptide (131-GTLVTVSSASTK-142) with a transition pair of m/z 575.9+2 to m/z 780.4 (y8+) was used as a reference peptide for normalization purposes. The IgG subclass distribution was quantitatively determined by nano LC-MRM analysis of tryptic peptides following removal of glycans from purified IgGs with PNGase F. Here, the m/z value of fragment ions for monitoring transition pairs was always larger than that of their precursor ions being multicharged to enhance the selectivity for unmodified targeted peptides and the reference peptide. All raw MRM data were processed using MultiQuant 2.1.1 (SCIEX). MRM peak areas were automatically integrated and manually inspected. In the event that automatic peak integration by MultiQuant failed, manual integration was performed using the MultiQuant software.
Preparation of Homogeneous Glycoforms of Monoclonal Antibodies.
The glycoforms of monoclonal antibodies 6A6 and FI6 were synthesized by the chemoenzymatic glycan remodeling method following a previously reported procedure (31).
SPR.
SPR experiments were performed with a Biacore T200 SPR system (Biacore, GE Healthcare) at 25 °C in HBS-EP+ buffer (10 mM Hepes, pH 7.4, 150 mM NaCl, 3.4 mM ethylenediaminetetraacetic acid, 0.005% [vol/vol] surfactant P20). For the measurement of human FcγR binding affinity, antibodies were immobilized on Series S Protein G sensor chip (GE Healthcare) at a density of 2,000 RU (response units). Serial two-fold dilutions of soluble, recombinant human FcγR ectodomains were injected as analytes. The FcγRs tested were FcγRI (10256-H08H, Sino Biological), FcγRIIaR131(10374-H08H1, Sino Biological), FcγRIIaH131 (10374-H08H1, Sino Biological), FcγRIIb (10259-H08H, Sino Biological), FcγRIIIaV158 (10389-H08H1, Sino Biological), and FcγRIIIaF158 (10389-H08H, Sino Biological). The FcγRs were injected through flow cells at a flow rate of 20 µL⋅min−1 with the concentration ranging from 0.488 to 250 nM (serial two-fold dilutions) for FcγRI and from 15.625 to 1,000 nM (serial two-fold dilutions) for all of the other FcγRs (except for FcγRIIIaV158; 500 nM was used as the maximum concentration). A concentration ranging from 175.6 to 3,000 nM (serial 1.5-fold dilutions) was used to test IgG2 binding affinity to FcγRIIIaF158 and FcγRIIIaV158. Association time was 60 s followed by a 300-s dissociation (900 s for FcγRI). At the end of each cycle, the sensor surface was regenerated with glycine HCl buffer (10 mM, pH 1.5) at a flow rate of 50 µL⋅min−1 for 30 s. Background binding to blank immobilized flow cells was subtracted. Data were analyzed using the BIA Evaluation software (GE Healthcare), and the steady-state affinity model was used to validate that the equilibrium had been reached under the selected concentration series of the analytes (RUmax vs. analyte concentration) and to calculate the affinity constants (KD). For the measurement of human FcRn binding affinity, antibodies were immobilized on a Series S Protein L sensor chip (GE Healthcare) at a density of 400 RU, and experiments were performed in HBS-EP+ buffer at pH 6.0 following the method described above. Human FcRn/β2m (Sino Biological) was tested at a concentration ranging from 17.5 to 450 nM (serial 1.5-fold dilutions).
In Vivo Cellular Depletion Assays.
The in vivo cytotoxic activity of Fc glycovariants of mAbs was assessed in either FcγR humanized or FcRn/FcγR humanized mice in models of mAb-mediated depletion of CD4+ T cells or platelets, following previously described protocols (34). For the T cell depletion model, mice were injected intraperitoneally with 50 μg of recombinant GK1.5 human IgG1 mAb Fc variants, and the abundance of CD4+ CD8− T cells was determined 48 h post monoclonal antibody administration in the blood and spleen by flow cytometric analysis. Baseline CD4+ CD8− T cell frequency was determined in blood samples obtained prior to mAb administration. For the platelet depletion model, mice were injected intravenously (i.v.) with the indicated dose of recombinant 6A6 human IgG1 mAb Fc variants. Mice were bled at the indicated time points before and after mAb administration, and platelet counts were measured using an automated hematologic analyzer (Advia 120 system or Heska HT5).
Cloning, Expression, and Purification of Recombinant IgG Antibodies.
Recombinant antibodies were generated following previously described protocols (41). Briefly, antibodies were generated by transient transfection of Expi293 cells with heavy- and light-chain expression plasmids. Prior to transfection, plasmid sequences were validated by direct sequencing (Genewiz). Recombinant IgG antibodies were purified from cell-free supernatants by affinity purification using Protein G or Protein A Sepharose beads (GE Healthcare). Purified proteins were dialyzed in phosphate-buffered saline (PBS) and filter-sterilized (0.22 μm), and purity was assessed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis followed by Coomasie blue staining. All antibody preparations were >90% pure, and endotoxin levels were <0.005 endotoxin units/mg, as determined by the Limulus Amebocyte Lysate assay.
Quantification of Serum and Tissue IgG Levels.
Blood from mice was collected in gel microvette tubes, and serum was fractionated by centrifugation (10,000 × g, 5 min). Fetal tissue lysates were prepared by mechanical homogenization in ice-cold lysis buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1% Y-30, pH 7.4) using gentleMACS M tubes. Lysates were clarified by centrifugation (10,000 × g, 10 min), and supernatants were stored at −20 °C. IgG levels in serum samples and tissue homogenates were determined by ELISA following previously published protocols (42). Briefly, high-binding 96-well microtiter plates (Nunc) were coated overnight at 4°C with Neutravidin (2 μg/mL in PBS). All sequential steps were performed at room temperature. Plates were blocked for 1 h with PBS/2% bovine serum albumin and incubated with biotinylated goat anti-human IgG antibodies for 1 h (5 μg/mL). Serum or tissue lysate samples were serially diluted and incubated for 1 h, followed by incubation with horseradish peroxidase-conjugated anti-human IgG (1:5,000). Plates were developed using the 3,3′,5,5′-tetramethylbenzidine (TMB) two-component peroxidase substrate kit (KPL), and reactions were stopped with the addition of 1 M phosphoric acid. Absorbance at 450 nm was immediately recorded using a SpectraMax Plus spectrophotometer (Molecular Devices), and background absorbance from negative control samples was subtracted. Detection was performed using the TMB two-component peroxidase substrate kit (KPL), and reactions were stopped with the addition of 2M phosphoric acid. Absorbance at 450 nm was immediately recorded using a SpectraMax Plus spectrophotometer (Molecular Devices), background absorbance from negative control samples was subtracted, and duplicate wells were averaged. Antibody half-life was calculated using the following formula: half-life t1/2 (h) = 0.693/((ln[IgG concentration on day 1]) – ln[IgG concentration on day 8])/168 (tinterval; h).
Statistical Analysis.
Results from multiple experiments are presented as mean ± SEM. One- or two-way ANOVA was used to test for differences in the mean values of quantitative variables, and where statistically significant effects were found, post hoc analysis using the Bonferroni multiple comparison test was performed. Data were analyzed with Graphpad Prism software and P values of <0.05 were considered to be statistically significant.
Data Availability.
All data for this paper are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
We thank P. Smith, E. Lam, R. Peraza, and M. Ye (Rockefeller University) for excellent technical help. We acknowledge support from Rockefeller University, Stanford University, and the Chan Zuckerberg Biohub. We thank Dr. Grant Dorsey (University of California, San Francisco), Professor Moses Kamya, the Infectious Diseases Research Collaboration, and the PROMOTE study team for provision of paired maternal and cord blood clinical samples. The Pf CSP protein was provided by Drs. David Narum and Patrick Duffy (NIH Laboratory of Malaria Immunology and Vaccinology). We thank members of the study team based at the Hospital de la Mujer Bertha Calderon Roque; the Hospital Infantil Manuel de Jesús Rivera; the Sócrates Flores Vivas, Edgar Lang, Francisco Buitrago, and Via Libertad Health Centers; the National Virology Laboratory in the Centro Nacional de Diagnóstico y Referencia; and the Sustainable Sciences Institute in Nicaragua for their dedication and high-quality work. We also thank the Nicaraguan Ministry of Health and especially the study participants. Research reported in this publication was supported by Bill and Melinda Gates Foundation Grant OPP1188461 (to J.V.R. and T.T.W.) and in part by National Institute of Allergy and Infectious Diseases Grants R01AI129795 (to J.V.R.), R01AI139119 (to T.T.W.), and U19AI118610 (to E.H.); and National Institute of General Medical Sciences Grant R01GM096973 (to L.-X.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing interest.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004325117/-/DCSupplemental.
References
- 1.Vaccaro C., Bawdon R., Wanjie S., Ober R. J., Ward E. S., Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc. Natl. Acad. Sci. U.S.A. 103, 18709–18714 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Firan M. et al., The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int. Immunol. 13, 993–1002 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Ghetie V., Ward E. S., FcRn: The MHC class I-related receptor that is more than an IgG transporter. Immunol. Today 18, 592–598 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Simister N. E., Jacobowitz Israel E., Ahouse J. C., Story C. M., New functions of the MHC class I-related Fc receptor, FcRn. Biochem. Soc. Trans. 25, 481–486 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Shields R. L. et al., High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J. Biol. Chem. 276, 6591–6604 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Kim J. K. et al., Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur. J. Immunol. 29, 2819–2825 (1999). [DOI] [PubMed] [Google Scholar]
- 7.West A. P. Jr., Bjorkman P. J., Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor. Biochemistry 39, 9698–9708 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Bakchoul T. et al., Inhibition of HPA-1a alloantibody-mediated platelet destruction by a deglycosylated anti-HPA-1a monoclonal antibody in mice: Toward targeted treatment of fetal-alloimmune thrombocytopenia. Blood 122, 321–327 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simmons L. C. et al., Expression of full-length immunoglobulins in Escherichia coli: Rapid and efficient production of aglycosylated antibodies. J. Immunol. Methods 263, 133–147 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Tao M. H., Morrison S. L., Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J. Immunol. 143, 2595–2601 (1989). [PubMed] [Google Scholar]
- 11.Bournazos S., Wang T. T., Dahan R., Maamary J., Ravetch J. V., Signaling by antibodies: Recent progress. Annu. Rev. Immunol. 35, 285–311 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T. T., IgG Fc glycosylation in human immunity. Curr. Top. Microbiol. Immunol. 423, 63–75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghetie V., Ward E. S., Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu. Rev. Immunol. 18, 739–766 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Einarsdottir H. K. et al., Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj. J. 30, 147–157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu C. et al., Placental antibody transfer efficiency and maternal levels: Specific for measles, coxsackievirus A16, enterovirus 71, poliomyelitis I-III and HIV-1 antibodies. Sci. Rep. 6, 38874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanaveeradej V. et al., Transplacentally transferred maternal-infant antibodies to dengue virus. Am. J. Trop. Med. Hyg. 69, 123–128 (2003). [PubMed] [Google Scholar]
- 17.van den Berg J. P. et al., Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr. Infect. Dis. J. 29, 801–805 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Stach S. C. et al., Placental transfer of IgG antibodies specific to Klebsiella and Pseudomonas LPS and to group B Streptococcus in twin pregnancies. Scand. J. Immunol. 81, 135–141 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Jennewein M. F. et al., Fc glycan-mediated regulation of placental antibody transfer. Cell 178, 202–215.e14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez D. R. et al., Fc characteristics mediate selective placental transfer of IgG in HIV-infected women. Cell 178, 190–201.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakuru A. et al., Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N. Engl. J. Med. 374, 928–939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagannathan P. et al., Dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria during pregnancy and risk of malaria in early childhood: A randomized controlled trial. PLoS Med. 15, e1002606 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balmaseda A. et al., Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc. Natl. Acad. Sci. U.S.A. 114, 8384–8389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T. T. et al., Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell 162, 160–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmeira P., Quinello C., Silveira-Lessa A. L., Zago C. A., Carneiro-Sampaio M., IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 985646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einarsdottir H. K. et al., On the perplexingly low rate of transport of IgG2 across the human placenta. PLoS One 9, e108319 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashira S., Okitsu-Negishi S., Yoshino K., Placental transfer of IgG subclasses in a Japanese population. Pediatr. Int. 42, 337–342 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Shields R. L. et al., Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Shinkawa T. et al., The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J. Biol. Chem. 278, 3466–3473 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Dekkers G. et al., Affinity of human IgG subclasses to mouse Fc gamma receptors. MAbs 9, 767–773 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li T. et al., Modulating IgG effector function by Fc glycan engineering. Proc. Natl. Acad. Sci. U.S.A. 114, 3485–3490 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C., Wang L. X., Chemoenzymatic methods for the synthesis of glycoproteins. Chem. Rev. 118, 8359–8413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsson M., Bruhns P., Frazier W. A., Ravetch J. V., Oldenborg P. A., Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood 105, 3577–3582 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith P., DiLillo D. J., Bournazos S., Li F., Ravetch J. V., Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc. Natl. Acad. Sci. U.S.A. 109, 6181–6186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwab I., Lux A., Nimmerjahn F., Pathways responsible for human autoantibody and therapeutic intravenous IgG activity in humanized mice. Cell Rep. 13, 610–620 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Zalevsky J. et al., Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 28, 157–159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bournazos S. et al., Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158, 1243–1253 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petkova S. B. et al., Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: Potential application in humorally mediated autoimmune disease. Int. Immunol. 18, 1759–1769 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Roopenian D. C., Christianson G. J., Sproule T. J., Human FcRn transgenic mice for pharmacokinetic evaluation of therapeutic antibodies. Methods Mol. Biol. 602, 93–104 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Wang T. T. et al., IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 355, 395–398 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bournazos S., DiLillo D. J., Goff A. J., Glass P. J., Ravetch J. V., Differential requirements for FcγR engagement by protective antibodies against Ebola virus. Proc. Natl. Acad. Sci. U.S.A. 116, 20054–20062 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weitzenfeld P., Bournazos S., Ravetch J. V., Antibodies targeting sialyl Lewis A mediate tumor clearance through distinct effector pathways. J. Clin. Invest. 129, 3952–3962 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data for this paper are included in the manuscript and SI Appendix.