Abstract
Objective
To evaluate the most commonly used medications and in-hospital morbidities and mortality in infants born 22–24 weeks of gestation.
Study design
Multicenter retrospective cohort study of infants born 22–24 weeks of gestation (2006–2016), without major congenital anomalies and with available medication data obtained from neonatal intensive care units managed by the Pediatrix Medical Group.
Results
This study included 7578 infants from 195 sites. Median (25th, 75th percentile): birthweight was 610 g (540, 680); the number of distinct medications used was 13 (8, 18); and different antimicrobial exposure was 4 (2, 5). The most common morbidities were BPD (41%) and grade III or IV IVH (20%), and overall survival varied from 46% (2006) to 57% (2016).
Conclusions
A large number of medications were used in periviable infants. There was a high prevalence of in-hospital morbidities, and survival of this population increased over the study period.
Introduction
The care of periviable infants presents substantial challenges in neonatal and perinatal medicine [1, 2]. Recent studies report survival of infants born at 22 weeks of gestation, leading to increased active resuscitation at this gestational age (GA) [3, 4]. The survival of infants born at 22–24 weeks GA varies greatly based on GA and hospital of birth [5–7]. Despite improvements in medical technology and the use of prenatal medications, such as antibiotics and corticosteroids, infants born at 22–24 weeks GA suffer from a high prevalence of morbidities and mortality [5, 8, 9]. During this vulnerable period of brain and organ development and maturation, periviable infants are exposed to numerous medical interventions and therapeutics. Most of the medications administered in the neonatal intensive care unit (NICU) are not labeled for use in premature infants [10, 11]. There are no data on the number and types of medications administered to this vulnerable population during their initial hospitalization. Furthermore, there are an insufficient number of studies on the distribution, disposition, and effects of medications on premature and periviable infants, which is largely due to their complicated physiology (including a larger extracellular fluid volume, immature renal and hepatic function, and a unique blood–brain barrier) [12–14], as well as the challenges involved with including this vulnerable population in clinical trials.
Infants born in the periviable window often require intensive resuscitation and long admissions to the NICU [3]. Given the increasing active resuscitation and variable outcomes in infants born at 22–24 weeks [4, 9, 15], there is a need to identify factors associated with improved outcomes and management in this population. In this study, we sought to report the most commonly administered medications, as well as the mortality and morbidities during the initial NICU hospitalization in infants born at 22–24 weeks GA.
Methods
Data source and study population
This was a multicenter retrospective cohort study evaluating the numbers and types of the most commonly reported medications used during the initial NICU hospitalization, as well as in-hospital morbidities and mortality in infants born at periviable GA. We obtained data from the Pediatrix Medical Group Clinical Data Warehouse, which prospectively captures clinical information entered into an electronic health record by clinicians at more than 300 NICUs in the United States [16]. The Duke University Institutional Review Board approved this study with a waiver of informed consent.
We included all inborn infants born between 22 weeks and 0/7 days and 24 weeks and 6/7 days gestation who were admitted to the NICU between 2006 and 2016 without major congenital anomalies. We excluded infants with missing or incomplete discharge data (including acute transfers), and infants discharged home at a postnatal age of <32 weeks. In order to eliminate concerns regarding inaccurate GA recorded in the database, we excluded all male and female infants with a birthweight (BW) > 869 and >828 g, respectively (>97th percentile at 24 weeks by the Olsen Growth curves) [17].
We collected the following infant characteristics: BW; GA; sex; race; maternal age; prenatal care; prolonged rupture of membranes; antenatal exposure to steroids; delivery mode; postnatal exposure to surfactant; 5-min Appearance, Pulse, Grimace, Activity, and Respiration score; length of hospital stay; medications used for treatment throughout the first hospitalization; patent ductus arteriosus (PDA) ligation; severe retinopathy of prematurity (ROP); intraventricular hemorrhage (IVH) grade III or IV; medical or surgical necrotizing enterocolitis (NEC); or bronchopulmonary dysplasia (BPD).
Definitions
GA was defined as the neonatologist’s best estimate of GA, based on obstetrical history (including last menstrual period), obstetrical examinations, prenatal ultrasound, or postnatal physical examinations. We excluded infants for whom GA by last menstrual period and neonatologist’s best estimate were more than 1 week different. We defined small for GA as a BW < 10th percentile for GA, based on Olsen growth curves [17]. We defined BPD as the receipt of supplemental oxygen or respiratory support (nasal cannula, continuous positive airway pressure, or mechanical ventilation) continuously from a corrected GA of 36 0/7 weeks to 36 6/7 weeks (designated as the test period) [18]. All surfactant products (beractant, calfactant, poractant alpha, colfosceril palmitate) were combined together as one medication. All nutritional supplements, vitamins (except Vitamin A), vaccines, eye drops, and topical medications were excluded. We excluded eye drops and topical medications because we were interested in systemic exposures. Intravenous nutrition is not captured in these data, so the decision was made to exclude vitamins and supplements. We considered vaccines to be a separate exposure because they are universally recommended; vaccines will be evaluated in a separate study. We examined the incidence of in-hospital outcomes including IVH grade III or IV according to the Papile criteria [19], severe ROP (receipt of surgical treatment, cryotherapy, laser, vitrectomy, scleral buckle, or intravitreal bevacizumab), medical or surgical NEC, ligation of the PDA, and the need for gastrostomy tube placement. We described a composite outcome of death or severe morbidity, where severe morbidity included BPD, IVH grade III or IV, ligation of PDA, severe ROP, and NEC.
Statistical analysis
The unit of observation for this analysis was an infant. We summarized the distribution of continuous variables and categorical variables using medians (25th and 75th percentiles) and counts (%), respectively. We noted infants who were discharged home or to convalescent care and excluded from mortality calculations infants who were transferred prior to discharge. We noted infants with missing data for BPD and severe ROP, but did not exclude those infants from the analysis. We used Wilcoxon rank-sum and chi-square tests as appropriate to compare the distribution of study variables between infants by GA. We determined the number of unique medication names that were reported for each infant, regardless of administration route. We used counts and proportions to describe medication use over time. We identified the 30 most commonly used medications in periviable infants. We reported the number of infants exposed to postnatal steroids (dexamethasone, hydrocortisone, or prednisolone) by GA, and the median number of distinct antimicrobials infants were administered during their first hospital stay. We calculated survival and the median number of medications per infant by site overall and survival for the sites with >20 infants. We performed the statistical analysis using Stata version 15.1 (College Station, TX).
Results
There were 784,186 infants born between 2006 and 2016 in the Pediatrix-managed neonatal units, 10,470 of whom were born at 22–24 weeks GA. After applying exclusion criteria, 7578 periviable infants from 195 sites were included in the analysis (Table 1). Median (25th, 75th percentile) BW was 610 g (540, 680); females comprised 47% of the sample. Most mothers giving birth to infants at 22–24 weeks’ GA had prenatal care 7062/7587 (93%), and 2758/7497 (37%) had a vaginal delivery. The prevalence of cesarean section increased over time and rose with GA (Supplementary Fig.). Overall, 5844/7578 (77%) infants were exposed to at least one dose of antenatal steroids (ANS). The use of ANS was higher in white infants (2241/2799 [80%]) vs. black infants (1989/2675 [74%]) and Hispanic infants (1100/1431 [77%]) (p < 0.001). Median length of stay was 91 days (7, 119), and varied by GA (p < 0.0001). Median age of death was postnatal day 6 (2, 17), and varied from day 2 (1, 8) for 22-week infants, to day 8 (3, 22) for 24-week infants. Infants who survived had a median stay of 116 days (102, 134).
Table 1.
Infant characteristics.
| Gestational age | 22 weeks | 23 weeks | 24 weeks | |||
|---|---|---|---|---|---|---|
| Overall (N = 329) |
Survived (N = 58) |
Overall (N = 2690) |
Survived (N = 1116) |
Overall (N = 4559) |
Survived (N = 2914) |
|
| Birthweight (g), | 511 | 530 | 570 | 590 | 646 | 655 |
| median (IQR) | (465, 560) | (498, 579) | (516, 625) | (530, 640) | (577, 710) | (590, 720) |
| Male (%) Race (%) | 57 | 53 | 53 | 49 | 52 | 49 |
| White | 39 | 37 | 36 | 34 | 40 | 39 |
| Black | 36 | 37 | 38 | 38 | 35 | 36 |
| Hispanic | 19 | 17 | 21 | 23 | 19 | 19 |
| Other | 5 | 9 | 5 | 5 | 6 | 6 |
| Cesarean section (%) |
35 | 35 | 55 | 56 | 70 | 70 |
| SGA (%) | - | - | 16 | 11 | 17 | 12 |
| Received surfactant (%) |
86 | 90 | 91 | 92 | 89 | 90 |
| ANS (%) | 43 | 53 | 69 | 78 | 84 | 87 |
| APGAR (5 min), median (IQR) |
5 (3, 6) | 5 (4, 7) | 6 (3, 7) | 6 (4, 7) | 7 (5, 8) | 7 (5, 8) |
| Maternal age, median (IQR) |
27 (23, 31) | 27 (23, 30) | 28 (23, 32) | 28 (23, 32) | 28 (23, 33) | 28 (23, 33) |
| Prenatal care (%) | 93 | 88 | 93 | 93 | 94 | 94 |
| PROM (%) | 20 | 22 | 18 | 21 | 19 | 21 |
| LOS (in days), median (IQR) |
3 (2, 27) | 137 (116, 154) |
25 (3, 119) | 124 (110, 144) |
98 (17, 120) | 112 (98, 130) |
ANS antenatal steroids, APGAR Appearance, Pulse, Grimace, Activity, and Respiration, IQR interquartile range, LOS length of stay, PROM prolonged rupture of membranes, SGA small for gestational age.
The three most commonly used medications in our cohort were ampicillin, gentamicin, and surfactant (Table 2). Among the ten most frequently prescribed medications, four (ampicillin, gentamicin, vancomycin, and fluconazole) were antimicrobials; the others included surfactant, caffeine citrate, dopamine, furosemide, fentanyl, and hydrocortisone. The number of medications used in infants born at 22–24 weeks’ GA was 13 (8, 18) overall, and 16 (12, 20) for surviving infants. The median number of medications per infant varied across sites (range 5–24, Fig. 1a) and by GA, from 7 (5, 13) for an infant born at 22 weeks’ GA to 13 (9, 18) for an infant born at 24 weeks’ GA. During their NICU stay, infants received four (2, 5) different antimicrobials. Overall, exposure to postnatal steroids varied by GA, with 134/329 (41%) of infants born at 22 weeks, 1505/2690 (56%) of infants born at 23 weeks, and 2622/4559 (58%) of infants born at 24 weeks receiving at least one steroid dose during their hospital stay. However, among survivors, the number of infants exposed to steroids was 46/58 (79%), 875/1116 (78%), and 1874/2914 (64%) for infants born at 22–24 weeks, respectively.
Table 2.
Top 30 most commonly used medications by gestational age (weeks) and the (%) of exposed infants.
| Ranking | 22 weeks GA (n = 329) | 23 weeks GA (n = 2690) | 24 weeks GA (n = 4559) | |||
|---|---|---|---|---|---|---|
| 1 | Ampicillin | 92% | Ampicillin | 96% | Ampicillin | 97% |
| 2 | Gentamicin | 90% | Gentamicin | 94% | Gentamicin | 96% |
| 3 | Surfactant | 86% | Surfactant | 91% | Surfactant | 89% |
| 4 | Dopamine | 60% | Dopamine | 68% | Caffeine Citrate | 80% |
| 5 | Caffeine Citrate | 40% | Caffeine Citrate | 63% | Vancomycin | 65% |
| 6 | Fluconazole | 40% | Vancomycin | 55% | Furosemide | 60% |
| 7 | Hydrocortisone | 36% | Fentanyl | 46% | Dopamine | 59% |
| 8 | Vancomycin | 36% | Furosemide | 45% | Fentanyl | 45% |
| 9 | Fentanyl | 29% | Hydrocortisone | 44% | Indomethacin | 43% |
| 10 | Insulin | 27% | Fluconazole | 42% | Fluconazole | 40% |
| 11 | Epinephrine | 26% | Insulin | 35% | Hydrocortisone | 40% |
| 12 | Dobutamine | 26% | Indomethacin | 34% | Albuterol | 34% |
| 13 | Furosemide | 25% | Midazolam | 30% | Midazolam | 33% |
| 14 | Indomethacin | 25% | Morphine | 27% | Dexamethasone | 29% |
| 15 | Morphine | 21% | Albuterol | 26% | Morphine | 29% |
| 16 | Midazolam | 17% | Epinephrine | 25% | Insulin | 25% |
| 17 | Phenobarbital | 17% | Dobutamine | 25% | Cefotaxime | 24% |
| 18 | Albuterol | 13% | Dexamethasone | 25% | Acetaminophen | 20% |
| 19 | Dexamethasone | 12% | Cefotaxime | 23% | Phenobarbital | 18% |
| 20 | Cefotaxime | 12% | Phenobarbital | 20% | Epinephrine | 17% |
| 21 | Lorazepam | 9% | Lorazepam | 15% | Dobutamine | 17% |
| 22 | Meropenem | 8% | Tobramycin | 14% | Chlorothiazide | 16% |
| 23 | Piperacillin + Tazobactam | 8% | Chlorothiazide | 14% | Budesonide | 16% |
| 24 | Amphotericin B | 8% | Vitamin A (retinyl palmitate) | 13% | Spironolactone | 16% |
| 25 | Epoetin Alpha | 8% | Budesonide | 13% | Ranitidine | 15% |
| 26 | Tobramycin | 7% | Acetaminophen | 13% | Vitamin A (retinyl palmitate) | 15% |
| 27 | Chlorothiazide | 6% | Nitric Oxide (iNO) | 12% | Lorazepam | 15% |
| 28 | Acetaminophen | 6% | Spironolactone | 12% | Tobramycin | 14% |
| 29 | Nitric Oxide (iNO) | 6% | Meropenem | 11% | Ceftazidime | 12% |
| 30 | Cefepime | 6% | Piperacillin + Tazobactam | 11% | Piperacillin + Tazobactam | 12% |
GA gestational age.
Fig. 1. Number of medications and survival by site.
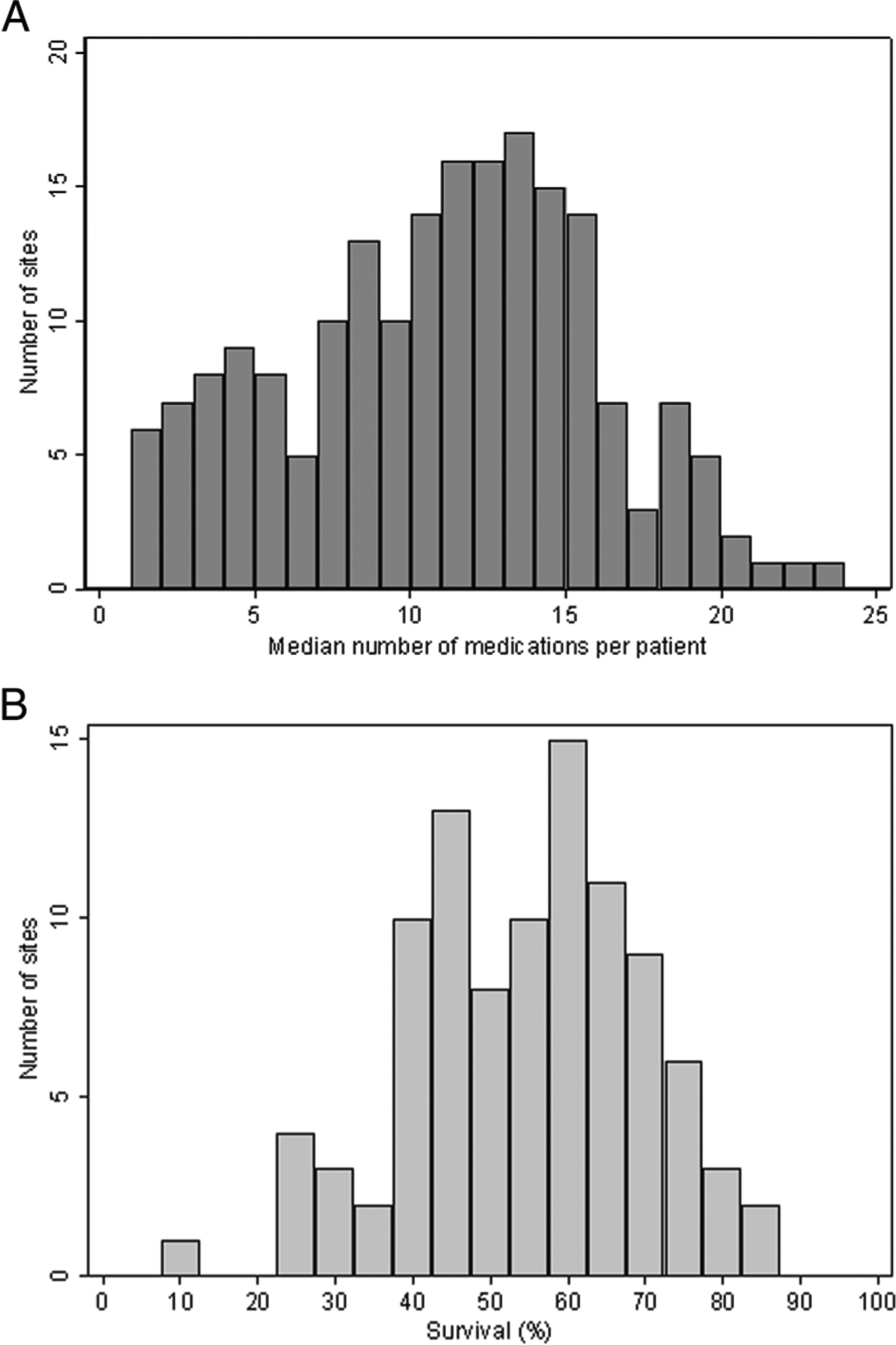
a medication use across sites: distribution of the median number of medications to which a periviable infant was exposed during the study period; and b survival among sites with >20 infants: distribution of the proportion of infants 22–24 weeks GA who survived to discharge. GA gestational age.
The most common morbidities reported were BPD, which affected 3082/7461 (41%) of infants; grade III or IV IVH in 1542/7578 (20%) infants; and PDA ligation in 1434/7578 (19%) infants. In total, 360/4088 (9%) infants were discharged with a gastrostomy tube. A total of 3490/7578 (46%) infants died, and 6910/7578 (91%) had the composite outcome of death or severe morbidity (Table 3).
Table 3.
Morbidities and mortality.
| Gestational age | 22 weeks | 23 weeks | 24 weeks | |||
|---|---|---|---|---|---|---|
| Overall (N = 329) |
Survived (N = 58) |
Overall (N = 2690) |
Survived (N = 1116) |
Overall (N = 4559) |
Survived (N = 2914) |
|
| PDA ligation (%) | 7 | 26 | 19 | 38 | 20 | 27 |
| BPD (%) | 14 | 77 | 34 | 80 | 48 | 73 |
| NEC medical (%) | 3 | 7 | 6 | 8 | 6 | 6 |
| NEC surgical (%) | 2 | 2 | 4 | 4 | 5 | 3 |
| IVH (grade III/ IV) (%) |
23 | 28 | 24 | 19 | 18 | 13 |
| Severe ROP (%) | 20 | 24 | 21 | 22 | 16 | 16 |
| Gastrostomy tube (%) |
2 | 9 | 5 | 11 | 5 | 8 |
| Died (%) | 82 | - | 59 | - | 36 | - |
| Death or any severe morbidity (%)a |
98 | 91 | 95 | 89 | 88 | 81 |
BPD bronchopulmonary dysplasia, IVH intraventricular hemorrhage, NEC necrotizing enterocolitis, PDA patent ductus arteriosus, ROP retinopathy of prematurity.
Severe morbidity included BPD, IVH grade III or IV, ligation of PDA, severe ROP and NEC.
Overall, 4088/7578 (54%) of infants born at 22–24 weeks’ GA in our cohort survived. Survival to discharge from the initial NICU stay varied across sites (Fig. 1b). Survival increased with GA, and also over time, from 263/566 (46%) in 2006 to 419/733 (57%) in 2016 (Fig. 2) (p < 0.001). There was no significant difference in survival across races (p = 0.22). Infants exposed to ANS were less likely to die than infants not exposed (2409/5844 [41%] vs. 1081/1734 [62%]; p < 0.001).
Fig. 2. Survival by GA.
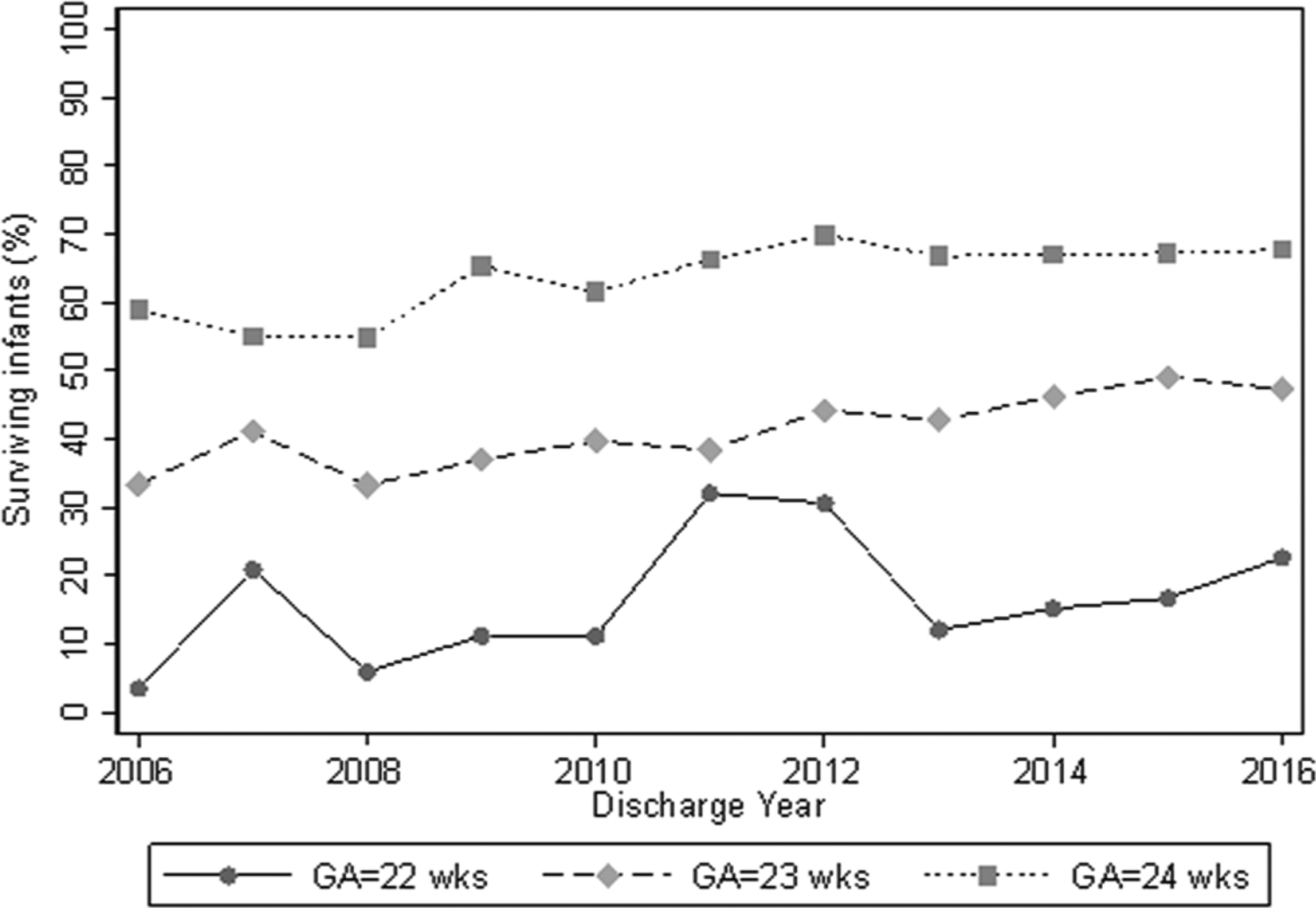
Survival rates among infants born at 22–24 weeks GA, from 2006 to 2016. GA gestational age.
Discussion
This is the largest report to date on medications used in infants born at 22–24 weeks of gestation. Periviable infants are exposed to multiple medications during their first NICU stay, and there is a wide variation by GA and hospital of birth. While survival increased over the study period, we report a high prevalence of in-hospital morbidities in infants born at the edge of viability.
As expected, given the infants’ high risk for infection, ampicillin and gentamicin are the two most commonly administered medications in periviable infants, just as they are the top medications used in infants of any GA admitted to the NICU [11, 20]. In the current analysis, four out of the top ten medications were antimicrobials. Out of the top ten medications administered to periviable infants, gentamicin, vancomycin, and surfactant are the only ones approved by the Food and Drug Administration for use in infants with BW < 1000 g [11]. Given that both animal-derived and synthetic surfactants are recommended in infants born < 30 weeks’ gestation as therapy for respiratory distress syndrome [21], we combined all surfactant products together, and this was the third most common medication prescribed for periviable infants. Caffeine citrate, used to treat apnea of prematurity, was the fourth most common drug prescribed in this group, and remains one of the most widely used off-label medications in infants <28 weeks of gestation [22]. Our results highlight the need for prospective studies of the safety and efficacy of drugs in this population.
Infants born at 22–23 weeks’ GA in the Japanese Neonatal Research Network from 2003 to 2007 (11,607 infants) had a lower mortality if they were exposed to ANS [23]. The exposure to ANS in our cohort (77%) was similar to the report of 10,541 infants born at 22–25 weeks’ GA in the National Institute of Child Health and Human Development Neonatal Research Network (NRN) between 1993 and 2009, in which 74% of mothers received ANS [24], but <88% in the recent study from the Vermont Oxford Network [25]. In the NRN study, the lowest administration of ANS occurred in black mothers (43%). In our study, the prevalence of ANS administration was statistically higher in white mothers (80%) vs. black (74%) or Hispanic (77%) mothers but the differences were small. In both our study and the NRN report, mortality was higher (62% and 56%, respectively) in infants born to mothers who did not receive ANS, than in infants with ANS exposure (41% and 36%, respectively) [24]. Antenatal corticosteroids given to mothers at 23–34 weeks’ gestation have been shown to dramatically reduce mortality and complications associated with premature birth [26, 27]. For infants born 22–25 weeks’ gestation, ANS administration reduces the risk of death similar to an additional 1.1 weeks GA [28, 29] and appears to reduce morbidities, as well [23, 24, 29]. Nevertheless, it is unclear if this relationship is causal, since the decision to give ANS may imply active treatment of the infant. We suspect that the lower ANS rates at the early GA are due to imminent deliveries, but details regarding these data are not captured in our dataset.
A challenge that obstetricians and neonatal providers face in the periviable period is the method of delivery. Cesarean section (63%) was much more common than vaginal delivery (37%) in our cohort, consistent with other studies of periviable infants that have found a prevalence of cesarean sections between 59 and 64% [5, 7]. With more interventions, and higher survival of periviable infants over time, cesarean delivery of periviable infants is becoming more common [30–32]. Some studies support the idea that delivery via cesarean section may provide benefits for the most immature infants, delivered at 22–25 weeks of gestation, independent of maternal risk factors for cesarean section [33–35]. However, in one recent study of cesarean sections done at this early GA, almost one in four women experienced a serious maternal complication, regardless of incision type [32]. There have been no randomized controlled trials comparing cesarean delivery with vaginal delivery in the periviable period. Any association of cesarean section with outcomes should be interpreted with caution, because this could be a sign of a different, more aggressive approach to obstetric management that is accompanied by other, unmeasured, interventions [36]. Similarly, in our study, the increased prevalence of cesarean sections over time may reflect a more active perinatal approach to the management of periviable infants.
In our cohort, 54% of the infants born at 22–24 weeks GA survived. The proportion of infants who survived until discharge varied by GA from 18% (in 22 weeks GA infants) to 64% (in the 24 weeks GA infants). In the NRN study of 9575 infants born alive from 2003 to 2007 at 22–28 weeks GA, overall survival was 72%, and in the periviable period, between 6% (for 22 weeks GA infants) and 55% (for 24 weeks GA infants) [5]. Our study began in 2006, and part of the increased survival over our sample period may be due to advancements in periviable infant care over the last decade. There was an increase in survival from the beginning of our study in 2006 (46%) to the end of our study in 2016 (57%). For infants born between 1993 and 2015 at 22–25 weeks GA in the NRN, survival increased over time from 7% (22 weeks GA infants), 28% (23 weeks GA infants), and 53% (24 weeks GA infants) during the 1993 to 1997 period to 9% (22 weeks GA infants), 49% (23 weeks GA infants), and 70% (24 weeks GA infants) during the 2013–2015 period [37, 38].
With increasing survival of periviable infants [5, 9, 39–42], clinicians are faced with multiple morbidities and more complex care in-hospital and after discharge. Recent studies debate whether the increasing survival for periviable infants is also leading to an increase in morbidities [9, 15, 43]. Of the infants included in our study, 41% were diagnosed with BPD, similar to a study of 8515 infants born 22–27 weeks GA from 2003 to 2007 that reported a prevalence of BPD of 42% [5]. In a Canadian study of 287 infants born at 23 weeks’ GA, all infants who survived to discharge had at least one morbidity and the prevalence of ROP was 58% [15]. The vast majority of infants in our cohort died or had severe morbidities, and this composite outcome varied by GA, from 88% in 24 weeks’ GA infants to 95% and 98% in 23 and 22 weeks’ GA infants, respectively. Knowledge of the incidence of morbidities and mortality can be helpful for clinicians and parents when deciding on the course of treatment for periviable infants, especially as survival rates continue to increase [42, 44–46].
The length of stay for periviable infants who survived to discharge from initial NICU stay in our cohort is inversely related to GA, and similar to a recent study of 59 periviable infants (23–25 weeks’ GA, 2012–2015, conducted in a single tertiary care, university-based NICU) where the reported average length of stay was 114 ± 11 days for infants born at 23 weeks’ GA, and 111 ± 13 days for infants born at 24 weeks’ GA [47].
Our study’s strengths include that it was a large, representative cohort of infants admitted to 195 NICUs, as well as the fact that we were able to report in-hospital outcomes and medications used in this population of periviable infants. Our examination also had several limitations. Our study is limited by the use of administrative data, derived from prospectively collected electronic medical documentation. We did not have access in this dataset to diagnosis criteria for NEC and PDA ligations, and the criteria likely differed among sites. In our study, the GA was established as the best neonatal and obstetric estimate and relied on documentation in the electronic health records. We did not have access to postdischarge data, so we were unable to determine neurodevelopmental outcome. The use of G-tube at discharge was used as a surrogate predictor for neurodevelopmental outcomes [48]. In this report, there are no data reflecting intrauterine demise, deaths at birth or in the delivery room, or decisions regarding perinatal palliative care. The decision to actively resuscitate the infant was also not available. Active intervention for infants born at or after 22 weeks of gestation varies. In the United States, both the American Academy of Pediatrics and the American Congress of Obstetricians and Gynecologists recommend that clinicians and families make individualized decisions about treating extremely preterm infants on the basis of parental preference and the most recent data available regarding survival and morbidity [42, 49]. In the current study, survival is likely higher than other reports due to our inability to account for any delivery room deaths prior to NICU admission.
In conclusion, we identified the most commonly used medications in periviable infants and showed a high prevalence of in-hospital morbidities in this vulnerable population. The survival rate has increased over the study period, which may reflect changes in the active treatment of periviable infants. Despite the improvements over time, there is a continuing need to identify factors that can enhance outcomes, including long-term neurodevelopment of periviable infants. With increased survival in this population, more frequent studies of medication use patterns should be conducted to facilitate the development of drug safety and efficacy studies in periviable infants. As clinical trials are rare and very difficult in this vulnerable population, frequent studies of medication use patterns should be performed, and systems to prospectively identify adverse effects should be developed. These data will help clinicians and researchers in setting priorities for studies.
Supplementary Material
Acknowledgements
PTN Steering Committee Members: Daniel K. Benjamin Jr., Christoph Hornik, Kanecia Zimmerman, Phyllis Kennel, and Rose Beci, Duke Clinical Research Institute, Durham, NC, USA; Chi Dang Hornik, Duke University Medical Center, Durham, NC, USA; Gregory L. Kearns, Independent; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC USA; Ian M. Paul, Penn State College of Medicine, Hershey, PA, USA; Janice Sullivan, University of Louisville, Louisville, KY, USA; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; Paula Delmore, Wichita Medical Research and Education Foundation, Wichita, KS, USA. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): Perdita Taylor-Zapata and June Lee. The Emmes Company, LLC (Data Coordinating Center): Ravinder Anand, Gaurav Sharma, Gina Simone, Kim Kaneshige, and Lawrence Taylor. PTN Publications Committee: Chaired by Thomas Green, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
Funding This work was funded under National Institute of Child Health and Human Development (NICHD) contract (HHSN275201 000003I) for the Pediatric Trials Network (PI Daniel K. Benjamin Jr.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by Duke Clinical Research Institute’s R25 Summer Training in Academic Research (STAR) Program (grant #5R25HD076475-07).
Footnotes
Conflict of interest NY received support from the NIH (K23DK12 0960). MP-D, DKB, KL, CH, KH, JM, AW, KOZ, KA, and RGG report no relevant disclosures.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
High school or college student affiliated with the Duke Clinical Research Institute Summer Training in Academic Research (STAR) Summer Program (5R25HD076475–07).
Supplementary information The online version of this article (https://doi.org/10.1038/s41372-020-0614-4) contains supplementary material, which is available to authorized users.
References
- 1.Ecker JL, Kaimal A, Mercer BM, Blackwell SC, deRegnier RA. American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, et al. Periviable birth: interim update. Am J Obstet Gynecol. 2016;215:B2–B12.e1. [DOI] [PubMed] [Google Scholar]
- 2.Raju TN, Mercer BM, Burchfield DJ, Joseph GF. Periviable birth: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Academy of Pediatrics, and American College of Obstetricians and Gynecologists. J Perinatol. 2014;34:333–42. [DOI] [PubMed] [Google Scholar]
- 3.Mercer BM. Periviable birth and the shifting limit of biability. Clin Perinatol. 2017;44:283–6. [DOI] [PubMed] [Google Scholar]
- 4.Brumbaugh JE, Hansen NI, Bell EF, Sridhar A, Carlo WA, Hintz SR, et al. Outcomes of extremely preterm infants with birth weight less than 400 g. JAMA Pediatr. 2019;173:434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372:1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker Edmonds B, McKenzie F, Macheras M, Srinivas SK, Lorch SA. Morbidity and mortality associated with mode of delivery for breech periviable deliveries. Am J Obstet Gynecol. 2015;213:70.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129:1019–26. [DOI] [PubMed] [Google Scholar]
- 9.Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and neurodevelopmental outcomes among periviable infants. N. Engl J Med 2017;376:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–87. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr, Smith PB. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31:811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu TW, Azhibekov T, Seri I. Transitional hemodynamics in preterm neonates: clinical relevance. Pediatr Neonatol. 2016;57:7–18. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez E, Perez R, Hernandez A, Tejada P, Arteta M, Ramos JT. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics. 2011;3:53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane JM, Magee LA, Lee T, Synnes A, von Dadelszen P, Dahlgren L, et al. Maternal and perinatal outcomes of pregnancies delivered at 23 weeks’ gestation. J Obstet Gynaecol Can. 2015;37:214–24. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system-tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. [DOI] [PubMed] [Google Scholar]
- 17.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24. [DOI] [PubMed] [Google Scholar]
- 18.Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. [DOI] [PubMed] [Google Scholar]
- 20.Nir-Neuman H, Abu-Kishk I, Toledano M, Heyman E, Ziv-Baran T, Berkovitch M. Unlicensed and off-label medication use in pediatric and neonatal intensive care units: no change over a decade. Adv Ther. 2018;35:1122–32. [DOI] [PubMed] [Google Scholar]
- 21.Polin RA, Carlo WA. Committee on fetus and newborn; American Academy of Pediatrics Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–63. [DOI] [PubMed] [Google Scholar]
- 22.Regent American, Inc. Caffeine citrate drug label information. DAILYMED. 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d17e3be5-514f-454a-8347-d804cd5ec37f. Accessed 5 July 2019.
- 23.Mori R, Kusuda S, Fujimura M. Neonatal Research Network Japan. Antenatal corticosteroids promote survival of extremely preterm infants born at 22 to 23 weeks of gestation. J Pediatr. 2011;159:110–4.e1. [DOI] [PubMed] [Google Scholar]
- 24.Carlo WA, McDonald SA, Fanaroff AA, Vohr BR, Stoll BJ, Ehrenkranz RA, et al. Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. J Am Med Assoc. 2011;306:2348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehret DEY, Edwards EM, Greenberg LT, et al. Association of antenatal steroid exposure with survival among infants receiving postnatal life support at 22 to 25 weeks’ gestation. JAMA Netw Open. 2018;1:e183235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts D, Brown J, Medley N, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. [DOI] [PubMed] [Google Scholar]
- 27.Travers CP, Clark RH, Spitzer AR, Das A, Garite TJ, Carlo WA. Exposure to any antenatal corticosteroids and outcomes in preterm infants by gestational age: prospective cohort study. BMJ. 2017;356:j1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wapner RJ. Antenatal corticosteroids for periviable birth. Semin Perinatol. 2013;37:410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. National Institute of Child Health Human Development Neonatal Research Network Intensive care for extreme prematurity-moving beyond gestational age. N Engl J Med. 2008;358:1672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitchen WH, Permezel MJ, Doyle LW, Ford GW, Rickards AL, Kelly EA. Changing obstetric practice and 2-year outcome of the fetus of birth weight under 1000 g. Obstet Gynecol. 1992;79:268–75. [PubMed] [Google Scholar]
- 31.Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. 2015;372:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawakita T, Reddy UM, Grantz KL, Landy HJ, Desale S, Igbal SN. Maternal outcomes associated with early preterm cesarean delivery. Am J Obstet Gynecol. 2017;216:312.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malloy MH. Impact of cesarean section on neonatal mortality rates among very preterm infants in the United States, 2000–2003. Pediatrics. 2008;122:285–92. [DOI] [PubMed] [Google Scholar]
- 34.Deulofeut R, Sola A, Lee B, Buchter S, Rahman M, Rogido M. The impact of vaginal delivery in premature infants weighing less than 1,251 grams. Obstet Gynecol. 2005;105:525–31. [DOI] [PubMed] [Google Scholar]
- 35.Holzer I, Lehner R, Ristl R, Husslein PW, Berger A, Farr A. Effect of delivery mode on neonatal outcome among preterm infants: an observational study. Wien Klin Wochenschr. 2017;129:612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bottoms SF, Paul RH, Iams JD, Mercer BM, Thom EA, Roberts JM, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960–6. [DOI] [PubMed] [Google Scholar]
- 37.Patel RM, Rysavy MA, Bell EF, Tyson JE. Survival of infants born at periviable gestational ages. Clin Perinatol. 2017;44:287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. J Am Med Assoc. 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M. Neonatal Research Network, Japan Outcomes of infants born at 22 and 23 weeks’ gestation. Pediatrics. 2013;132:62–71. [DOI] [PubMed] [Google Scholar]
- 40.Gunasekera H, Isaacs D. Outcome for 22–24 week gestation newborns. J Paediatr Child Health. 2017;53:1240. [DOI] [PubMed] [Google Scholar]
- 41.Moore T, Hennessy EM, Myles J, Johnson SJ, Draper ES, Costeloe KL, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ. 2012;345:e7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus no. 6: periviable birth. Obstet Gynecol. 2017;130:e187–99. [DOI] [PubMed] [Google Scholar]
- 43.Brunkhorst J, Weiner J, Lantos J. Infants of borderline viability: the ethics of delivery room care. Semin Fetal Neonatal Med. 2014;19:290–5. [DOI] [PubMed] [Google Scholar]
- 44.Guillén U, Weiss EM, Munson D, Maton P, Jefferies A, Norman M, et al. Guidelines for the management of extremely premature deliveries: a systematic review. Pediatrics. 2015;136:343–50. [DOI] [PubMed] [Google Scholar]
- 45.Chervenak FA, McCullough LB. Ethical issues in periviable birth. Semin Perinatol. 2013;37:422–5. [DOI] [PubMed] [Google Scholar]
- 46.Edmonds BT, McKenzie F, Hendrix KS, Perkins SM, Zimet GD. The influence of resuscitation preferences on obstetrical management of periviable deliveries. J Perinatol. 2015;35:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen KM, Smith B, Iliev I, Evans J, Werthammer J. Short term cost of care for the surviving periviable neonate. J Neonatal Perinat Med. 2017;10:191–4. [DOI] [PubMed] [Google Scholar]
- 48.Jadcherla SR, Khot T, Moore R, Malkar M, Gulati IK, Slaughter JL. Feeding methods at discharge predict long-term feeding and neurodevelopmental outcomes in preterm infants referred for gastrostomy evaluation. J Pediatr. 2017;181:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batton DG. Committee on Fetus and Newborn Clinical report—antenatal counseling regarding resuscitation at an extremely low gestational age. Pediatrics. 2009;124:422–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


