Significance
T cell coreceptors are often described as molecular switches that broadly regulate T cell activation by enhancing or repressing T cell receptor (TCR) signaling according to the immunological context. However, many coreceptors act more selectively by instructing or restricting specific T cell responses. The molecular mechanisms by which these subtle regulations occur remain incompletely defined. In this study, we show that CD5 coreceptors engage a multimeric signaling complex, which synchronize positive and negative feedback on TCR signaling to limit the induction of inopportune regulatory T cells during immune response. Our findings suggest that rather than exclusively acting as stimulators or inhibitors of TCR signaling, coreceptors may coordinate antagonist TCR signals that act together to promote specific T cell responses.
Keywords: T cells, signaling, coreceptors
Abstract
CD5 is characterized as an inhibitory coreceptor with an important regulatory role during T cell development. The molecular mechanism by which CD5 operates has been puzzling and its function in mature T cells suggests promoting rather than repressing effects on immune responses. Here, we combined quantitative mass spectrometry and genetic studies to analyze the components and the activity of the CD5 signaling machinery in primary T cells. We found that T cell receptor (TCR) engagement induces the selective phosphorylation of CD5 tyrosine 429, which serves as a docking site for proteins with adaptor functions (c-Cbl, CIN85, CRKL), connecting CD5 to positive (PI3K) and negative (UBASH3A, SHIP1) regulators of TCR signaling. c-CBL acts as a coordinator in this complex enabling CD5 to synchronize positive and negative feedbacks on TCR signaling through the other components. Disruption of CD5 signalosome in mutant mice reveals that it modulates TCR signal outputs to selectively repress the transactivation of Foxp3 and limit the inopportune induction of peripherally induced regulatory T cells during immune responses against foreign antigen. Our findings bring insights into the paradigm of coreceptor signaling, suggesting that, in addition to providing dualistic enhancing or dampening inputs, coreceptors can engage concomitant stimulatory and inhibitory signaling events, which act together to promote specific functional outcomes.
T cells have the ability to develop a wide variety of cellular responses following the stimulation of a single receptor, namely the T cell antigen receptor (TCR). The recognition by TCRs of self or foreign peptides bound to the major histocompatibility complex (pMHC) triggers multiple signaling pathways, which lead to the activation of specific effector proteins involved in the transmission of distinct signaling responses. The relative intensity and the persistency by which signals are transmitted in each pathway play a critical role in specifying and driving specific T cell responses. Because different pathways may have either synergistic or antagonist effects on these responses, their coordination in time and space (signaling patterns) is also critical to shape T cell effector profiles and determine specific outcomes.
Signals transmitted by the TCR can be regulated by coreceptors that are engaged differentially based on their relative expression on the T cell surface and on the availability of their cognate ligands in the extracellular environment. Initial work on coreceptor signaling led to the classification of these proteins into two main functional categories, depending on their overall effect on T cell activity: Stimulatory coreceptors—such as CD28, ICOS, or OX40—which promote naïve T cell activation and amplify effector T cell responses, and inhibitory coreceptors—such as CTLA-4, PD-1, or BTLA—which prevent the potential activation of T cells by self-antigens and contribute to terminate or tune down effector T cell responses following antigen clearance. More recent investigations indicate that many coreceptors act more selectively on specific signaling pathways and contribute to shape the effector profile of T cells according to the immunological context (1–3). Although the mechanisms by which coreceptors positively or negatively regulate T cell activity have been well documented (1, 4–7), the molecular processes by which they convey signals to selectively modulate T cell responses remain poorly understood.
CD5 is a type 1 transmembrane cell surface glycoprotein that is essentially expressed in T cells. Initial characterization of Cd5−/− mice indicated an inhibitory function for this receptor on TCR signaling (8). Later work showed that CD5 surface levels on thymocytes are correlated to the strength of TCR signaling that is dictated during positive selection by the affinity of the TCR for self-pMHC (9). The increased expression of CD5 in thymocytes that express TCRs with greater self-reactivity dampens TCR signals, possibly enabling some thymocytes that would otherwise be negatively selected to avoid activation-induced cell death and instead complete their maturation and be exported to peripheral lymphoid organs (10). Through this mechanism CD5 would enable the selection of T cells with higher self-reactivity, which are presumed to be more effective responders to foreign antigens (11).
Whereas the role of CD5 during thymic selection has been well characterized, its function in peripheral T cells is less clear. The expression level of CD5 remains correlated with the affinity of TCR for self-pMHC in peripheral T cells (11), suggesting that CD5 could be important for the maintenance of self-tolerance by dampening homeostatic TCR signals that could otherwise cause activation and autoimmunity. However, Cd5−/− mice do not exhibit signs of spontaneous autoimmune or inflammatory pathology and, in contrast, show a reduced susceptibility to active experimental autoimmune encephalomyelitis (12) and inflammatory bowel disease (13). This suggests that in the absence of CD5 compensatory mechanisms might prevent the expansion and the full activation of T cells expressing TCRs with relatively high affinity to self-pMHC. Notably, previous studies showed that the numbers and suppressive function of regulatory T (Treg) cells are increased in CD5-deficient mice (13, 14). However, more recent findings indicate that CD5 plays an instructive role in the generation of peripherally induced Treg cells (iTreg) in response to tolerizing antigens (15), suggesting that CD5 could have different influences on this T cell subset according to the immunological context.
Although several ligands have been reported for CD5 (16–18), it was shown that its extracellular domain is not required for negative regulation of TCR signaling in thymocytes (19), indicating that CD5 is engaged in a feedback loop that tunes down TCR signals following TCRs engagement. Accordingly, CD5 is constitutively associated with the TCR subunits at the cell surface (20) and contains several phospho-tyrosine binding (PTB) sites that are phosphorylated by SRC kinases following TCR engagement (21). Although many CD5-interacting partners have been reported, the relative importance of these interactions remains unclear because most of these binding partners were identified in independent studies or within distinct cellular models through approaches that do not always enable global comparisons of protein–protein interactions. Interestingly, whereas some of these proteins are well-characterized inhibitors of TCR signaling (22, 23), others are known to be positive effectors (24–27), suggesting that CD5-mediated feedback on TCR signaling might be more complex than what was initially presumed.
In this study, we combined quantitative mass spectrometry (MS) and mouse genetics to analyze the composition, the mode of assembly, and the molecular function of the CD5 transduction machinery in primary T cells. We found that CD5 coordinates the recruitment of a signaling complex composed of proteins with adapter functions (c-CBL, CIN85, and CRKL) that connect CD5 to positive (PI3K) and negative (UBASH3A and SHIP1) regulators of TCR signaling. The recruitment of this complex is entirely dependent on the Y429 of CD5, which is predominantly phosphorylated following TCR engagement and serves as a docking site for c-CBL. Disruption of Y429 phosphorylation site in primary T cells shows that this signaling complex promotes, on the one hand, AKT-mediated inhibition of FOXO1 and represses, on the other hand, ERK kinase activity to selectively dampen the transactivation of Foxp3 gene expression. Analysis of antigen-specific Treg cells in CD5-Y429F mutant mice show that CD5 signaling selectively represses the generation of these cells presumably to promote the development of optimal immune responses.
Results
MS Analysis of the CD5 Interactome.
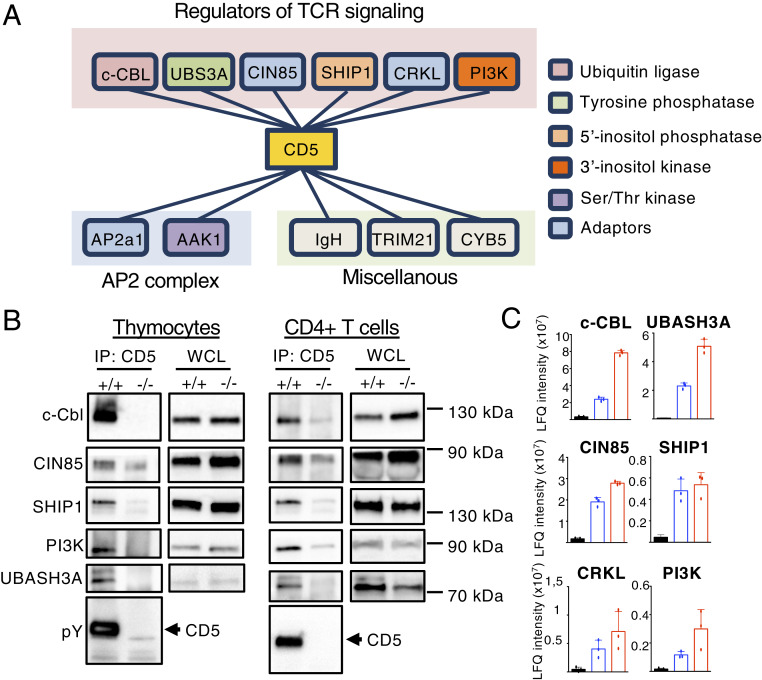
To investigate the molecular mechanism by which CD5 regulates TCR signaling, we performed an MS-based analysis of CD5-containing complexes in thymocytes. CD5 was immunoprecipitated from WT or Cd5−/− thymocytes that were treated with pervanadate for 1 min to induce widespread activation of protein tyrosine kinases. CD5 protein complexes were eluted, and the components of the different purified complexes were characterized by nanoflow liquid chromatography combined with tandem MS. To discriminate CD5-binding molecules from the background of contaminant proteins, a thorough quantitative comparison based on MS intensity values was performed for each identified protein between samples immunoprecipitated from WT versus Cd5−/− thymocytes. Candidate proteins were selected based on their significant enrichment in WT samples (fold-change > 2 and Student t test P < 0.001, n = 8 replicate experiments) (see Materials and Methods for details). On this basis, we identified 11 proteins as potential interacting partners of CD5 in thymocytes (Fig. 1A, SI Appendix, Fig. S1A, and Dataset S1). Among these, six proteins were previously identified as regulators of TCR-mediated signaling (c-CBL, UBASH3A/STS-2, CIN85/SH3KBP1, SHIP1, CRKL, and PI3K), two proteins are known components of the AP2 complex that is involved in clathrin-mediated internalization of CD5 (AP2a1, AAK1) (28), and three proteins have diverse reported functions not directly associated with TCR signaling (IGH, TRIM21, CYB5). The interactions of CD5 with c-CBL, UBASH3A, PI3K, CRKL, and AP2 were reported previously in independent studies (22, 24, 25, 28, 29) but not its interaction with SHIP1 and CIN85. Among these proteins, three are cytosolic adaptors (CRKL, CIN85, and AP2) and five are effector molecules with enzymatic function (c-CBL, UBASH3A, SHIP1, AAK1, and PI3K). The association of these proteins with CD5 was still detected after 10 min of stimulation and no additional interactors were identified at this later time of stimulation (SI Appendix, Fig. S1B and Dataset S2).
Fig. 1.
MS analysis of CD5 interactome. (A) Schematic of those proteins that preferentially interact with CD5 in thymocytes stimulated with pervanadate for 1 min. Layouts indicate the classification of these proteins according to their function (pink layout: TCR signaling; blue layout: AP2 complex; green layout: unknown function in T cell signaling). Data represent eight independent experiments with n = 8 mice per group. (B) Thymocytes and peripheral CD4+ T cells from Cd5+/+ (+/+) or Cd5−/− (−/−) mice were stimulated with pervanadate for 5 min. Samples were then subjected to immunoprecipitation (IP) with antibodies specific for CD5 and then analyzed by Western blotting with antibodies specific for the indicated proteins. WCL, whole cell lysate. (C) Relative abundance of principal CD5 interacting partners after co-IP in thymocytes either nonstimulated (black bars) or stimulated with anti-CD3 and anti-CD4 antibodies (blue bars) or pervanadate (red bars). Protein abundances were estimated using the LFQ metric calculated by MaxQuant based on MS peptide signals intensities. Data are means ± SD of three MS measurement and are representative of three independent experiments containing n = 1 mouse per group.
To estimate the relative abundance of these signaling partners in the immunoprecipitated samples, we used the intensity-based absolute quantification (iBAQ) metric, which corresponds to the sum of all of the peptide intensities divided by the number of theoretically observable tryptic peptides of a protein. Analysis of these interactions (with normalized iBAQ intensities) shows that UBASH3A (IBAQ = 604 × 104) and c-CBL (IBAQ = 560 × 104) are more abundantly recruited to CD5 than CIN85 (IBAQ = 147 × 104), CRKL (IBAQ = 143 × 104), SHIP1 (IBAQ = 55 × 104), and PI3K (IBAQ = 18 × 104), suggesting an essential role for c-Cbl and UBASH3A in CD5-mediated regulation of T cell activation (SI Appendix, Fig. S1A). Analysis by Western blot showed that CD5 interacts with c-CBL, UBASH3A, SHIP1, CIN85, and PI3K both in thymocytes and in peripheral CD4+ T cells, suggesting that CD5 engages similar signaling processes upon T cell development and primary T cell responses (Fig. 1B). Further analysis showed that c-CBL, UBASH3A, SHIP1, CRKL, CIN85, and PI3K interacted poorly with CD5 in resting cells but were recruited to CD5 upon TCR+CD4 cross-linking (Fig. 1C and Dataset S3), suggesting that CD5 contributes to TCR signaling through the activity of these proteins. Note that RAS-GAP (22), SHP-1 (23), CBL-b (30), and CK2 (27), which were previously reported to interact with CD5 in thymocytes or in T cell lines, were either undetected (Ras-GAP), detected at the same level in immunoprecipitated samples from WT and Cd5−/− controls (SHP-1), or inconsistently detected across biological replicates without a statistically significant enrichment ratio in immunoprecipitated samples versus controls (CBL-b and CK2). Thus, these proteins were not selected in the list of major CD5-interacting proteins (Dataset S1).
Tyrosine 429 of CD5 Is Essential for Assembly of the CD5 Signalosome.
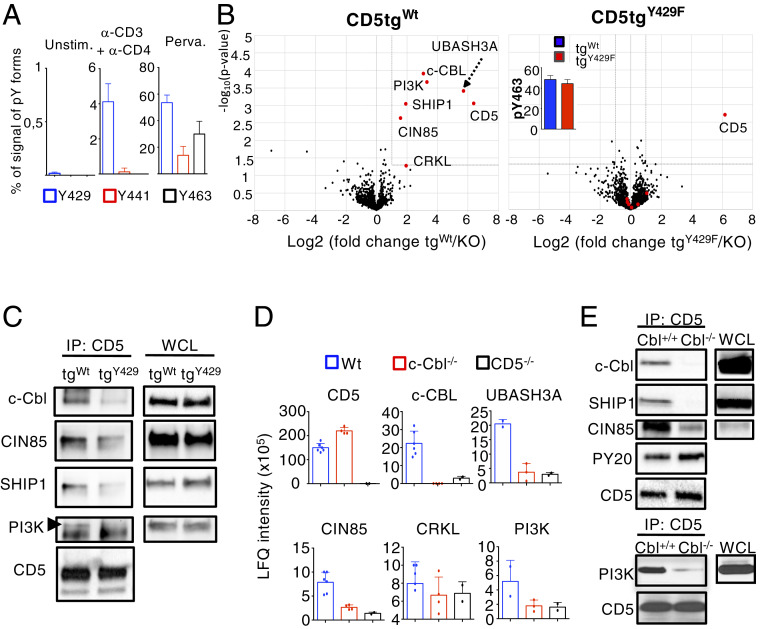
We next investigated the mechanism by which CD5 recruits these proteins following TCR stimulation. Previous studies performed on cell lines identified three potential tyrosine-phosphorylation sites on the intracytoplasmic domain of human CD5: Tyrosine 429 (pY429), tyrosine 441 (pY441), and tyrosine 463 (pY463) (31, 32). To address which of these tyrosines is preferentially phosphorylated following TCR engagement in primary thymocytes, we analyzed the relative MS intensity of CD5 phosphorylated peptides encompassing these three modification sites compared to their respective unmodified forms. It must be noted that this percentage does not strictly measure the phosphorylation stoichiometry of each site (as phosphorylated and unphosphorylated forms may have different ionization efficiencies in the mass spectrometer). We nevertheless used this metric to qualitatively illustrate the phosphorylation occupancy before and after stimulation. Whereas the phosphorylation of these residues was barely detectable in unstimulated thymocytes, we found that CD5 was nearly exclusively phosphorylated on Y429 after TCR cross-linking with CD4 upon different times of stimulation (Fig. 2A and Datasets S3 and S4). All three tyrosine residues of CD5 were phosphorylated following pervanadate treatment, suggesting that Y441 and Y463 might be phosphorylated independently of TCR engagement upon additional costimulatory signals (Fig. 2A).
Fig. 2.
c-CBL is recruited to CD5 Y429 and is connecting CD5 with its signaling partners. (A) MS analysis of CD5 phosphorylation sites. Thymocytes from WT mice were left unstimulated (Unstim.) or were stimulated with anti-CD3 + anti-CD4 antibodies or pervanadate (Perva.) for 1 min. Samples were then subjected to IP with antibodies specific for CD5. MS intensity of peptide ions containing the three main phosphotyrosine residues of the protein were used to calculate for each site the percentage of MS signal deriving from modified phosphotyrosine forms. MS intensity values of phosphorylated ions were averaged for MS replicate measurements of each sample. Data are the means ± SD and represent three independent experiments containing n = 1 mouse per group. (B) Volcano plots [−log10(Student t test P value) versus log2(fold-change)] showing abundances difference of proteins identified by MS analysis of samples obtained by IP of CD5 from cellular extracts of thymocytes stimulated with pervanadate. (Left) Comparison CD5tgWt vs. Cd5−/− thymocytes (n = 6 independent samples); (Right) comparison CD5tgWt versus Cd5−/− thymocytes (n = 5 independent samples). Protein labeled in red show significant enrichment in CD5tgWt samples compared to control Cd5−/− samples (fold-change > 2 and P < 0.05). The same proteins plotted in red show no significant enrichment in CD5tgY429F samples versus controls. Bar graph (Inset ) in the CD5tgY429F volcano plot represent the MS intensity (log10) of CD5 phosphorylated peptides containing the Y463 of CD5. (C) Thymocytes from CD5tgWt or CD5tgWt mice were stimulated with anti-CD3 + anti-CD4 for 1 min. Samples were then subjected to IP with antibodies specific for CD5 and then analyzed by Western blotting with antibodies specific for the indicated proteins. (D and E) CD5 was immunoprecipitated from cellular extracts of total thymocytes from the indicated genotypes stimulated with pervanadate. Samples were analyzed by MS (D) or Western blot (E). Abundance of specific interacting partners was estimated based on MS signal intensity. Data are means ± SD from at least two MS measurements and are representative of two experiments containing n = 1 mouse per group. Western blots are representative of two independent experiments. WCL, whole cell lysate.
To determine the role of Y429 in the context of CD5 signaling, we expressed a WT (CD5tgWt) or a mutated form of CD5, containing a tyrosine to phenylalanine substitution at position 429 (CD5tgY429F), as transgenes under the control of the T cell-specific hCD2 promoter/enhancer and crossed both transgenes into the Cd5−/− background (Cd5−/−;CD5tgWt or Cd5−/−;CD5tgY429F, hereafter designated CD5tgWt or CD5tgY429F, respectively). We verified that the WT and Y429F CD5 transgenes were similarly expressed in thymocyte subsets and in peripheral CD4+ and CD8+ T cells (SI Appendix, Fig. S2A). The surface levels of CD5 remained unchanged following stimulation with anti-CD3 antibodies and were comparable in CD5tgwt and CD5tgY429F thymocytes (SI Appendix, Fig. S2B). Moreover, the proportions and numbers of thymocytes and peripheral T cells in each subset were similar in CD5tgWt, CD5tgY429F, and Cd5−/− mice (SI Appendix, Fig. S2 C and D). Percentages of FOXP3+ T cells in the thymus and the spleen (SI Appendix, Fig. S2E) and surface levels of activation/memory markers, such as CD44, CD69, and PD-1 (SI Appendix, Fig. S2 F and G) were also comparable between these three lines. To investigate the impact of this mutation on the formation of CD5 signaling complexes, we compared the interactome of the WT and the mutated form of CD5 in thymocytes stimulated with pervanadate. We verified that c-CBL, UBASH3A, PI3K, CRKL, SHIP1, and CIN85 interacts with WT CD5 in CD5tgWt thymocytes similarly to what we observed in the C57BL/6 background (Fig. 2B and Dataset S5). IGH, TRIM21, CYB5 were not detected in this interactome, suggesting that they represent low affinity or nonspecific interactions. Notably, the mutation of tyrosine Y429 disrupted the association of c-CBL, UBASH3A, PI3K, CRKL, SHIP1, and CIN85 with CD5 following pervanadate treatment (Fig. 2B and Dataset S5) and TCR cross-linking (Fig. 2C), despite comparable phosphorylation of tyrosine Y463 of CD5Wt and CD5Y429F (Fig. 2B and Dataset S5). Quantitative analysis of the LFQ intensity metrics showed that the amount of each protein partner detected in the CD5tgY429F interactome was drastically decreased compared to CD5tgWt, and nearly comparable to those detected in Cd5−/− thymocytes (SI Appendix, Fig. S1C), suggesting that the mutation of Y429 almost entirely disrupted the assembly of the CD5 signalosome. No additional proteins were detected in the interactome of CD5 when tyrosine 429 was mutated (Fig. 2B).
c-CBL Harbors a Connective Function that Links CD5 to Other Regulators of TCR Signaling.
We next investigated the mechanism by which CD5 interacting proteins are recruited by tyrosine phosphorylation of a single residue (Y429) on the cytoplasmic tail of CD5. Y429 is included in a D(N/D)XpY motif predicted to be a potential binding site for the PTB domain of c-CBL (33). In addition, UBASH3A, SHIP1, CRKL, PI3K, and CIN85 were also previously characterized as direct or indirect c-CBL–interacting proteins, suggesting that c-CBL might be important to recruit these molecules to CD5 (34–36). To investigate this possibility, we immunoprecipitated CD5 in WT, c-Cbl−/−, and Cd5−/− thymocytes and evaluated if UBASH3A, SHIP1, CRKL, PI3K, and CIN85 were coimmunoprecipitated with CD5 by MS (Fig. 2D and Dataset S3) and Western blot (Fig. 2E). The amount (Fig. 2 D and E) and the phosphorylation level (Fig. 2E) of immunoprecipitated CD5 were slightly increased in c-Cbl−/− thymocytes compared to that in WT cells. Nevertheless, we found that the amounts of UBASH3A, SHIP1, CRKL, PI3K, and CIN85 that coimmunoprecipitated with CD5 were strongly reduced in c-Cbl−/− thymocytes compared to those in WT thymocytes and were similar to those observed in Cd5−/− thymocytes, suggesting that c-CBL is required to recruit these molecules to CD5. In addition, we found that CD5 was not required for the phosphorylation of c-CBL (SI Appendix, Fig. S3A) or for its interaction with PI3K, SHIP1, and CIN85 (SI Appendix, Fig. S3B), suggesting that CD5 is important for the recruitment of these molecules at the membrane but not for their assembly with c-CBL.
CD5 Integrates Both Costimulatory and Coinhibitory Signaling.
To elucidate the mechanism by which the CD5 regulates TCR signals, we next focused our study on c-CBL, which has well characterized inhibitory functions on TCR signaling (37). It has been proposed that c-CBL, an E3 ubiquitin-protein ligase, negatively regulates TCR signals by targeting TCRs and other components of the TCR signaling machinery to lysosomal or proteasomal degradation through ubiquitination. Consequently, TCR surface expression is increased in c-Cbl−/− CD4+CD8+ thymocytes (referred to as double-positive [DP] thymocytes) compared to WT (c-Cbl+/+) DP thymocytes (SI Appendix, Fig. S3C). Contrasting with this observation, we found that CD5 deficiency did not affect TCR surface expression in DP thymocytes, suggesting that CD5 is not required for c-CBL–mediated TCR turnover. Supporting this conclusion, the degradation of the TCR ζ-chain, which is induced following TCR cross-linking, was reduced in c-Cbl−/− thymocytes but not in Cd5−/− thymocytes (SI Appendix, Fig. S3 D and E). Expression of LCK, which was previously identified as a target of c-CBL in T cells (38), was slightly enhanced in c-Cbl−/− thymocytes but was also unaffected in Cd5−/− thymocytes (SI Appendix, Fig. S3F). Finally, we found that PI3K, SHIP1, and CIN85 are expressed similarly in WT, c-Cbl−/−, and Cd5−/− thymocytes, suggesting that c-CBL does not drive the degradation of the other CD5-interacting proteins (SI Appendix, Fig. S3F).
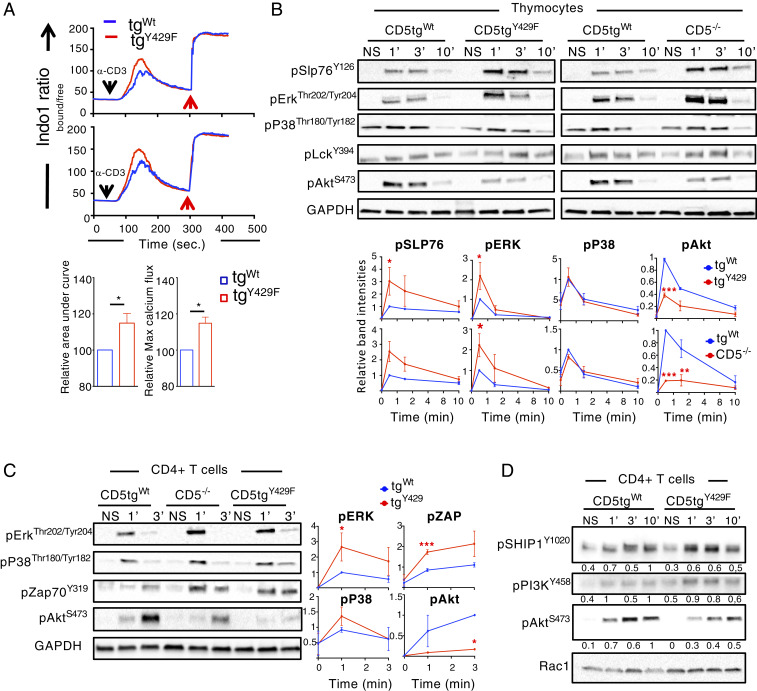
We next hypothesized that CD5 could use c-CBL, CIN85, and CRKL adapter functions to recruit positive (PI3K) and negative (UBASH3A, SHIP1) regulators of TCR signaling that would act more selectively to regulate specific signaling pathways. Recent studies show that CIN85 negatively regulates the phosphorylation of ZAP-70, SLP-76, and ERK by recruiting UBASH3A into TCR microclusters (39). CIN85 also interacts with SHIP1, which also exerts an inhibitory effect on ZAP-70 activity through the proteins Dok-1 and Dok-2 (40) that presumably act through CSK to inhibit SRC kinases activity (41, 42). This suggested that SHIP1 and UBASH3A could act together to repress ZAP-70 activity. In addition, SHIP1 negatively regulates calcium responses (43) and the phosphorylation of Tec kinases (44, 45), which play an essential role in PLCγ1 and ERK kinases activation. PI3K may interact directly with c-Cbl (46) or indirectly through CRKL (47). The corecruitment of SHIP1 and PI3K by CD5 was surprising since these two proteins were reported to have antagonistic effect on AKT activity (40, 48). To analyze whether these effector proteins are involved in CD5 signaling function, we stimulated thymocytes or peripheral CD4+ T cells from CD5tgWt, CD5tgY429F, and Cd5−/− mice with anti-CD3 and anti-CD4 antibodies and compared the intracellular increase of calcium and the phosphorylation of several of their downstream signaling targets. The calcium response was increased in CD5tgY429F DP thymocytes compared to that in CD5tgWt DP thymocytes (Fig. 3A). Accordingly, Western blot analysis showed that the phosphorylation of ERK and SLP-76 was increased in CD5tgY429F thymocytes and peripheral CD4+ T cells similarly to what was observed in Cd5−/− cells (Fig. 3 B and C). Although we could not detect significant ZAP-70 phosphorylation in CD5tgWt thymocytes with the stimulation conditions used in these experiments, we found that TCR stimulation induced phosphorylation of ZAP-70 was increased in both CD5tgY429F and Cd5−/− CD4+ T cells compared to that in CD5tgWt CD4+ T cells (Fig. 3C). In contrast, the phosphorylation of AKT was strikingly reduced in thymocytes and peripheral CD4+ T cells from CD5tgY429F and Cd5−/− mice compared to that in similar T cell subsets from CD5tgWt mice (Fig. 3 B and C). In comparison, the phosphorylation of LCK and P38 were similar in thymocytes and CD4+ T cells from the three different lines of mice (Fig. 3 B and C). Finally, we found that the phosphorylation of CD5-interacting proteins, such as SHIP1 and PI3K, were not significantly impaired in CD5tgY429F CD4+ T cells despite a consistent decreased of Akt phosphorylation in those cells, suggesting that CD5 is important to relocate those proteins close to their molecular targets rather than directly controlling their activity (Fig. 3D). Altogether, these results suggest that CD5 acts through c-CBL binding proteins to exert both enhancing and inhibitory effects on specific TCR-generated signals.
Fig. 3.
CD5 coordinates TCR signals in thymocytes and peripheral T cells. (A) Analysis of calcium flux in DP thymocytes from CD5tgWt and CD5tgY429F mice stimulated with anti-CD3 antibodies at 5 or 10 µg/mL (Upper and Lower graph, respectively) in the presence of anti-CD4 antibodies. Red arrows indicate maximum responses following stimulation with ionomycine. Bar graphs represents the area under the curve and the maximum peak intensity of calcium flux normalized to the values obtain in DP thymocytes from CD5tgWt mice. Data are means ± SD and are representative of two independent experiments with n = 1 to 2 mice per group. (B) Thymocytes from CD5tgWt, CD5tgY429F, and Cd5−/− mice were stimulated with anti-CD3 + anti-CD4 antibodies for the indicated times. Total cytoplasmic extracts of the cells were then analyzed by Western blotting with antibodies against phosphorylated forms of SLP76, ERK, P38, LCK, and AKT. Curves show the relative abundances of the indicated phosphorylated proteins as determined by calculating the ratios of the intensities of the bands corresponding to the phosphorylated proteins to those corresponding to GAPDH, the loading control. The y axes represent means ± SD of the relative values calculated after normalization to the highest value in the CD5tgWt cells. Data are representative of three to five independent experiments, each including one mouse of the indicated genotype. (C and D) Peripheral CD4+ T cells from CD5tgWt, CD5tgY429F and Cd5−/− mice were stimulated with anti-CD3 antibodies for the indicated times. Total cytoplasmic extracts of the cells were then analyzed by Western blotting with antibodies against phosphorylated forms of SLP76, ERK, P38, ZAP-70, PI3K, SHIP1, and AKT. Curves or values next to the blots show the relative abundances of the indicated phosphorylated proteins calculated as in B. Data are representative of two to four independent experiments each including one mouse of the indicated genotype. Unpaired two-tailed t test. *P < 0.05; **P < 0.01; ***P < 0.001.
CD5-Mediated Signaling Negatively Regulates CD4+ T Cell Activation and Restrains the Generation of Treg Cells Induced by Foreign Antigens.
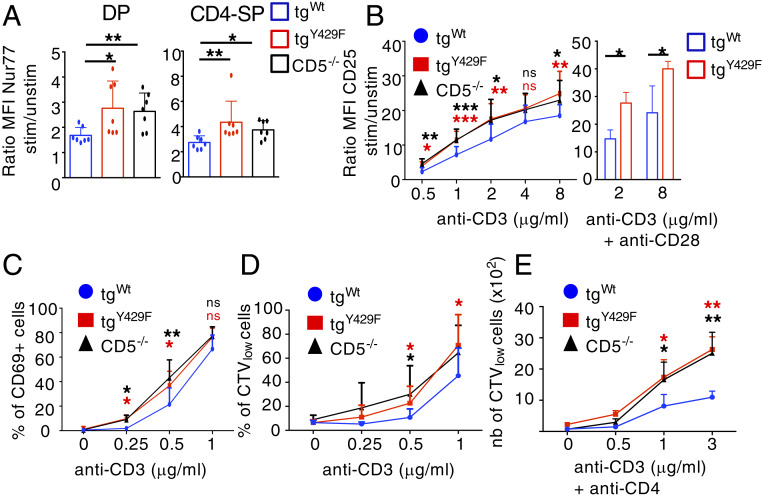
We next analyzed the functional consequences of these CD5-mediated signaling modulations on thymocyte and naïve CD4+ T cell activation. We found that expression of Nur77, a quantitative sensor of TCR signal strength (49), was enhanced in CD5tgY429F and Cd5−/− DP and CD4-SP thymocytes as compared to that in CD5tgWt thymocytes following TCR cross-linking (Fig. 4A). TCR stimulation-induced surface expression of CD25 on CD4+ T cells and the proportions of CD4+ T cells expressing CD69 were also similarly augmented in CD5tgY429F and Cd5−/− T cells as compared to those in CD5tgWt thymocytes (Fig. 4 B and C). The up-regulation of CD25 surface expression was also increased in CD5tgY429F CD4+ T cells when cells were costimulated with anti-CD28 antibodies, indicating that CD28 signaling does not compensate for CD5 signaling deficiency (Fig. 4B). Finally, we found that CD5tgY429F and Cd5−/− CD4+ T cells proliferated more than CD5tgwt CD4+ T cells in response to anti-CD3 stimulation alone (Fig. 4D) or with anti-CD4 antibodies (Fig. 4E), suggesting that CD5 negatively regulates peripheral CD4+ T cell activation independently of CD4 costimulation.
Fig. 4.
The CD5 signalosome negatively regulates the activation and the proliferation of CD4+ T cells. (A) Thymocytes from CD5tgWt, CD5tgY429F, and Cd5−/− mice were stimulated or not with anti-CD3 and anti-CD28 antibodies for 4 h. Nur77 protein abundance was then analyzed by flow cytometry in DP and CD4-SP cells after intracytoplasmic staining with anti-Nur77 antibodies. Bar graphs represents the ratio of mean fluorescent intensities (MFI) of Nur77 in stimulated cells to unstimulated cells. Data are means ± SD and are representative of two independent experiments, with n = 3 mice per group. (B) Peripheral CD4+ T cells from CD5tgWt, CD5tgY429F, and Cd5−/− mice were stimulated with the indicated doses of anti-CD3 antibodies alone (curves) or with anti-CD28 antibodies (bar graphs) for 24 h. Curves and bar graphs represents the ratio of MFI of CD25 in stimulated cells to unstimulated cells. Data are mean ± SD and represent, for anti-CD3 alone, four independent experiments with n = 12 mice per group or, for anti-CD3 + anti-CD28, two experiments with n = 4 mice per group. (C) Peripheral CD4+ T cells from CD5tgWt, CD5tgY429F, and Cd5−/− mice were stimulated with the indicated doses of anti-CD3 antibodies for 24 h. Curve graph represents the percentages of CD69+ cells. Data are mean ± SD and represent three independent experiments with n = 4 to 8 mice per group. (D and E) Peripheral CD4+ T cells from CD5tgWt, CD5tgY429F, and Cd5−/− mice previously stained with cell trace violet (CTV) were stimulated with anti-CD3 antibodies alone (D) or with anti-CD4 antibodies (E) for 72 h. Curve graphs represent the percentages (D) or the numbers (E) of CTVlow CD4+ T cells following stimulation with variable doses of anti-CD3 antibodies for 72 h. Data are mean ± SD and represent three independent experiments with n = 7 to 11 mice per group. Unpaired two-tailed Mann–Whitney t test. *P < 0.05; **P < 0.01; ***P < 0.001.
The analysis of TCR signaling suggested that, rather than repressing broadly TCR signals, CD5 acts more selectively by promoting AKT and repressing ERK kinases and calcium response activities through a dedicated set of effector molecules. We thus suspected that CD5 signaling could exert a more selective effect on T cell responses in addition to its role in controlling the threshold for T cell activation. Among the many reported effects of AKT in CD4+ T cells, it is well known that strong AKT-mediated signals reduce their ability to differentiate into FOXP3+ Treg cells in presence of TGF-β (50). One proposed mechanism to explain this effect is that AKT stimulates the cytoplasmic retention of FOXO1 through the phosphorylation of two residues (Thr24 and Ser256), inhibiting the translocation of FOXO1 to the nucleus and the subsequent transactivation of Foxp3 gene (51). The activation of ERK kinases was shown, in similar experimental settings, to have positive effect on Foxp3 transactivation in vitro (52), suggesting that CD5 could coordinate these signaling pathways to selectively repress the induction of Treg cells.
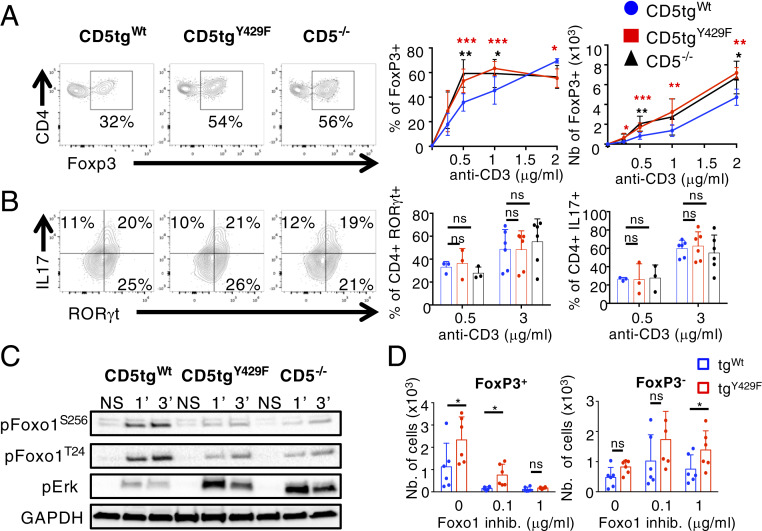
To address this possibility, we first compared FOXP3 expression in CD5tgWt, CD5tgY429F, and Cd5−/− CD4+ T cells stimulated with different doses of anti-CD3 antibodies in the presence of TGF-β. We found that the percentages of CD5tgY429F and Cd5−/− CD4+ T cells expressing FOXP3 were enhanced, compared to that in CD5tgWt CD4+ T cells (Fig. 5A), upon weak but not high TCR stimulations, suggesting that CD5 may contribute to enhance the threshold at which the development of iTreg is engaged. By comparison, we found that CD5tgWt, CD5tgY429F, and Cd5−/− CD4+ T cells differentiated similarly into RORγt+IL-17+ cells following TCR stimulation under Th17 polarizing conditions (Fig. 5B). Numbers of CD5tgY429F and Cd5−/− FOXP3+ cells were enhanced independently of the concentration of anti-CD3 antibodies (Fig. 5A), suggesting that CD5 operates both by controlling the ability of CD4+ T cells to differentiate into iTreg and by repressing the proliferation/survival of conventional T cells prior or during their engagement into the Treg lineage. Accordingly, the percentages of cells expressing FOXP3 were higher in nondivided (CTVhi) CD5tgY429F CD4+ T cells compared to the same cell populations from CD5tgWt CD4+ T cells (SI Appendix, Fig. S4). Confirming the positive effect of CD5 on AKT activity, we found that the phosphorylation of FOXO1 on Thr24 and Ser256 was impaired in CD5tgY429F and Cd5−/− CD4+ T cells (Fig. 5C). The phosphorylation of ERK, which is also correlated with efficient generation of induced Treg cells (52), was enhanced in CD5tgY429F and Cd5−/− CD4+ T cells compared to that in CD5tgWt CD4+ T cells, showing further that CD5 delivers both costimulatory and coinhibitory signals to modulate a specific T cell response, namely the induction of Foxp3 and the generation of iTreg cells. Finally, the numbers of FOXP3+ cells were similar in CD5tgWt and CD5tgY429F CD4+ T cells when cells were incubated with high concentrations of FOXO1 inhibitors in in vitro assays, suggesting that the increased activity of ERK observed in CD5tgY429F CD4+ T cells was not sufficient alone to enhance the generation of Treg cells (Fig. 5D). In comparison, the numbers of CD5tgY429F CD4+FOXP3− T cells, reflecting T cell expansion, remained increased compared to those in CD5tgWt CD4+FOXP3− T cells when cells were treated with similar doses of FOXO1 inhibitors (Fig. 5D). Altogether, these results suggested that CD5 signaling shape TCR signals to selectively repress FOXP3 expression.
Fig. 5.
CD5 signaling restrains the generation of induced Treg cells by promoting AKT-mediated inhibition of FOXO1 and repressing ERK activity. (A) Naïve CD62LhiCD25lo CD4+ T cells from CD5tgWt, CD5tgY429F, and Cd5−/− mice were stimulated with anti-CD3 antibodies in presence of TFG-β for 3 d. Contour plots represent CD4 vs. FOXP3 staining profiles of CD4+ T cells from the indicated genotypes. Curve graphs represent the percentages and numbers of FOXP3+ CD4+ T cells from mice of the indicated genotypes stimulated with the indicated doses of anti-CD3 antibodies in presence of TGF-β. Data are means ± SD and represent two independent experiments with n = 6 to 9 mice per group. (B) Naïve CD62LhiCD25lo CD4+ T cells from CD5tgWt, CD5tgY429F, and Cd5−/− mice were stimulated with anti-CD3 antibodies in presence of TFG-β and IL-6 for 5 d. Contour plots represent CD4 versus RORγt staining profiles of CD4+ T cells from the indicated genotypes. Bar graphs represent the percentages of RORγt+ and IL-17+ CD4+ T cells from mice of the indicated genotypes stimulated with the indicated doses of anti-CD3 antibodies in presence of TGF-β and IL-6. Data are means ± SD and represent two independent experiments, with n = 3 (0.5 μg/mL anti-CD3) and n = 6 (3 μg/mL anti-CD3) mice per group. (C) Peripheral CD4+ T cells from CD5tgWt, CD5tgY429F, and Cd5−/− mice were stimulated or not (NS) with anti-CD3 antibodies for the indicated times. Total cytoplasmic extracts of the cells were then analyzed by Western blotting with antibodies against phosphorylated forms of FOXO1 and ERK. Western blots are representative of two independent experiments. (D) Naïve CD62LhiCD25lo CD4+ T cells from CD5tgWt and CD5tgY429F mice were stimulated with anti-CD3 antibodies in presence of TFG-β with or without variable doses of FOXO1 inhibitors for 3 d. The expression FOXP3 was then analyzed by flow cytometry after intracytoplasmic staining of the cells with anti-FOXP3 antibodies. Bar graphs represent the numbers of FOXP3+ (Left) and FOXP3− CD4+ T cells from mice of the indicated genotypes stimulated with anti-CD3 antibodies in presence of TGF-β with or without the indicated doses of FOXO1 inhibitors. Data are means ± SD and represent two experiments with n = 6 mice per group. Unpaired two-tailed Mann–Whitney t test, except for D, where paired t test with Welch’s correction was performed. *P < 0.05; **P < 0.01; ***P < 0.001.
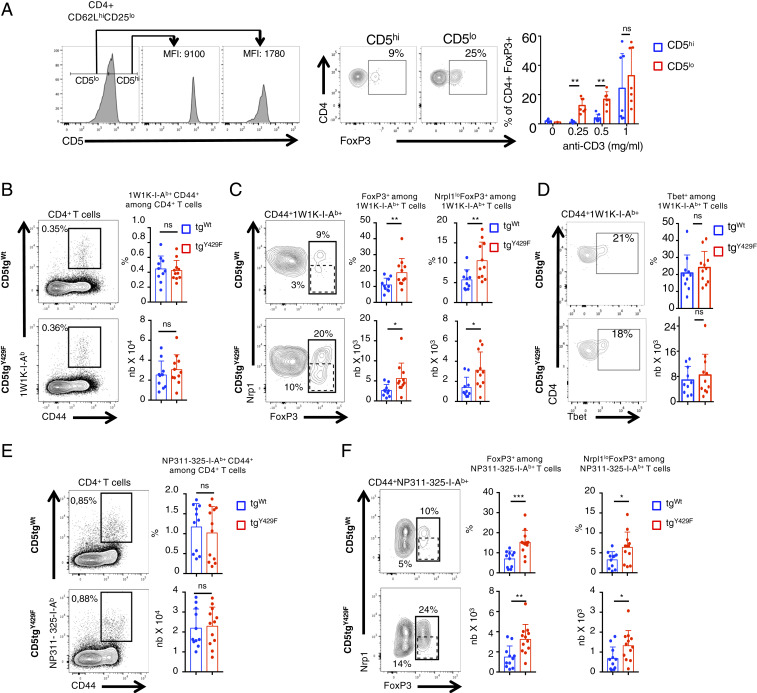
The surface level of CD5 correlates with TCR signal intensity, which is dictated by the affinity of the TCR for self-ligands (9). To determine whether quantitative variations of CD5 surface expression within normal physiological ranges influences the generation of induced FOXP3+ cells, we next sorted naïve CD62Lhi CD25− CD4+ T cells expressing either high or low surface levels of CD5 and compared their ability to differentiate into FOXP3+ cells in Treg-cell polarizing conditions. We found that CD5loCD4+ T cells differentiate more efficiently into FOXP3+ cells than CD5hiCD4+ T cells under identical TCR stimulation conditions, suggesting that physiologically high levels of CD5 on naïve CD4+ T cells might reduce the ability of these cells to differentiate into Treg cells upon antigenic recognition (Fig. 6A). Contrasting with those results, previous studies suggested that CD5 promotes, rather than represses, the differentiation of peripherally induced Treg cells when those cells are generated in a tolerogenic context such as the gut mucosa (15). Accordingly, we found that the percentages of FOXP3+CD4+ T cells and of FOXP3+NeuropilinlowCD4+ T cells, which are essentially composed of iTreg cells (53), were decreased in the Peyer patches of CD5−/− and CD5tgY429F mice as compared to those in CD5tgWt mice (SI Appendix, Fig. S5). We thus hypothesized that CD5 signalosome might be influenced by the environment in which T cells are localized and that CD5 may operate differently in a nontolerogenic environment when CD4+ T cells encounter foreign antigens or pathogens. To examine this possibility, we first immunized CD5tgWt and CD5tgY429F mice with a peptide variant (EAWGALANKAVDKA, called 1W1K peptide hereafter) of the I‐E α-chain immunodominant peptide 52‐68 in the presence of incomplete Freunds adjuvant (IFA), which was shown to favor the polarization of iTreg cells (54). To follow antigen-induced FOXP3+ T cells, we stained cells from the draining lymph nodes with 1W1K-pMHCII tetramer and analyzed FOXP3 expression in tetramer+CD4+ T cells. We found that the percentages of CD44+tetramer+CD4+ T cells were similar in CD5tgWt and CD5tgY429F mice, indicating that CD5 signaling does not significantly impact the clonal expansion of antigen-specific CD4+ T cells in this experimental setting (Fig. 6B). We observed that the percentages and numbers of tetramer+FOXP3+CD4+ T cells were increased in CD5tgY429F mice compared to those in CD5tgWt mice (Fig. 6C). In comparison, the percentages and numbers of tetramer+T-BET+CD4+ T cells were similar in CD5tgWt and CD5tgY429F mice (Fig. 6D). Proportions and numbers of tetramer+FOXP3+NeuropilinlowCD4+ T cells were also higher in CD5tgY429F mice compared to those in CD5tgWt mice, suggesting that CD5 signaling restrains the generation of iTreg cells following immunization with foreign antigens.
Fig. 6.
CD5-mediated signaling restrain the generation of peripherally induced Treg cells following immunization with foreign antigens and viral infection. (A) CD5lo and CD5hi naïve CD62LhiCD25lo CD4+ T cells from C57BL/6 mice were sorted prior to stimulation with anti-CD3 antibodies in presence of TFG-β for 3 d. Histograms show the sorting strategy. Contour plots represent CD4 versus FOXP3 staining profiles of CD5lo and CD5hi CD4+ T cells. Bar graphs represent the percentages of FOXP3+ CD4+ T cells following stimulation with the indicated doses of anti-CD3 antibodies in presence of TGF-β. Data are means ± SD and represent two independent experiments with n = 7 mice per group. (B–D) CD5tgWt and CD5tgY429F mice were immunized with 1W1K peptide in presence of IFA. Draining lymph nodes were collected after 7 d and analyzed by flow cytometry after staining of the cells with 1W1K-conjugated MHC tetramers. (E and F) CD5tgWt and CD5tgY429F mice were intranasally infected with PR8 virus. Draining lymph nodes were collected after 5 d and analyzed by flow cytometry after staining of the cells with NP311-325-conjugated MHC tetramers. (B and E) Contour plots represents CD44 versus tetramer staining profile on gated CD4+ T cells. Bar graphs represent the percentages and absolute numbers of tetramer+CD44+ T cells among CD4+ T cells. (C and F) Contour plots represents FoxP3 vs. Neuropilin1 (Nrp1) staining profile on gated tetramer+CD44+ CD4+ T cells. Bar graphs represent the percentages and absolute numbers of either FoxP3+ (solid gate) or FoxP3+ neuropilinlow (dashed gate) among tetramer+CD44+ CD4+ T cells. Data are representative of two independent experiments and are means ± SD of at least 10 mice for each genotype. Nonparametric t test *P < 0.05; **P < 0.01; ***P < 0.001.
To confirm this observation in a more pathophysiological model, we next analyzed whether CD5 signaling could repress the generation of Treg cells following mice infection with the respiratory virus influenza A, which was shown to drive important antigen-specific Treg cell responses (55). Mice were infected intranasally and pathogen-specific Treg were analyzed with NP311-325-IAb tetramer in the draining lymph nodes 5 d following infection. We found that the percentages of virus-specific CD4+ T cells were comparable in CD5tgWt and CD5tgY429F mice, indicating that CD5 signaling does not influence the overall expansion of antigen-specific CD4+ T cell following infection (Fig. 6E). The proportions and numbers of virus-specific FOXP3+CD4+ T cells and of FOXP3+NeuropilinlowCD4+ T cells were increased in CD5tgY429F mice as compared to those in CD5tgWt, showing that CD5 represses the development of those cells during pathogenic infection (Fig. 6F).
Discussion
In this study, we identified a multimeric signaling complex that acts through CD5 to limit the induction of Treg cells. This complex is composed of proteins with adaptor functions (c-CBL, CIN85, CRKL) that connect CD5 to distinct effector proteins (UBASH3A, SHIP1, PI3K), which acts cooperatively by repressing ERK and promoting AKT activity to inhibit the transactivation of Foxp3. This signalosome is recruited to CD5 Y429 following TCR engagement, suggesting that it is part of a feedback loop that is differentially engaged according to the strength of TCR signals that gradually regulates CD5 expression at the cell surface. It is generally admitted that stimulation of CD4+ T cells with a high dose of strong agonist exerts a repressive effect on the generation of iTreg cells, presumably because it promotes strong AKT-mediated signals that prevent the transactivation of Foxp3 (50). A molecular model to explain this effect was that strong, but not weak, TCR signals repress the expression of the PtdIns(3,4,5)P3 phosphatase and tensin homolog (PTEN), which is an inhibitor of the AKT signaling pathway (56). Our study also suggests that CD4+ T cells could finely tune AKT signaling by titrating the surface expression of CD5 to reduce the generation of iTreg cells upon TCR stimulation with a relatively high dose of high-affinity ligands. It was shown that CD5hiCD4+ T cells respond better than CD5loCD4+ T cells to diverse foreign antigens (11). This was explained by the enrichment, in the CD5hi population, of CD4+ T cells with greater self-reactivity, which exhibit enhanced TCR signaling potential (57). An additional explanation inferred from our study is that CD5 could act by reducing the emergence of inopportune antigen-specific Treg cells that might occur during the recognition of foreign antigens and which may lead to the development of ineffective immune responses. This interpretation could also bring an explanation to the reduced development of active autoimmunity observed in CD5 deficient mice models (12).
The modes of interaction of c-CBL with UBASH3A, SHIP, CIN85, CRKL, and PI3K have been extensively studied particularly in the context of EGF receptor (EGFR) signaling for which a similar molecular machinery as the one described in this study for CD5 has been described (36, 58, 59). PI3K can bind directly to c-CBL through a PTB-site located at the C-terminal end of c-Cbl (Y731) (60), or indirectly, through CRKL, which binds to two PTB sites located in the same region (Y700 and Y774) (61). CIN85 binds directly with SHIP1 and was shown to compete with UBASH3A for binding to c-CBL, suggesting that two distinct CD5 signaling subsets might coexist in T cells (62). Previous studies have shown that CIN85/c–CBL complexes drive EGFR internalization and degradation in lysosomal compartments, whereas UBASH3A/c-CBL complexes prevent these processes and sustain EGFR-mediated signals (36, 58). Thus, these distinct complexes could contribute to regulate the turnover of CD5 in addition to their regulatory function on TCR signaling. Although c-CBL was shown to mediate CD5 ubiquitylation, resulting in its degradation in lysosomes following TCR/CD5 cocross-linking (63), we found that the substitution of tyrosine 429 by phenylalanine does not significantly modify CD5 surface expression either before or after TCR engagement, suggesting that the direct engagement of CD5 by external ligands might be required for its degradation.
Several proteins previously identified as CD5-interacting proteins, such as Ras-Gap (22), SHP-1 (23), Vav1 (25), and ZAP70 (64), were not detected as CD5 interactors in our MS analysis. Although initial studies suggested that SHP-1 interacts with CD5 in thymocytes (65) and Jurkat cells (23), several studies since then have failed to reproduce this interaction (22, 66). Moreover, both the phosphatase activity associated to CD5 immunoprecipitates and the reduction in positive selection conferred by CD5 overexpression were shown to be unaffected by SHP-1 deficiency, suggesting that CD5 operates independently of this phosphatase (67). CK2 (26) and Cbl-b (30), also previously described as CD5-binding proteins, were recruited to CD5 but not as robustly as other interactors, suggesting that CD5 could engage these proteins but in other molecular or cellular contexts than the one used in this study. It was shown that CD5 enhances Th17 responses when cross-linked to the TCR but not when the TCR is engaged alone (68, 69), suggesting that CD5 could recruit distinct signaling effectors whether or not it is engaged by extracellular ligands. Accordingly, the deletion of a CK2 binding site on CD5 impairs the polarization of CD4+ T cells into Th17 cells following stimulation with antigen-presenting cells but not following anti-CD3 antibodies (69).
We show that CD5 exerts a combined effect on TCR signaling, reducing ZAP-70 and ERK activity, presumably through the joint action of UBASH3A and SHIP1, which are known regulators of these signaling molecules (40, 44, 45, 70, 71), and enhancing AKT activity, likely through PI3K, which facilitates the retention of FOXO1 in the cytoplasm and prevents the consequent transactivation of Foxp3 (51). This signaling complex does not detectably modulate the expression of RORγt or T-BET in activated CD4+ T cells in vitro and in vivo, respectively, indicative of a selective effect of CD5 on the generation of iTreg cells. Remarkably, studies using SHIP1- and PI3K-deficient murine models identified repressive functions for both proteins on the generation of induced Treg cells (72, 73). Whether UBASH3A is also directly involved in the control of this population has not yet been studied. However, a recent investigation performed in human T cells has shown that it represses NF-κB signaling (74), a key pathway for the generation of induced Treg cells (75). Thus, CD5 could act as a scaffold that selectively engages specific signaling effectors involved in the generation of peripheral Treg cells to selectively control the expansion of this population during immune responses.
Previous studies have shown that CD5 deficiency on the BALB/c but not on the C57BL/6 genetic background leads to increased numbers of thymic Treg cells, suggesting that CD5 may also repress the generation of these cells according to the molecular context in which CD5 operates in the thymus (13, 14). CD5 is highly expressed at the surface of thymic Treg cells, indicating that the ability of CD5 to negatively regulate FoxP3 expression in the thymus could also be overcome by additional signals (such as high TCR affinity for self-ligand) that impose FoxP3 expression despite the opposite force exerted by CD5. We thus speculate that CD5-mediated inhibition may be sufficient to block Foxp3 expression and Treg lineage commitment in thymocytes that express TCRs that bind with intermediate affinity to self-ligands, preventing their commitment to the Treg lineage. In addition, coreceptors, such as GITR, OX40, and TNFR2, are highly expressed by thymic Treg progenitors and were also shown to trigger costimulatory signals that induce thymic Treg differentiation (76). In contrast, these coreceptors are poorly expressed by naïve peripheral CD4 T cells, suggesting that the negative effect of CD5 on FoxP3 expression may become predominant in this subset.
A recent study reported an effect of CD5 opposite to that shown in our study on the generation of peripherally induced Treg cells in a mouse model in which tolerance to experimental autoimmune encephalomyelitis is induced by direct delivery of encephalitogenic peptides to antigen-presenting dendritic cells in the absence of adjuvant (15). Those authors found that CD5 prevents the inhibition of Treg cell induction potentially mediated by effector cell cytokines, such as IL-6, IL-4, and IFN-γ. Although they do not provide a clear signaling mechanism by which CD5 operates here, they show that CD5 promotes the generation of Treg cells by inhibiting IL-6–mediated AKT signaling leading to mammalian target of rapamycin activation. One potential explanation for the apparent discrepancy between those results and our current findings is that CD5 might exert opposite effects on PI3K/AKT signaling in tolerized and nontolerized T cells. A recent proteomic study showed that CD5 interacts with both c-CBL and CBL-b in peripheral CD4+ T cells (30), suggesting that distinct pools of CD5, connected either to a c-CBL or CBL-b signalosome, could be preferentially assembled under specific stimulation conditions. Whereas c-CBL has known positive effects on PI3K/AKT signaling (46), CBL-b blocks this pathway by inducing PI3K ubiquitylation (77) and by blocking Nedd4-mediated ubiquitylation of PTEN (78). Because CBL-b expression is up-regulated in CD4+ T cells after tolerizing signals (79) and degraded in CD4+ T cells that have received appropriate costimulatory signals (80), we speculate that CD5 signaling pools may vary in size or composition, tuning up or down the PI3K/AKT signaling axis according to the immunological context in which cells are stimulated.
Altogether, the findings of our study suggest that CD5 engages a polymorphic signaling machinery that can transduce both stimulatory and inhibitory signals to selectively control specific T cell responses depending on the immunological context. Our results also provide new insights into the paradigm of coreceptor signaling, suggesting that, in addition to providing classic enhancing or dampening inputs, coreceptors coordinate TCR signals that may have antagonist effects to promote specific functional outcomes, such as the generation of iTreg cells.
Materials and Methods
Full details of materials and methods, including mice, antibodies, cell stimulation, immunoprecipitations, MS analysis, calcium flux, Western blot, immunization and influenza virus infection, statistical analysis are provided in SI Appendix.
Mice.
All the experiments were conducted with sex- and age-matched mice between 6- and 12-wk-old housed under specific pathogen-free conditions at the INSERM Zootechnie US-006 animal facility, which is accredited by the French Ministry of Agriculture to perform experiments on live mice (accreditation number A-31 55508). All experimental protocols were approved by the local ethics committee and are in compliance with the French and European Union regulations on care and protection of laboratory animals (EC Directive 2010/63).
Data Availability Statement.
The data generated or analyzed during this study are included in the article and its SI Appendix or dataset files. The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD017343. Raw data from Figs. 1 A and C and 2 A, B, and D and SI Appendix, Fig. S1 A–C can be found in Datasets S1–S3 and S5.
Supplementary Material
Acknowledgments
We thank L. Dupré for critical reading of the manuscript; F.-E. L’Faqihi-Olive, V. Duplan-Eche, and A.-L. Iscache for technical assistance at the flow-cytometry facility of INSERM U1043; the personnel of the Centre Régional d’Exploration Fonctionnelle et de Ressources Expérimentales for expert animal care; and L. Guennec for administrative assistance. This work was supported by INSERM and Sanofi (Avenir grant to R.L.); the Association pour la Recherche sur le Cancer; the Intramural Research Program of the Eunice Kennedy Shriver, National Institute of Child Health and Human Development; a Marie Curie International Reintegration Grant (to R.L.); the French Ministry of Higher Education and Research (PhD fellowship to G.B.); the Région Midi-Pyrénées, European funds (Fonds Européens de Développement Régional), Toulouse Métropole, and the French Ministry of Research with the ‘Investissement d’Avenir Infrastructures Nationales en Biologie et Santé program’ (ProFI, Proteomics French Infrastructure project, ANR-10-INBS-08) (to O.B.-S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (ID PXD017343).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917182117/-/DCSupplemental.
References
- 1.Chen L., Flies D. B., Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13, 227–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coquet J. M., Rausch L., Borst J., The importance of co-stimulation in the orchestration of T helper cell differentiation. Immunol. Cell Biol. 93, 780–788 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Anderson A. C., Joller N., Kuchroo V. K., Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acuto O., Michel F., CD28-mediated co-stimulation: A quantitative support for TCR signalling. Nat. Rev. Immunol. 3, 939–951 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Parry R. V., Riley J. L., Ward S. G., Signalling to suit function: Tailoring phosphoinositide 3-kinase during T-cell activation. Trends Immunol. 28, 161–168 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Rudd C. E., Taylor A., Schneider H., CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 229, 12–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker L. S. K., PD-1 and CTLA4: Two checkpoints, one pathway? Sci. Immunol. 2, eaan3864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarakhovsky A. et al., A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science 269, 535–537 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Azzam H. S. et al., CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188, 2301–2311 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzam H. S. et al., Fine tuning of TCR signaling by CD5. J. Immunol. 166, 5464–5472 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Mandl J. N., Monteiro J. P., Vrisekoop N., Germain R. N., T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 38, 263–274 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axtell R. C., Webb M. S., Barnum S. R., Raman C., Cutting edge: Critical role for CD5 in experimental autoimmune encephalomyelitis: Inhibition of engagement reverses disease in mice. J. Immunol. 173, 2928–2932 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Dasu T. et al., CD5 plays an inhibitory role in the suppressive function of murine CD4(+) CD25(+) T(reg) cells. Immunol. Lett. 119, 103–113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordoñez-Rueda D. et al., Increased numbers of thymic and peripheral CD4+ CD25+Foxp3+ cells in the absence of CD5 signaling. Eur. J. Immunol. 39, 2233–2247 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Henderson J. G., Opejin A., Jones A., Gross C., Hawiger D., CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity 42, 471–483 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Van de Velde H., von Hoegen I., Luo W., Parnes J. R., Thielemans K., The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature 351, 662–665 (1991). [DOI] [PubMed] [Google Scholar]
- 17.Vera J. et al., The CD5 ectodomain interacts with conserved fungal cell wall components and protects from zymosan-induced septic shock-like syndrome. Proc. Natl. Acad. Sci. U.S.A. 106, 1506–1511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C. et al., CD5 binds to interleukin-6 and induces a feed-forward loop with the transcription factor STAT3 in B cells to promote cancer. Immunity 44, 913–923 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhandoola A. et al., CD5-mediated inhibition of TCR signaling during intrathymic selection and development does not require the CD5 extracellular domain. Eur. J. Immunol. 32, 1811–1817 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Osman N., Lazarovits A. I., Crumpton M. J., Physical association of CD5 and the T cell receptor/CD3 antigen complex on the surface of human T lymphocytes. Eur. J. Immunol. 23, 1173–1176 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Burgess K. E., Yamamoto M., Prasad K. V., Rudd C. E., CD5 acts as a tyrosine kinase substrate within a receptor complex comprising T-cell receptor zeta chain/CD3 and protein-tyrosine kinases p56lck and p59fyn. Proc. Natl. Acad. Sci. U.S.A. 89, 9311–9315 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennehy K. M., Broszeit R., Ferris W. F., Beyers A. D., Thymocyte activation induces the association of the proto-oncoprotein c-cbl and ras GTPase-activating protein with CD5. Eur. J. Immunol. 28, 1617–1625 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Perez-Villar J. J. et al., CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: Involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol. Cell. Biol. 19, 2903–2912 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennehy K. M. et al., Thymocyte activation induces the association of phosphatidylinositol 3-kinase and pp120 with CD5. Eur. J. Immunol. 27, 679–686 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Gringhuis S. I., de Leij L. F., Coffer P. J., Vellenga E., Signaling through CD5 activates a pathway involving phosphatidylinositol 3-kinase, Vav, and Rac1 in human mature T lymphocytes. Mol. Cell. Biol. 18, 1725–1735 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raman C., Kimberly R. P., Differential CD5-dependent regulation of CD5-associated CK2 activity in mature and immature T cells: Implication on TCR/CD3-mediated activation. J. Immunol. 161, 5817–5820 (1998). [PubMed] [Google Scholar]
- 27.Raman C., Kuo A., Deshane J., Litchfield D. W., Kimberly R. P., Regulation of casein kinase 2 by direct interaction with cell surface receptor CD5. J. Biol. Chem. 273, 19183–19189 (1998). [DOI] [PubMed] [Google Scholar]
- 28.Lu X. et al., AP2 adaptor complex-dependent internalization of CD5: Differential regulation in T and B cells. J. Immunol. 168, 5612–5620 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Voisinne G. et al., Quantitative interactomics in primary T cells unveils TCR signal diversification extent and dynamics. Nat. Immunol. 20, 1530–1541 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voisinne G. et al., Co-recruitment analysis of the CBL and CBLB signalosomes in primary T cells identifies CD5 as a key regulator of TCR-induced ubiquitylation. Mol. Syst. Biol. 12, 876 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dennehy K. M. et al., Determination of the tyrosine phosphorylation sites in the T cell transmembrane glycoprotein CD5. Int. Immunol. 13, 149–156 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Vilà J. M. et al., Residues Y429 and Y463 of the human CD5 are targeted by protein tyrosine kinases. Eur. J. Immunol. 31, 1191–1198 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Lupher M. L. Jr., Songyang Z., Shoelson S. E., Cantley L. C., Band H., The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J. Biol. Chem. 272, 33140–33144 (1997). [DOI] [PubMed] [Google Scholar]
- 34.van Leeuwen J. E., Paik P. K., Samelson L. E., Activation of nuclear factor of activated T cells-(NFAT) and activating protein 1 (AP-1) by oncogenic 70Z Cbl requires an intact phosphotyrosine binding domain but not Crk(L) or p85 phosphatidylinositol 3-kinase association. J. Biol. Chem. 274, 5153–5162 (1999). [DOI] [PubMed] [Google Scholar]
- 35.Take H. et al., Cloning and characterization of a novel adaptor protein, CIN85, that interacts with c-Cbl. Biochem. Biophys. Res. Commun. 268, 321–328 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Kowanetz K. et al., Suppressors of T-cell receptor signaling Sts-1 and Sts-2 bind to Cbl and inhibit endocytosis of receptor tyrosine kinases. J. Biol. Chem. 279, 32786–32795 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Naramura M., Kole H. K., Hu R. J., Gu H., Altered thymic positive selection and intracellular signals in Cbl-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 95, 15547–15552 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao N. et al., Negative regulation of Lck by Cbl ubiquitin ligase. Proc. Natl. Acad. Sci. U.S.A. 99, 3794–3799 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong M. S. et al., Inhibition of T cell activation and function by the adaptor protein CIN85. Sci. Signal. 12, eaav4373 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Dong S. et al., T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J. Exp. Med. 203, 2509–2518 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Slyke P. et al., Dok-R mediates attenuation of epidermal growth factor-dependent mitogen-activated protein kinase and Akt activation through processive recruitment of c-Src and Csk. Mol. Cell. Biol. 25, 3831–3841 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao M., Janas J. A., Niki M., Pandolfi P. P., Van Aelst L., Dok-1 independently attenuates Ras/mitogen-activated protein kinase and Src/c-myc pathways to inhibit platelet-derived growth factor-induced mitogenesis. Mol. Cell. Biol. 26, 2479–2489 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Q. et al., The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signaling. J. Exp. Med. 188, 1333–1342 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomlinson M. G., Heath V. L., Turck C. W., Watson S. P., Weiss A., SHIP family inositol phosphatases interact with and negatively regulate the Tec tyrosine kinase. J. Biol. Chem. 279, 55089–55096 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Scharenberg A. M. et al., Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: A target for SHIP-mediated inhibitory signals. EMBO J. 17, 1961–1972 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thien C. B. et al., c-Cbl promotes T cell receptor-induced thymocyte apoptosis by activating the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 285, 10969–10981 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sattler M. et al., Steel factor induces tyrosine phosphorylation of CRKL and binding of CRKL to a complex containing c-kit, phosphatidylinositol 3-kinase, and p120(CBL). J. Biol. Chem. 272, 10248–10253 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Deane J. A. et al., T-cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood 109, 2894–2902 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moran A. E. et al., T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M. O., Rudensky A. Y., T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat. Rev. Immunol. 16, 220–233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fabre S. et al., Stable activation of phosphatidylinositol 3-kinase in the T cell immunological synapse stimulates Akt signaling to FoxO1 nuclear exclusion and cell growth control. J. Immunol. 174, 4161–4171 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Lu L. et al., Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J. Immunol. 184, 4295–4306 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss J. M. et al., Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J. Exp. Med. 209, 1723–1742 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korn T. et al., IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. U.S.A. 105, 18460–18465 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Betts R. J. et al., Influenza A virus infection results in a robust, antigen-responsive, and widely disseminated Foxp3+ regulatory T cell response. J. Virol. 86, 2817–2825 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez-Rodriguez J. et al., Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J. Exp. Med. 211, 529–543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Persaud S. P., Parker C. R., Lo W. L., Weber K. S., Allen P. M., Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat. Immunol. 15, 266–274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soubeyran P., Kowanetz K., Szymkiewicz I., Langdon W. Y., Dikic I., Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416, 183–187 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Avraham R., Yarden Y., Feedback regulation of EGFR signalling: Decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Hunter S., Burton E. A., Wu S. C., Anderson S. M., Fyn associates with Cbl and phosphorylates tyrosine 731 in Cbl, a binding site for phosphatidylinositol 3-kinase. J. Biol. Chem. 274, 2097–2106 (1999). [DOI] [PubMed] [Google Scholar]
- 61.Andoniou C. E., Thien C. B., Langdon W. Y., The two major sites of cbl tyrosine phosphorylation in abl-transformed cells select the crkL SH2 domain. Oncogene 12, 1981–1989 (1996). [PubMed] [Google Scholar]
- 62.Peruzzi G. et al., The adaptor molecule CIN85 regulates Syk tyrosine kinase level by activating the ubiquitin-proteasome degradation pathway. J. Immunol. 179, 2089–2096 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Demydenko D., c-Cbl mediated ubiquitylation and regulation of cell surface exposure of CD5. Biochem. Biophys. Res. Commun. 392, 500–504 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Gary-Gouy H., Lang V., Sarun S., Boumsell L., Bismuth G., In vivo association of CD5 with tyrosine-phosphorylated ZAP-70 and p21 phospho-zeta molecules in human CD3+ thymocytes. J. Immunol. 159, 3739–3747 (1997). [PubMed] [Google Scholar]
- 65.Pani G., Fischer K. D., Mlinaric-Rascan I., Siminovitch K. A., Signaling capacity of the T cell antigen receptor is negatively regulated by the PTP1C tyrosine phosphatase. J. Exp. Med. 184, 839–852 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gary-Gouy H. et al., The pseudo-immunoreceptor tyrosine-based activation motif of CD5 mediates its inhibitory action on B-cell receptor signaling. J. Biol. Chem. 275, 548–556 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Dong B. et al., CD5-mediated inhibition of TCR signaling proceeds normally in the absence of SHP-1. Int. J. Mol. Med. 38, 45–56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Wit J. et al., CD5 costimulation induces stable Th17 development by promoting IL-23R expression and sustained STAT3 activation. Blood 118, 6107–6114 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Sestero C. M. et al., CD5-dependent CK2 activation pathway regulates threshold for T cell anergy. J. Immunol. 189, 2918–2930 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carpino N. et al., Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity 20, 37–46 (2004). [DOI] [PubMed] [Google Scholar]
- 71.San Luis B., Sondgeroth B., Nassar N., Carpino N., Sts-2 is a phosphatase that negatively regulates zeta-associated protein (ZAP)-70 and T cell receptor signaling pathways. J. Biol. Chem. 286, 15943–15954 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kashiwada M. et al., Downstream of tyrosine kinases-1 and Src homology 2-containing inositol 5′-phosphatase are required for regulation of CD4+CD25+ T cell development. J. Immunol. 176, 3958–3965 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Sauer S. et al., T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U.S.A. 105, 7797–7802 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ge Y., Paisie T. K., Newman J. R. B., McIntyre L. M., Concannon P., UBASH3A mediates risk for type 1 diabetes through inhibition of T-cell receptor-induced NF-κB signaling. Diabetes 66, 2033–2043 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh H. et al., An NF-kappaB transcription-factor-dependent lineage-specific transcriptional program promotes regulatory T cell identity and function. Immunity 47, 450–465 e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahmud S. A. et al., Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 15, 473–481 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang D., Liu Y. C., Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat. Immunol. 2, 870–875 (2001). [DOI] [PubMed] [Google Scholar]
- 78.Guo H. et al., E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell Rep. 1, 472–482 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeon M. S. et al., Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 21, 167–177 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Yang B. et al., Nedd4 augments the adaptive immune response by promoting ubiquitin-mediated degradation of Cbl-b in activated T cells. Nat. Immunol. 9, 1356–1363 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated or analyzed during this study are included in the article and its SI Appendix or dataset files. The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD017343. Raw data from Figs. 1 A and C and 2 A, B, and D and SI Appendix, Fig. S1 A–C can be found in Datasets S1–S3 and S5.