Significance
AhR, which is expressed in many epithelial, mucosal, and immune cells, represents a vital cellular sensor of environmental factors. Immune regulation by AhR can be cell, tissue, and ligand specific. Langerhans cells (LC), professional antigen presenting cells in the epidermis, govern the immunological barrier against environmental threats. How the AhR in LC regulates epicutaneous immune responses remains unclear. Experimenting with Langerin-specific AhR−/− mice, we discovered that AhR-deficient epidermal LC were reduced in number and, following epicutaneous protein (Ova) immunization, promoted T helper type-2 (Th2) and T regulatory type-1 (Tr1) responses, leading to increased Ova-specific IgE in the blood. Thus, AhR represents a critical sensor regulating LC activation and LC-mediated T cell polarization.
Keywords: Langerhans cells, aryl hydrocarbon receptor, epicutaneous protein sensitization, Tr1, Th2
Abstract
The aryl hydrocarbon receptor (AhR) represents an environmental sensor regulating immune responses. In the skin, AhR is expressed in several cell types, including keratinocytes, epidermal Langerhans cells (LC), and dermal dendritic cells (DC). The mechanisms how AhR activates or inhibits cutaneous immune responses remain controversial, owing to differences in the cell-specific functions of AhR and the different activating ligands. Therefore, we sought to investigate the role of AhR in LC and langerin+ and negative DC in the skin. To this aim, we generated Langerin-specific and CD11c-specific knockout (−/−) mice lacking AhR, respectively, in LC and Langerin+ dermal DC and in all CD11c+ cells. These were then tested in an epicutaneous protein (ovalbumin, Ova) sensitization model. Immunofluorescence microscopy and flow cytometry revealed that Langerin-AhR−/− but not CD11c-AhR−/− mice harbored a decreased number of LC with fewer and stunted dendrites in the epidermis as well as a decreased number of LC in skin-draining lymph nodes (LN). Moreover, in the absence of AhR, we detected an enhanced T helper type-2 (Th2) [increased interleukin 5 (IL-5) and interleukin 13 (IL-13)] and T regulatory type-1 (Tr1) (IL-10) response when LN cells were challenged with Ova in vitro, though the number of regulatory T cells (Treg) in the LN remained comparable. Langerin-AhR−/− mice also exhibited increased blood levels of Ova-specific immunoglobulin E (IgE). In conclusion, deletion of AhR in langerin-expressing cells diminishes the number and activation of LC, while enhancing Th2 and Tr1 responses upon epicutaneous protein sensitization.
Langerhans cells (LC) were discovered by Paul Langerhans in 1868 (1), but it was not until 1978 that they were found to regulate the immune system through antigen presentation (2). LC are professional antigen presenting cells that govern both adoptive and adaptive immune responses. By demonstrating the critical role of LC in tolerance induction by the hapten 2,4-dinitrothiocyanobenzene (DNTB), Gomez et al. have suggested that strategies targeting LC might be valuable in the prevention of cutaneous allergy (3). On the other hand, the use of an epicutaneous protein (ovalbumin, Ova) to immunize the skin of conditional knockout mice with EpCAM-deficient LC increased the induction of type-2 Ova-specific antibodies as well as the proliferation of Ova-reactive T cells that occurred together with an increased accumulation of LC in skin-draining lymph nodes (LN) (4). Over the past 10 years, several LC-ablation mouse models have been developed that allowed researchers to study the complex and delicate roles LC play in orchestrating immune responses (5, 6). Using murine Langerin-DTR (diphtheria toxin receptor) mice that enable the inducible depletion of LC and langerin+ dermal dendritic cells (DC) (7), we previously demonstrated that arsenic, an environmental carcinogen, mobilizes LC migration through CCL21and induces a T helper type-1 (Th1) response during epicutaneous Ova sensitization (8). Moreover, we discovered a dose-dependent relationship between another carcinogen, cigarette smoke, and adult-onset atopic dermatitis (9), which was triggered by the aryl hydrocarbon receptor (AhR) ligand benzopyrene (BP), a major polyaromatic hydrocarbon (PAH) present in cigarette fumes.
In the skin, AhR is expressed in several cells, including keratinocytes (10), lymphocytes, and LC (11). In keratinocytes, AhR signaling contributes to many physiological functions, including keratinocyte differentiation, skin barrier restoration, skin pigmentation, and oxidative stress response (12). AhR senses environmental stimuli and can evoke an atopic dermatitis-like pathology by inducing the neurotrophic factor artemin (13). During lymphocyte development, AhR regulates the differentiation of regulatory T cells (Treg) and T helper type-17 (Th17) cells (14). Abnormal activation of AhR by ligands mediates the development of allergies and autoimmunity (15). Specifically, AhR activation mediates its nuclear translocation and binding to aryl hydrocarbon receptor nuclear translocator (ARNT), which in turn induces cytochrome P450 1A1 (CYP1A1), activates DNA-responsive elements, and induces immune responses (15). At the same time, AhR activation is negatively regulated by the AhR repressor (AhRR).
In contrast to its role in keratinocytes and T cells, the function of AhR in LC- and dermal DC-mediated immune regulation is less well understood. Furthermore, how AhR regulation promotes or dampens immune responses remains controversial (12). Brandstatter et al. showed that cell type-specific expression of AhR differently regulates the balance of intestinal and systemic inflammation, depending on varying microbial and environmental stimuli (16). AhRR counteracts the anti-inflammatory effects of AhR in systemic inflammation, while AhR and AhRR act in concert to dampen gut inflammation. One study of ultraviolet radiation (UVR)-mediated immunosuppression of contact hypersensitivity (CHS) found that the AhR antagonist 3-methoxy-4-nitroflavone reduced UVR-mediated immunosuppression and induction of Tregs (17), while, similar to UVR, AhR activation by the agonist 4-n-nonylphenol (NP) induced antigen-specific Tregs and suppressed CHS. These discrepancies may result from distinct AhR cell type-specific functions and different immunogens. In fact, the role of AhR in LC is not well understood.
Jux et al. reported that LC in AhR-deficient mice (AhR−/−, total knockout) as compared to control mice are smaller, contain fewer granules, and remain immature and are unable to up-regulate costimulatory molecules like CD40, CD80, and CD24a during in vitro maturation, possibly due to reduced granulocyte-macrophage colony-stimulating factor (GM-GSF) levels (18). LC maturation and CHS against fluorescein-5-isothiocyanate (FITC) are impaired in AhR−/− mice (18). In contrast, a recent study by Bieber et al. demonstrated that AhR activation by the ligand FICZ (6-formylindolo[3,2-b]carbazole) reduces high-affinity immunoglobulin E (IgE) receptor (FcεRI) and up-regulates expression of indoleamine 2,3 dioxygenase (IDO) in LC, suggesting that an AhR-driven anti-inflammatory feedback mechanism may dampen allergen-induced inflammation in atopic dermatitis (11). In fact, transcriptome analysis using AhR−/− mice revealed that there is little overlap of AhR-dependent transcripts found in splenic CD4 and CD8 T cells, and DC (19). This finding again stresses that AhR-dependent transcription and its functional outcomes are regulated in a ligand-, cell-type- and context-specific manner (20). Here, we sought to investigate the role of AhR specifically in LC. To this aim, we analyzed Langerin- and CD11c-specific AhR knockout mice in an epicutaneous protein (ovalbumin, Ova) sensitization model.
Materials and Methods
Mice.
C57BL/6 (B6) and loxP-flanked AhR mice (Ahrfx or Ahrloxp) (21) were obtained from, respectively, Taiwan’s National Laboratory Animal Center-Tainan Facility and the Jackson Laboratory. Langerin-Cre mice (22) were kindly provided by Dr. Björn E. Clausen and CD11c-Cre mice (23) by Dr. Shau-Ku Huang (John Hopkins University). Primer sequences for AhR-loxP were: Forward:5′-GGT ACA AGT GCA CAT GCC TGC-3′; Reverse:5′-CAG TGG GAA TAA GGC AAG AGT GA-3′. Primer sequences for Cre were: Forward:5′-CCG GTC ATG CAA CGA GTG A-3′; Reverse:5′-GGC CCA AAT GTT GCT GGA TA-3′. DNA (100 ng/uL) of 1 uL was mixed with forward primer (10 μM) of 0.5 μL, reverse primer (10 μM) of 0.5 μL, H2O of 8 μL, and Master mix of 10 μL, followed by the PCR following manufacturer’s instructions (Thermo Fisher). Mice were housed in a specific pathogen-free animal facility and at the age of 8 to 12 wk treated following experimental protocols approved by the Animal Care and Use Committee of Kaohsiung Chang Gung Memorial Hospital (IACUC2016031704).
Validation of LC-Specific AhR Deletion.
To validate the selective deletion of AhR in langerin-expressing cells, LC from epidermal sheets were enriched by major histocompatibility complex class II (MHC-II) magnetic cell sorting according to Kadow et al. (24). The complementary DNA (cDNA) was prepared and analyzed by PCR for the presence of AhR as described by Wallisser et al. (21) and Jux et al. (18).
Epicutaneous Ova Sensitization.
Mice were immunized with Ova as previously described (25). Briefly, 20 mL of Ova in phosphate-buffered saline (PBS) (100 mg/mL) were placed on the disk of a Finn chamber (Epitest), which was then fixed onto shaved back skin of mice (20 μL per mouse per day). For each round of immunizations, freshly prepared Ova- or PBS-patches were applied on 5 consecutive days. For the sensitization study, the skin and LN were collected 96 h after the last patch application.
Preparation of Epidermal Sheets.
Skin was soaked in 20 mM ethylenediaminetetraacetic acid (EDTA)/PBS at 37 °C for 4 h. Epidermal sheets were separated using tweezers and fixed in acetone at −20 °C for 5 min. After washing with PBS, the epidermal sheets were blocked with 1% fetal bovine serum (FBS)/PBS (Sigma) for 30 min and then incubated with epithelial cell adhesion molecule (EpCAM)-fluorescein isothiocyanate (FITC) (1:100, Biolegend) and/or CD207-phycoerythrin (PE) (1:100, eBioscience) antibodies at 4 °C for 60 min, followed by washing with PBS, 4′,6-diamidino-2-phenylindole (DAPI) staining at room temperature for 20 min, washing with reverse osmosis (RO) water, air drying, and finally mounting. Images were captured to analyze the number of positively stained cells by NIH Image J (five random high-power fields).
For isolation of LC for flow cytometry, epidermal sheets were explanted in Roswell Park Memorial Institute (RPMI)-1640 for 72 h at 4 °C and then transferred to 20% FBS/RPMI1640 at 37 °C for centrifuging at 60 rpm for 3 h to release LC into the medium. The medium was then pelleted at 400 g for 10 min. The cells were resuspended and washed by 1% FBS/PBS before staining for flow cytometry to identify LC (MHC-IIhigh and CD207high).
For the experiments to confirm specific deletion of AhR in langerin-expressing cells (SI Appendix, Fig. S1) and to measure expression of costimulatory molecules (CD40, CD80, OX40L) and high-affinity IgE receptor (FcεRI) of LC after tumor necrosis factor (TNF)-α treatment (SI Appendix, Fig. S2), the LC were enriched with MHC-II magnetic beads.
Flow Cytometry.
Six skin-draining LN (axillary, subscapular, and inguinal) were removed 96 h after the start of the immunization regimen. LN cells were prepared by digestion of isolated LN with 2.5 mg mL/L collagenase (Roche Applied Science) for 30 min at 37 °C, followed by isolation of CD11c+ cells by MACS (Miltenyi). Cells were stained with various combinations of antibodies, including epithelial cell adhesion molecule (EpCAM, BioLegend) and CD11c (e-Bioscience) along with appropriate isotype controls. Intracellular staining of langerin (e-Bioscience) was performed as previously described (8). The complete list of antibodies used to identify LC is shown in the supplementary data (SI Appendix, Table S1).
Cytokine Measurements.
Pooled LN cells (1 × 106) were cultured in the presence or absence of Ova at 100 μg/mL for 96 h. Supernatants were harvested 48 h later and stored at −80 °C. IL-10, IL-5, IL-13, and interleukin 17 (IL-17) levels were measured by standard sandwich enzyme-linked immunosorbent assay (ELISA). The limit of detection for IL-5, IL-10, and IL-13 was 10 pg/mL. For IL-17A, it was 50 pg/mL.
The expression of these cytokines in Th1, Th2, and Tr1 LN cells was measured by flow cytometry. Th1 cells were identified as CD4+CXCR3+ cells, and the percentage of cells expressing IL-13 was calculated. Th2 cells were identified as CD4+GATA+ cells and the frequency of cells expressing IL-13 was calculated. Tr1 cells were identified as CD4+CD49b+LAG3+ cells or CD4+CD49b+c-maf+ cells. The frequency among these cells expressing IL-10 was calculated. Tregs were identified as CD4+CD25+foxp3+ cells. Additional antibodies used in these experiments are listed in the supporting information (SI Appendix, Table S1).
Statistical Analysis.
Statistical significance between two groups was determined using Student’s t test or Mann–Whitney U test in case of a nonparametric distribution. Statistical significance among several groups was calculated by ANOVA with postcomparison Scheffé test. The immunohistochemical pictures were analyzed using NIH image J for the number of positive cells among high power field (HPF), staining colocalization, and dendritic morphology. All statistical operations were performed using the SPSS software package (version 14). A P value of less than 0.05 was considered statistically significant.
Data Availability.
The published article contains all datasets generated during this study. Requests for further information and resources should be directed to the corresponding author.
Results
Generation of Conditional Langerin-Specific AhR Knockout Mice.
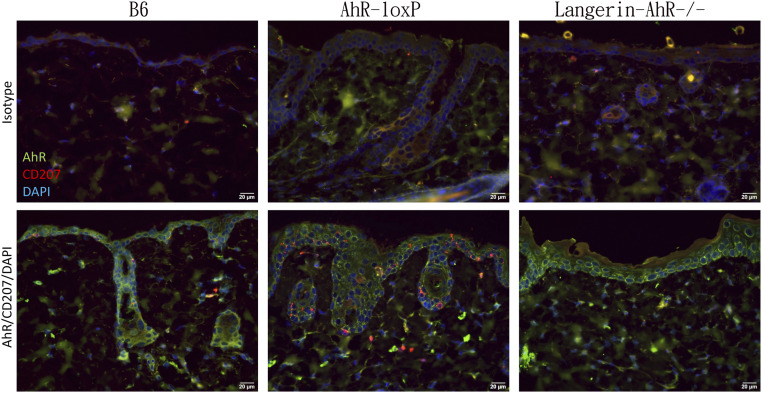
We crossbred Langerin-Cre (22) loxP-flanked AhR mice (Ahrfx or AhrloxP) (21) to generate Langerin-AhR−/− as confirmed by genomic PCR (SI Appendix, Fig. S1). To further validate the selective deletion of AhR in LC, we followed a method previously reported by Kadow et al. (24). Briefly, we identified the excised AhR product in MHC-II magnetic-activated cell sorting (MACS)-enriched LC, in epidermal sheets, or in the liver of Langerin-AhR−/− mice and Cre-negative controls. Our results confirmed that only LC from Langerin-AhR−/− mice showed the characteristic AhR excision product (SI Appendix, Fig. S1C), indicating that we had successfully obtained Langerin-AhR−/−. When we measured expression of the AhR in the epidermis by immunofluorescence microscopy (Fig. 1), in B6 and AhR-loxP mice, AhR-positive cells were clearly evident throughout the skin, including some langerin+ (CD207+) LC in the basal epidermis. In contrast, there was a significant decrease in the number of epidermal LC in Langerin-AhR−/− mice (Fig. 1). Together these results confirmed the specific knockout of AhR in langerin-expressing cells in Langerin-AhR−/− mice.
Fig. 1.
Selective depletion of AhR in langerin-expressing cells. Vertical sections of back skin from B6, AhR-loxP, and Langerin-AhR−/− mice were stained with AhR-FITC and CD207-PE (or corresponding isotype antibodies) along with a nuclear DAPI staining. The result showed the uniform expression of AhR in the epidermis of all three strains and dendritic appearance of CD207-expressing cells in B6 and AhR-loxP mice. However, there was a reduction of CD207-expressing cells in the Langerin-AhR−/− mice (representative photos of one out of three independent experiments with n = 5 mice each are depicted).
Less LC with Impaired Dendrite Formation in the Epidermis of Langerin-AhR−/−.
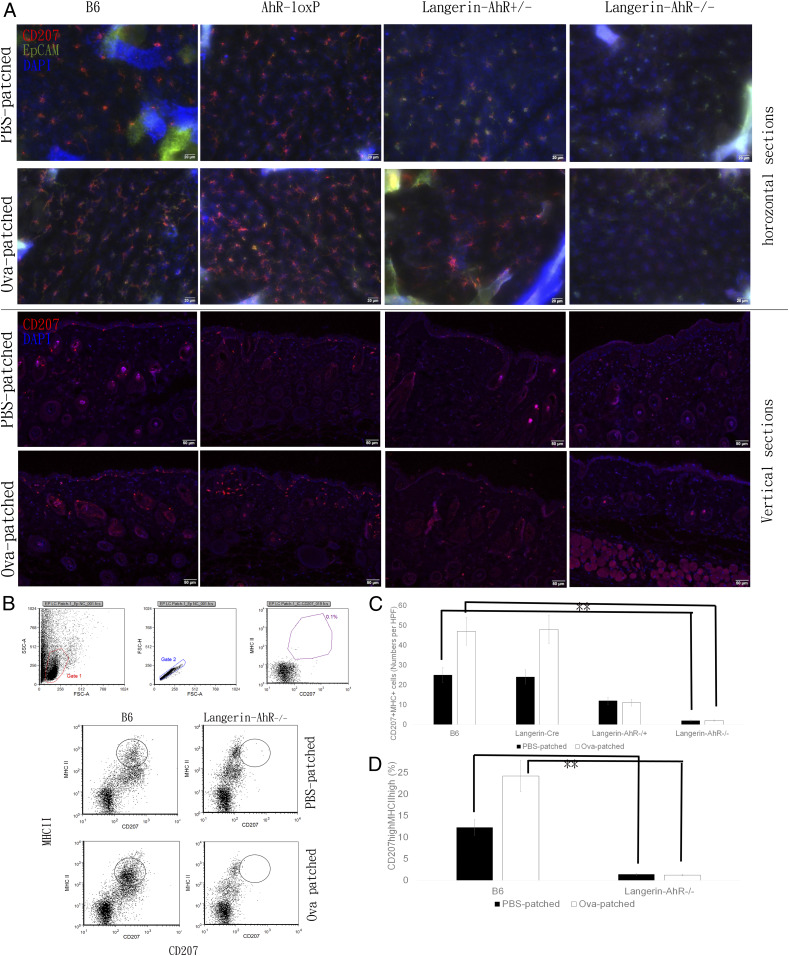
We used epicutaneous protein (Ova) sensitization to investigate the dynamic function of LC. We patched the shaved backs of mice with Ova daily for five consecutive days and then killed the animals after another 96 h. Epidermal sheets were stained with CD207 (langerin) and EpCAM to highlight LC. We found the typical network of LC, characterized by their dendrites, in epidermal sheets obtained from B6 and AhR-loxP mice (Fig. 2 A, Upper). In contrast, the number of LC was significantly diminished in Langerin-AhR+/− mice and further reduced in Langerin-AhR−/−. Moreover, AhR-deficient LC revealed a decreased branching and fewer dendrites than wild type cells (Fig. 2 A, Upper).
Fig. 2.
Deletion of AhR in langerin-expressing cells diminished the number of epidermal LC and dermal DC. (A) Mice were patched on the shaved back skin with Ova or PBS once a day for five consecutive days and killed 96 h after the last patching. Epidermal sheets were stained with CD207 and EpCAM in B6, AhR-loxP, Langerin-AhR+/−, and Langerin-AhR−/− mice. Vertical skin sections were stained with CD207 to characterize epidermal LC and langerin+ dermal DC (representative pictures of one out of three independent experiments with n = 5 mice each are depicted). Quantitative data are presented in C. (B) Epidermal sheets were explanted and cultured for 72 h at 4 °C, and subsequently centrifuged at 60 rpm for 3 h to release LC into medium, which were identified as MHC-IIhigh and CD207high cells. Representative dot plots of three independent experiments are depicted. Quantitative data are shown in D. **P < 0.01.
To characterize the presence of dermal DC (Fig. 2 A, Lower), we stained vertical skin sections with CD207 and detected a significantly decreased number of CD207-expressing cells in both the epidermis and the dermis of Langerin-AhR−/− mice (quantification in Fig. 2C). In addition to the semiquantitative data obtained by immunohistochemical examination, we recovered the cells from epidermal sheets, isolated LC by MHC-II magnetic cell sorting, and measured the number of CD207highMHC-IIhigh LC using flow cytometry. This analysis confirmed the significant decrease in the number of LC in Langerin-AhR−/− mice (Fig. 2 B and D).
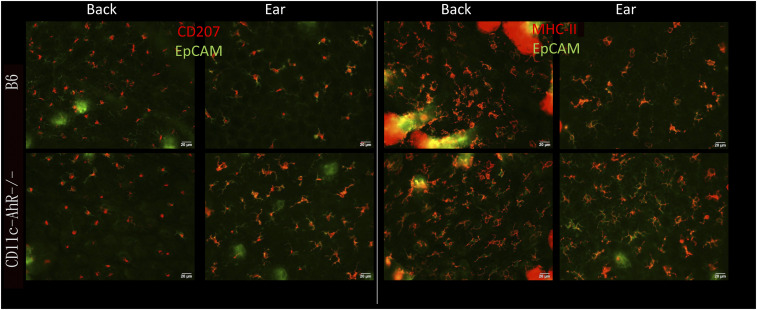
Next, to determine the specific function of the AhR in LC and langerin-expressing dermal DC, we analyzed mice with a conditional AhR deletion in all CD11c-expressing cells (Fig. 3). In contrast to Langerin-AhR−/−, these CD11c-AhR−/− mice displayed epidermal LC (highlighted as CD207+EpCAM+ cells) similar in number and morphology to those of B6 mice, indicating that langerin-specific but not CD11c-specific deletion of the AhR affected the number of LC in the epidermis (Fig. 3). Given the reduction in the number of LC in Langerin-AhR−/− mice, we remained interested in the functional capacities of LC after selective AhR depletion in langerin-expressing cells. Therefore, we isolated LC from epidermal sheets by MHC-II magnetic cell-sorting, treated them with TNF-α and measured the expression of costimulatory molecules, including CD40, CD80, and OX40L, along with the high-affinity IgE receptor, FcεRI (SI Appendix, Fig. S2). As expected, we observed a profound reduction of CD207highMHC-II+ LC in the epidermal sheets of Langerin-AhR−/− mice, which was not changed in response to TNF-α (left). On the other hand, there was a significant increase in the percentage of OX40L-positive cells among the CD207highMHC-II+ cells in Langerin-AhR−/− mice (right). Expression of CD40, CD80, and FcεRI was similar to the corresponding isotype control, even after TNF-α stimulation. Almost all LC remaining in the Langerin-AhR−/− expressed high amounts of OX40L. TNF-α treatment did not appear to change these percentages in vitro.
Fig. 3.
Depletion of AhR in CD11c-expressing cells did not affect the number of epidermal LC. The back and ear skin of B6 and CD11c-AhR−/− mice was removed, epidermal sheets were prepared and incubated with EpCAM-FITC and MHC-II-PE (Right) or CD207-PE (Left) antibodies, followed by DAPI nuclear staining. Image analysis was performed to measure the number of positively stained cells by NIH Image J in five random high-power fields. The B6 and CD11c-AhR−/− mice displayed similar numbers of LC with their typical dendritic morphology. Representative pictures of one out of two experiments are shown.
Decreased Number of LC in Skin-Draining LN of Langerin-AhR−/− Mice.
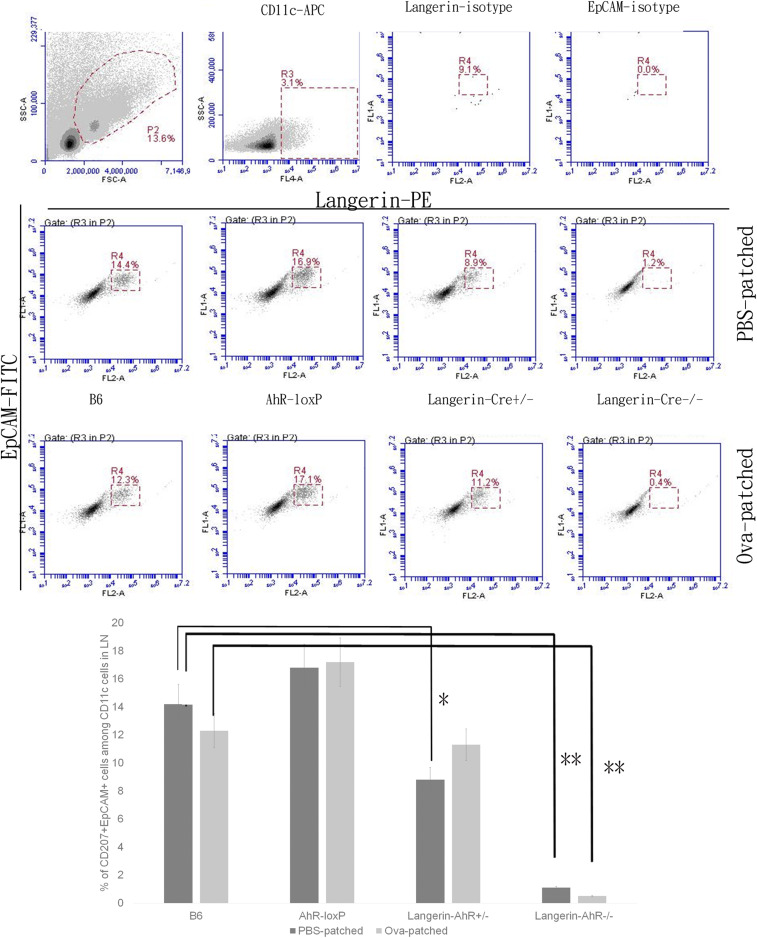
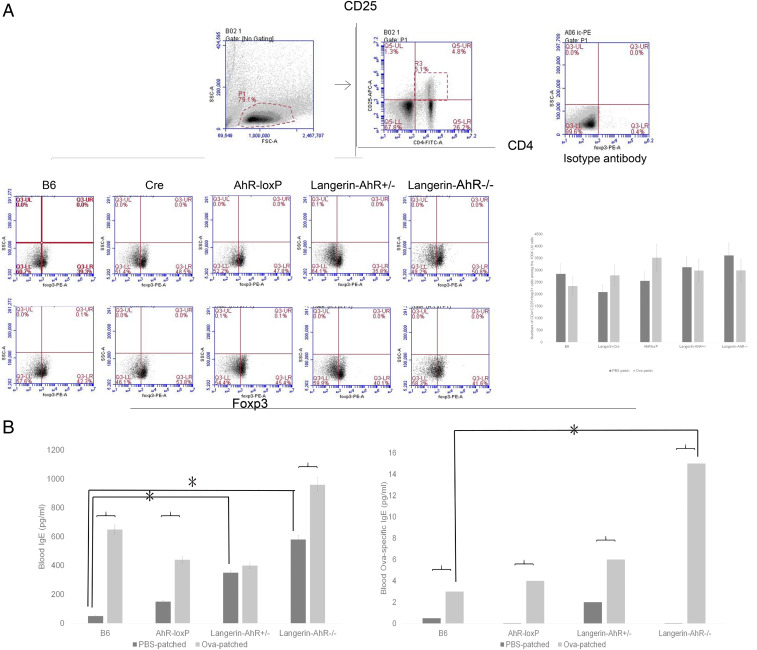
Since there were fewer LC present in the skin of Langerin-AhR−/− mice, we sought to investigate the number of migratory LC in the skin-draining LN. Therefore, we obtained the LN cells 96 h after the last patch immunization and quantified the number of CD207+EpCAM+ LC among the CD11c+ cells. As expected and similar to the results in the skin, there was a significant decrease in the number of CD207-expressing cells in the LN collected from Langerin-AhR+/− and Langerin-AhR−/− mice as compared to B6 wild type controls and irrespective of Ova patching (Fig. 4).
Fig. 4.
The number of LC in skin draining LN were significantly decreased in Langerin-AhR−/− mice. Six skin draining LN were collected 96 h after the last patching with Ova or PBS. LN cells were stained with CD11c, CD207, and EpCAM to characterize LC by flow cytometry. Representative dot plots of one out of three independent experiments with n = 5 mice each (Upper), and the percentage of CD207+EpCAM+ LC among the CD11c+ LN cells (Lower) are shown. *P < 0.05 and **P < 0.01.
Increased Production of IL-5, IL-13, and IL-10 Following In Vitro Restimulation of LN Cells of Langerin-AhR−/− Mice.
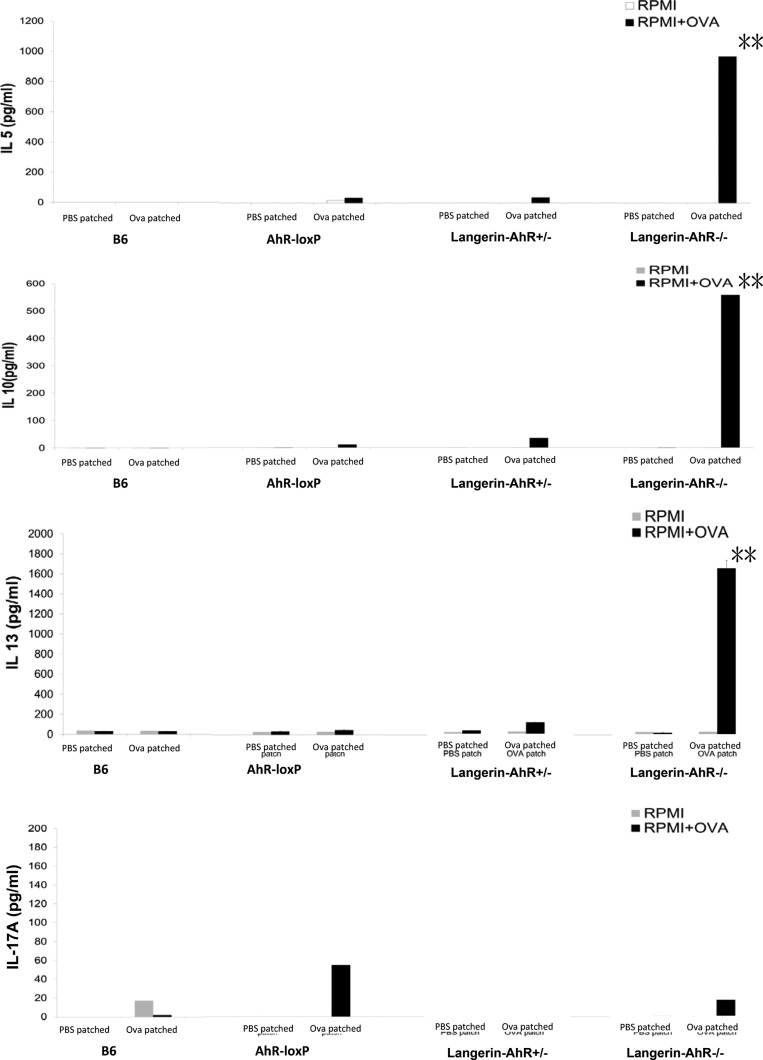
Although there was a significant decrease in the number of migratory LC in Langerin-AhR−/− mice, we wondered whether LN cells obtained from Ova-patched Langerin-AhR−/− and wild type mice would differ with regard to their T cell polarization capacity. Therefore, we restimulated LN cells with Ova in vitro. After 96 h, the supernatants were collected to measure the cytokines IL-4, IL-5, IL-13, IL-10, and IL-17A. Intriguingly, the Th2 cytokines IL-5 and IL-13 were dramatically increased in supernatants collected from LN cells of Langerin-AhR−/− mice as compared to controls, together with a parallel increase in the amount of IL-10, a Tr1-related cytokine (Fig. 5). In contrast, interleukin 4 (IL-4) was below the detection limit by ELISA in most of the experimental groups, except for the Langerin-AhR−/−. Here, the IL-4 levels averaged 16 and 64 pg/mL in the PBS-patched and the Ova-patched groups, respectively (SI Appendix, Fig. S2C). The level of IL-4 was far below that of IL-5 and IL-13, both of which was in the range of 1000 to 2000 pg/mL. Flow cytometric analysis did not reveal any significant changes in the frequency or in the absolute number of CD4+CD25+foxp3+ Tregs in LN obtained from Langerin-AhR−/− mice (Fig. 6A). Taken together, these findings suggest that AhR deficiency in langerin-expressing cells results in a profound reduction of LC in the skin and skin-draining LN as well as a preferential Th2 and possibly Tr1 response.
Fig. 5.
Increased IL-5, IL-13, and IL-10 production after in vitro restimulation of Langerin-AhR−/− LN cells. Mice were patched with PBS or Ova daily for 5 consecutive days. The LN were harvested 96 h after the last patching. Pooled LN cells (1 × 106) were cultured in the presence or absence of Ova at 100 μg/mL for 96 h. Supernatants were harvested 48 h later to measure IL-10, IL-5, IL-13, and IL-17 levels by ELISA. Three independent experiments were performed with five mice in each group. **P < 0.01.
Fig. 6.
Similar Treg numbers but increased systemic Ova-specific IgE in Ova-patch immunized Langerin-AhR−/− mice. (A) Following epicutaneous Ova sensitization, LN cells were harvested to characterize CD4+CD25+foxp3+ Treg. Quantitative data for the number of Tregs are shown on the Right. (B) Systemic levels of total and Ova-specific IgE in B6, AhR-loxP, Langerin-AhR+/−, and Langerin-AhR−/− mice were measured by ELISA. Ova-specific IgE was significantly increased in the peripheral blood of Langerin-AhR−/− mice (three independent experiments, n = 5 mice per group). *P < 0.05.
Increased Blood Levels of Ova-Specific IgE in Langerin-AhR−/− Mice.
Since we observed a Th2 response in the LN of Langerin-AhR−/− mice after Ova patch immunization, we tested whether this would be recapitulated systemically in the peripheral blood of these mice. Thus, we measured serum levels of total and Ova-specific IgE in B6, AhR-loxP, Langerin-AhR+/−, and Langerin-AhR−/− mice by ELISA. Both total and Ova-specific blood IgE were increased after Ova patching in each mouse strain (Fig. 6B). Notably, baseline total IgE levels were already elevated in Langerin-AhR−/−. Furthermore, blood levels of Ova-specific IgE were significantly increased in Langerin-AhR−/− as compared to B6 wild type mice (Fig. 6B). These findings confirmed that the Ova-specific humoral response (Th2) was enhanced when the AhR was selectively lacking in langerin-expressing cells.
Preferential Th2 and Tr1 Responses in Langerin-AhR−/− Mice In Vivo.
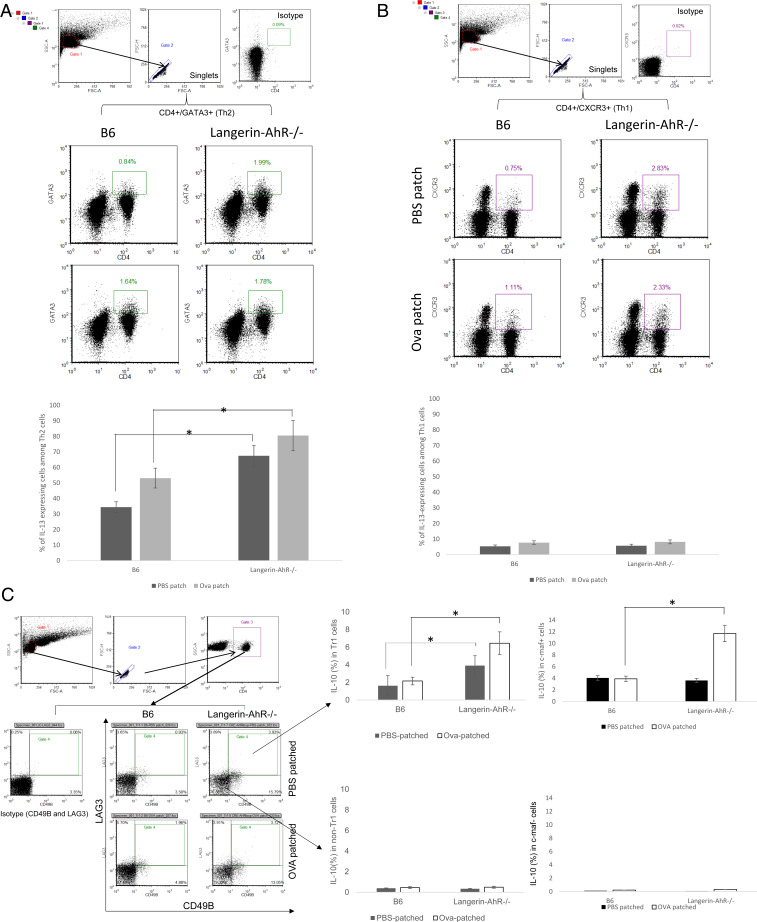
In light of our in vitro cytokine data (Fig. 5), we next asked whether Th2 and Tr1 cells might also contribute to elevated in vivo levels of IL-13 and IL-10, respectively. Therefore, we isolated CD4+GATA+ Th2 and CD4+CXCR3+ Th1 cells from LN and measured the percentage of IL-13-expressing cells. While the frequency of IL-13-producing cells among the Th2 cells (but not Th1 cells) was significantly increased in Langerin-AhR−/− mice and was further augmented after Ova patching (Fig. 7A), this was not the case for the Th1 cells (Fig. 7B). Expression of IL-5 among the Th1 and Th2 cells was undetectable in Langerin-AhR−/− and B6 mice. Next, we focused on CD4+CD49b+c-maf+ Tr1 cells and revealed that the percentage of IL-10-expressing cells was significantly elevated in Langerin-AhR−/− mice with or without Ova patching (Fig. 7C). Notably, the percentage of IL-10-producing cells was only increased within the CD4+CD49b+LAG3+ Tr1 population but not on Tr1 cells in Langerin-AhR−/− (Fig. 7C). The raised frequency of IL-10+ cells after Ova patching of Langerin-AhR−/− mice was also detected among CD4+CD49b+ LN cells coexpressing c-maf, an essential transcription factor for Tr1 development. The significant difference found in LAG3+ cells was mirrored in c-maf+ cells, at least for Langerin AhR−/−. Taken together, these data suggest that the selective knockout of AhR in LC promotes Th2 (IL-13) and Tr1 (IL-10) responses.
Fig. 7.
Th2 and Tr1 cells were the main producers of, respectively, IL-13 and IL-10 following Ova-patch immunization of Langerin-AhR−/− mice. (A) Frequency of IL-13-producing CD4+GATA+ Th2 cells. (B) Frequency of IL-13-expressing cells among CD4+CXCR3+ Th1 cells. (C) Tr1 cells were identified as CD4+CD49b+LAG3+ cells (Left) or CD4+c-maf+ (Right) and analyzed for the percentage of cells expressing IL-10 as compared to non-Tr1 cells. Representative data out of two independent experiments (n = 5 mice in each group) are shown. *P < 0.05.
Discussion
In this study, we establish that conditional deletion of the AhR in langerin-expressing cells leads to a decrease in the number of LC and a loss of the typical dendritic morphology of the remaining LC in the skin. Although the former also results in a diminished number of migratory LC in skin-draining LN, epicutaneous protein sensitization of Langerin-Ahr−/− mice elicits exacerbated Th2 and Tr1 responses.
Kadow et al. reported that the AhR is critical for the homeostasis of invariant epidermal γδ T cells in total but not in keratinocyte- and langerin-specific AhR knockout mice (24). In this study, the morphology of LC seemed to be unaffected in Langerin-AhR−/−, which is in contrast to our current observation that the epidermis of Langerin-AhR−/− contains fewer LC with dramatically impaired dendrite formation. Notably, we also detect a gene dosage-dependent effect in the reduction of epidermal LC between heterozygous Langerin-AhR+/− and homozygous Langerin-AhR−/− mice, suggesting a linear link between AhR signaling strength and the number of LC present in the epidermis. On the other hand, LC numbers were not reduced in CD11c-AhR−/− mice. Thus, while this study provides data regarding the role of AhR in LC homeostasis and morphology, the reason for these inconsistent findings remains elusive.
Nakajima et al. detected impaired Th2 responses upon epicutaneous protein sensitization of LC-depleted Langerin-DTR mice, suggesting that LC are essential for causing the Th2 response and blood IgE levels characteristic for this model (26). Our observation that the conditional knockout of AhR in LC (Langerin-AhR−/−) results in an elevated Th2 response with increased Ova-specific IgE levels in the blood, identifies absent AhR activation of LC as a molecular mechanism that promotes their ability to elicit Th2 responses. Furthermore, our results reveal that this mechanism overrides the expected weakened Th2 immunity given the reduced number of AhR-deficient LC that migrate to the skin-draining LN after topical Ova immunization. Consistent with our findings of an enhanced Th2 response in Langerin-AhR−/− mice, Bogaard et al. reported that coal tar-activated AhR dampens Th2 cytokine signaling via dephosphorylation of STAT6, leading to skin barrier repair in atopic dermatitis (27). Another recent study indicated that AhR−/− mice develop enhanced pulmonary Th2 immune reactions in an Ova-induced asthma model, which was driven at least in part by increased AhR-deficient lung DC activation (28). Taken together, all of these studies, in agreement with our data, prove that AhR signaling in DC regulates Th2 responses, which may open immunotherapeutic strategies to overcome Th2-mediated pathologies.
In this study, we found increased blood levels of Ova-specific IgE in Langerin-AhR−/− mice. Moreover, LN cells of Langerin-AhR−/− mice, when restimulated with Ova, showed increased IL-5 and IL-13 levels (ranging from 1000 to 2000 pg/mL), while IL-4 was barely detectable (under 70 pg/mL). However, IL-4 is one of the major Th2 cytokines that regulates class switching to IgE. It has previously been demonstrated that IL-13 is another T cell-derived cytokine that efficiently directs naive human B cells to switch to IgE production (29). In fact, IL-4 and IL-13 share the same receptor, IL-4Rα, the activation of which induces Th2 responses and IgE production (30). In agreement, targeting IL-4R (31) or IL-13 (32) both result in clinical improvement of Th2-mediated diseases, including atopic dermatitis and allergic asthma.
Beyond preferential Th2 differentiation, our study revealed that selective AhR deletion in LC leads to a Tr1 response with c-maf expression and elevated IL-10 production. It is well known that AhR activation by immunosuppressive ligands promotes the development of Foxp3+ Treg cells (33). However, much is unknown concerning the development of AhR-induced Tr1 cells (34). One study showed that Tr1 but not Foxp3+ Treg cells suppress immune activation via an IL-10-dependent mechanism (35). Another study reported that AhR signaling is essential for Tr1 cell development and IL-10 production in a humanized mouse model of asthma (36). Moreover, our data demonstrating increased IL-10 production among c-maf-expressing cells are in line with a previous study showing that AhR interacts with c-maf to promote Tr1 differentiation (37).
In human atopic dermatitis, FcεRI is up-regulated on LC (38). In contrast, following Ova patch immunization, there was no difference between FcεRI expression on LC of Langerin-AhR−/− and B6 control mice. Instead, we detected an increased percentage of OX40L expression among the reduced number of LC in our Langerin-AhR−/− mice. Interestingly, IL-10-producing and OX40L-expressing mature LC are mandatory for ultraviolet B (UVB)-induced immunosuppression (39). Intriguingly, OX40L-transgenic mice exhibit a significant increase in hapten-induced T cell-mediated ear swelling responses (40). Thus, the augmented OX40L upon selective deletion of AhR in LC may be responsible for the elevated Th2 and/or Tr1 responses.
There are some limitations of this study. First, although we detected an increased percentage of OX40L expression in epidermal LC from Langerin-AhR−/− mice, an unbiased transcriptome analysis by RNA sequencing would be helpful to identify additional molecular changes/pathways that are altered in AhR-deficient LC/DC, endowing them with their enhanced capacity to promote Th2 and Tr1 differentiation. Second, long-term Ova patch experiments (for more than 5 d) may be required to investigate immune responses during the elicitation phase rather than the current experiments representing the sensitization phase. Third, the langerin-specific deletion of the AhR also targets langerin+ dermal DC, which are functionally distinct from LC (6). Future experiments may want to exploit the known radio resistance of epidermal LC and use lethally irradiated Langerin-AhR−/− mice reconstituted with wild type bone marrow (and vice versa) to dissect the differential contribution of AhR to govern the function of these two skin DC populations.
In summary, our analysis of Langerin-AhR−/− mice revealed that conditional deletion of AhR in langerin-expressing cells decreased the number of LC present in the epidermis and impaired the ability of the remaining LC to acquire their typical dendritic morphology. Moreover, despite attenuated LC migration to draining LN, AhR-deficient LC triggered enhanced Th2 and Tr1 responses following epicutaneous Ova immunization. Thus, our data identify AhR as a pivotal molecular switch governing the regulatory function of LC, which may facilitate the development of improved DC-based immunotherapeutic strategies in the future.
Supplementary Material
Acknowledgments
We thank Dr. Jau-Ling Suen for providing expert technical advice with cell sorting and flow cytometry. This work was supported by Taiwan’s Ministry of Science and Technology (MOST 105-2314-B-010-054-MY3 and 108-2314-B-010-045-MY3 to C.-H.H., and 107-2314-B-182A-082-MY3 to C.-H.L.), Chang Gung Memorial Foundation (CMRPG8K0291 and CMRPG8I0361 to C.-H.L.), and Kaohsiung Veterans General Hospital (VGHKS108-139 to C.-H.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917479117/-/DCSupplemental.
References
- 1.Langerhans P., Uber die Nerven der menschlichen Haut. Virchows Arch Pathol Anat 44, 325–327 (1868). [Google Scholar]
- 2.Stingl G., Katz S. I., Clement L., Green I., Shevach E. M., Immunologic functions of Ia-bearing epidermal Langerhans cells. J. Immunol. 121, 2005–2013 (1978). [PubMed] [Google Scholar]
- 3.Gomez de Agüero M. et al., Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. J. Clin. Invest. 122, 1700–1711 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouchi T., Nakato G., Udey M. C., EpCAM expressed by murine epidermal langerhans cells modulates immunization to an epicutaneously applied protein antigen. J. Invest. Dermatol. 136, 1627–1635 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan D. H., Kissenpfennig A., Clausen B. E., Insights into Langerhans cell function from Langerhans cell ablation models. Eur. J. Immunol. 38, 2369–2376 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Clausen B. E., Stoitzner P., Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front. Immunol. 6, 534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett C. L. et al., Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 169, 569–576 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C. H. et al., Arsenic mobilizes langerhans cell migration and induces Th1 response in epicutaneous protein sensitization via CCL21: A plausible cause of decreased langerhans cells in arsenic-induced intraepithelial carcinoma. Biochem. Pharmacol. 83, 1290–1299 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Lee C. H. et al., Lifetime exposure to cigarette smoking and the development of adult-onset atopic dermatitis. Br. J. Dermatol. 164, 483–489 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong C. H., Lee C. H., Yu H. S., Huang S. K., Benzopyrene, a major polyaromatic hydrocarbon in smoke fume, mobilizes Langerhans cells and polarizes Th2/17 responses in epicutaneous protein sensitization through the aryl hydrocarbon receptor. Int. Immunopharmacol. 36, 111–117 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Koch S. et al., AhR mediates an anti-inflammatory feedback mechanism in human Langerhans cells involving FcεRI and IDO. Allergy 72, 1686–1693 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Napolitano M., Patruno C., Aryl hydrocarbon receptor (AhR) a possible target for the treatment of skin disease. Med. Hypotheses 116, 96–100 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Hidaka T. et al., The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 18, 64–73 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Quintana F. J. et al., Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Esser C., Rannug A., Stockinger B., The aryl hydrocarbon receptor in immunity. Trends Immunol. 30, 447–454 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Brandstätter O. et al., Balancing intestinal and systemic inflammation through cell type-specific expression of the aryl hydrocarbon receptor repressor. Sci. Rep. 6, 26091 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navid F. et al., The Aryl hydrocarbon receptor is involved in UVR-induced immunosuppression. J. Invest. Dermatol. 133, 2763–2770 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Jux B., Kadow S., Esser C., Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J. Immunol. 182, 6709–6717 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Frericks M., Temchura V. V., Majora M., Stutte S., Esser C., Transcriptional signatures of immune cells in aryl hydrocarbon receptor (AHR)-proficient and AHR-deficient mice. Biol. Chem. 387, 1219–1226 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Rothhammer V., Quintana F. J., The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 19, 184–197 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Walisser J. A., Glover E., Pande K., Liss A. L., Bradfield C. A., Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proc. Natl. Acad. Sci. U.S.A. 102, 17858–17863 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahner S. P. et al., Conditional deletion of TGF-βR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J. Immunol. 187, 5069–5076 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Caton M. L., Smith-Raska M. R., Reizis B., Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadow S. et al., Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J. Immunol. 187, 3104–3110 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Wang L. F. et al., Cross-priming with an epicutaneously introduced soluble protein antigen generates Tc1 cells. Eur. J. Immunol. 36, 2904–2911 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Nakajima S. et al., Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J. Allergy Clin. Immunol. 129, 1048–1055 e6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Bogaard E. H. et al., Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Invest. 123, 917–927 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thatcher T. H. et al., Endogenous ligands of the aryl hydrocarbon receptor regulate lung dendritic cell function. Immunology 147, 41–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punnonen J. et al., Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc. Natl. Acad. Sci. U.S.A. 90, 3730–3734 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bacharier L. B., Geha R. S., Molecular mechanisms of IgE regulation. J. Allergy Clin. Immunol. 105, S547–S558 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Olesen C. M. et al., Treatment of atopic dermatitis with dupilumab: Experience from a tertiary referral centre. J. Eur. Acad. Dermatol. Venereol. 33, 1562–1568 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Corren J. et al., Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 365, 1088–1098 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Schulz V. J. et al., Aryl hydrocarbon receptor activation affects the dendritic cell phenotype and function during allergic sensitization. Immunobiology 218, 1055–1062 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Ehrlich A. K. et al., AhR activation increases IL-2 production by alloreactive CD4+ T cells initiating the differentiation of mucosal-homing Tim3+ Lag3+ Tr1 cells. Eur. J. Immunol. 47, 1989–2001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Y. et al., Tr1 cells, but not Foxp3+ regulatory T cells, suppress NLRP3 inflammasome activation via an IL-10-dependent mechanism. J. Immunol. 195, 488–497 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Tousa S. et al., Activin-A co-opts IRF4 and AhR signaling to induce human regulatory T cells that restrain asthmatic responses. Proc. Natl. Acad. Sci. U.S.A. 114, E2891–E2900 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Apetoh L. et al., The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida K. et al., Distinct behavior of human Langerhans cells and inflammatory dendritic epidermal cells at tight junctions in patients with atopic dermatitis. J. Allergy Clin. Immunol. 134, 856–864 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Yoshiki R. et al., The mandatory role of IL-10-producing and OX40 ligand-expressing mature Langerhans cells in local UVB-induced immunosuppression. J. Immunol. 184, 5670–5677 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Sato T. et al., Consequences of OX40-OX40 ligand interactions in langerhans cell function: Enhanced contact hypersensitivity responses in OX40L-transgenic mice. Eur. J. Immunol. 32, 3326–3335 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article contains all datasets generated during this study. Requests for further information and resources should be directed to the corresponding author.