Abstract
We report on the presentation and course of 33 children with multisystem inflammatory syndrome in children and confirmed severe acute respiratory syndrome coronavirus 2 infection. Hemodynamic instability and cardiac dysfunction were prominent findings, with most patients exhibiting rapid resolution following anti-inflammatory therapy.
Keywords: kawasaki disease, adolescents, coronary aneurysm, inflammation
Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; IVIG, Intravenous immunoglobulin; LV, Left ventricular; LVEF, Left ventricular ejection fraction; MIS-C, Multisystem inflammatory syndrome in children; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
See related article, p 24
Since the first descriptions of coronavirus disease 2019 (COVID-19) due to acute infections from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in November 2019, reports have identified a relatively low incidence of acute infections in children, who predominantly manifest mild respiratory symptoms, with few requiring hospitalization or intensive care.1 , 2 A report from 46 North American intensive care units identified only 48 children receiving critical care between March 14 and April 3, 2020, with 4% of children coming to medical attention with circulatory failure.2 Multiple reports from Europe alerted the medical community to a new clinical syndrome associated with SARS-CoV-2 infection in children.3 , 4 Patients were primarily presenting with a febrile inflammatory disease with some features of Kawasaki disease and toxic shock syndrome, with profound cardiovascular involvement. Evidence suggests that this disease is an immunologically mediated inflammatory syndrome associated with a previous SARS-CoV-2 infection.3
The Centers for Disease Control and Prevention (CDC) published a case definition for this syndrome, termed the multisystem inflammatory syndrome in children (MIS-C), associated with COVID-19;5 meanwhile, the World Health Organization issued a different case definition.6 Here we describe a large cohort of children with this COVID-19–related inflammatory syndrome, focusing on clinical manifestations, disease severity, therapeutic interventions, and early outcomes.
Methods
This case series was approved by the Northwell Health Institutional Review Board. No case included in this report has been published previously in the medical literature or as part of a multicenter registry. This is a single-center retrospective study of pediatric patients admitted to Cohen Children's Medical Center in Queens, New York. New York state has been the epicenter of COVID-19 in the US, and Queens County has had the highest number of cases of COVID-19 of any county in New York.7
All sequentially patients hospitalized between April 17, 2020, and May 13, 2020, with fever and an inflammatory illness who met the CDC case definition for MIS-C were included.5 Importantly, all included patients were required to have a positive test for SARS-CoV-2 by detection of serum antibodies or nucleic acid from a nasopharyngeal specimen. Patients with COVID-like lower respiratory tract involvement were excluded.
Data were collected from the Enterprise electronic health record (Sunrise Clinical Manager, Allscripts, Chicago, Illinois), and all analyses were performed using Excel (Office Professional +13, Microsoft, Redmond, Washington). Data included patient demographic information, presenting symptoms, respiratory support requirements, use of vasoactive medications, and initial laboratory and other test results, including markers of inflammation and cardiac function. Acute kidney injury was defined by the KDIGO criteria,8 and liver dysfunction was defined as an alanine aminotransferase level of >80 U/L.
Left ventricular (LV) dysfunction was defined as a LV ejection fraction (LVEF) of <55% based on Boston z-scores. Mild LV dysfunction was defined as LVEF 45%-54% (z-score, −2.0 to −4.0); moderate, as LVEF 35%-44% (z-score, −4.0 to −6.0); and severe, as LVEF <35% (z-score <-6.0). Aneurysm and dilation of a coronary artery (right coronary artery and/or left anterior descending artery) were defined by a z-score ≥2.5 and a z-score of 2.0-2.49, respectively.8
Continuous variables are summarized as median and IQR, and categorical variables are presented as frequency.
Results
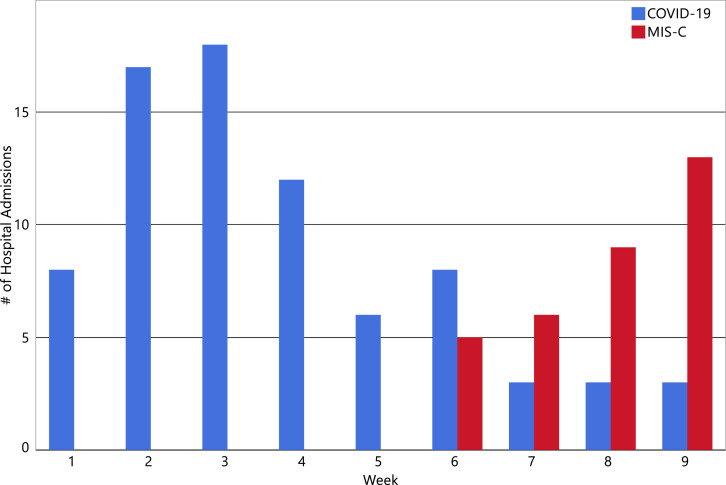
We identified 33 patients who met the CDC criteria for MIS-C5 and had laboratory evidence of SARS-CoV-2 infection. All 33 patients also met the World Health Organization criteria for multisystem inflammatory syndrome in children and adolescents.6 The peak of hospitalizations occurred approximately five weeks after the peak of hospitalizations with acute COVID-19 (Figure ). The cohort was predominantly male (n = 20; 61%), and non-Hispanic (n = 24; 73%), with a median age of 8.6 years (IQR, 5.5-12.6 years) (Table ). Most patients were previously healthy, with the exception of 2 patients who were overweight (6%) and 12 who had obesity (39%). The racial/ethnic distribution compares closely with our general in-hospital population (2019 data) of 71% non-Hispanic and, by race, 37% white non-Hispanic, 21% Black non-Hispanic,15% Asian, and 24% other. Our region has a childhood obesity rate of 18%.
Figure.
Weekly acute COVID-19– and MIS-C–related hospital admissions.
Table.
Demographics, clinical characteristics, and hospital course
| Variables | Value |
|---|---|
| Demographic characteristics | |
| Patients, n | 33 |
| Age, y, median, IQR | 8.6 (5.5-12.6) |
| Age, y, range | 2.2-17.0 |
| Female sex, n (%) | 13 (39) |
| Race, n (%) | |
| White | 3 (9) |
| Black | 8 (24) |
| Asian | 3 (9) |
| Other/multiracial | 15 (45) |
| Unknown/declined | 4 (12) |
| Ethnicity, n (%) | |
| Hispanic | 9 (27) |
| Non-Hispanic | 24 (73) |
| Clinical characteristics | |
| No underlying medical conditions (excluding obesity), n, % | 26 (79) |
| Asthma or reactive airway disease, n, % | 5 (15) |
| Other, n, %∗ | 2 (6) |
| Weight status categories, n, % | |
| Underweight (<5th percentile) | 3 (9) |
| Normal weight (5th-<85th percentile) | 15 (45) |
| Overweight (85th-<95th percentile) | 2 (6) |
| Obese (≥95th percentile) | 13 (39) |
| Presenting signs/symptoms | |
| Duration of fever, d, median (IQR) | 4 (3-5) |
| Neurocognitive symptoms (headache, irritability, lethargy), n, % | 19 (58) |
| Gastrointestinal symptoms (vomiting, diarrhea, abdominal pain), n, % | 32 (97) |
| Respiratory symptoms (cough, congestion, dyspnea, sore throat), n, % | 17 (52) |
| Shock (requiring vasoactive), n, % | 25 (76) |
| Complete Kawasaki disease, n, % | 21 (64) |
| with shock, n/N (% of category) | 16/21 (76) |
| Hospitalization | |
| Pediatric intensive care unit admission, n (%) | 26 (79) |
| Length of stay, d, median (IQR) | 4 (4-8) |
| Initial laboratory results | |
| White blood cell count, K/μL, median (IQR) | 9.14 (7.19-12.33) |
| Absolute lymphocyte count, K/μL, median (IQR) | 0.80 (0.49-1.42) |
| Lymphopenia, n, % | 27 (82) |
| Hemoglobin, g/dL, median, IQR | 11.2 (10.5-12.0) |
| Platelet count, K/μL, median (IQR) | 154 (104-205) |
| C-reactive protein (ref: <5), mg/L, median (IQR) | 206 (122-291) |
| D-dimer (ref: <230), ng/mL, median (IQR) | 1700 (958-2410) |
| Fibrinogen, mg/dL, median (IQR) | 736 (619-870) |
| Ferritin (ref: 15-150), ng/mL, median (IQR) | 640 (313-1192) |
| Lactate dehydrogenase (ref: 135-225), U/L, median (IQR) | 320 (263-419) |
| INR, median (IQR) | 1.31 (1.20-1.51) |
| Pro-BNP (ref: <300), pg/mL, median (IQR) | 3325 (640-6776) |
| Troponin T (ref: <14), ng/L, median (IQR) | 31 (6-78) |
| Procalcitonin (ref: <0.10), ng/mL, median (IQR) | 12.05 (2.87-24.96) |
| Sodium, mmol/L, median (IQR) | 133 (131-135) |
| ALT, U/L, median (IQR) | 38 (30-64) |
| AST, U/L, median (IQR) | 54 (36-76) |
| Total bilirubin, mg/dL, median (IQR) | 0.5 (0.4-0.6) |
| Albumin, g/dL, median (IQR) | 3.4 (3.0-3.7) |
| SARS-CoV-2 testing, n (%) | |
| IgG positive and NAA positive | 6 (18) |
| IgG positive and NAA negative | 24 (73) |
| NAA positive, serology test unavailable | 3 (9) |
| Organ dysfunction | |
| Acute liver injury (ALT > 80 U/L), n, % | 7 (21) |
| Acute kidney injury (KDIGO), n, %† | 23 (70) |
| Requirement for oxygen or positive pressure, n, % | 17 (52) |
| Mechanical ventilation, n, % | 6 (18) |
| Intubation days, median (IQR) | 3 (2-4) |
| Maximal vasoactive infusion score, median (IQR)‡ | 10 (5-20) |
| Echocardiography findings | |
| Any coronary abnormality, n (%) | 16 (48) |
| Left anterior descending/right coronary artery findings, n (%) | |
| z-score ≥2.5, n (%) | 5 (15) |
| z-score 2-2.49, n (%) | 3 (9) |
| Lack of tapering (z-score <2), n (%) | 8 (24) |
| Any LV dysfunction, n, % | 19 (58) |
| Mild (LVEF 45%-54%) | 11 (33) |
| Moderate (LVEF 35%-44%) | 8 (24) |
| Severe (LVEF < 35%) | 0 (0) |
| Medications for MIS-C, n (%) | |
| IVIG | 33 (100) |
| Second dose IVIG | 11 (33) |
| Methylprednisolone | 23 (70) |
| Aspirin | 29 (88) |
| Anakinra | 4 (12) |
| Tocilizumab | 3 (9) |
| Infliximab | 1 (3) |
| Enoxaparin | 14 (42) |
| Disposition | |
| Cardiac function at discharge, n (%) | |
| Always normal | 14 (42) |
| Depressed then normalized | 10 (30) |
| Mildly depressed | 9 (27) |
| Status | |
| Discharged alive, n, % | 33 (100) |
INR, International Normalized Ratio; BNP, brain natriuretic peptide; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NAA, nucleic acid amplification; Pro-BNP, Pro-B-type natriuretic peptide; IgG, immunoglobulin G.
Patients with other diagnoses included 1 patient with hemodynamically insignificant ventricular septal defect and 1 patient with renal tubular acidosis.
Creatinine >50% increase from baseline or absolute increase of 0.3 mg/dL.7
Vasoactive infusion score = dopamine (μg/kg/min) + dobutamine (μg/kg/min) + 100∗epinephrine (μg/kg/min) + 100∗norepinephrine (μg/kg/min) + 10∗milrinone (μg/kg/min) + 10, 000∗vasopressin (U/kg/min).
Patients presented with a median of 4 days (IQR, 3-5 days) of fever, and almost all (n = 32; 97%) had gastrointestinal symptoms as well as involvement of other organ systems. Twenty-one patients (64%) had signs and symptoms meeting the complete criteria for Kawasaki disease. The majority of patients meeting all the criteria had shock as well (n = 16; 76%).
Tests for inflammation showed markedly elevated values (Table). All patients had evidence of SARS-CoV-2 infection, including a positive serology in 30 patients; the remaining 3 came to attention before the availability of serology testing with a positive viral nucleic acid test. Blood cultures were negative in all patients; multiplex nucleic acid amplification test for multiple respiratory pathogens was negative in all patients with the exception of 1 patient positive for influenza virus. During hospitalization, 26 patients (79%) required an intensive level of care, and 6 (18%) required mechanical ventilation. Hemodynamic dysfunction was common; 58% of the patients had myocardial dysfunction and 76% required vasoactive medications. Coronary artery aneurysm and dilation were detected in 5 (15%) and 3 (9%) patients, respectively. All patients received intravenous immunoglobulin (IVIG), 88% received aspirin, and 70% received a corticosteroid. After incomplete response to these initial therapies, 24% received therapy with a biologic modifying medication. Most patients exhibited rapid clinical improvement. There were no deaths, and the median length of hospital stay was 4 days (IQR, 4-8 days). At the time of hospital discharge, mild cardiac dysfunction was still present in 9 of 19 patients who had impaired function during hospitalization.
Discussion
Here we report 33 cases of a newly recognized inflammatory syndrome in a single US children's medical center that exhibits some clinical and laboratory features of Kawasaki disease and appears related to antecedent COVID-19.9 The association with COVID-19 is supported by 2 lines of evidence: (1) all patients had COVID-19, as evidenced by the detection of SARS-CoV-2 serum antibodies or SARS-CoV-2 nasal RNA; and (2) the onset and peak occurrence of cases followed the peak in the number of children with COVID-19 admitted to the same hospital by approximately 3 and 5.5 weeks, respectively (Figure). The latent period between the peak of pediatric cases of COVID-19 and MIS-C suggests that MIS-C has a postinfectious, possibly immunologically mediated pathogenesis.10 , 11
Our case series shares many similarities with the smaller international case series reported as Kawasaki-like disease from Italy3 and the hyperinflammatory shock syndrome reported from the United Kingdom.4 Similar features include a patient population composed of previously healthy children of similar median age manifesting fever and gastrointestinal symptoms, with the development of shock with cardiac dysfunction in most patients and detection of coronary abnormalities in some patients. In addition, many patients in our cohort were overweight or obese, similar to a previous smaller case series.4 Similar to the report of Verdoni et al, we found a spectrum of severity, with 21% of our patients not requiring intensive care.3 Across studies, most patients had antibodies against SARS-CoV-2 virus, suggestive of a postinfectious, immunologically mediated pathophysiology.
Because our patients with MIS-C demonstrated some clinical and laboratory similarities with Kawasaki disease, we applied the principles of therapy for Kawasaki disease to our patients. The regimen included initial treatment with IVIG and aspirin, with the addition of corticosteroid therapy for those with shock or at greater risk of coronary artery aneurysm.12 Biological modifying medication was also given to those who were unresponsive to IVIG and corticosteroids.13 This treatment protocol was associated with rapid clinical improvement and reduction in inflammatory markers in most patients, with no mortality. Considering the severity of cardiocirculatory manifestations, the median length of hospitalization of only 4 days is remarkable and likely unique to MIS-C associated with SARS-CoV-2. Although almost all patients had organ dysfunction, most had resolved by hospital discharge; however, a subset of patients had coronary abnormalities and decreased cardiac function at hospital discharge. The incidence of coronary artery aneurysms and cardiac function in convalescence will be informed by follow-up evaluations.
Although the clinical presentation had features in common with Kawasaki disease, notable differences include the predominance of gastrointestinal symptoms and older age range, with a median age of 8.6 years in the patients with MIS-C, compared with the median age of 2.5 years for patients with Kawasaki disease previously admitted to our hospital.14 The most significant clinical differences are the markedly elevated measurements of inflammation and the higher incidence of shock and/or impaired cardiac function (76%) in our patients with MIS-C compared with the <3% rate of shock reported in patients with Kawasaki disease.14 , 15 Thus, as discussed by Shulman,16 MIS-C may be a syndrome distinct from Kawasaki disease.
None of the patients in our cohort developed a recognized thrombotic event, such as a pulmonary embolus or stroke. Hypercoagulability has been widely reported in adults with COVID-19,17 and patients with Kawasaki disease are at risk for coronary artery thrombosis. Our patients were treated with aspirin, as is routine for children with Kawasaki disease; in addition, enoxaparin was administered to patients with significantly elevated d-dimer and fibrinogen levels along with LV dysfunction, coronary artery involvement, or electrocardiographic changes.
A potential limitation of this study is that some patients included in this case series may in fact have had acute COVID-19 with “cytokine storm” rather than MIS-C, given the difficulty in differentiating these clinical entities. However, we attempted to avoid including patients with acute COVID-19 by limiting our cases to a time period after the peak of acute COVID-19 at our center and excluding patients with lower respiratory tract involvement, a hallmark of acute COVID-19.18 It is also possible that some pediatric patients diagnosed with acute COVID-19 and cytokine storm may in fact have had MIS-C. In addition, for clarity in reporting, only cases with confirmed coronavirus infection were included; 3 additional patients who were hospitalized during the study period and met the criteria for MIS-C but did not have a positive SARS-CoV-2 test were not included.
It is likely this newly described inflammatory syndrome is related to recent COVID-19 infection. A large proportion of patients developed shock requiring vasoactive medications, but with supportive intensive care and anti-inflammatory therapy, most experienced clinical improvement. Further studies to elucidate the pathophysiological basis of MIS-C, optimize treatment regimens, and determine the sequelae of this syndrome are of paramount importance.
Data Statement
Data sharing statement available at www.jpeds.com.
Acknowledgments
We thank Irene Anzalone, RN, BSN, Melissa Belfiore, MS, Michelle Cavataio, RN, MSN, Brianna Concannon, LPN, MHA, Heather DiScala, RN, BSN, and George Reeder, MS, RN, CEN, CPHQ for their assistance with data collection and management.
Supplementary Data
References
- 1.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. e20200702. [DOI] [PubMed] [Google Scholar]
- 2.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., Riggs B.J., Ross C.E., McKiernan C.A. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1948. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Disease Control and Prevention Health Alert Network (HAN). Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) https://emergency.cdc.gov/han/2020/han00432.asp
- 6.World Health Organization Multisystem inflammatory syndrome in children and adolescents with COVID-19. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19
- 7.New York State Department of Health NYSDOH COVID-19 Tracker. https://www.covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker
- 8.Kellum J.A., Lameire N., Aspelin P., Barsoum R.S., Burdmann E.A., Goldstein S.L. Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 9.McCrindle B.W., Rowley A.H., Newburger J.W., Burns J.C., Bolger A.F., Gewitz M. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 10.Rowley A.H., Shulman S.T. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6:374. doi: 10.3389/fped.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esper F., Shapiro E.D., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dionne A., Burns J.C., Dahdah N., Tremoulet A.H., Gauvreau K., de Ferranti S.D. Treatment intensification in patients with Kawasaki disease and coronary aneurysm at diagnosis. Pediatrics. 2019;143:e20183341. doi: 10.1542/peds.2018-3341. [DOI] [PubMed] [Google Scholar]
- 13.Kanegaye J.T., Wilder M.S., Molkara D., Frazer J.R., Pancheri J., Tremoulet A.H. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–e789. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baer A.Z., Rubin L.G., Shapiro C.A., Sood S.K., Rajan S., Shapir Y. Prevalence of coronary artery lesions on the initial echocardiogram in Kawasaki syndrome. Arch Pediatr Adolesc Med. 2006;160:686–690. doi: 10.1001/archpedi.160.7.686. [DOI] [PubMed] [Google Scholar]
- 15.Schuster J.E., Palac H.L., Innocentini N., Rowley A.H., Young L.T., Shulman S.T. Hyponatremia is a feature of Kawasaki disease shock syndrome: a case-control study. J Pediatric Infect Dis Soc. 2017;6:386–388. doi: 10.1093/jpids/piw081. [DOI] [PubMed] [Google Scholar]
- 16.Shulman S.T. Pediatric corona virus disease-2019-associated multi-system inflammatory syndrome (PMIS) [Editorial] J Pediatric Infect Dis Soc. 2020;9:285–286. doi: 10.1093/jpids/piaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T., Sun L.X., Feng R.E. Comparison of clinical and pathological features between severe acute respiratory syndrome and coronavirus disease 2019. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:496–502. doi: 10.3760/cma.j.cn112147-20200311-00312. [DOI] [PubMed] [Google Scholar]
- 18.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.