Summary
The rapid growth of the coronavirus disease 2019 (COVID-19) pandemic, limited availability of personal protective equipment, and uncertainties regarding transmission modes of severe acute respiratory syndrome coronavirus-2 have heightened concerns for the safety of healthcare workers (HCWs). Systematic studies of occupational risks for COVID-19 in the context of community risks are difficult and have only recently started to be reported. Ongoing quality improvement studies in various locales and within many affected healthcare institutions are needed. A template design for small-scale quality improvement surveys is proposed. Such surveys have the potential for rapid implementation and completion, are cost-effective, impose little administrative or workforce burden, can reveal occupational risks while taking community risks into account, and can be repeated easily with short time intervals between repetitions. This article describes a template design and proposes a survey instrument that is easily modifiable to fit the particular needs of various healthcare institutions in the hope of beginning a collaborative effort to refine the design and instrument. These methods, along with data management and analytic techniques, can be widely useful and shared globally. The authors' goal is to facilitate quality improvement surveys aimed at reducing the risk of occupational infection of HCWs during the COVID-19 pandemic.
Keywords: Quality improvement, Infection control, Nested case–control study, SARS-CoV-2, COVID-19, Occupational health, Healthcare workers, Epidemiology, Questionnaire
Introduction and background
A critical mission of infection control programmes in healthcare institutions is to reduce the risk of occupationally acquired infections among healthcare workers (HCWs). The importance of this mission has increased along with the magnitude of the coronavirus disease 2019 (COVID-19) pandemic. Massive surges in the numbers of hospitalized patients, evolving understanding of the transmissibility of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), limited availability of personal protective equipment (PPE), and limited availability of diagnostic testing have contributed to concerns about the inadequacies of institutional programmes for the protection of HCWs' health. This article proposes a template design for quality improvement of infection control programmes in healthcare institutions in order to support the efforts of these programmes to identify preventable HCW exposures and implement remedial actions.
In the absence of effective vaccines or treatments for COVID-19, protection of HCWs with consistent implementation of infection control procedures is necessary. These may include, as appropriate to the clinical situation, careful hand hygiene, engineering controls (e.g. ventilation), administrative controls (e.g. planned cohorting of patients), PPE (such as N95 face masks, gloves, gowns, face shields and goggles) and ensuring that patients wear facemasks. Large numbers of HCW infections have been reported in many countries, and adequate protection of HCWs has proven to be challenging, as reported in studies from Italy, Spain and India [[1], [2], [3]]. Risk factors for incident SARS-CoV-2 infection, reported in a preprint (not yet peer reviewed) from the UK and the USA, found significantly elevated hazard ratios among HCWs compared with the general public associated with re-use of PPE, inadequate access to PPE and caring for patients with COVID-19, even in the context of adequate access to PPE [4]. A study of 41 HCWs in Singapore (85% wore surgical masks, 15% wore N95 masks) who had close contact with a single patient with COVID-19 during an endotracheal intubation reported that none of the HCWs became infected as a result [5]. Newspaper reports of SARS-CoV-2 infections and COVID-19 deaths among HCWs underscore health concerns [[6], [7], [8]]. In sum, while adequate PPE supplies and infection control guidelines are important, the quality of protection for HCWs has to be monitored, and failures need rigorous and prompt investigation.
Quality improvement surveys to identify and reduce occupational risks of SARS-CoV-2 infection among HCWs are warranted. Potential risks of SARS-CoV-2 infection among HCWs that must be considered in such surveys include risks from patients, patients' visitors (if these are permitted), other HCWs, contaminated surfaces and PPE, and HCWs' non-occupational activities in the community, including commuting between work and home. As SARS-CoV-2 may be transmissible by air, studies need to address the possibility of virus dispersal over longer distances, and persistence in air over greater periods of time, than if transmission was solely via droplets. Persistence of the virus in places that lack adequate ventilation must also be considered [9].
Risk factors for SARS-CoV-2 infection among HCWs are likely to vary substantially among institutions and geographic areas. As such, the authors considered that a widely useful design should be developed for use by medical institutions as a template, and that this template design could be easily refined and improved cooperatively over time, while simultaneously being adapted and customized for specific local situations.
In the context of varying rates of growth in the numbers of cases of COVID-19 in different institutions and locales, the following criteria are proposed for evaluation of quality improvement study designs:
-
•
potential for rapid implementation and completion;
-
•
cost-effectiveness;
-
•
minimization of administrative and manpower burden required from stressed HCWs and healthcare institutions;
-
•
effectiveness of and potential for revealing occupational risks;
-
•
ease of implementation and potential for repetition to identify new risks over time, and ability to identify possible slippage or inefficiency in management of known risks;
-
•
ease of statistical analysis;
-
•
ability to identify risks with modest-to-large odds ratios;
-
•
ability to monitor risks associated with well-known transmission routes over time; and
-
•
ability to utilize small numbers of subjects to identify novel, unexpected transmission routes.
Small-scale studies that require few incident cases are proposed, because small studies can be repeated frequently over time to identify newly emergent problems and the success or failure of previous remediation efforts.
Proposed design
A template design for quality improvement case–control surveys of the risk of SARS-CoV-2 infection among HCWs is proposed. The proposed design of a small-scale case–control study seems to be the right choice as it satisfies all of the criteria listed above.
Assumptions regarding institutional resources and cooperation
It is assumed that occupational health services and/or human resource departments (OHS/HR) exist within each institution, and the infection control office/officer and OHS/HR of the institution are informed expeditiously of all documented or presumed SARS-CoV-2 infections and are able to create a list of HCWs who have ever had COVID-19. It is also assumed that OHS/HR possess a list of all employees, and it is possible to concatenate the list of employees who have ever had COVID-19 with the list of all employees to create a list of at-risk, probably uninfected potential controls. It is assumed that adequate measures are taken in the institution to protect privacy and personal information, as required by law. It is assumed that the investigators conducting the proposed quality improvement studies are able to obtain support and cooperation from the senior administrators (in their roles as gatekeepers), OHS/HR and information services to permit conduct of the requisite computerized matching of SARS-CoV-2-infected individuals (obtained from OHS office) against a list of all employees (obtained from HR). Finally, it is assumed that emails can be transmitted to each potential subject without revealing to the survey staff any names or other personal identifiers of the subjects participating in the quality improvement survey.
Inclusion/exclusion criteria for cases and controls
Cases in the quality improvement case–control surveys are HCWs with incident COVID-19, while controls are at-risk HCWs not known to have had COVID-19 at any time prior to enrolment in the study. Inclusion criteria for cases are:
-
•
employed by the institution and worked in one of the institution's buildings for at least 1 day per week during the week prior to the presumed date of SARS-CoV-2 infection; and
-
•
infected with SARS-CoV-2 on the basis of clinical presentation or laboratory tests. Most cases will be incident infections during the study period, but prevalent infections at the time of study commencement would be accepted if their likely date of infection was within 2 weeks of study commencement.
Inclusion criteria for controls are:
-
•
employed by the institution and worked in one of the institution's buildings for at least 1 day per week during the week preceding the index case's date of infection;
-
•
free of physician diagnosis of COVID-19; and
-
•
free of symptoms of COVID-19 or with a negative COVID-19 test (in the context of an institutional screening programme) during the 2 weeks prior to study enrolment.
It is proposed that all employees who recently worked on-site at the institution should be eligible for inclusion as cases or controls, irrespective of whether their duties involved contact with patients or soiled patient materials. Inclusion of employees who had no contact with patients or soiled patient materials will allow estimation of community risks and risks associated with transmission through air ducts or within shared common facilities (e.g. elevators, dining halls, hallways, restrooms).
The proposed study design can be considered a nested case-control study, with the underlying, dynamic cohort being all employees in the institution. To increase statistical precision, three to four or more potential controls per case will be selected at random from the list of potential at-risk controls. Controls will be selected at random from employees who have remained COVID-19-free up to the date of each case's enrolment. Temporal matching of control to case questionnaire responses will be attempted by sending invitations to index cases and their matched controls on the same dates. Date matching will control for possibly important, unmeasured determinants of the risk of infection that may have varied strongly by date, such as local prevalence of individuals shedding SARS-CoV-2, local policies and access to PPE, community access to and use of face masks, and access to SARS-CoV-2 diagnostic tests.
It is estimated that two-thirds of potential controls would participate, and thus the power estimates (see below) are based on two-to-one matching of controls to cases (assuming invitations are sent to three controls per case). Institutions that elect to invite four or more controls for each case may achieve greater precision, including more precise estimates of odds ratios.
Solicitation of participation
Cases and controls will be sent e-mail invitations to participate in the case–control study at the time when OHS/HR learns of the index, incident case infection. E-mails will include a short explanation of the study and a link to an online survey created with a suitable database platform, such as the REDCap data management program which is widely available in US healthcare institutions [10]. The e-mail invitation will provide the following options: refuse participation; accept participation; or, if the subject's health is poor, request to postpone participation. The first paragraph of the survey instrument will contain information usually found in an informed consent form appropriate for a quality improvement survey. The survey questionnaire will be self-administered by most respondents on either a computer or smartphone. Surrogate respondents will not be interviewed when subjects are too ill to respond themselves as it is not expected that surrogates would have the knowledge and insights needed to provide accurate answers. It is suggested that cases whose health improves should be allowed to participate when they are well enough, if they wish to do so. Survey responses will be anonymous; names, telephone numbers, e-mail addresses and internet protocol addresses will not be recorded.
Risk factors to assess
The present authors have developed a sample questionnaire, building on questionnaires of the World Health Organization [11] and a questionnaire used to investigate the SARS epidemic in Toronto, Canada in 2003 [12]. When creating the questionnaire, the intention was to keep it brief in recognition of the many other burdens that HCWs are facing during the present epidemic. In pilot testing, the questionnaire has taken approximately 20 min to complete.
The proposed questionnaire gathers information on demographics (job category, age, sex, race, ethnicity, number of people sharing residence, and number of children sharing residence), and occupational and community risk factors for infection with SARS-CoV-2. In addition, open-ended questions are included, asking how the index case thinks he/she became infected, why the controls think they have remained uninfected, and observations of risky behaviours among colleagues. The open-ended questions may provide a means to discover risk behaviours or situations that were not previously considered to be important, or were not suspected to be associated with meaningful risk.
Several examples of risk factors that can be explored with the proposed questionnaire are:
-
•
occupation;
-
•
storing a used face mask in a paper bag followed by re-use;
-
•
age;
-
•
years of experience in current job;
-
•
months of experience in the COVID-19 infection control environment;
-
•
close quarter (<2 m) exposures for >10 min to asymptomatic co-workers who are not wearing face coverings;
-
•
close quarter exposure to a patient talking without wearing a mask; and
-
•
failure to wear a mask in hallways outside patients' rooms.
In developing the proposed questionnaire, a challenge was identification of the relevant time span of behaviours that may have caused infection with SARS-CoV-2. Behaviours may change after symptom onset. Symptoms have been reported to appear anywhere from 4 to 14 days after infection. It is estimated that 0–6 days may pass between onset of symptoms and employee evaluation by the OHS, at which time an invitation to participate in the survey will be e-mailed to the potential case. Therefore, the questionnaire asks for average behaviours in the 7-day period from 4 to 11 days prior to either onset of symptoms or (for those who experienced no symptoms) sample donation resulting in the subject's first positive COVID-19 test result. Controls are asked for average behaviours in the period 7–14 days prior to interview. Investigators adopting this template study design can modify the template questionnaire to fit local epidemic conditions.
As mentioned above, the quality improvement survey and the present template protocol could be mounted on a journal's or academic institution's website, and opened for refinement and use by health institutions and investigators. The template questionnaire is included in the appendix (see online supplementary material); it can be modified by investigators based on local institutional needs.
Repeated conduct
The proposed case–control approach is designed to use very limited numbers of participants to provide rapid estimates of potentially strong associations that could quickly identify the most important targets for improved measures of infection control. At the same time, it is designed to minimize time requirements of administrators, clinicians, allied health employees and support staff, all of whom may be overworked during the local peak of the epidemic. It is expected that risks may change as community and hospital prevalence rates of infectious status change, stores of PPE increase or decrease, guidelines for preventive measures change, and workload and prevalence rates of other contributing risk factors change. The proposed approach can be conducted repeatedly during the pandemic. However, in later stages of the pandemic, the survey tool can be adapted accordingly. If major non-compliance issues and unexpected routes of exposures for HCWs have been corrected, cases of infection will become isolated rare events. At that time, investigators may want to strive for better matching of times of exposure relative to the presumed dates of infection of index cases.
Data analyses for the proposed template study can be conducted using classical categorical techniques and conditional logistic regression or Cox regression for nested case–control studies.
Qualitative data and free-text responses
It is proposed that open-ended questions on how participants believe they became infected, why they believe they have remained uninfected, and risky situations or behaviours they have observed should be included in the survey form. These responses could provide the basis for interventions, even in the absence of corresponding data from a comparison group, as would be required for statistical analysis. Behaviours or situations may pose risks that are obvious enough that they should be remediated without a need for a demonstrated, significant association with infection, much as it has long been recognized that some measures for the prevention of adverse health outcomes do not require clinical trials [13]. Responses to the open-ended questions may also provide insights that could be used to modify the survey form in the future.
Sample size
In the situation of 20 HCW infections and a control group of 40 uninfected controls, the study could detect odds ratios ≥5.0 for a risk factor present in 20% of controls at an alpha level of significance of 0.05 and power of 80% [14]. A retrospective study of HCWs involved with patients requiring intubation during the 2003 SARS-CoV epidemic in Toronto found several risk factors with odds ratios >5 [6]. The detected risk factors that are easily addressed would be corrected even if their odds ratios (estimating the strengths of association) had wide confidence intervals due to small sample sizes. The aim of this survey and data analysis is rapid identification of strong effects of possibly preventable exposures so remediation can begin promptly. Larger sample sizes could be accrued in large institutions. Analyses using small numbers of participants, however, might be preferable because they could be repeated within a healthcare system every few weeks, depending on numbers of new cases, to capture changing risks. In addition, small sample size investigations can generate useful data and hypotheses to be used to design more comprehensive research.
Ethical considerations
The clinical, practical or administrative uses for these measurements are intended to improve the delivery of health care. The study's purpose and design meet the criteria for quality improvement surveys, and so, in the USA, can reasonably be interpreted not to fall under US Department of Health and Human Services' regulations for the protection of human subjects in research (45 CFR Part 46) [15]. As such, the study could be implemented in most US institutions without the need for review or approval by an institutional review board (IRB). If an investigative team wished to view the study as research (i.e. a systematic investigation designed to develop or contribute to generalizable knowledge), IRB review would be required but this can typically be done swiftly during the pandemic. Regulatory requirements will differ by country. The World Health Organization has posted advice on ethical standards for research and development during the COVID-19 epidemic [16].
Limitations
Possible limitations of the proposed quality improvement studies include:
-
•
odds ratios will be biased towards the null if many controls are, in fact, infected with SARS-CoV-2, but are asymptomatic or in the early stages of the disease when antigens and antibodies may be undetectable; and
-
•
participation rates, especially among controls, may be low if COVID-19 patient loads are high, emotional and physical exhaustion are common, and HCWs are being asked to participate in numerous other studies. However, it is anticipated that HCWs would be interested in the study, and it is not considered that the findings will be biased substantially due to poor participation.
Quality improvement studies such as those proposed can reduce SARS-CoV-2 infection of HCWs, thereby protecting the general population from the risks associated with loss of HCWs due to illness and death. The payoffs for the public will be at least two-fold: first, in the greater availability of HCWs needed by the public during the pandemic; and second, in the lessened chance of HCWs unknowingly spreading disease to patients, co-workers and community contacts in the absence of high-quality protection of HCWs. With modifications, the template study design and questionnaire proposed here could be applied to other industries, such as meatpacking, warehousing or transit, where risky conditions or behaviours appear to be resulting in excess cases of SARS-CoV-2 infection.
Summary
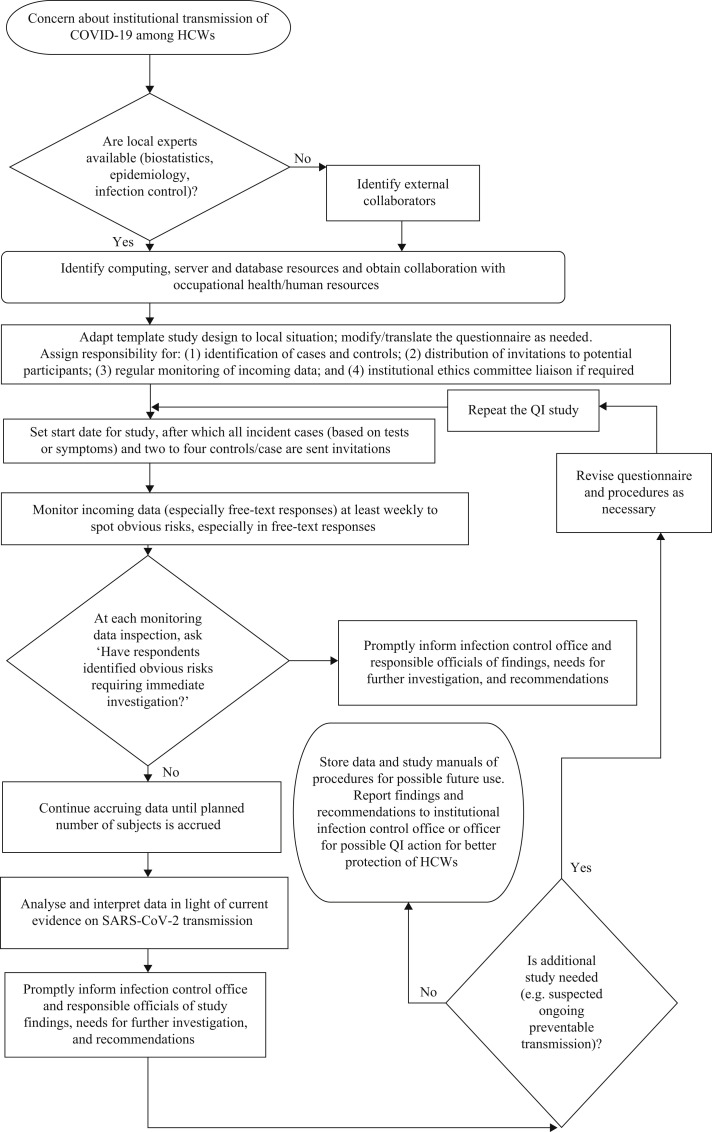
The proposed study design scores well on the criteria for template designs. Steps to be followed in conducting the study have been suggested, both in outline (Table I ) and flowchart formats (Figure 1 ). The design can be implemented rapidly and the study completed promptly if the rate of SARS-CoV-2 infection is high, particularly in institutions with large numbers of employees. Participation rates and the rapidity of implementation will be enhanced by the anonymous nature of the surveys and the quality improvement purpose of the study, obviating the need for delays associated with submission to IRBs unless research teams choose to view the investigations as research projects. Financial resources required would be modest, as the standardized survey can be developed using REDCap or a similar platform and made available as open source code, subjects will do their own data entry, and data analysis will use simple categorical or logistic regression methods. Administrative burdens will be limited to time incurred by collaborating individuals in OHS/HR. The studies will have adequate power to reveal strong occupational risks, should they exist, may point towards risks with elevated but imprecisely estimated odds ratios, and qualitative, free-text responses may identify previously unrealized but dangerous situations where immediate remediation may be required.
Table I.
Outline of proposed study
|
|
|
|
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID, coronavirus disease 2019; HCWs, healthcare workers.
It is the authors' hope that a central repository can be created for questionnaires from different institutions in various languages used in quality improvement studies of SARS-CoV-2 infection control in healthcare institutions and non-healthcare institutions, such as labour unions of essential workers, meatpacking houses and warehouses. This repository could include code for computer and smartphone administration of questionnaires, manuals of procedure, reports of findings, and reports of experiences with remediation programs.
Figure 1.
Flowchart of proposed quality improvement study. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; COVID-19, coronavirus disease 2019; HCWs, healthcare workers; QI, quality improvement.
Acknowledgements
The authors wish to thank Dr Allison McGeer, Mount Sinai Hospital, Toronto, Ontario, Canada, for sharing questionnaires used to investigate the 2003 SARS epidemic.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.06.012.
Contributor Information
M. Marmor, Email: michael.marmor@nyulangone.org.
Y. Shao, Email: yongzhao.shao@nyulangone.org.
Conflict of interest statement
None declared.
Funding sources
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bellizzi S., Fiamma M., Arru L., Farina G., Manca A. COVID-19: The daunting experience of healthcare workers in Sardinia, Italy. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legido-Quigley H., Mateos-Garcia J.T., Campos V.R., Gea-Sanchez M., Muntaner C., McKee M. The resilience of the Spanish health system against the COVID-19 pandemic. Lancet Public Health. 2020;5 doi: 10.1016/S2468-2667(20)30060-8. e251–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee P., Anand T., Singh K.J., Rasaily R., Singh R., Das S. Healthcare workers & SARS-CoV-2 infection in India: a case–control investigation in the time of COVID-19. http://www.ijmr.org.in/preprintarticle.asp?id=285520 Epub ahead of print. Available at: [last accessed June 2020] [DOI] [PMC free article] [PubMed]
- 4.Nguyen LH, Drew DA, Joshi AD, Guo C-G, Ma W, Mehta RS, et al. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv. 10.1101/2020.04.29.20084111. [DOI]
- 5.Ng K., Poon B.H., Kiat Puar T.H., Shan Quah J.L., Loh W.J., Wong Y.J. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020 doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuters . Reuters; New York: 2020. U.S. nurses who can't get tested fear they are spreading COVID-19.https://www.reuters.com/article/us-health-coronavirus-usa-nurses/u-s-nurses-who-cant-get-tested-fear-they-are-spreading-covid-19-idUSKCN21Q33D Available at: [last accessed July 2020] [Google Scholar]
- 7.Baumbach J., LaRocca P., Schwartz D., Peddie S. Newsday, LLC; Melville, NY: 2020. Hospital workers on LI contracting virus; pp. 2–3. [Google Scholar]
- 8.Hirji Z. Thousands of US health care workers have been infected by the coronavirus. This is how each state stacks up. Buzzfeed News. 9 April 2020 [Google Scholar]
- 9.Ferioli M., Cisternino C., Leo V., Pisani L., Palange P., Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev. 2020;29:200068. doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO; Geneva: 2020. Household transmission investigation protocol for coronavirus disease 2019 (COVID-19), Version 2.https://apps.who.int/iris/bitstream/handle/10665/331464/WHO-2019-nCoV-HHtransmission-2020.3-eng.pdf Available at: [last accessed April 2020] [Google Scholar]
- 12.Raboud J., Shigayeva A., McGeer A., Bontovics E., Chapman M., Gravel D. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith G.C., Pell J.P. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ. 2003;327:1459–1461. doi: 10.1136/bmj.327.7429.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hintze J. NCSS, LLC; Kaysville, UT: 2011. PASS 2008. [Google Scholar]
- 15.Casarett D., Karlawish J.H., Sugarman J. Determining when quality improvement initiatives should be considered research: proposed criteria and potential implications. JAMA. 2000;283:2275–2280. doi: 10.1001/jama.283.17.2275. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . WHO; Geneva: 2020. Ethical standards for research during public health emergencies: distilling existing guidance to support COVID-19 R&D.https://apps.who.int/iris/bitstream/handle/10665/331507/WHO-RFH-20.1-eng.pdf Available at: [last accessed April 2020] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.