Abstract
IMPORTANCE
Functional neurological disorders (FND) are common sources of disability in medicine. Patients have often been misdiagnosed, correctly diagnosed after lengthy delays, and/or subjected to poorly delivered diagnoses that prevent diagnostic understanding and lead to inappropriate treatments, iatrogenic harm, unnecessary and costly evaluations, and poor outcomes.
OBSERVATIONS
Functional Neurological Symptom Disorder/Conversion Disorder was adopted by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, replacing the term psychogenic with functional and removing the criterion of psychological stress as a prerequisite for FND. A diagnosis can now be made in an inclusionary manner by identifying neurological signs that are specific to FNDs without reliance on presence or absence of psychological stressors or suggestive historical clues. The new model highlights a wider range of past sensitizing events, such as physical trauma, medical illness, or physiological/psychophysiological events. In this model, strong ideas and expectations about these events correlate with abnormal predictions of sensory data and body-focused attention. Neurobiological abnormalities include hypoactivation of the supplementary motor area and relative disconnection with areas that select or inhibit movements and are associated with a sense of agency. Promising evidence has accumulated for the benefit of specific physical rehabilitation and psychological interventions alone or in combination, but clinical trial evidence remains limited.
CONCLUSIONS AND RELEVANCE
Functional neurological disorders are a neglected but potentially reversible source of disability. Further research is needed to determine the dose and duration of various interventions, the value of combination treatments and multidisciplinary therapy, and the therapeutic modality best suited for each patient.
Functional neurological disorders (FND) are among the most common causes of neurological disability.1 These conditions have had a long and difficult history of mind-body dualism and have now reached better-defined pathophysiological and neurobiological bases that challenge old assumptions of psychological abnormalities as their sole cause (in other words, their status as a psychogenic illness). The term functional, adopted by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as the primary descriptor in the term functional neurological symptom disorder, provides causative neutrality and may also increase patient understanding and acceptance.2,3 The term conversion disorder has been retained in the DSM-5 from previous DSM iterations as an alternative expression that acknowledges unconscious processes in patients (but is used less often in neurology because of its implicit inference of causative psychological stressors, which are not always present or may not be readily identifiable).4
The term dissociative, which is used in the International Classification of Diseases, Tenth Revision, implies compartmentalization or detachment of neurological functioning from normal awareness. Seizures in the context of FNDs are termed psychogenic and/or nonepileptic seizures (and often abbreviated to PNES or NES) or, less commonly, nonepileptic attack disorder or dissociative seizures. These terms have replaced the term pseudoseizures, which could imply feigned symptoms. Functional neurological disorders are distinct from symptoms that are intentionally produced, as in malingering and factitious disorder. Although there are no tests currently capable of demonstrating whether symptoms are willfully produced, and there may not be a clear categorical difference between voluntary and involuntary symptoms, intentionally produced symptoms are relatively rare and will not be considered further here.
Epidemiology of FND
Functional neurological disorders have an incidence of 4 to 12 per 100 000 population per year (4 to 5 per 100 000 population per year for motor FND; 1.5 to 4.9 per 100 000 per year for video electroencephalography–confirmed cases of PNES)5–7 and a prevalence of 50 per 100 000 population based on a community registry.1 In a well-designed consecutive series of 3781 outpatients of neurology clinics, 5.4% had a primary diagnosis of FND, and 30% had symptoms that were described as only somewhat or not at all explained by disease.7 Women are more frequently affected and are estimated to be 60% to 75% of the patient population, although specific presentations such as functional myoclonus or Parkinsonism appear to have similar or greater frequency in men.8 A crucial concern has been fear of misdiagnosis. However, in a review of 27 studies of FND (with a total study population of 1466), the frequency of misdiagnosis was consistently low (4%) after a mean of 5 years of follow-up.9 In a subsequent large, prospective cohort study of patients referred from primary care to specialized neurology clinics with diagnoses that were not at all or only somewhat explained by what the authors termed organic disease, only 4 of 1030 patients (0.4%) had been revised to (or acquired) a recognized neurological diagnosis at 18 months of follow-up.10 Thus, FNDs are common and can be accurately diagnosed by neurologists.
Phenomenology and Diagnosis
Diagnosis by DSM-5 no longer requires identifying precipitating stressors, because these are not always found despite recent and historical stressors being more common in patients with FNDs than in healthy and clinical control participants.11 Positive signs are essential in supporting a phenotype-based diagnosis that does not depend on excluding other disorders (Box 1 and Figure). However, it should be acknowledged that the specificity and sensitivity of these maneuvers may be biased by several factors, including lack of gold standards against which to compare them and unblinded assessments in most studies.12
Box 1. Positive Signs in Categories of Functional Movement Disorders.
Functional Poverty of Movement (Weakness and Slowness)
General Features
Extreme slowness and fatigue
Giveway weakness
Inconsistency in performance
Leg Weakness
Hoover sign
Hip abductor signa
Able to stand on toes or ankles despite weak plantarflexion or dorsiflexion on bed
Arm Weakness
Drift without pronation
Finger abduction signb
Able to remove objects from bag or put on clothes inconsistent with upper limb examination
Parkinsonism
Lack of speed or amplitude decrement on repetitive tapping (sequence effect)
Variable resistance during passive manipulation (Gegenhalten)
Functional Excess of Movement
Tremor
Variability in frequency
Entrainment or full suppressibilityc
Tonic coactivation of antagonistic muscles at tremor onset
Pause during contralateral ballistic movements
Whack-a-mole signd
Myoclonus
Entrainment or full suppressibility
Variability in duration and or distribution of jerks or of their latency (if stimulus sensitive)
Predominance of axial or facial jerks
Dystonia
Fixed dystonia at onset
Variable resistance to passive manipulation
Lack of sensory trick
Lack of overflow
Face: tonic pulling of the lips or jaw to 1 side; closed eyelids resist retraction by examiner
Tics
Not fully stereotypical
Interference with speech or voluntary actions
Lack of premonitory urge
Inability to voluntarily suppress tics
Functional Axial Manifestations
Gait
Knee buckling
Dragging gait with forefoot in contact with ground
Excessive slowness or a gait similar to walking on ice
Posture
Variability of positions over time
Inconsistent, uneconomic postures
Balance
No or controlled falls despite excessive swaying when walking
Swaying and imbalance lessened with dual tasks
Speech
Effortful speech
Sudden onset of dysphonia, stuttering, or dysprosody
Foreign accent
Hip abductor sign is weakness of hip abduction in a paretic leg that resolves with contralateral hip abduction against resistance in the normal leg.
Finger abduction sign is weakness of fingers abduction that resolves with contralateral finger abduction against resistance.
Entrainment or ceasing of tremor to externally cued rhythmic movement or an inability to copy movement.
Whack-a-mole sign is the emergence or worsening of an involuntary movement in a separate body part when the initially affected body part is suppressed by someone holding it down.
Figure.
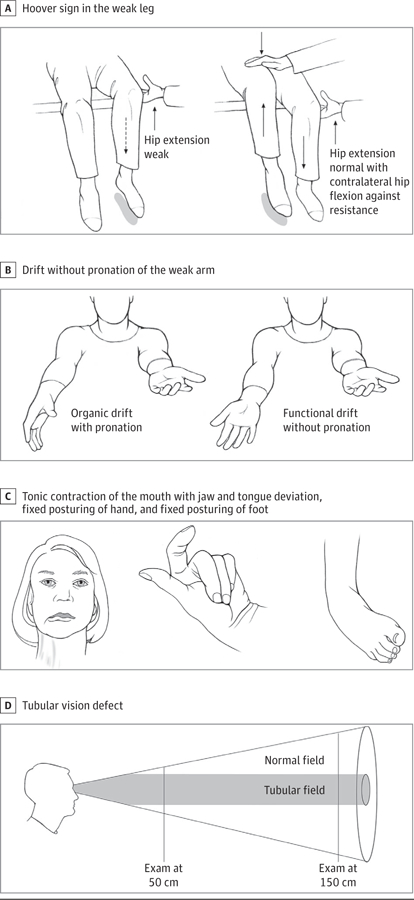
Clinical Signs in Selected Functional Neurological Disorders
A, Hoover sign in functional leg weakness is present if a weak hip extension is corrected when the patient flexes the contralateral hip against resistance. B, Drift without pronation sign in functional weakness is present when the affected outstretched arm, held in supination at the outset, fails to pronate when drifting. C, Fixed dystonia phenotypes expressed as fixed posturing of the jaw (unilateral deviation, often with ipsilateral platysma activation, resistant to passive manipulation), hand (with preservation of pincer function), and foot (ankle inversion with plantar flexion). D, Tubular vision in functional blindness is positive when the area of visual field defect remains unchanged despite moving away from the visual target
Motor FND
Suggestive clinical features include sudden onset, disappearance with distraction, increase with attention, and excessive fatigue or demonstration of effort. The diagnosis is based on ascertaining specific elements of the neurological examination demonstrating inconsistency (ie, changing patterns over time with susceptibility to distraction) and/or incongruence (ie, a clinical picture incompatible with known organically determined patterns).13 Within this context, the examination can establish a positive rather than exclusionary diagnosis for disorders of movement (Box 1) and epileptiform disorders (Table 1).
Table 1.
Ictal Semiology in Nonepileptic vs Epileptic Seizures
| Examination Features | Common in PNES, Rare in ES | Common in ES, Rare in PNES | May Be Present in Either |
|---|---|---|---|
| Eyelids and pupils | |||
| Closed | Yes | No | No |
| Open | No | Yes | No |
| Fluttering | No | No | Yes |
| Resistance to eyelid opening | Yes | No | No |
| Absent light reflex | No | Yes | No |
| Preserved pupil reflex | No | No | Yes |
| Eyes rolled up | No | No | Yes |
| General phenotype | |||
| Duration longer than 2 min | Yes | No | No |
| Opisthotonus | Yes | No | No |
| Asynchronous limb movements | Yes | No | No |
| Side-to-side head shaking | Yes | No | No |
| Prolonged event with falling down and lying still with eyes closed | Yes | No | No |
| Guttural cry at onset | No | Yes | No |
| Visible tongue bite | No | Yes | No |
| Synchronous limb movements | No | Yes | No |
| Clonic jerking | No | Yes | No |
| Highly stereotyped movementsa | No | Yes | No |
| Self-injury | No | No | Yes |
| Urinary incontinence | No | No | Yes |
| Report of tongue biting | No | No | Yes |
| Nocturnal seizures | No | No | Yes |
| Postictal behaviors | Yes | No | No |
| Rapid reorientation | Yes | No | No |
| Prolonged atonia | Yes | No | No |
| Whispering | Yes | No | No |
| Crying | Yes | No | No |
| Slow reorientation | No | Yes | No |
| Stertorous breathing | No | Yes | No |
| Impaired communication | No | No | Yes |
Abbreviations: ES, epileptic seizures; PNES, psychogenic nonepileptic seizures.
In patients with PNES, events can be stereotypical in up to one-third of patients, even if the collective range of events is wider in this population.
Functional weakness is recognized by variability in severity over time and discordant performance between assessments, especially during the same examination. Functional weakness may be global or limited to one side of the body, mimicking a stroke. A pattern of giveway weakness may involve inability to rotate the head toward the paralyzed limb (with the atypical sternocleidomastoid muscle affected),14 Hoover sign of the weak leg (Figure A),15 and drift without pronation of the weak arm (Figure B).16
Functional tremor is characterized by variable frequency and a characteristic response to externally cued rhythmic movements (known as entrainment test). Functional parkinsonism manifests as excessive slowness without decrement and fatigue as well as variable resistance to passive manipulation (inconsistent rigidity), with normal speed for spontaneous movements.17 Concurrent functional tremor may encourage the misdiagnosis of Parkinson disease. Functional dystonia manifests either in paroxysms or with fixed plantar flexion and inversion of the feet. Sudden onset and the presence of pain are common in functional (fixed) dystonia13 and rare in organic dystonia with the exception of cervical dystonia.18 Functional dystonia of the cranial region includes tonic contraction of the mouth pulled to one side, unilateral or bilateral platysma contraction, jaw and tongue deviation (Figure C), and, when involving eye closure, elevation of the contralateral rather than ipsilateral eyebrow.19,20
FND With Sensory Manifestations
Positive but less reliable diagnostic features of functional somatosensory impairments include precise midline splitting of vibration sense across the single bones of the forehead or sternum and sharply demarcated sensory loss at the groin or shoulder.21 Visual field testing may show a tubular (Figure D) or missing half defect (a complete hemianopia on testing with both eyes open, contrasted with a normal visual field in the unaffected eye).22
Persistent perceptual postural dizziness (PPPD) is a new consensus term for functional dizziness, which emerged from previous concepts including phobic postural vertigo, visual vertigo, and chronic subjective dizziness. This condition is defined as persistent disequilibrium or nonspinning dizziness provoked by upright posture, active or passive motion, and exposure to moving visual stimuli or complex visual patterns. It is usually triggered by an episode of acute dizziness, such as vestibular neuronitis or panic attack. Symptoms persist because of failure of vestibular and brain readaptation. Secondary anxiety and functional gait disorder are common accompaniments.23
While pain is not part of DSM-5–defined FND, it is a common co-morbidity, especially in the form of associated fibromyalgia, chronic spinal pain, complex regional pain syndrome, or migraine. As with sudden onset, pain is common in fixed dystonia but rare in organic dystonias apart from cervical dystonia.
Axial FND
Functional axial disturbances include disorders of gait and posture.24,25 Some gait patterns are frequently represented in patients with FNDs, such as excessive gait slowness, astasia-abasia, and knee buckling.24 Excessive demonstration of effort during ambulation (also referred to as huffing and puffing sign) is poorly sensitive but highly specific for functional gait disorders.26 Functional trunk postural abnormalities may manifest as myoclonic jerks affecting the trunk (often diagnosed as propriospinal myoclonus)27 and more rarely as fixed forward–flexion of the thoracolumbar spine, which is also known as camptocormia.25
Speech FND
Common functional speech disorders include dysfluency similar to stuttering, articulation deficits, visible demonstration of excessive effort, and prosodic abnormalities (including foreign accent), often with overlapping features.28 Functional voice disorders include aphonia or dysphonia in which vocal cord or neurological pathology is absent or insufficient to account for the nature and severity of the voice disorder.
Paroxysmal FND Including Seizures/Attacks
While most FNDs fluctuate in severity over time, some are strictly episodic. At one end of the spectrum, there are patients with paroxysmal akinesia, typically evaluated for cardiogenic syncope by cardiologists. Typical positive features include long-duration attacks with closed eyes.29 At the other end of paroxysmal FNDs are a variety of paroxysmal hyperkinesias (eg, tremor, dystonia, and jerks) with no apparent alteration in awareness or convulsive events with falls and variable or complete impairment of consciousness (ie, PNES).30,31 Helpful clinical clues distinguishing PNES from epileptic seizures are the long duration of shaking episodes (>3 minutes, placing patients at risk of being mismanaged for status epilepticus), fluctuating course of seizures, asynchronous limb movements, pelvic thrusting, side-to-side head movements, closed eyes, ictal crying, and recall of ictal events (Table 1).32,33 The co-occurrence of interictal functional symptoms (eg, functional tremor) is also supportive of the interpretation of the paroxysmal movements as a manifestation of an FND. Simultaneous recording of video and physiological parameters (eg, cortical, muscle, and cardiac electrical activity, or blood pressure and oxygenation) helps exclude recognized neurological or medical conditions (such as epilepsy or syncope) to reach a documented or laboratory-supported level of diagnostic certainty for PNES.34
Diagnostic Criteria
Diagnostic clinical criteria have been developed for PNES34and have high interrater reliability when using video electroencephalography,32 serving to provide a laboratory-supported definite diagnosis for PNES. In contrast, diagnostic criteria for functional movement disorders13,35 may have poor interrater reliability when applied to clinically uncertain movement disorders.36 The Gupta-Lang criteria for functional movement disorders introduced a laboratory-supported, definite diagnostic category based on electrophysiological findings in functional myoclonus (which uses electromyography [EMG] and electro-encephalography back-averaging for assessment of premovement potential, or Bereitschaftspotential) and functional tremor (surface EMG to document, among other features, entrainment, which is also termed coherence, and coactivation signs).35 Probable and possible diagnostic categories for functional movement disorders are unhelpful, because they force clinicians to apply an exclusionary approach to the diagnosis by requiring investigations to exclude other disorders; a definite category permits an inclusionary examination–based diagnosis, with separate investigations only in selected cases for laboratory-supported confirmation.
Pathophysiology
Psychological Perspectives
The traditional model has been that of conversion, where psychological distress is converted into a physical symptom. The idea has existed for centuries, and Briquet provided experimental evidence37 that prior experiences of abuse increased the risk of l’hysterie. Freud changed the focus from events to thoughts and suggested that unacceptable, usually sexual, drives were repressed from conscious awareness with the resulting psychic energy converted into physical symptoms.38 Contemporary psychodynamic models emphasize that a symptom may either suppress an emotion or serve to resolve dilemmas, support important interpersonal relationships, or escape interpersonal conflicts.39
While aversive experiences may increase the risk of FNDs, these cannot fully account for their development given several limitations: (1) the current lack of an explanation of the neurophysiologic mechanism of conversion, (2) the fact that similar exposures create different symptoms in different patients, (3) the long latency between exposure and FND onset in some patients (termed the why now? dilemma), and (4) absence of aversive events in many patients, although this may be a factor of how carefully the history is taken40 or how comfortable the patient is about disclosing sensitive information in brief or limited medical encounters. Suggested answers to the why now? question include the sensitizing effect of recent events, such as trauma, medical illness, or physiological or psychophysiological events, which can form robust somatosensory experiences embedded into strong ideas and expectations about these events.41
Attentional dysregulation is a major feature in FNDs. General hierarchical information flow in the nervous system is based on the integration between bottom-up sensory information and top-down predictions about the nature of the expected sensory information. Differential weighting of the 2 streams of information leads to abnormal predictions about sensory data with abnormal body-focused attention driving abnormal perceptions or movements.42 Electrophysiological and psychophysical studies have provided additional support for the predictions of this model.42,43 The role of attention is also underscored by the suppression of symptoms with distraction, a core diagnostic feature in FNDs.44 Used in reverse, the practice of deflecting attention away from the affected area has provided a basis for novel physiotherapies.45,46
More recent psychological theories have focused more on how FND symptoms are produced rather than why they may develop. These theories have been elaborated for FNDs in general, as well as for PNES.42,47 The integrative cognitive model for PNES proposes that factors such as inherent responses to emotions, ideas about illness, and illness models contribute to the formation of a symptom scaffold which, especially in the context of deficient inhibition, may be activated by arousal or internal or external stimuli perceived as threatening.47 These models explain why the manifestations of FNDs conform to factually incorrect beliefs of what symptoms should look like and how the nervous system might go wrong.48 One example is a tubular visual field, which is incompatible with the laws of optics. This also explains why functional symptoms may be internally inconsistent, such as the walking on ice gait, where the patient’s expression of imbalance is manifested by a lurching, oscillating axial deviation on a narrow base, inconsistent with truly impaired balance function. These symptoms may be further facilitated by illness awareness, health anxiety, and excessive threat vigilance (a recognized sequelae of abusive experiences).49 Regardless of the initiating mechanism, once the functional symptoms are formed, phobic avoidance, affective disorders, and, in time, brain plasticity may be perpetuating features.50
Neurobiological Perspectives
Functional neuroimaging has elucidated dysfunction in FNDs at the level of brain network activity, connectivity, and specific anatomic areas of altered metabolic demand during tasks. Early research found differences between feigned and functional weakness.51 In feigned weakness, no activation was seen when participants were given the command to move. In the functional variant, normal activation within areas associated with movement preparation was accompanied by activation within the prefrontal cortex that would be unexpected during voluntary movement activation. In patients with functional tremor, dystonia, or gait abnormalities, there was hypoactivation of the supplementary motor area, a key structure involved in action selection and movement preparation, and abnormal connectivity between the supplementary motor area and limbic areas.52 These abnormalities, activated via negative emotional stimuli, may be associated with abnormally increased activation in areas involved in emotion recognition and self-awareness (primarily the amygdalae and cingulate gyrus53) and in networks related to emotion processing and theory of mind (ability to represent mental states to understand others’ intentions and predict future intentional social interaction).54 In patients with PNES, resting-state functional magnetic resonance imaging has shown strong functional connectivity between areas involved in emotion (insula), executive control (inferior frontal gyrus and parietal cortex), and movement (precentral sulcus).55
A deficit in the sense of agency for movements is an appealing explanation for how movements that appear voluntary in nature (because they are altered by distraction) are experienced as involuntary. Because of the relative disconnection between supplementary motor area and areas that would usually select or inhibit movement (eg, prefrontal cortex), movements occur without a normal feeling of sense of agency. Further supporting the role of impaired agency, a study of patients with functional tremor who acted as their own control participant by also voluntarily mimicking their tremor, there was hypoactivation of the temporoparietal junction, an area associated with the sense of agency for movements.56
An Integrated Perspective
Ultimately, we need a model that integrates these neurobiological and psychological perspectives into a biopsychosocial framework. This framework should include predisposing, precipitating, and perpetuating factors involving genetics, neural networks, temperament, cognition, emotion, and environmental influences.57
Treatment
Treatment of FNDs is a process that starts with explaining the diagnosis in a way that helps the patient understand and gain confidence in it. This in turn enhances the odds of adherence to and success from therapeutic strategies. Although randomized clinical trial evidence is limited, promising data are emerging from cohort and pilot randomized studies to support specific treatments (Table 2). How best to select patients for specific treatments and predictors of response are important knowledge gaps, but certain general principles are known to apply (Box 2).
Table 2.
Randomized Clinical Trials of Therapeutic Interventions for Functional Neurological Disordersa
| Source | FND Type | Active Interventions | Control Treatment | Active Arm Sample Size | Control Sample Size | Main Result |
|---|---|---|---|---|---|---|
| Goldstein et al,58 2010 | PNES | 12 Sessions of CBT modified for PNES, plus SMC | SMCb alone | 33 | 33 | The active arm had lower PNES frequency at end of treatment; differences were nonsignificant at 6 mo and with respect to 3-mo seizure freedom. |
| LaFrance et al,59 2014 | PNES | 12 CBT-informed sessions of psychotherapy alone; 12 CBT-informed sessions of psychotherapy and flexible-dose sertraline; Flexible-dose sertraline alone | SMCb alone | Psychotherapy: 9; Psychotherapy and sertraline: 10; Sertraline: 9 | 10 | The psychotherapy group had 51.4% lower PNES frequency; the psychotherapy and sertraline group had 59.3% lower PNES frequency; those receiving sertraline only had a nonsignificant finding of 26.5% lower PNES; and the control group had a nonsignificant finding of 33.8% lower PNES. |
| Nielsen et al,45 2017 | Functional motor disorder | Specialized physical therapy for functional motor disorder (5-d outpatient program)60 | Generic neurophysiotherapy | 30 | 30 | The active arm had 72% rated symptoms improvement at 6 mo and SF-36 physical function adjusted mean difference of 19.8 (vs the control group). In the control arm, rated symptoms improved 18% at 6 mo. |
| Jordbru et al,61 2014 | Functional motor disorder | Inpatient adapted physical therapy | Delayed start design | 30 | 30 | The active arm had a mean difference on functional independence measure 8.4 points greater than control arm (scale 18–125; sustained at 1 y). |
| Moene et al,62 2002 | Functional motor disorder | Eight 1-h sessions of manualized hypnosis, plus inpatient group psychotherapy and other multidisciplinary team input | 8 Nonspecific 1-h therapy sessions, plus inpatient group psychotherapy and other multidisciplinary team input | 24 | 21 | The study found overall improvement in all outcome measures in both groups. No additional effect on outcome in the active arm. |
| Moene et al,63 2003 | Functional motor disorder | Outpatient manualized hypnosis (10 sessions over 3 mo) | Waiting list | 20 | 25 | There was a greater improvement on Video Rating Scale for Motor Conversion Symptoms in the active arm. |
| Dallocchio et al,64 2016 | Functional motor disorder | CBT, plus adjunctive physical activity; CBT alone | SMCb | CBT and activity: 15; CBT alone: 14 | 8 | In both active arms, there was significant Psychogenic Movement Disorder Rating Scale improvement; no differences were observed between active groups, and no improvement in the control arm. |
| Sharpe et al,65 2011 | Mixed FND | CBT-based guided self-help in 4 30-min sessions, plus SMCb | SMCb | 64 | 63 | The active arm had greater improvement on self-rated 5-point CGI at 3 mo than the control group. |
| Hubschmid et al,66 2015 | Functional motor disorder or PNES | 4–6 Sessions of interdisciplinary psychotherapeutic intervention, plus 2 sessions with a neurologist and psychiatrist | SMCb | 11 | 12 | The active arm had greater improvement on SDQ-20, CGI, mental health scores on SF-36, and depression at 12 mo than the control group. |
Abbreviations: CBT, cognitive behavioral therapy; CGI, Clinical Global Impression Scale; FND, functional neurological disorders; PNES, psychogenic nonepileptic seizures; SDQ-20, 20-item somatoform dissociation questionnaire; SF-36, 36-item short form health survey; SMC, standard psychiatric/medical care.
All included studies had total sample sizes of 20 or more participants.
Standard psychiatric/medical care differed between studies.
Box 2. General Treatment Principles of Therapy for Functional Neurological Disorders.
General Principles
Diagnosis should be established prior to starting therapy and clearly communicated to the patient within a biopsychosocial framework.
Encourage transparency, especially regarding positive diagnostic features.
Explore and address unhelpful illness beliefs and behaviors.
Ensure that the patient understands potential for reversibility and is motivated to change.
Foster independence and self-management during treatment.
Involve the family and caregivers in treatment.
Functional Movement Disorder–Specific Principles
Establish the treatment goal of relearning normal motor control.
Motor retraining begins by establishing elementarymovements (eg, weight-shifting) and consecutively adding more complex movements.
Visual feedback during motor relearning from mirrors and video can be helpful.
Emphasis is placed on the quality ofmovement instead of the quantity.
Avoid excessive attention to abnormal movements.
Treatment adjuncts may enhance movement relearning: treadmill training, electrical stimulation, electromyography biofeedback, and transcranial magnetic stimulation.
Nonepileptic Seizure–Specific Principles
Learn techniques to avert episodes, if there are warning symptoms.
Foster cognitive awareness of triggers when these are present.
Develop a self-management and relapse plan.
Learn to challenge safety or avoidance behaviors around episodes.
Delivery of Diagnosis
A critical first step in increasing favorable outcomes is the discussion of the diagnosis with the patient, encompassing these critical points: (1) there is an established diagnosis; (2) in most cases, the diagnosis can be made definitively with a neurological examination, rather than as a diagnosis of exclusion; and (3) the disorder is potentially reversible with treatment. The effectiveness of the diagnostic delivery can be helped by disclosing to patients how the diagnosis was made, including the specific features on examination that make it clinically definite, when such level of certainty can be achieved (eg, demonstration of the Hoover sign or tremor entrainment).67 Success in this step ensures that the patient leaves with (1) a validation of the neurological symptoms and/or disability, (2) confidence in the diagnosis, preventing the need to seek alternative medical opinions, (3) a sense of partnership with the neurologist, and (4) an understanding of the rationale for tailored multidisciplinary management, which may include psychological interventions.
Functional Overlay
It is important to note the possibility of other concurrent or future medical comorbidities (eg, comorbid epilepsy or Parkinson disease).68,69 A properly explained diagnosis should not alienate patients from requesting a reevaluation if they develop new symptoms. This also mandates a mature response from health care professionals, who should not be dismissive (ie, not all manifestations are solely because of a patient’s FND). Treatment options for FNDs need to reflect the diversity of symptom phenotypes as well as the heterogeneity and comorbidity within the patient population; there is no one-size-fits-all approach.
Psychological Treatments of FND
Psychological interventions have traditionally been considered the treatment of choice. Studies of psychological therapies have been undertaken in subpopulations with FNDs, with particularly promising results for disorder-adapted cognitive behavioral therapy for PNES and functional movement disorders, and for multimodal cognitive behavioral–informed psychotherapy for PNES, as well as cognitive behavioral therapy–oriented self-management and interdisciplinary psychodynamic interpersonal therapy for a wider range of FNDs (Table 2).58,59,62–66 Cognitive behavioral therapy is a structured, time-limited therapy that helps patients identify how thinking affects emotional states or specific behaviors. Cognitive behavioral therapy and other psychotherapies (designed for PNES) include components such as education, skills in gaining control of seizures, recognizing triggers, changing cognitions and behaviors associated with seizures, and widening therapy to other aspects of interpersonal functioning.
Physical Treatment of FND
Recently, a greater role for physical therapy has been recognized when motor symptoms predominate (Table 2).45,61,70,71 However, while many such studies focus on the physical treatment of FNDs, most successful programs incorporate psychotherapeutic modalities (eg, cognitive behavioral, psychoeducation, stress reduction techniques), underscoring the importance of addressing the brain and behavioral elements. Motor rehabilitation strategies aim to help the patient to establish normal control of movement through physiotherapy, occupational therapy or speech therapy, informed by an understanding of FNDs (Box).
Motor retraining begins with establishing basic movement patterns (eg, weight-shifting), and the complexity of movement is sequentially increased toward normal movement patterns. The focus lies on function and automatic movement (eg, walking) rather than specific impairments (eg, weakness) and controlled movement, such as strengthening exercises. Reduced symptom severity with distraction can be used as part of motor retraining by redirecting the patient’s focus of attention toward the goal of the movement (eg, transferring from bed to chair) and away from the individual components of the movement (eg, knee extension). Encouraging movements that are initiated more automatically, such as rhythmical weight-shifting, or novel movement, such as walking backward, can trigger normal movement patterns. Unhelpful cognitions (eg, thinking “my nerves are damaged”) and behaviors (eg, acting as though moving could cause more damage) should be addressed as part of the treatment to integrate important aspects of psychological treatment.60 Chronic pain and fatigue are common comorbidities in FNDs and, when these are present, patients may benefit from specific interventions to address these issues, and treatment approach may need to be adapted (eg, lower intensity or domiciliary treatment). Outpatient interventions with a physical or neurorehabilitation focus may be beneficial for patients with less severe symptoms, and treatment success can sometimes be achieved in short periods.45,61,70
Other Treatments
Comorbid depression and anxiety as well as pain may be treated pharmacologically. However, treatments directed at symptoms (eg, antitremor medications) are not appropriate. Furthermore, favorable responses to any pharmacological treatment can occur because of positive effects on mood, coexisting disease, or placebo response. Multidisciplinary treatment for severely affected patients has shown promise in single-center studies. Many nonpharmacological interventions have been used in single cases or small case-series, as ancillary treatments for FNDs. These include transcutaneous electrical stimulation, transcranial magnetic stimulation, and therapeutic sedation with propofol.72 The mechanism of action of central and peripheral neuromodulation treatments is uncertain, although this is likely to be mediated by a cognitive-behavioral effect rather than by a modification of cortical excitability.73
Prognosis
Partly because of underrecognition or poorly delivered FND diagnoses and a lack of availability of knowledgeable therapists, the prognosis of FND remains collectively poor, with disability persisting or even worsening over time. Many patients with good understanding and acceptance of the diagnosis continue to have severe symptoms despite treatment. Ongoing or anticipated litigation or disability proceedings may act as conflicts of interest, affecting the likelihood of success in some patients. Consistent negative predictors include long duration of symptoms (with accrual of severe disability and secondary gain) before diagnosis and personality disorders, whereas good outcomes are associated with young age and early diagnosis.72,74 Even in unfavorable scenarios, treatment of comorbidities and limitation of iatrogenic harm are important management strategies, which can be accomplished if the diagnosis is accurately conveyed to the referring physician and the patient’s other health care clinicians. There are currently no established clinical factors or ancillary testing to guide which patients may benefit from which treatment modalities and over what period.
Conclusions
Functional neurological disorders are common and disabling but potentially reversible. A positive or inclusionary diagnosis can be made with a high level of certainty. Treatment includes psychological interventions, such as cognitive-behavioral therapy, behavioral therapy, or psychodynamic therapy and, for functional motor disorder, rehabilitation strategies. While the level of evidence has increased for some of these interventions, further research is needed to determine the dose and duration of various approaches, the value of combination and multidisciplinary therapy, and the appropriate therapeutic modality for each patient. Therapeutic success hinges on diagnostic delivery that validates the patient’s symptoms and disability and allows full understanding and acceptance of the diagnosis by the patient.
Acknowledgments
Funding/Support: This article presents independent research part-funded by the National Institute for Health Research, Maudsley Biomedical Research Centre at the South London, and Maudsley National Health Service Foundation Trust and King’s College London.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Espay is supported by the National Institutes of Health and has received grant support from CleveMed/Great Lakes Neurotechnologies, Davis Phinney Foundation, and the Michael J Fox Foundation; personal compensation as a consultant or scientific advisory board member for Solvay, Abbott, Chelsea Therapeutics, TEVA, Impax, Merz, Lundbeck, and Eli Lilly; honoraria from TEVA, UCB, the American Academy of Neurology, and the Movement Disorders Society; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer. Dr Aybek is supported by a Swiss National Foundation Ambizione Grant (grant PZ00P3–147997). Dr Carson acknowledges submitting paid testimony in court actions on a range of neuropsychiatric topics and receiving payment as editor of Journal Neurology, Neurosurgery, and Psychiatry and royalties from BMJ press and Elsevier for books. Dr Edwards has received research funding from the Medical Research Council, National Institute for Health Research, and the Guarantors of Brain; honoraria from Merz Pharma, TEVA, and UCB; and publishing royalties from Oxford University Press. Dr Goldstein has received research funding from the National Institute of Health Research and publishing royalties from Wiley and Routledge. Dr Hallett serves as Chair of the Medical Advisory Board for and may receive honoraria and funding for travel from the Neurotoxin Institute. He might accrue revenue on US patent #6,780,413 B2 (issued August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US patent #7,407,478 (issued August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the National Institutes of Health (from Brainsway) for licensing of this patent. He is on the Medical Advisory Board of Cala Health. He is on the editorial board of approximately 20 journals, and received royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, and Elsevier. Dr Hallett’s research at the National Institutes of Health is largely supported by the National Institutes of Health Intramural Program. Supplemental research funds have been granted by Merz for treatment studies of focal hand dystonia, Allergan for studies of methods to inject botulinum toxins, Medtronic Inc for a study of deep brain stimulation for dystonia, and Cala Health for studies of a device to suppress tremor. Dr LaFaver has received honoraria from Pfizer, Teva, the American Academy of Neurology, and the Movement Disorders Society and is on the medical advisory board for FND Hope, a patient organization for functional neurological disorders. Dr LaFrance has served on the editorial boards of Epilepsia, Epilepsy & Behavior, Journal of Neuropsychiatry and Clinical Neurosciences and Journal of Neurology, Neurosurgery and Psychiatry; receives editor’s royalties from the publication of Gates and Rowan’s Nonepileptic Seizures, 3rd ed. (Cambridge University Press, 2010) and 4th ed. (2018); author’s royalties for Taking Control of Your Seizures: Workbook and Therapist Guide (Oxford University Press, 2015); has received research support from the National Institutes of Health/National Institute of Neurological Disorders and Stroke(grant 5K23NS45902), Rhode Island Hospital, the American Epilepsy Society, the Epilepsy Foundation, Brown University, the Siravo Foundation, and the Department of Defense (grant W81XWH-17–1-0619); serves on the Epilepsy Foundation Professional Advisory Board; has received honoraria for the American Academy of Neurology Annual Course; has served as a clinic development consultant at University of Colorado Denver, Cleveland Clinic, Spectrum Health, and Emory University; and has provided medicolegal expert testimony. Dr Lang has served as an advisor for AbbVie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Ceregene, Cipla, InteKrin, Lilly, Medtronic, Merck, Novartis, NeuroPhage Pharmaceuticals, Teva, and UCB; received honoraria from Medtronic, Teva, UCB, and AbbVie; received grants from Brain Canada, the Canadian Institutes of Health Research, the Edmond J. Safra Philanthropic Foundation, The Michael J Fox Foundation, the Ontario Brain Institute, National Parkinson Foundation, Parkinson Society Canada, Physicians Services Incorporated, Tourette Syndrome Association, W Garfield Weston Foundation; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry. Dr Nicholson receives research funding from the National Institute for Health Research. Dr Nielsen receives research funding from the National Institute for Health Research. Dr Reuber receives payment as Editor in Chief of Seizure: European Journal of Epilepsy; royalties from Oxford University Press; an unrestricted research grant from UCB; and speaker fees from Eisai, Livanova, and UCB. Dr Voon is a Medical Research Council Senior Fellow (grant MR/P008747/1). Dr Stone receives royalties from UpToDate Inc, BMJ Publications, and Elsevier, and acknowledges paid testimony in court in relation to functional neurologic disorders. He is supported by NHS Scotland Career Fellowship and runs the free website https://www.neurosymptoms.org/ for patients with functional neurologic disorders. Dr Morgante has received honoraria as a Consultant and Advisory Board member from Medtronic, UCB, and Chiesi. She has received honoraria for speaking from UCB Pharma, Medtronic, Zambon, Chiesi, and Abbvie. She serves on the editorial boards of Movement Disorders Clinical Practice and Frontiers in Movement Disorders. She receives royalties from the publication of Disorders of Movement (Springer, 2016).
Disclaimer: The views expressed are those of the author and not necessarily those of the National Health Service, the National Institute of Health Research, or the Department of Health and Human Services.
Contributor Information
Alberto J. Espay, University of Cincinnati Gardner Neuroscience Institute, Gardner Family Center for Parkinson’s Disease and Movement Disorders, Department of Neurology, University of Cincinnati, Cincinnati, Ohio..
Selma Aybek, Department of Neurology, University Hospital Inselspital, Bern, Switzerland.
Alan Carson, Neuropsychiatry, Centre for Clinical Brain Studies, University of Edinburgh, Edinburgh, United Kingdom.
Mark J. Edwards, Motor Control and Movement Disorders Group, Institute of Molecular and Clinical Sciences, St George’s University of London, London, United Kingdom.
Laura H. Goldstein, Department of Psychology, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom.
Mark Hallett, Human Motor Control Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland.
Kathrin LaFaver, Department of Neurology, University of Louisville, Louisville, Kentucky.
W. Curt LaFrance, Jr, Department of Psychiatry, Alpert Medical School of Brown University, Providence, Rhode Island; Department of Neurology, Alpert Medical School of Brown University, Providence, Rhode Island.
Anthony E. Lang, Morton and Gloria Shulman Movement Disorders Centre, Toronto Western Hospital and Edmond J. Safra Program in Parkinson Disease, University of Toronto, Toronto, Ontario, Canada.
Tim Nicholson, Section of Cognitive Neuropsychiatry, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, United Kingdom.
Glenn Nielsen, Motor Control and Movement Disorders Group, Institute of Molecular and Clinical Sciences, St George’s University of London, London, United Kingdom.
Markus Reuber, Academic Neurology Unit, University of Sheffield, Royal Hallamshire Hospital, Sheffield, United Kingdom.
Valerie Voon, Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom.
Jon Stone, Centre for Clinical Brain Sciences, University of Edinburgh, Western General Hospital, Edinburgh, United Kingdom.
Francesca Morgante, Motor Control and Movement Disorders Group, Institute of Molecular and Clinical Sciences, St George’s University of London, London, United Kingdom; Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy.
REFERENCES
- 1.Carson A, Lehn A. Epidemiology. Handb Clin Neurol 2016;139:47–60. [DOI] [PubMed] [Google Scholar]
- 2.Ding JM, Kanaan RA. Conversion disorder: A systematic review of current terminology. Gen Hosp Psychiatry. 2017;45:51–55. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 4.Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med 2016;46(12):2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szaflarski JP, Ficker DM, Cahill WT, Privitera MD. Four-year incidence of psychogenic nonepileptic seizures in adults in hamilton county, OH. Neurology. 2000;55(10):1561–1563. [DOI] [PubMed] [Google Scholar]
- 6.Binzer M, Andersen PM, Kullgren G. Clinical characteristics of patients with motor disability due to conversion disorder: a prospective control group study. J Neurol Neurosurg Psychiatry. 1997;63(1):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010; 112(9):747–751. [DOI] [PubMed] [Google Scholar]
- 8.Cubo E, Hinson VK, Goetz CG, et al. Transcultural comparison of psychogenic movement disorders. Mov Disord 2005;20(10):1343–1345. [DOI] [PubMed] [Google Scholar]
- 9.Stone J, Smyth R, Carson A, et al. Systematic review of misdiagnosis of conversion symptoms and “hysteria”. BMJ 2005;331(7523):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone J, Carson A, Duncan R, et al. Symptoms ‘unexplained by organic disease’ in 1144 new neurology out-patients: how often does the diagnosis change at follow-up? Brain. 2009;132(pt 10):2878–2888. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig L, Pasman JA, Nicholson T, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry. 2018;5(4): 307–320. [DOI] [PubMed] [Google Scholar]
- 12.Gasca-Salas C, Lang AE. Neurologic diagnostic criteria for functional neurologic disorders. Handb Clin Neurol 2016;139:193–212. [DOI] [PubMed] [Google Scholar]
- 13.Espay AJ, Lang AE. Phenotype-specific diagnosis of functional (psychogenic) movement disorders. Curr Neurol Neurosci Rep 2015;15(6):32. [DOI] [PubMed] [Google Scholar]
- 14.Horn D, Galli S, Berney A, Vingerhoets F, Aybek S. Testing head rotation and flexion is useful in functional limb weakness. Mov Disord Clin Pract (Hoboken). 2017;4:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McWhirter L, Stone J, Sandercock P, Whiteley W. Hoover’s sign for the diagnosis of functional weakness: a prospective unblinded cohort study in patients with suspected stroke. J Psychosom Res 2011;71(6):384–386. [DOI] [PubMed] [Google Scholar]
- 16.Daum C, Aybek S. Validity of the “drift without pronation” sign in conversion disorder. BMC Neurol. 2013;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic J. Diagnosis and treatment of psychogenic parkinsonism. J Neurol Neurosurg Psychiatry. 2011;82(12):1300–1303. [DOI] [PubMed] [Google Scholar]
- 18.Ganos C, Edwards MJ, Bhatia KP. The phenomenology of functional (psychogenic) dystonia. Mov Disord Clin Pract (Hoboken). 2014;1 (1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaski D, Bronstein AM, Edwards MJ, Stone J. Cranial functional (psychogenic) movement disorders. Lancet Neurol 2015;14(12):1196–1205. [DOI] [PubMed] [Google Scholar]
- 20.Fasano A, Valadas A, Bhatia KP, et al. Psychogenic facial movement disorders: clinical features and associated conditions. Mov Disord 2012;27(12):1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone J, Vermeulen M. Functional sensory symptoms. Handb Clin Neurol 2016;139:271–281. [DOI] [PubMed] [Google Scholar]
- 22.Dattilo M, Biousse V, Bruce BB, Newman NJ. Functional and simulated visual loss. Handb Clin Neurol 2016;139:329–341. [DOI] [PubMed] [Google Scholar]
- 23.Dieterich M, Staab JP. Functional dizziness: from phobic postural vertigo and chronic subjective dizziness to persistent postural-perceptual dizziness. Curr Opin Neurol 2017;30(1):107–113. [DOI] [PubMed] [Google Scholar]
- 24.Baik JS, Lang AE. Gait abnormalities in psychogenic movement disorders. Mov Disord 2007;22(3):395–399. [DOI] [PubMed] [Google Scholar]
- 25.Skidmore F, Anderson K, Fram D, Weiner W. Psychogenic camptocormia. Mov Disord 2007;22 (13):1974–1975. [DOI] [PubMed] [Google Scholar]
- 26.Laub HN, Dwivedi AK, Revilla FJ, Duker AP, Pecina-Jacob C, Espay AJ. Diagnostic performance of the “huffing and puffing” sign in psychogenic (functional) movement disorders. Mov Disord Clin Pract 2015;2(1):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erro R, Edwards MJ, Bhatia KP, Esposito M, Farmer SF, Cordivari C. Psychogenic axial myoclonus: clinical features and long-term outcome. Parkinsonism Relat Disord 2014;20(6): 596–599. [DOI] [PubMed] [Google Scholar]
- 28.Duffy JR. Functional speech disorders: clinical manifestations, diagnosis, and management. Handb Clin Neurol 2016;139:379–388. [DOI] [PubMed] [Google Scholar]
- 29.Blad H, Lamberts RJ, van Dijk GJ, Thijs RD. Tilt-induced vasovagal syncope and psychogenic pseudosyncope: Overlapping clinical entities. Neurology 2015;85(23):2006–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuber M, Howlett S, Khan A, Grünewald RA. Non-epileptic seizures and other functional neurological symptoms: predisposing, precipitating, and perpetuating factors. Psychosomatics 2007;48(3):230–238. [DOI] [PubMed] [Google Scholar]
- 31.Reuber M, Kurthen M. Consciousness in non-epileptic attack disorder. Behav Neurol 2011; 24(1):95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syed TU, LaFrance WC Jr, Kahriman ES, et al. Can semiology predict psychogenic nonepileptic seizures? a prospective study. Ann Neurol 2011;69(6):997–1004. [DOI] [PubMed] [Google Scholar]
- 33.Reuber M, Pukrop R, Mitchell AJ, Bauer J, Elger CE. Clinical significance of recurrent psychogenic nonepileptic seizure status. J Neurol 2003;250(11):1355–1362. [DOI] [PubMed] [Google Scholar]
- 34.LaFrance WCB Jr, Baker GA, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia 2013;54(11): 2005–2018. [DOI] [PubMed] [Google Scholar]
- 35.Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol 2009;22(4):430–436. [DOI] [PubMed] [Google Scholar]
- 36.Morgante F, Edwards MJ, Espay AJ, Fasano A, Mir P, Martino D; DISMOV-SIN Study Group on Psychogenic Movement Disorders. Diagnostic agreement in patients with psychogenic movement disorders. Mov Disord 2012;27(4):548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briquet P Traité Clinique et Thérapeutique de L’hysterie. Paris, France: J B Ballière; 1859. [Google Scholar]
- 38.Breurer JE, Freud S. Studien über Hysterie. Leipzig, Germany: Franz Deuticke, 1895. [Google Scholar]
- 39.Carson A, Ludwig L, Welch K. Psychologic theories in functional neurologic disorders. Handb Clin Neurol 2016;139:105–120. [DOI] [PubMed] [Google Scholar]
- 40.Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153(1):57–63. [DOI] [PubMed] [Google Scholar]
- 41.Stone J, Carson A, Aditya H, et al. The role of physical injury in motor and sensory conversion symptoms: a systematic and narrative review. J Psychosom Res 2009;66(5):383–390. [DOI] [PubMed] [Google Scholar]
- 42.Edwards MJ, Adams RA, Brown H, Pareés I, Friston KJ. A Bayesian account of ‘hysteria’. Brain. 2012;135(pt 11):3495–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macerollo A, Chen JC, Pareés I, Kassavetis P, Kilner JM, Edwards MJ. Sensory attenuation assessed by sensory evoked potentials in functional movement disorders. PLoS One. 2015;10(6): e0129507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pareés I, Saifee TA, Kassavetis P, et al. Believing is perceiving: mismatch between self-report and actigraphy in psychogenic tremor. Brain. 2012;135(pt 1):117–123. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen G, Buszewicz M, Stevenson F, et al. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J Neurol Neurosurg Psychiatry. 2017;88(6):484–490. [DOI] [PubMed] [Google Scholar]
- 46.Espay AJ, Edwards MJ, Oggioni GD, et al. Tremor retrainment as therapeutic strategy in psychogenic (functional) tremor. Parkinsonism Relat Disord 2014;20(6):647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown RJ, Reuber M. Towards an integrative theory of psychogenic non-epileptic seizures (PNES). Clin Psychol Rev 2016;47:55–70. [DOI] [PubMed] [Google Scholar]
- 48.Stone J, Carson A. Functional neurologic symptoms: assessment and management. Neurol Clin 2011;29(1):1–18, vii. vii. [DOI] [PubMed] [Google Scholar]
- 49.Bakvis P, Roelofs K, Kuyk J, Edelbroek PM, Swinkels WA, Spinhoven P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia 2009; 50(5):1001–1011. [DOI] [PubMed] [Google Scholar]
- 50.Stone J, Gelauff J, Carson A. A “twist in the tale”: altered perception of ankle position in psychogenic dystonia. Mov Disord 2012;27(4): 585–586. [DOI] [PubMed] [Google Scholar]
- 51.Spence SA, Crimlisk HL, Cope H, Ron MA, Grasby PM. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet 2000;355(9211): 1243–1244. [DOI] [PubMed] [Google Scholar]
- 52.Voon V, Brezing C, Gallea C, et al. Emotional stimuli and motor conversion disorder. Brain. 2010; 133(pt 5):1526–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aybek S, Nicholson TR, O’Daly O, Zelaya F, Kanaan RA, David AS. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One. 2015;10(4):e0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Espay AJ, Maloney T, Vannest J, et al. Impaired emotion processing in functional (psychogenic) tremor: A functional magnetic resonance imaging study. Neuroimage Clin 2017;17:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Kruijs SJ, Bodde NM, Vaessen MJ, et al. Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J Neurol Neurosurg Psychiatry. 2012;83(3):239–247. [DOI] [PubMed] [Google Scholar]
- 56.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74(3):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voon V, Cavanna AE, Coburn K, Sampson S, Reeve A, LaFrance WC Jr; on behalf of the American Neuropsychiatric Association Committee for Research. Functional neuroanatomy and neurophysiology of functional neurological disorders (conversion disorder). J Neuropsychiatry Clin Neurosci 2016;28(3):168–190. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein LH, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology. 2010; 74(24):1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.LaFrance WC Jr, Baird GL, Barry JJ, et al. ; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71(9):997–1005. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen G, Stone J, Matthews A, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry. 2015;86(10):1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jordbru AA, Smedstad LM, Klungsøyr O, Martinsen EW. Psychogenic gait disorder: a randomized controlled trial of physical rehabilitation with one-year follow-up. J Rehabil Med 2014;46(2):181–187. [DOI] [PubMed] [Google Scholar]
- 62.Moene FC, Spinhoven P, Hoogduin KAL, van Dyck R. A randomised controlled clinical trial on the additional effect of hypnosis in a comprehensive treatment programme for in-patients with conversion disorder of the motor type. Psychother Psychosom 2002;71(2):66–76. [DOI] [PubMed] [Google Scholar]
- 63.Moene FC, Spinhoven P, Hoogduin KAL, van Dyck R. A randomized controlled clinical trial of a hypnosis-based treatment for patients with conversion disorder, motor type. Int J Clin Exp Hypn 2003;51(1):29–50. [DOI] [PubMed] [Google Scholar]
- 64.Dallocchio C, Tinazzi M, Bombieri F, Arnó N, Erro R. Cognitive behavioural therapy and adjunctive physical activity for functional movement disorders (conversion disorder): a pilot, single-blinded, randomized study. Psychother Psychosom 2016;85(6):381–383. [DOI] [PubMed] [Google Scholar]
- 65.Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology. 2011;77(6):564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hubschmid M, Aybek S, Maccaferri GE, et al. Efficacy of brief interdisciplinary psychotherapeutic intervention for motor conversion disorder and nonepileptic attacks. Gen Hosp Psychiatry. 2015;37(5):448–455. [DOI] [PubMed] [Google Scholar]
- 67.Stone J, Edwards M. Trick or treat? Showing patients with functional (psychogenic) motor symptoms their physical signs. Neurology. 2012;79(3):282–284. [DOI] [PubMed] [Google Scholar]
- 68.Wissel BD, Dwivedi AK, Merola A, et al. Functional neurological disorders in Parkinson disease [published online March 16, 2018]. J Neurol Neurosurg Psychiatry. pii:jnnp-2017-317378. doi: 10.1136/jnnp-2017-317378. [DOI] [PubMed] [Google Scholar]
- 69.Wissel BD, Dwivedi AK, Gaston TE, et al. Which patients with epilepsy are at risk for psychogenic nonepileptic seizures (PNES)? A multicenter case-control study. Epilepsy Behav 2016;61:180–184. [DOI] [PubMed] [Google Scholar]
- 70.Czarnecki K, Thompson JM, Seime R, Geda YE, Duffy JR, Ahlskog JE. Functional movement disorders: successful treatment with a physical therapy rehabilitation protocol. Parkinsonism Relat Disord 2012;18(3):247–251. [DOI] [PubMed] [Google Scholar]
- 71.Nielsen G, Stone J, Edwards MJ. Physiotherapy for functional (psychogenic) motor symptoms: a systematic review. J Psychosom Res 2013;75(2):93–102. [DOI] [PubMed] [Google Scholar]
- 72.Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85(2):220–226. [DOI] [PubMed] [Google Scholar]
- 73.Garcin B, Mesrati F, Hubsch C, et al. Impact of transcranial magnetic stimulation on functional movement disorders: cortical modulation or a behavioral effect? Front Neurol 2017;8:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gelauff J, Stone J. Prognosis of functional neurologic disorders. Handb Clin Neurol 2016;139: 523–541. [DOI] [PubMed] [Google Scholar]


