Abstract
The ongoing pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is one of the most devastating outbreaks witnessed in the last 100 years. The outbreak started in China and spread rapidly to almost every country, culminating in woefully overwhelmed health-care systems in most countries. The only approved diagnostic test to accompany radiographic evaluation is reverse transcription PCR. However, the applicability of this test in diagnosis and surveillance is challenged by a global shortage of reagents and the lack of well-equipped laboratories with specialized staff in several low- and middle-income countries. Loop-mediated isothermal amplification and CRISPR-based diagnostic assays have developed and expected to play a role however, their accuracy is still inferior to the recommended PCR approach. The need for the development of accurate and rapid diagnostic assays became apparent. Immunodiagnostic tests and other molecular approaches were developed and tested. Other recently developed point-of-care molecular tests are expected to be helpful in pandemic management as no particular skills are required from the operator. Fortunately, a number of serological tests have been granted authorization for use under the emergency situation by the US FDA for the diagnosis of SARS-CoV-2. The majority of recently authorized serological tests detect IgG and IgM in blood of infected individuals by on ELISA, chemiluminescence platforms or lateral flow cassettes.
Keywords: Antigen, Coronavirus disease 2019, Isothermal amplification, Nasopharyngeal swab, Real-time RT-PCR, Serology
Introduction
Medically important coronaviruses were identified from the nasal secretions of patients with mild colds for the first time in the 1960s. Generally, coronaviruses are spherical (80–160 nm), pleomorphic, enveloped, single-stranded, positive-sense RNA viruses [1]. Their helical nucleocapsid surrounds a non-segmented genome (27–32 kb) characterized by a 5ʹ-end containing genes important for viral replication and pathogenesis in the host cell [2]. The 3ʹ-end genomic region harbours genes for nucleocapsid and membrane proteins [3]. In 2003, the animal coronavirus severe acute respiratory syndrome coronavirus (SARS-CoV) was described; it crossed the species barrier and resulted in the epidemic of severe acute respiratory syndrome (SARS) in China and nearby countries. Middle East respiratory syndrome coronavirus (MERS-CoV) was described in 2012, when an outbreak took place in the Middle East of a similar disease called Middle East respiratory syndrome (MERS) [4]. A third animal coronavirus to be described, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), originated from the Chinese city of Wuhan and led to the ongoing pandemic of coronavirus disease 2019 (COVID-19) [5]. SARS-CoV-2 seems to have spilled over from the bat population through an intermediate host (most likely the pangolin) [6].
Since the official announcement of this novel coronavirus (known then as 2019-nCoV) in China in December 2019, the global confirmed cases had reached 6 663 304 in more than 190 countries and territories with a total of 392 802 deaths as of 6 June 2020 (10:00 CEST) [7]. Similar to SARS-CoV and MERS-CoV, infections of SARS-CoV-2 predominate in adults and men without a confirmed underlying cause [8]. The majority of SARS-CoV-2 infections are subclinical, whereas symptomatic patients usually present with cough, fever, fatigue and ground-glass appearance of lungs on radiographic imaging [9]. Admission to intensive care units is common because of the bad prognosis in individuals with co-morbidities and in the elderly population [10,11]. The clinical management of SARS-CoV-2 from a physician's perspective is best reviewed elsewhere [12,13].
The laboratory-based approach to the diagnosis of SARS-CoV-2 is real-time reverse transcription PCR (rRT-PCR) approved by the WHO and by the US CDC. Due to the pandemic situation, testing laboratories were overwhelmed and shortage of reagents became a global issue. The introduction of accurate serological tests will undoubtedly facilitate pandemic management, in addition to reducing time, costs and workloads in national laboratories and health-care systems. For accurate diagnosis, rRT-PCR should be accompanied by computed tomography (CT) radioimaging [14]. Indeed, patients were found to develop ground-glass appearance on chest CT before the successful detection of viral RNA. Generally, rRT-PCR results turn positive 4–6 days after lung manifestations become apparent on radiographs [15].
Next-generation sequencing and microarray analyses are not currently applied for diagnosis of SARS-CoV-2; however, these techniques are important research tools for the scrutiny of mutations and virus evolution [16]. The aim of this review is to provide a concise view of the currently known diagnostic tests for SARS-CoV-2. Laboratory staff should be aware of the importance of continually consulting up-to-date laboratory interim guides published by WHO and other regional centres for infectious diseases as information becomes available or more accurate data are obtained regarding the disease and diagnostics.
Considerations before laboratory testing
As of 12 May 2020, the WHO defines a suspected case as ‘A patient with any acute respiratory illness and having been in contact with a confirmed or probable COVID-19 case in the last 14 days before symptom onset’ [17]. However, this definition is not valid in countries where SARS-CoV-2 is spreading widely in a community transmission pattern. In such communities, acquiring the infection may occur by contact with asymptomatic untested cases. Hence, an individual with relevant clinical manifestations not fully explained by other aetiology should be considered as a suspected case [12].
Diagnosis of suspected and asymptomatic cases is of paramount importance in the management and control of outbreaks. When testing is not possible, specimens should be shipped, according to WHO instructions, to the nearest reference laboratory [18]. It should be noted that a negative result does not rule out the infection and the tested person may be still in the first days of infection when viral RNA load in respiratory specimens is still undetectable. Consequently, caution and follow-up testing are recommended for highly suspected individuals. It should be noted that most SARS-CoV-2 tests are currently approved to be implemented only under the current emergency situation and manufacturer-independent evaluation for test performance is mostly lacking. In other words, clinical correlation with patient history and radiographic imaging is necessary to determine the correct infection status.
Diagnostic specimens: types and times
For general procedures for specimen collection, interim guides from the WHO and CDC are the best to consult [18,19]. Notably, different respiratory specimens (from upper and lower parts of the tract) were found to have different detection rates of SARS-CoV-2. Indeed, variations occur between patients and even in the same individual during the course of the illness; the pattern of viral shedding is not fully understood [[20], [21], [22]]. For instance, nasopharyngeal specimens have shown negative results, while viral RNA was detected in sputum specimens from the same patients [23]. Additionally, the accuracy of the test depends on specimen quality and quantity, time of collection during the course of the disease, and the inherent quality of the test kit [15].
Swab specimens are not possible from bedridden patients with severe respiratory involvement or those undergoing mechanical ventilation. Invasive procedures to obtain endotracheal aspirates, sputum and bronchial lavage are possible ways to obtain specimens from such patients. Throat swabs were found to result in false-negative results as viral RNA was recovered from sputum samples of patients after viral load in the throat had decreased to undetectable levels [24,25]. As course of illness progresses, lower respiratory tract specimens become the best choice [22,26]. However, collection of specimens from the lower respiratory tract may increase infection risk to health-care workers owing to aerosol generation during collection procedures [27]. Saliva and nasal wash specimens have been found to be good alternatives, especially when laboratory biosafety measures cannot be met [[28], [29], [30]]. Stool and blood specimens were found to contain SARS-CoV-2 and viral RNA has been successfully detected from such specimens by RT-PCR; however, their diagnostic value has not been studied thoroughly [31]. Lastly, minimum essential medium, sterile phosphate buffer, and 0.9% saline solutions were all found to be alternatives to viral transport media [32].
Specimen processing
Upon receiving the specimen, immediate testing procedures should be performed in a biosafety cabinet of class II or higher. Care should be paid to minimizing aerosol generation during specimen manipulation. In addition to biosafety precautions, the testing operator should be aware of technical factors affecting result accuracy [33]. For nucleic acid amplification assays, the processing kit reagents should be the recommended match with the PCR platform to be used [33]. Moreover, reagent preparation and storage should be in accordance with the manufacturer's instructions. For RNA extraction phase, various commercially available extraction kits (from Qiagen, Roche and bioMérieux) have been validated by the CDC to work properly when manufacturer's instructions are followed strictly [33].
Specimens should quickly be diluted in lysis buffer containing an inactivating agent (guanidinium-based compounds to inactivate virions) and non-denaturing detergent to disrupt the lipid envelopes of respiratory epithelial cells and the virus. Additionally, lysis buffer also prevents viral RNA degradation [34]. When self-enclosed automated systems are used, success of this step is optimally guaranteed. The currently used systems are ID NOW™ (Abbott, Abbott Park, IL, USA), Cobas Liat® (Roche Molecular Systems, Basel, Switzerland) and GeneXpert® (Cepheid, Sunnyvale, CA, USA). Extracted RNA should be stored at or below –70°C if subsequent steps of analysis are to be performed later.
Detection of SARS-CoV-2 nucleic acid
There are two available categories for RNA amplification tests for SARS-CoV-2; rRT-PCR and loop-mediated isothermal amplification (LAMP). For SARS-CoV-2 detection, protocols of rRT-PCR were approved by WHO and the US Food and Drug Administration (FDA), whereas the isothermal amplification assays have not been authorized. For diagnosis of RNA virus infections, RT-PCR is the most common tool due to its accuracy and popularity [35]. Additionally, less common amplification assays other than PCR are also used in the detection of RNA viruses. Nonetheless, the accuracy of nucleic acid amplification tests is ultimately affected by mutations in the sequences targeted by test primers [36].
Real-time RT-PCR
Different qualitative rRT-PCR protocols for SARS-CoV-2 diagnosis were developed by different countries [33,34,[37], [38], [39]]. Two diagnostic rRT-PCR-based assays are accepted for procurement under Emergency Use Listing procedure; Genesig Real-Time PCR Coronavirus (Primerdesign Ltd, Southampton, UK) and Cobas SARS-CoV-2 6800/8800 system (Roche Molecular Systems). The Cobas 6800 SARS-CoV-2 system from Roche was recently evaluated by Roche-independent researchers and found to be reliable for detecting SARS-CoV-2 RNA in nasopharyngeal specimens [40].
The first rRT-PCR protocol developed outside China incorporated primers targeting genes of the envelope protein (E), nucleocapsid (N) and RNA polymerase (RdRp) [39]. The primers of E and RdRp were the most sensitive and were rapidly and widely adopted in Europe [41]. However, RdRp primers were found to cross-react with RNA of SARS-CoV [42]. A point worth mentioning is that reagent design has the most influential role on assay performance, therefore, well-optimized targets are expected to arise from different genomic sequences of SARS-CoV-2.
Currently, numerous primers are designed to target various RNA sequences in six genes of SARS-CoV-2 for diagnostic purposes: ORF1a/b, ORF1b-nsp14 (5′-UTR), RdRp (RNA-dependent RNA polymerase), S (spike protein), E (envelope), N1/N2/N3 (nucleocapsid) and RdRp/Hel (RNA-dependent RNA polymerase/helicase). Of note, a recent study found nucleocapsid N2 and envelope E genes to be the most sensitive singleplex reactions and no significant change in cycle threshold (Ct) was noted when both assays were combined [43]. The recommended rRT-PCR diagnostic panel by CDC includes primers for (i) two specific regions of SARS-CoV-2 nucleocapsid (N) gene and (ii) human RNase P gene (RP) in a one-step qualitative RT-PCR-based detection [33].
Real-time RT-PCR tests have been positive in convalescent patients after earlier negative RT-PCR tests performed in the same laboratory with the same testing kit and same technician [[44], [45], [46], [47]]. False results in rRT-PCR may be attributed to various factors. Variations in viral load kinetics may lead to collecting specimens with less viral load resulting in false-negative results. False-negative results for RT-PCR were also reported in cases of typical CT findings of SARS-CoV-2 infection [48,49]. Indeed, some researchers found the sensitivity of rRT-PCR to be around 70% in SARS-CoV-2 diagnosis [49]. Additionally, studies have indicated that the SARS-CoV-2 genome is undergoing an evolutionary process through mutations and active genetic recombination [50,51]. This observation is expected because RNA viruses lack efficient proofreading machinery to ensure fidelity of RNA replication [52]. Mutations in primer and probe targeted sequences may lead to false-negative results, but can be reduced by targeting two or three sequences within the viral genome.
Isothermal amplification assays
Loop-mediated isothermal amplification is based on the technology of autocycling strand displacement DNA synthesis via special DNA polymerase (Bst) (Fig. 1). In such tests, the positive reaction is detected visually or by simple turbidity measurement [53]. Incorporation of fluorescent intercalating dyes to these tests is possible, allowing for real-time monitoring of the reaction [54]. The technique was further developed to enable RNA detection by reverse transcription with successful application in detection of numerous RNA viruses including H7N9 influenza virus, MERS-CoV, West Nile virus and Zika virus [[55], [56], [57], [58]]. However, these tests are awaiting authorization by the WHO or FDA to be performed even under emergency situations. Successful application of real-time LAMP tests in the SARS-CoV-2 pandemic will positively contribute to pandemic management because this test is rapid (results are available in less than 1 hour) and can be performed using a small instrument at general laboratories in hospitals, at bedsides or in the field. Nonetheless, primer designing for LAMP tests is more difficult than in PCR assays.
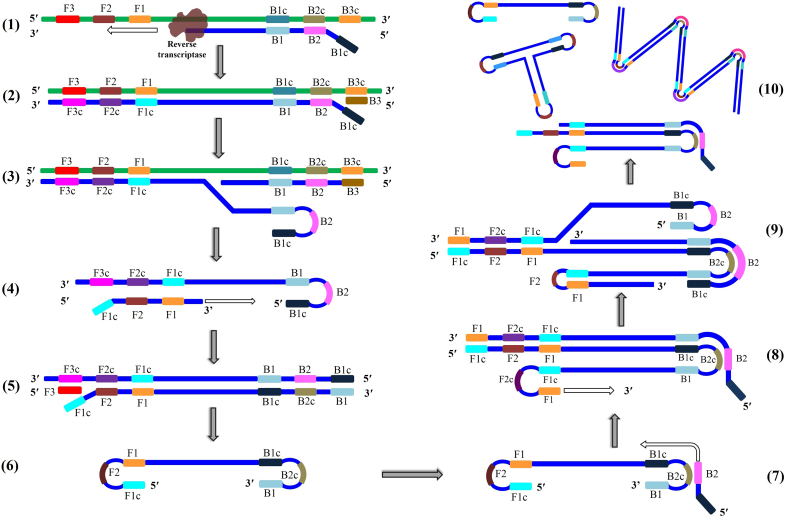
Fig. 1.
Principle of reverse transcription loop-mediated isothermal amplification (RT-LAMP). B2 backwards inner primer (BIP) anneals to the target RNA forming a start point for reverse transcriptase to synthesize a complementary DNA strand (cDNA) (1). The outer primer B3 anneals to a sequence outside that of B2 to initiate synthesis of a new cDNA (2). During synthesis of the second cDNA, the former strand is displaced by the reverse transcriptase (3). The displaced strand is released with a loop at the 5′ end. Forward inner primer F2 anneals to the 3′ end of the newly released cDNA strand and DNA polymerase starts synthesis of a complementary DNA strand (4). Forwards outer primer F3 anneals to the 3′ end of the strand formed by the F2 primer and DNA polymerase initiates the synthesis of new strand and simultaneous displacing of the former (5). The recently displaced cDNA strand contains complementary sequences at both ends, hence it forms a dumbbell-shaped structure (6). This structure is the starting material for loop-mediated amplification cycles. It contains multiple sites for amplification initiation and is rapidly converted to stem-loop DNA structures in response to self-primed synthesis at the 3′ end (7). At the same time, backwards inner primer B2 to the single-stranded loop region to start DNA synthesis culminating in long concatemers (8 and 9). Further synthesis leads to dumbbell-shaped structures and accumulation of amplification dsDNA products (10).
A handful of studies have developed, optimized and tested RT-LAMP assays for the diagnosis of SARS-CoV-2 using primers for different genes [[59], [60], [61], [62], [63], [64]]. Park et al. found primers of the nucleocapsid gene to be the most sensitive, detecting as low as 100 RNA copy/reaction [60]. This level of detection is still considered high for sensitive diagnosis of suspected cases. Indeed, the primer sets of another study targeting Orfl1ab and S genes achieved a detection level as low as 20 copies/reaction [59]. Moreover, high sensitivity and specificity of RT-LAMP were also found comparable to that of PCR [60].
Next-generation assays
Applications of CRISPR-Cas technology in diagnostic microbiology and biomedicine is growing rapidly [65]. Within the past 2 years, researchers invented new diagnostic sensitive assays based on the CRISPR-Cas system for detection of infectious agents with minimal or no equipment to perform the tests [66].
In April 2020, American scientists developed an RT-LAMP-based CRISPR-Cas assay on lateral flow strips to detect the RNA of SARS-CoV-2 in extracts from nasopharyngeal specimens [67]. The assay is performed by dipping the test strip into the RNA extract solution of the treated specimen and after 40 minutes the results are visually read. The Cas12, protein component of CRISPR-Cas, is guided by specially designed associated RNA (gRNA) to base-pair with a specific RNA sequence of SARS-CoV-2. When the Cas12–gRNA complex recognizes the targeted sequence, a labelled single-stranded DNA reporter probe is cleaved by Cas12 to liberate a fluorescent molecule visible to naked eye. However, its current sensitivity is lower than of rRT-PCR. As in nucleic acid amplification assays, CRISPR-based diagnostics are also expected to generate false results if mutations have occurred in the targeted sequences.
Immunodiagnostic approaches
Immunodiagnostic point-of-care tests generating rapid results (in less than 1 hour) are less complex than molecular tests. Based on current evidence, seroconversion for SARS-CoV-2 was found to occurs between 7 and 11 days after onset of symptoms [68]. Consequently, antibody detection assays might be impractical for diagnosis of acute (current) infection at the early stage. Nonetheless, these tests may be useful in epidemiological surveillance (retrospective evaluation), contact tracing and research studies addressing neutralizing antibodies.
At the time of revision of this article, 19 serodiagnostic tests have been granted Emergency Use Authorization from the FDA [69]. Six of these tests are to be performed only in laboratories certified to perform high complexity tests, whereas the remaining panel can be performed in laboratories with requirement of moderate complexity tests. Antibody detecting tests are either run on ELISA, chemiluminescence (Cobas analysers platforms) or rapid-test lateral flow cassettes. On the other hand, a single lateral flow cassette (Sofia 2 SARS antigen FIA® from Quidel, San Diego, CA, USA) was authorized to be used to detect nucleocapsid antigen in nasopharyngeal and nasal swabs. Other antibody-detecting tests exploit recombinant nucleocapsid antigens to detect/quantify anti-SARS-CoV-2 antibodies in blood. However, dozens have been developed and introduced to markets with limited independent validation.
Due to high nucleotide sequence similarity with SARS-CoV, cross-reactivity is expected in such tests and their sensitivity is expected to range from 34% to 80% [[70], [71], [72]]. Some lateral flow rapid test kits have the capacity to detect IgM and IgG or viral antigen, which makes these tests suitable for detecting current and past SARS-CoV-2 infections. Theoretically, such assays will decrease the time, costs and labour of testing in comparison to nucleic acid amplification assays. The majority of available immunodiagnostic tests are based on the principle of immunochromatography (i.e. lateral flow) (Fig. 2). However, sampling variations and variations between individuals in terms of viral load are important factors affecting the accuracy of such assays [70,71].
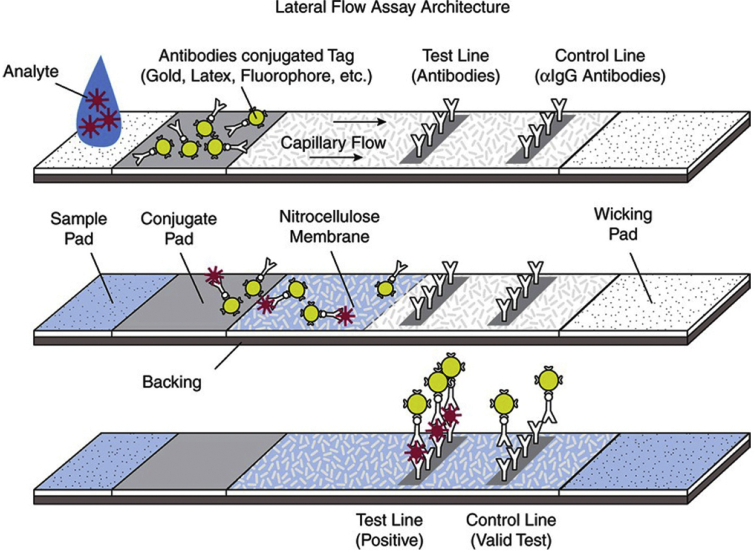
Fig. 2.
Principle of immunochromatography. This drawing shows lateral flow to detect an antigen. The specimen containing the antigen (Analyte) is placed on the sample pad. The antigen, with the fluid, moves to the conjugate pad where it is bound by a labelled antibody specific to the targeted antigen. The conjugate pad also contains labelled antibodies non-specific to the antigen to be detected. Antigen–antibody complexes migrate through the nitrocellulose membrane and reach the ‘Test line’ area. In this area, antigen-specific antibodies are immobilized to catch the antigen–antibody complexes. When antigen–antibody complexes accumulate in this area, the line becomes visible to the naked eye. The non-specific antibodies also migrate and pass the ‘Test line’ to reach the ‘Control line’ area and that line also becomes visible. The test is considered positive only when the two lines (T and C) are visible. (Reproduced from Paulini et al. [82]).
Antigen detection tests
Rapid antigen detection kits are generally characterized by suboptimal sensitivity and specificity [73]. Nonetheless, unique and conserved domains of proteins in SARS-CoV-2 could be exploited to develop sensitive testing kits. Two approaches are expected to increase the sensitivity of such tests: (i) prior treatment to concentrate the targeted antigen and (ii) use of monoclonal antibodies to different epitopes of the antigen to be detected. Two recent pre-print studies reported a sensitivity range of 93%–100% and 100% specificity of the immunochromatography SARS-CoV-2 antigen tests targeting N protein [74,75] (accessed 13 May 2020).
Serological tests
Many commercially available serological kits have been granted Emergency Use Licenses from the FDA to detect antibodies against SARS-CoV-2 [69]. Such tests are mostly based on the principles of immunochromatography, chemiluminescence or ELISA to detect IgG or IgG and IgM in serum. Serodiagnosis is believed to be useful in convalescent patients with negative PCR findings, as the accuracy of molecular assays is influenced by viral shedding dynamics [20]. However, cross-reactivity with other antibodies is a major challenge to serological tests. In fact, the profile of the humoral immune response to SARS-CoV-2 is still partially unknown [76,77]. From the second week of symptoms, IgM levels are detectable by commercially available assays. In the third week after onset of symptoms, the IgM titre peaks and then gradually decreases whereas IgG stabilises around 4 weeks [68,77,78]. It should be noted that interference from other antibodies, mounted against other phylogenetically related viruses, is to be evaluated.
With the exception of immunodeficient individuals, infections of SARS-CoV-2 result in cell-mediated immunity and humoral responses by IgM, IgA and IgG uniformly in all patients. IgM is usually the first class to be produced within the first week of onset followed by robust IgG immunity [77]. IgM and IgG against SARS-CoV-2 were detected by quantitative ELISA and qualitative immunochromatography assays with an increase in detection rates as the course of the illness progressed [79,80]. However, a recent study (of a small sample size and still as a preprint) from Oxford University has found ELISA for IgM and IgG to be more accurate than lateral flow strips with high sensitivity for IgG from day 10 after onset [81]. Another immunodiagnostic test for quantitative measurement of interleukin-6 (Elecsys IL-6 from Roche Diagnostics) has also been granted an Emergency Use Authorization (2 June 2020). This test is helpful in determining the risk of intubation with mechanical ventilation as it measures the level of inflammatory response in individuals with SARS-CoV-2.
Conclusions
The choice of specimen type for SARS-CoV-2 diagnosis is subject to patient condition and stage of the disease course. Specimens from upper respiratory tract are the best choice during the early days of the illness, wheras sputum is the most sensitive at later stages. Owing to occasional false results with rRT-PCR, CT radiography should be obtained to reach an accurate diagnosis and for proper management. As in other RNA viruses, mutations and other genetic changes are likely to occur, which may result in pitfalls of nucleic acid amplification assays. Genomic homology of SARS-CoV-2 with other coronaviruses is also a challenge to serological and antigen detection tests. However, improvements in such point-of-care tests are expected to aid in better management of the pandemic as they are rapid and simple to perform. Epidemiological studies will be easily conducted once accurate serological tests become available.
Funding
This work is a personal effort and did not receive any specific grant from funding agencies in the public, commercial and not-for-profit sectors.
Conflict of interest
The author declares the absence of any potential or actual conflict of interest.
References
- 1.Saif L.J., Wang Q., Vlasova A.N., Jung K., Xiao S. Coronaviruses. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., Zhang J., editors. Diseases in swine. 7th ed. John Wiley & Sons, Ltd; Chichester: 2019. pp. 488–523. [Google Scholar]
- 2.Yoshimoto F.K. The proteins of severe acute respiratory syndrome coronavirus-2 (SARS CoV-2 or n-COV19), the cause of COVID-19. Protein J. 2020;39:198–216. doi: 10.1007/s10930-020-09901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang T, Wu Q, Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . WHO; Geneva: 2020. Coronavirus disease (COVID-19) situation report—138.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200606-covid-19-sitrep-138.pdf?sfvrsn=c8abfb17_4 [Google Scholar]
- 8.Del Rio C., Malani P.N. COVID-19-new insights on a rapidly changing epidemic. JAMA. 2020;323:1339–1340. doi: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- 9.Abduljalil J.M., Abduljalil B.M. Epidemiology, genome, and clinical features of the pandemic SARS-CoV-2: a recent view. New Microbe. New Infect. 2020;35:100672. doi: 10.1016/j.nmni.2020.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 11.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . WHO; Geneva: 2020. Clinical management of COVID-19. [Google Scholar]
- 13.World Health Organization . WHO; Geneva: 2020. Clinical management of severe acute respiratory infection when COVID-19 is suspected (v1.2) pp. 1–21. [Google Scholar]
- 14.Wang Y., Kang H., Liu X., Tong Z. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J Med Virol. 2020;92:538–539. doi: 10.1002/jmv.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B., Si H.-R., Zhu Y., Yang X.-L., Anderson D.E., Shi Z.-L. Discovery of bat coronaviruses through surveillance and probe capture-based next-generation sequencing. MSphere. 2020;5:e00807–e00819. doi: 10.1128/mSphere.00807-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . WHO; Geneva: 2020. Global surveillance for COVID-19 caused by human infection with COVID-19 virus.https://apps.who.int/iris/rest/bitstreams/1272502/retrieve [Google Scholar]
- 18.World Health Organization . 2020. Technical guidance.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance [Google Scholar]
- 19.Centers for Disease Control and Prevention . 2020. Clinical specimens: novel coronavirus (2019-nCoV)https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html [Google Scholar]
- 20.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;1–4 doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020:1–2. doi: 10.1016/S1473-3099(20)30232-2. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winichakoon P., Chaiwarith R., Liwsrisakun C., Salee P., Goonna A., Limsukon A. Negative nasopharyngeal and oropharyngeal swabs do not rule out COVID-19. J Clin Microbiol. 2020;58:1–2. doi: 10.1128/JCM.00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . WHO; Geneva: 2020. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) [Google Scholar]
- 25.Joynt G.M., Wu W.K. Understanding COVID-19: what does viral RNA load really mean? Lancet Infect Dis. 2020:1–2. doi: 10.1016/s1473-3099(20)30237-1. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azzi L., Carcano G., Gianfagna F., Grossi P., Gasperina D.D., Genoni A. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Y.-W., Schmitz J.E., Persing D.H., Stratton C.W. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol. 2020 doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.To K.K.-W., Tsang O.T.-Y., Yip C.C.-Y., Chan K.-H., Wu T.-C., Chan J.M.-C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodino K.G., Espy M.J., Buckwalter S.P., Walchak R.C., Germer J.J., Fernholz E. Evaluation of saline, phosphate buffered saline and minimum essential medium as potential alternatives to viral transport media for SARS-CoV-2 testing. J Clin Microbiol. 2020 doi: 10.1128/JCM.00590-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2020. CDC real-time RT-PCR diagnostic panel. [Google Scholar]
- 34.Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin D.W., Gibson C.J., Lipp E.K., Riley K., Paul J.H., Rose J.B. Detection of viral pathogens by reverse transcriptase PCR and of microbial indicators by standard methods in the canals of the Florida Keys. Appl Environ Microbiol. 1999;65:4118–4125. doi: 10.1128/aem.65.9.4118-4125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bustin S.A., Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155–166. [PMC free article] [PubMed] [Google Scholar]
- 37.Nao N., Shirato K., Katano H., Matsuyama S., Takeda M. 2020. Detection of second case of 2019-nCoV infection in Japan. Tokyo. [Google Scholar]
- 38.Chinese Center for Disease Control and Prevention . Chinese CDC; Beijing: 2020. Emergency response technical centre. Laboratory testing for COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poljak M., Korva M., Knap Gašper N., Fujs Komloš K., Sagadin M., Uršič T. Clinical evaluation of the cobas SARS-CoV-2 test and a diagnostic platform switch during 48 hours in the midst of the COVID-19 pandemic. J Clin Microbiol. 2020 doi: 10.1128/JCM.00599-20. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reusken C.B.E.M., Broberg E.K., Haagmans B., Meijer A., Corman V.M., Papa A. Vol. 25. Eurosurveillance; 2020. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries. January 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan J.F.-W., Yip C.C.-Y., To K.K.-W., Tang T.H.-C., Wong S.C.-Y., Leung K.-H. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waggoner J.J., Stittleburg V., Pond R., Saklawi Y., Sahoo M.K., Babiker A. Triplex real-time RT-PCR for severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis J. 2020;26 doi: 10.3201/eid2607.201285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing Y., Mo P., Xiao Y., Zhao O., Zhang Y., Wang F. vol. 25. Eurosurveillance; 2020. (Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen D., Xu W., Lei Z., Huang Z., Liu J., Gao Z. Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int J Infect Dis. 2020;93:297–299. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Yan K., Ye H., Lin J., Zheng J., Cai T. SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standard for discharge. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong H.Y.F., Lam H.Y.S., Fong A.H.-T., Leung S.T., Chin T.W.-Y., Lo C.S.Y. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiol n.d. 2011:60. doi: 10.1148/radiol.2020201160. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020:200432. doi: 10.1148/radiol.2020200432. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun J., He W.-T., Wang L., Lai A., Ji X., Zhai X. COVID-19: epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol Med. 2020;26:483–495. doi: 10.1016/j.molmed.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81:104260. doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanjuán R., Domingo-Calap P. Mechanisms of viral mutation. Cell Mol Life Sci. 2016;73:4433–4448. doi: 10.1007/s00018-016-2299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Notomi T., Mori Y., Tomita N., Kanda H. Loop-mediated isothermal amplification (LAMP): principle, features, and future prospects. J Microbiol. 2015;53:1–5. doi: 10.1007/s12275-015-4656-9. [DOI] [PubMed] [Google Scholar]
- 54.Oscorbin I.P., Belousova E.A., Zakabunin A.I., Boyarskikh U.A., Filipenko M.L. Comparison of fluorescent intercalating dyes for quantitative loop-mediated isothermal amplification (qLAMP) Biotechniques. 2016;61:20–25. doi: 10.2144/000114432. [DOI] [PubMed] [Google Scholar]
- 55.Huang P., Wang H., Cao Z., Jin H., Chi H., Zhao J. A rapid and specific assay for the detection of MERS-CoV. Front Microbiol. 2018;9:1101. doi: 10.3389/fmicb.2018.01101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chotiwan N., Brewster C.D., Magalhaes T., Weger-Lucarelli J., Duggal N.K., Rückert C. Rapid and specific detection of Asian- and African-lineage Zika viruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aag0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao Z., Wang H., Wang L., Li L., Jin H., Xu C. Visual detection of West Nile Virus using reverse transcription loop-mediated isothermal amplification combined with a vertical flow visualization strip. Front Microbiol. 2016;7:554. doi: 10.3389/fmicb.2016.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ge Y., Wu B., Qi X., Zhao K., Guo X., Zhu Y. Rapid and sensitive detection of novel avian-origin influenza a (H7N9) virus by reverse transcription loop-mediated isothermal amplification combined with a lateral-flow device. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan C., Cui J., Huang L., Du B., Chen L., Xue G. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park G.-S., Ku K., Baek S.-H., Kim S.-J., Kim S Il, Kim B.-T. Development of reverse transcription loop-mediated isothermal amplification assays targeting SARS-CoV-2. J Mol Diagn. 2020 doi: 10.1016/j.jmoldx.2020.03.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu R., Wu X., Wan Z., Li Y., Zuo L., Qin J. Development of a novel reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Virol Sin. 2020 doi: 10.1007/s12250-020-00218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baek Y.H., Um J., Antigua K.J.C., Park J.-H., Kim Y., Oh S. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg Microbe. Infect. 2020;9:998–1007. doi: 10.1080/22221751.2020.1756698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu L., Wu S., Hao X., Dong X., Mao L., Pelechano V. Rapid detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu R., Wu X., Wan Z., Li Y., Jin X., Zhang C. A Novel Reverse transcription loop-mediated isothermal amplification method for rapid detection of SARS-CoV-2. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foss D.V., Hochstrasser M.L., Wilson R.C. Clinical applications of CRISPR-based genome editing and diagnostics. Transfusion. 2019;59:1389–1399. doi: 10.1111/trf.15126. [DOI] [PubMed] [Google Scholar]
- 66.Chiu C. Cutting-edge infectious disease diagnostics with CRISPR. Cell Host Microbe. 2018;23:702–704. doi: 10.1016/j.chom.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J. CRISPR–Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020:1–5. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 69.Food and Drug Administration . 2020. Emergency use authorizations.https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations#covid19ivd [Google Scholar]
- 70.Bruning A.H.L., Leeflang M.M.G., Vos J.M.B.W., Spijker R., de Jong M.D., Wolthers K.C. Rapid tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1026–1032. doi: 10.1093/cid/cix461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Döhla M., Boesecke C., Schulte B., Diegmann C., Sib E., Richter E. Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity. Public Health. 2020;182:170–172. doi: 10.1016/j.puhe.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 doi: 10.1002/jmv.25727. n/a:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merckx J., Wali R., Schiller I., Caya C., Gore G.C., Chartrand C. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med. 2017;167:394–409. doi: 10.7326/M17-0848. [DOI] [PubMed] [Google Scholar]
- 74.Porte L., Legarraga P., Vollrath V., Aguilera X., Munita J.M., Araos R. Evaluation of novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. SSRN Electron J. 2020 doi: 10.2139/ssrn.3569871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diao B., Wen K., Chen J., Liu Y., Yuan Z., Han C. Diagnosis of acute respiratory syndrome coronavirus 2 infection by detection of nucleocapsid protein. MedRxiv. 2020 doi: 10.1101/2020.03.07.20032524. [DOI] [Google Scholar]
- 76.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 78.Xiao A.T., Gao C., Zhang S. Profile of specific antibodies to SARS-CoV-2: the first report. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jääskeläinen A.J., Kekäläinen E., Kallio-Kokko H., Mannonen L., Kortela E., Vapalahti O. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.18.2000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pan Y., Li X., Yang G., Fan J., Tang Y., Zhao J. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adams E.R., Ainsworth M., Anand R., Andersson M.I., Auckland K., Baillie J.K. MedRxiv; 2020. Antibody testing for COVID-19: a report from the national COVID scientific advisory panel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paulini I., Siqueira-Silva J., Thomaz L., Rocha L., Harsi C., Bellei N. Development of a prototype immunochromatographic test for rapid diagnosis of respiratory adenovirus infection. Braz J Infect Dis. 2017;21:500–506. doi: 10.1016/j.bjid.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]