Highlights
-
•
The CURB-65 scores and pneumonia severity index (PSI) are widely used to predict mortality in patients with community-acquired pneumonia.
-
•
There is no scoring system to predict mortality in patients with COVID-19.
-
•
The PSI scores performed significantly better than the CURB-65 scores in predicting 30-day mortality, with a discriminatory ability of 91%.
-
•
Adding CRP levels to PSI scores did not improve their performance, as has also been observed in community-acquired pneumonia.
Keywords: COVID-19, Pneumonia, CURB-65, Pneumonia severity index, Prognosis, Mortality
Abstract
Objective
The aim of the study was to analyze the usefulness of CURB-65 and the pneumonia severity index (PSI) in predicting 30-day mortality in patients with COVID-19, and to identify other factors associated with higher mortality.
Methods
A retrospective study was performed in a pandemic hospital in Istanbul, Turkey, which included 681 laboratory-confirmed patients with COVID-19. Data on characteristics, vital signs, and laboratory parameters were recorded from electronic medical records. Receiver operating characteristic analysis was used to quantify the discriminatory abilities of the prognostic scales. Univariate and multivariate logistic regression analyses were performed to identify other predictors of mortality.
Results
Higher CRP levels were associated with an increased risk for mortality (OR: 1.015, 95% CI: 1.008–1.021; p < 0.001). The PSI performed significantly better than CURB-65 (AUC: 0.91, 95% CI: 0.88–0.93 vs AUC: 0.88, 95% CI: 0.85–0.90; p = 0.01), and the addition of CRP levels to PSI did not improve the performance of PSI in predicting mortality (AUC: 0.91, 95% CI: 0.88–0.93 vs AUC: 0.92, 95% CI: 0.89–0.94; p = 0.29).
Conclusion
In a large group of hospitalized patients with COVID-19, we found that PSI performed better than CURB-65 in predicting mortality. Adding CRP levels to PSI did not improve the 30-day mortality prediction.
Introduction
The novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has become a major health concern worldwide. According to the World Health Organization, as of May 31, 2020, there had been 5 934 936 confirmed cases and 367 166 deaths (WHO, 2020). Respiratory failure is the leading cause of mortality in patients with COVID-19 (Ruan et al., 2020). Myocardial injury, kidney or liver injury, and multi-organ dysfunction are among the other complications leading to death (Yang et al., 2020). Several prognostic factors, such as older age, male gender, presence of comorbidities, and smoking, have been found to be associated with severe disease or death (Zhou et al., 2020, Zheng et al., 2020). In addition, deceased patients are more likely to have had leukocytosis, lymphopenia, higher levels of lactate dehydrogenase, C-reactive protein (CRP) (Yan et al., 2020), interleukin (IL)-6 (Aziz et al., 2020), troponin (Du et al., 2020), and D-Dimer (Zhang et al., 2020), and an elevated neutrophil-to-lymphocyte ratio (Liu et al., 2020c).
Turkey has a comprehensive public healthcare system, with all residents receiving medical treatment free of charge in public and private hospitals during the COVID-19 outbreak. According to the Health Ministry guidelines, any suspected case who is over 50 years old or has any comorbidity should be hospitalized irrespective of vital signs, laboratory results, and computed tomography (CT) findings (Bilim Kurulu, 2020) Thus, a large proportion of patients with COVID-19 meet the criteria for admission as inpatients. This might lead to over-hospitalization, resulting in many problems, such as psychological disturbances, lack of sleep, and accidental falls (Zuk and Zawora, 2003, Hitcho et al., 2004)
CURB-65 and the pneumonia severity index (PSI) are widely used in predicting 30-day mortality in community-acquired pneumonia (Shah et al., 2010). CURB-65 has also been found to be useful in predicting 14-day mortality in hospital-acquired pneumonia (Oktariani et al., 2019). However, these tools have not been assessed in patients with COVID-19. A simple predictive tool for estimating the risk of 30-day mortality, and to stratify patients with COVID-19 as high or low risk for poor outcome at the time of hospital admission, would be useful.
This study aimed to assess whether CURB-65 or the PSI is a useful tool in predicting 30-day mortality, and to identify other factors associated with higher mortality in patients with COVID-19.
Materials and methods
Study design and setting
We performed a retrospective cohort study at Gaziosmanpasa Research and Training Hospital, University of Health Sciences, Istanbul, Turkey. Our hospital has been working as a pandemic hospital since the outbreak began.
Our study was conducted in line with the Declaration of Helsinki. The local institutional ethics committee approved the study protocol (ethics approval number: 59/05.2020) and waived the requirement for written informed consent.
Study population
The first case was reported on March 11, 2020 in Turkey. Management strategies have been revised and updated during the outbreak. With favipiravir treatment becoming a suggested therapeutic option for COVID-19 patients with severe pneumonia on April 2, 2020, we retrospectively enrolled patients who had been diagnosed with COVID-19 pneumonia at our center between April 2, 2020 and May 1, 2020. All patients over 18 years old with COVID-19 confirmed by PCR on nasopharyngeal swab, and who were hospitalized in our hospital, were included in the study. Pregnant patients were excluded.
In line with Health Ministry guidelines, any suspected case who was over 50 years old, or had any comorbidity including cardiopulmonary disease, diabetes mellitus, hypertension, chronic renal disease, immunosuppressive conditions or malignancy, or with tachycardia (pulse >125/min), tachypnea (respiratory rate >22/min), hypotension (<90/60 mmHg), or hypoxemia (Spo2 < 93%) were hospitalized (Bilim Kurulu, 2020).
Severe cases were defined as those with any of the following: (1) respiratory distress (>30 breaths/min), (2) oxygen saturation lower than <90% at rest, or (3) arterial partial pressure of oxygen/fraction of inspired oxygen ≤300 mmHg (Bilim Kurulu, 2020).
Data collection
Demographic characteristics, comorbidities, presenting symptoms, triage vitals (including fever, blood pressure, respiratory rate, oxygen saturation at rest, heart rate), initial laboratory parameters, and time to death were collected from electronic medical records.
Variables
Our primary outcome was 30-day mortality, defined as documented death from any cause during hospitalization or within 30 days of admission to our emergency department. The CURB-65 and PSI scores at hospital admission were calculated as shown in Table 1, Table 2. CURB-65 scores range from 0 to 4. A score of 0–1 indicates a low risk for mortality, whereas scores of 2 or higher are associated with higher mortality (Table 1). PSI scores are classified into groups I, II, III, IV, and V. Patients are stratified into two levels of risk: low risk (groups I–III) and high risk (groups IV–V) (Table 2).
Table 1.
CURB-65 scoring system.
| Clinical feature | Points |
|---|---|
| Confusion | 1 |
| Urea > 7 mmol/L | 1 |
| Respiratory rate ≥ 30 | 1 |
| Systolic blood pressure ≤ 90 mmHg or diastolic blood pressure ≤ 60 mmHg | 1 |
| Age over 65 years | 1 |
| CURB-65 score | Risk |
|---|---|
| 0–1 | Low risk |
| ≥ 2 | Moderate and high risk |
Table 2.
Pneumonia severity index.
| Factor | Score |
|---|---|
| Patient age | |
| Male | Age |
| Female | Age-10 |
| Long-term care facility resident | +10 |
| Accompanying disease | |
| Neoplastic disease | +30 |
| Liver disease | +20 |
| Congestive heart failure | +10 |
| Cerebrovascular disease | +10 |
| Chronic kidney disease | +10 |
| Symptoms at diagnosis | |
| Acute psychosis | +20 |
| Breathing rate ≥ 30/min | +20 |
| Systolic pressure < 90 mmhg | +15 |
| Body temperature < 35 °C or ≥ 40 °C | +15 |
| Heart rate ≥ 125/min | +10 |
| Laboratory measurements | |
| Arterial blood pH < 7.73 | +30 |
| Blood urea nitrogen ≥ 30 mg/dL | +20 |
| Serum sodium < 130 meq/L | +20 |
| Serum glucose > 250 mg/dL | +10 |
| Hemoglobin < 9 mg/dL | +10 |
| Partial pressure of oxygen < 60 mmhg | +10 |
| Pleural effusion | +10 |
| PSI group | PSI score | Risk |
|---|---|---|
| I | Age < 50, none from comorbidities, physical and laboratory findings | Low risk |
| II | ≤70 | |
| III | 71–90 | |
| IV | 91–130 | High risk |
| V | >130 |
Treatment
All hospitalized patients were treated according to the COVID-19 Diagnosis and Treatment Protocol issued by the Turkish Ministry of Health (Bilim Kurulu, 2020). The recommended hydroxychloroquine regimen for all hospitalized patients was a loading dose of 400 mg twice on day 1, followed by 400 mg daily for 4 additional days. In addition, azithromycin at a dose of 500 mg on day 1 and then 250 mg daily for 4 more days was also used cautiously, with QT interval monitoring. Favipiravir was initiated in patients with severe pneumonia or in those with ongoing fever, despite hydroxychloroquine and/or azithromycin treatment, at a loading dose of 1600 mg twice on day 1, followed by 600 mg twice a day for 4 additional days. Tocilizumab was used at a dose of 8 mg/kg in patients with elevated inflammatory markers and ongoing hypoxemia despite favipiravir treatment. In cases of inadequate clinical response, a second dose of tocilizumab was considered within 24–48 h after the initial dose. A prophylactic dose of enoxaparin was initiated in all patients unless there was a contraindication. A therapeutic dose of enoxaparin was used in the following conditions: severe pneumonia, D-dimer level ≥ 1000 ng/mL, body mass index ≥ 40 kg/m2, and acute venous thromboembolism.
Data analysis and statistical methods
We used descriptive statistics to define variables. Categorical data were reported as proportions and counts, and continuous data were presented as medians and interquartile ranges (IQR) unless the data were normally distributed. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of CURB-65 ≥ 2 and PSI ≥ 4 were calculated using the standard two-by-two tables. Univariate and multivariate logistic regression analyses were performed to identify independent predictors of 30-day mortality. Variables that were components of CURB-65 and PSI were not taken into account in multivariate analysis. The discrimination capability of the combination of each prognostic scoring system with other independent factors was evaluated in the receiver-operating-characteristic analysis. The areas under the curves (AUC) of the prediction models were compared using the Delong and Clarke-Pearson approach (DeLong et al., 1988). A p-value < 0.05 was accepted as statistically significant. The analyses were computed using IBM SPSS Statistics 23.
Results
681 patients were included in the study. Mean ± SD age was 56.9 ± 15.7 years, and 49% of the patients were female. 370 patients (54.3%) had at least one comorbidity. The most common comorbidity was hypertension, followed by diabetes mellitus, asthma, chronic obstructive lung disease, ischemic heart disease, hyperlipidemia, chronic renal disease, and congestive heart failure. The most common clinical presentations were fever (32.5%) and respiratory tract symptoms, including cough (71.2%) and dyspnea (27.3%) (Table 3 ).
Table 3.
Comparison of demographic, clinical, and laboratory findings between alive and deceased patients.
| Variable | All patients (n = 681) | Alive patients (n = 626) | Deceased patients (n = 55) | p-Value | ||
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 56.9 ± 15.7 | 56.1 ± 15.8 | 65.8 ± 12.0 | <0.001 | ||
| Female, n (%) | 334 (49) | 312 (49.8) | 22 (40) | 0.2 | ||
| Comorbidities, n (%) | ||||||
| Any comorbidity | 370 (54.3) | 332 (53) | 38 (69.1) | 0.02 | ||
| Hypertension | 234 (34.4) | 206 (32.9) | 28 (50.9) | 0.01 | ||
| Diabetes mellitus | 191 (28) | 168 (26.8) | 23 (41.8) | 0.02 | ||
| Malignity | 9 (1.3) | 6 (1) | 3(5.5) | 0.03 | ||
| COPD | 28 (4.1) | 27 (4.3) | 1 (1.8) | 0.71 | ||
| Asthma | 43 (6.3) | 42 (6.7) | 1 (1.8) | 0.24 | ||
| Ischemic heart disease | 62 (9.1) | 54 (8.6) | 8 (14.5) | 0.14 | ||
| Hyperlipidemia | 34 (5) | 31 (5) | 3 (5.5) | 0.74 | ||
| Chronic renal disease | 24 (3.5) | 20 (3.2) | 4 (7.3) | 0.12 | ||
| Congestive heart failure | 19 (2.8) | 16 (2.6) | 3 (5.5) | 0.19 | ||
| Symptoms, n (%) | ||||||
| Cough | 485 (71.2) | 450 (71.9) | 35 (63.6) | 0.21 | ||
| Fever | 221 (32.5) | 199 (31.8) | 22 (40) | 0.23 | ||
| Dyspnea | 186 (27.3) | 166 (22.5) | 20 (36.4) | 0.11 | ||
| Myalgia | 76 (11.2) | 72 (11.5) | 4 (7.3) | 0.5 | ||
| Nausea and/or diarrhea | 45 (6.6) | 44 (7) | 1 (1.8) | 0.16 | ||
| Headache | 29 (4.3) | 23 (3.7) | 6 (10.9) | 0.02 | ||
| Physical findings, n (%) | ||||||
| Respiratory rate ≥ 30/min | 51 (7.5) | 22 (3.5) | 29 (52.7) | <0.001 | ||
| Partial pressure of oxygen < 60 mmHg | 135 (19.8) | 95 (15.2) | 40 (72.7) | <0.001 | ||
| Heart rate ≥ 125/min | 20 (2.9) | 13 (2.1) | 7 (12.7) | 0.001 | ||
| SBP < 90 mmHg or | 50 (7.3) | 13 (2.1) | 37 (67.3) | <0.001 | ||
| DBP < 60 mmHg | ||||||
| Laboratory findings, median (IQR) | ||||||
| Lymphocyte count (cells/mm3) | 1280 (940–1740) | 1325 (970–1752) | 925 (660–1335) | 0.001 | ||
| Platelet count (103/mm3) | 195 (159–241) | 195 (159–241) | 199 (163–226) | 0.07 | ||
| Neutrophil count (cells/mm3) | 4260 (3140–5860) | 4060 (2990–5590) | 6100 (4780–9614) | <0.001 | ||
| BUN (mg/dl) | 14.7 (11.4–20.9) | 14.7 (11.4–19.5) | 24.7 (13.8–38.0) | <0.001 | ||
| Ferritin (ng/L) | 159.2 (77.7–354.6) | 150.1 (74.9–337.4) | 390.5(177.5–745.4) | 0.02 | ||
| CRP (mg/L) | 34 (11.6–88.3) | 28.8 (10.9–28.8) | 147 (71–210) | <0.001 | ||
| Fibrinogen (g/dL) | 355 (311.5–401) | 349 (309–396) | 399 (349–469) | 0.07 | ||
| D-dimer (ng/mL) | 920 (534.2–1572.5) | 858 (492.5–1385) | 1480 (874–3090) | 0.17 | ||
| Troponin (ng/L) | 4 (2.3–8.1) | 3.8 (2.2–7.1) | 13 (7–53.5) | 0.03 | ||
| Disease status | ||||||
| Non-severe | 546 (80.2) | 531 (84.8) | 15 (27.3) | <0.001 | ||
| Severe | 135 (19.8) | 95 (15.2) | 40 (72.7) | |||
Abbreviations: COPD: chronic obstructive lung disease, SBP: systolic blood pressure, DBP: diastolic blood pressure, BUN: blood urea nitrogen, CRP: C-reactive protein. *Bold values indicate significant p values (<0.05).
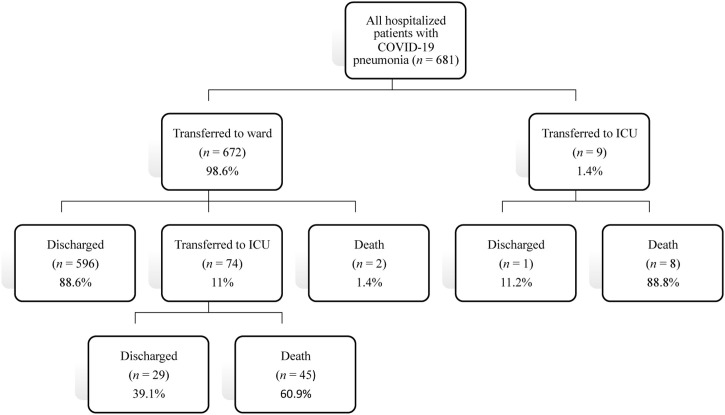
Among the 681 patients hospitalized with COVID-19, 672 patients (98.6%) had been initially transferred to the ward. Of these, 596 (88.6%) were discharged, 74 (11%) were transferred to intensive care unit (ICU), and two died in the ward. Among the 74 patients transferred to ICU, 45 patients died and 29 were discharged. Among the nine patients who were initially transferred to ICU, eight died and one was discharged (Figure 1 ).
Figure 1.
Patient flow chart.
Overall, 55 patients (8%) died within 30 days of admission to the hospital. The median time from admission to death was 9.5 days (IQR: 6–22 days). Deceased patients were older, more hypoxic, tachycardic, tachypneic, and hypotensive at admission. They were more likely to have at least one comorbidity. Regarding laboratory parameters, they had higher neutrophil counts, and higher levels of blood urea nitrogen, ferritin, CRP, and troponin, as well as lower lymphocyte counts (Table 3).
CURB-65
A total of 550 (80.8%) patients had a CURB-65 score of 0 or 1. Of these, 15 patients (2.7%) died within 30 days. 131 patients (19.2%) had a CURB-65 score of ≥2. Of these, 40 patients (30.5%) died within 30 days. A CURB-65 score of ≥2 had a fair discriminatory ability to predict 30-day mortality with a sensitivity of 73%, specificity of 85%, PPV of 31%, and NPV of 97% (AUC: 0.79, 95% CI 72–86; p < 0.001) (Table 4 ).
Table 4.
Discriminative accuracy of CURB-65 and PSI in predicting 30-day mortality.
| Value % (95% CI) | CURB-65a | PSIb |
|---|---|---|
| Sensitivity | 73 (59–83) | 80 (67–90) |
| Specificity | 85 (82–88) | 89 (86–91) |
| PPV | 31 (26–36) | 39 (33–45) |
| NPV | 97 (96–98) | 98 (97–99) |
| AUC | 79 (72–86) | 85 (78–90) |
Abbreviations: CI: confidence interval, PPV: positive predictive value, NPV: negative predictive value, AUC: area under curve.
CURB-65 score 0 or 1 versus 2, 3, or 4.
PSI group I, II, or III versus IV or V.
PSI
182 patients (26.7%) were in group I, 249 (36.6%) were in group II, 136 (20%) in group III, 82 (12%) in group IV, and 31 (4.7%) in group V. There were no deaths among the patients in group I. The mortality rate was 2% in group II, 4.4% in group III, 28% in group IV, and 65.6% in group V. The PSI ≥ 4 group had a good discriminatory ability to predict 30-day mortality, with a sensitivity of 80%, specificity of 89%, PPV of 39%, NPV of 98% (AUC = 0.85, 95% CI 78–90; p < 0.001) (Table 4).
Other independent variables predicting 30-day mortality in COVID-19 pneumonia
The univariate analysis revealed that levels of ferritin, CRP, and troponin, as well as lymphocyte count, were associated with 30-day mortality. After multivariate analysis, only elevated CRP values (OR: 1.015, 95% CI 1.008–1.021; p < 0.001) were significantly associated with 30-day mortality (Table 4).
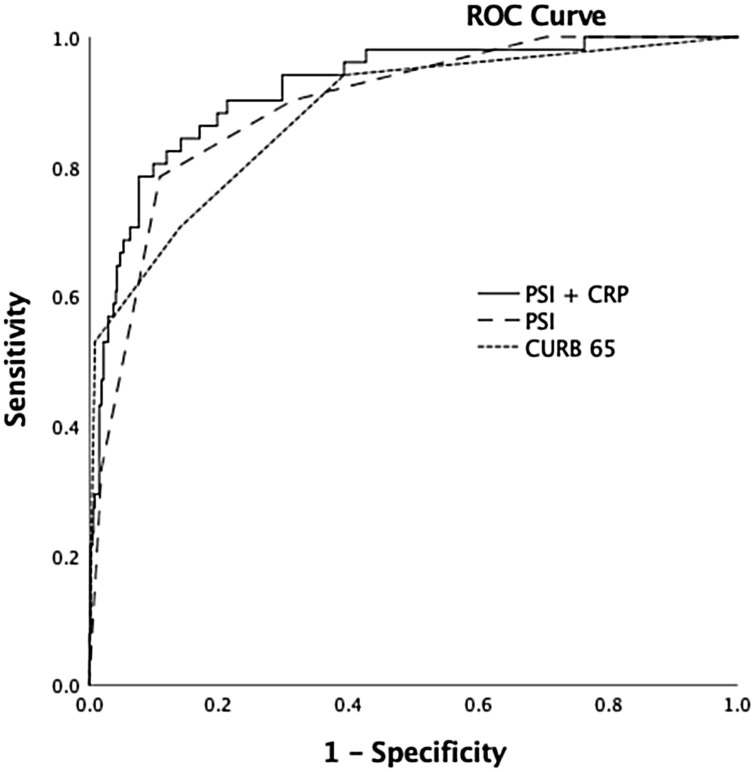
AUCs for the 30-day mortality prediction of CURB-65 alone, PSI alone, and PSI with CRP were 0.88, with 95% CI from 0.85 to 0.90 (p < 0.001), 0.91 with 95% CI from 0.88 to 0.93 (p < 0.001), and 0.92 with 95% CI from 0.89 to 0.94 (p < 0.001), respectively (Figure 2 ). Comparing the AUCs for 30-day mortality prediction of CURB-65 alone, PSI alone, and the model including PSI and CRP levels showed that the two-variable model and PSI alone predicted 30-day mortality significantly better than CURB-65 alone (p = 0.01 and p = 0.04, respectively). However, the discriminatory abilities of PSI and the two-variable model including PSI and CRP were similar (p = 0.29).
Figure 2.
ROC curve for PSI, CURB-65, and PSI with CRP in predicting 30-day mortality.
Discussion
In this study, we assessed the abilities of two prognostic scoring systems to predict 30-day mortality and evaluated independent predictive factors of mortality in a large group of patients with COVID-19. The 30-day mortality rate in our study was 8%. The PSI ≥ 4 group showed better sensitivity (80% vs 73%) and specificity (89% vs 85%), but a similar negative predictive value (98% vs 97%) in predicting death compared with a CURB-65 score of ≥ 2. Only elevated levels of CRP were independently associated with 30-day mortality. The PSI scores alone and the two-variable model including PSI scores and CRP levels performed better than the CURB-65 scores, whereas the PSI scores alone and the two-variable model had similar discriminatory abilities in predicting 30-day mortality.
The mortality rate of COVID-19 has been reported at between 11.7% and 28.2%. (Cao et al., 2020, Giacomelli et al., 2020, Huang et al., 2020, Liu et al., 2020b, Wu et al., 2020, Zhou et al., 2020). This variation in mortality rate may be due to heterogeneity in patient characteristics, treatment strategies, and mortality measures (e.g. in-hospital or 30-day measures). In our study, the mortality rate was somewhat lower than previously reported, although our cohort had similar demographic features and comorbidities to those in these earlier studies (Cao et al., 2020, Giacomelli et al., 2020, Huang et al., 2020, Liu et al., 2020b, Wu et al., 2020, Zhou et al., 2020). The hospitalization criteria in Turkey may be a possible explanation for this finding. As discussed in the introduction above, a considerable number of non-severe patients were hospitalized because of their older age and/or coexisting comorbidities. Thus, our cohort might represent less severe COVID-19 patients. For instance, the proportion of severe cases at admission was 21.1% in our cohort, while in the study by Zhou et al. it was 63%, with a mortality rate of 28% (Zhou et al., 2020). On the other hand, a retrospective study including only non-severe cases at admission showed that 20 (5%) of the 348 patients became severe during hospitalization, and 40% of them received only conventional oxygen therapy (Duan et al., 2020).
There have been ongoing attempts to develop a prognostic scoring system that can predict a poor outcome for patients with COVID-19 (Wynants et al., 2020). CURB-65 scores have been found to be significantly higher in deceased patients with COVID-19 (Zhou et al., 2020). Liu et al. compared the clinical characteristics and outcomes of elderly and young patients with COVID-19 and showed that PSI scores were higher in the elderly compared with young patients (Liu et al., 2020a). As far as we know, ours is the first study to evaluate the performance of CURB-65 and PSI in the prediction of mortality. In our study, in predicting 30-day mortality, CURB-65 scores of ≥ 2 had a sensitivity of 73% and specificity of 85%, while the PSI ≥ 4 group had a sensitivity of 80% and specificity of 89%. When we analyzed the prognostic scoring systems as continuous variables, we found that PSI scores alone predicted mortality significantly better than CURB-65 scores (p = 0.04). Finally, we included CRP levels with PSI scale in order to improve prognostic performance; however, this did not perform better than PSI scores alone. A better discriminatory ability of PSI scale was an expected finding because the PSI scale considers several parameters, such as age, comorbidities, and hypoxemia, that were found to be associated with increased risk of mortality in patients with COVID-19. More surprising was the finding that CRP levels did not add prognostic information beyond PSI scores alone. However, adding CRP to the PSI scale has been shown not to increase the prognostic performance of PSI in hospitalized patients with community-acquired pneumonia (Lee et al., 2011).
Since our first aim was to assess the performance of two prognostic scoring systems and to find additional variables that could improve their performance, we did not include variables that were components of these tools in the multivariate analysis. Non-surviving patients had increased levels of CRP, troponin, and ferritin, lower lymphocyte counts and higher neutrophil counts compared with surviving patients. After multivariate analysis, elevated CRP levels were significantly associated with increased risk of mortality, and this finding was consistent with previous studies. Elevated CRP levels have also been reported to predict progression to severe illness and to correlate with the radiological extent of disease (Duan et al., 2020, Wang, 2020).
Our study had some limitations. First, we did not calculate the prognostic scores prospectively. However, the Turkish hospitals had collected the clinical data in a standard format during the outbreak. Regarding laboratory results, other than for D-dimer levels, there were no missing data because all the laboratory parameters were part of the routine evaluation of all hospitalized patients. Second, among the previously reported risk factors for mortality in COVID-19, our analysis did not take into account potential risk factors such as body mass index, IL-6 levels, or radiological findings.
In conclusion, this single-center retrospective study, including a large cohort of COVID-19 patients, showed that PSI is a powerful tool for predicting mortality in patients with COVID-19. It performed significantly better than CURB-65, while the addition of CRP levels to PSI scale did not improve the performance of PSI. During the outbreak, PSI can help physicians to stratify patients on admission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare no conflicts of interest.
Ethics
This study was conducted in accordance with the Declaration of Helsinki on Ethical Principles and was approved by the ethics committee of Gaziosmanpasa Research and Training Hospital, University of Health Sciences, Istanbul, Turkey (approval number: 59/05.2020).
References
- Aziz M., Fatima R., Assaly R. Elevated interleukin-6 and severe COVID-19: a meta analysis [published online ahead of print, 2020 Apr 28] J Med Virol. 2020 doi: 10.1002/jmv.25948. NLM (Medline) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilim Kurulu Çalışması. 2020. COVID-19 (SARS-CoV-2 enfeksiyonu) Rehberi. TC. Sağlık Bakanlığı Halk Sağlığı Genel Müdürlüğü (2 Nisan 2020) Ankara.https://covid19rehberi.com/wp-content/uploads/2020/04/COVID19_Eriskin_Hasta_Tedavisi_02042020.pdf [Google Scholar]
- Cao J., Tu W.J., Cheng W., Yu L., Liu Y.K., Hu X. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China [published online ahead of print, 2020 Apr 2] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARSCoV- 2: a prospective cohort study. Eur Respir J. 2020;55(May (5)) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J., Wang X., Chi J., Chen H., Bai L., Hu Q. Correlation between the variables collected at admission and progression to severe cases during hospitalization among COVID-19 patients in Chongqing. J Med Virol. 2020;(May) doi: 10.1002/jmv.26082. http://www.ncbi.nlm.nih.gov/pubmed/32470186 [Cited 1 June 2020]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;(May) doi: 10.1016/j.phrs.2020.104931. https://linkinghub.elsevier.com/retrieve/pii/S1043661820312391 [Cited 1 June 2020]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitcho E.B., Krauss M.J., Birge S., Dunagan W.C., Fischer I., Johnson S. Characteristics and circumstances of falls in a hospital setting: a prospective analysis. J Gen Intern Med. 2004;19:732–739. doi: 10.1111/j.1525-1497.2004.30387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(February (10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Kim J., Kim K., Jo Y.H., Rhee J.E., Kim T.Y. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care. 2011;26(June (3)):287–294. doi: 10.1016/j.jcrc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. 2020;80:e14–e18. doi: 10.1016/j.jinf.2020.03.005. W.B. Saunders Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(May (9)):1025–1031. doi: 10.1097/CM9.0000000000000744. http://journals.lww.com/10.1097/CM9.0000000000000744 [Cited 1 June 2020]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktariani, Pitoyo C.W., Singh G., Mansjoer A. CURB 65 score as a predictor of early mortality in hospital-acquired pneumonia. Egypt J Chest Dis Tuberc. 2019;68(2):231. [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B.A., Ahmed W., Dhobi G.N., Shah N.N., Khursheed S.Q., Haq I. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2010;52(1):9–17. [PubMed] [Google Scholar]
- Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50(June (4)):332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2020. Novel coronavirus (2019-nCoV) situation report 132.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200531-covid-19-sitrep-132.pdf?sfvrsn=d9c2eaef_2 [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;(March) doi: 10.1001/jamainternmed.2020.0994. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2763184 [Cited 1 June 2020]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynants L., Van Calster B., Bonten M.M.J., Collins G.S., Debray T.P.A., De Vos M. Prediction models for diagnosis and prognosis of covid-19 infection: Systematic review and critical appraisal. BMJ. 2020;(April):369. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Zhang H.-T., Goncalves J., Xiao Y., Wang M., Guo Y. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2(May (5)):283–288. [Google Scholar]
- Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020;(April) doi: 10.1002/jmv.25891. http://doi.wiley.com/10.1002/jmv.25891 [Cited 1 June 2020]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;S0163-4453(20):30234–30236. doi: 10.1016/j.jinf.2020.04.021. W.B. Saunders Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(March (10229)):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M., Zawora J. Essential psychological problems of hospitalized patients. Ann Univ Mariae Curie Sklodowska Med. 2003;58(2):425–430. [PubMed] [Google Scholar]