Coronavirus disease 2019 (COVID-19) is an acute respiratory syndrome that emerged in the city of Wuhan and rapidly spread throughout the world causing a global pandemic.1 The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been identified as its causal agent.1 Factors such as older age or presence of comorbidities are frequently identified as variables with a negative effect on patients’ prognosis.2 If we focus on the preexistent respiratory conditions, a higher risk of developing a severe infection has been reported in patients with chronic obstructive pulmonary disease.3 However, there is controversial evidence regarding the prevalence of asthma in patients diagnosed as having COVID-19 (eTable 1) or the effect of asthma and its treatment on the clinical evolution of COVID-19.
We report 2 patients with severe asthma on treatment with benralizumab, an antieosinophil monoclonal antibody, who have been affected by COVID-19.
A 56-year-old woman who has been followed at our severe asthma unit for late-onset, severe, eosinophilic asthma with bronchiectasis without criteria for asthma–chronic obstructive lung disease overlap syndrome. Her asthma was controlled with high-dose ICS, long-acting β2-agonist, montelukast, ipratropium, and benralizumab. On March 8, 2020, she went to the emergency department owing to a 24-hour episode of fever, arthralgia, myalgia, dyspnea, and brownish expectoration. On physical examination, no wheezing was found. Complementary tests revealed a unilobar opacity in the right lung, a slightly increased C-reactive Protein and lactate dehydrogenase (Table 1 ), and a positive polymerase chain reaction result for SARS-CoV-2. A dose of levofloxacin 500 mg for 14 days and systemic corticosteroids (1 mg/kg) were administered owing to the brownish expectoration and history of bronchiectasis (lopinavir/ritonavir and hydroxychloroquine were not started according to the hospital’s protocol, at that moment, because the patient did not have hypoxemia). The patient was discharged on the fourth day of admission owing to clinical stability, which was maintained without oral corticosteroids. After 1 week, the patient was asymptomatic. Notably, 4 of her relatives also received a diagnosis of COVID-19.
Table 1.
Laboratory Data Reported at the Emergency Department
| Laboratory data | Patient 1 | Patient 2 |
|---|---|---|
| Neutrophils | N (1.9 × 1000 cell/μL) | N (3.3 × 1000 cell/μL) |
| Lymphocytes | N (1.3 × 1000 cell/μL) | ↓ (1.1 × 1000 cell/μL) |
| Eosinophils | N (0.0 × 1000 cell/μL) | N (0.0 × 1000 cell/μL) |
| Platelets | N (245 × 1000 cell/μL) | N (226 × 1000 cell/μL) |
| Hemoglobin | N (14.4 g/dL) | N (14.8 g/dL) |
| CRP | ↑ (2.83 mg/dL) | ↑ (26.19 mg/dL) |
| ALT | N (25 U/L) | N (28 U/L) |
| AST | ↑ (30 U/L) | N (33 U/L) |
| CK | N (90 U/L) | NA |
| LDH | ↑ (242 U/L) | ↑ (266 U/L) |
| D-dimer | NA | N (367 ng/mL) |
| Ferritin | N (216 ng/mL) | NA |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatinine kinase; CRP, C-reactive protein; N, normal; NA, not available; LDH, lactate dehydrogenase.
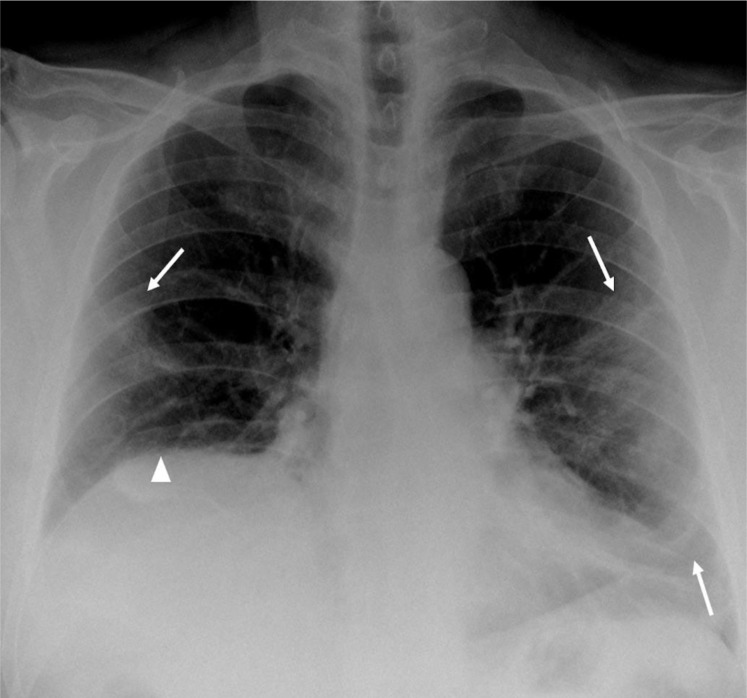
The other case is a 62-year-old man with severe eosinophilic asthma on treatment with benralizumab since July 2018. Previously, he had received treatment with omalizumab and mepolizumab, which were both discontinued because of poor response. As comorbidities, he had moderate obstructive sleep apnoea, chronic rhinosinusitis with nasal polyps, bronchiectasis, and obesity (body mass index of 33 kg/m2). He did not fulfill the criteria of asthma–COPD overlap syndrome. On March 25, 2020, he experienced cough, fever, and darker and thicker expectoration than his usual, therefore he self-medicated with a dose of levofloxacin 500 mg for 3 days. Owing to a lack of improvement in symptoms, he was evaluated at a primary care where a chest X-ray examination was performed, which revealed peripheral and bilateral opacities, more evident in mid/lower lung areas, compatible with COVID-19 pneumonia (Fig 1 ); thus, he was referred to the emergency department. One of his relatives, who lived with him, had the same symptoms. Complementary test results revealed lymphopenia with increased levels of lactate dehydrogenase, C-reactive protein, D-dimers, and fibrinogen (Table 1) and a baseline partial pressure of oxygen of 59 mm Hg. The diagnosis of SARS-CoV-2 pneumonia was assumed considering the epidemic context, symptoms, radiologic and laboratory findings, and following the recommendations of the Spanish authorities at that moment. The patient requested his voluntary discharge. He was placed at home isolation and was monitored by his primary care physician. He was treated with a dose of azithromycin 500 mg (3 days), hydroxychloroquine 200 mg twice a day (5 days), and amoxicillin-clavulanic acid 875/125 mg (7 days). After 1 week, he had no symptoms, and he completed 14 days more of isolation.
Figure 1.
Peripheral parenchymal opacities in the middle and lower areas in both lungs, which is more extensive in the left lung (arrows). Elevation of the right hemidiaphragm (arrowhead).
Owing to the respiratory nature of COVID-19, it could have been reasonable to expect that patients with asthma, especially severe ones, could have a worse prognosis. However, both patients had a good response to the infection, which could provide some support to the recommendations made concerning COVID-19 and asthma that encourage the continuation of maintenance therapies in patients with asthma. For example, the recent 2020 Global Initiative for Asthma report recommends advising patients with asthma to continue taking their prescribed asthma medications.4
In our opinion, the treatment with benralizumab and the rest of the maintenance asthma medications may have had a protective effect on our patients. A recent consensus paper highlights the importance of maintaining asthma control in the context of this pandemic.5 The same publication states that there is no evidence of an impaired immune response to this infection in patients with asthma on treatment with monoclonal antibodies.5
In contrast, a positive effect of ICS in the defense and against SARS-CoV-2 infection cannot be ruled out. Possible mechanisms have already been listed, such as the in vitro inhibition of the replication of SARS-CoV-2 by inhaled ciclesonide6 and budesonide7 and the reduction of expression of angiotensin-converting enzyme 2 receptor in atopic subjects utilized by protein S of the virus.8 In addition, some reports describe the important role of macrophage infiltration in the deterioration of patients with COVID-19.9 ICS, such as budesonide, suppress the synthesis of the granulocyte macrophage-colony stimulating factor.10 The potential advantages of ICS do not apply to systemic corticosteroids; data reveal potential harm with increased time for viral clearance and no evidence of clinical benefit.11
Recent data have revealed that an important proportion of patients with COVID-19 developed eosinopenia during the infection, and it has been suggested that an increase in eosinophils might indicate a clinical improvement in this disease.12 Because benralizumab has a cytotoxic effect on eosinophils mediated by the NK cells, it seems unlikely that an increase in eosinophils could have taken place in our patients, although we do not have the analytical data to confirm this fact. This suggests that the rise in eosinophil levels may not be necessary for a successful COVID-19 recovery.
To the best of our knowledge, this is the first report of patients with severe asthma and biologic treatment who have been affected by COVID-19. It would be necessary to have a higher number of cases and a deeper understanding of this viral infection to the potential relevance of asthma and its treatment with corticosteroids and biologics in the evolution of the infection. Despite these limitations, we believe that our data encourage the continuation of maintenance therapy and biologic treatment of patients with asthma in the context of this pandemic.
Footnotes
Disclosures: Dr. García-Moguel is a consultant, speaker, and researcher promoted by GlaxoSmithKline, AstraZeneca, Teva Pharmaceuticals, Mundipharma, Chiesi Farmaceutici S.p.A, Novartis, and Leti. Dr. Díaz Campos is a consultant, speaker, and researcher promoted by GlaxoSmithKline, AstraZeneca, Teva Pharmaceuticals, and Chiesi. Dr. Fernández Rodríguez is a consultant, speaker, and researcher promoted by Novartis and AstraZeneca. The remaining authors have no conflicts of interest to report.
Funding: The authors have no funding sources to report.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.anai.2020.06.012.
Supplementary Data
eTable 1.
Prevalence of Asthma Among Patients With Coronavirus Disease 2019
| Country | Total patients n | Asthma n (%) | COPD n (%) | Patient characteristics | |
|---|---|---|---|---|---|
| Arentz et al1 | US | 21 | 2 (9.1) | 7 (33.3) | Patients admitted to ICU |
| Bhatraju et al2 | US | 24 | 3 (14) | 1 (4) | Patients admitted to ICU |
| Borobia et al3 | Spain | 2226 (460 deaths and 1766 live discharges) | 115 (5.2) - 3.7% of deaths and 5.5% of live discharges | 153 (6.9) -14.1% of deaths and 5.0% of live discharges | Admitted to hospitalb |
| Garg et al4 | US | 160a | 28 (17.5) | 17 (10.6) | Hospitalized patients |
| Goyal et al5 | US | 393-130 requiring IMV | 49 (12.5) - 13.1% of those requiring IMV | 20 (5.1) - 5.4% of those requiring IMV | Admitted to hospital |
| Guan et al6 | China | 1590 | 0 (0) | 24 (1.5) | Hospitalized patients with laboratory-confirmed COVID-19 |
| Richardson et al7 | US | 5700 | 479 (9) | 287 (5.4) | Admitted to hospital and with confirmed SARS-CoV-2 infection by PCR |
| Zhang et al8 | China | 140 (58 were severe) | 0(0) | 2 (1.4) (both were severe) | Hospitalized patients with laboratory-confirmed COVID-19 |
Abbreviations: COPD, chronic obstructive lung disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IMV, invasive mechanical ventilation; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The full report refers to a higher number of patients, but comorbidities are only reported on these.
Of the total number of patients, 75 were admitted to the ICU, and of those, 4 (5.3%) had asthma.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC COVID-19 Response Team, US Center for Disease Control and Prevention Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12-March 28, 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6913e2.htm Available at: [DOI] [PMC free article] [PubMed]
- 3.Lippi G., Henry B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma Global strategy for asthma management and prevention (pocket guide, 2020 update) https://ginasthma.org/pocket-guide-for-asthma-management-and-prevention/ Available at:
- 5.Shaker M.S., Oppenheimer J., Grayson M. COVID-19: pandemic contingency planning for the allergy and Immunology Clinic. J Allergy Clin Immunol Pract. 2020;8(5):1477–1488.e5. doi: 10.1016/j.jaip.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuyama S., Kawase M., Nao N. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv. 2020;03:987016. doi: 10.1128/JVI.01648-20. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaya M., Nishimura H., Deng X. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58(3):155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson D.J., Busse W.W., Bacharier L.B. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2 [e-pub ahead of print]. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.04.009 accessed April 22, 2020. [DOI] [PMC free article] [PubMed]
- 9.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelaia G., Vatrella A., Busceti M.T. Molecular and cellular mechanisms underlying the therapeutic effects of budesonide in asthma. Pulm Pharmacol Ther. 2016;40:15–21. doi: 10.1016/j.pupt.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(1022 3):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F., Xu A., Zhang Y. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
eReferences
- 1.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borobia A.M., Carcas A.J., Arnalich F., Alvarez-Sala R., Montserrat J., Quintana M. A cohort of patients with COVID-19 in a major teaching hospital in Europe. medRxiv. 2020;04.29:20080853. doi: 10.3390/jcm9061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 states, 2March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Liang W.H., Zhao Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [e-pub ahead of print]. Allergy. https://doi.org/10.1111/all.14238 accessed February 19, 2020. [DOI] [PubMed]