Abstract
Nonvalvular atrial fibrillation (AF) is a relatively frequent arrhythmia in cancer patients; it is possibly due to direct effect of cancer or consequence of cancer therapies. AF creates important problems for both therapeutic management and prognosis in cancer patients. The anticoagulation of cancer patients presenting AF is a main issue because of the difficult balance between thromboembolic and bleeding risks, both elevated in this clinical setting. A comprehensive echo Doppler examination is mandatory to identify the eventual sources of emboli in left atrial (LA) cavity, mainly the transesophageal echocardiography (TEE), but also to predict the subsequent development of heart failure. This evaluation is particularly important to graduate anticoagulation and to prevent and manage symptoms/signs of heart failure. The performance of a TEE precardioversion is highly encouraged to detect possible thrombi in LA appendage. A careful assessment of LA size (LA volume index) and function (LA emptying fraction and/or LA strain) should always be planned to predict the possible recurrence of AF paroxysmal episodes. This is in fact a key action, not only from the cardiologic point of view but also for the oncologic perspectives in individual situations. Patients with larger left atrium and more impaired LA function should be addressed toward a less aggressive cancer treatment, with drugs which are not associated or are poorly related with the risk of AF development. A correct and comprehensive echocardiographic assessment could even induce the oncologist to change the cancer management balancing the oncologic and the cardiac risk.
Keywords: Anticoagulation, atrial fibrillation, cancer, echocardiography, left atrial strain, left atrial volume index
INTRODUCTION
Patients affected by cancer may develop several arrhythmias, including nonvalvular atrial fibrillation (AF).[1,2] Information on the prevalence of AF in this clinical setting is poor. In an epidemiological study enrolling 24,125 patients, 2.4% of patients presented long-lasting, persistent AF at the time of cancer diagnosis and 1.8% developed new-onset AF during cancer therapy.[3] Of interest, cancer patients with AF had a two-fold increased risk of thromboembolic events and a six-fold higher risk of heart failure, even after adjustment for cardiovascular risk factors.[3]
Although persistent AF may be evident before cancer diagnosis (long-lasting AF according to the European Society of Cardiology [ESC] guidelines),[1] various neoplasms may be associated with paroxysmal, persistent, or permanent AF in cancer patients.[4] AF may appear as a direct effect of cancer (extracardiac compression or intracardiac localization), or, more frequently, after surgery, in particular as complication of thoracic (lung and esophageal cancer) or abdominal (colon cancer) surgery.[5] AF may have its onset also during/after cancer therapies (chemotherapy and radiotherapy). This latter condition is underevaluated because information on drug-induced AF substantially derives from isolated reports, and comprehensive data on the real prevalence of cancer drug-induced AF are lacking.[2,5,6]
AF creates important problems for both therapeutic management and prognosis in cancer patients.[3,7,8] The anticoagulation of cancer patients presenting AF is a main problem because of the difficult balance between thromboembolic and bleeding risks. Both these risks are elevated in cancer patients, due to the cancer itself and the effect of cancer therapy. A 2016 ESC position paper on anticancer therapies and cardiovascular toxicity has highlighted the difficulties in the pharmacologic management of cancer patients presenting nonvalvular AF.[2]
Cancer medications may produce nonvalvular AF through various mechanisms. The most common factors include release of proinflammatory substances (cytokines), calcium homeostasis alterations, and direct damage on the myocardium.[9] Anthracyclines reduce the antioxidant effect on cardiomyocytes[5,6] and increase vagal and adrenergic tones, because of hypotension, myocardial ischemia, and electrolyte abnormalities. These mechanisms are also induced by alkylating agents, gemcitabine, 5-fluorouracil, antimetabolites, docetaxel, rituximab, paclitaxel, and alemtuzumab.[9]
MANAGEMENT OF NONVALVULAR ATRIAL FIBRILLATION IN CANCER PATIENTS
Nonvalvular AF in cancer patients needs a multidisciplinary approach. First, it is mandatory to identify patients more prone to AF development, such as those affected by arterial hypertension, diabetes, or coronary artery disease. Patients at elevated risk for AF should be treated by cancer drugs which are known to be less aggressive and poorly associated with AF.
When AF occurs during cancer therapy, the decision whether to continue, adjust the dosage, or withdraw cancer medications is fundamental. Particular attention will be taken to achieve an optimal control of heart rate and to restore normal sinus rhythm with antiarrhythmic drugs, mainly amiodarone. A complete echo Doppler will be performed before starting antiarrhythmic treatment to unmask possible left ventricular (LV) systolic and/or diastolic dysfunction and/or coexisting valvular heart disease.[10] This echocardiographic assessment is also important to determine the pretest probability of sinus rhythm restoration and establish optimal cardiac treatment choice.
THE STEPS OF ECHO ASSESSMENT IN CANCER PATIENTS WITH ATRIAL FIBRILLATION
A comprehensive echo Doppler examination is mandatory in any kind of AF, including cancer patients who present this arrhythmia. The echocardiographic assessment is important to identify the eventual sources of emboli in left atrial (LA) cavity, mainly the transesophageal echocardiography (TEE), but also to predict the subsequent development of heart failure. This evaluation is particularly important in cancer patients undergoing cancer therapy to graduate anticoagulation and to prevent and manage symptoms/signs of heart failure. The main measurements to be quantified in the report include LV mass index, ejection fraction (EF), and, whenever available, global longitudinal strain as parameters of LV systolic function and E velocity deceleration time, E/e' ratio, LA volume index (LAVi), and tricuspid regurgitation velocity as the main parameters of diastolic function. Among these parameters, considering the poor reproducibility of both LV mass and LV-EF measurements,[11,12] E/e' ratio, which has a good interobserver variability,[13] and LAVi [Figure 1], which is the best and direct expression of LA remodeling and reflects the chronic burden of both LA function and pressure,[14] should always be preferred. Despite very promising in this clinical setting,[15,16,17] LA strain [Figure 2] is actually limited to reference echo laboratories by the poor availability of speckle tracking echocardiography software needed for its calculation. Several prospective studies tested the predictive value of different echocardiographic parameters, independently of clinical risk profile, versus the incidence of thromboembolic events.[18,19,20,21,22,23] TEE-determined LA appendage abnormalities, such as presence of thrombi, dense spontaneous LA echo-contrast and low appendage flow velocities, have shown strong predictive values against thromboembolic events at follow-up.[24,25,26]
Figure 1.
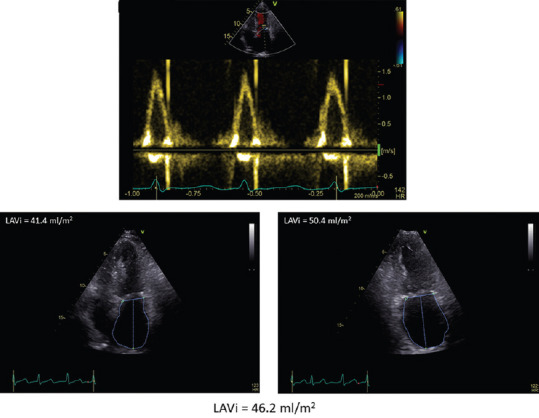
Left atrial dilation in a breast cancer patient with paroxysmal atrial fibrillation. In the top, transmitral pattern showing the absence of A velocity due to atrial fibrillation. In the bottom, left atrial volume in apical four-chamber (left) and two-chamber (right) part. Left atrial volume index is 46.2 ml/m2
Figure 2.
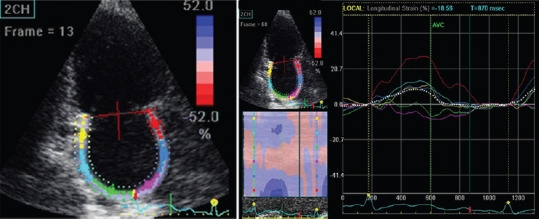
Reduction of left atrial strain in apical two-chamber view in a patient with chronic lymphatic leukemia experiencing paroxysmal atrial fibrillation during ibrutinib therapy
Some echo parameters have been applied to cancer patients experiencing AF. Among these, atrial electromechanical delay has been successfully used to predict AF in 53 patients with breast cancer (48 ± 8 years old) who received 240 mg/m2 of Adriamycin, 2400 mg/m2 of cyclophosphamide, and 960 mg/m2 of paclitaxel.[27] Notably, in this study, left intra-atrial and interatrial electromechanical intervals were prolonged and LV diastolic function was impaired. The authors concluded that impaired LV relaxation and LA electrical conduction could be contributing to the development of AF in this clinical setting.
Most of echocardiographic studies in cancer patients with AF involved the prediction of postsurgery AF. In 134 patients undergoing lobectomy or pneumonectomy for lung cancer, at the multivariate analysis, only LA area enlargement was an independent prognostic factor for postoperative AF.[28] In 126 patients with lung cancer who underwent a lobectomy, the area under the receiver operating characteristic curve for E/e' ratio to predict postoperative AF after pulmonary resection for lung cancer was 0.83 (95% confidence interval: 0.74–0.92; P < 0.001).[29] It is also of interest that in 191 neoplastic patients who had undergone anatomic lung or esophageal resection, the incidence of postoperative AF in the presence of LAVi ≥34 mL/m2 was 37% (11/30) and greater than in those with LAVi <34 mL/m2 (14%, 22/160; P = 0.002).[30] Older age was significantly associated with greater β-blocker use and LA volume and lower LA emptying fraction. On multivariate analysis, lower LA emptying fraction and preoperative use of β-blockers were the only independent risk factors associated with postoperative AF. Combined, these studies demonstrate that echo predictors of LA mechanical dysfunction might have clinical usefulness in stratifying the risk of patients in whom postoperative AF is more likely to develop and would benefit from prevention strategies.
Accordingly, in cancer patients experiencing AF, a careful assessment of LA size and function should always be planned to predict the possible recurrence of AF paroxysmal episodes. Dealing with this issue, the possible role of LA strain, despite its limited availability, should not be underestimated. Of note, LA remodeling as characterized by LA strain and myocardial calibrated integrated backscatter, suggestive of myocardial fibrosis, occurs in adult survivors of childhood cancers, thus demonstrating the capacity in predicting new-onset nonvalvular AF in this clinical setting.[31]
ELECTRICAL CARDIOVERSION IN CANCER PATIENTS WITH ATRIAL FIBRILLATION
According to the ESC guidelines,[32] electrical cardioversion can be considered also in cancer patients with paroxysmal AF. Pretreatment with amiodarone per os during the anticoagulation period can increase this procedure efficacy.[32] In relation with the cancer-related high thromboembolic risk, the performance of a TEE precardioversion is highly encouraged in this clinical setting to detect possible thrombi in LA appendage. The performance of a preelectrical cardioversion TEE is particularly advisable when choosing an anticoagulant with fast onset, e.g., 4 h after a single dose of a new oral anticoagulant drug. This approach has recently been validated for rivaroxaban[33] and is now available also using edoxaban with the same strategy.[34] This approach could be particularly promoted in cancer patients who develop new-onset paroxysmal AF in relation with the use of ibrutinib, an inhibitor of Bruton kinases, recently approved for the treatment of chronic lymphatic leukemia, Waldenstrom's macroglobulinemia, and second-line therapy of mantle cell lymphoma. This drug has been found to be responsible for AF but has also shown to easily increase the bleeding risk, because of a platelet function defect, in three different clinical trials.[35,36,37] Under these circumstances, the adoption of a fast and short anticoagulation could be a winning strategy to restore sinus rhythm, thus allowing to restart cancer therapy with the same drug but, possibly, at a lower dosage.
CONCLUSIONS
The echocardiographic evaluation has a pivotal importance in cancer patients affected from any kind of nonvalvular AF. This is due to several reasons which should correspond to subsequent, well-established items [Table 1], also following European Association of Cardiovascular Imaging (EACVI) standardization of the echo report.[38] First, the echo examination, in particular TEE, is fundamental to identify the possible sources of systemic embolism in a clinical setting, which is very prone to the thrombotic risk. In addition, some echo LA parameters such LA size (mainly LAVi) and function (LA emptying fraction or, better, LA strain) become even more important than in cancer-free AF patients in the prediction of sinus rhythm restoration and/or AF recurrence in patients who have recovered their normal rhythm. This is in fact a key action, not only from the cardiologic point of view but also for the oncologic perspectives in individual situations. Patients with larger left atrium and more impaired LA function should be addressed toward a less aggressive cancer treatment, with drugs which are not associated or are poorly related with the risk of AF development. A correct and comprehensive echocardiographic assessment could even induce the oncologist to change the cancer management balancing the oncologic and the cardiac risk, taking well into account that the thrombotic and the bleeding complications exert an equal burden in this delicate clinical setting.
Table 1.
Main items of the echo Doppler examination in cancer patients with nonvalvular atrial fibrillation
| 1. Search cardiac sources of systemic emboli (use TEE before electrical cardioversion of paroxysmal AF) |
| 2. Measure LV mass and relative wall thickness to provide information on LV geometry |
| 3. Measure LV-EF and, whenever available, GLS to provide information on LV systolic function |
| 4. Measure transmitral E/A ratio, E velocity DT, e’ velocity of mitral annulus, E/e’ ratio, and TR velocity to classify LV diastolic function and degree of LV filling pressures |
| 5. Measure LAVi, LA emptying fraction and, whenever available, LA strain to provide information on LA size and function |
| 6. Search concomitant valvular heart disease |
| 7. Consider all these measurements to address the choice of oncologist (dose adjustment of cancer therapy, temporary interruption, and definitive withdrawn) |
DT=Deceleration time, EF=Ejection fraction, GLS=Global longitudinal strain, LA=Left atrial, LV=Left ventricular, TR=Tricuspid regurgitation, TEE=Transesophageal echocardiography, AF=Atrial fibrillation, LAVi=LA volume index
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zamorano JL. Specific risk of atrial fibrillation and stroke in oncology patients. Eur Heart J. 2016;37:2747–8. doi: 10.1093/eurheartj/ehw385. [DOI] [PubMed] [Google Scholar]
- 2.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: The task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 3.Hu YF, Liu CJ, Chang PM, Tsao HM, Lin YJ, Chang SL, et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165:355–7. doi: 10.1016/j.ijcard.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: Atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–53. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: Chemotherapy-induced arrhythmia. Europace. 2009;11:1579–86. doi: 10.1093/europace/eup300. [DOI] [PubMed] [Google Scholar]
- 6.Tamargo J, Caballero R, Delpón E. Cancer chemotherapy and cardiac arrhythmias: A review. Drug Saf. 2015;38:129–52. doi: 10.1007/s40264-014-0258-4. [DOI] [PubMed] [Google Scholar]
- 7.Lardaro T, Self WH, Barrett TW. Thirty-day mortality in ED patients with new onset atrial fibrillation and actively treated cancer. Am J Emerg Med. 2015;33:1483–8. doi: 10.1016/j.ajem.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tufano A, Galderisi M, Esposito L, Trimarco V, Sorriento D, Gerusalem G, et al. Anticancer drug-related nonvalvular atrial fibrillation: Challenges in management and antithrombotic strategies. Semin Thromb Hemost. 2018;44:388–96. doi: 10.1055/s-0038-1648229. [DOI] [PubMed] [Google Scholar]
- 9.Suter TM, Ewer MS. Cancer drugs and the heart: Importance and management. Eur Heart J. 2013;34:1102–11. doi: 10.1093/eurheartj/ehs181. [DOI] [PubMed] [Google Scholar]
- 10.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–93. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottdiener JS, Livengood SV, Meyer PS, Chase GA. Should echocardiography be performed to assess effects of antihypertensive therapy. Test-retest reliability of echocardiography for measurement of left ventricular mass and function? J Am Coll Cardiol. 1995;25:424–30. doi: 10.1016/0735-1097(94)00375-z. [DOI] [PubMed] [Google Scholar]
- 12.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Kotecha D, Mohamed M, Shantsila E, Popescu BA, Steeds RP. Is echocardiography valid and reproducible in patients with atrial fibrillation? A systematic review. Europace. 2017;19:1427–38. doi: 10.1093/europace/eux027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, et al. Prediction of cardiovascular outcomes with left atrial size: Is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–23. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 15.Her AY, Kim JY, Kim YH, Choi EY, Min PK, Yoon YW, et al. Left atrial strain assessed by speckle tracking imaging is related to new-onset atrial fibrillation after coronary artery bypass grafting. Can J Cardiol. 2013;29:377–83. doi: 10.1016/j.cjca.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Imanishi J, Tanaka H, Sawa T, Motoji Y, Miyoshi T, Mochizuki Y, et al. Left atrial booster-pump function as a predictive parameter for new-onset postoperative atrial fibrillation in patients with severe aortic stenosis. Int J Cardiovasc Imaging. 2014;30:295–304. doi: 10.1007/s10554-013-0346-z. [DOI] [PubMed] [Google Scholar]
- 17.Sade LE, Atar I, Özin B, Yüce D, Müderrisoǧlu H. Determinants of new-onset atrial fibrillation in patients receiving CRT: Mechanistic insights from speckle tracking imaging. JACC Cardiovasc Imaging. 2016;9:99–111. doi: 10.1016/j.jcmg.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Asinger RW, Hart RG, McBride R, Pearce LA, Rothbart RM. Predictors of thromboembolism in atrial fibrillation: Features of patients at risk. Ann Int Med. 1992;116:6–12. [Google Scholar]
- 19.Paciaroni M, Agnelli G, Falocci N, Caso V, Becattini C, Marcheselli S, et al. Prognostic value of trans-thoracic echocardiography in patients with acute stroke and atrial fibrillation: Findings from the RAF study. J Neurol. 2016;263:231–7. doi: 10.1007/s00415-015-7957-3. [DOI] [PubMed] [Google Scholar]
- 20.Buber J, Luria D, Sternik L, Raanani E, Feinberg MS, Goldenberg I, et al. Left atrial contractile function following a successful modified maze procedure at surgery and the risk for subsequent thromboembolic stroke. J Am Coll Cardiol. 2011;58:1614–21. doi: 10.1016/j.jacc.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 21.Ezekowitz M, Laupacis A, Boysen G. Echocardiographic predictors of stroke in patients with atrial fibrillation: A prospective study of 1066 patients from 3 clinical trials. Arch Intern Med. 1998;158:1316–20. doi: 10.1001/archinte.158.12.1316. [DOI] [PubMed] [Google Scholar]
- 22.Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026–33. doi: 10.1016/j.jacc.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Gupta DK, Giugliano RP, Ruff CT, Claggett B, Murphy S, Antman E, et al. The prognostic significance of cardiac structure and Function in atrial fibrillation: The ENGAGE AF-TIMI 48 echocardiographic substudy. J Am Soc Echocardiogr. 2016;29:537–44. doi: 10.1016/j.echo.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke prevention in atrial fibrillation III investigators. J Am Coll Cardiol. 1998;31:1622–6. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 25.Leung DY, Black IW, Cranney GB, Hopkins AP, Walsh WF. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1994;24:755–62. doi: 10.1016/0735-1097(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 26.Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The stroke prevention in atrial fibrillation investigators committee on echocardiography. Ann Intern Med. 1998;128:639–47. doi: 10.7326/0003-4819-128-8-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 27.Yaylali YT, Saricopur A, Yurtdas M, Senol H, Gokoz-Dogu G. Atrial function in patients with breast cancer after treatment with anthracyclines. Arq Bras Cardiol. 2016;107:411–9. doi: 10.5935/abc.20160146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anile M, Telha V, Diso D, De Giacomo T, Sciomer S, Rendina EA, et al. Left atrial size predicts the onset of atrial fibrillation after major pulmonary resections. Eur J Cardiothorac Surg. 2012;41:1094–7. doi: 10.1093/ejcts/ezr174. [DOI] [PubMed] [Google Scholar]
- 29.Nojiri T, Maeda H, Takeuchi Y, Funakoshi Y, Maekura R, Yamamoto K, et al. Predictive value of preoperative tissue Doppler echocardiographic analysis for postoperative atrial fibrillation after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2010;140:764–8. doi: 10.1016/j.jtcvs.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 30.Raman T, Roistacher N, Liu J, Zhang H, Shi W, Thaler HT, et al. Preoperative left atrial dysfunction and risk of postoperative atrial fibrillation complicating thoracic surgery. J Thorac Cardiovasc Surg. 2012;143:482–7. doi: 10.1016/j.jtcvs.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 31.Li VW, Lai CT, Liu AP, Cheuk DK, Cheung YF. Left atrial mechanics and integrated calibrated backscatter in anthracycline-treated long-term survivors of childhood cancers. Ultrasound Med Biol. 2017;43:1897–905. doi: 10.1016/j.ultrasmedbio.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 33.Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY, et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014;35:3346–55. doi: 10.1093/eurheartj/ehu367. [DOI] [PubMed] [Google Scholar]
- 34.Lip GY, Merino J, Ezekowitz M, Ellenbogen K, Zamoryakhin D, Lanz H, et al. Aprospective evaluation of edoxaban compared to warfarin in subjects undergoing cardioversion of atrial fibrillation: The edoxabaN vs. warfarin in subjects UndeRgoing cardiovErsion of atrial fibrillation (ENSURE-AF) study. Am Heart J. 2015;169:597–604. doi: 10.1016/j.ahj.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 35.McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-akt signaling. Blood. 2014;124:3829–30. doi: 10.1182/blood-2014-10-604272. [DOI] [PubMed] [Google Scholar]
- 36.Gustine JN, Meid K, Dubeau TE, Treon SP, Castillo JJ. Atrial fibrillation associated with ibrutinib in Waldenström macroglobulinemia. Am J Hematol. 2016;91:E312–3. doi: 10.1002/ajh.24366. [DOI] [PubMed] [Google Scholar]
- 37.Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): A randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–11. doi: 10.1016/S1470-2045(15)00465-9. [DOI] [PubMed] [Google Scholar]
- 38.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18:1301–10. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]


