Abstract
Background:
Underlying blood pressure is that observed in the absence of antihypertensive treatment or, among those treated, the estimate of that which would be observed without treatment. This study aims to examine the relationships between diabetes or obesity and underlying systolic blood pressure adjusted for antihypertensive treatment by several methods.
Methods:
Data from two population studies were analyzed– an American Indian community in Arizona and the National Health and Nutrition Examination Surveys. Antihypertensive treatment was accounted for using: no adjustment; antihypertensive use as a covariate; blood pressure dichotomized into normotension and hypertension; addition of a fixed treatment effect; non-parametric algorithm; and censored normal regression.
Results:
The magnitude of association at each time point differed by adjustment method particularly where there was a difference in prevalence of antihypertensive use between people with and without diabetes or obesity. The common methods of ignoring antihypertensive treatment or including it as a covariate in a regression model underestimated the effects of diabetes and obesity on underlying blood pressure, compared to the recommended method of the censored normal regression.
Conclusion:
Proper accounting for antihypertensive treatment is needed in interpreting variables that affect blood pressure.
Keywords: antihypertensive treatment, blood pressure, diabetes, obesity
1. BACKGROUND
Hypertension is a global health challenge estimated to affect approximately 1.56 billion people worldwide by 2025 [1]. Epidemiological studies investigating the relationship between diabetes and obesity with elevated blood pressure require careful adjustment for variables that confound this relationship. Antihypertensive treatment is often included as a covariate in statistical models in these studies, but this approach is flawed in part because it equates the observed (measured) blood pressure with the underlying blood pressure—the blood pressure that would be observed if the person was not receiving antihypertensive treatment. Furthermore, since both antihypertensive treatment and the observed blood pressure are influenced by the underlying blood pressure, which may itself be influenced by the presence of diabetes and obesity, antihypertensive treatment is not a true confounder, and traditional analytical approaches that treat it as a confounder will reduce power and are also likely to increase bias. Tobin et al [2] illustrated the loss of power and excessive bias that occurred when inappropriate adjustments for antihypertensive treatment were applied to a cross-sectional examination of the relationship between the APOE gene and systolic blood pressure, and they proposed several more appropriate analytical approaches to address this issue. How these various adjustment methods perform in different settings, including longitudinal studies, is not known. Thus, the aim of this study was to compare commonly used and more novel adjustment methods for antihypertensive treatment effect in the relationship between diabetes or obesity and systolic blood pressure in two different populations each spanning approximately 15 years. The primary purpose of this study was to demonstrate the importance of properly accounting for antihypertensive use in analyses of blood pressure and its determinants, and to highlight appropriate existing statistical methods to do so, which are currently underutilized such analyses.
2. METHODS
2.1. American Indian community study
A longitudinal study of diabetes in an American Indian community in the Southwestern U.S. began in 1965. Subjects aged five years and over were examined approximately every two years until 2007. The examination included a medical history, physical examination, and review of outpatient and inpatient medical records of the sole medical care provider located in the community [3]. The current study included participants aged 20 years and over who were examined between 1993 and 2007, when detailed information on medication use was recorded. The study was divided into three five-year periods: 1993–1997; 1998–2002; and 2003–2007. Participants were restricted to contributing data from only one research examination for each five-year period. For participants with more than 1 examination in a particular five-year period, the first examination was chosen. Each participant could contribute to more than one five-year period. This study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). All participants provided written consent.
At each examination, height and weight were measured with the subject wearing light clothing and no shoes, and blood pressure was measured to the nearest 2 mmHg with subjects resting in the supine position. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m)2 and obesity was defined as BMI ≥30 kg/m2. Venous blood was obtained two hours following oral ingestion of 75 gm of carbohydrate (Glucola, Ames Co., Elkhart, IN, or Dexcola, Custom Laboratories, Baltimore, MD). Diabetes diagnoses were based on a two-hour post-load plasma glucose concentration of ≥200 milligrams per deciliter (mg/dl) [4]. Information on current medication use was collected using an interviewer-administered questionnaire.
2.2. National Health and Examination Survey
The National Health and Examination Survey (NHANES) is a series of cross-sectional surveys designed to evaluate the health and nutritional status of noninstitutionalized adults and children in the United States. Since 1999, the survey became a continuous program which examines a nationally representative sample of approximately 5,000 persons each year. Each of the surveys followed a stratified multistage probability design in which a sample of the US population was selected [5, 6]. Beginning in 2005, an oral glucose tolerance test (OGTT) was added to the protocol for participants who attended the morning examination sessions. The current study included adults aged 20 years and over who were surveyed between 2005 and 2011 and had an OGTT.
Weight and height were measured using a standard protocol and BMI was calculated as weight (kg) divided by the square of height (m)2. Obesity was defined as BMI ≥30 kg/m2. At each survey, blood pressure was measured after 5 minutes of resting with participants in a seated position. Procedures for blood collection and processing have been described [7]. Diabetes was defined as two-hour post-load plasma glucose concentration of ≥200 mg/dl [4]. Information on current medication use was collected using an interviewer-administered questionnaire.
2.3. Statistical analysis
Age-adjusted mean systolic blood pressure was calculated using a mixed model with individuals as a random effect. Six previously described methods for adjusting for antihypertensive treatment effect were included in this study [2]: (i) assuming underlying and observed blood pressure are equal thus making no adjustment for potential treatment effect; (ii) treatment included as a covariate in a linear regression model; (iii) blood pressure dichotomized into normotension and hypertension, defined as systolic blood pressure ≥140 mmHg or use of antihypertensive treatment; (iv) adding a fixed treatment effect of 15 mmHg to the observed blood pressure of treated individuals based on previous recommendations [2, 8]; (v) non-parametric algorithm; and (vi) censored normal regression. For methods (i), (ii) and (iv), linear regression was used to calculate the beta coefficients and standard error (SE) for the relationship between diabetes and systolic blood pressure in each five-year period, with the beta coefficient representing the estimated difference in underlying systolic blood pressure (mmHg) between those with and without diabetes. For method (iii), logistic regression was used to calculate the odds ratio and 95% confidence interval for the relationship between diabetes and hypertension. The odds ratio indicates the odds of hypertension in those with diabetes compared to those without diabetes. For method (v) the nonparametric method of Levy et al was used [9]; the residuals of all observed values, based on the sample mean, were from a ‘null’ regression, and the adjusted value for each treated individual was calculated as the mean of the individual’s residual and that of all higher residuals in the sample. The adjusted values were then analyzed in a linear regression model. For method (vi), censored normal regression, a modified form of the model proposed by James Tobin [10], was used where observations for treated individuals were considered right censored; thus, the underlying blood pressure is estimated by integration over all values higher than the observed value, assuming a normal distribution of underlying blood pressure. The multiple regression models were adjusted for age. Because of significant effect modification by sex identified by an interaction between sex and diabetes, all analyses were stratified by sex. To test whether the relationship between diabetes or obesity and systolic blood pressure was modified by time, an interaction term was included between diabetes and time, and between obesity and time.
To account for the complex probability sampling design of NHANES, all linear and logistic regression analyses using NHANES data were weighted using the OGTT sample weights and adjusted for stratification and clustering. The censored normal regression analysis was weighted, but we were unable to adjust for stratification and clustering. This is expected to affect our variance estimates but not the beta coefficients.
2.4. Sensitivity analyses
A sensitivity analysis was performed excluding those with macroalbuminuria (urinary albumin:creatinine ratio ≥300 mg/g), to test whether more complicated diabetes may explain some of the changes over time in the relationship between diabetes and systolic blood pressure.
To test the robustness of our assumptions, sensitivity analyses were performed for methods (iii) and (iv). For (iii), the threshold at which hypertension was defined was varied to 130 mmHg or use of antihypertensive treatment, and for (iv), the fixed treatment effect was varied to 5 mmHg and 10 mmHg, as previously described [2, 11, 12]. To check whether including those with impaired glucose tolerance in the reference group influenced the results, a sensitivity analysis was performed where those with diabetes were compared to those with normal glucose tolerance, defined as two-hour post-load glucose <140 mg/dl.
A sensitivity analysis was also performed to test for the effect of some individuals appearing in multiple five-year periods in the American Indian study, by restricting each individual to one five-year period only, which was chosen at random. This was not done for the analysis using NHANES data as each NHANES survey was independently sampled and while it is possible that a small number of people participated in more than one survey, we are unable to determine who these persons are.
3. RESULTS
Table 1 presents the characteristics of the two study populations according to year and sex. Over the three five-year periods in the American Indian study, 2,881 participants contributed to one five-year period, 1,416 contributed to two five-year periods, and 1,072 contributed to all three five-year periods. A total of 3,687 individuals were analyzed for NHANES (range 761 to 1,053 per survey). The American Indian study population was younger and had much higher prevalences of diabetes and obesity than the NHANES survey populations. The mean age-adjusted observed systolic blood pressures according to sex and diabetes status for each study is presented in Appendix A.
Table 1.
Characteristics of the Study Populations According to Sex.
| American Indian study | National Health and Nutrition Examination Survey | ||||||
|---|---|---|---|---|---|---|---|
| 1993–1997 | 1998–2002 | 2003–2007 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | |
| Men | n=1,035 | n=1,019 | n=1,432 | n=360 | n=448 | n=444 | n=402 |
| Age (years) | 36 (28–48) | 35 (27–44) | 36 (27–45) | 53 (43–66) | 52 (41–63) | 54 (40–66) | 55 (42–63) |
| Diabetes | 33.0 | 29.9 | 33.9 | 8.7 | 8.3 | 7.3 | 6.3 |
| Obesity | 61.6 | 69.0 | 66.1 | 36.0 | 29.6 | 41.7 | 36.9 |
| Antihypertensive use | 14.5 | 17.1 | 22.1 | 48.5 | 46.0 | 47.0 | 50.0 |
| Women | n=1,672 | n=1,631 | n=2,140 | n=401 | n=524 | n=609 | n=499 |
| Age (years) | 36 (28–48) | 36 (27–44) | 37 (27–47) | 50 (37–64) | 50 (38–64) | 52 (38–64) | 51 (37–64) |
| Diabetes | 39.7 | 37.6 | 42.9 | 7.6 | 5.1 | 8.9 | 6.4 |
| Obesity | 73.4 | 78.7 | 77.8 | 36.0 | 32.7 | 36.3 | 31.4 |
| Antihypertensive use | 12.4 | 18.3 | 28.4 | 37.4 | 38.5 | 40.2 | 41.4 |
Data presented as median (interquartile range) or %.
Obesity defined as body mass index ≥30 kg/m2.
The estimated differences in underlying blood pressure between individuals with and without diabetes, adjusted for age and with various adjustments for the effect of antihypertensive treatment on systolic blood pressure, are presented in Figures 1 and 2. The estimated differences in underlying blood pressure between obese and non-obese individuals are presented in Figures 3 and 4. Positive beta coefficients indicate higher blood pressure in those with diabetes compared to those without diabetes, or in those who were obese compared to those non-obese. The effect sizes are tabulated in the appendix.
Figure 1.
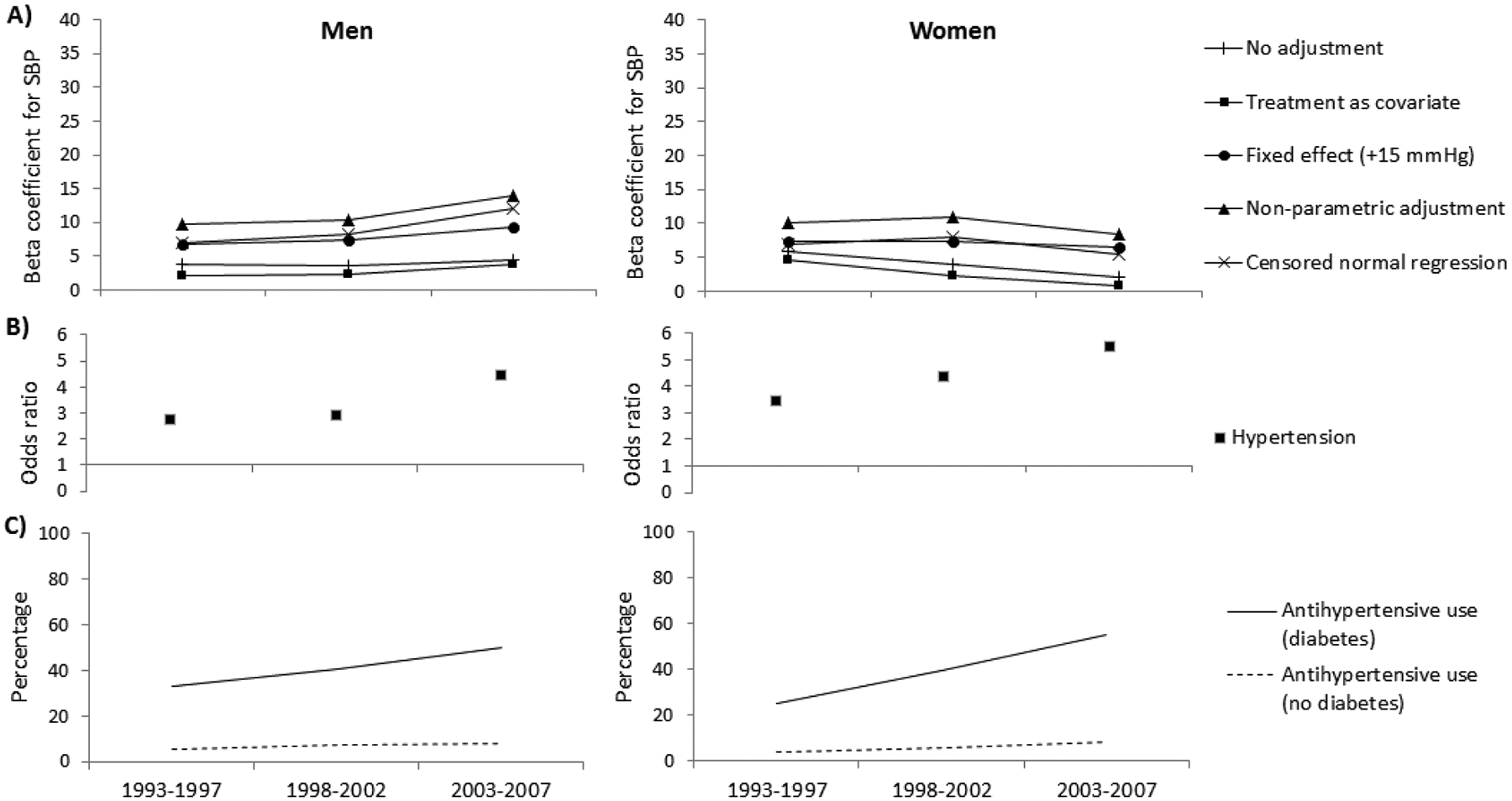
(A and B) The relationship between systolic blood pressure (SBP) or hypertension and diabetes, adjusted for age and using various adjustment methods for antihypertensive use, and (C) the percentage using antihypertensive therapy in the American Indian study. The beta coefficients represent the difference in SBP (mmHg) between persons with and without diabetes. The odds ratios represent the odds for hypertension in persons with diabetes compared to those without diabetes.
Figure 2.
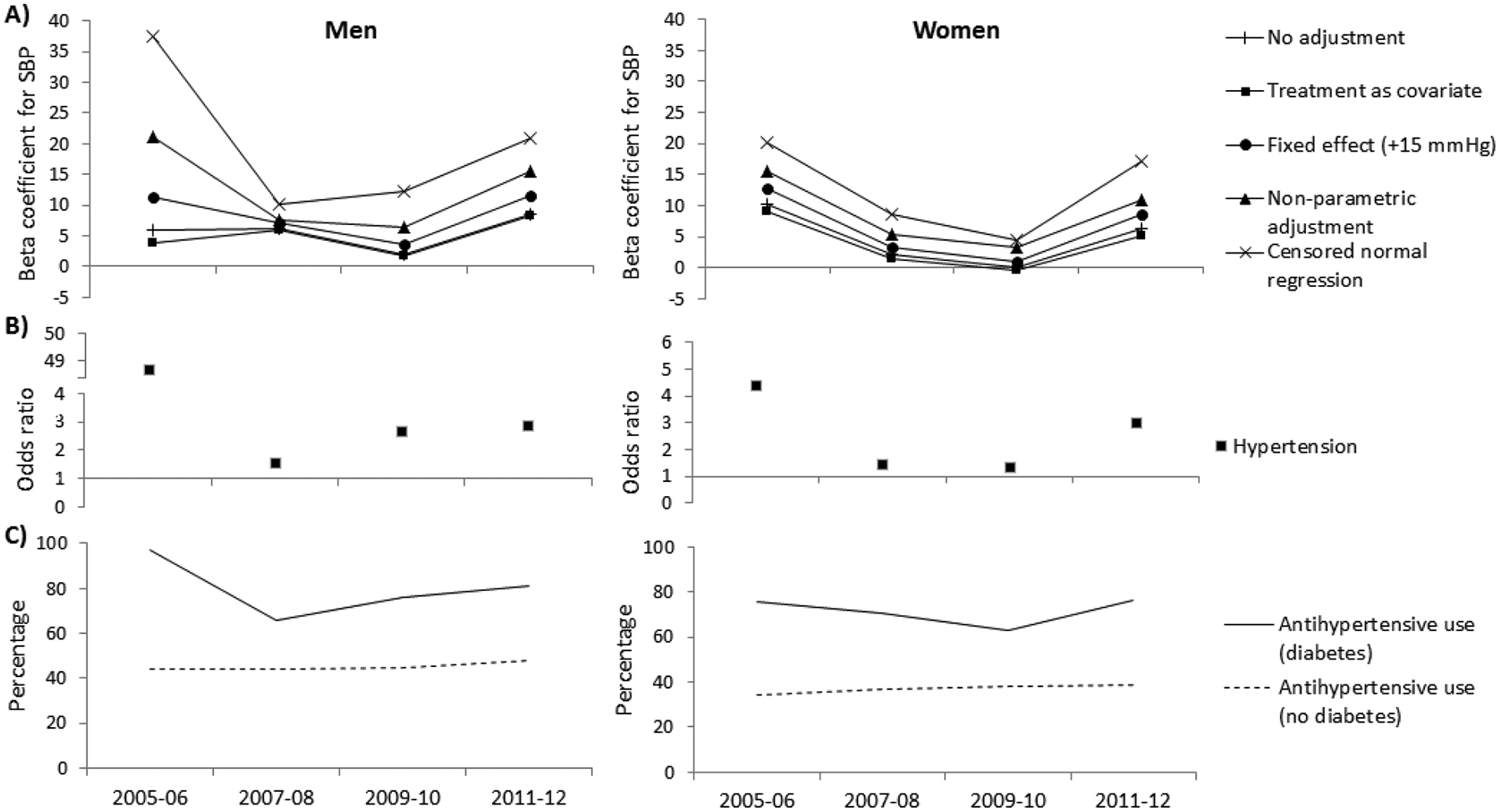
(A and B) The relationship between systolic blood pressure (SBP) or hypertension and diabetes, adjusted for age and using various adjustment methods for antihypertensive use, and (C) the percentage using antihypertensive therapy in the National Health and Nutrition Examination Survey. The beta coefficients represent the difference in SBP (mmHg) between persons with and without diabetes. The odds ratios represent the odds for hypertension in persons with diabetes compared to those without diabetes.
Figure 3.
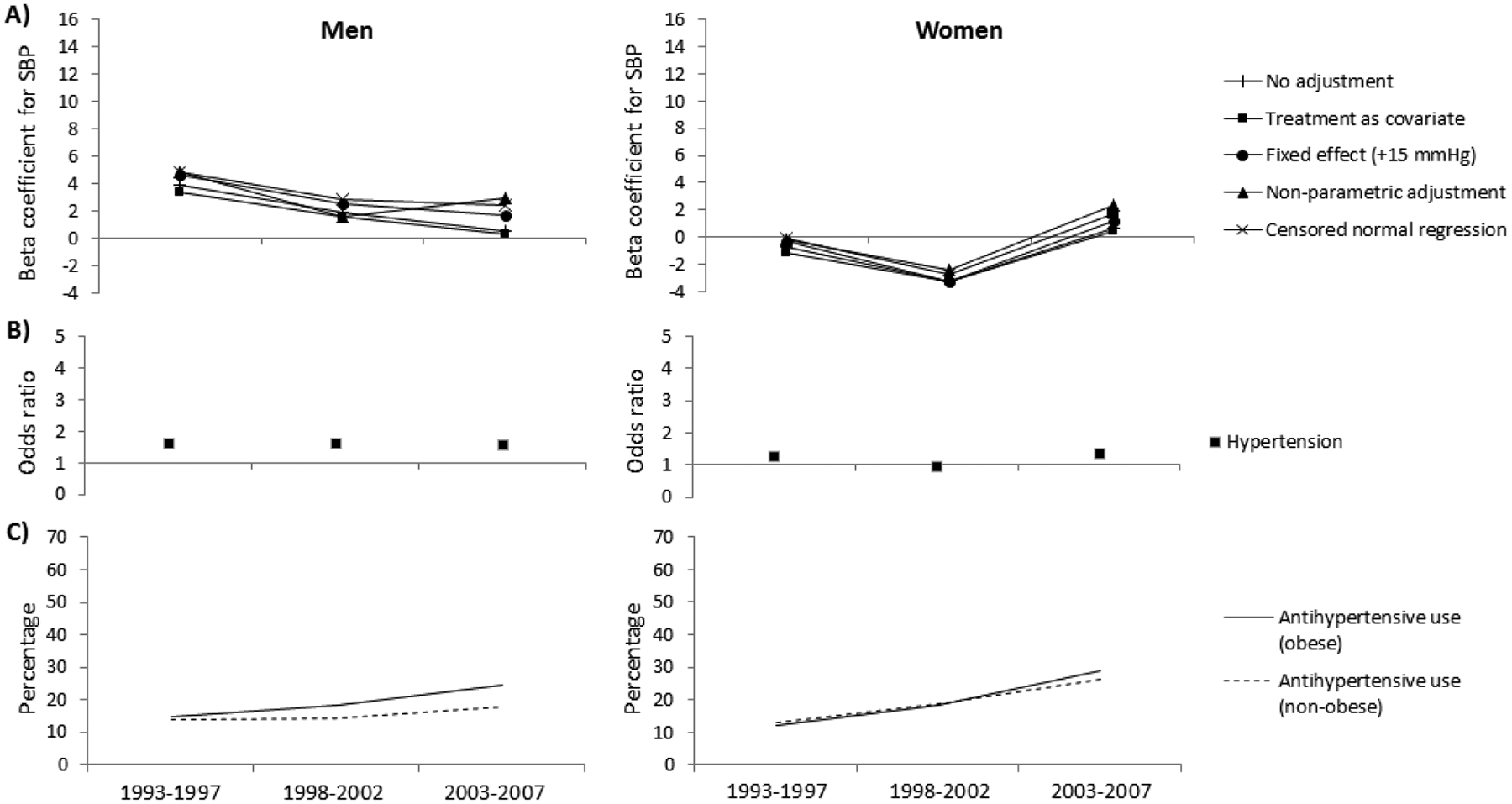
(A and B) The relationship between systolic blood pressure (SBP) or hypertension and obesity, adjusted for age and using various adjustment methods for antihypertensive use, and (C) the percentage using antihypertensive therapy in the American Indian study. The beta coefficients represent the difference in SBP (mmHg) between persons who are and are not obese. The odds ratios represent the odds for hypertension in obese persons compared to those not obese.
Figure 4.
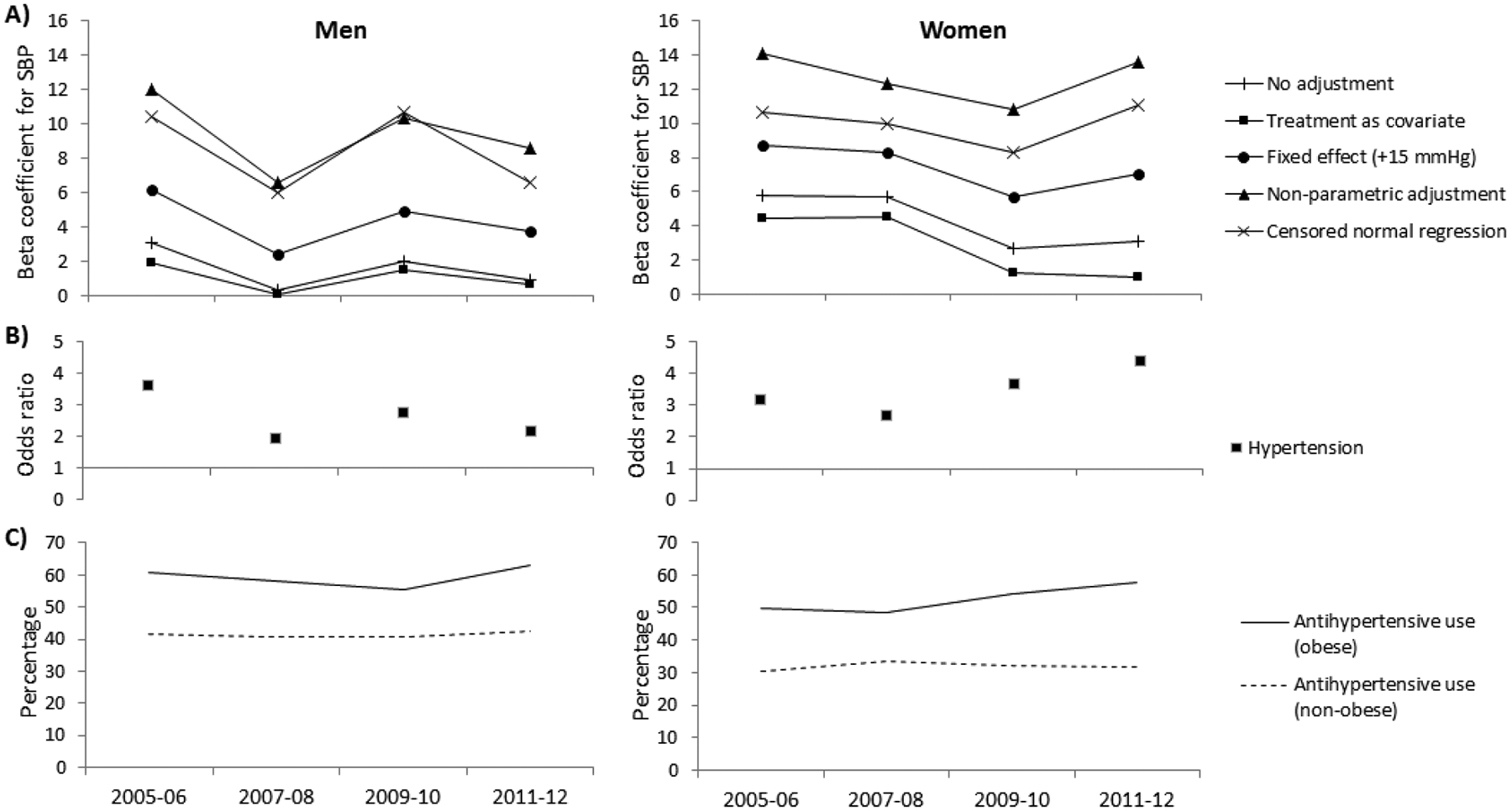
(A and B) The relationship between systolic blood pressure (SBP) or hypertension and obesity, adjusted for age and using various adjustment methods for antihypertensive use, and (C) the percentage using antihypertensive therapy in the National Health and Nutrition Examination Survey. The beta coefficients represent the difference in SBP (mmHg) between persons who are and are not obese. The odds ratios represent the odds for hypertension in obese persons compared to those not obese.
The primary difference in results produced by the various adjustment methods was in the parameter estimates at each time point. In particular, a difference in beta coefficients was found when antihypertensive treatment was related to the explanatory variable (i.e. diabetes and obesity). The prevalence of antihypertensive drug use in persons with diabetes in the American Indian study was consistently higher than in those without diabetes (Figure 1C). Conversely, the prevalence of antihypertensive drug use in the obese population of the American Indian study was not different to their non-obese counterparts (Figure 3C). Consequently, the beta coefficients for systolic blood pressure in relation to diabetes differed depending on the method of adjustment for treatment effect (Figure 1A), but the beta coefficients for systolic blood pressure in relation to obesity were similar across all methods of adjustment (Figure 3A). The beta coefficients produced when no adjustment for treatment effect was made or when treatment was included as a covariate was commonly lower compared to other adjustment methods.
The mean difference in systolic blood pressure between men with and without diabetes in the American Indian study increased over time regardless of the adjustment method used, though an interaction between diabetes and time was found only when using the non-parametric algorithm or the censored normal regression (Figure 1A, Appendix B). Conversely, the relationship between diabetes and systolic blood pressure in women in the American Indian study differed over time depending on the method of adjustment used. When no adjustment for treatment was made, when treatment was included as a covariate or when a fixed effect of treatment was added, the mean difference in systolic blood pressure between women with and without diabetes decreased over time. However, when using the non-parametric algorithm or the censored normal regression, this difference increased over time. An interaction between diabetes and time was observed in women only when no adjustment for treatment effect was made, when treatment was included as a covariate or when a fixed effect of treatment was added. In NHANES, the mean difference in systolic blood pressure between those with and without diabetes generally followed a U-shaped pattern over time in both men and women. This relationship between diabetes and systolic blood pressure was not modified by time in either sex and across all adjustment methods (Figure 2A, Appendix C).
The mean difference in systolic blood pressure between those obese and non-obese decreased over time in men in the American Indian study and NHANES, and in women in NHANES, but generally increased in women in the American Indian study (Figures 3A and 4A, Appendices D and E). No interaction between obesity and time was found for either sex and across all adjustment methods, in both the American Indian study and NHANES.
The odds ratio for hypertension in those with diabetes compared to those without increased over time in the American Indian study but not in NHANES (Figures 1B and 2B, Appendices B and C). An interaction was found between diabetes and time in both sexes in the American Indian study. For obesity, the odds ratios for hypertension decreased in obese men versus non-obese men in both the American Indian study and NHANES, and increased in women in both studies (Figure 3B and 4B, Appendices D and E). No interaction between obesity and time was found in either study.
3.1. Sensitivity analysis
Similar results were observed when those with macroalbuminuria or those with IGT were excluded, when the threshold at which hypertension was defined was varied to 130 mmHg or use of antihypertensive treatment, and when the fixed treatment effect was varied to 5 mmHg and 10 mmHg. When we restricted each individual to appearing only in one randomly chosen five-year period in the American Indian study, our sample size decreased to 1,631 in 1993–1997, 1,407 in 1998–2002 and 2,331 in 2003–2007. This did not significantly alter our results.
4. DISCUSSION
This paper illustrates the relationships between two risk factors for hypertension, diabetes and obesity, and systolic blood pressure, accounting for antihypertensive treatment. The magnitude of the association between each risk factor and systolic blood pressure differed depending on the analytical method used to account for effects of antihypertensive treatment. The common methods of no adjustment or including treatment as a covariate in a regression consistently produced very similar results and were generally lower than those produced by more suitable methods [2] such as the non-parametric algorithm or censored normal regression. The larger the difference in prevalence of antihypertensive use between persons with and without diabetes and persons who were obese compared to non-obese, the more the conventional adjustment methods underestimated the magnitudes of the relationships.
The flaws of commonly used methods to account for treatment use have been discussed in detail by Tobin et al [2]. When antihypertensive treatment is included as a covariate in a regression that evaluates a blood pressure outcome, antihypertensive treatment is incorrectly treated as a confounder. In relationships between blood pressure and its determinants, antihypertensive treatment is not a confounder but rather part of the outcome, given that high blood pressure is a determinant of antihypertensive use [2]. Dichotomizing blood pressure into normotension and hypertension recognizes that individuals on treatment may have an observed systolic blood pressure below the hypertension cut-point but should still be classified as hypertensive. While correct, this approach results in a loss of power and should be avoided when the available data permit more rigorous adjustment. Results from this method should be interpreted with care, as the resulting odds ratios represent either the relationship with elevated blood pressure, antihypertensive treatment, or a combination of both.
Adding a fixed effect for antihypertensive treatment is a robust method of adjustment in various settings [8, 11–13]. However, this method relies heavily on assumptions about the expected magnitude of effect. Treatment for hypertension has advanced over time with large increases in both the availability and use of antihypertensives, and with changes in types of antihypertensive treatments on the market. Consequently, several separate assumptions of treatment effect may be required depending on the time period being analyzed and types of drugs used in the study population. Where detailed information is available, a preferred approach would be to impute underlying blood pressure based on the antihypertensive drug class and the number of antihypertensive drugs used. An algorithm to account for up to two drugs has been devised by Wu et al and extended for use with ≥3 drugs by Rana et al [14, 15]. Where reasonable assumptions about the treatment effect are difficult, the censored normal regression method and the non-parametric algorithm are appropriate. Both assume right-censoring of blood pressure. However, the non-parametric algorithm does not make assumptions about the distribution, while the censored normal regression assumes a normal probability distribution for underlying blood pressure of treated individuals, which is to the right of the observed blood pressure. The censored normal regression method deals with shrinkage bias slightly better than the non-parametric algorithm [2], and is preferred when reasonable assumptions about normality can be made, as is the case in our study.
Differences in results between different adjustment methods for treatment effect was predominantly driven by the relationship between the risk factor for hypertension (diabetes or obesity) and antihypertensive treatment use. Not surprisingly, where there was little difference in treatment use between people with and without the risk factor, as was the case for antihypertensive use in obese vs. non-obese persons in the American Indian study, similar relationships were seen between systolic blood pressure and its risk factor across all adjustment methods for treatment. Where the difference in prevalence of antihypertensive use between groups diverged or converged over time, similar divergence or convergence were observed for the beta coefficients. Thus appropriate adjustment for treatment effect in analysis of blood pressure and its determinants is particularly important when there is a strong relationship between treatment use and the blood pressure determinant.
When adjusting for antihypertensive treatment use with the censored normal regression, our preferred method for adjustment, the difference in systolic blood pressure between those with and without diabetes increased over time in the American Indian study in men, but not in women. An increase in blood pressure difference remained even after those with more complicated diabetes (macroalbuminuria) were excluded, indicating that it was not a consequence of more advanced disease in the later time period. For obesity, when using the censored normal regression, the difference in systolic blood pressure between people with and without obesity did not change over time in both the American Indian study and in NHANES. This is in contrast with previous reports of a change in the relationship between obesity and blood pressure over time [16, 17]. Notably, these studies accounted for antihypertensive treatment using the flawed method of including antihypertensive treatment as a covariate in a regression. Using the same method, we also found suggestions of a change in the relationship between obesity and systolic blood pressure in the American Indian study (P for interaction between obesity and time = 0.053 for men and 0.054 for women; Appendix D), though not in NHANES. This further highlights the importance of appropriately accounting for antihypertensive treatment use as different methods may lead to different conclusions.
The self-reported nature of medication use is a potential limitation as it may have resulted in underreporting of medicines. Prescribed but untaken medications may also have been missed or misclassified. However, this should not differ by time period, and is expected to have minimal effect on the trends reported in our study.
5. CONCLUSIONS
The previous study by Tobin et al compared different adjustment methods for antihypertensive treatment effect in a cross-sectional examination of the relationship between the APOE gene and systolic blood pressure [2]. Of the ten methods examined, the authors recommended the addition of a fixed treatment effect and the censored normal regression. Here we illustrated the implications of different adjustment methods for treatment on the estimated relationship between diabetes or obesity and systolic blood pressure over time. The commonly used methods of no adjustment for treatment and including treatment as a covariate underestimated the difference in underlying blood pressure between those with and without diabetes and between those obese and non-obese, when compared to the censored normal regression approach recommended by Tobin et al [2]. No one analytical approach is clearly superior to the others, as the best method for a given scenario depends on the assumptions that can most reasonably be made. We prefer the censored normal regression model for the current examples, and, in agreement with Tobin et al [2], suggest that ignoring treatment variables or including them as covariates in regression models is rarely appropriate.
7. ACKNOWLEDGEMENTS
We are grateful to the NIDDK clinic staff and the study volunteers. We thank the NHANES investigators for the use of their dataset.
8. FUNDING SOURCE
This work was supported by the Intramural Research Program of the NIDDK.
6. LIST OF ABBREVIATIONS
- BMI
body mass index
- NHANES
National Health and Examination Survey
- OGTT
oral glucose tolerance test
- SBP
systolic blood pressure
Appendix A.
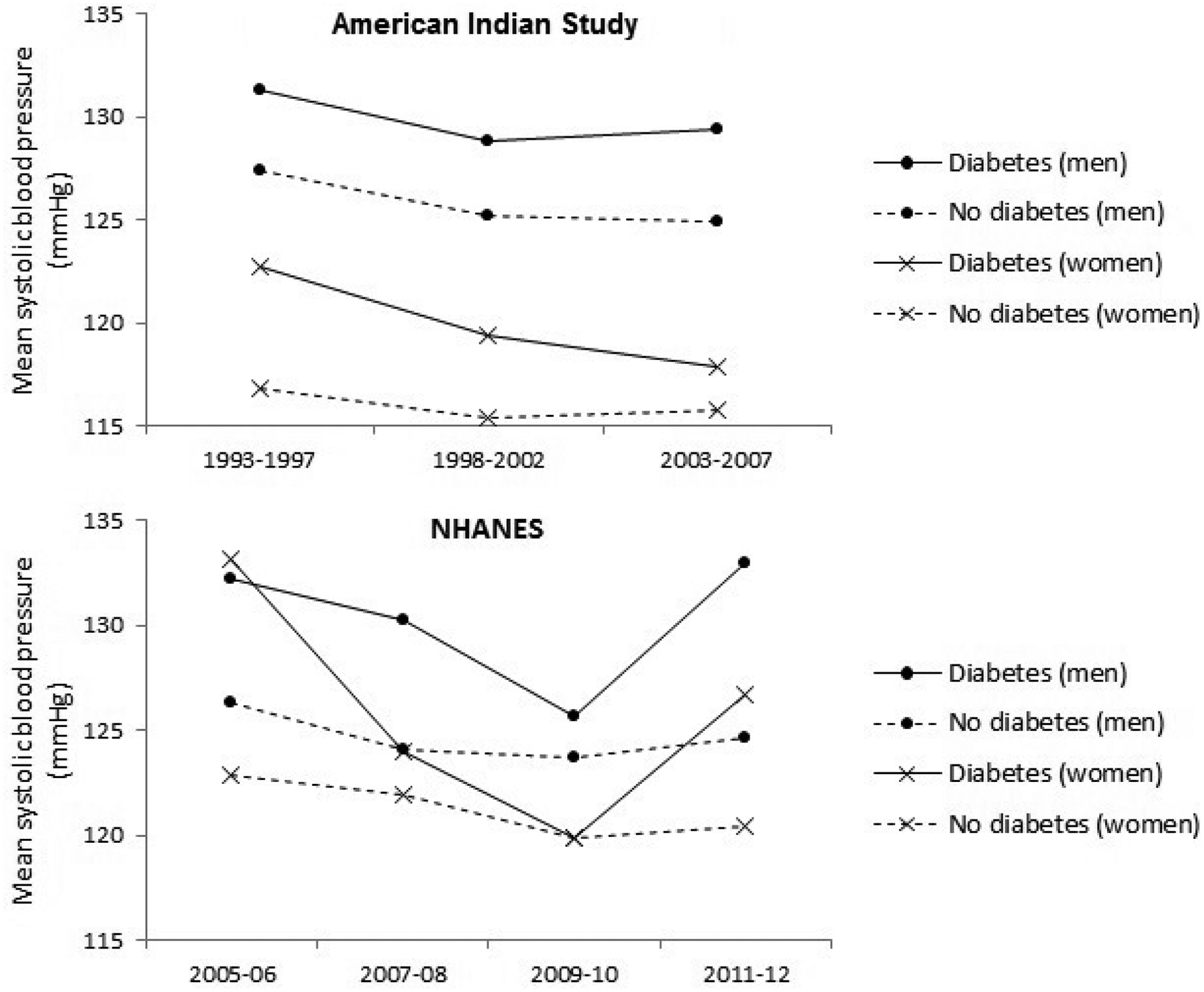
Mean age-adjusted systolic blood pressure in men and women with and without diabetes in the American Indian study and the National Health and Nutrition Examination Survey (NHANES). The results are for observed blood pressure, not accounting for antihypertensive treatment.
Appendix B.
Analysis of Different Methods to Adjust for Blood Pressure Treatment in the Relationship Between Systolic Blood Pressure And Diabetes in the American Indian Study.
| 1993–1997 | 1998–2002 | 2003–2007 | |||||
|---|---|---|---|---|---|---|---|
| Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | P value* | |
| Men | n=1035 | n=1019 | n=1432 | ||||
| No adjustment | 3.86 | 1.55, 6.17 | 3.65 | 1.34, 5.95 | 4.47 | 2.37, 6.57 | 0.37 |
| Treatment as covariate | 2.04 | −0.31, 4.39 | 2.32 | −0.08, 4.73 | 3.76 | 1.50, 6.02 | 0.15 |
| Fixed effect (+15 mmHg) | 6.77 | 4.24, 9.31 | 7.32 | 4.83, 9.81 | 9.30 | 7.08, 11.52 | 0.49 |
| Non-parametric adjustment | 9.78 | 6.72, 12.84 | 10.32 | 7.45, 13.19 | 14.04 | 11.50, 16.57 | 0.04 |
| Censored normal regression | 6.89 | 4.32, 9.45 | 8.24 | 5.65, 10.82 | 12.09 | 9.62, 14.55 | 0.01 |
| HypertensionϮ | 2.78 | 2.01, 3.85 | 2.92 | 2.11, 4.02 | 4.50 | 3.46, 5.84 | 0.02 |
| Women | n=1672 | n=1631 | n=2140 | ||||
| No adjustment | 5.82 | 4.04, 7.60 | 4.00 | 2.25, 5.75 | 2.17 | 0.67, 3.68 | <0.001 |
| Treatment as covariate | 4.54 | 2.80, 6.29 | 2.27 | 0.47, 4.07 | 0.83 | −0.75, 2.42 | <0.001 |
| Fixed effect (+15 mmHg) | 7.28 | 5.31, 9.24 | 7.40 | 5.48, 9.32 | 6.56 | 4.92, 8.20 | 0.02 |
| Non-parametric adjustment | 10.17 | 7.74, 12.59 | 10.97 | 8.83, 13.12 | 14.07 | 12.11, 16.03 | 0.06 |
| Censored normal regression | 6.96 | 5.03, 8.89 | 7.89 | 5.92, 9.85 | 8.33 | 6.54, 10.12 | 0.47 |
| HypertensionϮ | 3.50 | 2.47, 4.95 | 4.41 | 3.28, 5.94 | 5.51 | 4.28, 7.09 | 0.01 |
Analyses were adjusted for age. Beta coefficients are difference in systolic blood pressure (mmHg) between those with diabetes compared to without diabetes.
Results are odds ratio (95% CI).
P value for interaction between diabetes and time.
Appendix C.
Analysis of Different Methods to Adjust for Blood Pressure Treatment in the Relationship Between Systolic Blood Pressure And Diabetes in the National Health and Nutrition Examination Survey.
| 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | P value* | |
| Men | n=360 | n=448 | n=444 | n=402 | |||||
| No adjustment | 5.94 | −0.22, 12.09 | 6.12 | −3.47, 15.70 | 2.04 | −3.66, 7.73 | 8.39 | −0.21, 17.00 | 0.95 |
| Treatment as covariate | 3.91 | −3.29, 11.10 | 6.03 | −3.56, 15.62 | 1.74 | −4.05, 7.53 | 8.19 | −0.46, 16.85 | 0.90 |
| Fixed effect (+15 mmHg) | 11.37 | 4.68, 18.06 | 7.00 | −2.55, 16.54 | 3.71 | −3.41, 10.83 | 11.55 | 3.54, 19.56 | 0.72 |
| Non-parametric adjustment | 21.17 | 13.89, 28.45 | 7.49 | −1.90, 16.88 | 6.48 | −3.37, 16.33 | 15.51 | 7.87, 23.16 | 0.17 |
| Censored normal regression† | 37.58 | − | 10.17 | − | 12.24 | − | 20.93 | − | − |
| HypertensionϮ | 48.67 | 5.31, 446.33 | 1.55 | 0.54, 4.44 | 2.67 | 0.52, 13.64 | 2.86 | 1.11, 7.33 | 0.12 |
| Women | n=401 | n=524 | n=609 | n=499 | |||||
| No adjustment | 10.33 | 0.92, 19.73 | 2.13 | −4.47, 8.72 | 0.04 | −5.51, 5.59 | 6.32 | −2.25, 14.90 | 0.22 |
| Treatment as covariate | 9.17 | 0.33, 18.00 | 1.52 | −5.29, 8.33 | −0.42 | −5.83, 5.00 | 5.13 | −4.39, 14.64 | 0.25 |
| Fixed effect (+15 mmHg) | 12.69 | 2.52, 22.86 | 3.27 | −3.59, 10.13 | 1.01 | −4.99, 7.00 | 8.50 | 0.48, 16.53 | 0.22 |
| Non-parametric adjustment | 15.49 | 4.22, 26.75 | 5.44 | −2.35, 13.23 | 3.40 | −3.36, 10.16 | 10.99 | 2.74, 19.24 | 0.21 |
| Censored normal regression† | 20.05 | − | 8.53 | − | 4.47 | − | 17.17 | − | − |
| HypertensionϮ | 4.41 | 1.43, 13.64 | 1.45 | 0.60, 3.51 | 1.36 | 0.80, 2.34 | 3.02 | 1.27, 7.21 | 0.40 |
Analyses were adjusted for age and weighted using NHANES analytical weights. Beta coefficients are difference in systolic blood pressure (mmHg) between those with diabetes compared to without diabetes.
Results are beta coefficients. 95% confidence intervals are not reported as variance estimates could not be adjusted for stratification and clustering in the sampling design, thus considered unreliable.
Results are odds ratio (95% CI).
P value for interaction between diabetes and time.
Appendix D.
Analysis of Different Methods to Adjust for Blood Pressure Treatment in the Relationship Between Systolic Blood Pressure And Obesity in the American Indian study.
| 1993–1997 | 1998–2002 | 2003–2007 | |||||
|---|---|---|---|---|---|---|---|
| Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | P value* | |
| Men | n=1035 | n=1019 | n=1432 | ||||
| No adjustment | 3.87 | 1.83, 5.91 | 1.85 | −0.27, 3.97 | 0.54 | −1.38, 2.47 | 0.06 |
| Treatment as covariate | 3.36 | 1.36, 5.36 | 1.56 | −0.54, 3.67 | 0.27 | −1.66, 2.20 | 0.053 |
| Fixed effect (+15 mmHg) | 4.67 | 2.42, 6.92 | 2.55 | 0.23, 4.86 | 1.64 | −0.43, 3.71 | 0.10 |
| Non-parametric adjustment | 4.84 | 2.11, 7.57 | 1.58 | −1.12, 4.27 | 2.98 | 0.58, 5.37 | 0.43 |
| Censored normal regression | 4.84 | 2.60, 7.08 | 2.87 | 0.56, 5.18 | 2.41 | 0.23, 4.58 | 0.15 |
| HypertensionϮ | 1.61 | 1.16, 2.24 | 1.64 | 1.17, 2.31 | 1.57 | 1.21, 2.04 | 0.95 |
| Women | n=1672 | n=1631 | n=2140 | ||||
| No adjustment | −0.73 | −2.49, 1.04 | −3.23 | −5.10, −1.37 | 0.65 | −0.91, 2.22 | 0.07 |
| Treatment as covariate | −1.17 | −2.88, 0.54 | −3.24 | −5.06, −1.41 | 0.48 | −1.07, 2.04 | 0.054 |
| Fixed effect (+15 mmHg) | −0.26 | −2.22, 1.70 | −3.23 | −5.30, −1.17 | 1.18 | −0.55, 2.91 | 0.14 |
| Non-parametric adjustment | −0.15 | −2.58, 2.28 | −2.41 | −4.75, −0.07 | 2.34 | 0.21, 4.46 | 0.07 |
| Censored normal regression | −0.11 | −2.03, 1.80 | −2.72 | −4.80, −0.65 | 1.73 | −0.12, 3.57 | 0.14 |
| HypertensionϮ | 1.30 | 0.92, 1.83 | 0.95 | 0.68, 1.33 | 1.37 | 1.03, 1.83 | 0.85 |
Analyses were adjusted for age. Beta coefficients are difference in systolic blood pressure (mmHg) between those obese compared to those non-obese.
Results are odds ratio (95% CI).
P value for interaction between obesity and time.
Appendix E.
Analysis of Different Methods to Adjust for Blood Pressure Treatment in the Relationship Between Systolic Blood Pressure And Obesity in the National Health and Nutrition Examination Survey.
| 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | Beta coefficient or odds ratio | 95% CI | P value* | ||
| Men | n=360 | n=448 | n=444 | n=402 | ||||||
| No adjustment | 3.12 | −0.28, 6.52 | 0.32 | −3.71, 4.34 | 1.96 | −0.44, 4.37 | 0.88 | −2.36, 4.12 | 0.49 | |
| Treatment as covariate | 1.95 | −1.30, 5.20 | 0.10 | −3.68, 3.88 | 1.50 | −1.26, 4.25 | 0.63 | −2.67, 3.92 | 0.48 | |
| Fixed effect (+15 mmHg) | 6.19 | 2.65, 9.73 | 2.39 | −2.35, 7.14 | 4.92 | 0.83, 9.02 | 3.79 | 0.23, 7.35 | 0.56 | |
| Non-parametric adjustment | 12.02 | 7.26, 16.77 | 6.56 | 0.24, 12.87 | 10.30 | 2.78, 17.83 | 8.56 | 4.03, 13.09 | 0.52 | |
| Censored normal regression† | 10.44 | − | 6.01 | − | 10.67 | − | 6.62 | − | − | |
| HypertensionϮ | 3.64 | 1.96, 6.77 | 1.95 | 0.97, 3.92 | 2.78 | 0.92, 8.38 | 2.22 | 1.45, 3.41 | 0.38 | |
| Women | n=401 | n=524 | n=609 | n=499 | ||||||
| No adjustment | 5.74 | 1.36, 10.13 | 5.71 | 2.97, 8.45 | 2.63 | −1.30, 6.57 | 3.10 | −0.35, 6.55 | 0.19 | |
| Treatment as covariate | 4.42 | 0.01, 8.82 | 4.50 | 1.47, 7.52 | 1.27 | −2.46, 5.00 | 0.97 | −2.36, 4.30 | 0.15 | |
| Fixed effect (+15 mmHg) | 8.69 | 4.55, 12.82 | 8.28 | 5.06, 11.50 | 5.70 | 1.16, 10.23 | 7.04 | 2.63,11.45 | 0.43 | |
| Non-parametric adjustment | 14.06 | 8.44, 19.68 | 12.28 | 6.59, 17.98 | 10.83 | 5.54, 16.12 | 13.63 | 7.24, 20.03 | 0.87 | |
| Censored normal regression† | 10.61 | 10.01 | 8.31 | 11.03 | − | |||||
| HypertensionϮ | 3.21 | 1.42, 7.24 | 2.69 | 1.18, 6.11 | 3.68 | 2.14, 6.35 | 4.43 | 2.11, 9.33 | 0.33 | |
Analyses were adjusted for age. Beta coefficients are difference in systolic blood pressure (mmHg) between those obese compared to those non-obese.
Results are beta coefficients. 95% confidence intervals and p-value for difference over time are not reported as variance estimates could not be adjusted for stratification and clustering in the sampling design, thus considered unreliable.
Results are odds ratio.
The relationship between obesity and systolic blood pressure differed significantly over time.
P value for interaction between obesity and time.
Footnotes
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. [DOI] [PubMed] [Google Scholar]
- 2.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–35. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. 1978;108(6):497–505. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015;38 Suppl:S8–S16. [DOI] [PubMed] [Google Scholar]
- 5.Curtin LR, Mohadjer LK, Dohrmann SM, et al. The National Health and Nutrition Examination Survey: Sample design, 1999–2006 National Center for Health Statistics; Vital Health Stat 2012. [PubMed] [Google Scholar]
- 6.Curtin LR, Mohadjer LK, Dohrmann SM, et al. National Health and Nutrition Examination Survey: Sample Design, 2007–2010 National Center for Health Statistics; Vital Health Stat 2013. [PubMed] [Google Scholar]
- 7.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: Plan and operations, 1999–2010 National Center for Health Statistics; Vital Health Stat, 2013. [PubMed] [Google Scholar]
- 8.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326(7404):1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy D, DeStefano AL, Larson MG, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension. 2000;36(4):477–83. [DOI] [PubMed] [Google Scholar]
- 10.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26(1):24–36. [Google Scholar]
- 11.Cui J, Hopper JL, Harrap SB. Genes and family environment explain correlations between blood pressure and body mass index. Hypertension. 2002;40(1):7–12. [DOI] [PubMed] [Google Scholar]
- 12.Neaton JD, Grimm RH Jr., Prineas RJ, et al. Treatment of Mild Hypertension Study. Final results. Treatment of Mild Hypertension Study Research Group. JAMA. 1993;270(6):713–24. [PubMed] [Google Scholar]
- 13.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41(2):207–10. [DOI] [PubMed] [Google Scholar]
- 14.Rana BK, Dhamija A, Panizzon MS, et al. Imputing observed blood pressure for antihypertensive treatment: impact on population and genetic analyses. Am J Hypertens. 2014;27(6):828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Kraja AT, Oberman A, et al. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens. 2005;18(7):935–42. [DOI] [PubMed] [Google Scholar]
- 16.Danon-Hersch N, Chiolero A, Shamlaye C, Paccaud F, Bovet P. Decreasing association between body mass index and blood pressure over time. Epidemiology. 2007;18(4):493–500. [DOI] [PubMed] [Google Scholar]
- 17.Adler C, Schaffrath Rosario A, Diederichs C, Neuhauser HK. Change in the association of body mass index and systolic blood pressure in Germany - national cross-sectional surveys 1998 and 2008–2011. BMC Public Health. 2015;15:705. [DOI] [PMC free article] [PubMed] [Google Scholar]


