“I looked, and there before me was a pale horse! Its rider was named Death, and Hades was following close behind him. They were given power over a fourth of the earth to kill by sword, famine and plague, and by the wild beasts of the earth.”
(The Fourth Horseman of the Apocalypse) - Revelation 6.8
The Coronavirus Disease (Covid-19) pandemic is caused by a strain from the coronavirus family and named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was first identified in Wuhan, China, in December 2019. Its global spread was rapid, prompting the World Health Organization (WHO) to declare it as a Public Health Emergency of International Concern by 30 January 2020. The relentless march of the disease led to the condition being declared a pandemic on 11 March 2020.
Little is known about the virus as it is an entirely new mutation and hitherto unknown. The vast majority of infections are asymptomatic. In others, it may manifest as an influenza-like illness with a variable course. The usual incubation period is approximately five days, though it may be as long as 14 days after acquiring the infection. The illness usually presents with fever, a dry cough, breathlessness, malaise and sometimes with anosmia. Spontaneous recovery is the rule, though, in approximately 5% of infected people, and it follows a more fulminant course. In its severe form, it presents as an acute respiratory distress syndrome with a radiological picture suggestive of extensive pneumonia and may require mechanical ventilation. There is no known treatment, and management is mostly supportive. Not surprisingly, mortality rates in the severe form of the condition are high. Mortality rates are higher with advancing age and comorbidities, with approximately 50% of deaths occurring in octogenarians.
The principal mode of spread is by contact and through droplets and aerosolization of virus particles during coughing, sneezing or talking. The virus survives on surfaces for a variable period ranging from a day on cardboard to four days on plastic surfaces. As no viricidal agents or vaccines that act against this virus are available as yet, the principle mode of control is by prevention. The main preventive measures are frequent hand washing, use of alcohol hand gels and by self-isolation at the first sign of infection. The recommended duration of self-isolation is seven days for symptomatic individuals and fourteen days for household contacts.
As the cornerstone of control is prevention, it is unsurprising that testing for the presence of the infection occupies a critical role in managing the infection both at an individual level and at a community and global level. The diagnostic tests available are broadly classified as reverse transcriptase-polymerase chain reaction (RT-PCR) tests to detect the presence of virus RNA, antigen tests and antibody tests. The frequency and indications for use have not been defined consistently. They are primarily left to the discretion of individual national health authorities to determine the type of test and the nature of the population to be tested.
In this article, we describe and compare the tests, their clinical use and implementation in two diverse nations. We have chosen the United Kingdom, a developed country with a population of only 66.5 million, and India, a vast and populous developing nation home to 1.3 billion, to understand better the use of these tests in diverse settings.
Diagnostic tests for COVID-19
Viral infections are primarily diagnosed by molecular tests and immunoassays. The molecular test detects the nucleic acid from the virus and helps in the diagnosis of active infection. Immunoassays, on the other hand, detect the presence of either virus-specific antigens or antibodies produced in response to infection. SARS-CoV-2 being a novel coronavirus, different immunodiagnostic tests developed so far are recommended to be used only for surveillance and research purpose with their potential diagnostic utility still being studied.
The sequence of the viral genome was published in early January 2020,1 enabling designing of primers for molecular diagnostics. These primers are used to amplify tiny fragments of viral nucleic acid and further detection by RT-PCR technology. The test is recommended on respiratory tract specimens – nasal or pharyngeal swabs and sputum if obtainable. The RT-PCR based test is highly sensitive and considered as the gold standard frontline test for COVID-19 testing. This test can detect the virus early, and the turnaround time is from a few hours to two days. At the moment, the test is being used worldwide for case confirmation and isolation guidance. The test requires high-quality specimens to avoid false-negative results. Other significant limitations of these tests are its high cost, biosafety concerns and requirement of specialized molecular laboratories with a trained workforce.
Owing to the limited capacity of laboratory-based molecular testing, a large number of immunoassay based diagnostic kits have been developed by multiple manufacturers. These tests are comparatively cheap, easy to perform and do not require sophisticated laboratory infrastructure. Antibody based tests detect IgM and IgG antibodies specific to SARSCoV- 2 proteins in the serum of the patient. Currently, antibody tests are recommended only for epidemiological studies and surveillance purposes. It remains to be seen if antibody tests have a role in determining individual and population immunity to the disease. Antigen-based immunoassays to detect viral-specific antigens are being developed as rapid and point-of-care immunodiagnostic tests. These tests can be performed on respiratory specimens but have low sensitivity as often there is not enough antigen present in the specimens to be detectable. At present, the WHO recommends these antigen-based tests to be used only for research purpose. 2
The pandemic has seen even the developed nations struggling to scale up the testing requirements. Nonavailability of sufficient kits and consumables due to worldwide demand is also a constraint. Some countries are considering the feasibility of using pooled samples for surveillance to increase the capacity of screening. Modification of conventional RT-PCR is recommended for small healthcare setups as they are easy to perform, have rapid turnaround time and require minimal infrastructure. The major limitation of these tests is their high cost. Cartridge Based Nucleic Acid Amplification Test (CBNAAT) using Cepheid Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, United States of America) on GeneXpert machines and TrueNAT Beta CoV test (Molbio Diagnostics Private Limited, India) on Truelab workstation are two such platforms. The latter is recommended only for screening purpose in India.
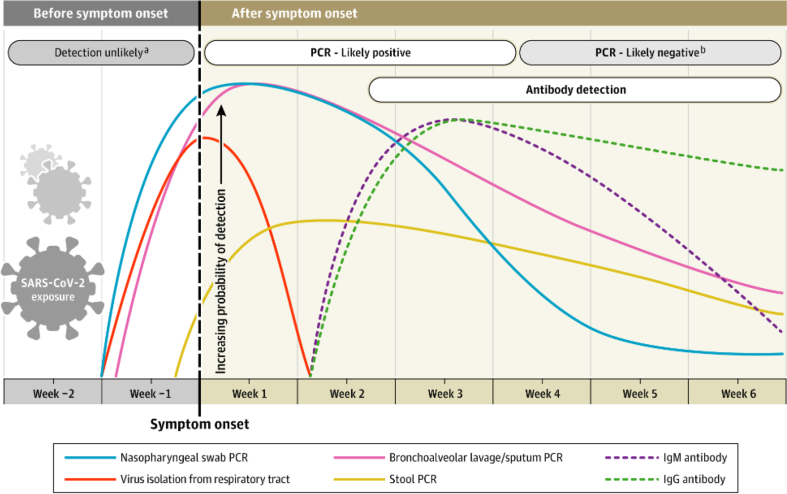
Understanding the timeline of the diagnostic tests described previously will help the clinician interpret the results.3 (Fig. 1).
Fig. 1.
Estimated variation over time in diagnostic tests for detection of SARS-CoV-2 infection relative to symptom onset. Estimated time intervals and rates of viral detection are based on data from several published reports. Because of variability in values among studies, estimated time intervals should be considered approximations and the probability of detection of SARS-CoV-2 infection is presented qualitatively. SARS-CoV-2 indicates severe acute respiratory syndrome coronavirus 2; PCR, polymerase chain reaction. aDetection only occurs if patients are followed up proactively from the time of exposure. bMore likely to register a negative than a positive result by PCR of a nasopharyngeal swab. Reproduced from: Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. Published online May 06, 2020. https://doi.org/10.1001/jama.2020.8259.
Testing for SARS-CoV-2 in the UK
The ongoing COVID-19 pandemic spread to the United Kingdom in late January 2020.4 Transmission within the UK was first documented on 28 February.5 According to the latest government statistics, by 08 May there had been 211,364 confirmed COVID-19 cases and 31,241 deaths.6 (Table 1).
Table 1.
Cumulative Testing Figures of COVID-19 tests (RT-PCR) in the UK on 08 May 2020.
| Tests for Covid | Pillar 1 | Pillar 2 | Pillar 4 | Total |
|---|---|---|---|---|
| People tested | 728,115 | 478,954 | – | 1,207,069 |
| Tests | 941,508 | 656,407 | 33,646 | 1,631,561 |
| Positive | 162,192 | 49,172 | – | 211,364 |
Pillar 1: swab testing in PHE labs and NHS hospitals for those with a clinical need and health and care workers.
Pillar 2: swab testing for essential workers and their households, as well as other groups that meet the eligibility criteria as set out in government guidance.
Pillar 4: serology and swab testing for national surveillance supported by PHE, ONS, Biobank, universities and other partners to learn more about the prevalence and spread of the virus and for other testing research purposes, for example on the accuracy and ease of use of home testing.
RT-PCR, reverse transcriptase-polymerase chain reaction; NHS, National Health Service; PHE, Public Health England; ONS, Office for National Statistics.
In response, the Department of Health and Social Care in England immediately launched a public health information campaign to help slow the virus's spread and introduced the Health Protection (Coronavirus) Regulations 2020.7 The government outlined a four-pronged strategy to tackle the outbreak: contain, delay, research and mitigate.8
The fundamental principle underpinning the overall strategy is identifying infected individuals and isolating them from the unaffected population. The availability of reliable and accurate tests to identify infected patients in the face of this rapidly spreading pandemic has been a challenge throughout the world, and the UK has been no exception. It follows that the UK has had to prioritize its testing capacity and adapt according to the evolving epidemic.
Five pillars of testing
The UK testing strategy is described in an article, ‘Coronavirus (COVID-19) Scaling up the testing programmes,’ published on 04 April 2020.9
This document describes five ‘pillars’ of the testing strategy.
The first pillar: Scaling up National Health Service swab testing for SARS-CoV-2 RNA for those with a medical need and, where possible, the most critical key workers.
This type of testing allows the National Health Service (NHS) to identify and effectively care for those who are most severely ill – saving lives by helping clinicians decide the most effective treatment options and for whom. These swab tests are delivered mainly through the Public Health England's (PHE) regional laboratory network and, since the beginning of March, the NHS and approved private laboratories across the country.
The second pillar: Mass swab testing for SARS-CoV-2 RNA in critical workers in the NHS, social care and other sectors.
This approach will allow organizations to release more staff back to the frontline, who are well enough to work but currently self or household isolating. In turn, this would boost capacity at a time when public services are under pressure. This strategy was implemented on 15 April 2020.
The third pillar: Mass antibody testing in the blood to help determine if people have immunity to coronavirus.
Antibody tests could inform people whether they have had the virus and are now immune. Such tests are performed by taking a blood sample and looking for the presence of the right COVID-19 antibodies.
However, such tests are currently being validated and are not yet approved for routine diagnostic use in the UK. The key issues that need resolution are the accuracy of the tests and importantly whether the presence of antibodies reflects immunity of the individual to COVID-19 infection.
The fourth pillar: Surveillance testing to learn more about the disease and help develop new tests and treatments.
Robust population surveillance programmes are essential to understand the rate of infection and how the virus is spreading across the country. They help us to assess the impact of measures taken so far to contain the virus, to inform current and future actions and to develop new tests and treatments.
Since the end of February, the UK has established a national surveillance programme for population blood testing, using a high accuracy antibody test operated by PHE at Porton Down, to find out what proportion of the population has had the virus.
Public Health England is testing samples of the population to test around 5000 swabs for SARS-CoV-2 RNA per week. The UK government aims to roll out a national mass population sample over the coming months. Participants will be invited to take part in a study involving at-home immunity (antibody) testing every 4 weeks over a 6- to 12-month period. The aim is to enrol 16,000 to 20,000 people who will undergo repeat testing. This study will add to the population data, as well as provide information about immunity and how long it lasts.
The fifth pillar: Spearheading a Diagnostics National Effort to build a mass testing capacity at an entirely new scale.
This pillar aims to develop resilient, diagnostic capability in the UK capable of meeting the testing demands over the coming months and years.
Progress of testing of patients in the hospitals and community
In the early stages of the epidemic in the UK till early March 2020, the testing of patients was concentrated on travellers with symptoms of COVID-19 infection and their contacts who returned from affected (high risk) countries such as China, South East Asia and Italy. In mid-March, it became apparent that the infection had spread to people who had no history of travel to high-risk countries or direct contact with those who had been. At this stage, NHS hospitals were directed to test all patients presenting with symptoms of COVID-19 infection (high fever, persistent cough or severe acute respiratory infection).
On 15 April, the government in England had offered to test all symptomatic essential workers including the NHS (refer the full list of essential workers). Testing is also provided to those aged 65 years and older with symptoms and to symptomatic individuals who cannot work from home (for example, construction workers, shop workers, plumbers and delivery drivers), as well as anyone with symptoms who lives with those mentioned earlier. In addition, testing is being offered to social care workers and residents in care homes (with or without symptoms). The purpose is both to investigate outbreaks and, following successful pilots, as part of a rolling programme to test all care homes NHS workers and patients without symptoms, in line with NHS England guidance.,
On 23 April, all patients to be discharged to a care home will be tested before discharge to ascertain their COVID-19 status. In the first week of May, accident and emergency departments were directed to screen all patients admitted to the hospital. Rapid point-of-care tests have also become available now in some hospitals to facilitate improved segregation of patients.
As the outbreak is beginning to recede, several NHS and private hospitals are advised to screen asymptomatic patients admitted for elective procedures.
Challenges in testing
The critical challenge in testing has been the availability of reliable tests and testing equipment. In the early days of the outbreak, there was minimal capacity. Only the central PHE laboratory was able to perform the tests. As the epidemic progressed, the capacity of this laboratory was being overwhelmed. In mid-March, several laboratories in the NHS and the private sector developed the ability to test for SAR-CoV-2 viral RNA. Until recently, laboratory capacity was limited owing to reagent and testing platform shortages, as many are imported from the United States of America. The time taken for testing was another limitation. One procedure that prolongs the time taken for the test is the extraction of viral RNA required to identify the infection. A UK laboratory has discovered a process that eliminates the need for extraction and reduces the time taken for the test.10 This innovation has enabled laboratories to increase their capacity.
In summary, testing for COVID-19 infection in the UK has increased substantially in the last three months. Over 1.6 million tests have been performed in approximately 1.2 million patients.6 Currently, nearly 80, 000 to 100,000 tests are performed each day. The tests are provided to a wide range of patients and essential workers in hospitals, care homes and the community. This extensive testing together with isolation and containment of affected patients underpins UK's current strategy for controlling the COVID-19 pandemic.
COVID-19 testing in India
India is home to more than 1.3 billion people living in twenty-eight states and nine union territories. There are challenges of overcrowded cities, high levels of internal migration, inadequate hygiene and sanitation, economic constraints and an overburdened health sector. The delivery of health care largely rests on respective states and union territories, public health care being a state matter. The first coronavirus case was reported in India on 30 January 2020 in the state of Kerala. The number of confirmed COVID-19 cases started rising by early to mid-March, prompting the Government of India to draw up plans to contain the spread of disease. With the experience of managing the Nipah virus outbreak in 2018, a cluster containment strategy was adopted for breaking the chain of transmission. The responsibility of formulating guidelines, coordination and monitoring of COVID-19 testing was given to the Indian Council of Medical Research (ICMR), the apex health research body of the country.
Owing to multiple factors, including limited laboratory network and paucity of testing kits, the initial guidelines the ICMR issued on 9 and 17 March recommended tests to be performed only on symptomatic individuals with an international travel or contact history and healthcare workers treating patients with severe acute respiratory illness.11 The strategy was soon revised to include patients admitted to the hospital with severe acute respiratory illness. Among asymptomatic individuals, testing was recommended only for direct and high-risk contacts of a confirmed COVID-19 case (version 3 dated 20 March 2020)12 Later on, patients in hotspots and containment zones with influenza-like illness regardless of travel or contact history were added to the ambit (version 4 dated 09 April 2020).13 Separate guidelines on 20 April 2020 recommended testing even for asymptomatic pregnant women residing in cluster and containment areas, large migration gatherings and evacuee centres and presenting in labour or likely to deliver within the next five days.14
The laboratory network is continuously being strengthened to cover the vast area of the country. Until early March, the National Institute of Virology in Pune, Maharashtra, was the only laboratory in India performing the COVID-19 testing. As on 07 May, a total of 339 government and 123 private laboratories have been approved by the ICMR for COVID-19 testing. Of these, 380 (government 273 and private 107) are approved for real-time RT PCR, 53 (government 52 and private 01) for TrueNat test and 29 (government 14 and private 15) CBNAAT test for COVID-19. A total of 15, 23, 213 samples have been tested till 08 May confirming 59,662 cases, including 1981 deaths and 17,847 recovered patients.15
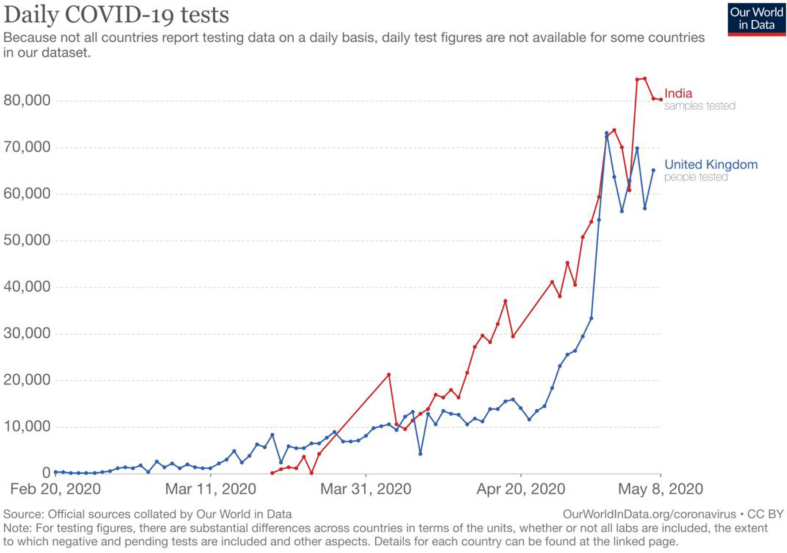
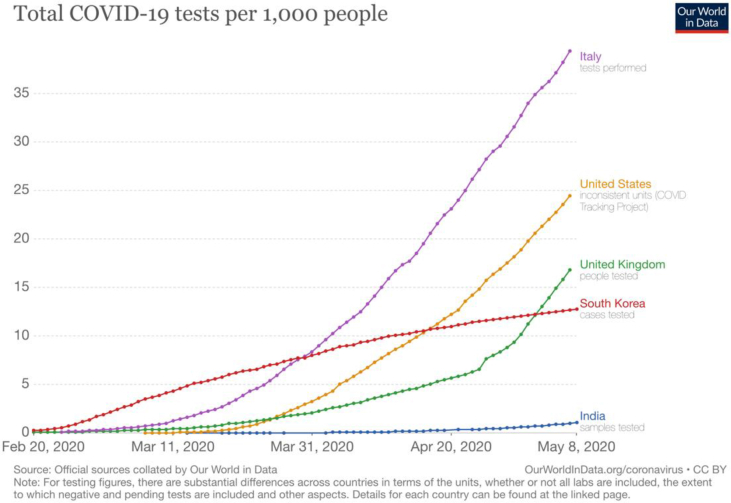
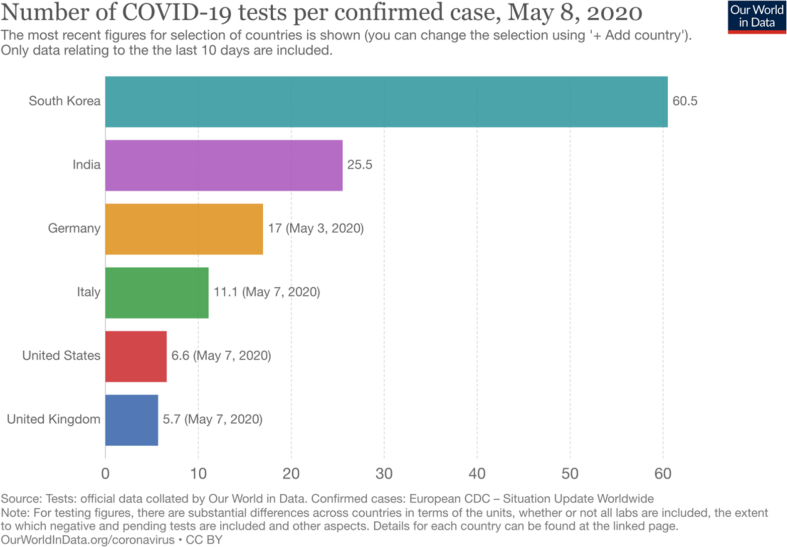
The testing capacity is continuously expanding in India. India aims to do a minimum of 100,000 tests per day by the end of May 2020. Questions have been raised whether India is testing adequately to get an accurate picture of COVID-19. Currently, India performs more tests per day than in the UK (Fig. 2).16 However, despite having processed around 1.5 million COVID-19 samples, India still has one of the lowest testing rates (1.04 tests per 1000 of the population) in the world. Putting it another way, the number of tests needed (NNT) to detect a case is 25.5 in India, second only to South Korea (Fig. 3).16 In comparison, in the USA and UK, the NNT to detect a case is 6.6 and 5.7, respectively (Fig. 4).16 These data suggest that the prevalence of the infection in India is substantially lower than in these countries.
Fig. 2.
Daily COVID-19 tests (India vs United Kingdom).
Fig. 3.
Total COVID-19 tests per 1000 population (India vs other countries).
Fig. 4.
Number of COVID-19 tests performed per confirmed case (India vs other countries).
Given the limited resources and the need to target testing effectively, the entire nation has been colour coded into red, orange and green zones based on district profiling of hotspots. The testing is prioritized accordingly with a focus on the hotspots and containment zones.
Conclusion
The UK and India have both been affected by the COVID-19 pandemic to a varying degree. Testing strategies in the two countries have mainly been driven by the availability of reliable tests and the severity of the pandemic in the two countries. Both countries faced a shortage of tests initially but have progressively increased their capacity for testing.
The UK is one of the most severely affected countries in the world. The testing was initially restricted to hospitalised patients. However, with the greater availability of the tests and increased capacity, the UK has cast its net wide to test symptomatic and asymptomatic patients admitted to hospitals, hospital staff, essential workers such as care home staff and their families. Seroprevalence studies have also started to establish the extent of spread of the infection in the community.
In contrast, India appears to have a relatively lower prevalence of the infection. However, the large size of the population, and importantly, the relatively insufficient availability of tests, has necessarily led the country to adopt a different testing strategy. The testing is currently focused on high-risk groups for acquiring the infection in the community and the hospitals.
Owing to the rapidly, evolving nature of the pandemic, it remains to be seen which of the two strategies for testing will be effective. Nevertheless, one size does not fit all. Solutions for controlling the pandemic should be pragmatic and country specific based on local circumstances.
Disclosure of competing interest
The authors have none to declare.
References
- 1.SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) Sequences [Internet]. [cited 2020 May 9]. Available from: https://www.ncbi.nlm.nih.gov/genbank/sars-cov-2-seqs/.
- 2.Advice on the Use of Point-Of-Care Immunodiagnostic Tests for COVID-19 [Internet]. [cited 2020 May 27]. Available from: https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19..
- 3.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.8259. Published online May 06. [DOI] [PubMed] [Google Scholar]
- 4.Hunt for contacts of coronavirus-stricken pair in York | News | Times [Internet]. [cited 2020 May 27]. Available from: https://www.thetimes.co.uk/article/hunt-for-contacts-of-coronavirus-stricken-pair-in-york-dh363qf8k.
- 5.Coronavirus: Latest Patient Was First to Be Infected in UK - BBC News [Internet]. [cited 2020 May 27]. Available from: https://www.bbc.co.uk/news/uk-51683428.html.
- 6.Number of Coronavirus (COVID-19) Cases and Risk in the UK - GOV.UK [Internet]. [cited 2020 May 27]. Available from: https://www.gov.uk/guidance/coronavirus-covid-19-information-for-the-public.
- 7.Department of Health and Social Care. [cited 2020 May 27]. Available from: http://www.legislation.gov.uk/uksi/2020/129/pdfs/uksiem_20200129_en.pdf.
- 8.Department of Health and Social Care Coronavirus Action Plan: A Guide to what You Can Expect across the UK - GOV.UK [Internet]. [cited 2020 May 27]. Available from: https://www.gov.uk/government/publications/coronavirus-action-plan/coronavirus-action-plan-a-guide-to-what-you-can-expect-across-the-uk.
- 9.Department of Health and Social Care . Coronavirus (COVID-19) Scaling up Our Testing Programmes [cited 2020 May 27]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/878121/coronavirus-covid-19-testing-strategy.pdf..
- 10.Grant P.R., Turner M.A., Shin G.Y., Nastouli E., Levett L.J. Extraction-free COVID-19 (SARS-CoV-2) diagnosis by RT-PCR to increase capacity for national testing programmes during a pandemic. bioRxiv. 2020 Apr 8 19:2020.04.06.028316. [Google Scholar]
- 11.Indian Council of Medical Research [Internet]. India [cited 2020 May 27]. Available from:https://www.icmr.gov.in/pdf/covid/strategy/Strategy_COVID19_testing_India. pdf.
- 12.Indian Council of Medical Research [Internet]. India [cited 2020 May 27]. Available from: https://www.icmr.gov.in/pdf/covid/strategy/2020-03-20_covid19_test_v3.pdf.
- 13.Indian Council of Medical Research [Internet]. India [cited 2020 May 27]. Available from: https://www.icmr.gov.in/pdf/covid/strategy/Strategey_for_COVID19_Test_v4_09042020.pdf.
- 14.Indian Council of Medical Research [Internet]. India [cited 2020 May 27]. Available from: https://www.icmr.gov.in/pdf/covid/strategy/COVID19_Testing_Strategy_for_Pregnant_Women.pdf..
- 15.India Coronavirus: 62,808 Cases and 2,101 Deaths - Worldometer [Internet]. [cited 2020 May 27]. Available from: https://www.worldometers.info/coronavirus/country/india/.
- 16.Coronavirus (COVID-19) Testing - Statistics and Research - Our World in Data [Internet]. [cited 2020 May 27]. Available from: https://ourworldindata.org/coronavirus-testing#different-types-of-tests-for-covid-19.