Abstract
BACKGROUND
A key challenge in the medical treatment of brain tumors is the limited penetration of most chemotherapeutic agents across the blood–brain barrier (BBB) into the tumor and the infiltrative margin around the tumor. Magnetic resonance-guided focused ultrasound (MRgFUS) is a promising tool to enhance the delivery of chemotherapeutic agents into brain tumors.
OBJECTIVE
To review the mechanism of FUS, preclinical evidence, and clinical studies that used low-frequency FUS for a BBB opening in gliomas.
METHODS
Literature review.
RESULTS
The potential of externally delivered low-intensity ultrasound for a temporally and spatially precise and predictable disruption of the BBB has been investigated for over a decade, yielding extensive preclinical literature demonstrating that FUS can disrupt the BBB in a spatially targeted and temporally reversible manner. Studies in animal models documented that FUS enhanced the delivery of numerous chemotherapeutic and investigational agents across the BBB and into brain tumors, including temozolomide, bevacizumab, 1,3-bis (2-chloroethyl)-1-nitrosourea, doxorubicin, viral vectors, and cells. Chemotherapeutic interventions combined with FUS slowed tumor progression and improved animal survival. Recent advances of MRgFUS systems allow precise, temporally and spatially controllable, and safe transcranial delivery of ultrasound energy. Initial clinical evidence in glioma patients has shown the efficacy of MRgFUS in disrupting the BBB, as demonstrated by an enhanced gadolinium penetration.
CONCLUSION
Thus far, a temporary disruption of the BBB followed by the administration of chemotherapy has been both feasible and safe. Further studies are needed to determine the actual drug delivery, including the drug distribution at a tissue-level scale, as well as effects on tumor growth and patient prognosis.
Keywords: Focused ultrasound, Therapy, Brain tumor, Glioma, Blood–brain barrier, Drug delivery
ABBREVIATIONS
- BBB
blood-brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- Da
daltons
- DNA
deoxyribonucleic acid
- FUS
focused ultrasound
- GBM
glioblastoma
- MRgFUS
magnetic resonance-guided focused ultrasound
- MRI
magnetic resonance imaging
- MGMT
O6-methylguanine-DNA methyltransferase
- TMZ
temozolomide
The prognosis for malignant gliomas remains poor relative to other cancers. There has been limited success in improving the prognosis for patients with glioblastoma (GBM) despite extensive efforts on multiple fronts. The median survival for patients with GBM who undergo gross total surgical resection followed by adjuvant therapy with temozolomide (TMZ) chemotherapy concurrent with radiotherapy still remains approximately 15 mo and rarely exceeds 2 yr after diagnosis.1 Low-grade gliomas can be successfully controlled over prolonged periods with surgical resection, radiotherapy, and chemotherapy; however, tumor progression is often inevitable.2 Poor prognosis for glioma patients is, in part, because the penetration of systemic chemotherapeutic agents into the central nervous system (CNS) is largely restricted by the blood–brain barrier (BBB).
The BBB plays a critical role in the maintenance of brain homeostasis by protecting the brain from both exogenous and endogenous substances that can be potentially damaging. The BBB is a complex and highly selective semipermeable system that is mainly formed by capillary endothelial cells connected via tight junctions.3,4 Other important supporting cells of the BBB are vascular smooth muscle cells, pericytes, immune cells, glial cells, and neural cells.5 Transport of various molecules across the BBB is accomplished via passive diffusion of lipid soluble molecules that have molecular weight of less than approximately 400 daltons (Da) or via active transport systems.3
The BBB is an obstacle to the effective treatment of brain tumors because of its limitations of drug delivery and penetration.6,7 One of the factors limiting the transfer of chemotherapeutic agents used for the treatment of gliomas across the BBB is their large molecular weight, eg, doxorubicin ∼540 Da and bevacizumab 149 kDa. TMZ, usually the primary treatment option for gliomas, is a relatively small lipophilic molecule of 194 Da and, therefore, can cross the BBB.8 It has been shown that TMZ levels in the brain and cerebrospinal fluid (CSF) reach up to 20% of the drug plasma concentration.9 However, the therapeutic potential of TMZ for glioma treatment is limited by a short half-life (1.8 h), which requires continuous drug administration to maintain the therapeutic concentration of the drug in tumor tissue and to optimize therapeutic potential. However, this is precluded by systemic side effects of TMZ as well as by the propensity of glioma cells to develop a resistance to TMZ.10 To overcome these limitations, there is an increasing interest in developing nanocarrier delivery systems of TMZ; however, they are often limited by a reduced transfer across the BBB.10,11
In many malignant brain tumors, the BBB is dysfunctional, and its integrity is variable. BBB within tumors comprises both existing and newly formed blood vessels that lack normal physiological structure and are “leaky”, because their formation occurs as a result of abnormal angiogenesis.12,13 Hence, the abnormal permeability of the BBB in brain tumors may allow extravasation of larger molecules.13-15 However, the permeability of the BBB is also characterized by significant differences between tumors and spatial heterogeneity within different areas of infiltrative brain tumors.13,14 Evidence of BBB disruptions in high-grade gliomas is documented by the accumulation of radiographic contrast material within brain tumor tissue, which normally does not penetrate into the brain with an intact BBB. Dynamic contrast enhanced magnetic resonance imaging (MRI) allows a quantitative estimation of vascular permeability by measuring the transport constant of contrast molecules across different contrast-enhancing regions.16 Convincing evidence from surgical and autopsy series indicates that glioma is a whole-brain disease that extends well beyond the radiographically defined tumor borders using contrast enhancement areas on T1-weighted MRI sequences as well as beyond the T2-weighted/fluid-attenuated inversion-recovery signal abnormality.17-19 Therefore, the BBB is largely nondisrupted in a significant fraction of the total volume of high-grade gliomas and in brain regions of distant invading tumor cells and in virtually all low-grade gliomas that do not enhance with an intravenous administration of contrast agents.
Because of both intertumor and intratumor heterogeneities of BBB permeability, the penetration of chemotherapeutic agents into gliomas is largely unpredictable. This poses an important therapeutic dilemma, as the localized concentration and spatial distribution of systematically administered drug within the tumor volume is unclear; hence, it is difficult to accurately estimate whether the drug penetration into the tumor mass is sufficient to reach a sufficient localized drug concentration that is needed to control the tumor growth and achieve therapeutic goals.13,20 Preclinical studies documented that the tumor tissue-to-plasma ratio of antiglioma agents is lower in nonenhancing areas of gliomas when compared to an enhancing tumor.21,22 Within the peritumor tissue, local tissue concentrations of chemotherapeutic agents, such as carboplatin and paclitaxel, are up to 40 times lower than those at the tumor center.6,22 Heterogeneity of drug distribution within the tumor can also contribute to cancer cell reprogramming, leading to the emergence of chemotherapy-resistant cell clones even in the absence of pre-existent resistant cells.23,24
Numerous methods have been tested with the goal of transiently disrupting the BBB and enhancing drug delivery into brain tumors.13,15 Approaches include chemical disruption involving the administration of vasoactive compounds, mannitol, polymeric nanoparticles and microparticles, radiation therapy, and convection-enhanced delivery. Despite improved drug concentrations at the target, present limitations of direct intracranial injection or convection-enhanced delivery relate to the risk of surgery as well as the difficulty in performing repeated administrations. Chemical BBB disruption can cause an unpredictable generalized BBB disruption that can pose risks to healthy brain tissue and systemic side effects.25 With regard to the modification of therapeutics to bypass the BBB via transcytosis by coupling it with a monoclonal antibody against the BBB cellular target (for example, human insulin receptors), concerns relate to low spatial specificity and off-target effects.26 Radiotherapy has also been shown to open the BBB, but it can be temporally unpredictable and cause damage to healthy brain tissues. Despite these efforts, BBB opening strategies are not routinely used in neuro-oncology clinical practice outside of research protocols because of the limited clinical experience regarding the efficacy and safety of these interventions.
Focused ultrasound (FUS) is a promising intervention for BBB disruption that has been widely studied across preclinical brain tumor models with promising results, and initial studies in brain tumor patients are underway. Here, we will discuss the mechanism of FUS, preclinical evidence, and clinical studies that used low-frequency FUS for BBB in the treatment of gliomas. The role of FUS for BBB in metastatic brain disease was recently reviewed elsewhere.27
FOCUSED ULTRASOUND
The biological effect of FUS is a function of ultrasound energy, frequency, intensity, treatment duration, and target volume. Recent technological advances have substantially improved the spatial accuracy, monitoring, safety, and clinical efficacy of transcranial magnetic resonance-guided FUS (MRgFUS), and opened doors for a more widespread use of FUS in clinical practice. High-intensity MRgFUS uses 650-Hz-frequency ultrasound energy, which can increase tissue temperature to 65°C, causing a spatially precise thermal ablation of targeted tissues.28,29 The method was clinically approved for thalamotomy for essential tremor in 2016,30 for tremor-dominant Parkinson disease in 2018,31 and is under investigation for other functional applications.32 Thermal ablation of tumor using high-intensity MRgFUS showed initial promise for the treatment of brain tumors but was largely abandoned because of the limited-treatment envelope and the time required to ablate a significant volume of tumor.33-35 With high-intensity ablation, ultrasound energy is delivered through the skull without overheating the bone by employing active cooling of the scalp and by widely distributing ultrasound energy over the skull. Precise targeting of ultrasound energy within the brain is achieved by using stereotactic targeting systems. MRgFUS systems are steerable and compensate for tissue structures, such as skull thickness variability, allowing precise targeting of different brain and tumor areas.36,37
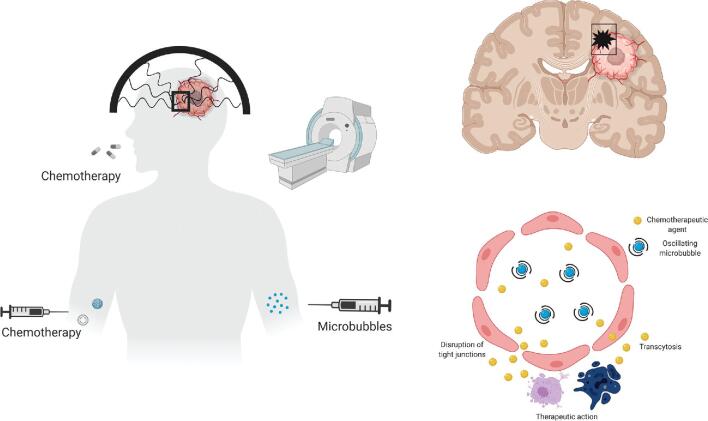
Low-intensity MRgFUS is an emerging technology, which allows us to perform a temporally and spatially predictable, controllable, and safe disruption of the BBB (Figure 1).38,39 Substantially lower intensity ultrasound energy used for BBB disruption does not cause irreversible tissue damage. Instead, low-intensity ultrasound bursts are combined with circulating microbubbles, which concentrate the ultrasound effects on the vasculature, resulting in a temporary and local disruption (permeabilization) of the BBB. Exogenous microbubbles are lipid spheres encapsulating a perfluorocarbon gas, which are commonly used as ultrasound contrast agents, eg, Definity® (Lantheus Medical Imaging Inc). Microbubbles can also be composed of proteins and polymers with other gases, resulting in different physiochemical properties.40 The introduction of the microbubbles reduces the exposure level needed by orders of magnitude compared to those used for thermal ablation, removing the skull-related limitations of high-intensity FUS.40,41 Another important advantage of modern MRgFUS systems is the real-time monitoring of biological effects of ultrasound energy delivered to the CNS with either thermometry for measuring local heating effects (used for high-intensity thermal ablation) or acoustic monitoring of emissions from oscillating microbubbles (used for BBB opening). Based on the spectral content of the emissions, the sonication can be monitored in near real time.42,43 Broadband emissions indicate inertial cavitation, which may potentially cause vascular damage (petechiae).44 Research in animal models using imaging biomarkers and a histological confirmation of BBB opening documented that the duration of BBB opening may last for up to 24 h after a single treatment session, and this time depends on the size of the administered agent (Figure 2).45,46 Confirmation of BBB opening using noninvasive imaging biomarkers in close temporal proximity to FUS therapy is important to ensure the efficacy of MRgFUS therapy. In clinical studies, BBB opening is confirmed with gadolinium-enhanced T1w brain MRI (Figure 3).39,47 Furthermore, the conjugation of chemotherapeutic agents with MRI contrast agents can help us directly visualize the penetration of a chemotherapeutic agent into the tumor.48 However, this approach remains investigational.
FIGURE 1.
Mechanism of action increased BBB permeability using the FUS. Made in © BioRender – biorender.com.
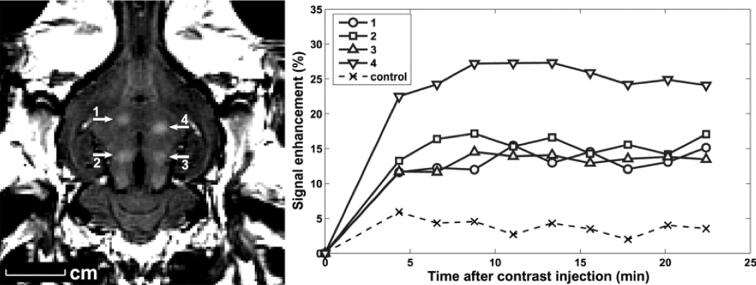
FIGURE 2.
Focal BBB disruption in the rabbit brain using FUS. Left panel, Localized extravasation of Magnevist (MRI contrast agent) in areas of blood-barrier disruption as seen on contrast enhanced T1-weighted brain MRI. Right panel, Change in contrast enhancement over time in the 4 sonicated brain regions and in nonsonicated (control) brain region. Reprinted with permission from Jolesz FA, McDannold N. Current status and future potential of MRI‐guided focused ultrasound surgery. Journal of Magnetic Resonance Imaging. 2008;27(2):9. (c) 2008 Wiley-Liss, Inc.
FIGURE 3.
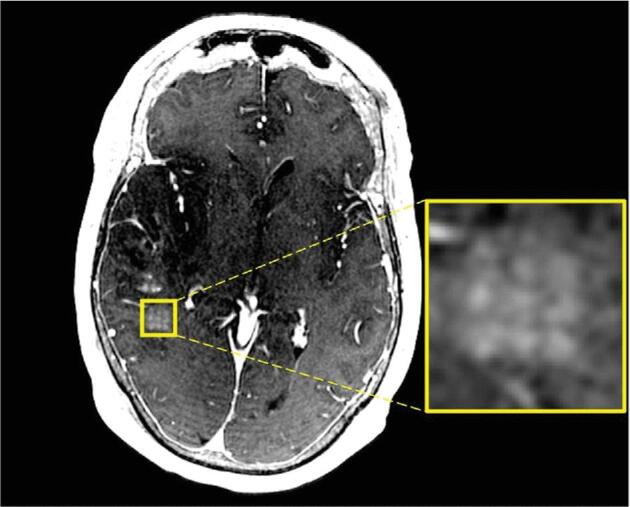
T1-weighted gadolinium-enhanced axial brain MRI immediately after BBB disruption with MRgFUS that demonstrates contrast extravasation in a discrete and precise grid pattern (enlarged panel) in regions where sonication was performed. Figure is reprinted from Mainprize T, et al (2019). Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Scientific Reports, 9(1), 321. https://doi.org/10.1038/s41598-018-36340-0. The figure is licensed under the Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/.
The hypothesized biological mechanisms underlying the BBB opening with low-energy FUS include the following: disruption of endothelial cell tight junctions, potentiation of transcytosis,49 and suppression of P-glycoprotein drug efflux (Figure 1).50 The exact mechanism or mechanisms that produce these effects remain unclear but are thought to be related to mechanical effects on the vasculature, resulting from the microbubble oscillations in the ultrasound fields. It is important to ensure that the microbubbles undergo the so-called “stable cavitation” instead of “inertial cavitation.”
With stable cavitation, microbubbles oscillate within the ultrasound field, impinging forces on the vessel wall and producing shear stress caused by the streaming of the surrounding fluid. At higher intensities, the microbubbles grow because of gas diffusion and eventually collapse violently because of the inertia of the surrounding medium. The bubble collapse during inertial cavitation can cause shock waves and violent jetting, which can damage blood vessels. This damage is manifested as petechiae, and in extreme cases, ischemia is due to the loss of blood supply.
FUS-induced BBB opening and permeability of molecule size correlates positively with acoustic pressures.51 For example, it was shown that acoustic pressures of 0.31 MPa caused a BBB opening size smaller than 3 kDa (2.3 nm), whereas acoustic pressures of 0.84 MPa caused a BBB opening for molecules up to 2000 kDa (54.4 nm).51 Stable cavitation was associated with a smaller BBB opening size (up to 70 kDa), whereas a larger BBB opening size (above 500 kDa) was achieved with inertial cavitation, which is usually associated with tissue damage. Other parameters, including the frequency, microbubble diameter, burst length, burst frequency, and sonication duration, influence the “magnitude” of the disruption and the amount of agent that is delivered to the brain.52
PRECLINICAL STUDIES
Numerous studies in small and large animal glioma models have evaluated the safety and efficacy of BBB opening with FUS. In animal studies, BBB opening is immediate, repeatable, resolves within 6 to 8 h, and does not cause axonal or neuronal injury. In addition to this, an enhanced delivery of various drugs has been shown in small to large animal models. These drugs include trastuzumab,53 doxorubicin,52 TMZ,54 methotrexate,55 and carboplatin.56 In addition, this approach has been used to deliver viruses57 and cells58 (Table 1).
TABLE 1.
Chemotherapeutic Agents of Gliomas Tested With FUS BBB Disruption in Animal Models
| Chemotherapeutic agent | Main FUS effects/findings | References |
|---|---|---|
| TMZ | Improved penetration to CSF, brain, and tumor tissue | 54, 59, 62 |
| Slower tumor growth rate | ||
| Better tumor control | ||
| Longer survival of treated animals | ||
| Enhanced delivery of MGMT inactivator liposomal O6BTG | ||
| Bevacizumab | Greater CNS penetration with expected antiangiogenic effects | 64 |
| Slower tumor growth | ||
| Prolonged animal survival | ||
| BCNU | Better CNS penetration | 95 |
| Better tumor control | ||
| Longer animal survival | ||
| Doxorubicin | Improved CNS penetration | 42, 70 |
| Better tumor control rate | 73, 96 | |
| Improved animal survival | ||
| Carboplatin | Improved CNS penetration | 56 |
| Better tumor control rate | ||
| Improved animal survival |
Chemotherapeutic Agents
Temozolomide
TMZ is a deoxyribonucleic acid (DNA)-alkylating chemotherapeutic agent that is presently the first-line option for the treatment of gliomas. Despite its limited clinical efficacy, oral TMZ remains the mainstay treatment for O6-methylguanine-DNA methyltransferase (MGMT)-methylated high-grade gliomas and is also often used in the management of MGMT-unmethylated high-grade gliomas as well as low-grade gliomas.1
Studies in rat and mouse glioma models reported that the BBB opening with FUS was associated with increased tissue concentrations of TMZ, which translated into better tumor control rates and prolonged survival of animals. In Fisher rats implanted with 9-L glioma cells, the BBB opening with FUS after the administration of TMZ relative to TMZ alone was associated with a higher TMZ CSF/plasma ratio, reduced 7-d tumor progression ratio, and an improved survival of TMZ-FUS-treated animals by 38% relative to controls.54 Another study in nude mouse implanted with U87 human glioma cells and treated with different doses of TMZ found that the BBB opening with FUS increased TMZ accumulation in the brain tissue, increased TMZ degradation in the tumor core, slowed down tumor progression, and prolonged animal survival.59
The clinical benefit of TMZ is minimal in gliomas with an unmethylated promoter region of the MGMT gene.60 Hence, there is an increasing interest in MGMT-gene-modulation strategies in order to overcome the resistance to TMZ.61 A recent study explored low-intensity, microbubble-enhanced MgFUS for increasing the brain delivery of MGMT inactivator liposomal O6-(4-bromothenyl)guanine (O6BTG) in mouse bearing TMZ-resistant gliomas. MgFUS facilitated liposomal O6BTG delivery, which was associated with reversed MGMT resistance and resulted in MGMT depletion in Vivo, which was associated with a reduced tumor growth and prolonged survival of glioma-bearing mouse.62
These data suggest that FUS could be an effective strategy for facilitating the delivery of TMZ as well as liposomal MGMT inactivators in small animal models and, therefore, has a therapeutic potential in the management of glioma patients by optimizing TMZ delivery to brain tumors and modulating tumor resistance to TMZ.
Bevacizumab
Bevacizumab, a humanized monoclonal antibody that specifically binds to and inhibits vascular endothelial growth factor, is commonly used as a second-line agent for the treatment of recurrent gliomas.63 It was shown to be efficacious for controlling peritumoral edema and improving quality of life; however, the effects of bevacizumab for improving patient prognosis remain less clear.60
A study in a U87 glioma mouse model found that FUS with microbubbles increased bevacizumab penetration in the CNS by 5.7 to 56.77-fold.64 Furthermore, animals treated with bevacizumab and FUS had a lower tumor vessel density and lesser vascular distribution in highly proliferative tumor rims. Animals treated with bevacizumab and FUS had a slower tumor growth rate and a longer overall survival compared to control animals and animals treated with bevacizumab or FUS alone.
BCNU
1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) can be used as a second-line agent for the treatment of recurrent GBMs; however, it is associated with only a modest improvement in survival when used as a single agent65 or in combination with other adjuvant chemotherapy agents.66 Furthermore, a systemic administration of BCNU often causes significant systemic toxicity, which prevents continued therapy. Alternatively, it has been shown that BCNU wafers implanted in the GBM resection cavity can improve the survival of GBM patients with an acceptable safety profile.66
The addition of FUS to BCNU therapy in a rat C6 glioma model enhanced the penetration of BCNU through the BBB by 202%.64 Treatment with FUS before BCNU administration was associated with a suppressed tumor growth, better tumor control, and longer animal survival compared to BCNU alone.
Doxorubicin
Doxorubicin is an anthracycline antibiotic that blocks topoisomerase II and inhibits DNA and ribonucleic acid synthesis. It has been shown to be an effective treatment option across solid tumors. However, doxorubicin has only limited clinical efficacy in glioma patients mainly because of poor bioavailability to the brain.67 High doses of doxorubicin are associated with systemic toxicity and can be neurotoxic. However, there remains an interest in doxorubicin for glioma treatment, because it has been shown that doxorubicin can potentiate the TMZ effect.68 Furthermore, an intratumoral administration of doxorubicin via an Ommaya reservoir was safe and associated with a durable tumor response.69
Studies in animal models indicate that FUS can be an effective intervention that can increase the penetration of doxorubicin across the BBB and improve the tumor control rate and animal survival.42,70 A study in a mouse GBM model using cerebral microdialysis found that FUS opening was associated with a 2.35-fold greater tumor-to-normal brain doxorubicin ratio, with a 10 times greater peak doxorubicin concentration.71 Encapsulation of doxorubicin in liposomes reduces side effects and prolongs circulation. FUS can deliver liposomal doxorubicin across the BBB despite its large size (80-85 nm)72 and improve survival in a rat glioma model.73
Viral Therapy
Viral therapy can be promising in the management of glioma patients.74,75 However, the BBB poses a significant barrier for the CNS transfer of some viral vectors.76 In order to overcome the BBB, viral vectors are usually implanted via open surgery or using stereotactic surgical techniques. Furthermore, despite their ability to replicate and infect tumor cells, local delivery can still result in uneven spatial coverage of tumor volume. MRgFUS could be a promising technique for guided viral vector transmission in brain tumors and allow delivery of the vector to the whole tumor because of their ability to infect cells. In mouse and rat glioma models, MRgFUS combined with intravenously injected microbubbles facilitated delivery of a recombinant adeno-associated viral vector into brain parenchyma and was associated with transgene expression in Vivo.77
Cell Therapy
Given the limited clinical efficacy of existing adjuvant therapeutic approaches, cell therapies are being actively investigated as possible alternatives for treatment of gliomas. Chimeric antigen receptor T cell therapy showed promising results in hematologic malignancies and initial experience suggest that T cell engineering can be a promising therapeutic option of gliomas.78 However, limitations of T cell trafficking into the CNS usually requires direct intraventricular administration. FUS was shown to facilitate transfer of immune and neural stem cells across the BBB.58,79
Immunomodulation
Another important biological action of high-intensity FUS is immunomodulation.80 Proposed mechanisms underlying immunomodulatory actions of high-intensity FUS include destruction of tumor cells with high-intensity FUS, uncovering tumor antigen epitopes, and activating heat shock proteins and adenosine triphosphate that stimulate innate immunity and increase tumor immunogenicity. Cavitation-induced BBB opening facilitates transfer of immune cells from blood into tumor, and FUS suppresses tumor-induced immunosuppression via modulation of immune system activity, including increased cytotoxic T lymphocyte activity, decrease of anti-inflammatory cytokine levels, NK cell stimulation, and other mechanisms.80
CLINICAL EVIDENCE IN GLIOMA PATIENTS
Despite abundant preclinical evidence in glioma animal models indicating efficacy of BBB disruption using low-intensity FUS for enhanced delivery of various chemotherapeutic agents and viral vectors to the CNS,51 evidence regarding safety and efficacy of this treatment method in patients with brain tumors remains limited.
One published study to date evaluated safety and efficacy of transcranial MRgFUS for drug delivery in glioma patients. A small phase I, single arm, open label study of 5 patients with high-grade glioma investigated transcranial low-intensity MRgFUS with the ExAblate Neuro system (InSightec Tirat Carmel, Israel) with microbubble (Definity®; Lantheus Medical Imaging, North Billerica, MA, USA) injection for BBB opening in conjunction with systemic administration of subtherapeutic dose of chemotherapy (liposomal doxorubicin n = 1 or temozolomide n = 4) that was administered one hour prior MRgFUS.39 Surgical tumor resection was performed the next day. The procedure was well-tolerated, with successful opening of the BBB based on increased gadolinium enhancement. Tissue liquid-chromatography mass spectrometry analysis demonstrated greater concentration of liposomal doxorubicin and oral TMZ in brain regions where BBB disruption occurred compared to areas without BBB disruption.
A recent single-center trial (NCT02253212) investigated safety and efficacy of implantable, low-intensity, pulsed ultrasound device (SonoCloud-1; CarThera, Paris, France) with microbubble injection in 21 patients with recurrent GBM.81,82 At least 1 sonication was achieved in 19 patients. BBB disruption was evaluated with contrast-enhanced T1-weighted brain MRI and was visible after 52 out of 65 ultrasound sessions. The treatment was safe without serious adverse events or carboplatin-related neurotoxicity. Patients with documented BBB disruption (n = 11) relative to patients without or with poor BBB disruption (n = 8) had longer progression free survival (4.11 vs 2.73 mo) and overall survival (12.94 vs 8.64 mo).
Clinical trials evaluating BBB disruption in glioma patients using different FUS systems currently are ongoing (Table 2). The ExAblate Neuro™ system (Isightec Ltd, Haifa, Israel) recently approved by the Food and Drug Administration for thalamotomy provides a focal therapy that can penetrate through the skull to target tissues and create BBB disruption in small, discrete areas. Two phase I clinical trials are currently recruiting. The system combines FUS delivery with a conventional diagnostic 1.5T or 3T MRI scanner.
TABLE 2.
Ongoing BBB Disruption Clinical Trials in Glioma Patients
| Study title | ClinicalTrials.gov identifier | Study location(s) | Intervention | Condition | Status |
|---|---|---|---|---|---|
| Safety and Efficacy of Transient Opening of the Blood-brain Barrier (BBB) With the SonoCloud-9 (SC9-GBM-01) | NCT03744026 | Hôpital Neurologique Pierre Wertheimer, Bron, France | SonoCloud-9 (CarThera, Paris, France) | GBM | Recruiting |
| ExAblate Blood-Brain Barrier Disruption for Glioblastoma in Patients Undergoing Standard Chemotherapy | NCT03712293 | Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea | ExAblate Model 4000 Type 2.0 (Insightec Tirat Carmel, Israel) | GBM | Recruiting |
| Safety of BBB Disruption Using NaviFUS System in Recurrent Glioblastoma Multiforme (GBM) Patients | NCT03626896 | Linkou Chang Gung Memorial Hospital, Taoyuan City, Taiwan | NaviFUS System (NaviFUS, Taiwan) | GBM, brain tumor | Completed |
| Assessment of Safety and Feasibility of ExAblate Blood-Brain Barrier (BBB) Disruption for Treatment of Glioma | NCT03616860 | Sunnybrook Health Sciences Centre, Toronto, Canada | ExAblate (Insightec Tirat Carmel, Israel) | GBM | Recruiting |
| ExAblate (Magnetic Resonance-guided Focused Ultrasound Surgery) Treatment of Brain Tumors | NCT01473485 | Sunnybrook Health Sciences Centre, Toronto, Canada | ExAblate Transcranial System (Insightec Tirat Carmel, Israel) | Gliomas, metastatic brain cancer | Recruiting |
| ExAblate Blood Brain Barrier Disruption (BBBD) for Planned Surgery in Suspected Infiltrating Glioma | NCT03322813 | University of Maryland Medical System, Baltimore, Maryland | ExAblate 4000 Type 2 (Insightec Tirat Carmel, Israel) | Glioma | Recruiting |
| Assessment of Safety and Feasibility of ExAblate Blood-Brain Barrier (BBB) Disruption for Treatment of Glioma | NCT03551249 | Brigham Women's Hospital and University of Maryland, Baltimore, Maryland | ExAblate (Insightec Tirat Carmel, Israel) | GBM | Recruiting |
Safety
Abundant preclinical and growing clinical evidence suggest that low-intensity MRgFUS can be administered safely, as it uses only a small fraction of energy compared to FUS-based thermal ablation.83 There were no serious FUS-related adverse events in 1 study that used low-intensity MRgFUS in 5 glioma patients within 24 h window after MRgFUS treatment preceding scheduled tumor resection surgery.39 However, 1 patient had to abort the procedure because of back pain while on the MRI table, and 2 patients experienced minor self-limiting headaches at the helmet attachment site. The most concerning side effects associated with MRgFUS include brain hemorrhage and edema. Immediate intracranial complications can be detected early with MRI scanning that is also used to confirm BBB opening. Optimal and clinically meaningful therapeutic strategies of MRIgFUS as well as safety profile of repeated MRgFUS BBBD treatment sessions remain to be established for treatment of brain tumors.84 Microbubble intravenous injection is generally accepted as safe with low risk of serious adverse events.85
Clinical experience with repeated application of FUS with microbubble injection is limited. Studies in mice,86 rats, pigs,87,88 dogs,89 and nonhuman primates90-92 have evaluated the safety profile of repeated FUS-induced BBB disruption. Repeated BBB disruption can be achieved safely over multiple sessions without significant damage detected by histology or behavioral testing. However, such studies have also revealed the potential damage that can occur with repeated BBB disruption. The most commonly reported damage is vascular injury in the form of petechiae, presumably resulting from inertial cavitation. Others have shown that repeated BBB opening can be associated with inflammatory response, apoptosis, and tissue damage when using excessive energy.93-96
CONCLUSION
BBB opening using FUS is an emerging treatment modality for brain tumors that is expected to facilitate transfer of chemotherapeutic and other agents across the BBB and, hence, optimize exposure to brain tumor and limit systemic toxicity. Extensive research in animal models have documented that FUS facilitates transfer of TMZ, bevacizumab, doxorubicin, and BCNU across the BBB and improves tumor control rates and animal survival. Initial experience with brain tumor patients documented that MRgFUS is a safe treatment method; larger studies evaluating possible clinical efficacy and feasibility of MRgFUS are underway.
Disclosures
This work was supported by National Institute of Mental Health under Grant R01MH116858 and National Center for Image Guided Therapy under Grant P41 EB015898. Dr MacDannold receives research support from Insightec. Dr Golby is principal investigator of a clinical trial of blood brain barrier disruption sponsored by Insightec. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ et al.. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 2. Hottinger AF, Hegi ME, Baumert BG. Current management of low-grade gliomas. Curr Opin Neurol. 2016;29(6):782-788. [DOI] [PubMed] [Google Scholar]
- 3. Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1)13-25. [DOI] [PubMed] [Google Scholar]
- 4. Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72(5):648-672. [DOI] [PubMed] [Google Scholar]
- 5. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663-1674. [DOI] [PubMed] [Google Scholar]
- 7. Achrol AS, Rennert RC, Anders C et al.. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5. [DOI] [PubMed] [Google Scholar]
- 8. Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5(2):144-151. [DOI] [PubMed] [Google Scholar]
- 9. Ostermann S, Csajka C, Buclin T et al.. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10(11):3728-3736. [DOI] [PubMed] [Google Scholar]
- 10. Berrocal A, Perez Segura P, Gil M et al.. Extended-schedule dose-dense temozolomide in refractory gliomas. J Neurooncol. 2010;96(3):417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee CY. Strategies of temozolomide in future glioblastoma treatment. Onco Targets Ther. 2017;10:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1-12. [DOI] [PubMed] [Google Scholar]
- 14. Karim R, Palazzo C, Evrard B, Piel G. Nanocarriers for the treatment of glioblastoma multiforme: current state-of-the-art. J Control Release. 2016;227:23-37. [DOI] [PubMed] [Google Scholar]
- 15. Oberoi RK, Parrish KE, Sio TT, Mittapalli RK, Elmquist WF, Sarkaria JN. Strategies to improve delivery of anticancer drugs across the blood–brain barrier to treat glioblastoma. Neuro Oncol. 2016;18(1):27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao Y, Sundgren PC, Tsien CI, Chenevert TT, Junck L. Physiologic and metabolic magnetic resonance imaging in gliomas. J Clin Oncol. 2006;24(8):1228-1235. [DOI] [PubMed] [Google Scholar]
- 17. Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34(6):463-469. [DOI] [PubMed] [Google Scholar]
- 19. Sahm F, Capper D, Jeibmann A et al.. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch Neurol. 2012;69(4):523. [DOI] [PubMed] [Google Scholar]
- 20. Lockman PR, Mittapalli RK, Taskar KS et al.. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104(3):629-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarkaria JN, Hu LS, Parney IF et al.. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hata AN, Niederst MJ, Archibald HL et al.. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22(3):262-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vargas-Garcia CA, Torre EA, Herlyn M et al.. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature. 2017;546(7658):431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Askoxylakis V, Arvanitis CD, Wong CSF, Ferraro GB, Jain RK. Emerging strategies for delivering antiangiogenic therapies to primary and metastatic brain tumors. Adv Drug Deliv Rev. 2017;119:159-174. [DOI] [PubMed] [Google Scholar]
- 26. Ohshima-Hosoyama S, Simmons HA, Goecks N et al.. A monoclonal antibody-GDNF fusion protein is not neuroprotective and is associated with proliferative pancreatic lesions in parkinsonian monkeys. PLoS One. 2012;7(6):e39036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng Y, Suppiah S, Surendrakumar S, Bigioni L, Lipsman N. Low-intensity MR-guided focused ultrasound mediated disruption of the blood-brain barrier for intracranial metastatic diseases. Front Oncol. 2018;8:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meng Y, Suppiah S, Mithani K, Solomon B, Schwartz ML, Lipsman N. Current and emerging brain applications of MR-guided focused ultrasound. J Ther Ultrasound. 2017;5(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang TR, Dallapiazza R, Elias WJ. Neurological applications of transcranial high intensity focused ultrasound. Int J Hyperthermia. 2015;31(3):285-291. [DOI] [PubMed] [Google Scholar]
- 30. Elias WJ, Lipsman N, Ondo WG et al.. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med. 2016;375(8):730-739. [DOI] [PubMed] [Google Scholar]
- 31. Bond AE, Shah BB, Huss DS et al.. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease. JAMA Neurol. 2017;74(12):1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jung HH, Kim SJ, Roh D et al.. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive-compulsive disorder: a proof-of-concept study. Mol Psychiatry. 2015;20(10):1205-1211. [DOI] [PubMed] [Google Scholar]
- 33. McDannold N, Clement GT, Black P, Jolesz F, Hynynen K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery. 2010;66(2):323-332;discussion 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ram Z, Cohen ZR, Harnof S et al.. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery. 2006;59(5):949-955;discussion 955-6. [DOI] [PubMed] [Google Scholar]
- 35. Coluccia D, Fandino J, Schwyzer L et al.. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound. 2014;2(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monteith S, Sheehan J, Wintermark M et al.. Transcranial MRI-guided focused ultrasound: a review of the technologic and neurologic applications. Am J Roentgenol. 2015;205(1):150-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moser D, Zadicario E, Schiff G, Jeanmonod D. MR-guided focused ultrasound technique in functional neurosurgery: targeting accuracy. J Ther Ultrasound. published online: 2013 (doi:10.1186/2050-5736-1-3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640-646. [DOI] [PubMed] [Google Scholar]
- 39. Mainprize T, Lipsman N, Huang Y et al.. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. 2019;9(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu HL, Fan CH, Ting CY, Yeh CK. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics. 2014;4(4):432-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu HL, Chen PY, Yang HW et al.. In vivo MR quantification of superparamagnetic iron oxide nanoparticle leakage during low-frequency-ultrasound-induced blood-brain barrier opening in swine. J Magn Reson Imaging. 2011;34(6):1313-1324. [DOI] [PubMed] [Google Scholar]
- 42. Sun T, Zhang Y, Power C et al.. Closed-loop control of targeted ultrasound drug delivery across the blood–brain/tumor barriers in a rat glioma model. Proc Natl Acad Sci USA. 2017;114(48):E10281-E10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O’Reilly MA, Hynynen K. Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions–based controller. Radiology. 2012;263(1):96-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51(4):793-807. [DOI] [PubMed] [Google Scholar]
- 45. Jolesz FA, McDannold N. Current status and future potential of MRI-guided focused ultrasound surgery. J Magn Reson Imaging. 2008;27(2):391-399. [DOI] [PubMed] [Google Scholar]
- 46. Marty B, Larrat B, Van Landeghem M et al.. Dynamic study of blood–brain barrier closure after its disruption using ultrasound: a quantitative analysis. J Cereb Blood Flow Metab. 2012;32(10):1948-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lipsman N, Meng Y, Bethune AJ et al.. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 2018;9(1):2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thanou M, Gedroyc W. MRI-guided focused ultrasound as a new method of drug delivery. J Drug Deliv. 2013;2013:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30(7):979-989. [DOI] [PubMed] [Google Scholar]
- 50. Cho H, Lee H-Y, Han M et al.. Localized down-regulation of P-glycoprotein by focused ultrasound and microbubbles induced blood-brain barrier disruption in rat brain. Sci Rep. 2016;6(1):31201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen H, Konofagou EE. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J Cereb Blood Flow Metab. 2014;34(7):1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aryal M, Vykhodtseva N, Zhang Y-Z, McDannold N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood–brain barrier disruption: a safety study. J Control Release. 2015;204:60-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci. 2006;103(31):11719-11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wei K-C, Chu P-C, Wang H-YJ et al.. Focused ultrasound-induced blood–brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS One. 2013;8(3):e58995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mei J, Cheng Y, Song Y et al.. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med. 2009;28(7):871-880. [DOI] [PubMed] [Google Scholar]
- 56. Dréan A, Lemaire N, Bouchoux G et al.. Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J Neurooncol. 2019;144(1):33-41. [DOI] [PubMed] [Google Scholar]
- 57. Thévenot E, Jordão JF, O’Reilly MA et al.. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther. 2012;23(11):1144-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6(11):e27877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu H-L, Huang C-Y, Chen J-Y, Wang H-YJ, Chen P-Y, Wei K-C. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS One. 2014;9(12):e114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mariani L, Gorlia T, Hainfellner JA et al.. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003. [DOI] [PubMed] [Google Scholar]
- 61. Hegi ME, Liu L, Herman JG et al.. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26(25):4189-4199. [DOI] [PubMed] [Google Scholar]
- 62. Papachristodoulou A, Signorell RD, Werner B et al.. Chemotherapy sensitization of glioblastoma by focused ultrasound-mediated delivery of therapeutic liposomes. J Control Release. 2019;295:130-139. [DOI] [PubMed] [Google Scholar]
- 63. Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5(11):610-620. [DOI] [PubMed] [Google Scholar]
- 64. Liu H-L, Hsu P-H, Lin C-Y et al.. Focused ultrasound enhances central nervous system delivery of bevacizumab for malignant glioma treatment. Radiology. 2016;281(1):99-108. [DOI] [PubMed] [Google Scholar]
- 65. Walker MD, Green SB, Byar DP et al.. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303(23):1323-1329. [DOI] [PubMed] [Google Scholar]
- 66. Cardona AF, Rojas L, Wills B et al.. A comprehensive analysis of factors related to carmustine/bevacizumab response in recurrent glioblastoma. Clin Transl Oncol. 2019;21(10)1364-1373. [DOI] [PubMed] [Google Scholar]
- 67. Hau P, Fabel K, Baumgart U et al.. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer. 2004;100(6):1199-1207. [DOI] [PubMed] [Google Scholar]
- 68. Villodre ES, Kipper FC, Silva AO, Lenz G, Lopez PL, da C. Low dose of doxorubicin potentiates the effect of temozolomide in glioblastoma cells. Mol Neurobiol. 2017;55(5):4185-4194. [DOI] [PubMed] [Google Scholar]
- 69. Voulgaris S, Partheni M, Karamouzis M, Dimopoulos P, Papadakis N, Kalofonos HP. Intratumoral doxorubicin in patients with malignant brain gliomas. Am J Clin Oncol. 2002;25(1):60-64. [DOI] [PubMed] [Google Scholar]
- 70. Kovacs Z, Werner B, Rassi A, Sass JO, Martin-Fiori E, Bernasconi M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Control Release. 2014;187:74-82. [DOI] [PubMed] [Google Scholar]
- 71. Lin Y-L, Wu M-T, Yang F-Y. Pharmacokinetics of doxorubicin in glioblastoma multiforme following ultrasound-induced blood-brain barrier disruption as determined by microdialysis. J Pharm Biomed Anal. 2018;149:482-487. [DOI] [PubMed] [Google Scholar]
- 72. Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int J Cancer. 2007;121(4):901-907. [DOI] [PubMed] [Google Scholar]
- 73. Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38(10):1716-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Peruzzi P, Chiocca EA. Viruses in cancer therapy—from benchwarmers to quarterbacks. Nat Rev Clin Oncol. 2018;15(11):657-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Desjardins A, Gromeier M, Herndon JE et al.. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med. 2018;379(2):150-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lawler SE, Speranza M-C, Cho C-F, Chiocca EA. Oncolytic viruses in cancer treatment. JAMA Oncol. 2017;3(6):841. [DOI] [PubMed] [Google Scholar]
- 77. Noroozian Z, Xhima K, Huang Y et al.. MRI-guided focused ultrasound for targeted delivery of rAAV to the brain. Methods Mol Biol. 2019;1950:177-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Migliorini D, Dietrich P-Y, Stupp R, Linette GP, Posey AD, June CH. CAR T-cell therapies in glioblastoma: a first look. Clin Cancer Res. 2018;24(3):535-540. [DOI] [PubMed] [Google Scholar]
- 79. Alkins R, Burgess A, Ganguly M et al.. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73(6):1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cohen-Inbar O, Xu Z, Sheehan JP. Focused ultrasound-aided immunomodulation in glioblastoma multiforme: a therapeutic concept. J Ther Ultrasound. 2016;4(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Idbaih A, Canney M, Belin L et al.. Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res. 2019;25(13):3793-3801 [DOI] [PubMed] [Google Scholar]
- 82. Carpentier A, Canney M, Vignot A et al.. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8(343):343re2-343re2. [DOI] [PubMed] [Google Scholar]
- 83. Arvanitis CD, McDannold N. Drug delivery to the brain via focused ultrasound. In: A Golby, ed. Image-Guided Neurosurgery, 1st ed. Academic Press; 2015;441-474. [Google Scholar]
- 84. McMahon D, Poon C, Hynynen K. Evaluating the safety profile of focused ultrasound and microbubble-mediated treatments to increase blood-brain barrier permeability. Expert Opin Drug Deliv. 2019;16(2):129-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Herzog CA. Incidence of adverse events associated with use of perflutren contrast agents for echocardiography. JAMA. 2008;299(17):2023-5. [DOI] [PubMed] [Google Scholar]
- 86. Olumolade OO, Wang S, Samiotaki G, Konofagou EE. Longitudinal motor and behavioral assessment of blood-brain barrier opening with transcranial focused ultrasound. Ultrasound Med Biol. 2016;42(9):2270-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang Y, Alkins R, Schwartz ML, Hynynen K. Opening the blood-brain barrier with MR Imaging-guided focused ultrasound: preclinical testing on a trans-human skull porcine model. Radiology. 2017;282(1):123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wei K-C, Tsai H-C, Lu Y-J et al.. Neuronavigation-guided focused ultrasound-induced blood-brain barrier opening: a preliminary study in swine. AJNR Am J Neuroradiol. 2013;34(1):115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. O’Reilly MA, Jones RM, Barrett E, Schwab A, Head E, Hynynen K. Investigation of the safety of focused ultrasound-induced blood-brain barrier opening in a natural canine model of aging. Theranostics. 2017;7(14):3573-3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Downs ME, Buch A, Sierra C et al.. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS One. 2015;10(5):e0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Horodyckid C, Canney M, Vignot A et al.. Safe long-term repeated disruption of the blood-brain barrier using an implantable ultrasound device: a multiparametric study in a primate model. J Neurosurg. 2017;126(4):1351-1361. [DOI] [PubMed] [Google Scholar]
- 92. McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72(14):3652-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kovacs ZI, Kim S, Jikaria N et al.. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci USA. 2017;114(1):E75-E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McMahon D, Hynynen K. Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics. 2017;7(16):3989-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu H-L, Hua M-Y, Chen P-Y et al.. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255(2):415-425. [DOI] [PubMed] [Google Scholar]
- 96. Aryal M, Vykhodtseva N, Zhang Y-Z, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood–tumor and blood–brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169(1-2):103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]