Abstract
Background
LABA (long-acting β2-agonists) and/or LAMA (long-acting muscarinic antagonists) represent the first treatment options for patients with symptomatic COPD. Although both display different mechanisms of activity, in combination they have a stronger broncho-dilating effect than monotherapy; hence, a combination of both LABA and LAMA is particularly recommended for patients whose symptoms cannot be sufficiently improved by a single active ingredient. To date, only few data have been collected regarding the therapeutic outcomes of approved LABA/LAMA fixed-dose combinations (FDCs) under everyday (real-life) conditions in non-clinical trial settings.
Objective and Methods
The main objective of the DETECT study was to investigate the impact of aclidinium/formoterol (AB/FF, b.i.d.), glycopyrronium/indacaterol (GLY/IND, q.d.) and umeclidinium/vilanterol (UME/VL, q.d.) in patients with COPD in daily clinical practice. Therefore, a prospective, non-randomized, 12-month, observational study was implemented to assess the effectiveness of these treatments in patients who had been switched to FDC within the last 3 months or for whom such a changeover was intended. Changes in lung function were analyzed by the forced expiratory volume (FEV1) and forced vital capacity (FVC) measures. Quality of life and well-being were evaluated by the COPD Assessment Test (CAT™). Furthermore, a number of exacerbations and early morning COPD symptoms were documented.
Results
In total, 3653 patients were enrolled. FEV1 and FVC values significantly improved during the study with AB/FF (increase by 0.09 ± 0.40 L and 0.10 ± 0.57 L, respectively; p<0.0001), GLY/IND (0.06±0.38/0.05±0.51 L; p<0.0001 and p=0.0025) and UME/VL (0.12±0.39/0.10±0.52 L; p<0.0001). CAT scores decreased indicating improved COPD (AB/FF, 4.17±8.30; GLY/IND, 3.66±7.88; UME/VL, 4.06±7.96; p<0.0001). Moreover, the number of exacerbations as well as early morning COPD symptoms similarly diminished in all treatment groups. A comparable proportion of patients with adverse drug reactions was recorded: AB/FF, 4.07% of patients; GLY/IND, 3.52%; UME/VL, 3.64%.
Conclusion
In summary, AB/FF, GLY/IND and UME/VL provided clinical benefits in lung function, quality of life and early morning COPD symptoms in a broad cohort of COPD patients under routine medical practice conditions. All three treatments were well tolerated.
Keywords: chronic obstructive pulmonary disease, combination therapy, bronchodilators, chronic bronchitis, long-acting muscarinic antagonists, long-acting β2-agonists
Introduction
COPD (chronic obstructive pulmonary disease) represents a serious pulmonary condition, a chronic lung disease characterized by obstructed airflow from the lungs. Primary symptoms include chronic coughing, mucus production, wheezing and dyspnea.1 A feared consequence of COPD includes exacerbations, which accounts for the greatest proportion of total COPD burden on the healthcare system.2 Furthermore, hospitalization due to COPD exacerbations is usually associated with poor prognosis and increased risk of death.3 COPD usually develops over many years and is most frequently provoked by tobacco smoking but also by passive smoking and air pollution. Currently, COPD is ranked the fourth most common cause of chronic morbidity and mortality worldwide.4
In 2001, the Global Initiative for Obstructive Lung Disease (GOLD) was launched.5 The goals of GOLD are to increase awareness of COPD and decrease morbidity and mortality from this disease by implementing an optimized procedure for prevention, diagnosis and treatment.6 Its treatment guidelines were last revised in 2019,2 but since 2013, besides classic spirometry measurements, patient questionnaires have been integrated into the guideline to assess COPD symptoms.7 The main goals of current treatment strategies are to slow the progression of the disease and to improve the control of underlying symptoms. The keystones of COPD therapy are bronchodilators. Long-acting muscarinic antagonists (LAMA) and/or long-acting β2-agonists (LABA) are recommended as first therapy options for COPD patients.8 The National Institute for Health and Care Excellence (NICE) guidelines and GOLD strategy recommend therapy with combined bronchodilators of different pharmacological classes in COPD patients due to higher efficacy and reduction of adverse events in comparison to increasing doses of monotherapy.9–16 An advantage is the different mechanism of activity as a combination formula of LABA and LAMA can achieve stronger bronchodilating effect than treatment with one single active ingredient.17–21 Since bronchodilation, exacerbations and quality of life have been reported to be substantially improved by combined LABA/LAMA medication,10,15,22-25 they are recommended for bronchodilating maintenance therapy and symptomatic relief in patients with moderate or more severe COPD whose symptoms cannot be improved sufficiently by one long-acting bronchodilator alone.8 This has led to an increased use of LABA/LAMA fixed-dose medications, which show a further benefit in the fact that patient compliance can be substantially improved in COPD patients.19 The FDCs aclidinium/formoterol (AB/FF, Duaklir® Genuair®), glycopyrronium/indacaterol (GLY/IND, Ultibro® Breezhaler®) and umeclidinium/vilanterol (UME/VL, Anoro®) are approved combination preparations for the treatment of COPD. To date, only few data have been collected regarding their therapeutic outcomes under everyday (real-life) conditions in non-clinical trial settings. Thus, the main objectives of the non-interventional DETECT-study were to gain information on exacerbations, lung function, quality of life/well-being, early morning symptoms and tolerability of FDCs under medical routine in the practices of pneumologists, internists and general practitioners in Germany.
Methods
Study Design and Ethical Statements
Only adult patients who were capable of understanding the nature, significance and implications of the study and of forming a rational intention in the light of the facts were enrolled in the study. Each patient provided signed consent to study participation prior to being accepted in the study. The current study was a non-interventional observational trial according to the definition § 4, (23), sentence 3 of the German Medicinal Products Act (AMG). This prospective, multicenter, non-interventional, observational study was best suited to gain information on the therapeutic behaviour of physicians and patients in routine medical practice without accompanying conditions or other influences. The enrollment of patients into the non-interventional observational study was based solely on the physicians’ assessment of medical usefulness and necessity. Before implementing the study, the protocol and all other appropriate documents were reviewed and approved by the ethics committee of the federal state of Hessen. Recognized principles for conducting a non-interventional study were observed.26 Planning, conduction and evaluation of the study was based on the relevant combined recommendation and conclusion of The Federal Institute for Drugs and Medical Devices (BfArM) and Paul-Ehrlich Institute (PEI). The study was performed in accordance with International Conference on Harmonisation (ICH) Good Clinical Practice Guidelines and the Declaration of Helsinki. This trial was registered at ClinicalTrials.gov (registration number NCT02552160). Sharing individual deidentified participant data is not intended.
Participants
Suitable COPD patients were recruited by the physicians (pneumologists, internists, and specialized general practitioners) from August 2015 to December 2016. As part of the inclusion criteria, included patients had to be diagnosed with COPD and at least 40 years of age. As a prerequisite for study participation, the physicians’ decision to prescribe AB/FF, GLY/IND or UME/VL prior to survey begin and independently of study participation was mandatory. FDCs were prescribed according to the usual therapeutic procedure and within the authorized indication described in the local summary of product characteristics (SmPC). The first patient was enrolled in March 2015 (retrospectively) and the last visit of the last patient was in January 2018.
Study Conduct and Assessment
Planned observational period per patient was 1 year. Five time points for data collection were set. The exact examination dates were determined individually by the physician’s decision. Data were collected at baseline examination (Visit 1, V1), at three different intermediate examinations (V2, after about 3 months; V3, after about 6 months; V4, after about 9 months) and at one final examination (V5, after about 12 months).
Recording of COPD Exacerbations
An exacerbation of COPD is defined as an acute worsening of respiratory symptoms that results in additional therapy.27–30 At all five visits during FDC treatment, physicians questioned patients on the number and nature of exacerbations suffered after their last visit. Exacerbation was recorded as moderate if the patient was indicated to have received antibiotics or oral steroid as a result of the worsening of COPD symptoms. If the patient indicated to have been hospitalized, the exacerbation was recorded as severe.
Lung Function Tests
Data on pulmonary function were likewise collected at each visit during the entire FDC treatment by measuring the forced expiratory volume in the first second (FEV1) and the forced vital capacity (FVC) values of each patient. These measurements were only recorded as part of the medical routine assessment.
CAT-COPD Assessment Test™
COPD status was evaluated using the validated CAT questionnaire, which involves an evaluation of COPD symptoms with respect to well-being and quality of life. This questionnaire is part of the COPD treatment guideline of the Global Initiative for Obstructive Lung Disease2 as a supplement to the classification of the disease by spirometry.
CAT includes eight questions on a 5-point scale that are related to cough, expectoration, dyspnoea, tightness of chest, breathlessness while going up hills/stairs, confidence in leaving home, limitations of activity at home, energy levels, and COPD-associated impact on sleep. Total scores ranged from 0 to 40, with higher scores indicating a more severe impact of COPD on an individual’s life.22,31
Questionnaire on Early Morning COPD Symptoms (EMSCI)
To gain further information on COPD status, a second patient questionnaire was utilized. Several studies have shown that COPD symptoms fluctuate during the day, with those in the early morning being the most severe.32,33 The EMSCI questionnaire was developed to evaluate early morning COPD symptoms34 and includes a question about the overall severity of COPD symptoms in the morning that can be rated with values between 0 (no symptoms) and 4 (very severe).
Tolerability of FDCs
Tolerability was determined by recording all adverse events (AEs) during the entire study period. The participating physicians were required to document all AEs of which they became aware. In addition to the description of the event, the physician had to evaluate its intensity, seriousness, and its relationship with the medication and report the details known to him such as the outcome of the event.
Statistical Analyses
Data processing and analysis were performed with the SAS™ program system (Version 9.4) at the Clinical Research Organisation (CRO) ANFOMED GmbH. Statistical evaluation was carried out in a descriptive and explorative way. Nominal and ordinal variables were expressed as frequencies and percentages. Continuous variables were expressed as means and standard deviation. For variables that had been documented once in the course of the study, missing values were not replaced. For variables that had been noted several times in the course of the investigation, an analysis according to the Last Observation Carried Forward Method (LOCF) was performed. In the LOCF method, missing values in the course of a study are simply replaced with the last assessed observation. A condition for the application of this method was the existence of a baseline value and a value after baseline. No statistical hypotheses were formulated in advance. The p-values reported are two-tailed and an alpha level of 0.05 was used to draw a conclusion on the statistical significance of observed outcomes. No correction of the alpha level for multiple testing was performed. Tests used were Bhapkar’s test of marginal homogeneity and Wilcoxon’s signed-rank test. AEs and adverse drug reactions (ADRs) were coded according to MedDRA (Version 20.1).
Results
Patient Demographics and Clinical Baseline Characteristics
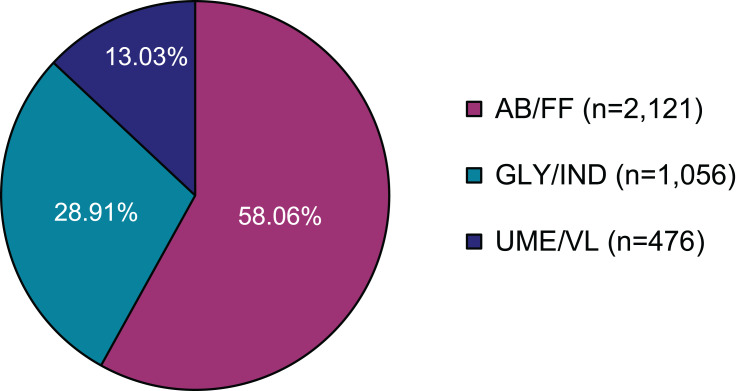
In total, 3653 eligible COPD patients from 254 study sites were enrolled. The mean number of patients per centre was 14.38 (±23.77, median 8.50). From all 3653 patients, full data from all five visits were available for 2369 patients (64.85% of all patients). For all patients, the sum of the observed patient-years was 3129 years. Mean duration of observation was 11.03 (±4.13, median 11.97) months with a range of 4 days to 31 months. 58.06% of patients were treated with AB/FF, followed by GLY/IND (28.91%) and UME/VL (13.03%) therapy (Figure 1). As for demographic data (age, BMI, gender), no significant differences were detected between the three treatment groups (Table 1). The majority of patients were current smokers (AB/FF, 47.85%; GLY/IND, 49.15%; UME/VL, 49.58%). The number of non-smokers were 13.50%, 13.26% and 12.39%, respectively. In addition, anamnestic data were largely comparable between treatment groups. Most frequent prior COPD medications were selective β2-adrenoceptor agonists (AB/FF, 46.06%; GLY/IND, 40.06%; UME/VL 39.08%) and/or anticholinergics (AB/FF, 29.89%; GLY/IND, 31.72%; UME/VL, 22.27%; Table 1). Furthermore, 37.62% of AB/FF, 32.58% of GLY/IND and 38.03% UME/VL FDC treated patients suffered from accompanying disease(s), with hypertension and coronary artery diseases being the most common. Most of the patients with concomitant therapy were treated with agents acting on the renin-angiotensin system (AB/FF, 18.67%; GLY/IND, 24.02%; UME/VL 18.91%). Ramipril, an ACE-inhibitor and acetylsalicylic acid were the most frequently documented concomitant medications (AB/FF, 8.16%, 6.88%; GLY/IND, 5.97%, 5.40%; UME/VL, 10.08%, 5.88%). AB/FF patients had on average 0.75 (±1.40), GLY/IND patients 0.57 (±1.21), and UME/VL patients 0.71 (±1.28) exacerbations within the last 24 months prior to the study.
Figure 1.
LABA/LAMA fixed-dose therapy at baseline (n=3653).
Abbreviations: LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists.
Table 1.
Patients' Demographic and Anamnestic Data (AB/FF, n=2121; GLY/IND, n=1056; UME/VL, n=476)
| Characteristics | AB/FF | GLY/IND | UME/VL | |
|---|---|---|---|---|
| Mean age ± SD, years (n) | 65.50±10.52 (2121) | 65.69±10.42 (1055) | 65.44±10.50 (476) | |
| Mean BMI ± SD, kg/m2 (n) | 27.61±5.88 (2120) | 27.58±5.80 (1056) | 27.27± 5.47 (476) | |
| Gender | Female, n (%) | 916 (43.19) | 406 (38.45) | 225 (47.27) |
| Male, n (%) | 1205 (56.81) | 650 (61.55) | 251 (52.73) | |
| Smoking status | Smoker, n (%) | 1014 (47.85) | 519 (49.15) | 236 (49.58) |
| Non-smoker, n (%) | 286 (13.50) | 140 (13.26) | 59 (12.39) | |
| Ex-smoker, n (%) | 819 (38.65) | 397 (37.59) | 181 (38.03) | |
| Prior COPD treatment (chemical subgroup), patients, n (%) | ||||
| Selective β2-adrenoceptor agonists | 977 (46.06) | 423 (40.06) | 186 (39.08) | |
| Anticholinergics | 634 (29.89) | 335 (31.72) | 106 (22.27) | |
| Adrenergics and other anti-asthmatics | 345 (16.27) | 135 (12.78) | 85 (17.86) | |
| Adrenergics in combination with anticholinergics | 235 (11.08) | 103 (9.75) | 60 (12.61) | |
| Glucocorticoids | 223 (10.51) | 69 (6.53) | 37 (7.77) | |
| Corticosteroids | 50 (2.36) | 5 (0.47) | 9 (1.89) | |
| Other anti-asthmatics for systemic use | 28 (1.32) | 17 (1.61) | 8 (1.68) | |
| Xanthines | 17 (0.80) | 12 (1.14) | 5 (1.05) | |
| Mucolytics | 6 (0.28) | 4 (0.38) | 0 | |
| Leukotriene receptor antagonists | 4 (0.19) | 1(0.09) | 1 (0.21) | |
| Medical gases (oxygen) | 4 (0.19) | 2 (0.19) | 2 (0.42) | |
| Macrolides | 0 | 2 (0.19) | 0 | |
| Other inhalants | 0 | 2 (0.19) | 0 | |
| Ace inhibitors, plain | 1 (0.05) | 0 | 0 | |
| Anti-asthmatics | 1 (0.05) | 0 | 0 | |
| Opium alkaloids and derivatives | 1 (0.05) | 0 | 0 | |
| Platelet aggregation inhibitors excluding heparin | 1 (0.05) | 0 | 0 | |
| Concomitant diseases, patients, n (%) | ||||
| Without concomitant disease | 1323 (62.38) | 712 (67.42) | 295 (61.97) | |
| With concomitant disease | 798 (37.62) | 344 (32.58) | 181 (38.03) | |
| Most frequent concomitant diseases, patients, n (%) | ||||
| Hypertension | 521 (24.56) | 218 (20.64) | 123 (25.84) | |
| Coronary artery disease | 123 (5.80) | 56 (5.30) | 25 (5.25) | |
| Diabetes mellitus | 97 (4.57) | 40 (3.79) | 20 (4.20) | |
| Hypercholesterolaemia | 67 (3.16) | 21 (1.99) | 22 (4.62) | |
| Atrial fibrillation | 44 (2.07) | 23 (2.18) | 20 (4.20) | |
| Most frequent concomitant therapies, patients, n (%) | ||||
| Agents acting on the renin-angiotensin system | 396 (18.67) | 148 (14.02) | 90 (18.91) | |
| Beta blocking agents | 280 (13.20) | 110 (10.42) | 64 (13.45) | |
| Antithrombotic agents | 215 (10.14) | 83 (7.86) | 51 (10.71) | |
| Exacerbations within the last 24 months | ||||
| Without exacerbations, n (%) | 1338 (63.08) | 736 (69.76) | 315 (66.18) | |
| With exacerbations, n (%) | 783 (36.92) | 319 (30.24) | 161 (33.82) | |
| Mean number ± SD (n) | 0.75±1.40 (2121) | 0.57±1.21 (1055) | 0.71±1.28 (476) | |
Notes: Glucocorticoids = ATC-chemical subgroup R03BA. This chemical subgroup includes Beclometason, Budesonid, Flunisolid, Betamethason, Fluticason, Triamcinolon, Mometason, Ciclesonid, Fluticasonfuroat and Dexamethason; Corticosteroids = ATC-chemical subgroup R01AD (mainly Prednisolon in this study).
Abbreviations: BMI, body mass index; SD, standard deviation.
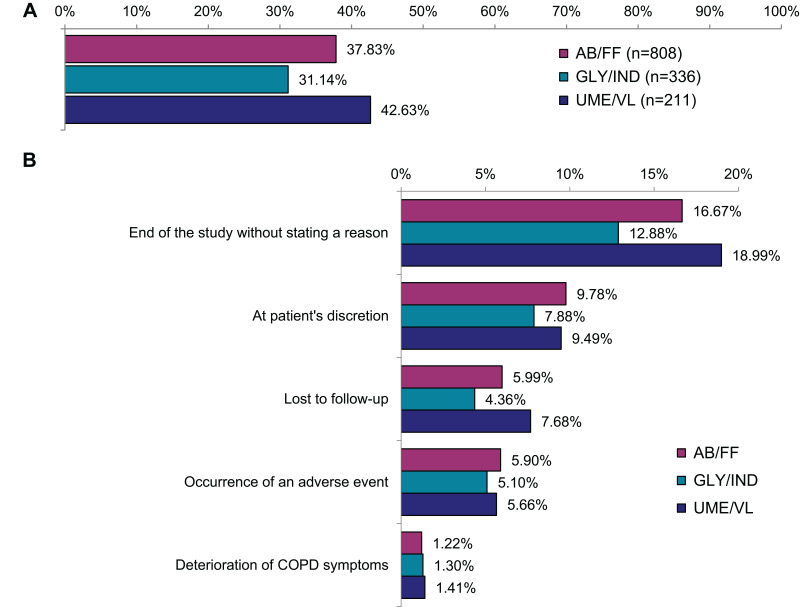
In total, 36.52% of patients prematurely discontinued the study or treatment. The proportion of patients prematurely discontinuing the study or FDC therapy was highest in the UME/VL group (42.63%), whereas 37.83% AB/FF and 31.14% GLY/IND patients discontinued the study or FDC therapy, respectively (Figure 2A). In some cases (AB/FF, 16.67%; GLY/IND, 12.88% and UME/VL, 18.99%), no reason was specified for premature discontinuation (Figure 2B). Patients’ decision at one’s own discretion represented the second most common answer (9.78% in AB/FF group, 7.88% in GLY/IND group and 9.49% in UME/VL group) for premature discontinuation (Figure 2B), some patients (5.58%) prematurely discontinued due to AEs (Figure 2B) and few patients (1.29%) raised deterioration of COPD symptoms as the main reason. In addition, a proportion of patients (5.80% in total) were lost to follow-up according to physicians’ information.
Figure 2.
Premature termination of treatment or study (AB/FF, n=2136; GLY/IND, n=1079; UME/VL, n=495). (A) Number of patients who discontinued therapy or study. (B) Most frequent reasons for termination.
Effectiveness of FDC Therapy
In order to document the effectiveness of the different FDC therapies, exacerbation frequency and occurrence, changes in lung function, well-being and quality of life, as well as the severity of early morning COPD symptoms were recorded during the entire study period.
Frequency and Severity of Exacerbations
Across all groups (AB/FF, GLY/IND and UME/VL) a similar proportion of patients (7.02%, 6.45% and 8.15%, respectively) reported moderate or severe exacerbations (Table 2). For each study group the number of patients with severe exacerbations was low (AB/FF, 1.48%; GLY/IND, 2.15%; UME/VL, 2.88%) and no significant difference in severity was recorded across groups (Table 2). Moreover, the mean number of exacerbations was also largely comparable: 0.10 (±0.42), AB/FF; 0.08 (±0.36), GLY/IND; 0.12 (±0.46), UME/VL (Table 2).
Table 2.
Frequency and Severity of Exacerbations During the Study (Patients with Follow-Up Visits; AB/FF, n=1969; GLY/IND, n=1000; UME/VL, n=439)
| Exacerbations | AB/FF | GLY/IND | UME/VL |
|---|---|---|---|
| Without exacerbations, n (%) | 1762 (92.98) | 913 (93.55) | 383 (91.85) |
| With exacerbations, n (%) | 133 (7.02) | 63 (6.45) | 34 (8.15) |
| ● Moderate exacerbations | 105 (5.54) | 42 (4.30) | 22 (5.28) |
| ● Severe exacerbations* | 28 (1.48) | 21 (2.15) | 12 (2.88) |
| Mean number ± SD (n) | 0.10±0.42 (1912) | 0.08±0.36 (981) | 0.12±0.46 (421) |
Note: *Including patients experiencing moderate and severe exacerbations.
Abbreviation: SD, standard deviation.
Pulmonary Function
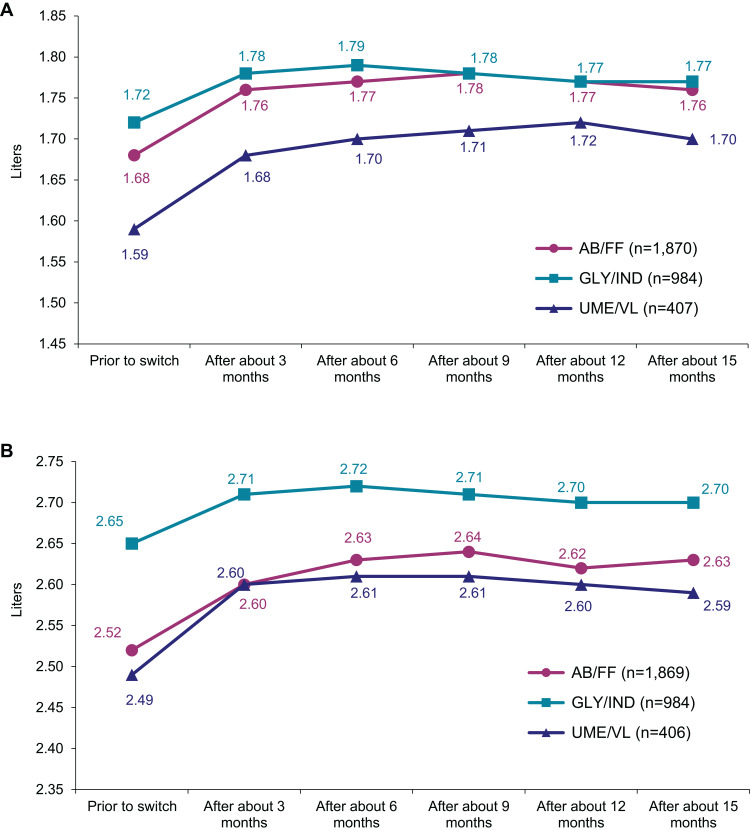
Data on lung function measurements (FEV1 and FVC values) were recorded at every visit as part of the medical routine. Prior to medication switch, mean FEV1 values were 1.68 (±0.65) L, 1.72 (±0.69) L and 1.59 (±0.61) L for AB/FF, GLY/IND and UME/VL, respectively. After 15 months of therapy, a significant improvement (p<0.0001) in FEV1 values was recorded across all groups (AB/FF FEV1 increase by 0.09 (±0.40) L, GLY/IND by 0.06 (±0.38) L and UME/VL by 0.12 (±0.39) L), leading to final recorded FEV1 values of 1.76 L (±0.68), 1.77 (±0.72) L and 1.70 (±0.64) L for AB/FF, GLY/IND and UME/VL, respectively (Figure 3A). Furthermore, FEV1 in percent of predicted normal value had increased (AB/FF: +3.23%; GLY/IND: +1.85%; UME/VL: +3.42%; data not shown). Compared to values prior to treatment start with FDC, FVC values were enhanced by 0.10 (±0.57) L (p<0.0001), 0.05 (±0.51) L (p=0.0025) and 0.10 (±0.52) L (p<0.0001) to final FVC values of 2.63 (±0.85) L, 2.70 (±0.88) L and 2.59 (±0.85) L (AB/FF, GLY/IND and UME/VL), respectively (Figure 3B). FVC values in percent of target (predicted normal value) were higher than 75% in all treatment groups at the final documentation (increased by 3.22%, 1.24% and 3.12%; data not shown).
Figure 3.
Lung function during the observational time (LOFC values). (A) Mean FEV1. (B) Mean FVC.
Abbreviations: LOCF, Last Observation Carried Forward Method; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Quality of Life, Well-Being and Early Morning COPD Symptoms
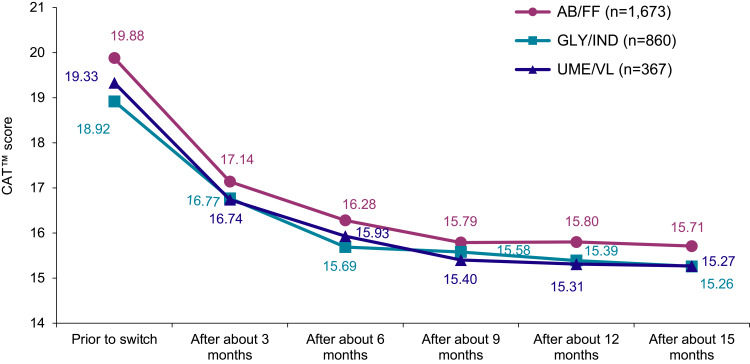
Evaluation of quality of life and well-being, as well as early morning COPD symptoms (EMSCI) as measures to determine COPD severity, revealed a comparable and significant decrease of symptoms across all treatment groups. Each of the eight CAT-questions showed a significantly reduced mean score (p<0.0001) at the final visit indicating improved COPD during the observational period (data not shown). Specifically, mean CAT score was reduced from 19.9 (±7.60, prior to switch) to 15.7 (±7.67) points after about 15 months on AB/FF therapy, from 18.9 (±7.64) to 15.3 (±7.42) points with GLY/IND and from 19.3 (±7.90) to 15.3 (±7.30) points with UME/VL treatment (AB/FF, reduction by 4.17±8.30; GLY/IND, 3.66±7.88 and UME/VL, 4.06±7.96, respectively; Figure 4).
Figure 4.
Well-being and quality of life. Mean CAT™ score during the study (LOCF).
Note: 0=normal – 40=very strong symptoms.
Abbreviations: CAT™, COPD Assessment Test; LOCF, Last Observation Carried Forward Method.
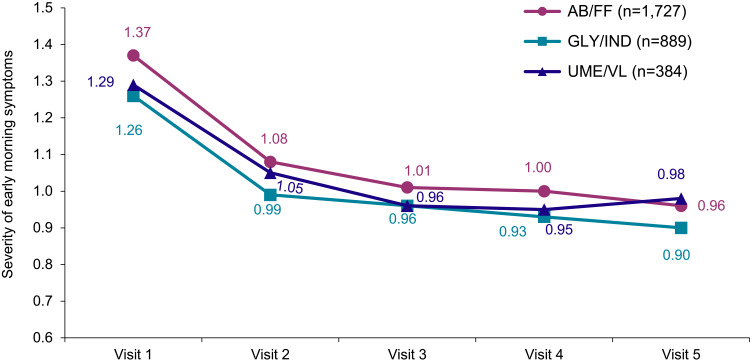
The severity of early morning COPD symptoms at Visit 1 was comparable (AB/FF 1.37±0.99, GLY/IND 1.26±0.96, UME/VL 1.29±0.96) and improved significantly in all three treatment groups (p<0.0001; AB/FF decrease from Visit 1 to Visit 5 by 0.42 ±1.09, GLY/IND by 0.36 ±1.01, UME/VL by 0.31 ±1.02; Figure 5).
Figure 5.
Overall severity of early morning COPD symptoms during the course of the study (LOCF).
Note: 0=no symptoms – 4=very severe.
Abbreviation: LOCF, Last Observation Carried Forward Method.
Tolerability of LABA/LAMA FDC
Overall, all three medications were well tolerated and a comparable number of AEs, as well as ADRs, were reported amongst patients in each group, regardless of LABA/LAMA study medication taken. In total, AEs were reported in 283 patients (13.25%) treated with AB/FF, 142 patients (13.16%) under GLY/IND therapy and in 63 patients (12.73%) receiving UME/VL (Table 3). As for events with causal relation to the study medications, the following number of affected patients was reported: AB/FF, 87 patients (4.07%); GLY/IND, 38 patients (3.52%); and UME/VL, 18 patients (3.64%). Likewise, an almost similar percentage of patients suffered from serious ADRs, 4 patients (0.19%), 3 patients (0.28%) and 1 patient (0.20%), respectively. In detail, nine serious ADRs were recorded in four patients with AB/FF (2x dyspnoea, 1x tachycardia, 3x cough, 1x dizziness, 1x palpitations, 1x COPD), three serious ADRs in patients with GLY/IND (1x condition aggravated, 1x drug intolerance, 1x muscle spasms) and one serious ADR in patients with UME/VL (1x COPD). None of these cases was fatal. Most commonly reported ADRs in patients under AB/FF were dyspnoea (16 events in 15 patients, 0.70%), tremor (10 events in 9 patients, 0.42%), tachycardia (8 events in 8 patients, 0.37%), ineffective drug (7 events in 7 patients, 0.33%), and cough (6 events in 6 patients, 0.28%). ADRs noted in patients with GLY/IND treatment included most often cough (12 events in 12 patients, 1.11%) and dyspnoea (3 events in 3 patients, 0.28%), while UME/VL patients most frequently reported an ineffectiveness of study drug (3 events in 3 patients, 0.61%; Table 3).
Table 3.
Category of Adverse Events and Frequency of Patients Affected (AB/FF, n=2136; GLY/IND, n=1079; UME/VL, n=495)
| Category | AB/FF | GLY/IND | UME/VL |
|---|---|---|---|
| AE, n (%) | 283 (13.25) | 142 (13.16) | 63 (12.73) |
| Non-serious AE, n (%) | 211 (9.88) | 91 (8.43) | 48 (9.70) |
| Serious AE, n (%) | 91 (4.26) | 59 (5.47) | 23 (4.65) |
| Fatal, n (%) | 16 (0.75) | 14 (1.30) | 5 (1.01) |
| ADR, n (%) | 87 (4.07) | 38 (3.52) | 18 (3.64) |
| Non-serious ADR, n (%) | 83 (3.89) | 36 (3.34) | 17 (3.43) |
| Serious ADR, n (%) | 4 (0.19) | 3 (0.28) | 1 (0.20) |
| Fatal, n (%) | 0 | 0 | 0 |
| Preferred term of most common ADRs, n (%) | |||
| Cough | 6 (0.28) | 12 (1.11) | 2 (0.40) |
| Dyspnoea | 15 (0.70) | 3 (0.28) | 2 (0.40) |
| Ineffective drug | 7 (0.33) | 2 (0.19) | 3 (0.61) |
| Tremor | 9 (0.42) | 2 (0.19) | 0 |
| Chronic obstructive pulmonary disease | 2 (0.09) | 0 | 2 (0.40) |
| Productive cough | 2 (0.09) | 2 (0.19) | 2 (0.40) |
| Stomatitis | 0 | 0 | 2 (0.40) |
| Tachycardia | 8 (0.37) | 0 | 0 |
| Muscle spasm | 5 (0.23) | 1 (0.09) | 1 (0.20) |
| Headache | 5 (0.23) | 0 | 1 (0.20) |
| Pruritus | 5 (0.23) | 0 | 0 |
Abbreviations: AE, adverse event; ADR, adverse drug reaction.
Discussion
COPD is a public health problem of global importance with patients suffering from COPD and its associated symptoms enduring a high economic and social burden. Although COPD is a preventable and treatable disease, the prevalence of COPD is increasing.27 It is assumed that COPD will be the third leading cause of death by 202035 as continuing exposure to COPD risk factors and aging of the population contributes to its growing prevalence.28 Amongst the many symptoms associated with COPD, main symptoms such as increased breathlessness, frequent coughing and sputum production are reported to be nonspecific and of gradual onset outside exacerbations.36 Ascertaining COPD symptoms is not only important for monitoring and treatment of the disease but also enables to estimate when an intervention is necessary. Over the last few years, LABA/LAMA inhalers have been regarded as a useful addition to ICS (inhaled corticosteroid)/LABA inhalers for the treatment of COPD.
The aim of this study was to analyze the effectiveness and tolerability of LABA/LAMA fixed-dose combinations AB/FF, GLY/IND and UME/VL in patients with COPD in routine practical care. Lung function measurements as well as different questionnaires involving patient assessment of COPD symptoms (patient-reported outcomes) were used as measuring instruments to assess changes in pulmonary function, quality of life and early morning COPD symptoms.
Demographic and Baseline Characteristics
Regarding patient demographics, no appreciable difference of sex and age was detected between the three study groups (AB/FF, GLY/IND or UME/VL; Table 1).
The current study population seems to be representative in evaluating the effectiveness of the various treatments as similar demographic and epidemiologic data have been reported in other studies on the effectiveness of, eg AB on COPD in Germany.22,37 Furthermore, about half of the patients included in the current study were either smokers (about 50%) or had been smokers in the past (about 40%). Smoking has been reported as the highest COPD risk factor.38 However, smokers are not the only target, as non-smokers are also diagnosed with COPD resulting from eg secondary smoking (inhalation of smoke as a result of being in close proximity to a smoker) or air pollution, such as biomass fuel.39 Although the number of non-smokers investigated in the current study (AB/FF, 13.50%; GLY/IND, 13.26%; UME/VL, 12.39%) may seem high, this figure actually represents a lower percentage compared to the numbers reported by the Third National Health and Nutrition Examination Survey, which reported 23% of airway obstruction in US adults in persons without a smoking history.40 Our finding is comparable to the findings by Meyer et al, who reported that 16.7% of estimated 225,400 adults, who died with COPD, had never smoked.41 Current study data revealed that most patients suffered from concomitant diseases. Overall, hypertension was most commonly mentioned in all treatment groups. This result is not surprising as hypertension is likely to be the most frequently occurring comorbidity in patients with COPD and, notably, may have implications for prognosis.42,43 Premature discontinuation of the study or FDC treatment was high (36.52% of all patients) and highest in the UME/VL group (42.63%). In most of the cases, no reason was stated. Partly discontinuation was due to loss of follow-up. Specific reasons mentioned by the patients themselves were AEs and worsening of COPD symptoms. Often the study was discontinued at one’s own discretion.
Effectiveness of FDC Therapy
A number of clinical trials have shown that different LABA/LAMA FDCs result in comparable FEV1 improvements with enhancements exceeding 0.1 L when compared with placebo-treatment.13,44-47 Smaller (below 0.1 L), but statistically significant enhancements in FEV1 values were achieved when compared with LABA and LAMA monotherapy, respectively.13,44,48,49 Furthermore, a recent meta-analysis (15 randomized controlled trials with 23,168 patients suffering from COPD) demonstrated the effectiveness of LABA/LAMA FDCs in comparison to LABA or LAMA monotherapy.50 FDCs have been shown to be superior to LABA or LAMA monotherapy regarding lung function, breathlessness and quality of life. No significant differences in the effectiveness of different LABA/LAMA FDCs were seen in these studies, but a small gradient in efficacy was previously reported with minimally greater improvements in pulmonary function for GLY/IND and UME/VL in comparison to AB/FF.50 However, due to the lack of controlled comparative studies, these data cannot be used for a conclusive evaluation.
The data collected in our study from all three different COPD treatments (AB/FF, GLY/IND and UME/VL) indicated an almost similar improvement of pulmonary function, which is demonstrated by increased FEV1 and FVC values.
For all treatment groups, increased FEV1 and FVC values were observed at various visits during the observational period compared to the values recorded prior to medication switch. FEV1 values increased most in patients under UME/VL therapy. However, a difference of mean FEV1 baseline values (prior to medication switch) was noted between the treatment groups with the lowest baseline value in UME/VL patients. Interestingly, baseline values documented in this study, were somewhat higher compared to the initial average FEV1 value of 1.41 L (± 0.50 L) from the ACLIFORM-COPD study, a multicenter, randomized trial, which analyzed the efficacy and safety of AB/FF FDC versus monotherapy in 1729 COPD patients.44
Early morning COPD symptoms, predominantly pronounced in patients with severe COPD, additionally contribute to the burden of disease, and therefore significantly influence patients’ quality of life. Our study data show that all three fixed-dose COPD medications led to a decrease in early morning COPD symptoms with significant improvements of a score of 0.42 in AB/FF patients, 0.36 in GLY/IND patients and 0.31 in UME/VL patients, indicative of a better COPD symptom control. Quality of life as well as well-being likewise positively changed. Evaluation of CAT showed a comparable score reduction in all treatment groups during the course of the underlying study. Notably, the greatest improvement of CAT scores was registered within the first 3 months. After about 15 months, a significant reduction of about 4 points was achieved with all analyzed FDC treatments. These results are in concordance to those of other COPD studies, eg the FLIGHT1/FLIGHT2 trials (indacaterol/glycopyrrolate versus monocomponents)51 or the large observational DACCORD study within the German COPD National Prospective Registry. Its “real life” data, published from an interim analysis including approximately 4000 patients who had either newly initiated LAMA/LABA FDC or who had switched to a regimen including a LABA/LAMA FDC, showed clinically relevant improvements in CAT score.52 Moreover, further evaluation of study results showed that patients who changed from mono-bronchodilator to dual bronchodilation had the greatest CAT total score improvement.53
Tolerability
In total, about 4% of patients experienced ADRs during the course of the study. This value is low compared to outcomes in the Marth-NIS examining LAMA AB, which reported 6.9% ADRs.37 Though in contrast to the Marth-study where no serious events occurred, a total of eight patients suffered from serious ADRs (AB/FF, 0.19%; GLY/IND, 0.28%; UME/VL, 0.20%) in this study. However, reported ADR rates were low and all three FDCs were generally safe. The low ADR rate may be due to the phenomenon of under-reporting in non-interventional trials as physicians may tend to under-report events already listed in the SmPC.54 Interestingly, the ACLIFORM-COPD study revealed no evidence for additive AEs when combining the two different drugs AB and FF.44 As for AB/FF treatment alone, low ADR rates were recorded in the current NIS. This is in concordance to other previous studies, which reported FF as a well-tolerated drug.55 Furthermore, AB has low systemic exposure, hence, minimising typical LAMA AEs44,56,57 and the recently conducted ASCENT-COPD clinical trial that specifically enrolled patients with elevated cardiovascular risk found no increase in cardiovascular risk with AB.58
Limitations
This study design represents an important instrument for collecting real-life data concerning therapy with LABA/LAMA FDC in patients with multiple accompanying illnesses and co-medications as such patients are usually not allowed to participate in controlled clinical studies. A main advantage of NIS is in general a better transfer of knowledge to non-selected patients in a non-study setting. However, given the fact that a considerable number of enrolled patients received additional concomitant medication, including respiratory therapeutics, and due to the lack of a reference group, efficiency and tolerability data could not be mathematically isolated.
Conclusion
In conclusion, the results of the current non-interventional study provide evidence for the effectiveness of LABA/LAMA fixed dose combinations aclidinium/formoterol (AB/FF), glycopyrronium/indacaterol (GLY/IND) and umeclidinium/vilanterol (UME/VL) in the treatment of COPD. The patient cohort analyzed can be considered representative of COPD patients from Germany regarding demographic and anamnestic data. All three treatments were effective and well tolerated under everyday medical routine conditions in a characteristic sample of patients.
Acknowledgments
This study was sponsored by AstraZeneca. Medical writing support was provided by Corina Hutterer and Tanja Burkard of the Clinical Research Organisation (CRO) ANFOMED GmbH in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461-464). This writing support was funded by AstraZeneca. The abstract of this paper was presented at the 60th Congress of the German Society of Pulmonology (DGP) 2019 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Pneumologie 2019; 73(S 01); DOI: 10.1055/s-0039-1678325.
Disclosure
TP and FWF are employees of AstraZeneca GmbH. JB is an employee of insaf – Respiratory Research Institute GmbH and has also received consultation fees from AstraZeneca, Berlin Chemie/Menarini, Pohl Boskamp, Chiesi and participated in scientific advisory boards that were funded by Astra Zeneca, Chiesi. The authors report no other conflicts of interest in this work.
References
- 1.Abholz H, Gillissen A, Magnussen H, et al. Nationale versorgungsleitlinie COPD [National Disease Management Guideline COPD]. German. 2012. Available from: http://www.versorgungsleitlinien.de. Accessed August22, 2019.
- 2.The Global Strategy for the Diagnosis, Management and Prevention of COPD. Global initiative for chronic obstructive lung disease. GOLD. 2019. Available from: http://www.goldcopd.org. Accessed August22, 2019.
- 3.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization; ebrary, Inc. World Health Statistics 2008. Geneva, Switzerland: World Health Organization;; 2008:110 ISBN: 978 92 4 0682740 Available from: https://www.who.int/gho/publications/world_health_statistics/EN_WHS08_Full.pdf. Accessed August22, 2019. [Google Scholar]
- 5.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 6.Pauwels R. Global initiative for chronic obstructive lung diseases (GOLD), time to act. Eur Respir J. 2001;18(6):901–902. doi: 10.1183/09031936.01.0027401 [DOI] [PubMed] [Google Scholar]
- 7.Tashkin DP, Ferguson GT. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res. 2013;14:49. doi: 10.1186/1465-9921-14-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl R, Greefhorst LA, Nowak D, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):778–784. doi: 10.1164/ajrccm.164.5.2007006 [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care (Partial Update). London: National Clinical Guideline Centre; 2010. Available from: http://www.nice.org.uk/CG101. Accessed August22, 2019. [Google Scholar]
- 10.The Global Strategy for the Diagnosis, Management and Prevention of COPD. Global initiative for chronic obstructive lung disease. GOLD. 2018. Available from: https://goldcopd.org/archived-reports/. Accessed August22, 2019.
- 11.Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23(4):257–267. doi: 10.1016/j.pupt.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 12.Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy, the SHINE study. Eur Respir J. 2013;42(6):1484–1494. doi: 10.1183/09031936.00200212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62. 5/25mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi: 10.1016/j.rmed.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 14.Toy EL, Beaulieu NU, McHale JM, et al. Treatment of COPD, relationships between daily dosing frequency, adherence, resource use, and costs. Respir Med. 2011;105(3):435–441. doi: 10.1016/j.rmed.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 15.van Noord JA, Aumann J-L, Janssens E, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26(2):214–222. doi: 10.1183/09031936.05.00140404 [DOI] [PubMed] [Google Scholar]
- 16.Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6(1):17–25. doi: 10.1080/15412550902724073 [DOI] [PubMed] [Google Scholar]
- 17.Jones PW, Leidy NK, Hareendran A, Lamarca R, Chuecos F, Garcia Gil E. The effect of aclidinium bromide on daily respiratory symptoms of COPD, measured using the evaluating respiratory symptoms in COPD (E-RS, COPD) diary: pooled analysis of two 6-month Phase III studies. Respir Res. 2016;17(1):61. doi: 10.1186/s12931-016-0372-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD. 2012;9(2):90–101. doi: 10.3109/15412555.2012.661492 [DOI] [PubMed] [Google Scholar]
- 19.Beier J, Kirsten A-M, Mróz R, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled phase IIIb study. COPD. 2013;10(4):511–522. doi: 10.3109/15412555.2013.814626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leidy NK, Wilcox TK, Jones PW, et al. Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient-reported outcome (PRO) measure. Value Health. 2010;13(8):965–975. doi: 10.1111/j.1524-4733.2010.00772.x [DOI] [PubMed] [Google Scholar]
- 21.Sethi S, Kerwin E, Watz H, et al. AMPLIFY, A randomized, Phase III study evaluating the efficacy and safety of aclidinium/formoterol vs monocomponents and tiotropium in patients with moderate-to-very severe symptomatic COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:667–682. doi: 10.2147/COPD.S189138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 23.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 24.van Noord JA, Aumann J-L, Janssens E, et al. Combining tiotropium and salmeterol in COPD, effects on airflow obstruction and symptoms. Respir Med. 2010;104(7):995–1004. doi: 10.1016/j.rmed.2010.02.017 [DOI] [PubMed] [Google Scholar]
- 25.Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol–fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE), A randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. doi: 10.1016/S2213-2600(12)70052-8 [DOI] [PubMed] [Google Scholar]
- 26.De la Haye R, Herbold M. Anwendungsbeobachtungen: Leitfaden für die praktische Durchführung [Observational study: Guideline for practical implementation]. German: Verlag ECV Editio Cantor; 2000. [Google Scholar]
- 27.Wedzicha JA, Seemungal TAR. COPD exacerbations, defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/S0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032 [DOI] [PubMed] [Google Scholar]
- 29.Hurst JR, Wedzicha JA. What is (and what is not) a COPD exacerbation, thoughts from the new GOLD guidelines. Thorax. 2007;62(3):198–199. doi: 10.1136/thx.2007.077883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J. Supplement. 2003;41(Supplement 41):46s–53s. doi: 10.1183/09031936.03.00078002 [DOI] [PubMed] [Google Scholar]
- 31.Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. doi: 10.1183/09031936.00177210 [DOI] [PubMed] [Google Scholar]
- 32.Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264–272. doi: 10.1183/09031936.00051110 [DOI] [PubMed] [Google Scholar]
- 33.Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25(8):2043–2048. doi: 10.1185/03007990903103006 [DOI] [PubMed] [Google Scholar]
- 34.Palsgrove A, Houghton K. The development of the early morning symptoms of COPD instrument (EMSCI). Int J Chron Obstruct Pulmon Dis. 2018;13:1633–1645. doi: 10.2147/COPD.S152087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207. doi: 10.1183/09031936.06.00024505 [DOI] [PubMed] [Google Scholar]
- 36.Walters J. COPD - diagnosis, management and the role of the GP. Aust Fam Physician. 2010;39(3):100–103. [PubMed] [Google Scholar]
- 37.Marth K, Schuller E, Pohl W. Improvements in patient-reported outcomes: a prospective, non-interventional study with aclidinium bromide for treatment of COPD. Respir Med. 2015;109(5):616–624. doi: 10.1016/j.rmed.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 38.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD, systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605 [DOI] [PubMed] [Google Scholar]
- 39.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi: 10.1016/S0140-6736(09)61303-9 [DOI] [PubMed] [Google Scholar]
- 40.Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers, results from the third National Health and Nutrition Examination Survey. Am J Med. 2005;118(12):1364–1372. doi: 10.1016/j.amjmed.2005.06.041 [DOI] [PubMed] [Google Scholar]
- 41.Meyer PA, Mannino DM, Redd SC, Olson DR. Characteristics of adults dying with COPD. Chest. 2002;122(6):2003–2008. doi: 10.1378/chest.122.6.2003 [DOI] [PubMed] [Google Scholar]
- 42.Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31(1):204–212. doi: 10.1183/09031936.00114307 [DOI] [PubMed] [Google Scholar]
- 43.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. doi: 10.1164/rccm.201201-0034OC [DOI] [PubMed] [Google Scholar]
- 44.Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD), s multicentre, randomised study. BMC Pulm Med. 2014;14:178. doi: 10.1186/1471-2466-14-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312–1319. doi: 10.1016/j.rmed.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 46.D’Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum AR, Caracta CF. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate, the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15:123. doi: 10.1186/s12931-014-0123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Urzo A, Rennard S, Kerwin E, et al. A randomised double-blind, placebo-controlled, long-term extension study of the efficacy, safety and tolerability of fixed-dose combinations of aclidinium/formoterol or monotherapy in the treatment of chronic obstructive pulmonary disease. Respir Med. 2017;125:39–48. doi: 10.1016/j.rmed.2017.02.008 [DOI] [PubMed] [Google Scholar]
- 48.Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J. 2015;45(4):969–979. doi: 10.1183/09031936.00136014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK), a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi: 10.1016/S2213-2600(13)70052-3 [DOI] [PubMed] [Google Scholar]
- 50.Calzetta L, Rogliani P, Matera MG, Cazzola M. A Systematic Review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest. 2016;149(5):1181–1196. doi: 10.1016/j.chest.2016.02.646 [DOI] [PubMed] [Google Scholar]
- 51.Mahler DA, Kerwin E, Ayers T, et al. FLIGHT1 and FLIGHT2, efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(9):1068–1079. doi: 10.1164/rccm.201505-1048OC [DOI] [PubMed] [Google Scholar]
- 52.Worth H, Buhl R, Criee C-P, Kardos P, Lossi NS, Vogelmeier CF. GOLD 2017 treatment pathways in ‘real life’, an analysis of the DACCORD observational study. Respir Med. 2017;131:77–84. doi: 10.1016/j.rmed.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 53.Buhl R, Criee C-P, Kardos P, et al. Dual bronchodilation vs triple therapy in the “real-life” COPD DACCORD study. Int J Chron Obstruct Pulmon Dis. 2018;13:2557–2568. doi: 10.2147/COPD.S169958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32(1):19–31. doi: 10.2165/00002018-200932010-00002 [DOI] [PubMed] [Google Scholar]
- 55.Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;10:CD010177. [DOI] [PubMed] [Google Scholar]
- 56.Jansat JM, Lamarca R, Garcia Gil E, Ferrer P. Safety and pharmacokinetics of single doses of aclidinium bromide, a novel long-acting, inhaled antimuscarinic, in healthy subjects. Int J Clin Pharmacol Ther. 2009;47(7):460–468. doi: 10.5414/CPP47460 [DOI] [PubMed] [Google Scholar]
- 57.Sentellas S, Ramos I, Alberti J, et al. Aclidinium bromide, a new, long-acting, inhaled muscarinic antagonist: in vitro plasma inactivation and pharmacological activity of its main metabolites. Eur J Pharm Sci. 2010;39(5):283–290. doi: 10.1016/j.ejps.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 58.Wise RA, Chapman KR, Scirica BM, et al. Effect of aclidinium bromide on major cardiovascular events and exacerbations in high-risk patients with chronic obstructive pulmonary disease, the ASCENT-COPD randomized clinical trial. JAMA. 2019;321(17):1693–1701. doi: 10.1001/jama.2019.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Abholz H, Gillissen A, Magnussen H, et al. Nationale versorgungsleitlinie COPD [National Disease Management Guideline COPD]. German. 2012. Available from: http://www.versorgungsleitlinien.de. Accessed August22, 2019.
- The Global Strategy for the Diagnosis, Management and Prevention of COPD. Global initiative for chronic obstructive lung disease. GOLD. 2019. Available from: http://www.goldcopd.org. Accessed August22, 2019.
- The Global Strategy for the Diagnosis, Management and Prevention of COPD. Global initiative for chronic obstructive lung disease. GOLD. 2018. Available from: https://goldcopd.org/archived-reports/. Accessed August22, 2019.