Abstract
Background.
Opioid abuse remains a significant public health challenge. With continuing emergence of novel psychoactive substances (e.g., synthetic cannabinoids found in “K2” or “spice” preparations), the co-administration of opioids and other novel drugs is likely to become more prevalent, which might increase the risk for abuse and other adverse effects. This study examined whether the synthetic cannabinoid receptor agonist JWH-018 alters the reinforcing effectiveness of the mu opioid receptor agonist remifentanil in rhesus monkeys (n=4) using economic demand analyses.
Methods.
Lever presses delivered i.v. infusions of a drug or drug mixture according to a fixed ratio schedule. For each condition, the ratio progressively increased in quarter-log unit steps across sessions yielding a demand curve: consumption (infusions obtained) was plotted as a function of price (fixed ratio value).
Results.
When available alone, remifentanil (0.00032 mg/kg/infusion) occasioned the highest consumption at the lowest cost and highest essential value, while JWH-018 (0.0032 mg/kg/infusion) alone occasioned lower unconstrained demand and essential value. Unconstrained demand for a mixture of remifentanil and JWH-018 was lower than for remifentanil alone, but essential value of the mixture was not significantly different from that of remifentanil alone.
Conclusion.
These data indicate that synthetic cannabinoids such as JWH-018 might alter some aspects of opioid self-administration (i.e., decreased consumption at the lowest price) but do not enhance reinforcing effectiveness as measured by sensitivity of consumption to increasing costs. Opioid/cannabinoid mixtures do not appear to have greater abuse potential compared with an opioid alone.
Keywords: opioid, cannabinoid, JWH-018, remifentanil, drug mixture, economic demand, rhesus monkey
1. Introduction
Opioid abuse remains a significant public health challenge (Hedegaard et al. 2018), and many factors, including polydrug abuse (e.g., Hassan and Le Foll 2018), contribute to its persistence. One particular concern is co-administration of opioids and cannabinoids, which could occur both because of increased legalization of cannabis and because of availability of synthetic cannabinoid receptor agonists commonly found in preparations known as “K2” or “spice”. Understanding the consequences of co-administering opioids and cannabinoids could aid in the development of more effective prevention and treatment strategies.
Cannabinoids enhance some but not other effects of opioids, and the pattern of interactions appears to indicate no greater risk of adverse effects of opioid/cannabinoid mixtures compared with opioids alone. For example, while cannabinoids enhance the antinociceptive effects of opioids (Li et al. 2008; Maguire et al. 2013b; Maguire et al. 2014; Maguire et al. 2018a; Nilges et al. in press), they do not enhance other effects of opioids, including ventilatory depression (Weed et al. 2018), discriminative-stimulus effects (Li et al. 2008; Maguire et al. 2013b; Maguire and France 2016a), effects on cognition and impulsivity (Minervini and France 2018; 2020), or physical dependence (Gerak and France 2016). Moreover, cannabinoids do not enhance the reinforcing effects of opioids under several conditions (Li et al. 2012; Maguire et al. 2013b; Maguire and France 2016b; Maguire and France 2018b; Gerak et al. 2019), indicating that opioid/cannabinoid mixtures might not have greater abuse potential compared with an opioid alone. However, given the public health implications of using opioid/cannabinoid mixtures either therapeutically (e.g., treating pain) or recreationally (e.g., co-abuse), it is important to examine interactions under a broad range of conditions.
This study used a behavioral economic approach to compare reinforcing effects of an opioid/cannabinoid mixture with effects of the constituents alone. Behavioral economics offers an approach different from, and complementary to, approaches used previously to characterize abuse-related effects of opioid/cannabinoid mixtures. One such method is economic demand analysis, which relates consumption of a commodity to its price (e.g., Hursh, 1991). Consumption of a commodity decreases as price increases, and this relationship is described well by the following exponential demand equation:
| (Equation 1) |
where Q is consumption, P is price, Q0 is consumption at a minimal cost, k is a scaling constant, and α is a free parameter that reflects demand elasticity (Hursh and Silberberg 2008). Because α is inversely related to reinforcing effectiveness, it has been transformed to essential value (EV), which is directly related to reinforcing effectiveness, using the following equation (e.g., Hursh and Roma 2016; Zanettini et al. 2018; Maguire et al. 2020b):
| (Equation 2) |
Other things being equal, drugs for which consumption is less sensitive to changes in price (i.e., lower α or higher EV) would have greater likelihood of being abused compared with drugs that are more sensitive to price (e.g. higher α or lower EV; Hursh et al. 2005). Demand analyses have been invaluable for quantifying abuse potential of novel drugs of abuse as well as candidate therapeutics (e.g., Schwartz et al. 2019).
A recent study investigating interactions between opioids and cannabinoids in rhesus monkeys (Gerak et al. 2019) showed that the synthetic cannabinoid receptor agonist JWH-018 did not alter the reinforcing effects of the mu opioid receptor agonist remifentanil under a drug-drug choice procedure or heroin-primed reinstatement of opioid-maintained responding, suggesting that synthetic cannabinoids like JWH-018 might not enhance abuse-relative effects of opioids. The current study tests the generality of interactions between opioids and cannabinoids with regard to reinforcing effects by quantifying demand for remifentanil and JWH-018 as well as a mixture of remifentanil and JWH-018.
2. Material and Methods
2.1. Subjects
Two male (9.5–10.6 kg) and two female (6.0–8.6 kg) adult rhesus monkeys were housed individually in stainless steel cages in colony rooms maintained under a 14/10-hr light/dark cycle. Chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI, USA), fresh fruit, peanuts, and treats were provided daily, and water was continuously available in the home cage. All monkeys had extensive experimental history including self-administration of opioids but not cannabinoids; monkeys did not participate in an experiment and did not receive any drug for at least 2 months prior to the start of this study. Experiments were conducted in accordance with guidelines set forth by the Guide for the Care and Use of Laboratory Animals (8th edition; 2011) and protocols were approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee.
2.2. Surgery
A 5-fr polyurethane catheter (Access Technologies, Skokie, IL, USA) was secured into a vein (e.g., jugular or femoral), tunneled subcutaneously (s.c.) to the back, and attached to a s.c. access port (Access Technologies) as described previously (Maguire et al. 2019, 2020a).
2.3. Apparatus
Monkeys were seated in chairs and positioned in light- and sound-attenuating operant conditioning chambers facing a stainless steel instrument panel containing two horizontally aligned levers, each located below a translucent disk that could be illuminated green or red. Drug infusions were delivered i.v. through the s.c. access port attached via Huber point needle and catheter extension to a syringe mounted in a pump (Med Associates, Inc., St. Albans, VT) located outside the chamber. Other details of the apparatus have been described previously (Maguire et al. 2019, 2020a).
2.4. Self-administration procedure
Sessions began with illumination of the red light over one lever (active) for 5 sec and non-contingent delivery of the solution available for self-administration that day. After the infusion, the green light over the same lever was illuminated, signaling the beginning of a response period. Completion of the response requirement turned off the green light, turned on the red light for 5 sec, activated the syringe pump to deliver an infusion of drug, and initiated a 30-sec timeout during which all lights were off and responding had no programmed consequence. After the timeout, the green light was illuminated, signaling a response period. Sessions lasted 90 min.
2.5. Experimental design
Demand curves were doubly determined for each of four self-administered solutions, as follows: vehicle alone (5% ethanol, 5% emulphor, and 90% saline), remifentanil alone (0.00032 mg/kg/infusion), JWH-018 alone (0.0032 mg/kg/infusion), and a mixture of remifentanil and JWH-018. Remifentanil was used because its short duration of action (< 10 min) limits drug accumulation under conditions in which drug intake can be quite high (e.g., low response requirements). Aside from a short duration of action, the pharmacological effects of remifentanil, including measures of reinforcing effectiveness as determined through demand procedures, are nearly indistinguishable from other longer-acting opioid receptor agonists such as fentanyl (e.g., Ko et al. 2002). In rhesus monkeys, this unit dose of remifentanil is at or near the peak of the self-administration dose-effect curve (Maguire et al. 2013a) and maintains choice of drug over food (Maguire and France 2018); in addition, this dose served as the comparator dose in the previous study investigating interactions between remifentanil and JWH-018 (Gerak et al. 2019). The unit dose of JWH-018 was used because it was tested in the choice and reinstatement experiments in the previous study (Gerak et al. 2019) where it failed to alter choice of remifentanil or heroin-primed reinstatement. Because self-administration of JWH-018 has not been demonstrated in rhesus monkeys, it is unclear where this unit dose might fall on a self-administration dose-effect curve. In monkeys trained to discriminate 0.1 mg/kg of JWH-018 from vehicle (Rodriguez and McMahon 2014), a single injection of 0.0032 mg/kg given i.v. failed to produce drug-lever responding; however, a 3-fold larger dose produced approximately 50% drug-lever responding, indicating that only a few injections of this unit dose would be sufficient to produce behavioral effects.
Solutions were presented in a mixed order across monkeys. Infusions were delivered according to a fixed-ratio (FR) schedule whereby each infusion required a set number of responses for the entire session; responses on the other lever during the response period reset the response counter. Initially, infusions were available according to an FR 10 schedule that remained in effect until the number of infusions obtained in each of two consecutive sessions did not vary by more than ± 20% of the mean of those sessions. Thereafter, the FR value increased in quarter-log unit steps (i.e., 18, 32, 56, 100, 180…) every two sessions until no infusions were obtained for both sessions under a particular FR. This procedure has been used effectively to determine demand curves in nonhuman primates (e.g., Winger et al. 1996).
2.6. Drugs
Remifentanil hydrochloride and JWH-018 (National Institute on Drug Abuse Drug Supply Program, Bethesda, MD, USA) alone and in combination were dissolved in the vehicle and administered i.v. in a volume of approximately 0.32 ml per 10 kg of body weight.
2.7. Data analyses
Infusions obtained across both sessions under each FR were averaged; those means were log transformed and the curve was fit with Equation 1 using the method of least-squares regression (Motulsky and Christopolous, 2004; Maguire et al. 2020b). Q0 was set to the mean number of infusions obtained under FR 10; k was set equal to the log of the maximum number of infusions obtained under any FR for a particular determination. The parameter α remained free to vary, and was transformed to essential value using Equation 2. Q0 and EV were averaged across determinations of the demand curve for individual monkeys, yielding one value per solution per monkey. Differences in Q0 and EV across solutions were analyzed using a one-way, repeated-measures ANOVA; a Geisser-Greenhouse adjustment was used to correct for potential violations of sphericity. Post-hoc comparisons among solutions were conducted using Dunnett’s test; p<.05 was considered significant. Analyses were conducted using GraphPad Prism (version 8.3.0; GraphPad Software, LLC; San Diego, CA, USA) and NCSS (version 10.0.13; NCSS; Kaysville, UT, USA).
3. Results
At the lowest response requirement, monkeys obtained on average (± 1 SEM) of 48.7 (12.7) infusions of vehicle, 96.8 (21.2) infusions of remifentanil alone, 21.0 (2.9) infusions of JWH-018 alone, and 29.9 (7.2) infusions of the remifentanil/JWH-018 mixture. For all solutions, the number of infusions decreased with increasing FR value (Figure 1). Across individual curve fits, the median R2 value was 0.90 (range: 0.49 to 0.99). There was a significant effect of solution on Q0 (F[1.53, 4.59]=7.3, p=.041), with remifentanil alone being significantly higher than JWH-018 alone or remifentanil in a mixture with JWH-018 (Figure 2A). Although not statistically significant at the group level, Q0 in all individual monkeys was higher for remifentanil than for vehicle (Figure 2B). There was a significant effect of solution on essential value (F[1.67, 5.02]=7.39, p=.034), with remifentanil alone being significantly higher than vehicle and remifentanil alone or in a mixture with JWH-018 being higher than JWH-018 alone (Figure 2C). Essential value for remifentanil alone was not significantly different from the mixture at the group level; for 3 of 4 monkeys, essential value of the mixture was either not different from or lower than for remifentanil alone (inset, Figure 2D).
Figure 1.
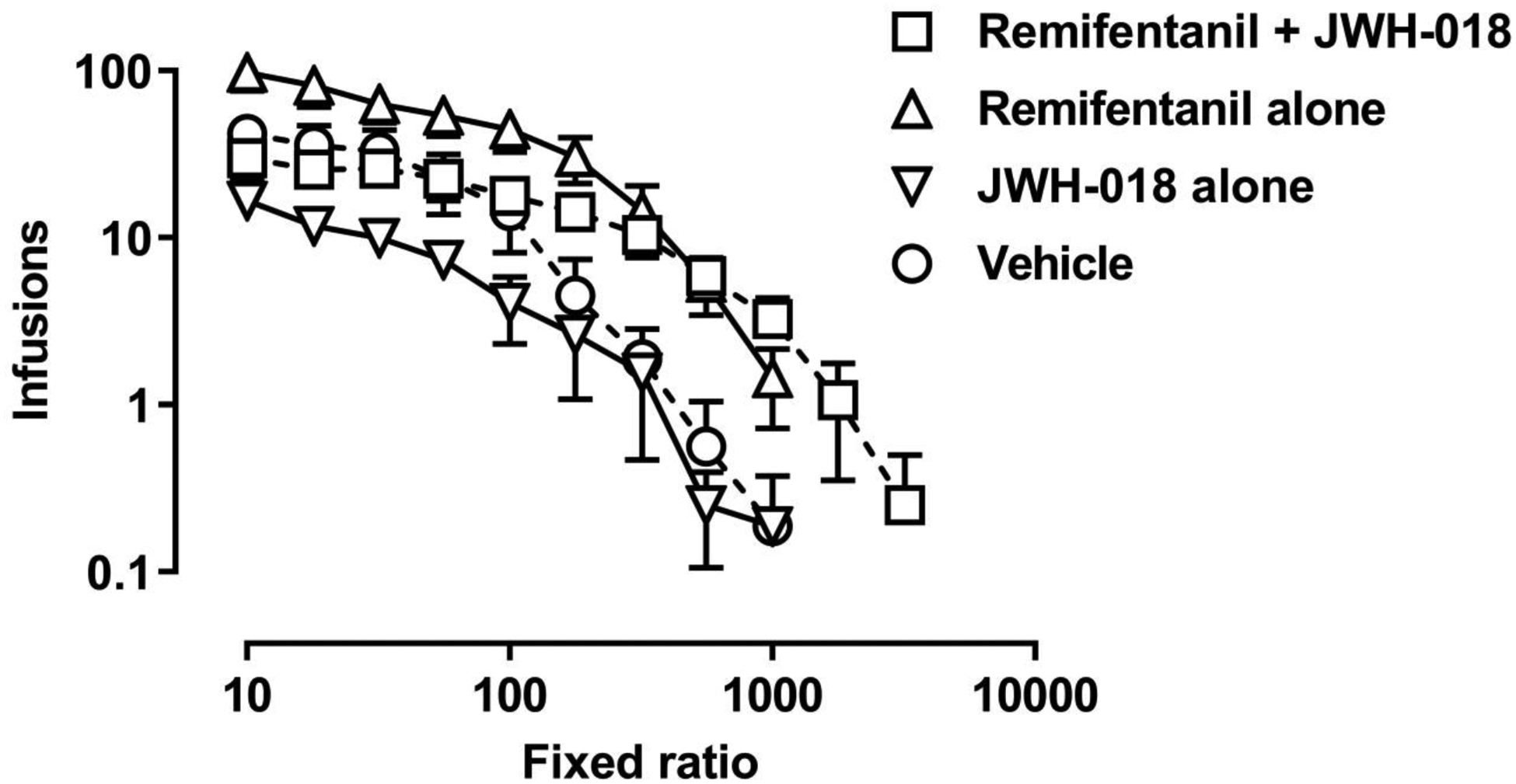
Demand curves for remifentanil in combination with JWH-018, 0.00032 mg/kg/infusion remifentanil alone, 0.0032 mg/kg/infusion JWH-018 alone, and vehicle in rhesus monkeys (n=4). Number of infusions is plotted as a function of the fixed-ratio requirement. Symbols indicate the mean, and error bars indicate ± 1 standard error of the mean. The abscissa and ordinate use logarithmic scaling.
Figure 2.
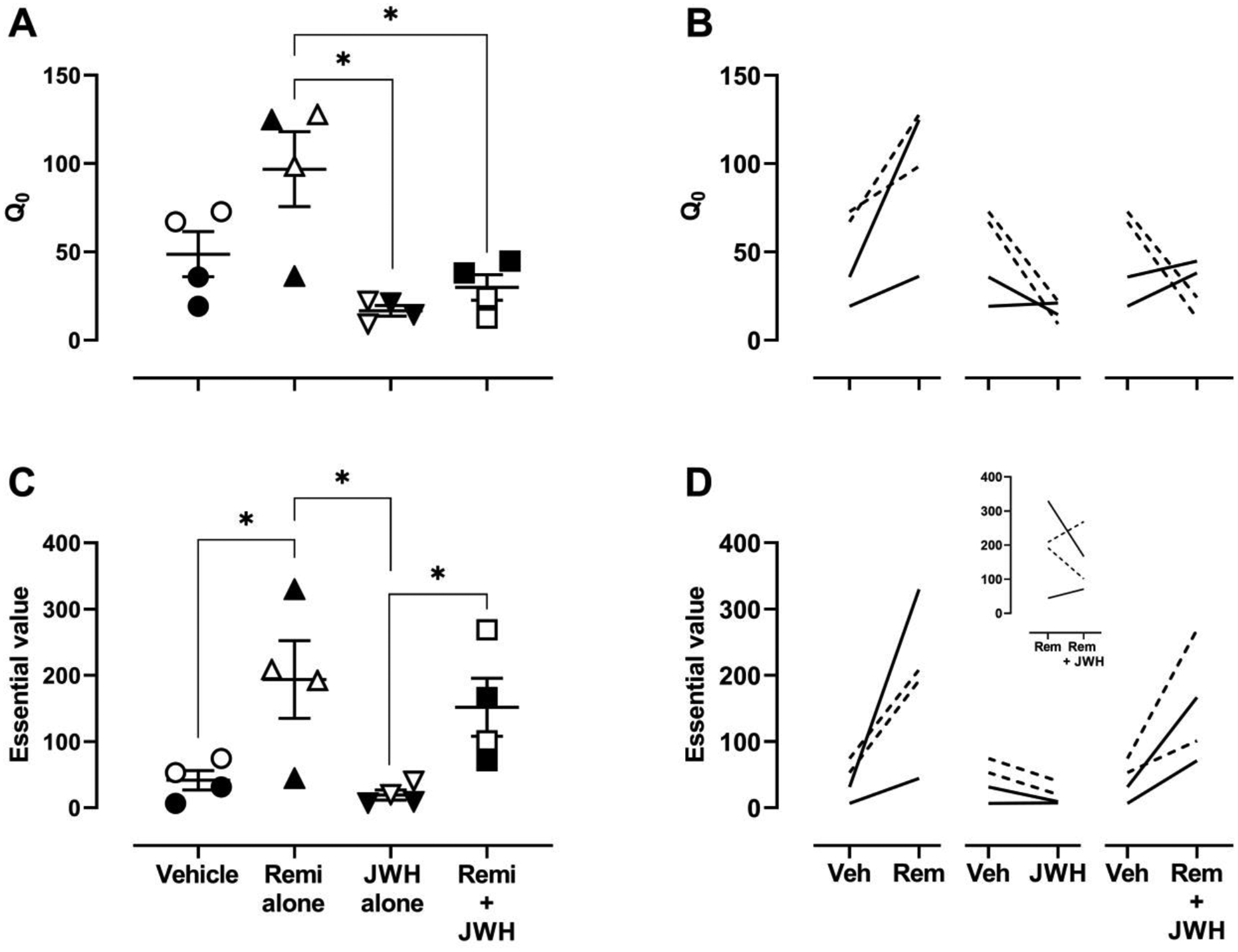
Parameters derived from the demand curve fits for each of the solutions available for self-administration. Estimates of consumption at a minimal cost (Q0; Equation 1) are plotted in the top panels whereas estimates of essential value (EV; Equation 2) are plotted in the bottom panels. In panels A and C, each data point indicates the mean value for an individual monkey across two determinations. The longer horizontal lines indicate the mean for the group, and error bars indicate ± 1 standard error of the mean. Filled symbols show data from males and unfilled symbols show data from females. Brackets with an asterisk indicate pairs of conditions that are statistically different (p<.05). Panels B and D show parameters for vehicle compared with each of the other solutions for individual monkeys; the inset in panel D shows a comparison of essential value between remifentanil alone and in combination with JWH-018. Solid lines show data from males and dashed lines show data from females.
4. Discussion
Whether for therapeutic (e.g., treating pain) or recreational (e.g., legalization of cannabis) purposes, it is likely that opioids and cannabinoids will increasingly be administered in combination; therefore, it is important to understand potential adverse interactions that might occur with their co-administration. In nonhuman primates, cannabinoids enhance some (e.g. antinociceptive) but not all (e.g., ventilatory depressant, discriminative stimulus, cognitive, and impulsive) effects of opioids. In addition, cannabinoids do not appear to enhance the reinforcing effects of opioids (Li et al. 2012; Maguire and France 2018; Gerak et al. 2019), which suggests that cannabinoids would not increase the abuse of opioids. However, given the severity of the opioid epidemic, increasing legalization of cannabis, and increasing availability of novel psychoactive substances, including synthetic cannabinoid receptor agonists, it is important to examine opioid/cannabinoid interactions under a broad range of conditions. The current study examined demand for a mixture of the opioid receptor agonist remifentanil and the cannabinoid receptor agonist JWH-018 to determine whether the mixture has greater reinforcing effectiveness compared with the opioid alone.
For all solutions, the number of infusions obtained (consumption) decreased as a function of the fixed-ratio value (price), and the resulting curves were described well by an exponential demand function (Hursh and Silberberg 2008). When available alone, remifentanil had a higher essential value than JWH-018 or vehicle. Although consumption of the mixture of remifentanil and JWH-018 was lower than that of remifentanil alone at the smallest response requirement (Q0), essential value of the mixture was not significantly different from that of remifentanil alone. These data indicate that, at the unit doses studied, JWH-018 did not enhance the reinforcing effectiveness of remifentanil, and are consistent with previous studies showing that cannabinoids do not enhance the reinforcing effects of opioids under choice procedures (Maguire and France 2018b; Gerak et al. 2019).
Although JWH-018 did not alter demand for remifentanil, the number of infusions of the remifentanil/JWH-018 mixture obtained at the lowest response requirement was significantly lower than when remifentanil was available alone. Reduced responding for an opioid/cannabinoid mixture under the single-lever procedure used in the current study is similar to effects reported previously in monkeys self-administering mixtures of heroin and Δ9-THC under a fixed-ratio 30 schedule (Li et al. 2012). Decreased responding in both studies likely reflects generalized rate-decreasing effects of the cannabinoid (or the opioid/cannabinoid mixture) rather than attenuation of the reinforcing effects of the opioid per se (see Maguire and France 2018b for a discussion). At the maximum level of intake (i.e., under the lowest response requirement), monkeys obtained, on average, between 10 and 46 infusions of 0.0032 mg/kg of JWH-018 per session, resulting in a cumulative intake of 0.032 to 0.15 mg/kg. Bolus injections of JWH-018 in this range of doses have robust discriminative stimulus effects in monkeys trained to discriminate JWH-018 or Δ9-tetrahydrocannabinol from vehicle (e.g., Ginsburg et al. 2012; Rodriquez and McMahon 2014). Taken together, these data indicate that JWH-018 was studied at behaviorally active levels of intake. Notably, when available alone JWH-018 did not maintain responding above vehicle levels, demonstrating that this unit dose did not function as a reinforcer under these conditions. Historically, the positive reinforcing effects of cannabinoids have been difficult to establish in rhesus monkeys, and the failure of JWH-018 to maintain responding in the current study is consistent with this literature, although reinforcing effects might become apparent after varying the unit dose and/or other experimental conditions (e.g., John et al. 2017).
The failure of JWH-018 to enhance the reinforcing effectiveness of remifentanil in the current study is consistent with a series of studies in nonhuman primates demonstrating failure of cannabinoids to enhance the reinforcing effects of opioids under a wide range of conditions. Results from studies in other species have been less consistent. For example, in rodents, cannabinoid receptor agonists have been shown to attenuate (e.g., Braida et al. 2001; Nquyen et al. 2019) or enhance (e.g., Solinas et al. 2005) self-administration of opioids, with studies varying along several dimensions including route of administration, schedule of reinforcement, and whether drugs were given as pretreatments or available for self-administration as mixtures. Far fewer studies have been conducted in humans, and results have similarly been mixed. For example, one study (Cooper et al. 2018) reported that smoked cannabis failed to enhance the reinforcing effects of oral oxycodone while a more recent study (Babalonis et al. 2019) reported that dronabinol enhanced abuse-related effects of oxycodone. These studies, as well, differed along several dimensions; thus, whether results in nonhuman primates will translate to humans has yet to be determined, owing largely to a paucity of studies.
In summary, using an economic demand analysis, this study examined effects of the synthetic cannabinoid receptor agonist JWH-018 on the reinforcing effectiveness of the mu opioid receptor agonist remifentanil in rhesus monkeys. Under the smallest response requirement, consumption of a mixture of remifentanil and JWH-018 was lower than that of remifentanil alone; however, essential value of the mixture was not significantly different. These data indicate that synthetic cannabinoids such as JWH-018 might alter some aspects of opioid self-administration (i.e., consumption at a particular price) but do not enhance reinforcing effectiveness as measured by sensitivity of consumption to increasing costs. Moreover, these data provide another source of convergent evidence that opioid/cannabinoid mixtures do not appear to have greater abuse potential compared with an opioid alone.
Highlights.
Opioid/cannabinoid mixtures could be used for therapeutic or recreational purposes
This study examined demand for remifentanil and JWH-018 alone and in combination
JWH-018 attenuated remifentanil intake at a low cost
JWH-018 did not enhance essential value of remifentanil
Cannabinoids do not appear to enhance the abuse liability of opioids
Acknowledgements
The authors thank M. Deande, C. Earlywine, S. Howard, J. Juarez, K. Martinez, A. Nelson, E. Spolarich, and S. Womack for expert technical assistance.
Role of Funding Source
This work was supported by the United States National Institutes of Health [grant number R01DA005018] and by the Welch Foundation [grant number AQ-0039]. Funding sources were not involved in this study beyond providing financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Maguire DR and France CP
The authors have nothing to disclose.
Conflict of Interest
No conflict declared
References
- Babalonis S, Lofwall MR, Sloan PA, Nuzzo PA, Fanucchi LC, Walsh SL, 2019. Cannabinoid modulation of opioid analgesia and subjective drug effects in healthy humans. Psychopharmacology (Berl). 236, 3341–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Pozzi M, Paroloaro D Sala M, 2001. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur. J. Pharmacol 413, 227–234. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Bedi G, Ramesh D, Balter R, Comer SD, Haney M, 2018. Impact of co-administration of oxycodone and smoked cannabis on analgesia and abuse liability. Neuropsychopharmacology. 43, 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, France CP, 2016. Combined treatment with morphine and Δ9-tetrahydrocannabinol in rhesus monkeys: antinociceptive tolerance and withdrawal. J. Pharmacol. Exp. Ther 357, 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Weed PF, Maguire DR, France CP, 2019. Effects of the synthetic cannabinoid receptor agonist JWH-018 on abuse-related effects of opioids in rhesus monkeys. Drug. Alcohol. Depend 202, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR, 2012. JWH-018 and JWH-073: Δ9-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J. Pharmacol. Exp. Ther 340, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AN, Le Foll B, 2019. Polydrug use disorders in individuals with opioid use disorder. Drug. Alcohol. Depend 198, 28–33. [DOI] [PubMed] [Google Scholar]
- Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M, 2018. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep. 67, 1–12. https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_09-508.pdf. Accessed 30 December 2019. [PubMed] [Google Scholar]
- Hursh SR, 1991. Behavioral economics of drug self-administration and drug abuse policy. J. Exp. Anal. Behav 56, 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH, 2005. The economics of drug abuse: a quantitative assessment of drug demand. Mol. Interv 5, 20–28. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Roma PG, 2016. Behavioral economics and the analysis of consumption and choice. Manag. Dec. Econ 37, 224–238. [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychol. Rev 115, 186–198. [DOI] [PubMed] [Google Scholar]
- John WS, Martin TJ, Nader MA, 2017. Behavioral determinants of cannabinoid self-administration in old world monkeys. Neuropsychopharmacology. 42, 1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Terner S, Hursh S, Woods JH, Winger G, 2002. Relative reinforcing effects of three opioids with different durations of action. J. Pharmacol. Exp. Ther 301, 698–704. [DOI] [PubMed] [Google Scholar]
- Li JX, Koek W, France CP, 2012. Interactions between Δ9-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav. Pharmacol 23, 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL, France CP, 2008. Interactions between Δ 9-tetrahydrocannabinol and μ opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology (Berl) 199, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2014. Impact of efficacy at the μ-opioid receptor on antinociceptive effects of combinations of μ-opioid receptor agonists and cannabinoid receptor agonists. J. Pharmacol. Exp. Ther 351, 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2016a. Interactions between cannabinoid receptor agonists and mu opioid receptor agonists in rhesus monkeys discriminating fentanyl. Eur. J. Pharmacol 784, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2016b. Effects of daily delta-9-tetrahydrocannabinol treatment on heroin self-administration in rhesus monkeys. Behav. Pharmacol 27, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2018a. Antinociceptive effects of mixtures of mu opioid receptor agonists and cannabinoid receptor agonists in rats: Impact of drug and fixed-dose ratio. Eur. J. Pharmacol 819, 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, France CP, 2018b. Reinforcing effects of opioid/cannabinoid mixtures in rhesus monkeys responding under a food/drug choice procedure. Psychopharmacology (Berl). 235, 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP, 2013a. Effect of delay on self-administration of remifentanil under a drug versus drug choice procedure in rhesus monkeys. J. Pharmacol. Exp. Ther 347, 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Cami-Kobeci G, Husbands SM, France CP, Belli B, Flynn P, 2020a. OREX-1019: a novel treatment of opioid use disorder and relapse prevention. J. Pharmacol. Exp. Ther 372, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Woods JH, Husbands SM, Disney A, France CP, 2019. Long-lasting effects of methocinnamox on opioid self-administration in rhesus monkeys. J. Pharmacol. Exp. Ther 368, 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Minervini V, Dodda V, France CP, 2020b. Impact of order of fixed-ratio presentation on demand for self-administered remifentanil in male rats. Beh. Pharmacol 31, 216–220. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP, 2013b. Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration. J. Pharmacol. Exp. Ther 345, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervini V, France CP, 2018. Effects of morphine/CP55940 mixtures on an impulsive choice task in rhesus monkeys. Behav. Pharmacol 29, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minervini V, France CP, 2020. Effects of opioid/cannabinoid mixtures on impulsivity and memory in rhesus monkeys. Behav. Pharmacol 31, 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A 2004. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford: Oxford University Press. [Google Scholar]
- Nguyen JD, Grant Y, Creehan KM, Hwang CS, Vandewater SA, Janda KD, Cole M, Taffe MA, 2019. Δ9-tetrahydrocannabinol attenuates oxycodone self-administration under extended access conditions. Neuropharmacology. 151, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilges MR, Bondy ZA, Grace JA, Winsauer PJ, in press Opioid-enhancing antinociceptive effects of delta-9-tetrahydrocannabinol and amitriptyline in rhesus macaques. Exp. Clin. Psychopharmacol Available online 29 August 2019. doi: 10.1037/pha0000313 [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, McMahon LR, 2014. JWH-018 in rhesus monkeys: Differential antagonism of discriminative stimulus, rate-decreasing, and hypothermic effects. Eur. J. Pharmacol 740, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz LP, Roma PG, Henningfield JE, Hursh SR, Cone EJ, Buchhalter AR, Fant RV, Schnoll SH, 2019. Behavioral economic demand metrics for abuse deterrent and abuse potential quantification. Drug. Alcohol. Depend 198, 13–20. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR 2005. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 30, 2046–2057. [DOI] [PubMed] [Google Scholar]
- Weed PF, Gerak LR, France CP, 2018. Ventilatory-depressant effects of opioids alone and in combination with cannabinoids in rhesus monkeys. Eur. J. Pharmacol 833, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Woods JH, Hursh SR, 1996. Behavior maintained by alfentanil or nalbuphine in rhesus monkeys: fixed-ratio and time-out changes to establish demand curves and relative reinforcing effectiveness. Exp. Clin. Psychopharmacol 4, 131–140. [Google Scholar]
- Zanettini C, Wilkinson DS, Katz JL, 2018. Behavioral economic analysis of the effects of N-substituted benztropine analogs on cocaine self-administration in rats. Psychopharmacology (Berl). 235, 47–58. [DOI] [PubMed] [Google Scholar]


