Abstract
Introduction
Elevated serum uric acid (SUA) is associated with cardiovascular risk factors, which often contribute to dementia and dementia-like morbidity, yet several cross-sectional studies have shown protective associations with cognition, which would be consistent with other work showing benefits of elevated SUA through its antioxidant properties.
Methods
We studied 11,169 participants free of dementia and cardiovascular disease from the Atherosclerosis Risk in Communities (ARIC) cohort. SUA was measured in blood samples collected in 1990–92, baseline for this study (age range 47–70 years). Incident dementia was ascertained based on clinical assessments in 2011–13 and 2016–17, surveillance based on dementia screeners conducted over telephone interviews, hospitalization discharge codes, and death certificates. Cognitive function was assessed up to four times between 1990–92 and 2016–17. We estimated the association of SUA, categorized into quartiles, with incidence of dementia using Cox regression models adjusting for potential confounders. The association between cognitive decline and SUA was assessed using generalized estimating equations.
Results
Over a median follow-up period of 24.1 years, 2,005 cases of dementia were identified. High baseline SUA was associated with incident dementia (HR, 1.29; 95% CI, 1.12, 1.47) when adjusted for sociodemographic variables. However, after further adjustment including cardiovascular risk factors, this relationship disappeared (HR, 1.03; 95% CI, 0.88, 1.21). Elevated baseline SUA was associated with faster cognitive decline even after further adjustment (25-year global z-score difference, −0.149; 95% CI, −0.246, −0.052)
Conclusion
Higher levels of mid-life SUA were associated with faster cognitive decline, but not necessarily with higher risk of dementia.
Keywords: dementia, cognitive decline, uric acid, cohort study, epidemiology
1. INTRODUCTION
Prevalence estimates in the United States suggest that 22% of persons over age 70 have some level of cognitive impairment and 13% over the age of 70 have dementia.[1] As more of the global population advances into old age, cognitive decline and dementia will become a progressively more prominent public health concern. These figures highlight the need to identify risk factors and preventive interventions to reduce the burden of dementia in the population.
Recent studies have investigated the involvement of serum uric acid (SUA), a product of purine metabolism, in the progression of cognitive decline and dementia with conflicting results.[2, 3] Antioxidants, such as SUA, are thought to exert neuroprotective influences, particularly against neurodegeneration, in theory, by protecting the blood brain barrier against oxidative stress.[4, 5] Supporting this, in chronic kidney disease patients, SUA levels were shown to be inversely associated with mild cognitive dysfunction.[6] Similarly, in the Rotterdam study, elevated SUA was associated with slower cognitive decline and reduced risk of dementia, even after controlling for cardiovascular risk factors.[7] Discrepancies in the literature, however, have made it difficult to come to consensus on the impact of SUA on cognitive decline. For example, elevated SUA can induce glomerulosclerosis and renal fibrosis, causing kidney injury,[8] which has long been associated with faster cognitive decline.[9–11] Other studies have implicated elevated SUA as exhibiting pro-oxidant properties by inducing microvascular dysfunction,[12, 13] mitochondrial dysfunction and triglyceride accumulation.[14] Overall, prior literature is mixed.[15, 16]
Evidence also suggests it may be important to consider sex- and race-based effect modification in the association of SUA with cognitive decline. The association between SUA and cardiovascular disease (CVD) is stronger in women compared to men.[17] Women also have a higher risk of Alzheimer’s disease in old age compared to men,[18] but whether it is due more to women’s longer life expectancies rather than other neurobiological factors is undecided. In the context of race, Black Americans are consistently at higher risk of dementia,[19] and tend to have higher rates of hyperuricemia compared to their white counterparts.[20]
Thus, while SUA’s antioxidant properties may potentially be protective against dementia and cognitive decline, there is also evidence that it may induce cognitive dysfunction. In light of the conflicting literature, we examined the relationship between mid-life SUA and the risk of incident dementia and cognitive decline over the course of 25 years within a community-based cohort. We also aimed to characterize the influence of race and sex on this relationship.
2. METHODS
The Atherosclerosis Risk in Communities (ARIC) study is an ongoing, community-based cohort study in the US with first study visit in 1987–1989.[21] The study recruited participants from four U.S. communities: Forsyth County, North Carolina; Jackson, Mississippi; selected Minneapolis suburbs in Minnesota; and Washington County, Maryland. Participants were also later examined through 5 subsequent follow-up visits that were available at the time of this study. The Jackson cohort are exclusively black, while the racial distribution in the other centers represents the underlying population (predominantly white in Washington County and Minneapolis sites, white and black in Forsyth County). Cognitive functioning was assessed at visits 2 (1990–92), 4 (1996–98), 5 (2011–13), and 6 (2016–17). Visit 2 is baseline for this analysis. All participants in the ARIC cohort have provided written informed consent for each study visit, and the institutional review boards at all ARIC study sites have approved of the study.
2.1. Blood Measurements
All blood analytes, including SUA, were measured in fasting blood samples from visit 2 collected at all four centers. Blood was drawn into vacuum tubes containing either serum separator gels for chemistries or EDTA for lipids, and then centrifuged for 10 minutes at 4°C at 3,000g. They were then packed into −70°C freezers and shipped to one of two laboratories in the U.S. for analysis: the Central Chemistry Laboratory or the Central Lipid Laboratory. Uric acid was measured using the uricase enzymatic method. The reliability coefficient for SUA, as assessed by repeat measurements in 40 participants taken at least one week apart, was 0.91, and the coefficient of variation was 7.2%.[22]
2.2. Incident Dementia
Determination of incident dementia was based on criteria recommended by the National Institute of Aging - Alzheimer’s Association workgroups.[23] In stage 1 of visit 5 (2011–13), all participants underwent neurocognitive exams and in-person examiner assessments at either the examination centers or in the participants’ homes, and if there was evidence of cognitive impairment, those individuals were selected to move onto stages 2 and 3. Of those without indication of cognitive impairment, a random sampling plan was implemented for selection onto stages 2 and 3. In visit 6 (2016–17), similar protocols were followed except for the inclusion of cognitively intact participants; data from visit 5 demonstrated that the inclusion of cognitively normal participants did not return additional cases of dementia that might have been missed in stage 1.[24]
Diagnosis of dementia was based on adjudication by an expert committee for participants who attended in-person evaluations at visit 5 or visit 6, using telephone and informant interviews for those that were alive during the follow-up (including participants who missed study visits), and using ICD-9/10-CM dementia hospitalization discharge codes and death certificates prior to visit 6 for all those that did not attend visits 5 and 6 nor complete the phone interviews. Of those individuals that were diagnosed with dementia based on in-person neurocognitive assessment at visit 5 or visit 6, date of diagnosis was defined as the date of the assessment. If participants were hospitalized for dementia prior to the visit 5 in-person assessment, date of diagnosis was defined as date of the hospitalization. If participants were diagnosed with dementia without having attended the in-person examinations, date of diagnosis was defined as the earliest date from the following: date of dementia screeners and/or informant interview conducted via telephone, date of dementia hospitalization discharge, or six months prior to date of death on death certificate. Six months were subtracted from date of death to account for lag in ascertainment of dementia status. In-depth details of the diagnostic process have been described elsewhere.[25] Person-years of follow-up were defined from visit 2 to dementia diagnosis, loss to follow-up, or administrative censoring up to December 31, 2017.
2.3. Cognitive Decline
Assessment of cognitive decline was based on three neurocognitive exams administered at baseline and the follow-up visits. The Delayed Word Recall Test (DWRT), Digit Symbol Substitution Test (DSST), and Word Fluency Test (WFT) evaluates verbal learning and short-term memory, executive functioning, and expressive language, respectively. The testing procedures have been described in detail elsewhere.[25]
Z-scores for the three tests were calculated at every visit and standardized to the visit 2 mean and standard deviation. Additionally, global composite z-scores were generated to assess global decline by averaging the z-scores of the tests, subtracting the mean global z-score from visit 2, and dividing by the global z-score standard deviation from visit 2.
2.4. Covariates
For all analyses, all covariates were obtained from data collected at visit 2, except for diet scores and education, which were assessed at visit 1. The following covariates were included in our minimally adjusted model: age, sex, race-center (Forsyth/White, Forsyth/Black, Washington/White, Minneapolis/White, Jackson/Black), and educational attainment (less than high school, high school without a degree, high school graduate, vocational school, college, graduate or professional school). Model 2 builds upon model 1 by also adjusting for waist-to-hip ratio, systolic and diastolic blood pressure (mmHg), antihypertensive diuretic use (antihypertensive diuretic, non-diuretic antihypertensive, no antihypertensive use), and diabetes status based off of a fasting blood glucose cutoff of 126 mg/dL, non-fasting blood glucose of ≥200 mg/dL, self-reported physician diagnosis of diabetes, or use of diabetic medication (diabetes/no diabetes), smoking status (ever smoked/never smoked), total and HDL cholesterol (mg/dL), estimated glomerular filtration rate (mL/min/1.73m2), C-reactive protein (mg/L), presence of the apolipoprotein ɛ4 (ApoE4) allele (allele/no allele), and diet scores. Diet scores assessing “Western” dietary patterns (characterized by processed foods high in fats and sugar) and “prudent” dietary patterns (characterized by fruits, vegetables, lean meats, etc.) were generated from consumption of 32 food groups via principal-components analysis.[26]
2.5. Analytic Cohort
The initial cohort, based in visit 2, consisted of 14,348 individuals. Participants were excluded if they refused consent for genetic research for ApoE4 genotyping (n=45), or if they presented with one or more of the following at visit 2: prevalent dementia (n=6); prevalent myocardial infarction (MI) as defined by self-report at visit 1 and adjudicated MI events between visit 1 and visit 2, prevalent MI as confirmed by electrocardiogram, prevalent coronary heart disease (CHD) (n=1,138); prevalent stroke at (n=184). Mid-life CVD is associated with elevated SUA and cognitive decline in later life,[27] so in order to assess the impact of SUA on dementia and cognitive decline in the absence of mid-life CVD, we excluded persons with baseline CVD from the analytic cohort. As the ARIC cohort is predominantly black and white, those of other ethnic/racial descents were excluded due to low counts (n=38). Additionally, blacks from Minneapolis and Washington County were excluded due to low counts compared to whites (n=46). Individuals with severe kidney disease, as indicated by an eGFR of less than 15 mL/min/1.73m2, were also excluded from analysis (n=12). Those with missing covariate information at visit 2 were likewise excluded from analysis (n=1710). Figure 1 presents a flowchart of study participants for the final analytic cohort.
Figure 1:
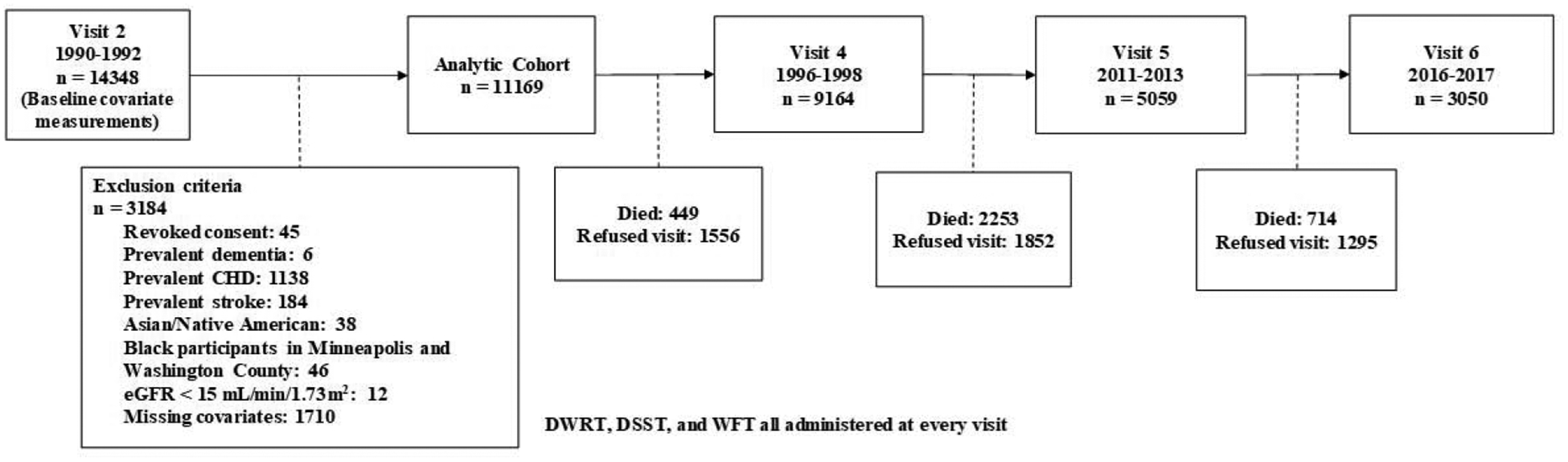
Flow diagram of analytic cohort selection and participant visit attendance for visits 2 through 6.
2.6. Statistical Analysis
We estimated the association of SUA, categorized into quartiles, with incidence of dementia by estimating hazard ratios (HR) with 95% confidence intervals (CI) using Cox proportional hazard models adjusting for potential confounders.
The association between cognitive decline and SUA was estimated using linear models and fitted with generalized estimating equations (GEE) with an unstructured correlation matrix to estimate changes in cognitive functioning from visit 2 up through visit 6 while accounting for correlated test scores among each participant. Because rates of cognitive decline tend to be more pronounced in later years, time was modeled using two linear spline terms with a knot at 6 years and 21 years - approximately corresponding to the time between visit 2 and visits 4 and 5, respectively - to better account for different slopes of decline. Interaction terms between these time splines and covariates were incorporated into the model to account for decline over time. Additionally, we estimated cross-sectional differences in cognitive function at baseline across SUA concentrations.
We also stratified the incident dementia and cognitive decline analyses by race and sex and added interaction terms to our models to test for associations of race-SUA and sex-SUA with incident dementia and cognitive decline.
Cognitive decline and the likelihood of attending later visits are linked.[28, 29] To account for attrition over the 25-year follow-up period since visit 2 we utilized multiple imputation by chained equations (MICE) to impute missing values, generating 20 imputed datasets for cognitive decline analysis.[30] Participation rates in the follow-up visits within our analytic cohort were about 80% at visit 4, 45% at visit 5, and 28% at visit 6. Neurocognitive scores were imputed at the median visit date for each visit after baseline for all those that were missing (alive or dead) using a dementia diagnosis indicator, dementia surveillance information derived from telephone screenings and informant interviews during visits 5 and 6, and all covariates used in model 2. All cognitive decline results presented have been obtained after multiple imputation.
Because elevated SUA has shown to be associated with CVD risk factors[31] and may contribute to mortality,[32] as a sensitivity analysis, we imputed scores at each visit for all participants that were missing, and then excluded visits at which the participants were confirmed to have died.
All analyses were performed using SAS 9.4 (Cary, NC; SAS Institute Inc).
3. RESULTS
After applying our exclusion criteria (Figure 1), the incident dementia cohort included 11,169 participants free of dementia, prevalent coronary heart disease, or stroke, and with available SUA at visit 2 [mean age: 56.7 years (SD: 5.7), 58.7% female, 24.4% black]. Subjects were followed for a median of 24.1 years (25th, 75th percentiles: 18.3, 25.5 years).
Table 1 presents the baseline characteristics of the cohort by baseline SUA quartile. Participants with higher SUA were older and more likely to be male and black. Educational attainment showed no clear trend between the quartiles. Of 11,169 participants, 2,005 (18.0%) developed dementia during follow-up. Patients who developed dementia during the follow-up were slightly older, more likely to be female, more likely to be black, and less educated than their cognitively intact counterparts (data not shown).
Table 1.
Baseline characteristics by baseline SUA quartile, ARIC 1990–92.
| Characteristics | Uric Acid Quartiles (mg/dL) | |||
|---|---|---|---|---|
| 1st (<4.20) | 2nd (4.20 – 5.07) | 3rd (5.08 – 5.95) | 4th (>5.95) | |
| N | 2645 | 2910 | 2619 | 2995 |
| Age, years | 55.9 (5.7) | 56.7 (5.6) | 57.1 (5.7) | 57.0 (5.7) |
| Female | 87.1% | 68.5% | 48.2% | 33.5% |
| Black | 20.6% | 23.7% | 24.0% | 28.8% |
| Education | ||||
| Less than High School | 5.3% | 7.5% | 7.5% | 9.1% |
| High School, but no degree | 12.1% | 11.8% | 12.7% | 14.0% |
| High School graduate | 37.4% | 35.1% | 31.9% | 28.7% |
| Vocational School | 8.3% | 9.2% | 9.1% | 8.0% |
| College | 27.5% | 25.5% | 27.8% | 28.9% |
| Graduate School or Professional School | 9.5% | 10.9% | 11.1% | 11.4% |
| Waist-to-Hip Ratio | 0.87 (0.08) | 0.91 (0.08) | 0.94 (0.07) | 0.96 (0.06) |
| Ever smoked | 51.7% | 56.0% | 61.1% | 64.0% |
| Diabetes | 10.3% | 12.1% | 12.9% | 17.7% |
| Systolic BP, mmHg | 116.5 (17.4) | 119.6 (18.3) | 122.2 (18.5) | 125.7 (18.4) |
| Diastolic BP, mmHg | 69.5 (9.7) | 71.3 (9.9) | 72.9 (10.1) | 74.9 (10.3) |
| Total Cholesterol, mg/dL | 206.9 (37.3) | 209.1 (39.0) | 209.7 (38.6) | 212.2 (41.0) |
| HDL Cholesterol, mg/dL | 58.4 (16.9) | 52.4 (16.4) | 47.7 (15.3) | 43.7 (14.5) |
| Antihypertensive medication | ||||
| Diuretic | 7.1% | 10.1% | 14.0% | 26.0% |
| Non-diuretic, antihypertensive | 10.0% | 13.8% | 13.7% | 16.2% |
| No antihypertensive medication | 82.9% | 76.2% | 72.3% | 57.8% |
| eGFR - Creatinine and Cystatin-C (mL/min/1.73m2) | 102.8 (14.2) | 98.4 (14.6) | 94.3 (15.0) | 89.5 (17.5) |
| ApoE e4 allele | 31.0% | 30.5% | 29.8% | 30.2% |
| C-reactive Protein, mg/L | 3.4 (6.1) | 4.1 (6.3) | 4.3 (6.1) | 4.9 (7.5) |
| Western Diet Score | −0.22 (0.88) | −0.10 (0.96) | 0.04 (1.01) | 0.15 (1.04) |
| Prudent Diet Score | 0.10 (1.03) | 0.03 (0.99) | −0.04 (0.95) | −0.05 (0.97) |
SUA, serum uric acid; eGFR, estimated glomerular filtration rate; ApoE, apolipoprotein E.
All variables presented either as mean (standard deviation) or as proportion.
3.1. Incident Dementia and SUA
The highest quartile of SUA was associated with a 29% higher rate of incident dementia (HR, 1.29; 95% CI, 1.12, 1.47) when adjusted for age, race, sex, and education level. (Table 2, model 1) However, after further adjustment for cardiovascular risk factors, (Table 2, model 2) this relationship disappeared (HR, 1.03; 95% CI, 0.88, 1.21); the addition of diabetes status, waist-to-hip ratio, eGFR, and antihypertensive medication use into the model seemed to be primarily responsible for the attenuation of the association (data not shown).
Table 2.
Hazard ratios (HR) and 95% confidence intervals (95% CI) for incident dementia by baseline SUA, ARIC 1990–2017
| Person Years of Follow-Up | Number developing dementia | IR‡ | Model 1* HR (95% CI) | Model 2** HR (95% CI) | |
|---|---|---|---|---|---|
| SUA, per 1 mg/dL | 235,055 | 2,005 | 8.53 | 1.07 (1.03, 1.12) | 1.00 (0.95, 1.05) |
| SUA, quartiles | |||||
| Quartile 1 | 58,149 | 446 | 7.67 | 1 (Referent) | 1 (Referent) |
| Quartile 2 | 62,558 | 549 | 8.78 | 1.12 (0.99, 1.27) | 1.06 (0.93, 1.21) |
| Quartile 3 | 54,592 | 468 | 8.57 | 1.07 (0.93, 1.22) | 0.96 (0.83, 1.10) |
| Quartile 4 | 59,756 | 542 | 9.07 | 1.29 (1.12, 1.47) | 1.03 (0.88, 1.21) |
| P for Trend*** | 0.001 | 0.96 |
SUA, serum uric acid; IR, incidence rate.
Crude incidence rate, per 1000 person-years.
Cox proportional hazards model adjusted for age, race-center, sex, and education
Adjusted for Model 1, plus smoking, total and HDL cholesterol, waist-to-hip ratio, diabetes status, apolipoprotein E4 allele, systolic and diastolic blood pressure, diuretic antihypertensive medication use, estimated glomerular filtration rate, western and prudent diet scores, and C-reactive protein
Wald Chi-Square, modeling SUA continuously
In the race- and sex-stratified analyses, associations between SUA and incident dementia were stronger in whites than blacks, (Supplemental Table 1) and in women than men (Supplemental Table 2). These relationships also disappeared after adjustment for model 2 covariates.
3.2. Cognitive Decline and SUA
Elevated SUA was associated with poorer cognitive functioning at baseline, (Supplemental Table 3) along with faster cognitive decline in all neurocognitive domains when adjusted for all model 1 covariates. (Table 3) However, after adjustment for cardiovascular risk factors, diet, and medication use, elevated SUA at baseline was associated with higher cognitive functioning at baseline in the DWRT only [Q4t=0 − Q1t=0 (95% CI), 0.092 (0.033, 0.151)]. Decline over 25 years was slightly attenuated but remained significant for all tests except for the DSST. (Table 3, model 2) Compared to the lowest baseline SUA quartile, those in the highest quartile experienced an additional decline of −0.149 (95% CI, −0.246, −0.052) in global z-scores over 25 years after adjustment for model 2 covariates. The association between the highest SUA concentrations and increased cognitive decline remained statistically significant, even after excluding visits in which participants were confirmed to have died. (Supplemental Table 4)
Table 3.
Difference in 25-year cognitive change by baseline SUA quartile, ARIC 1990–2017.
| Test Domain | Quartiles | Model 1* | P for trend*** | Model 2** | P for trend*** |
|---|---|---|---|---|---|
| Global | 0.0002 | 0.002 | |||
| 1 | 0 (Referent) | 0 (Referent) | |||
| 2 | −0.076 (−0.140, −0.012) | −0.066 (−0.131, −0.001) | |||
| 3 | −0.106 (−0.178, −0.035) | −0.092 (−0.166, −0.018) | |||
| 4 | −0.175 (−0.264, −0.086) | −0.149 (−0.246, −0.052) | |||
| DWRT | 0.01 | 0.007 | |||
| 1 | 0 (Referent) | 0 (Referent) | |||
| 2 | −0.077 (−0.167, 0.013) | −0.082 (−0.179, 0.014) | |||
| 3 | −0.053 (−0.170, 0.063) | −0.066 (−0.190, 0.059) | |||
| 4 | −0.205 (−0.345, −0.064) | −0.228 (−0.382, −0.074) | |||
| DSST | 0.02 | 0.28 | |||
| 1 | 0 (Referent) | 0 (Referent) | |||
| 2 | −0.040 (−0.086, 0.006) | −0.027 (−0.072, 0.017) | |||
| 3 | −0.057 (−0.118, 0.005) | −0.034 (−0.096, 0.028) | |||
| 4 | −0.083 (−0.155, −0.011) | −0.039 (−0.109, 0.030) | |||
| WFT | <.0001 | 0.004 | |||
| 1 | 0 (Referent) | 0 (Referent) | |||
| 2 | −0.049 (−0.110, 0.012) | −0.036 (−0.097, 0.025) | |||
| 3 | −0.135 (−0.187, −0.082) | −0.111 (−0.167, −0.055) | |||
| 4 | −0.121 (−0.182, −0.059) | −0.075 (−0.145, −0.005) |
SUA, serum uric acid; DWRT, delayed word recall test; DSST, digit symbol substitution test; WFT, word fluency test.
Results from Generalized Estimating Equations adjusted for age, race-center, sex, education, and interactions of all covariates with time. Time modeled as spline term with knots at 6 years and 21 years.
Adjusted for Model 1, plus smoking, total and HDL cholesterol, waist-to-hip ratio, diabetes status, apolipoprotein E4 allele, systolic and diastolic blood pressure, diuretic antihypertensive medication us e, estimated glomerular filtration rate, western and prudent diet scores, C-reactive protein, and interactions of all covariates with time. Time modeled as spline terms with knots at 6 years and 21 years.
Wald Chi-Square, modeling SUA continuously
When stratified by race, the trends observed in cognitive decline across SUA concentrations among the entire cohort held for white participants, but not for black participants, though there was no evidence of a race interaction (p-value for race-interaction in global z-score, 0.50). (Supplemental Table 5) Sex stratification saw similar results to race stratification, with the trends being observed in the entire cohort holding for women, but not for men, though there was no evidence of heterogeneity based on sex (p-value for sex-interaction in global z-score, 0.74). (Supplemental Table 6)
4. DISCUSSION
Within the ARIC cohort, higher mid-life SUA concentration was not associated with incident dementia, but it was associated with faster 25-year cognitive decline when accounting for lipid analytes, anthropomorphic measurements, medication usage, and lifestyle variables. The relationship between SUA and incident dementia attenuated once diabetes status, waist-to-hip ratio, eGFR, and antihypertensive medication use were introduced into the model, in part confirming SUA’s thoroughly scrutinized association with cardiovascular disease and metabolic dysfunction.[13, 33, 34] In contrast, elevated baseline SUA was associated with faster decline in each cognitive domain even after full adjustment, except in the DSST. There were markedly faster rates of decline at the highest levels of SUA within the domains of verbal learning, short-term memory, and expressive language. Overall, global cognitive decline seemed to be driven mainly by decline in DWRT scores. Additionally, the discrepancy between incident dementia and cognitive decline remained after excluding those who were confirmed to have been dead during the visits.
Stratification based on sex and race yielded similar results for whites and women, where an association was found with incident dementia at the highest baseline SUA quartile in the first model and later attenuating and losing significance after full adjustment. SUA was not associated with incident dementia among men or black participants in either model 1 or model 2. None of these sex or race differences were statistically significant.
Furthermore, while whites and women experienced the greatest cognitive decline and largely drove the general decline rates in the cohort, we did not find blacks to be inherently different from whites nor women from men when tested through interaction terms. Racial disparities in cardiovascular risk factors and dementia-related morbidities have been previously well-documented,[35–37] but whether these elements modify the relationship between SUA and dementia in blacks has not been explored as diligently as the racial disparities found in dementia prevalence and incidence studies.[19] Our findings do corroborate previous work in which race was not found to significantly account for the heterogeneity between studies examining the relationship between SUA and Alzheimer’s disease.[38] The lack of sex interaction in our decline rates, however, contrast previous work. For instance, higher baseline SUA has been shown to protect against executive dysfunction in middle aged-men[39] and visuospatial decline in older men,[40] whereas SUA in older women was associated with progressively poor working memory, but showed no relationship with visuospatial abilities nor global cognitive functioning.[41]
The discrepancy across studies may in part be explained by hormonal fluctuations evident during menopause. Post-menopausal women have been documented to have higher SUA than pre-menopausal women;[42, 43] such clear hormonal differences were not found in men.[44] Men on average have higher levels of SUA than women,[45] but these comparisons are often made in populations with large age ranges that include young and middle-aged adults. The average age at baseline in our cohort was 56 years; the average age of onset for menopause is 51 years[46] and lasts an average of 4 years.[47] It may be possible that menopause attenuates sex-based differences in cognitive decline, which in part explains the lack of heterogeneity between men and women in this study.
While we did not find an association in our second model between SUA and incident dementia, we did find disparate rates of cognitive functioning across mid-life SUA levels that may explain the discrepancy between incident dementia and cognitive decline. At baseline, after adjustment for model 2 covariates, those with elevated SUA were found to have higher cognitive functioning compared to those with lower SUA, within the domain of verbal learning and short-term memory. These same groups, however, also experienced the steepest rates of decline over the course of 25 years. It may be possible that while groups within this cohort start off at different levels of cognitive functioning, the time at which they reach the threshold to be diagnosed with dementia may not be different based on mid-life SUA.
The protective or detrimental properties of SUA may be dictated by age and length of time at which it is left elevated in the body. Chronically elevated SUA is associated with risk of stroke,[48] but several animal models provide evidence of SUA’s antioxidant properties in acute elevations. In rats subjected to induced cerebral ischemia, intravenous injection of uric acid was found to be protective against oxidative stress by upregulating neurotrophic factors[49] and preserving mitochondrial function,[50] which have been shown to be crucial in post-stroke recovery efforts.[51, 52] Clinical SUA interventions for post-stroke recovery in humans are less prevalent, but have also yielded similar conclusions regarding the efficacy of intravenous uric acid.[53] It is possible that elevated SUA in middle age facilitates greater cognitive reserve, but subsequent chronic elevation can induce SUA’s pro-oxidant properties, resulting in volatility of cognitive reserve.
The Rotterdam study found a decreased risk of incident dementia and better cognitive functioning among those with elevated baseline SUA in an elderly cohort in the Netherlands over the span of 10 years.[7] In contrast to our findings that saw an attenuation of significance for incident dementia once adjusted for the full range of covariates, the Rotterdam analysis found a notable trend once they adjusted for multiple potential confounders. It is important, however, to note a few key differences between these two studies when comparing results. The Rotterdam study limited their analytic cohort for cognitive functioning to those with available test scores at baseline and the fourth survey, excluding nearly two-thirds of their original cohort. Furthermore, their full model does not account for ApoE4 nor for antihypertensive medication use, both of which are heavily implicated in the progression of cognitive decline and dementia.[54–56] Third, the Rotterdam study was restricted to white Europeans, which may affect generalizability.
Strengths of our study include the ability to model the data longitudinally. Much of the available literature on the association between SUA and cognitive decline is cross-sectional in nature, and because dementia and cognitive decline are slow-developing diseases with no hard endpoints, the ability to track cognitive trajectories over an extended period of time is an especially valuable addition to the literature. Another strength of this study is the use of MICE to account for attrition over the 25-year span of the study. Loss-to-follow-up due to death or deteriorating health is common among older populations and can introduce significant bias into the model. The MICE method has been previously validated as a technique to account for attrition in the ARIC cohort.[57] Even after multiple sensitivity analyses, results remained robust. It is important to note, however, that while the MICE method is an acceptable procedure for loss-to-follow-up concerns, there is an increased probability of estimation errors for data over 50% missing,[58] and with over 70% of the original visit 2 participants missing by visit 6, results should be interpreted with caution. It may also be possible that those with elevated mid-life SUA could have died from complications due to cardiovascular disease in conjunction with dementia, thus masking the real burden of dementia and cognitive decline. Another limitation of this study is the one-time measurement of SUA at baseline, which impedes our ability to track the influence of blood analyte fluctuations on risk of dementia and cognitive decline.
5. CONCLUSION
In conclusion, we have substantiated previous reports claiming an association between elevated SUA and increased cognitive decline in whites and women, but have found the evidence for a relationship between SUA and formally diagnosed dementia to be inconclusive. Future endeavors in the study of cognitive decline may benefit from examining the influence of SUA fluctuations over an extended period of time in order to better understand SUA’s pro- and antioxidant properties in the study of dementia and cognitive decline.
Supplementary Material
HIGHLIGHTS.
Uric acid not associated with dementia adjusting for cardiovascular risk factors
Elevated uric acid associated with better baseline cognition but greater decline
Elevated uric acid associated with declines in multiple cognitive domains
Cognitive decline results remained robust after accounting for attrition
FUNDING
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. Additional support was provided by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K24HL148521. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF COMPETING INTERESTS
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Brookmeyer R, Evans DA, Hebert L, Langa KM, Heeringa SG, Plassman BL, et al. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duron E, Hanon O. Antihypertensive treatments, cognitive decline, and dementia. J Alzheimers Dis. 2010;20(3):903–14. [DOI] [PubMed] [Google Scholar]
- 3.Yap YW, Whiteman M, Cheung NS. Chlorinative stress: an under appreciated mediator of neurodegeneration? Cell Signal. 2007;19(2):219–28. [DOI] [PubMed] [Google Scholar]
- 4.Palmer AM. The blood-brain barrier. Neurobiol Dis. 2010;37(1):1–2. [DOI] [PubMed] [Google Scholar]
- 5.Lam V, Hackett M, Takechi R. Antioxidants and Dementia Risk: Consideration through a Cerebrovascular Perspective. Nutrients. 2016;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afsar B, Elsurer R, Covic A, Johnson RJ, Kanbay M. Relationship between Uric Acid and Subtle Cognitive Dysfunction in Chronic Kidney Disease. Am J Nephrol. 2011;34(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Euser SM, Hofman A, Westendorp RG, Breteler MM. Serum uric acid and cognitive function and dementia. Brain. 2009;132(Pt 2):377–82. [DOI] [PubMed] [Google Scholar]
- 8.Romi MM, Arfian N, Tranggono U, Setyaningsih WAW, Sari DCR. Uric acid causes kidney injury through inducing fibroblast expansion, Endothelin-1 expression, and inflammation. BMC Nephrol. 2017;18(1):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossola M, Antocicco M, Di Stasio E, Ciciarelli C, Luciani G, Tazza L, et al. Mini Mental State Examination over time in chronic hemodialysis patients. J Psychosom Res. 2011;71(1):50–4. [DOI] [PubMed] [Google Scholar]
- 10.Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009;73(12):920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CY, Lin CC, Tsai CF, Yang WC, Wang SJ, Lin FH, et al. Cognitive impairment and hippocampal atrophy in chronic kidney disease. Acta Neurol Scand. 2017;136(5):477–85. [DOI] [PubMed] [Google Scholar]
- 12.Prasad M, Matteson EL, Herrmann J, Gulati R, Rihal CS, Lerman LO, et al. Uric Acid Is Associated With Inflammation, Coronary Microvascular Dysfunction, and Adverse Outcomes in Postmenopausal Women. Hypertension. 2017;69(2):236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheepers L, Boonen A, Dagnelie PC, Schram MT, van der Kallen CJH, Henry RMA, et al. Uric acid and blood pressure: exploring the role of uric acid production in The Maastricht Study. J Hypertens. 2017;35(10):1968–75. [DOI] [PubMed] [Google Scholar]
- 14.Maarman GJ, Andrew BM, Blackhurst DM, Ojuka EO. Melatonin protects against uric acid-induced mitochondrial dysfunction, oxidative stress, and triglyceride accumulation in C2C12 myotubes. J Appl Physiol (1985). 2017;122(4):1003–10. [DOI] [PubMed] [Google Scholar]
- 15.Maetzler W, Stapf AK, Schulte C, Hauser AK, Lerche S, Wurster I, et al. Serum and cerebrospinal fluid uric acid levels in lewy body disorders: associations with disease occurrence and amyloid-beta pathway. J Alzheimers Dis. 2011;27(1):119–26. [DOI] [PubMed] [Google Scholar]
- 16.Cicero AF, Desideri G, Grossi G, Urso R, Rosticci M, D’Addato S, et al. Serum uric acid and impaired cognitive function in a cohort of healthy young elderly: data from the Brisighella Study. Intern Emerg Med. 2015;10(1):25–31. [DOI] [PubMed] [Google Scholar]
- 17.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283(18):2404–10. [DOI] [PubMed] [Google Scholar]
- 18.Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498–504. [DOI] [PubMed] [Google Scholar]
- 19.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13(1):72–83. [DOI] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, Law A, Maynard JW, Coresh J, Baer AN. Risk factors for incident hyperuricemia during mid-adulthood in African American and white men and women enrolled in the ARIC cohort study. BMC Musculoskelet Disord. 2013;14:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 22.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 23.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mi ld cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ARIC Visit 6 Manual 17: ARIC Neurocognitive Exam. 2017:6. [Google Scholar]
- 25.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild Cognitive Impairment and Dementia Prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117(6):754–61. [DOI] [PubMed] [Google Scholar]
- 27.Stephan BCM, Harrison SL, Keage HAD, Babateen A, Robinson L, Siervo M. Cardiovascular Disease, the Nitric Oxide Pathway and Risk of Cognitive Impairment and Dementia. Curr Cardiol Rep. 2017;19(9):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao C, Stawski RS, Hultsch DF, MacDonald SW. Selective attrition and intraindividual variability in response time moderate cognitive change. J Clin Exp Neuropsychol. 2016;38(2):227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegler IC, Botwinick J. A long-term longitudinal study of intellectual ability of older adults: the matter of selective subject attrition. J Gerontol. 1979;34(2):242–5. [DOI] [PubMed] [Google Scholar]
- 30.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Qiu L, Cheng XQ, Xu T, Wu W, Zeng XJ, et al. Hyperuricemia and clustering of cardiovascular risk factors in the Chinese adult population. Sci Rep. 2017;7(1):5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Iso H, Murakami Y, Miura K, Nagai M, Sugiyama D, et al. Serum Uric Acid and Mortality Form Cardiovascular Disease: EPOCH-JAPAN Study. J Atheroscler Thromb. 2016;23(6):692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Schaft N, Brahimaj A, Wen KX, Franco OH, Dehghan A. The association between serum uric acid and the incidence of prediabetes and type 2 diabetes mellitus: The Rotterdam Study. PLoS One. 2017;12(6):e0179482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Cosmo S, Viazzi F, Pacilli A, Giorda C, Ceriello A, Gentile S, et al. Serum Uric Acid and Risk of CKD in Type 2 Diabetes. Clin J Am Soc Nephrol. 2015;10(11):1921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heidemann DL, Joseph NA, Kuchipudi A, Perkins DW, Drake S. Racial and Economic Disparities in Diabetes in a Large Primary Care Patient Population. Ethn Dis. 2016;26(1):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limdi NA, Howard VJ, Higginbotham J, Parton J, Safford MM, Howard G. US Mortality: Influence of Race, Geography and Cardiovascular Risk Among Participants in the Population-Based REGARDS Cohort. J Racial Ethn Health Disparities. 2016;3(4):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen-Huynh MN, Hills NK, Sidney S, Klingman JG, Johnston SC. Race-ethnicity on blood pressure control after ischemic stroke: a prospective cohort study. J Am Soc Hypertens. 2017;11(1):38–44. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, Guo X, Huang R, Chen Y, Zheng Z, Shang H. Serum uric acid levels in patients with Alzheimer’s disease: a meta-analysis. PLoS One. 2014;9(4):e94084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baena CP, Suemoto CK, Barreto SM, Lotufo PA, Bensenor I. Serum uric acid is associated with better executive function in men but not in women: Baseline assessment of the ELSA-Brasil study. Exp Gerontol. 2017;92:82–6. [DOI] [PubMed] [Google Scholar]
- 40.Kueider AM, An Y, Tanaka T, Kitner-Triolo MH, Studenski S, Ferrucci L, et al. Sex-Dependent Associations of Serum Uric Acid with Brain Function During Aging. J Alzheimers Dis. 2017;60(2):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vannorsdall TD, Kueider AM, Carlson MC, Schretlen DJ. Higher baseline serum uric acid is associated with poorer cognition but not rates of cognitive decline in women. Exp Gerontol. 2014;60:136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung JH, Song GG, Lee YH, Kim JH, Hyun MH, Choi SJ. Serum uric acid levels and hormone therapy type: a retrospective cohort study of postmenopausal women. Menopause. 2018;25(1):77–81. [DOI] [PubMed] [Google Scholar]
- 43.Wingrove CS, Walton C, Stevenson JC. The effect of menopause on serum uric acid levels in non-obese healthy women. Metabolism. 1998;47(4):435–8. [DOI] [PubMed] [Google Scholar]
- 44.Mikkelsen WM, Dodge HJ, Valkenburg H. The Distribution of Serum Uric Acid Values in a Population Unselected as to Gout or Hyperuricemia: Tecumseh, Michigan 1959–1960. Am J Med. 1965;39:242–51. [DOI] [PubMed] [Google Scholar]
- 45.Heo SH, Lee SH. High levels of serum uric acid are associated with silent brain infarction. J Neurol Sci. 2010;297(1–2):6–10. [DOI] [PubMed] [Google Scholar]
- 46.te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–54. [DOI] [PubMed] [Google Scholar]
- 47.Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med. 2008;23(9):1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weir CJ, Muir SW, Walters MR, Lees KR. Serum urate as an independent predictor of poor outcome and future vascular events after acute stroke. Stroke. 2003;34(8):1951–6. [DOI] [PubMed] [Google Scholar]
- 49.Ya BL, Liu Q, Li HF, Cheng HJ, Yu T, Chen L, et al. Uric Acid Protects against Focal Cerebral Ischemia/Reperfusion-Induced Oxidative Stress via Activating Nrf2 and Regulating Neurotrophic Factor Expression. Oxid Med Cell Longev. 2018;2018:6069150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53(5):613–25. [DOI] [PubMed] [Google Scholar]
- 51.Lanfranconi S, Locatelli F, Corti S, Candelise L, Comi GP, Baron PL, et al. Growth factors in ischemic stroke. J Cell Mol Med. 2011;15(8):1645–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otsuka S, Sakakima H, Sumizono M, Takada S, Terashi T, Yoshida Y. The neuroprotective effects of preconditioning exercise on brain damage and neurotrophic factors after focal brain ischemia in rats. Behav Brain Res. 2016;303:9–18. [DOI] [PubMed] [Google Scholar]
- 53.Chamorro A, Amaro S, Castellanos M, Gomis M, Urra X, Blasco J, et al. Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int J Stroke. 2017;12(4):377–82. [DOI] [PubMed] [Google Scholar]
- 54.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3. [DOI] [PubMed] [Google Scholar]
- 55.Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, et al. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59(4):780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ronnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40-year follow-up of a population-based cohort. Dement Geriatr Cogn Disord. 2011;31(6):460–6. [DOI] [PubMed] [Google Scholar]
- 57.Rawlings AM, Sang Y, Sharrett AR, Coresh J, Griswold M, Kucharska-Newton AM, et al. Multiple imputation of cognitive performance as a repeatedly measured outcome. Eur J Epidemiol. 2017;32(1):55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson DR, Young R. Toward Best Practices in Analyzing Datasets with Missing Data: Comparisons and Recommendations. Journal of Marriage and Family. 2011;73(5):926–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


