Abstract
Background
Financial incentives for smoking cessation and use of evidence-based therapy may increase quitting rates and reduce health and economic disparities.
Methods
We randomized a low-income population of 182 hospitalized patients (mean age 58 years, 45% with high school education or less) to enhanced usual care, which included hospital-directed cessation care and Quitline referral, or enhanced usual care plus financial incentives. All patients received enhanced usual care, while participants randomized to the financial incentives group were also eligible to receive up to $550 for participation in Quitline counseling ($50), participation in a community-based cessation program ($50), use of pharmacotherapy ($50), and biochemically-confirmed smoking cessation at 2 months ($150) and 6 months ($250). Primary outcome was biochemically-confirmed smoking cessation at 6 months after hospital discharge.
Results
Total mean payment was $84 (SD=$133) in the incentive group. The 6-month rate of biochemically-confirmed smoking cessation was 19.6% in the incentive group and 8.9% in the enhanced usual care group (odds ratio, 2.56; 95% CI, 0.84 to 7.83, P=0.10). Participants in the incentive group had higher rates of nicotine replacement therapy use (57.3% versus 31.3%, P=0.002).
Financial incentives did not improve subjective social status but did increase financial stress.
Conclusions
Rates of bioconfirmed smoking cessation were higher among hospitalized patients randomized to financial incentives compared to usual care alone, but the difference was not significant. Considering the frequency of low payouts and the importance of assistance for successful quitting, future studies should explore the effectiveness of financial incentives sufficiently large to overcome barriers to evidence-based therapy.
Keywords: FIESTA, Smoking Cessation, Manhattan VA Hospital, Financial Incentives, Veterans
BACKGROUND
Smoking is the leading preventable cause of death and disease in the United States,1,2 and hospitalized patients who continue to smoke after discharge face higher risks of adverse health events compared to patients who quit.3–5 Prior studies of smoking cessation interventions have demonstrated that intensive counseling in the inpatient setting followed by supportive contact after discharge is effective, and evidence is also growing for pharmacotherapy-based approaches.6–8 Nonetheless, most smokers continue to smoke after discharge,6,9 and smokers often encounter substantial socioeconomic barriers that undermine the perceived feasibility of successful cessation.10 Moreover, smoking worsens health disparities and is associated with economic disparities.11–14 In light of these challenges, financial incentives designed to increase use of evidence-based therapy and promote abstinence–particularly when leveraging concepts from behavioral economics15–20-may improve health while simultaneously ameliorating economic challenges more prevalent among smokers.21–24 The Financial IncEntives for Smoking TreAtment (FIESTA) trial aimed to test the effectiveness of financial incentives for increasing evidence-based therapy and smoking cessation among hospitalized patients.
METHODS/DESIGN
Overall Design
We performed a randomized, controlled trial to compare the effects of two strategies—financial incentives plus enhanced usual care versus enhanced usual care alone—on smoking cessation and use of evidenced-based smoking cessation therapy among hospitalized patients. The protocol was approved by the institutional review board and a description of study procedures is available.25 The trial was funded by the Robert Wood Johnson Foundation (Grant 74140) and NIH (K24 DA038345).
Study Population
We enrolled hospitalized participants from the Veterans Affairs (VA) New York Harbor Healthcare System’s Manhattan campus from July 15, 2015 until March 27, 2018. Hospitalized patients were eligible for enrollment if they were at least 18 years old, smoked tobacco during the 30 days prior to hospitalization, had an active U.S. phone number, resided in the New York City area or had the ability to return to the Manhattan VA for at least one year, were contemplating smoking cessation as assessed by readiness to quit,26 and were able to provide consent in English. We excluded patients who had an anticipated discharge to an institution (i.e., a nursing home or long-term care facility) at which the patient would be subject to restrictions on smoking.
Randomization
Participants were randomized to financial incentives plus enhanced usual care or enhanced usual care alone with an allocation ratio of 1:1. We employed a computer-generated block randomization design, and research staff implemented the allocation sequence using numbered, sealed opaque envelopes.
Interventions
All participants received enhanced usual care, which included hospital-directed tobacco-use screening, counseling, education, and pharmacotherapy, all at the discretion of nursing and physician staff, and referral to a state Quitline (this component represented the enhancement). In addition to enhanced usual care, smokers randomized to financial incentives were also eligible to receive up to $550 for participating in counseling (both community-based counseling and state Quitline counseling), using smoking cessation pharmacotherapy, and achieving biochemically-confirmed smoking cessation at 2 months (expired carbon monoxide [CO] or salivary cotinine) and 6 months (salivary cotinine only) (Table 1).
Table 1.
Schedule of Incentives for Participants Randomized to Financial Incentive Group
| Activity | Time After Hospital Discharge | Incentive |
|---|---|---|
| Speaking with a coach from the New York Smoker’s Quitline |
2 weeks | $50 |
| Completion of community-based smoking cessation program | 2 weeks | $50 |
| Use of pharmacotherapy for smoking cessation | 2 weeks | $50 |
| Smoking cessation (bioconfirmed)* | 2 months | $150 |
| Smoking cessation (bioconfirmed)* | 6 months | $250 |
We considered participants to be abstinent at 2 months and 6 months if they self-reported abstinence from cigarettes for at least 7 days before the interview and had biochemical confirmation using salivary cotinine
The incentive intervention used goal-directed incentives (incentives weighted toward use of evidence-based therapies) and outcome-based incentives (incentives for successful achievement of an outcome, like successfully quitting).27 The first follow-up time point was early, at 2 weeks, because we found in a previous trial that smokers who abstain from tobacco during a hospitalization often relapse within the first 2 weeks of hospital discharge.8
All participants were compensated in U.S. dollars (USD) using ClinCards, a secure prepaid debit card system. To ensure that participants comprehended the targets for which they were being incentivized, we used the teach-back method, in which study participants are asked to repeat task-specific directions to staff in order to confirm understanding.28
Measures and Outcomes
Participants completed a baseline interview at the time of enrollment and follow-up interviews at 2 weeks, 2 months, 6 months, and 12 months. Baseline measures included sociodemographic characteristics; smoking history (i.e., smoking habits and home environment using items adapted from the California Tobacco Survey,29 nicotine dependence,30 and smoking cessation services received); exercise and nutrition habits; financial stress31; subjective social status32; quality of life based on the Veterans RAND 12-item Health Survey33 and the EuroQol-5D34,35; alcohol and substance use36–38; and healthcare utilization in the prior 6 months. The follow-up surveys also measured self-reported smoking cessation, quit attempts, use of nicotine replacement therapy (NRT)/pharmacotherapy, Quitline counseling participation, and use of e-cigarettes. Each participant received $20 after completing a follow-up survey and $50 after providing saliva samples.
Outcomes
The primary outcome was tobacco abstinence at 6 months after hospital discharge, defined as self-reported abstinence from cigarettes for at least 7 days before the 6-month follow-up interview and biochemical confirmation using salivary cotinine, a nicotine metabolite. Bioconfirmation was performed with Accutest® NicAlert™ strip kits; a cotinine concentration < 10 ng per milliliter was considered to indicate smoking cessation.39,40 Use of e-cigarettes was not considered use of cigarettes in determination of the primary outcome. Participants also underwent measurement of expired air carbon monoxide (with Covita piCO+ Smokerlyzer). At the 2-month follow-up interview, a carbon monoxide level ≤ 6 ppm among participants still using NRT was also considered to indicate smoking cessation. All bioconfirmation was performed in-person, usually at the hospital. We also assessed use of evidence-based tobacco therapy, including the Quitline and pharmacotherapy, and verified NRT and community-based counseling with receipts, letters from counselors, used products, and EHR records.
Statistical Analysis
All analyses were performed using an intent-to-treat approach. We summarized participants’ characteristics by group and compared use of evidence-based smoking cessation therapy using 2-sample t tests and Chi-squared tests. The primary analysis compared the difference in biochemically-confirmed smoking cessation rates between the incentive group and the enhanced usual care group at 6-month follow-up. As pre-specified in our protocol, this analysis was performed with multiple imputation.41 Multiple imputation by chained equations (MICE) was used with logistic regression models for smoking cessation status. We imputed 50 datasets to account for the proportion of missing outcome data.42,43 We then estimated the intervention effect using a logistic regression model with adjustment for a diagnosis of substance abuse because of the baseline between-group difference in prevalence.
For the power calculation, we estimated a sample of 182 hospitalized smokers would provide at least 80% power to detect a 20% absolute between-group difference in cessation rates, with 10% loss-to-follow-up rate at 6 months and α=0.05. A 2-sided P value of less than 0.05 was considered statistically significant. All analyses were performed using Stata (version 14, College Station, Texas).
RESULTS
Study Population
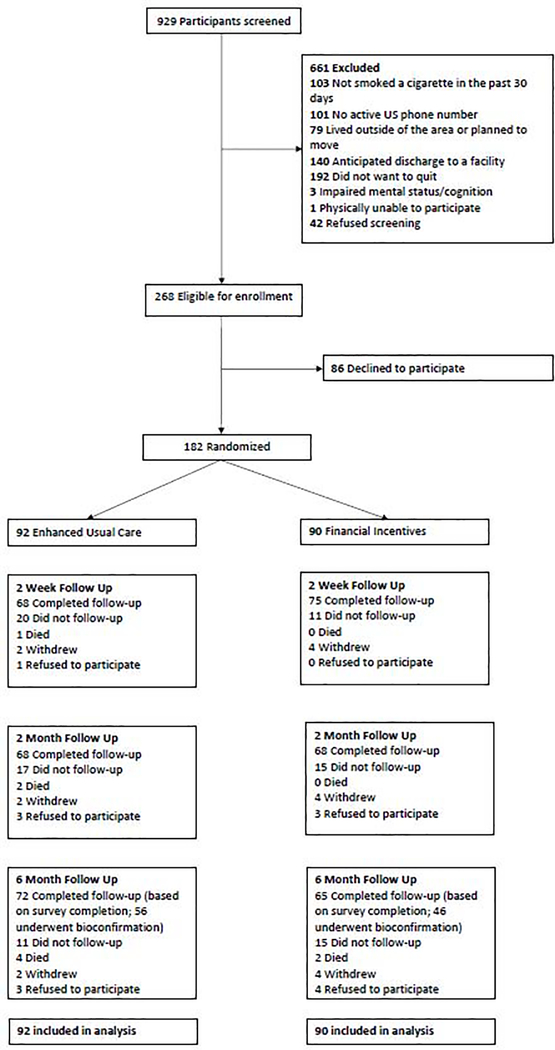
Overall, 182 hospitalized patients were enrolled (Figure 1). The proportion of patients who completed 6-month follow-up was 72% in the financial incentive group and 78% in the enhanced usual care group.
Figure.
Screening, Enrollment, and Follow-up of Study Participants
Participant Characteristics
The mean age of patients was 58 years, 95% of the patients were male, 27% were Hispanic, 41% were non-Hispanic black, and 45% had a high school education or less (Table 2). Participants reported smoking an average of 11 cigarettes per day (SD=8). The proportion of participants who were employed was 16% and 47% of participants reported being not at all satisfied with their financial status. In assessments of subjective social status, the mean score was 5.0 when socioeconomic status was compared to the general U.S. population and 6.1 when socioeconomic status was compared to participants’ communities (on a 1 to 10 scale, with 10 representing the highest status). Smoking-related comorbidities or mental health comorbidities included coronary heart disease in 18% of participants, chronic obstructive pulmonary disease in 25% of participants, alcohol abuse in 45%, and other current substance abuse in 51%. Median length of stay during the hospitalization was 6.5 days (interquartile range, 4 to 19 days).
Table 2.
Characteristics of Study Participants
| Characteristic | Enhanced Usual Care Group (N=92) | Financial Incentive Group (N=90) | P Value |
|---|---|---|---|
| Mean age (yr) | 56.8 | 59.2 | 0.165 |
| Male sex (%) Race/ethnicity (%)* |
94.6 | 94.4 | 0.971 |
| White, non-Hispanic | 22.8 | 17.8 | |
| Black, non-Hispanic | 40.2 | 42.2 | |
| Hispanic | 28.3 | 25.6 | |
| Other or unknown Education (%)† |
8.7 | 14.4 | 0.564 |
| High school or lower | 42.9 | 47.8 | |
| Some college | 50.6 | 42.2 | |
| College graduate | 6.6 | 10.0 | 0.460 |
| Employed outside home (%) | 15.2 | 16.7 | 0.789 |
| Married (%) Financial satisfaction (%)† |
17.4 | 14.4 | 0.587 |
| Not at all satisfied | 46.2 | 48.3 | |
| More or less satisfied | 33.0 | 38.2 | |
| Satisfied | 20.9 | 13.5 | 0.402 |
| Mean financial stress‡ | 2.9 | 2.9 | 1.0 |
| MacArthur Scale of Subjective Social Status†§ | |||
| Score relative to US population | 5.2 | 4.8 | 0.252 |
| Score relative to community | 6.3 | 6.0 | 0.544 |
| Housing | |||
| Low-income housing | 34.8 | 43.3 | |
| Non-low-income housing | 50.0 | 41.1 | |
| Homeless | 4.4 | 5.6 | |
| Other or unknown Smoking habits |
10.9 | 10.0 | 0.613 |
| Mean cigarettes per day | 11.8 | 11.1 | 0.540 |
| Electronic cigarette use (%)¶ | 14.1 | 14.4 | 0.952 |
| Illicit drug use (including marijuana) (%)|| | 55.4 | 57.8 | 0.750 |
| Prescription drug use recreationally (%)||
Self-reported health habits** |
22.8 | 22.2 | 0.922 |
| Mean days per week of exercise | 4.1 | 4.1 | 0.830 |
| Healthy diet (%) Comorbidities (%) |
70.9 | 73.8 | 0.687 |
| Hypertension | 54.4 | 52.2 | 0.774 |
| Diabetes | 25.0 | 26.7 | 0.797 |
| Dyslipidemia | 23.9 | 31.1 | 0.277 |
| Peripheral arterial disease | 4.4 | 3.3 | 0.722 |
| Cerebrovascular disease | 4.4 | 5.6 | 0.707 |
| Coronary heart disease | 16.3 | 18.9 | 0.647 |
| Congestive heart failure | 6.5 | 6.7 | 0.969 |
| Chronic obstructive pulmonary disease | 25.0 | 25.6 | 0.931 |
| Cancer | 12.0 | 11.1 | 0.858 |
| Depression | 39.1 | 36.7 | 0.732 |
| Alcohol abuse/dependence | 40.2 | 50.0 | 0.185 |
| Substance abuse/dependence | 43.5 | 58.9 | 0.038 |
| Surgical history (%) | |||
| Coronary angioplasty | 1.1 | 3.3 | 0.301 |
| Coronary artery bypass grafting | 2.2 | 4.4 | 0.391 |
| Peripheral vascular angioplasty/bypass | 3.3 | 0.0 | 0.084 |
| Inpatient smoking cessation pharmacotherapy (%) | |||
| Nicotine replacement therapy | 55.4 | 53.3 | 0.776 |
| Varenicline | 0.0 | 0.0 | - |
| Bupropion | 1.1 | 1.1 | 0.988 |
| Smoking cessation pharmacotherapy prescribed at discharge(%) | |||
| Any pharmacotherapy | 45.7 | 46.7 | 0.891 |
| Nicotine replacement therapy | 43.5 | 45.6 | 0.778 |
| Varenicline | 0.0 | 0.0 | - |
| Bupropion | 4.4 | 1.1 | 0.182 |
Race and ethnicity were self-reported
One participant in the usual care group did not report education, 1 participant in each group did not report financial satisfaction, 2 participants in each group did not report social status relative to US, and 4 participants in the usual care group and 2 participants in the incentives group did not report social status relative to their community
The Financial Stress Questionnaire has a range of 1 to 5, with higher scores indicating more financial stress.
The MacArthur Scale of Subjective Social Status has a range of 1 to 10, with higher scores indicating higher self-reported social status
Any use in past 1 month
Any use in past 12 months
Self-reported exercise was available from 81 participants in the usual care group and 79 participants in the incentive group. Self-reported consumption of a healthy diet was available from 79 participants in the usual care group and 80 participants in the incentive group. These questions were added to the study after 21 patients were already enrolled.
Note: Standard deviations for continuous measures in enhanced usual care and financial incentive group are, respectively: age 13.1 and 10.2, financial stress 1.0 and 1.1, cigarettes per day 8.0 and 8.0, days per week of exercise 2.8 and 2.9
Smoking Cessation
The 6-month rate of smoking cessation, based on biochemical confirmation, was 19.6% in the financial incentive group and 8.9% in the enhanced usual care group (odds ratio, 2.56; 95% confidence interval [CI], 0.84 to 7.83, P=0.10) (Table 3). The rate of self-reported smoking cessation was 54.7% in the financial incentive group and 37.1% in the enhanced usual care group (P=0.042). Saliva samples were not submitted for biochemical confirmation by 21.3% of patients who self-reported smoking cessation, and this difference was not significant between groups (P=0.732). We found no evidence for an interaction between the intervention effect and measures of financial stress or socioeconomic status.
Table 3.
Smoking Cessation After Hospital Discharge
| Smoking Cessation* | Enhanced Usual Care Group (N=92) | Financial Incentive Group (N=90) | P Value | ||
|---|---|---|---|---|---|
| n/N | Percent | n/N | Percent | ||
| Smoking cessation at 2 weeks (%) | |||||
| Self-reported | 27/67 | 40.3 | 35/75 | 46.7 | 0.445 |
| Smoking cessation at 2 months (%) | |||||
| Self-reported | 24/65 | 36.9 | 40/68 | 58.8 | 0.012 |
| Bioconfirmed | 12/48 | 25.0 | 14/40 | 35.0 | 0.306 |
| No saliva sample submitted† | 7/24 | 29.2 | 16/40 | 40.0 | 0.382 |
| Positive saliva sample submitted† | 5/24 | 20.8 | 10/40 | 25.0 | 0.703 |
| Smoking cessation at 6 months (%)‡ | |||||
| Self-reported | 26/70 | 37.1 | 35/64 | 54.7 | 0.042 |
| Bioconfirmed | 5/56 | 8.9 | 9/46 | 19.6 | 0.103 |
| No saliva sample submitted† | 5/26 | 19.2 | 8/35 | 22.9 | 0.732 |
| Positive saliva sample submitted† | 16/26 | 61.5 | 18/35 | 51.4 | 0.432 |
The denominator for self-reported smoking cessation indicates the number of participants who completed follow-up at each time point. The denominator for bioconfirmation indicates the number of participants who submitted a saliva sample or provided expired CO
Among participants with self-reported smoking cessation
P value for bioconfirmed smoking cessation at 6 months based on logistic regression model with multiple imputation for missing smoking cessation values
Note: At 6 months, 72 patients in the enhanced usual care group and 65 patients in the financial incentive group participated in the phone survey. Of these patients, 2 patients in the enhanced usual care group and 1 patient in the financial incentive group did not respond to questions about current smoking. After the 6 month phone survey, 56 patients in the enhanced usual care group and 46 patients in the financial incentive group subsequently presented for bioconfirmation.
Incentive Payments and Socioeconomic Measures
Participants in the financial incentive group received a total mean payment of $84 (SD=$133). This included $36 in incentive payments for goal-directed activities (e.g., speaking with a Quitline coach, completing a community-based smoking cessation program, and using cessation pharmacotherapy) and $48 for outcome-based activities (i.e., smoking cessation with biological confirmation). The proportion of patients in the financial incentive group receiving no incentives was 49%. Three patients received at least $500 in incentives (3.3%), 6 patients received at least $400 (6.7%), and 15 patients received at least $200 (16.7%).
There were no significant differences in participants’ reports of subjective social status at 6-month follow-up (scores relative to US population [P=0.75] and to the local community [P=0.50] were 4.8 and 5.8 in the financial incentive group versus 4.9 and 5.5 in the enhanced usual care group, respectively). Financial stress increased in the incentive group at 6-month follow-up compared to the enhanced usual care group (mean financial stress score of 3.17 versus 2.84, P=0.019), and this difference was attributable to higher financial stress reported by participants in the incentive group who did not achieve smoking cessation (mean financial stress score of 3.23, P=0.009 compared to enhanced usual care). Participants who did successfully quit smoking and earned the 6-month smoking cessation incentive did not report higher financial stress (mean financial stress score of 2.84, P=0.995 compared to enhanced usual care).
Use of Evidence-based Smoking Cessation Therapy
At 2-week follow-up, smoking cessation therapy rates were verified by study staff using New York State Quitline reports, medication prescription records, medication receipts, or other documentation provided by patients (Table 4). Participants in the incentive group were more likely to use NRT by 2 weeks (P=0.002) and no patients reported using varenicline at 2 weeks. By 6 months, 3 patients in the financial incentive group and 1 patient in the enhanced usual care reported using varenicline. There was no significant difference in Quitline use (44% vs 42%) or participation in community-based smoking cessation programs (4% vs. 0%) at 2-week follow-up between the incentive and enhanced usual care groups, respectively. The proportion of patients reporting use of e-cigarettes at 6-month follow-up was 11.11% in the financial incentive and 7.14% in the enhanced usual care group.
Table 4.
Use of Evidence-based Smoking Cessation Therapy After Hospital Discharge
| Evidence-based therapy* | Enhanced Usual Care Group (N=92) | Financial Incentive Group (N=90) | P Value | ||
|---|---|---|---|---|---|
| n/N | Percent | n/N | Percent | ||
| Quitline participation (%) | |||||
| At 2 week follow-up (verified) | 39/92 | 42.4 | 40/90 | 44.4 | 0.780 |
| At 6 month follow-up | 41/69 | 59.4 | 46/63 | 73.0 | 0.100 |
| Smoking cessation community-based program (%) | |||||
| At 2 week follow-up | 0/67 | 0.0 | 3/75 | 4.0 | 0.098 |
| At 6 month follow-up | 2/69 | 2.9 | 5/64 | 7.8 | 0.205 |
| Nicotine replacement therapy (%) | |||||
| At 2 week follow-up (self-reported) | 24/67 | 35.8 | 45/75 | 60.0 | 0.004 |
| At 2 week follow-up (verified) | 21/67 | 31.3 | 43/75 | 57.3 | 0.002 |
| At 6 month follow-up | 38/69 | 55.1 | 44/64 | 68.8 | 0.105 |
| Varenicline therapy (%) | |||||
| At 2 week follow-up | 0/67 | 0.0 | 0/75 | 0.0 | - |
| At 6 month follow-up | 1/69 | 1.5 | 3/64 | 4.7 | 0.275 |
| Bupropion therapy (%) | |||||
| At 2 week follow-up | 0/67 | 0.0 | 0/75 | 0.0 | - |
| At 6 month follow-up | 1/69 | 1.5 | 1/64 | 1.6 | 0.957 |
The denominator indicates the number of participants who completed follow-up at each time point. One participant in the enhanced usual care group completed 6 month follow-up but did not respond to questions about smoking cessation therapy and was not included in the denominator
DISCUSSION
We found that rates of bioconfirmed smoking cessation were higher among hospitalized patients randomized to financial incentives compared to hospitalized patients receiving enhanced usual care alone, but the difference was not significant. Financial incentives did increase self-reported smoking cessation and the rate of early NRT use in this patient population. Rates of other activities linked to incentives, including Quitline participation, were not significantly increased by financial incentives. To the best of our knowledge, this is the first clinical trial to evaluate the effectiveness of financial incentives for smoking cessation in a hospitalized patient population.
We designed FIESTA to improve health through increased smoking cessation and to improve economic well-being through substantial cash payments for healthy goal achievement. For these reasons, we purposely targeted a relatively low-income population, with the expectation that the marginal benefits of incentives would be larger. While patients could earn up to $550 in incentive payments over a 6-month period (likely representing a significant proportion of annual income for some of the participants), the mean payment in the financial incentive arm was a modest $84, and fewer than 1 in 10 patients earned at least $400. In light of this finding and the overall low rates of evidence-based smoking cessation therapy, it may have been beneficial to provide larger incentives for use of Quitline counseling and effective pharmacotherapy—particularly varenicline. Smokers often have preferences against using counseling or pharmacotherapy, and may cite concerns about side effects or overestimate their likelihood of successfully quitting without assistance.44–48 Financial incentives that are sufficiently large—in combination with behavioral economic strategies—may help more smokers overcome these barriers to evidence-based therapy.
Among patients earning higher incentives, financial stress at 6 months was unchanged compared to patients receiving enhanced usual care, but financial stress worsened among patients earning lower incentives or no incentives. This finding suggests that randomization to a financial incentive arm may have altered perceptions of financial stress adversely when patients had an opportunity to earn large incentives but were unsuccessful. Alternatively, the finding may have been due to chance, and analogous evaluations in other financial incentive studies will be informative. If this finding is reproduced, strategies to mitigate it should be developed.
A major unanswered question in the financial incentive literature is whether to use goal-directed incentives (incentives for use of evidence-based therapies, which are widely underutilized) or outcome-based incentives (incentives for successful achievement of an outcome, like successfully quitting) for health improvement.27,48 Most smoking cessation studies applying incentives have primarily targeted the outcome of smoking cessation. However, if incentives can be used to steer patients toward evidence-based therapies that also increase intrinsic motivation (e.g., motivational interviewing or successful use of pharmacotherapy),49,50 concerns that incentives engage extrinsic motivation at the expense of intrinsic motivation may be attenuated. The optimal design is unknown.
One major concern about financial incentives for smoking cessation is their long-term efficacy, with critics noting that financial incentives (extrinsic motivation) may crowd out intrinsic motivation51,52 and undermine durable smoking cessation. Others have noted, however, that levels of intrinsic motivation for activities we incentivize may already be low, leaving little motivation at risk for crowd out.53 The possibility that successfully quitting smoking itself may increase self-efficacy and intrinsic motivation further complicates the intrinsic-extrinsic motivation dynamic in the context of tobacco use.49,50 Our perspective is that the addiction component of smoking makes this particular habit amenable to incentives, whereas other activities, such as healthy eating or exercise, typically require more sustained, ongoing engagement and may be less amenable to incentives. Empirically, at least two randomized trials support durability of financial incentives for smoking cessation. In a study of 878 employees, Volpp et al followed smokers for 15 to 18 months and found that rates of smoking cessation at this point—up to 9 months after financial incentives were stopped—were higher in the incentive arm than the control arm.21 In a second study conducted outside of the workplace and involving 805 low-income smokers, continuous abstinence at 18 months—12 months after incentives ended—occurred at a higher rate in the incentive group than the control group.54
FIESTA has limitations. Based on the observed findings, the study had less power than we anticipated to detect a significant effect of incentives on bioconfirmed smoking cessation. FIESTA also enrolled from a VA hospital and more than 90% of our patients were male; it is unclear whether the study findings generalize to women. Patients in the VA also experience relatively high rates of post-traumatic stress disorder, depression, and other mood disorders that are associated with nicotine addiction and may decrease the likelihood of successful cessation.
FIESTA demonstrated that financial incentives for smoking cessation may be a promising adjunct to usual care among hospitalized patients with lower levels of income. Differences in bioconfirmed smoking cessation rates between the financial incentive and enhanced usual care groups were large but did not meet statistical significance. Future studies of financial incentives that similarly target a low-income population and potentially increase the incentive size for evidence-based smoking cessation therapy may improve the health and economic status of patients.
Clinical Significance.
Among hospitalized smokers, financial incentives totaling up to $550 (actual mean payment $84) increased 6-month biochemically-confirmed smoking cessation to 19.6% versus 8.9% among controls, but the difference was not significant.
Financial stress increased in the incentive group except among high incentive earners
To increase cessation rates and ameliorate economic burdens among hospitalized smokers, incentives need to be larger, more attainable, and more directed toward incentivizing evidence-based therapy.
Acknowledgements
We thank our research staff for their many contributions to the study: Katherine French, Sasha Gonzalez, Amy Chen, Alissa R. Link, Sadozai Zoe Malik, Saahil Jumkhawala, Briesny Tejada, and Andrew White. Not least of all, we thank our patients who generously participated in the study.
Funding
This study is funded by the Robert Wood Johnson Foundation (Grant 74140) (Drs. Ladapo and Sherman) and NIH K24 DA038345 (Dr. Sherman). The funding sources were not involved in the design of the study or collection, analysis, or interpretation of data or in writing the manuscript.
Funding: Robert Wood Johnson Foundation (Grant 74140) and NIH K24 DA038345
Primary Funding Source-Robert Wood Johnson Foundation (Grant 74140) and NIH (K24 DA038345).
Footnotes
Competing Interests
N/A
Data access: All authors had access to the data and a role in writing the manuscript.
Trial registration: ClinicalTrials.gov Identifier: NCT02506829
Trial registration: ClinicalTrials.gov Identifier: NCT02506829. Registered 1 July 2014, https://clinicaltrials.gov/ct2/show/NCT02506829
Disclosures: None
DECLARATIONS
Ethics approval and Consent to Participate
The protocol was approved by the Institutional Review Board of the Manhattan Campus of the VA NY Harbor Healthcare System, Protocol #01494. The principal investigators and research staff were responsible for obtaining informed consent from all study participants. Protocol amendments or amendments to informed consent forms were sent to the Institutional Review Board for approval.
Access to data with participants’ protected health information was limited to the investigators, research staff, and the Institutional Review Board. The full protocol may be requested by contacting the principal investigators.
Consent for publication
N/A
Availability of data and material
The datasets generated and/or analyzed during the current study and the analytic code are available from the corresponding author on reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Center for Chronic Disease P, Health Promotion Office on S, Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. [Google Scholar]
- 2.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. [DOI] [PubMed] [Google Scholar]
- 3.Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24(4):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290(1):86–97. [DOI] [PubMed] [Google Scholar]
- 5.Mills E, Eyawo O, Lockhart I, Kelly S, Wu P, Ebbert JO. Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med. 2011;124(2):144–154 e148. [DOI] [PubMed] [Google Scholar]
- 6.Rigotti NA, Clair C, Munafo MR, Stead LF . Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012(5):CD001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suissa K, Lariviere J, Eisenberg MJ, et al. Efficacy and Safety of Smoking Cessation Interventions in Patients With Cardiovascular Disease: A Network Meta-Analysis of Randomized Controlled Trials. Circ Cardiovasc Qual Outcomes. 2017;10(1). [DOI] [PubMed] [Google Scholar]
- 8.Sherman SE, Link AR, Rogers ES, et al. Smoking-Cessation Interventions for Urban Hospital Patients: A Randomized Comparative Effectiveness Trial. Am J Prev Med. 2016;51(4):566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streck JM, Chang Y, Tindle HA, et al. Smoking Cessation After Hospital Discharge: Factors Associated With Abstinence. Journal of hospital medicine. 2018;13(11):774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twyman L, Bonevski B, Paul C, Bryant J. Perceived barriers to smoking cessation in selected vulnerable groups: a systematic review of the qualitative and quantitative literature. BMJ Open. 2014;4(12):e006414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.C. D. C. Tobacco Free.Cigarette and Tobacco Use Among People of Low Socioeconomic Status. Centers for Disease Control and Prevention 2019; https://www.cdc.gov/tobacco/disparities/low-ses/index.htm. Accessed: April 5, 2019
- 12.Casetta B, Videla AJ, Bardach A, et al. Association Between Cigarette Smoking Prevalence and Income Level: A Systematic Review and Meta-Analysis. Nicotine Tob Res. 2017;19(12):1401–1407. [DOI] [PubMed] [Google Scholar]
- 13.Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–123. [DOI] [PubMed] [Google Scholar]
- 14.Prochaska JJ, Michalek AK, Brown-Johnson C, et al. Likelihood of Unemployed Smokers vs Nonsmokers Attaining Reemployment in a One-Year Observational Study. JAMA Intern Med. 2016;176(5):662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298(20):2415–2417. [DOI] [PubMed] [Google Scholar]
- 16.Volpp KG, Pauly MV, Loewenstein G, Bangsberg D. P4P4P: an agenda for research on pay-for-performance for patients. Health Aff (Millwood). 2009;28(1):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camerer C, Ho T-H. Experienced-weighted attraction learning in normal form games. Econometrica. 1999:827–874. [Google Scholar]
- 18.Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127(2):267–286. [DOI] [PubMed] [Google Scholar]
- 19.Connolly T, Butler D. Regret in economic and psychological theories of choice. Journal of Behavioral Decision Making. 2006;19(2):139–154. [Google Scholar]
- 20.Loewenstein G, Prelec D. Anomalies in Intertemporal Choice: Evidence and an Interpretation. The Quarterly Journal of Economics. 1992;107(2):573–597. [Google Scholar]
- 21.Volpp KG, Troxel AB, Pauly MV, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. [DOI] [PubMed] [Google Scholar]
- 22.Volpp KG, Gurmankin Levy A, Asch DA, et al. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiol Biomarkers Prev. 2006;15(1):12–18. [DOI] [PubMed] [Google Scholar]
- 23.Barbeau EM, Krieger N, Soobader MJ. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2004;94(2):269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halpern SD, French B, Small DS, et al. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372(22):2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.French KM, Gonzalez SZ, Sherman SE, et al. Financial IncEntives for Smoking TreAtment: protocol of the FIESTA trial and FIESTA Oral Microbiome Substudy. Trials. 2018;19(1):646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velicer WF, Redding CA, Sun X, Prochaska JO. Demographic variables, smoking variables, and outcome across five studies. Health Psychol. 2007;26(3):278–287. [DOI] [PubMed] [Google Scholar]
- 27.Ladapo JA, Prochaska JJ. Paying Smokers to Quit: Does It Work? Should We Do It? J Am Coll Cardiol. 2016;68(8):786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slater BA, Huang Y, Dalawari P. The Impact of Teach-Back Method on Retention of Key Domains of Emergency Department Discharge Instructions. J Emerg Med. 2017;53(5):e59–e65. [DOI] [PubMed] [Google Scholar]
- 29.California Tobacco Surveys. La Jolla: University of California, San Diego. University of California, San Diego; 1999. https://library.ucsd.edu/dc/collection/bb9353145q. Accessed February 24, 2018. [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. [DOI] [PubMed] [Google Scholar]
- 31.Corrigan A Financial Stress Questionnaire: Fast Track Project Technical Report. 2003. [Google Scholar]
- 32.Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med. 2005;67(6):855–861. [DOI] [PubMed] [Google Scholar]
- 33.Selim AJ, Rogers W, Fleishman JA, et al. Updated U.S. population standard for the Veterans RAND 12-item Health Survey (VR-12). Qual Life Res. 2009;18(1):43–52. [DOI] [PubMed] [Google Scholar]
- 34.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. [DOI] [PubMed] [Google Scholar]
- 35.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. [DOI] [PubMed] [Google Scholar]
- 36.Frank D, DeBenedetti AF, Volk RJ, Williams EC, Kivlahan DR, Bradley KA. Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. J Gen Intern Med. 2008;23(6):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 38.McNeely J, Strauss SM, Saitz R, et al. A Brief Patient Self-administered Substance Use Screening Tool for Primary Care: Two-site Validation Study of the Substance Use Brief Screen (SUBS). Am J Med. 2015;128(7):784 e789–784 e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 40.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 41.Little R, Rubin D. Statistical Analysis with Missing Data. 2nd ed. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 42.Bodner TE. What Improves with Increased Missing Data Imputations? Structural Equation Modeling: A Multidisciplinary Journal. 2008;15(4):651–675. [Google Scholar]
- 43.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30(4):377–399. [DOI] [PubMed] [Google Scholar]
- 44.Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res. 2004;6 Suppl 3:S303–310. [DOI] [PubMed] [Google Scholar]
- 45.Weinstein ND, Slovic P, Gibson G. Accuracy and optimism in smokers’ beliefs about quitting. Nicotine Tob Res. 2004;6 Suppl 3:S375–380. [DOI] [PubMed] [Google Scholar]
- 46.Hammond D, McDonald PW, Fong GT, Borland R. Do smokers know how to quit? Knowledge and perceived effectiveness of cessation assistance as predictors of cessation behaviour. Addiction. 2004;99(8):1042–1048. [DOI] [PubMed] [Google Scholar]
- 47.Shelley D, Tseng TY, Gonzalez M, et al. Correlates of Adherence to Varenicline Among HIV+ Smokers. Nicotine Tob Res. 2015;17(8):968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting Smoking Among Adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. [DOI] [PubMed] [Google Scholar]
- 49.Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns. 2009;76(2):174–180. [DOI] [PubMed] [Google Scholar]
- 50.Bandura A Social foundations of thought and action: A social cognitive theory Englewood Cliffs, NJ, US: Prentice-Hall, Inc; 1986. [Google Scholar]
- 51.Deci EL. Effects of externally mediated rewards on intrinsic motivation. J Pers Soc Psychol. 1971;18(1):105. [Google Scholar]
- 52.Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull. 1999;125(6):627–668; discussion 692–700. [DOI] [PubMed] [Google Scholar]
- 53.Promberger M, Marteau TM. When do financial incentives reduce intrinsic motivation? comparing behaviors studied in psychological and economic literatures. Health Psychol. 2013;32(9):950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etter JF, Schmid F. Effects of Large Financial Incentives for Long-Term Smoking Cessation: Randomized Trial. J Am Coll Cardiol. 2016;68(8):777–785. [DOI] [PubMed] [Google Scholar]