Abstract
Purpose
This research aimed to investigate the antibacterial activity and potential mechanism of luteolin against T. pyogenes.
Materials and Methods
The broth microdilution method was used to determine the minimum inhibitory concentrations (MICs) of luteolin against various T. pyogenes strains. The potential mechanism of action of luteolin was elucidated through testing and analysing the luteolin-induced alterations of T. pyogenes in several aspects, including cell wall, cell membrane, protein expression, nucleic acid content, topoisomerase activity and energy metabolism.
Results
The MIC values of luteolin against various T. pyogenes isolates and ATCC19411 were 78 µg/mL. The increased cell membrane permeability, destruction of cell wall integrity and TEM images after exposure to luteolin showed that the cell wall and membrane were damaged. The content of total protein and nucleic acid in T. pyogenes decreased significantly after treatment with luteolin (1/2 MIC) for 12, 24, and 36 h. Moreover, a hypochromic effect was observed in the absorption spectrum of luteolin when deoxyribonucleic acid (DNA) was added. In addition, after treatment with luteolin, a decrease in nicked or relaxed DNA content, which was catalysed by T. pyogenes-isolated DNA topoisomerase, was observed. In addition, the adenosine triphosphate (ATP) content in cells and the activity of succinate dehydrogenase (SDH) both decreased when T. pyogenes was exposed to different concentrations (1/4 MIC, 1/2 MIC, 1 MIC, 2 MIC) of luteolin for 1 h.
Conclusion
Luteolin showed distinct antibacterial activity against T. pyogenes by multiple actions, which mainly include destroying the integrity of the cell wall and cell membrane, influencing the expression of proteins, inhibiting nucleic acid synthesis, and interfering with energy metabolism.
Keywords: luteolin, Trueperella pyogenes, antibacterial activity, antibacterial mechanism
Introduction
T. pyogenes is a Gram-positive, rod-shaped, non-motile, non-spore-forming opportunistic pathogen with a broad range of virulence factors such as pyolisin (PLO), neuraminidases (NanH), collagen-binding protein (CbpA), fimbriae (FimA).1–3 It is commonly found on the skin, oropharynx, and in the upper respiratory, urogenital, and gastrointestinal tracts of livestock, and causes different clinical manifestations in domestic and wild animals.4–6 T. pyogenes can cause various diseases, including mastitis, endometritis, liver abscess, pneumonia, arthritis, and osteomyelitis, which are related to suppurative infections. Diseases caused by this pathogen are especially important in cattle and swine, since they cause substantial economic losses.6,7
Presently, antibiotic therapy continues to serve as the primary means to control infections caused by bacteria. However, because of the long-term use of antibiotics, many bacteria have developed varying degrees of antibiotic resistance. By examining the antimicrobial susceptibility of T. pyogenes isolated from domestic animals, researchers have found that the isolates have developed different degrees of resistance to some antimicrobial agents. For example, compared with β-lactams which T. pyogenes isolates are highly sensitive to, neomycin (MIC90 ≥ 16 µg/mL), oxytetracycline (MIC90 ≥ 16 µg/mL), sulfamethoxazole/trimethoprim (MIC90 ≥ 15.2/0.8 µg/mL), and tylosin (MIC90 ≥ 512 µg/mL) require higher concentrations for inhibiting bacterial growth.7
In a study on the tetracycline susceptibility of T. pyogenes isolated from dairy cattle with endometritis in China, 68.7% and 62.5% of the isolates were found to be resistant to tetracycline and doxycycline, respectively.8 In an antimicrobial susceptibility testing of T. pyogenes isolated from domestic and wild animals, a high percentage of strains resistant to ciprofloxacin, enrofloxacin, and tetracycline was noted, and the most tetracycline-resistant and enrofloxacin-resistant strains were found to originate from cattle.9 As the antibiotic resistance in T. pyogenes continues to increase in severity, it is extremely urgent to identify and develop new antibacterial drugs.
Natural plant products play an important role in drug discovery. Over the last 20 years, natural plant products have been increasingly used in antibacterial activity research to develop new antibacterial drugs. Many secondary metabolites produced by normal metabolic pathways of plants have potential antibacterial activities such as terpenes, alkaloids, flavonoids, and phenols. Natural compounds could concurrently address more than one bacterial target by enhancing membrane permeability, inhibiting the synthesis of enzymes, or blocking biochemical reactions.10
Luteolin (3,4,5,7-tetrahydroxyflavone) is a natural flavonoid and one of the most abundant secondary metabolites in plants. Luteolin is present in many medicinal plants and vegetables such as chrysanthemum, honeysuckle, thyme, broccoli, and cabbages.11–13 Studies have shown that luteolin has numerous pharmacological activities including anticancer, anti-inflammatory, antioxidant, anti-allergic, and antimicrobial.14–18
Luteolin has attracted extensive attention due to its excellent anticancer and anti-inflammatory activities. Luteolin exerts its anti-inflammatory effects partly by regulating inflammatory mediators and different cytokines, which inhibit the signal transduction pathway.19–22 Luteolin could hamper the progression of cancer through multiple mechanisms including the suppression of kinases, regulation of cell cycle, induction of apoptotic cell death, and reduction of transcription factors.23–26 Many researchers have focused on the anti-inflammatory and anti-tumour properties of luteolin; however, only minor attention has been paid to its good antibacterial activity. Nonetheless, luteolin has been demonstrated to exhibit good antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Pseudomonas fluorescens.18,27 The antibacterial mechanism of luteolin against Staphylococcus aureus involves inhibiting the synthesis of nucleic acid and protein, impairing bacterial cell membrane, inducing cell morphological alteration and inhibiting biofilm formation.27,28
To date, only few reports exist on the antibacterial activity of luteolin; however, no report has been published on the antibacterial activity and mechanism of luteolin against T. pyogenes.18,27–29 Thus, we attempted to detect the antibacterial activity of luteolin against T. pyogenes isolates and elucidate the mechanism of action of luteolin by examining its effect on the cell wall and cell membrane, protein, nucleic acid and energy metabolism of T. pyogenes, which could provide practical and scientific guidance for its potential application as a new antibacterial drug.
Materials and Methods
Antimicrobial Agent and Bacterial Strains
Luteolin was purchased from Shanghai Pureone Biotechnology Co., Ltd. (Shanghai, China) and the purity of luteolin was 98%.
The reference T. pyogenes strain, ATCC19411, was purchased from the American Type Culture Collection (USA). The T. pyogenes isolates (n = 17) were collected from dairy cattle diagnosed with mastitis in Liaoning, China, and identified by 16s rRNA gene sequencing. The sequencing data have been submitted to DNA Data Bank of Japan (DDBJ) with accession number LC523902, LC523903, LC523904, LC523905, LC523906, LC523907, LC523908 and LC523909. For the strains with the identical sequence, we only uploaded the sequence of one strain. The resistance phenotypes of T. pyogenes were showed in Table 1. All strains were stored in 20% glycerol at −80 ºC until use. The testing strains were activated in Mueller–Hinton Agar (MHA, AOBOX, Beijing, China) supplemented with 5% sterile defibrinated sheep blood under microaerophilic conditions (5% CO2) at 37 ºC for 24–48 h and inoculated in Nutrient Broth (NB, AOBOX, Beijing, China) supplemented with 8% fetal bovine serum (FBS, Gibco, USA) for culture.
Table 1.
The MICs of Luteolin Against T. pyogenes
| Strains | MICs (µg/mL) | Resistance Phenotypes |
|---|---|---|
| ATCC19411 | 78 | SUL |
| T001 | 78 | SUL |
| T002 | 78 | SUL |
| T003 | 78 | CHL/OXY/SUL |
| T004 | 78 | TIL/CLI/CIP/SUL |
| T005 | 78 | CIP/SUL |
| T006 | 78 | CIP/SUL |
| T007 | 78 | CIP/SUL |
| T008 | 78 | CIP/ENR/SUL |
| T009 | 78 | ENR/SUL |
| T010 | 78 | SUL |
| T011 | 78 | SUL |
| T012 | 78 | SUL |
| T013 | 78 | OXY/CIP/ENR/SUL |
| T014 | 78 | CHL/OXY/ERY/CLI/SUL |
| T015 | 78 | CIP/SUL |
| T016 | 78 | CHL/OXY/TET/TYL/ERY/TIL/CLI/FLO/CIP/SUL |
| T017 | 78 | CLI/SUL |
Notes: The resistance phenotypes were based on our previous research results.
Abbreviations: CHL, chlortetracycline; OXY, oxytetracycline; TET, tetracycline; TYL, tylosin; ERY, erythromycin; TIL, tilmicosin; CLI, clindamycin; FLO, florfenicol; CIP, ciprofloxacin; ENR, enrofloxacin; SUL, Sulfadimethoxine.
Antibacterial Activity Assay
Determination of Minimum Inhibitory Concentration (MIC)
The MICs values of luteolin against T. pyogenes (ATCC19411 and 17 isolates) were determined using the broth microdilution method according to Clinical Laboratory and Standards (CLSI) guidelines.30 Briefly, the bacterial suspension cultured to the logarithmic phase was diluted to 0.5 Mcfarland Standard (approximately 1.5×108 CFU/mL) and then diluted 150 times to 1×106 CFU/mL using Mueller–Hinton Broth (MHB, AOBOX, Beijing, China) containing 8% FBS. A 100-µL volume of serial twofold dilutions of luteolin with MHB was dispensed in U-bottom 96-well Microtiter plates (Corning, USA). Subsequently, an equal volume of adjusted inoculum (1×106 CFU/mL) was added to each well of the Microtiter plates up to a final volume of 200 µL. After incubation for 24 h at 37 ºC and 5% CO2, MIC was defined as the lowest concentration of luteolin that prevented visible bacterial growth. The determinations were performed in triplicate.
Determination of the Growth Curve
The effect of luteolin on the growth curve of T. pyogenes was evaluated according to the relevant literature, with partial modifications.31,32 One millilitre of T. pyogenes suspension (the strain used in this assay and the subsequent assays was ATCC19411) cultured to the logarithmic growth phase (approximately 1 × 108 CFU/mL) and luteolin (final concentrations, 1/2 MIC and 1/4 MIC) were simultaneously added to 100 mL of NB medium containing 8% FBS. The sample that was not treated with luteolin was employed as the control. The mixture was incubated at 37 ºC at a speed of 150 rpm and the absorption of the bacterial suspension at 600 nm was measured after 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, and 40 h of incubation. The bacterial growth curve was plotted to analyse the effect of luteolin on T. pyogenes growth.
Cell Wall Damage Assessment
Detection of Leakage of Alkaline Phosphatase (AKP)
To examine the effect of luteolin on the cell wall of T. pyogenes, 1 mL of T. pyogenes suspension cultured to the logarithmic phase (approximately 1 × 108 CFU/mL) and luteolin (final concentration, 1/2 MIC) were added to 100 mL NB. Thereafter, the bacterial suspension was incubated at 37 ºC and 150 rpm for 4, 8, 12, 24, 36, 48, and 72 h, respectively. The supernatant of the bacterial suspension was obtained by centrifugation at 4000 rpm for 10 min at 4 ºC. The AKP concentration in the supernatant was determined using an alkaline phosphatase assay kit (BioVision, USA).33,34
Detection of Uptake of N-Phenyl-1-Naphthylamine (NPN)
NPN, a type of hydrophobic fluorescent probe, was used to evaluate the effect of luteolin on the cell wall permeability of T. pyogenes. The bacterial suspension cultured to the logarithmic phase was centrifuged at 4000 rpm for 10 min at 4 ºC and washed twice with PBS (pH 7.4). After the concentration of the bacterial suspension was adjusted to OD600 nm = 0.2, it was mixed with different final concentrations of luteolin (0, 1/8 MIC, 1/4 MIC, 1/2 MIC, 1 MIC, and 2 MIC, respectively) and incubated at 37 ºC for 1 h. The bacterial cells were harvested and resuspended in PBS (pH 7.4). Thereafter, 200 µL of the bacterial suspension and 10 mM of the NPN (Sigma-Aldrich, Shanghai, China) solution were added to a 96-well plate (Corning, USA). The fluorescence intensity was immediately measured with a multimode plate reader (VICTOR Nivo, PerkinElmer, USA) at an excitation wavelength of 350 nm and an emission wavelength of 420 nm.35,36
Cell Membrane Damage Assessment
Detection of Leakage of β-Galactosidase
Using 2-Nitrophenyl β-D-galactopyranoside (ONPG) as the substrate, the release of cytoplasmic β-galactosidase from T. pyogenes into the culture medium was measured to determine the cell membrane permeability.37,38 T. pyogenes cells were cultured to the logarithmic phase in NB containing 2% lactose and harvested. The bacterial cells were washed and suspended in PBS (pH 7.4) until the OD600nm value reached 0.2. Thereafter, different concentrations of luteolin (0, 1/4 MIC, and 1/2 MIC) and 1.5 mM of ONPG (final concentration; Sigma-Aldrich) were added to the bacterial suspension, which was incubated at 37 ºC for 100 min. Every 10 min, the absorbance of the supernatant was recorded at 420 nm using a multimode plate reader (VICTOR Nivo, PerkinElmer, USA).
Detection of Uptake of Propidium Iodide (PI)
PI is often utilized as a DNA dye that can enter cells that possess a damaged cell membrane; PI cannot enter cells with an intact cell membrane. In brief, T. pyogenes cultured to the logarithmic phase were collected and resuspended in PBS (pH 7.4) after two rounds of washing. The bacterial suspensions were incubated with luteolin (final concentration, 1/2 MIC) at 37 ºC for 1 h. Thereafter, 2.0 μg/mL of PI (final concentration; Sigma-Aldrich) was added into the bacterial suspensions, which were maintained in a dark room for 30 min. Finally, the stained bacterial suspensions were deposited onto glass slides, covered with coverslips, and photographed with a fluorescence microscope (LeicaDM1000, Leica, Germany).39,40
Cell Membrane Potential Measurement
The membrane potential-sensitive dye, DiSC3(5) (AAT Bioquest, USA), was used to determine the cell membrane potential. The T. pyogenes suspension cultured to the logarithmic phase was centrifuged at 4000 rpm for 10 min at 4 ºC, washed twice with buffer (5 mM HEPES, pH 7.2; 5 mM glucose), and resuspended in the same buffer solution until the OD600nm value reached 0.2. The bacterial suspension was incubated with 4 µM DiSC3(5) in a dark room for 1.5 h until DiSC3(5) was fully absorbed by the bacteria. Luteolin at final concentrations of 1/2 MIC, 1 MIC, and 2 MIC was then added to the solution. Fluorescence was monitored with a multimode plate reader (VICTOR Nivo, PerkinElmer, USA) at an excitation wavelength of 622 nm and an emission wavelength of 670 nm.41,42
Transmission Electron Microscopy (TEM) Observation
T. pyogenes cells cultured to the logarithmic phase were diluted to form the bacterial suspension (approximately 1×106 CFU/mL) and treated with luteolin (final concentration, 1/2 MIC). The bacterial suspensions were incubated on a shaker at 37 ºC and 150 rpm for 12, 24, and 36 h, respectively. The bacterial cells were harvested by centrifugation at 3000 rpm for 10 min at 4 ºC, washed thrice with PBS. Then the specimens were fixed to the fibrous carbon film and observed using TEM (HT-7700, Hitachi, Japan).
Furthermore, the ultrastructure of bacteria was analysed by ultramicrotomy observation. After the bacterial cells were harvested and washed, the specimens were subjected to fixation with 2.5% glutaraldehyde at 4 ºC overnight. The bacterial cells were then pre-embedded with agar, washed thrice with PBS (10 min per wash) and post-fixed with 1% osmium tetroxide for 1 h. Thereafter, the cells were dehydrated with dehydrant (50% ethanol, 75% ethanol, 80% acetone, 90% acetone, 95% acetone and twice at 100% acetone, each time for 15 min), and then embedded and soaked with epoxy 812. Finally, the specimens were sectioned with an ultramicrotome, stained with uranyl acetate, and observed using TEM (HT-7700, Hitachi, Japan).
Effect of Luteolin on Protein
Quantification of Total Cell Protein
The effect of luteolin on the total cell protein content of T. pyogenes was evaluated by the bicinchoninic acid (BCA) method. Briefly, the T. pyogenes cultured to the logarithmic phase were diluted to form bacterial suspensions (approximately 1×106 CFU/mL) and mixed with luteolin (final concentration, 1/2 MIC); the bacterial suspensions that were not treated with luteolin were employed as control. After bacterial suspensions were incubated on a shaker at 37 ºC and 150 rpm for 12, 24, and 36 h respectively, bacterial cells were harvested, washed, and resuspended in PBS (pH 7.4) until the OD600nm value reached 0.8. Thereafter, 10 mL of the bacterial suspensions was centrifuged (4000 rpm, 4 ºC, 10 min), and the bacterial cells were fully mixed with 2 mL of bacterial protein extraction reagent (Sigma-Aldrich) and 20 µL of phenylmethanesulfonylfluoride (PMSF). The mixture was sonicated with an ultrasonic breaker (VCX150, SONICS, USA), and centrifuged at 12,000 rpm for 10 min at 4 ºC. The supernatant was retained as it contained the total cell protein of the bacteria. The protein samples were quantified using the BCA protein assay kit (Thermo Fisher, USA).
SDS-PAGE Analysis
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to analyse the changes in protein expression.43–45 The method described in “Quantification of total cell protein” was employed for the treatment of bacterial samples. The total cell proteins were denatured at 100 ºC for 10 min and electrophoresed on a 5.0% stacking gel at 80 V for 30 min, and then a 10% resolving gel at 110 V for 70 min. The gel was stained with Coomassie brilliant blue R-250 and decolorized until the protein bands were clear. The image of the protein bands was obtained with a gel imaging system (Azure Biosystems c300, USA).
Effect of Luteolin on Nucleic Acid
Quantification of Intracellular Nucleic Acid
T. pyogenes cells cultured to the logarithmic phase were diluted to form a bacterial suspension (approximately 1×106 CFU/mL) and mixed with luteolin (final concentration, 1/4 MIC and 1/2 MIC). After incubating on a shaker at 37 ºC and 150 rpm for 12, 24, and 36 h respectively, the bacterial cells were harvested, washed, and resuspended in PBS (pH 7.4) until the OD600nm value reached 0.6. Thereafter, 200 µL of the bacterial suspension was mixed with 600 µL of 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (Thermo Fisher) staining solution and incubated at room temperature in the dark for 30 min. A 200-µL volume of the bacterial suspension was then placed in a 96-well plate and the fluorescence intensity was determined at 454 nm after excitation at 364 nm. The bacterial cells stained by DAPI were also harvested, washed, and resuspended in PBS (pH 7.4). A 20-µL volume of the bacterial suspension was then used to create slides, which were observed with a fluorescence microscope (LeicaDM1000, Leica).46,47
UV Spectroscopic Test of the Interaction Between Luteolin and DNA
Luteolin (final concentration, 1/2 MIC) was fully mixed with pBR322 DNA (1 µg/mL, TAKARA, Dalian, China) and incubated at 37 ºC for 30 min. The absorption spectrum, which ranged from 300 to 420 nm, was determined by an ultraviolet spectrophotometer (U-3900/3900H, Hitachi, Japan).48,49
Determination of Topoisomerase Activity
The DNA topoisomerases (Topo I and Topo II) were extracted from T. pyogenes. After T. pyogenes were cultured to the logarithmic phase, they were centrifuged at 6000 rpm for 10 min at 4 ºC to retrieve the bacterial cells, which were washed twice with PBS (pH 7.4) and resuspended in TMN buffer (1 mM Tris-HCl pH 7.5, 1.5 mM MgCl2, 10 mM NaCl). Thereafter, the bacterial suspension was centrifuged at 4000 rpm for 10 min at 4 ºC and resuspended in crude enzyme extract buffer (100 mM NaCl, 1 mM KH2PO4, 5 mM MgCl2, 5 mM EDTA, 0.5 mM phenylmethanesulfonylfluoride, 1 mM dithiothreitol pH 6.4, 250 mM saccharose, 10% glycerol). The bacterial suspension was cooled on ice for 30 min, and then ultrasonicated at 80% strength for 10 s and then 30-s intervals, achieving a total of 10 cycles. The cell suspension was then centrifuged at 12,000 g for 10 min at 4 ºC and the supernatant was retained as the crude enzyme solution.50–52
A 2.5-μL volume of DNA despiralization buffer I/II (200 mM Tris-HCl pH 7.5, 340 mM KCl, 40 mM MgCl2, 20 mM dithiothreitol, 0.12 mg/mL Bovine Serum Albumin, 5 mM EDTA, and an extra 4 mM ATP to serve as the DNA despiralization buffer II), 0.5 μg pBR322 DNA, 4.0 μL of the crude enzyme solution, and different final concentrations (1/4 MIC, 1/2 MIC, 1 MIC, 2 MIC, 4 MIC and 8 MIC) of luteolin were added to Eppendorf tubes. Thereafter, sterile water was added to the mixture to obtain a final volume of 20 μL. After the mixture was incubated at 37 ºC for 30 min, 2 µL 10% SDS and 1 µL 10 mg/mL proteinase K were added. Incubation was then allowed to continue for 30 min at 37 ºC. Finally, DNA samples were electrophoresed on 1.0% agarose gel at a constant voltage of 80 V for 40 min and analysed using a gel imaging system (Azure Biosystems c300).28
Effect of Luteolin on Energy Metabolism
Quantification of Intracellular ATP
The ATP content of T. pyogenes was determined with an ATP assay kit (Sigma-Aldrich). Bacterial cells cultured to the logarithmic phase were harvested, washed, and resuspended in PBS (pH 7.4) until the OD600nm value reached 1.0. Luteolin at final concentrations of 1/4 MIC, 1/2 MIC, 1 MIC, and 2 MIC was respectively added to 20 mL of the prepared bacterial suspensions. The mixtures were then incubated at 37 ºC for 1 h. After the bacterial cells were harvested, their ATP content was determined with an ATP assay kit.53,54
Determination of the SDH Activity
Succinate dehydrogenase (SDH) is a key enzyme in the tricarboxylic acid cycle. To evaluate the effect of luteolin on SDH in T. pyogenes, the activity of SDH in bacterial cells was detected. The method described in “Quantification of intracellular ATP” was employed to treat the bacteria. The harvested bacterial cells were resuspended in 2 mL of PBS (pH 7.4) and ultrasonicated at a strength of 30% for 15 min (interval 3 s). The supernatant was then obtained by centrifugation at 7000 rpm for 5 min at 4 ºC, and the activity of SDH was determined using an SDH assay kit (Sigma-Aldrich).
Statistical Analysis
All assays were carried out in triplicate. The results are presented as mean ± standard deviation (SD). Statistical analysis was carried out using one-way of variance (ANOVA) on SPSS Statistics V17.0 and GraphPad Prism 6.0. Statistical significance was defined as *P < 0.05 and **P < 0.01.
Results
Antibacterial Activity of Luteolin
The results of luteolin’s antibacterial ability against T. pyogenes are presented in Table 1. Although the tested strains had different resistance phenotypes (see Table 1), the MICs of luteolin against all tested strains were 78 µg/mL. These data indicate that luteolin exerts significant antibacterial ability.
We further analysed the effect of luteolin on the growth curve of T. pyogenes. As shown in Figure 1, bacteria started the logarithmic growth after 4 h of the lag phase and could reach the maximum at 36 h in the control group, which showed a normal and typical growth curve. Compared with the control group, the growth of bacteria in the luteolin-treated group was significantly inhibited. The bacteria were found to start the logarithmic growth after 8 h, which was 4 h delay in comparison with the control group and grew slowly in this phase. Moreover, the absorbance of bacteria in the experimental group was consistently lower than that of the control group, thereby indicating that luteolin can evidently inhibit the growth of T. pyogenes.
Figure 1.
Effect of luteolin on the growth curve of T. pyogenes. Data are presented as mean (± SD) of three replicates.
Cell Wall Damage Assessment
AKP mainly exists between the cell wall and the cell membrane in bacteria. AKP leak out of the cell if the cell wall was damaged.33 Therefore, the influence of luteolin on the cell wall permeability can be indirectly revealed by detecting the changes in extracellular AKP. As shown in Figure 2A, compared with the control group, the extracellular AKP content evidently increased after luteolin was administered to T. pyogenes for 4 h and continuously increased with time. The experimental results indicate that luteolin can destroy the integrity of the cell wall of T. pyogenes and increase the extracellular AKP content.
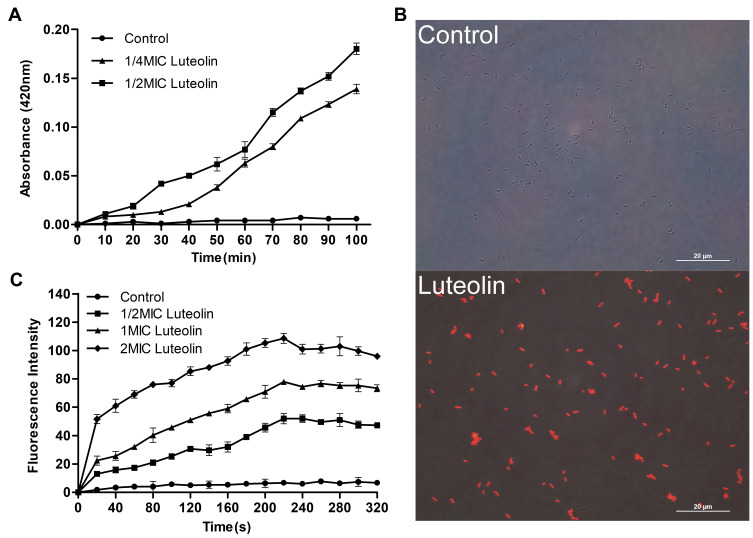
Figure 2.
Effect of luteolin on the cell wall integrity of T. pyogenes. (A) Leakage of AKP from T. pyogenes after treatment with luteolin. Data are presented as mean (± SD) of three replicates (compared with the control, ** P < 0.01). (B) Fluorescence intensity of NPN after treatment with different concentrations of luteolin. Data are presented as mean (± SD) of three replicates.
The integrity of cell wall was further detected using NPN. NPN is a hydrophobic probe, which remains quenched in an aqueous solution but can emit strong fluorescence in a hydrophobic environment. When the outer wall is damaged, NPN can enter the hydrophobic environment inside the cell membrane and emit strong fluorescence.55 As shown in Figure 2B, luteolin caused a significant increase in the fluorescence intensity of NPN in a concentration-dependent manner. Such findings indicate that the cell wall was at least partially disrupted, causing NPN to enter the cell membrane.
Cell Membrane Damage Assessment
The destructive effect of luteolin on the cell membrane was examined by ONPG. Generally, β-galactosidase in the cytoplasm cannot be detected unless the cell membrane is destroyed. ONPG can be catalysed by β-galactosidase to produce o-nitrophenol, which is absorbed at 420 nm.56 A difference in the absorbance value was observed when luteolin was added to the culture (Figure 3A). Evidently, the absorbance value increased after luteolin treatment. Moreover, the absorbance value in the 1/2 MIC luteolin group was greater than that of the 1/4 MIC luteolin group, indicating that luteolin could increase the permeability of the cell membrane, and the greater the concentration of luteolin, the greater the damage to the cell membrane.
Figure 3.
Effect of luteolin on the cell membrane of T. pyogenes. (A) Leakage of β-galactosidase from T. pyogenes after treatment with luteolin. Data are presented as mean (± SD) of three replicates. (B) Fluorescence microscopy images of T. pyogenes in control group and luteolin treatment group. (C) Cell membrane potential variation of T. pyogenes after treatment with luteolin. Data are presented as mean (± SD) of three replicates.
PI is a nucleic acid intercalating agent that can release red fluorescence after the insertion of a double-stranded DNA. Although PI cannot pass through the living cell membrane, it can penetrate the damaged cell membrane and stain the nucleic acid.57 Therefore, PI was used in this study to confirm the cell membrane integrity. As demonstrated by the results in Figure 3B, compared with T. pyogenes that were not treated with luteolin, those strains exposed to luteolin could emit strong red fluorescence. Such findings indicate that the integrity of the cell membrane and wall was damaged and after PI could pass through the cell wall and membrane.
Additionally, the effect of luteolin on membrane depolarization was evaluated by monitoring the fluorescence intensity change of the membrane-potential dependent probe, DiSC3(5). The fluorescent probe is quenched in the cytoplasmic membrane. However, when an antimicrobial agent disrupts the transmembrane electrostatic potential, the probe dissociates into the medium and fluoresces strongly.58 An increase in the fluorescence intensity indicates a reduction in the membrane potential. Based on the results presented in Figure 3C, adding luteolin induced a dramatic concentration-dependent increase in fluorescence, which indicates that luteolin could depolarize the cell membrane potential.
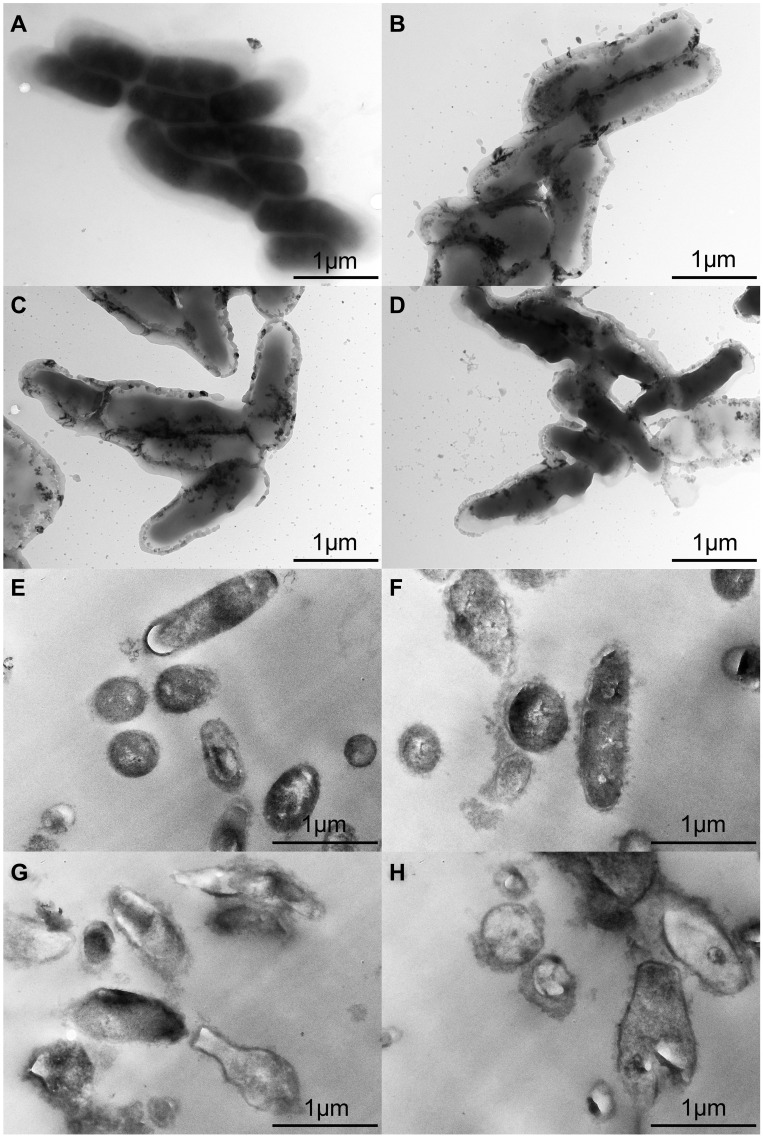
TEM Observation
To analyse the surface changes in the cells of T. pyogenes after luteolin treatment, we observed bacterial cells with TEM. As shown in Figure 4A, the cell surface of the control group was smooth and flat without any secretion, while cells of the experimental group (Figure 4B-D) had abundant secretion on their surface. The cell surface was severely damaged and a partial wall was missing. Thus, the results confirm that the cell membrane and wall are partial targets of luteolin.
Figure 4.
TEM images of T. pyogenes. (A and E) Bacteria untreated with luteolin. (B and F) Bacteria treated with 1/2 MIC luteolin for 12 h. (C and G) Bacteria treated with 1/2 MIC luteolin for 24 h. (D and H) Bacteria treated with 1/2 MIC luteolin for 36 h.
The ultrastructural changes in cells after treating T. pyogenes with luteolin were further observed. Based on the results presented in Figure 4E, T. pyogenes cells in the control group were plump, smooth, and compact with a clear structure. However, many intracellular components leaked out from the T. pyogenes cells treated with luteolin and the cell edges were rough (see Figure 4F-H). In addition, the cells were severely deformed after luteolin was administered to T. pyogenes for 24 h. These results reconfirm that luteolin can destroy the integrity of the membrane and wall of T. pyogenes.
Effect of Luteolin on Protein
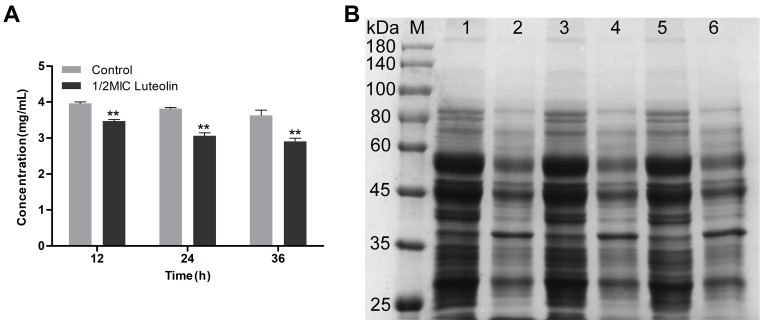
Protein is the material basis of life and the main bearer of life activities. Thus, it will have an important impact on the physiological function of bacterial cells when there is a change in protein content. Herein, we determined the effect of luteolin on the protein of T. pyogenes by the BCA assay and SDS-PAGE. As shown in Figure 5A, after luteolin was administered to T. pyogenes for 12, 24, and 36 h, the total cell protein content decreased by 12.45%, 19.76%, and 19.85% respectively. This finding was also confirmed by the SDS-PAGE profiles (Figure 5B) of the total bacterial proteins. Many protein bands of T. pyogenes after luteolin treatment appeared significantly shallower than those of the control group did. Notably, the protein band (approximately 38 kDa) became thicker after treatment with luteolin, which represented an up-regulation of protein expression level.
Figure 5.
Effect of luteolin on the expression of proteins in T. pyogenes. (A) Changes of total protein content after luteolin treatment. Data are presented as mean (± SD) of three replicates (compared with the control, ** P < 0.01). (B) SDS-PAGE profiles of total cell proteins. M: Molecular weight marker; 1, 3 and 5: T. pyogenes of control group cultured to 12, 24 and 36h, respectively; 2, 4 and 6: T. pyogenes treated with luteolin for 12, 24 and 36h, respectively.
Effect of Luteolin on Nucleic Acid
The changes of nucleic acid content were analysed using DAPI. DAPI can penetrate living cell membranes and produce a strong fluorescence signal when combined with double-stranded DNA. As shown in Figure 6A, compared with the control group, the nucleic acid content of T. pyogenes decreased by 36.9%, 35.27%, and 38.65% after treatment with the 1/4 MIC of luteolin, and 60.82%, 56.55%, and 56.04% after treatment with the 1/2 MIC of luteolin for 12, 24, and 36 h, respectively. These findings align with those depicted in the fluorescence microscopy images of T. pyogenes (Figure 6B). A strong blue fluorescence was emitted by the cells in the control group. However, cells treated with luteolin for 12, 24, and 36 h displayed an extremely weak florescence signal, which indicating the marked reduction in the nucleic acid content of cells.
Figure 6.
Effect of luteolin on the nucleic acid content in T. pyogenes. (A) Changes of fluorescence intensity of nucleic acid after treatment with luteolin. Data are presented as mean (± SD) of three replicates (compared with the control, ** P < 0.01). (B) Fluorescence microscopy images of T. pyogenes after 1/2 MIC luteolin treatment for 12 h, 24 h and 36 h, respectively.
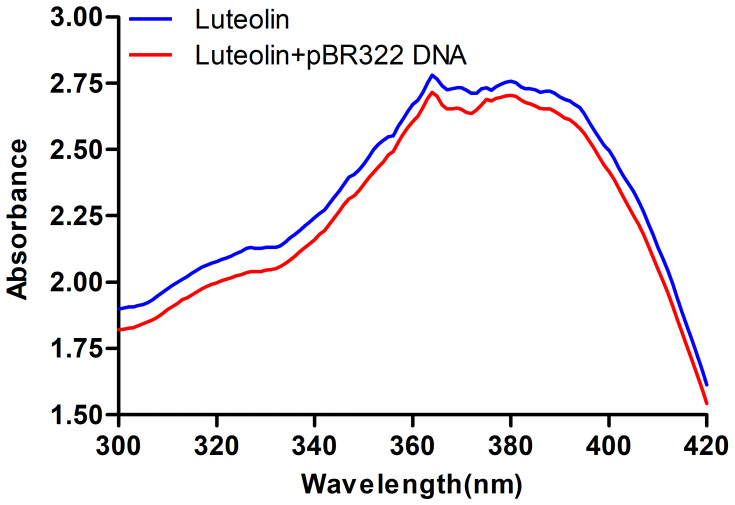
Next, the interaction between luteolin and DNA was analysed. Ultraviolet-visible absorption spectroscopy is often used to study the interaction mode between small molecular compounds and DNA. When a compound binds to DNA, the environment of its ligand changes, which causes its electronic structure to be intercepted by DNA. This binding also changes the compound’s absorption wavelength and intensity in its absorption spectrum.59 As shown in Figure 7, when DNA was added to luteolin, the hypochromic effect was demonstrated in its absorption spectrum, and its absorption intensity was evidently decreased. These findings suggest that luteolin may be embedded into DNA, which can inhibit the replication of DNA.
Figure 7.
UV spectra of interaction of luteolin and DNA.
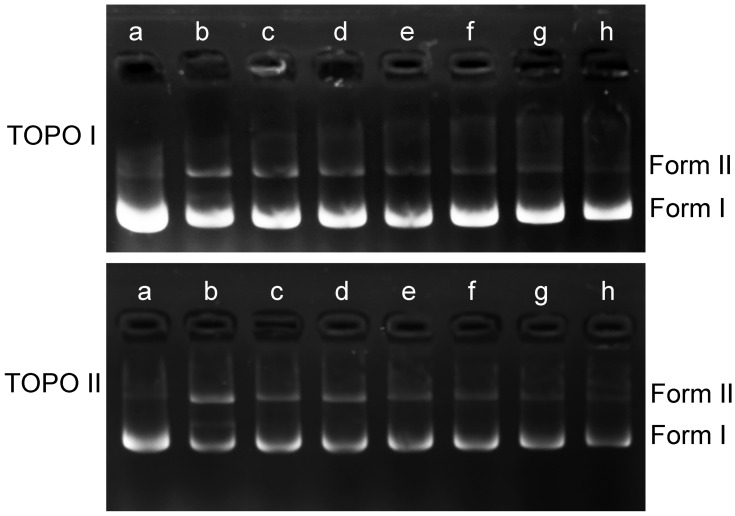
DNA topoisomerases are essential enzymes that mediate the topological adjustments required for DNA replication, transcription, recombination, repair, and chromatin assembly.60 Topoisomerase I affects DNA topology by passing a single DNA strand through a break in the opposing single strand through the use of an active site tyrosine residue to cleave the DNA strand. Conversely, topoisomerase II creates double-stranded breaks by using similar active site tyrosine residues, which allows the passing of another double-strand DNA segment.61 pBR322 DNA mainly exists as a superhelix (Form I) that can be unwound into the nicked or relaxed form (Form II). The effect of luteolin on the activity of topoisomerase was determined by enzyme-mediated supercoiled pBR322 DNA relaxation. Figure 8 showed that an increase in luteolin concentration led to a gradual decline in the amount of linear or nicked DNA, which demonstrated that luteolin could inhibit the activity of topoisomerases I and II. These results indicate that luteolin can inhibit the activity of key enzymes in the process of nucleic acid metabolism, which may be one of the reasons for the decrease of nucleic acid content.
Figure 8.
Effect of luteolin on topoisomerase I and II of T. pyogenes. a: pBR322; b–h: control and luteolin with different concentrations (1/4MIC,1/2MIIC, 1MIC, 2MIC, 4MIC, 8MIC).
Effect of Luteolin on Energy Metabolism
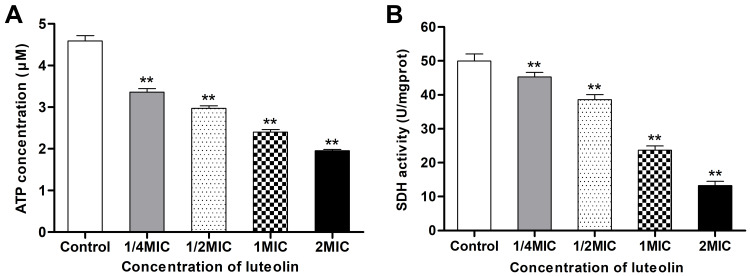
ATP plays an important role in energy metabolism in organisms. In fact, it functions as the direct energy source of metabolism. Thus, if luteolin affects the energy metabolism of T. pyogenes, it may affect energy production in bacteria. ATP is the direct energy supplier for the activities of cells. Under normal physiological conditions, the ATP content in cells is consistently in dynamic equilibrium. The ATP content in bacterial cells reflects their energy storage status. As shown in Figure 9A, when different concentrations (1/4 MIC, 1/2 MIC, 1 MIC, 2 MIC) of luteolin were administered to T. pyogenes for 1 h, the ATP content in cells decreased by 26.80%, 35.29%, 47.71%, and 57.44%, respectively. This finding aligns with the effect of Litsea cubeba essential oil on MRSA, which could also reduce ATP content in cells.48 Altogether, the results reveal that luteolin could interfere with the energy metabolism of T. pyogenes.
Figure 9.
Effect of luteolin on energy metabolism of T. pyogenes. (A) Effect of luteolin on the ATP content in T. pyogenes. Data are presented as mean (± SD) of three replicates (compared with the control, ** P < 0.01). (B) Effect of luteolin on the SDH activity in T. pyogenes. Data are presented as mean (± SD) of three replicates (compared with the control, ** P < 0.01).
We further analysed the effect of luteolin on the key enzyme activity in the energy metabolism. Under aerobic conditions, pyruvic acid is completely oxidised to carbon dioxide and water via the Krebs cycle, thereby serving as the main energy source for bacterial life activities. SDH is a key enzyme in the Krebs cycle. In addition, SDH is one of the hubs that link oxidative phosphorylation to electron transport. As a result, its activity is generally used to evaluate the operation of the tricarboxylic acid cycle. In the present study, we found that the activity of SDH decreased by 9.30%, 22.86%, 52.52%, and 73.50% respectively after T. pyogenes were exposed to different concentrations (1/4 MIC, 1/2 MIC, 1 MIC, 2 MIC) of luteolin for 1 h. Such findings (Figure 9B) demonstrate that luteolin can inhibit the activity of SDH in T. pyogenes.
Discussion
In recent years, there has been a rising interest in the discovery of new antibacterial compounds because of the alarming increase in the rate of infections with multidrug resistant bacteria. The evaluation of antibacterial activity and research on the antibacterial mechanism of natural plant products can provide theoretical and data support for the development of new drugs against drug-resistant bacteria. As a natural polyphenolic flavonoid compound, luteolin exhibited significant antibacterial activity against T. pyogenes in both sensitive and resistant strains (Table 1). Our previous research showed that the MICs of luteolin against Escherichia coli (ATCC25922), Salmonella (C7731), and Streptococcus (ATCC49619) were 2500 µg/mL, 1250 µg/mL, and 2500 µg/mL, respectively; these values were remarkably weaker than the MIC of luteolin against T. pyogenes.29 In addition, luteolin showed concentration-dependent activity based on the growth curve (Figure 1). Therefore, luteolin has broad prospects as a new drug for the treatment of T. pyogenes infections.
Many natural products have exhibited antibacterial activities by multiple mechanisms, including destroying the integrity of cell walls and cell membranes, inhibiting the expression of proteins, inhibiting the synthesis of nucleic acids, and affecting the energy metabolism of bacteria.62,63 Hence, in this research, we attempted to understand the mechanism of action of luteolin against T. pyogenes from the above four aspects.
The cell wall and membrane are important for sustaining cell life because they are able to prevent the leakage of intracellular components and function as a barrier.64 Our results demonstrated that luteolin could lead to the leakage of AKP, which is located between the cell wall and membrane, by destroying the integrity of the cell wall (Figure 2A). Moreover, the extracellular hydrophobic fluorescent probe NPN could penetrate the outer wall in a concentration-dependent manner after luteolin treatment; as the concentration of luteolin increased, the damage to the integrity of the cell wall increased (Figure 2B). In addition, luteolin could increase the permeability of the cell membrane of T. pyogenes; therefore, small molecules such as β-galactosidase can easily pass through the cell membrane and continuously accumulate in the extracellular environment. The PI assay further demonstrated that luteolin can destroy the integrity and barrier function of the cell membrane of T. pyogenes, resulting in extracellular substances being able to pass through the cell membrane.
We further detected changes in cell membrane potential after luteolin treatment with T. pyogenes. As shown in Figure 3C, the addition of luteolin depolarized the cell membrane potential in a concentration-dependent manner. A cascade of events occurred at the wall and membrane upon luteolin treatment, resulting in the easy passage of small molecules because of membrane destabilization. Furthermore, TEM observation revealed that luteolin could disrupt the cell wall and membrane of T. pyogenes cells, resulting in the leakage of intracellular components. Thus, the cell wall and membrane of T. pyogenes may be one of the targets of luteolin.
Protein is involved in a variety of biochemical reactions for catalysis, protein synthesis and expression and bacterial metabolism.44 In our study, SDS-PAGE and concentration of total bacterial protein were assayed. The results showed that luteolin can reduce the total cell protein content of T. pyogenes, indicating that the expression of some proteins in T. pyogenes was inhibited by luteolin. It is very interesting to note that the expression of some proteins was upregulated and the expression of some proteins was downregulated after luteolin treatment. The disorder of protein expression is bound to affect the normal physiological metabolism of T. pyogenes. In future studies, proteomics and transcriptomics technology will be used to further explore the effect of luteolin on protein expression in T. pyogenes.
DNA, a genetic material with relative stability, facilitates protein synthesis and controls organism metabolism and cell growth. Previous research has shown that luteolin could inhibit the nucleic acid synthesis of Staphylococcus aureus.28 In this study, luteolin showed the same effect and inhibited the synthesis of nucleic acids in T. pyogenes (Figure 6A and B). The inhibition of nucleic acid synthesis may also be one of the reasons that protein expression was affected. To explore the reason why nucleic acid synthesis was inhibited, we scanned the absorption spectrum of luteolin and analysed the effect of luteolin on DNA topoisomerase activity. The results illustrated that luteolin could inhibit the activity of topoisomerases I/II and may be embedded into DNA.
ATP plays an important role in energy metabolism. As shown in Figure 9A, ATP content in cells decreased significantly in a concentration-dependent manner after luteolin treatment. This may be caused by ATP leakage or the inhibition of key enzymes in the Krebs cycle, including SDH (Figure 9B). Almost all life activities in cells require energy, and the reduction in ATP content will definitely inhibit the normal life activities of T. pyogenes. Luteolin may interfere with the energy metabolism of T. pyogenes through several pathways. In this research, we only preliminarily detected several indicators related to energy metabolism, and the mechanism by which luteolin affects the energy metabolism of T. pyogenes was not deeply studied. In our future work, proteomics and transcriptomics technology will be used to further research this issue.
Compared with traditional antibiotics with a single target, luteolin has the advantage that it has multiple action targets on T. pyogenes, which is also the reason why natural products do not easily produce drug resistance. In general, this research has preliminarily clarified that luteolin may exhibit anti-T. pyogenes activity by destroying the integrity of the cell wall and cell membrane, affecting protein expression, inhibiting nucleic acid synthesis, and interfering with energy metabolism. However, the specific mechanism of action of luteolin on T. pyogenes remains to be further studied in the future.
Conclusion
In the present study, luteolin was demonstrated to exhibit significant antibacterial activity against T. pyogenes by disrupting the integrity of the cell membrane and cell wall, resulting in the leakage of cell contents and damage to the barrier function of the cell wall and membrane; influencing the expression of protein and interfering with the normal processes of T. pyogenes; interfering with the normal metabolism of nucleic acid, which may occur via an interaction with DNA and inhibiting the activity of key enzymes in nucleic acid metabolism; and reducing the ATP content in cells.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (No. 2016YFD0501309) and the National Natural Science Foundation of China (Nos. 31972736, 31572564).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Duarte VD, Dias RS, Kropinski AM, et al. A t4virus prevents biofilm formation by Trueperella pyogenes. Vet Microbiol. 2018;218:45–51. doi: 10.1016/j.vetmic.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 2.Huang T, Song XH, Zhao KL, et al. Quorum-sensing molecules N-acyl homoserine lactones inhibit Trueperella pyogenes infection in mouse model. Vet Microbiol. 2018;213:89–94. doi: 10.1016/j.vetmic.2017.11.029 [DOI] [PubMed] [Google Scholar]
- 3.Zastempowska E, Lassa H. Genotypic characterization and evaluation of an antibiotic resistance of Trueperella pyogenes (Arcanobacterium pyogenes) isolated from milk of dairy cows with clinical mastitis. Vet Microbiol. 2012;161(1–2):153–158. doi: 10.1016/j.vetmic.2012.07.018 [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro MG, Risseti RM, Bolaños CAD, et al. Trueperella pyogenes multispecies infections in domestic animals: a retrospective study of 144 cases (2002 to 2012). Vet Q. 2015;35(2):82–87. doi: 10.1080/01652176.2015.1022667 [DOI] [PubMed] [Google Scholar]
- 5.Jost BH, Billington SJ. Arcanobacterium pyogenes: molecular pathogenesis of an animal opportunist. Antonie Van Leeuwenhoek. 2005;88(2):87–102. doi: 10.1007/s10482-005-2316-5 [DOI] [PubMed] [Google Scholar]
- 6.Rzewuska M, Stefańska I, Osińska B, et al. Phenotypic characteristics and virulence genotypes of Trueperella (Arcanobacterium) pyogenes strains isolated from European bison (Bison bonasus). Vet Microbiol. 2012;160(1–2):69–76. doi: 10.1016/j.vetmic.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 7.Galán-Relaño Á, Gómez-Gascón L, Luque I, et al. Antimicrobial susceptibility and genetic characterization of Trueperella pyogenes isolates from pigs reared under intensive and extensive farming practices. Vet Microbiol. 2019;232:89–95. doi: 10.1016/j.vetmic.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 8.Zhang DX, Zhao JC, Wang QX, et al. Trueperella pyogenes, isolated from dairy cows with endometritis in Inner Mongolia, China: tetracycline susceptibility and tetracycline-resistance gene distribution. Microb Pathog. 2017;105:51–56. doi: 10.1016/j.micpath.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 9.Rzewuska M, Czopowicz M, Gawryś M, Markowska-Daniel I, Bielecki W. Relationships between antimicrobial resistance, distribution of virulence factor genes and the origin of Trueperella pyogenes isolated from domestic animals and European bison (Bison bonasus). Microb Pathog. 2016;96:35–41. doi: 10.1016/j.micpath.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 10.Martelli G, Giacomini D. Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur J Med Chem. 2018;158:91–105. doi: 10.1016/j.ejmech.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 11.Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–358. doi: 10.1016/j.jep.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 12.Rungsung S, Singh TU, Rabha DJ, et al. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine. 2018;110:333–343. doi: 10.1016/j.cyto.2018.03.042 [DOI] [PubMed] [Google Scholar]
- 13.Fan WC, Qian SH, Qian P, Li XM. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Res. 2016;220:112–116. doi: 10.1016/j.virusres.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 14.Zhang HX, Chen Y, Xu R, He QY. Nrf2 mediates the resistance of human A549 and HepG2 cancer cells to boningmycin, a new antitumor antibiotic, in vitro through regulation of glutathione levels. Acta Pharmacol Sin. 2018;39(10):1661–1669. doi: 10.1038/aps.2018.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funaro A, Wu X, Song MY, et al. Enhanced anti-inflammatory activities by the combination of luteolin and tangeretin. J Food Sci. 2016;81(5):H1320–H1327. doi: 10.1111/1750-3841.13300 [DOI] [PubMed] [Google Scholar]
- 16.Zhang RQ, Li DY, Xu TD, et al. Antioxidative effect of luteolin pretreatment on simulated ischemia/reperfusion injury in cardiomyocyte and perfused rat heart. Chin J Integr Med. 2017;23(7):518–527. doi: 10.1007/s11655-015-2296-x [DOI] [PubMed] [Google Scholar]
- 17.Sato A, Tamura H. High antiallergic activity of 5,6,4ʹ-trihydroxy-7,8,3ʹ-trimethoxyflavone and 5,6-dihydroxy-7,8,3ʹ,4ʹ-tetramethoxyflavone from eau de cologne mint (Mentha×piperita citrata). Fitoterapia. 2015;102:74–83. doi: 10.1016/j.fitote.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 18.Lv P-C, Li H-Q, Xue J-Y, Shi L, Zhu H-L. Synthesis and biological evaluation of novel luteolin derivatives as antibacterial agents. Eur J Med Chem. 2009;44(2):908–914. doi: 10.1016/j.ejmech.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 19.Kim A, Yun JM. Combination treatments with luteolin and fisetin enhance anti-inflammatory effects in high glucose-treated THP-1 cells through histone acetyltransferase/histone deacetylase regulation. J Med Food. 2017;20(8):782–789. doi: 10.1089/jmf.2017.3968 [DOI] [PubMed] [Google Scholar]
- 20.Cummins CB, Wang XF, Lopez ON, et al. Luteolin-mediated inhibition of hepatic stellate cell activation via suppression of the STAT3 pathway. Int J Mol Sci. 2018;19(6):1567. doi: 10.3390/ijms19061567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Elsaad N, El-Karef A. The falconoid luteolin mitigates the myocardial inflammatory response induced by high-carbohydrate/high-fat diet in Wistar rats. Inflammation. 2017;41(1):221–231. doi: 10.1007/s10753-017-0680-8 [DOI] [PubMed] [Google Scholar]
- 22.Yao ZH, Yao XL, Zhang Y, Zhang SF, Hu JC. Luteolin could improve cognitive dysfunction by inhibiting neuroinflammation. Neurochem Res. 2018;43(4):806–820. doi: 10.1007/s11064-018-2482-2 [DOI] [PubMed] [Google Scholar]
- 23.Huang LM, Jin KT, Lan HR. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol Lett. 2019;17(4):3842–3850. doi: 10.3892/ol.2019.10052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitakaze T, Makiyama A, Samukawa Y, Jiang S, Yamashita Y, Ashida H. A physiological concentration of luteolin induces Phase II drug-metabolizing enzymes through the ERK1/2 signaling pathway in HepG2 cells. Arch Biochem Biophys. 2019;663:151–159. doi: 10.1016/j.abb.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Wang HD, Jia Y, Pan H, Ding H. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother Pharmacol. 2017;79(5):1031–1041. doi: 10.1007/s00280-017-3299-4 [DOI] [PubMed] [Google Scholar]
- 26.Huang XC, Dai SJ, Dai JJ, et al. Luteolin decreases invasiveness, deactivates STAT3 signaling, and reverses interleukin-6 induced epithelial-mesenchymal transition and matrix metalloproteinase secretion of pancreatic cancer cells. OncoTargets Ther. 2015;8:2989–3001. doi: 10.2147/OTT.S91511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian W, Liu M, Fu Y, et al. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb Pathog. 2020;142:104056. doi: 10.1016/j.micpath.2020.104056 [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Xie MJ. Antibacterial activity and mechanism of Luteolin on Staphylococcus aureus. Acta Microbiol Sin. 2010;50(9):1180–1184. [PubMed] [Google Scholar]
- 29.Huang CC, Gao X, Sun TT, et al. The antimicrobial activity of luteolin against four bacteria in vitro. Chin J Vet Sci. 2017;37(8):1558–1561. [Google Scholar]
- 30.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100. 28th Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 31.Yi LH, Li X, Luo LL, et al. A novel bacteriocin BMP11 and its antibacterial mechanism on cell envelope of Listeria monocytogenes and Cronobacter sakazakii. Food Control. 2018;91:160–169. doi: 10.1016/j.foodcont.2018.03.038 [DOI] [Google Scholar]
- 32.Diao MM, Qi DP, Xu MM, et al. Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control. 2018;85:339–344. doi: 10.1016/j.foodcont.2017.10.019 [DOI] [Google Scholar]
- 33.He N, Wang PQ, Wang PY, Ma CY, Kang WY. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) lam. BMC Complement Altern Med. 2018;18(1):261. doi: 10.1186/s12906-018-2317-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang NN, Lan WQ, Wang Q, Sun XH, Xie J. Antibacterial mechanism of Ginkgo biloba leaf extract when applied to Shewanella putrefaciens and Saprophytic staphylococcus. Aquacult Fish. 2018;3(4):163–169. [Google Scholar]
- 35.Hong W, Zhao YN, Guo YR, et al. PEGylated self-assembled nano-bacitracin a: probing the antibacterial mechanism and real-time tracing of target delivery in vivo. ACS Appl Mater Interfaces. 2018;10(13):10688–10705. doi: 10.1021/acsami.8b00135 [DOI] [PubMed] [Google Scholar]
- 36.Xiang QS, Kang CD, Niu LY, Zhao DB, Li K, Bai YH. Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT-Food Sci Technol. 2018;96:395–401. doi: 10.1016/j.lwt.2018.05.059 [DOI] [Google Scholar]
- 37.Han FF, Gao YH, Luan C, Xie YG, Liu YF, Wang YZ. Comparing bacterial membrane interactions and antimicrobial activity of porcine lactoferricin-derived peptides. J Dairy Sci. 2013;96(6):3471–3487. doi: 10.3168/jds.2012-6104 [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Zhang YF, Wang XH, Ling JQ, He GH, Shen LR. Antibacterial activity and its mechanisms of a recombinant Funme peptide against Cronobacter sakazakii in powdered infant formula. Food Res Int. 2019;116:258–265. doi: 10.1016/j.foodres.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 39.Liang SN, Dang QF, Liu CS, et al. Characterization and antibacterial mechanism of poly(aminoethyl) modified chitin synthesized via a facile one-step pathway. Carbohydr Polym. 2018;195:275–287. doi: 10.1016/j.carbpol.2018.04.109 [DOI] [PubMed] [Google Scholar]
- 40.Wang F, Wei FY, Song CX, et al. Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind Crops Prod. 2017;109:358–366. doi: 10.1016/j.indcrop.2017.08.058 [DOI] [Google Scholar]
- 41.Dzoyem JP, Hamamoto H, Ngameni B, Ngadjui BT, Sekimizu K. Antimicrobial action mechanism of flavonoids from Dorstenia species. Drug Discov Ther. 2013;7(2):66–72. [PubMed] [Google Scholar]
- 42.Ma QQ, Dong N, Shan AS, et al. Biochemical property and membrane-peptide interactions of de novo antimicrobial peptides designed by helix-forming units. Amino Acids. 2012;43:2527–2536. doi: 10.1007/s00726-012-1334-7 [DOI] [PubMed] [Google Scholar]
- 43.Li YQ, Han Q, Feng JL, Tian WL, Mo HZ. Antibacterial characteristics and mechanisms of ε-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control. 2014;43:22–27. doi: 10.1016/j.foodcont.2014.02.023 [DOI] [Google Scholar]
- 44.Zhang Y, Wu YT, Zheng W, et al. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J Funct Foods. 2017;38:273–279. doi: 10.1016/j.jff.2017.09.047 [DOI] [Google Scholar]
- 45.Chang L, Wang J, Tong CY, Zhang XZ, Zhao L, Liu XM. Antibacterial mechanism of polyacrylonitrile fiber with organophosphorus groups against Escherichia coli. Fibers Polym. 2016;17(5):721–728. doi: 10.1007/s12221-016-5933-x [DOI] [Google Scholar]
- 46.Zhang YT, Feng RZ, Li LX, et al. The antibacterial mechanism of Terpinen-4-ol against Streptococcus agalactiae. Curr Microbiol. 2018;75(9):1214–1220. doi: 10.1007/s00284-018-1512-2 [DOI] [PubMed] [Google Scholar]
- 47.Cui HY, Zhao CT, Lin L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control. 2015;56:128–134. doi: 10.1016/j.foodcont.2015.03.026 [DOI] [Google Scholar]
- 48.Hu W, Li CZ, Dai JM, Cui HY, Lin L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind Crops Prod. 2019;130:34–41. doi: 10.1016/j.indcrop.2018.12.078 [DOI] [Google Scholar]
- 49.Zou N, Wang XL, Li GF. Spectroscopic and electrochemical studies on the interaction between luteolin and DNA. J Solid State Electrochem. 2016;20(6):1775–1782. doi: 10.1007/s10008-016-3174-y [DOI] [Google Scholar]
- 50.Chen BM, Chen JY, Kao M, Lin JB, Yu MH, Roffler SR. Elevated topoisomerase I activity in cervical cancer as a target for chemoradiation therapy. Gynecol Oncol. 2000;79(2):272–280. doi: 10.1006/gyno.2000.5947 [DOI] [PubMed] [Google Scholar]
- 51.Sullivan DM, Glisson BS, Hodges PK, Smallwood-Kentro S, Ross WE. Proliferation dependence of topoisomerase II mediated drug action. Biochemistry. 1986;25(8):2248–2256. doi: 10.1021/bi00356a060 [DOI] [PubMed] [Google Scholar]
- 52.Lin L, Gu YL, Li CZ, Vittayapadung S, Cui HY. Antibacterial mechanism of ε-poly-lysine against Listeria monocytogenes and its application on cheese. Food Control. 2018;91:76–84. doi: 10.1016/j.foodcont.2018.03.025 [DOI] [Google Scholar]
- 53.Cui HY, Zhang CH, Li CZ, Lin L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control. 2018;94:140–146. doi: 10.1016/j.foodcont.2018.07.007 [DOI] [Google Scholar]
- 54.Lv X, Du J, Jie Y, et al. Purification and antibacterial mechanism of fish-borne bacteriocin and its application in shrimp (Penaeus vannamei) for inhibiting Vibrio parahaemolyticus. World J Microbiol Biotechnol. 2017;33(8):156. doi: 10.1007/s11274-017-2320-8 [DOI] [PubMed] [Google Scholar]
- 55.Ning YW, Yan AH, Yang K, Wang ZX, Li XF, Jia YM. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017;228:533–540. doi: 10.1016/j.foodchem.2017.01.112 [DOI] [PubMed] [Google Scholar]
- 56.Liu F, Wang HM, Cao SS, Jiang CG, Hou JC. Characterization of antibacterial activity and mechanisms of two linear derivatives of bactenecin. LWT-Food Sci Technol. 2019;107:89–97. doi: 10.1016/j.lwt.2019.02.073 [DOI] [Google Scholar]
- 57.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1(3):1458–1461. doi: 10.1038/nprot.2006.238 [DOI] [PubMed] [Google Scholar]
- 58.Tague AJ, Putsathit P, Hammer KA, et al. Cationic biaryl 1,2,3-triazolyl peptidomimetic amphiphiles: synthesis, antibacterial evaluation and preliminary mechanism of action studies. Eur J Med Chem. 2019;168:386–404. doi: 10.1016/j.ejmech.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 59.Naing K, Takahashi M, Taniguchi M, Yamagishi A. Interactions of Enantiomeric Ruthenium (II) complexes with polynucleotides as studied by circular dichroism, electric dichroism measurements, and photolysis. Inorg Chem. 1995;34(1):350–356. doi: 10.1021/ic00105a054 [DOI] [Google Scholar]
- 60.Matsumoto H, Yamashita M, Tahara T, et al. Design, synthesis, and evaluation of DNA topoisomerase II-targeted nucleosides. Bioorg Med Chem. 2017;25(15):4133–4144. doi: 10.1016/j.bmc.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 61.Hevener KE, Verstak TA, Lutat KE, Riggsbee DL, Mooney JW. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm Sin B. 2018;8(6):844–861. doi: 10.1016/j.apsb.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aldulaimi OA. Pharmacognosy reviews general overview of phenolics from plant to laboratory, good antibacterials or not. Pharmacogn Rev. 2017;11(22):123–127. doi: 10.4103/phrev.phrev_43_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saritha K, Rajesh A, Manjulatha K. H.Setty O, Yenugu S. Mechanism of antibacterial action of the alcoholic extracts of Hemidesmus indicus (L.) R. Br. ex Schult, Leucas aspera (Wild.), Plumbago zeylanica L., and Tridax procumbens (L.) R. Br. ex Schult. Front Microbiol. 2015;6:577. doi: 10.3389/fmicb.2015.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schär-Zammaretti P, Ubbink J. The cell wall of lactic acid bacteria: surface constituents and macromolecular conformations. Biophys J. 2003;85(6):4076–4092. doi: 10.1016/S0006-3495(03)74820-6 [DOI] [PMC free article] [PubMed] [Google Scholar]