Abstract
There are limited data nationwide on the burden of systemic sclerosis (SSc)-related mortality. We aimed to determine recent trends in SSc and SSc-related pulmonary arterial hypertension (PAH) mortality overall and across population subgroups.
Using death certificate data from the National Center for Health Statistics, we computed the age-adjusted mortality rates of SSc and SSc-SSc−PAH, a lethal prevailing complication, across demographic groups, geographic regions and comorbid cardiorespiratory conditions, and used Joinpoint regression analysis to calculate the average annual percentage change (APC) in mortality.
From 2003 to 2016, 25 175 death records contained a code for SSc. Decedents were predominantly female (81%) and white (73%), with an average age of 66±14 years. The age-adjusted mortality rate decreased by 3% per year from 6.6 in 2003 to 4.3 per 1 000 000 population in 2016. Also, a decreasing trend was found when SSc was stratified by age, sex, race and geographic region. The prevalence of PAH was 23%. The odds of PAH were highest in female and black decedents, and in decedents with concomitant pulmonary embolism, cardiomyopathy and interstitial lung disease (ILD). SSc−PAH mortality remained stable from 2003 to 2008 then decreased by 3% per year from 2008 to 2016. In decedents with SSc−PAH, among all concomitant comorbidities, the mortality rate associated with ILD had the highest increase (average APC 6%, 95% CI 2%−10%).
The mortality rate from SSc decreased from 2003 to 2016. Decreases in mortality rates were similar across demographic groups and geographic regions. SSc−PAH-related mortality remained stable. The death rate for SSc−ILD and concomitant PAH increased during this period.
Short abstract
While SSc-PAH-related mortality remained stable, SSc-ILD and concomitant PAH mortality increased from 2003 to 2016 http://bit.ly/3d9G0pp
Introduction
Systemic sclerosis (SSc) is an autoimmune rheumatological disease characterised by fibrosis and vasculopathy of the dermis and visceral organs. Among subjects with SSc, pulmonary arterial hypertension (PAH) is a major cause of morbidity and mortality [1].
Although recent nationwide trends in SSc incidence and prevalence are not available in the general population, there have been several studies of survival completed over the past 50 years [2, 3]. In the most recent single-centre, observational study the 10-year cumulative survival improved from 53% in the 1970s to 67% in the 1990s [4]. In contrast, Krishnan and Furst [5] found a worsened survival over time by using US death certificate data from 1979 to 1998 and determined that age-adjusted mortality from SSc had increased 36% from 3.3 cases per million in 1979 to 4.5 cases per million in 1998. However, the impact of contributing causes of death, such as cardiopulmonary diseases in SSc, yearly mortality rates were not investigated and, to date, remains unknown at a national level in the USA.
National mortality data can reveal changing trends and patterns in vulnerable populations that can help inform on the importance of introducing screening health interventions, on monitoring the effect of targeted therapies and in promoting confirmatory studies of possible causal associations between risk factors and disease course. Thus, the present study aimed to determine the overall most recent changes in mortality trends attributed to SSc and SSc-related PAH stratified by demographic characteristics, geography and on the presence of comorbid conditions that may influence the clinical course and survival of SSc.
Methods
Annual mortality data for 2003−2016 was obtained from the National Centre for Health Statistics (NCHS). These files contain records of all deaths in the USA (approximately two million annually) that are reported to state vital statistics offices. The mortality data are based on information from all death certificates filed in the 50 states and the District of Columbia. Each death record includes a single underlying cause-of-death (UCOD), up to 20 additional multiple causes-of-death (MCOD) and demographic data. The World Health Organization (WHO) defines the UCOD as “the disease or injury which initiated the train of events leading directly to death, or the circumstances of the accident or violence which produced the fatal injury”. Analyses of SSc mortality based only on the UCOD derived from death certificates may underestimate disease burden [6]. Therefore, the primary aim was to describe temporal trends in SSc-related mortality, defined as death with the international classification of diseases, 10th revision (ICD-10) codes M34.0, M34.1, M34.8 or M34.9 listed among any of the 20 MCODs or as the UCOD. We also describe trends in death related to PAH (ICD-10 I27) among SSc decedents.
The analysis runs from 2003 to 2016, starting when “other secondary pulmonary hypertension” (ICD-10 I27.2), and “pulmonary heart disease, unspecified” (ICD-10 I27.9) were added to the ICD-10 and ending at the last year for which NCHS data were available at the time of the analyses. 2016 was the last year NCHS provided the data.
Statistical analysis
The yearly mortality rate from SSc was calculated stratified by sex, race, age-group, state of residence at the time of death and contributing causes of death. Crude and age-adjusted mortality rates were calculated per 1 000 000 population. Age-adjusted mortality rates were computed by the direct method to the projected year 2000 US population [7]. We used July 1st population projections obtained from the US Census Bureau to determine the denominators for corresponding yearly mortality rates (www.census.gov/en.html).
To evaluate the odds of the most lethal SSc complication, the dichotomic variable PAH (presence or absence of PAH) was compared among SSc subgroups (i.e. demographic characteristics and comorbidities) by logistic regression analysis. A p-value <0.05 was considered statistically significant. Data were analysed using Microsoft Office Excel 2018 16.16.4 (Microsoft Corporation, Redmond, WA, USA) and Stata/IC 14.1 (College Station, TX, USA). Heat maps of age-adjusted US mortality rates were generated using JMP 13.1 (SAS Institute, Cary, NC, USA).
The study period years, in which statistically significant change in mortality occurred (i.e. increased or decreased trend) or remained stable, the annual percentage change, and the average annual percentage change from 2003 to 2016 was computed with the National Cancer Institute's Joinpoint software (version 4.6.0; http://surveillance.cancer.gov/joinpoint). For each annual percentage change, 95% confidence intervals were calculated based on a t-distribution. Allowing as few as two observed time points, in the beginning, ending and middle line segments (including the joinpoints), a maximum of two joinpoints were searched for using the Grid search algorithm and the Bayesian Information Criterion test and an overall α level of 0.05 [8].
All data contained in these database files have been deidentified and are on public record; therefore, Institutional Review Board approval for this study was not required.
Results
All SSc mortality
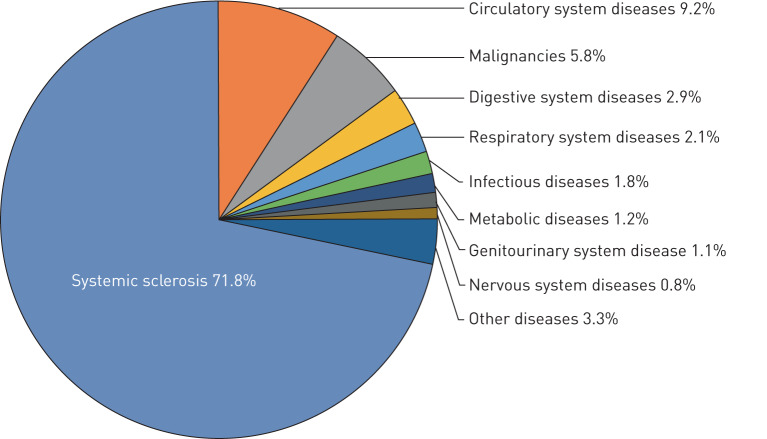
From 2003 to 2016, 25 175 death records contained a diagnostic code for SSc in the USA (table 1). The majority of these deaths (82.5%) occurred in a hospital, with small proportions occurring in the decedent's home (9.7%), in a long-term care facility (4.0%) or in an unknown location (3.7%). SSc was listed as the UCOD in 18 081 (72%) death certificates. The UCOD/MCOD proportion remained stable throughout the study period but tended to decline with increasing age (Figures S1and S2). The three most common UCOD other than SSc itself (72%) were diseases of the circulatory system (9%), malignant neoplastic disorders (6%), and diseases of the digestive system (3%) (figure 1).
TABLE 1.
Number of decedents, age-adjusted mortality rates and odds ratio of pulmonary hypertension by demographic characteristics and contributing cardiopulmonary causes-of-death among scleroderma decedents, 2003–2016
| Variable | Overall (N=25 175) | Age-adjusted mortality rates per 1 000 000 (95% CI) | Systemic sclerosis-PAH (N=5765) | Systemic sclerosis no PAH (N=19 410) | OR (95% CI) | p-value |
| Sex | ||||||
| Male | 4829 (19.2) | 2.05 (1.86 to 2.23) | 885 (15.4) | 3944 (20.3) | 0.71 (0.66 to 0.77) | <0.01 |
| Female | 20 346 (80.8) | 8.76 (8.17 to 9.34) | 4880 (84.6) | 15 466 (79.7) | 1.41 (1.30 to 1.52) | <0.01 |
| Race | ||||||
| White | 18 288 (72.6) | 5.90 (5.48 to 6.33) | 4165 (72.2) | 14 123 (72.8) | 0.97 (0.91 to 1.04) | 0.44 |
| Black | 3700 (14.7) | 5.86 (5.28 to 6.45) | 918 (15.92) | 2782 (14.3) | 1.13 (1.04 to 1.23) | <0.01 |
| Hispanic | 2356 (9.4) | 2.97 (2.67 to 3.26) | 509 (8.8) | 1847 (9.5) | 0.92 (0.83 to 1.02) | 0.11 |
| Race/sex | ||||||
| White men | 3410 (13.5) | 2.17 (1.97 to 2.37) | 652 (11.3) | 2758 (14.2) | 0.77 (0.70 to 0.84) | <0.01 |
| Black men | 877 (3.5) | 2.90 (2.51 to 3.29) | 152 (2.6) | 725 (3.7) | 0.70 (0.58 to 0.83) | <0.01 |
| Hispanic men | 400 (1.6) | 0.98 (0.81 to 1.15) | 56 (0.9) | 344 (1.8) | 0.54 (0.41 to 0.72) | <0.01 |
| White women | 14 878 (59.1) | 9.70 (9.08 to 10.31) | 3513 (60.9) | 11 365 (58.5) | 1.10 (1.04 to 1.17) | 0.01 |
| Black women | 2823 (11.2) | 8.61 (7.74 to 9.48) | 766 (13.3) | 2057 (10.6) | 1.29 (1.18 to 1.41) | <0.01 |
| Hispanic women | 1956 (7.8) | 5.11 (4.65 to 5.56) | 453 (7.9) | 1503 (7.7) | 1.02 (0.91 to 1.13) | 0.78 |
| Region | ||||||
| Northeast | 4873 (19.4) | 6.13 (5.19 to 7.08) | 1169 (20.3) | 3704 (19.1) | 1.08 (1.00 to 1.16) | 0.05 |
| South | 8828 (35.1) | 5.15 (4.83 to 5.47) | 1923 (33.4) | 6905 (35.7) | 0.91 (0.85 to 0.96) | <0.01 |
| Midwest | 5829 (23.1) | 6.09 (5.38 to 6.81) | 1376 (23.9) | 4453 (23.0) | 1.05 (0.98 to 1.13) | 0.14 |
| West | 5587 (22.2) | 5.54 (4.45 to 6.43) | 1286 (22.3) | 4301 (22.2) | 1.01 (0.94 to 1.08) | 0.81 |
| Age-groups years# | ||||||
| <35 | 614 (2.4) | 0.30 (0.27 to 0.33) | 110 (1.9) | 504 (2.6) | 0.73 (0.59 to 0.90) | <0.01 |
| 35–54 | 4621 (18.4) | 3.87 (3.53 to 4.20) | 1085 (18.8) | 3536 (18.2) | 1.04 (0.97 to 1.12) | 0.30 |
| 55–74 | 12 159 (48.3) | 15.30 (13.85 to 16.75) | 3150 (54.6) | 9009 (46.4) | 1.39 (1.31 to 1.48) | <0.01 |
| ≥75 | 7781 (30.9) | 29.79 (28.32 to 31.25) | 1420 (24.6) | 6361 (32.7) | 0.67 (0.63 to 0.72) | <0.01 |
| Contributing causes of death | ||||||
| Respiratory | ||||||
| Interstitial lung disease | 1010 (4.0) | 0.21 (0.20 to 0.22) | 257 (4.5) | 753 (3.9) | 1.16 (1.00 to 1.34) | 0.05 |
| COPD | 629 (2.5) | 0.13 (0.12 to 0.14) | 120 (2.1) | 509 (2.6) | 0.79 (0.65 to 0.97) | 0.02 |
| Pulmonary embolism | 351 (1.4) | 0.07 (0.07 to 0.08) | 102 (1.8) | 249 (1.3) | 1.39 (1.10 to 1.75) | 0.01 |
| Cardiovascular | ||||||
| Ischaemic heart disease | 2991 (11.9) | 0.64 (0.56 to 0.72) | 483 (8.4) | 3577 (13.4) | 0.62 (0.56 to 0.68) | <0.01 |
| Cardiomyopathy | 4028 (16.0) | 0.86 (0.81 to 0.91) | 1368 (23.7) | 2660 (13.7) | 1.96 (1.82 to 2.11) | <0.01 |
| Hypertension | 2626 (10.4) | 0.56 (0.52 to 0.60) | 387 (6.7) | 2239 (11.5) | 0.55 (0.49 to 0.62) | <0.01 |
| Valvular heart disease | 473 (1.9) | 0.10 (0.09 to 0.12) | 126 (2.2) | 347 (1.8) | 1.23 (1.00 to 1.51) | 0.055 |
Data are presented as n (%), unless otherwise stated. PAH: pulmonary arterial hypertension; COPD: chronic obstructive pulmonary disease. COPD (J40–J44); interstitial lung disease (J84.1, J84.9); pulmonary embolism (I26); ischaemic heart disease (I20-I25); cardiomyopathy (I42); hypertension (I10-I16); valvular heart disease (I35). #: Crude rates.
FIGURE 1.
Underlying causes of death among US decedents with systemic sclerosis, 2003−2016. Systemic sclerosis ICD-10 (International Classification of Diseases, 10th edition): M34.0, M34.1, M34.8, M34.9; circulatory system diseases ICD-10: I00-I99; malignancies ICD-10: C00-C96; digestive system diseases ICD-10: K00-K95; respiratory system diseases ICD-10: J00-J99; infectious diseases ICD-10: A00-A99, B00-B99; metabolic diseases ICD-10: E00-E89; genitourinary system diseases ICD-10: N00-N99; nervous system diseases ICD-10: G00-G99.
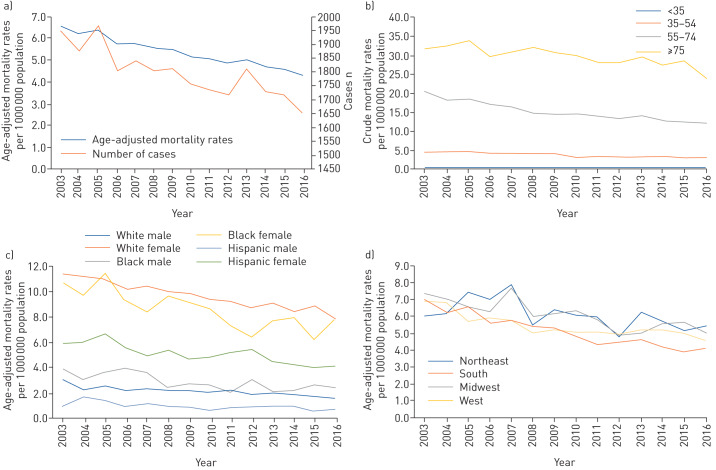
The age-adjusted SSc-related mortality rate decreased steadily from 6.6 per 1 000 000 in 2003 to 4.3 per 1 000 000 population in 2016 (figure 2). No joinpoints were identified in the SSc-related mortality rate trend during 2003−2016. Mortality rates from SSc declined at an average annual rate of 3% (95% CI −3.3% to −2.7%) per year during the study period.
FIGURE 2.
a) Age-adjusted mortality rates of systemic sclerosis per 1 000 000 population and number of cases from 2003 to 2016. b) Crude mortality rates of systemic sclerosis per 1 000 000 population from 2003 to 2016. c) Age-adjusted mortality rates of systemic sclerosis per 1 000 000 population by sex and race from 2003 to 2016. d) Age-adjusted mortality rates of systemic sclerosis per 1 000 000 population by region from 2003 to 2016.
Age-related mortality
The mean±sd age of decedents was 62±14 years for men and 67±14 years for women. Mortality rates increased with age. Decedents aged ≥75 years had the highest mortality rate due to SSc during 2003–2016 (29.8 per 1 000 000 population). In contrast with decedents aged ≥75, all other age groups had a decreased mortality trend during the study period (table 2). Notably, from 2003 to 2016, the magnitude of decline in mortality from SSc was significant in decedents aged 35–54 and 55–74 years (average annual percentage change −3.5%, 95% CI −4.2 to −2.9% and −3.7, 95% CI −4.2 to −3.3%, respectively).
TABLE 2.
Average annual percentage change of age-adjusted mortality rates from systemic sclerosis and systemic sclerosis−related pulmonary hypertension, 2003−2016
| Variable | Systemic sclerosis average annual change % (95% CI) | Systemic sclerosis−pulmonary hypertension average annual change % (95% CI) |
| Total | −3.0 (−3.3 to −2.7)# | 0.1 (−2.3 to 2.6)¶ |
| Sex | ||
| Male | −3.5 (−4.1 to −2.8) | −1.4 (−3.0 to 0.1) |
| Female | −2.7 (−3.0 to −2.4) | 0.5 (−2.1 to 3.2) |
| Race | ||
| White | −2.9 (−3.3 to −2.5) | 0.1 (−2.6 to 2.9) |
| Black | −3.6 (−4.7 to −2.5) | −0.4 (−3.1 to 2.4) |
| Hispanic | −3.5 (−4.7 to −2.2) | −0.9 (−3.1 to 1.3) |
| Race/sex | ||
| White men | −3.4 (−4.2 to −2.5) | −1.5 (−3.6 to 0.6) |
| Black men | −4.1 (−6.3 to −1.9) | −1.2 (−5.6 to 3.4) |
| Hispanic men | −4.1 (−6.9 to −1.1) | −1.5 (−12.2 to 10.3) |
| White women | −2.5 (−2.9 to −2.1) | 0.7 (−2.0 to 3.4) |
| Black women | −3.4 (−4.9 to −1.9) | −0.2 (−3.3 to 3.0) |
| Hispanic women | −3.2 (−4.3 to −2.0) | −0.9 (−3.0 to 1.1) |
| Region | ||
| Northeast | −1.9 (−3.5 to −0.3) | −0.2 (−3.6 to 3.2) |
| South | −4.2 (−4.9 to −3.5) | −1.0 (−3.4 to 1.3) |
| Midwest | −2.8 (−3.9 to −1.6) | 1.0 (−1.8 to 3.9) |
| West | −2.6 (−3.9 to −1.2) | −0.9 (−3.4 to 1.7) |
| Age-groups years | ||
| <35 | −0.8 (−3.4 to 1.8) | −1.4 (−7.1 to 4.7) |
| 35–54 | −3.5 (−4.2 to −2.9) | −1.7 (−4.6 to 1.4) |
| 55–74 | −3.7 (−4.2 to −3.3) | −1.2 (−2.7 to 0.3) |
| ≥75 | −1.1 (−2.7 to 0.6) | 3.6 (0.9 to 6.3) |
| Contributing causes of death | ||
| Respiratory | ||
| Interstitial lung disease | 1.2 (−0.2 to 2.6) | 6.0 (2.0 to 10.2) |
| COPD | −1.7 (−3.4 to 0) | 2.5 (−2.4 to 7.7) |
| Pulmonary embolism | −0.2 (−2.9 to 2.6) | −1.5 (−5.5 to 2.7) |
| Cardiovascular | ||
| Ischaemic heart disease | −4.9 (−5.7 to −4.1) | 0.2 (−2.9 to 3.4) |
| Cardiomyopathy | −1.7 (−2.7 to −0.8) | 2.7 (1.4 to 4.1) |
| Hypertension | −2.2 (−3.3 to −1.2) | 3.1 (0.6 to 5.7) |
| Valvular heart disease | −2.8 (−5.8 to 0.3) | 3.6 (−1.9 to 9.4) |
COPD: chronic obstructive pulmonary disease. #: no joinpoint identified.; ¶: one joinpoint identified at 2008: 2003–2008, APC 5.2% (95% CI −0.8 to 11.5); 2008–2016, APC −3.0% (−5.6 to −0.2%).
Sex and race
The age−adjusted SSc-related mortality rate decreased among women (from 10.3 per 1 000 000 in 2003 to 7.2 per 1 000 000 women in 2016) and men (from 2.7 per 1 000 000 in 2003 to 1.5 per 1 000 000 men in 2016). For any given year, women had higher age-adjusted mortality due to SSc compared with men by a 4:1 ratio. Overall, the highest age-adjusted mortality rate was seen in white (9.7 per 1 000 000 population) and black (8.6 per 1 000 000 population) women (table 1). All sex/racial groups had similar significant declines in the average annual percentage change in mortality rates during 2003–2016 (table 2).
Geographic changes in mortality
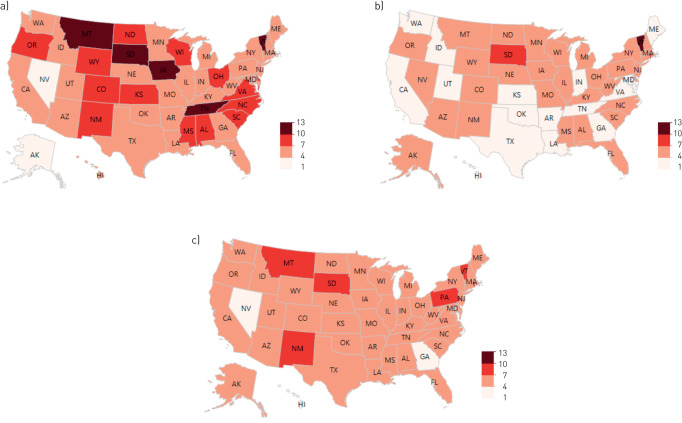
The Midwestern USA had the highest age-adjusted mortality from SSc during the study period (table 1). From 2003 to 2016, age-adjusted mortality uniformly decreased across US regions (table 2) and states (fig. 3). The three states with the highest average mortality rate were South Dakota (8.9 per 1 000 000 population), Montana (8.8 per 1 000 000 population) and Vermont (8.8 per 1 000 000 population).
FIGURE 3.
a) Age-adjusted mortality rates of systemic sclerosis per 1 000 000 population in 2003. b) Age-adjusted mortality rates of systemic sclerosis per 1 000 000 population in 2016. c) Average age-adjusted mortality rates of systemic sclerosis per 1 000 000 population from 2003 to 2016.
The impact of PAH
Among SSc decedents, the prevalence of PAH was 23% (n=5765). The odds of PAH were higher in female and black decedents, in the 55–74 age groups and decedents with pulmonary embolism, cardiomyopathy and interstitial lung disease (table 1).
Among subjects with SSc−PAH, joinpoint analysis showed that, in contrast to other respiratory or cardiovascular concomitant contributing causes-of-death, the mortality rate associated with interstitial lung disease (ILD) had the highest average annual percentage change increased during the study period (6%, 95% CI 2 to 10%).
The annual percentage change of SSc-related PAH mortality remained stable between 2003 and 2008 then decreased by 3% from 2008 to 2016. However, when the annual percentage change was averaged over the 2003–2016 period, there was no significant change (average annual percentage change 0.1%, 95% CI −2.3 to 2.6%, table 2).
Discussion
The most important finding emerging from this nationwide epidemiological study was the significant linear decrease in age-adjusted SSc-related mortality by approximately 3% per year from 2003 to 2016. Also, a decreasing trend was found when SSc was stratified by sex, race and geographic region. Deaths in SSc subjects aged 35−74 years decreased while death among subjects older than 75 years remained stable, supporting previous studies reporting that subjects with SSc might be living longer. Similarly, the mortality rate for PAH remained stable over this period, while an increasing trend was observed in decedents aged ≥75 years.
Despite the decreasing trends in overall SSc-related mortality, like other investigators, we also observed increased disease burden in female and black decedents and in older age groups [9, 10]. In contrast with our findings, using the same database Khrisnan and Furst [5] observed a rise in SSc mortality rates from 1978 to 1998. Their study was the first to use US death certificates and report SSc mortality that varied between races and sex: mortality rates were highest among female and black decedents. Although they observed a higher death rate in black compared with white decendents (6.5 per 1 000 000 versus 3.6 per 1 000 000, respectively), our data suggest that this difference has levelled off from 2003 to 2016 (table 2). This novel finding is further supported by the fact that the southern USA (with a 55% black population), experienced the largest drop in the average age-adjusted mortality (average annual percentage change −4.2%; 95% CI −4.9 to −3.5%) which may reflect long-term declines in SSc health disparities and gaps in access to care and treatment between black and white patients. Also, the odds of PAH-related mortality between black and white patients demonstrated similar trends. Further study is needed to clarify whether the ongoing interracial differences in mortality arise from different genetic predisposition for disease or differences in behavioural or environmental risk factors.
Our data mirrors other previous nationwide mortality analyses [11], in that the UCOD/MCOD ratio tended to decline with increasing age. Given the increased incidence of comorbid conditions with ageing, improve life expectancy compare to generations past, and the development and adoption of more effective treatments, comorbid complications involving SSc are expected to become more frequent. Thus, from a public health perspective, data enhancing our understanding of the interaction of comorbidities or complications and SSc will be increasingly relevant.
The decrease in the overall age-adjusted mortality over time may be attributed to improvements in primary and secondary coronary heart prevention as we saw ischaemic heart disease-related mortality among decedents with SSc decline with an average annual percentage change of −4.9%; 95% CI −5.7 to −4.1%.
Pulmonary vascular disease in commonly seen in SSc and leads to an increased mortality [12]. In SSc, PAH can be isolated (WHO group1), secondary to left heart disease (WHO group 2), due to severe ILD and/or chronic hypoxia (WHO group 3), or pulmonary veno-occlusive disease (WHO Group 1') [13]. In our study, only PAH with concomitant left heart disease (i.e. heart failure/cardiomyopathy) or pulmonary thromboembolic disease had increased odds of PAH−related death (table 1). We observed that overall SSc−PAH mortality rates remained stable during the 2003−2008 period and significantly decreased during the 2008–2016 period similar to other studies showing no change in survival following the introduction of PAH-directed therapies in 2002 [14, 15]. This finding also complements a recent large US multi-centre prospective cohort of subjects with SSc−PAH that demonstrated higher survival rates compared with older cohorts studies [16]. The decrease in the SSc−PAH mortality in recent years may reflect improved longevity due to increased availability of approved PAH-specific therapies, increased awareness, screening and diagnosis of PAH and increased use of combination pulmonary vasodilator therapy [17].
ILD was not a leading cause of mortality in our study and trends of SSc−ILD related mortality remained stable over the study period (average annual percentage change 1.2%; 95% CI −0.2 to 2.6%). This may be mainly because in contrast to ILDs associated with other underlying rheumatological diseases, the risk stratification and treatment for SSc−ILD is much better defined [1]. Indeed, to date, SSc−ILD is the only autoimmune rheumatological-related ILD that has been studied in phase III pharmacological treatment trials [18–20]. However, consistent with the results of other studies [21–23], the observed increase in mortality rate over time of subjects with SSc−ILD–PAH represents a prevailing challenge and an area of future research. Furthermore, we also observed that the odds of death associated with an ILD was 1.2-fold higher in SSc–PAH than in SSc without PAH (table 1). Considerably more work is needed to evaluate the effectiveness and safety of targeted therapies in this subgroup.
The major strength of our study lies in the generalisability of examining national-level data and capturing longitudinal mortality trends among the US population at large. In addition to the large sample size for answering the study question, sensitivity analyses using a different approach (i.e. UCOD) showed consistent results, suggesting the robustness of the study findings. Furthermore, despite differences in study designs, estimates of SSc from other studies are in the range of the results of the present study [24]. However, there are limitations. First, miscoding and under-reporting of SSc, SSc−ILD and SSc−PAH on the death certificate by certifying physicians is possible. However, decreasing mortality trends have occurred in the context of SSc diagnostic and treatment guidelines [25, 26]. It is unlikely that declining mortality trends would be largely due to reduced recognition and diagnosis of the disease. Furthermore, although recent studies on the incidence and prevalence of SSc in the USA are not available, prior estimates suggest that disease frequency has increased or remained stable over time. Secondly, despite the potential misclassification of contributing causes of death and not been able to precisely distinguish if the contributing causes of death was due to SSc (e.g. PAH associated to SSc and/or to concurrent ILD), the size of the study population, and particularly that of those identified with PAH was likely sufficient to detect excess risk among subgroups. Finally, we had no way to assess the fidelity of the diagnoses in this dataset; thus, results should be considered best estimates.
Despite these limitations, the study suggests that SSc-related mortality is decreasing in the USA. The finding that PAH, particularly in black women, and cardiovascular complications are the leading contributing causes of SSc death have significant implications for planning the future allocation of health resources. Although mortality trends in SSc–PAH appear to have decreased during 2008−2016, the increased mortality rate among those with PAH and concomitant ILD in the past decade highlights the importance of continued efforts aimed at the early detection and management of this complication.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: P. Ratanawatkul has nothing to disclose.
Conflict of interest: J.J. Solomon has nothing to disclose.
Conflict of interest: D. Kim has nothing to disclose.
Conflict of interest: M.P. George has nothing to disclose.
Conflict of interest: M. McGibbon has nothing to disclose.
Conflict of interest: K. Demorulle has nothing to disclose.
Conflict of interest: M. Maleki-Fischbach has nothing to disclose.
Conflict of interest: I. Amigues has nothing to disclose.
Conflict of interest: L. Kastsianok has nothing to disclose.
Conflict of interest: E.R. Fernández Pérez has nothing to disclose.
References
- 1.Solomon JJ, Olson AL, Fischer A, et al. . Scleroderma lung disease. Eur Respir Rev 2013; 22: 6–19. doi: 10.1183/09059180.00005512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayes MD, Lacey JV, Beebe-Dimmer J, et al. . Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum 2003; 48: 2246–2255. doi: 10.1002/art.11073 [DOI] [PubMed] [Google Scholar]
- 3.Silman AJ. Epidemiology of scleroderma. Curr Opin Rheumatol 1991; 3: 967–972. doi: 10.1097/00002281-199112000-00012 [DOI] [PubMed] [Google Scholar]
- 4.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 2007; 66: 940–944. doi: 10.1136/ard.2006.066068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan E, Furst DE. Systemic sclerosis mortality in the United States: 1979–1998. Eur J Epidemiol 2005; 20: 855–861. doi: 10.1007/s10654-005-2210-5 [DOI] [PubMed] [Google Scholar]
- 6.Fedeli U, Zoppini G, Goldoni CA, et al. . Multiple causes of death analysis of chronic diseases: the example of diabetes. Popul Health Metr 2015; 13: 21. doi: 10.1186/s12963-015-0056-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2000 Stat Notes 2001; 20: 1–9. [PubMed] [Google Scholar]
- 8.Kim HJ, Fay MP, Feuer EJ, et al. . Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335–351. doi: [DOI] [PubMed] [Google Scholar]
- 9.Gelber AC, Manno RL, Shah AA, et al. . Race and association with disease manifestations and mortality in scleroderma: a 20-year experience at the Johns Hopkins Scleroderma Center and review of the literature. Medicine (Baltimore) 2013; 92: 191–205. doi: 10.1097/MD.0b013e31829be125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerneis S, Boelle PY, Grais RF, et al. . Mortality trends in systemic sclerosis in France and USA, 1980–1998: an age-period-cohort analysis. Eur J Epidemiol 2010; 25: 55–61. doi: 10.1007/s10654-009-9403-2 [DOI] [PubMed] [Google Scholar]
- 11.Jamilloux Y, Maucort-Boulch D, Kerever S, et al. . Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J 2016; 48: 1700–1709. doi: 10.1183/13993003.00457-2016 [DOI] [PubMed] [Google Scholar]
- 12.Hinchcliff M, Fischer A, Schiopu E, et al. . Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS): baseline characteristics and description of study population. J Rheumatol 2011; 38: 2172–2179. doi: 10.3899/jrheum.101243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonneau G, Montani D, Celermajer DS, et al. . Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefevre G, Dauchet L, Hachulla E, et al. . Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum 2013; 65: 2412–2423. doi: 10.1002/art.38029 [DOI] [PubMed] [Google Scholar]
- 15.Rubenfire M, Huffman MD, Krishnan S, et al. . Survival in systemic sclerosis with pulmonary arterial hypertension has not improved in the modern era. Chest 2013; 144: 1282–1290. doi: 10.1378/chest.12-0653 [DOI] [PubMed] [Google Scholar]
- 16.Kolstad KD, Li S, Steen V, et al. . Long-term outcomes in systemic sclerosis-associated pulmonary arterial hypertension from the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma Registry (PHAROS). Chest 2018; 154: 862–871. doi: 10.1016/j.chest.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condliffe R, Kiely DG, Peacock AJ, et al. . Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. doi: 10.1164/rccm.200806-953OC [DOI] [PubMed] [Google Scholar]
- 18.Tashkin DP, Elashoff R, Clements PJ, et al. . Scleroderma Lung Study Research G. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006; 354: 2655–2666. doi: 10.1056/NEJMoa055120 [DOI] [PubMed] [Google Scholar]
- 19.Tashkin DP, Roth MD, Clements PJ, et al. . Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016; 4: 708–719. doi: 10.1016/S2213-2600(16)30152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Distler O, Highland KB, Gahlemann M, et al. . Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–2528. doi: 10.1056/NEJMoa1903076 [DOI] [PubMed] [Google Scholar]
- 21.Mathai SC, Hummers LK, Champion HC, et al. . Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum 2009; 60: 569–577. doi: 10.1002/art.24267 [DOI] [PubMed] [Google Scholar]
- 22.Launay D, Humbert M, Berezne A, et al. . Clinical characteristics and survival in systemic sclerosis-related pulmonary hypertension associated with interstitial lung disease. Chest 2011; 140: 1016–1024. doi: 10.1378/chest.10-2473 [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Gupta A, Rehman S, et al. . Pulmonary veno-occlusive disease is highly prevalent in scleroderma patients undergoing lung transplantation. ERJ Open Res 2019; 5: 00168-2018. doi: 10.1183/23120541.00168-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio-Rivas M, Royo C, Simeon CP, et al. . Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014; 44: 208–219. doi: 10.1016/j.semarthrit.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 25.van den Hoogen F, Khanna D, Fransen J, et al. . 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 2013; 72: 1747–1755. doi: 10.1136/annrheumdis-2013-204424 [DOI] [PubMed] [Google Scholar]
- 26.Smith V, Scire CA, Talarico R, et al. . Systemic sclerosis: state of the art on clinical practice guidelines. RMD Open 2018; 4: e000782. doi: 10.1136/rmdopen-2018-000782 [DOI] [PMC free article] [PubMed] [Google Scholar]