Abstract
Patients with short telomeres and interstitial lung disease may have decreased chronic lung allograft dysfunction (CLAD)-free survival following lung transplantation. The relationship between post-transplant telomere length and outcomes following lung transplantation has not been characterised among all recipients, regardless of native lung disease.
This was a single-centre prospective cohort study. Consenting transplant recipients had their telomere length measured using quantitative real-time PCR assays on peripheral blood collected at the time of surveillance bronchoscopy. We assessed the association between early post-transplant telomere length (as measured in the first 100 days) and CLAD-free survival, time to clinically significant leukopenia, cytomegalovirus (CMV) viraemia, chronic kidney disease, and acute cellular rejection. We also assessed the association between rate of telomere shortening and CLAD-free survival.
Telomere lengths were available for 98 out of 215 (45.6%) recipients who underwent lung transplant during the study period (median measurement per patient=2 (interquartile range, 1–3)). Shorter telomere length was associated with decreased CLAD-free survival (hazard ratio (HR)=1.24; 95% CI=1.03–1.48; p=0.02), leukopenia requiring granulocyte colony-stimulating factor (HR=1.17, 95% CI=1.01–1.35, p=0.03), and CMV viraemia among CMV-mismatch recipients (HR=4.04, 95% CI=1.05–15.5, p=0.04). Telomere length was not associated with acute cellular rejection or chronic kidney disease. Recipients with more rapid loss in telomere length (defined as the highest tertile of telomere shortening) did not have worse subsequent CLAD-free survival than those without rapid loss (HR=1.38, 95% CI=0.27–7.01, p=0.70).
Shorter early post-transplant telomere length is associated with decreased CLAD-free survival and clinically significant leukopenia in lung transplant recipients, regardless of native lung disease.
Short abstract
Shorter recipient telomere length following lung transplantation is associated with clinically significant leukopenia and decreased chronic lung allograft dysfunction-free survival https://bit.ly/2ytymXc
Introduction
Telomeres are repetitive nucleotide sequences that cap linear chromosomes. The normal telomere shortening that occurs with cellular division can eventually trigger cell senescence or apoptosis [1]. Individuals with short telomeres, with or without associated telomere-related mutations, are more susceptible to a range of premature organ dysfunctions, including interstitial lung disease (ILD) [2–6]. The most definitive treatment for ILD and other advanced lung diseases that progress despite standard therapy is lung transplantation.
Long-term survival following lung transplantation remains limited because of chronic lung allograft dysfunction (CLAD) [7]. While post-transplant events such as primary graft dysfunction or cytomegalovirus (CMV) pneumonitis are associated with CLAD, there are limited biomarkers to predict which patients are at highest risk for CLAD development [8–10]. Several case series and retrospective cohort studies have suggested that individuals with short telomeres and ILD have worse outcomes following lung transplantation, including decreased CLAD-free survival [11–14]. The mechanisms behind this association are unclear and may be related to an inability to tolerate immunosuppression because of limited bone-marrow reserve [11, 15]. It is unknown whether post-transplant telomere length and/or rate of telomere attrition are associated with worse outcomes or whether it is only pre-transplant short telomeres that are predictive. It is also unknown whether telomere length is associated with post-transplant course in diseases other than ILD.
The objective of this study was to assess the relationship between early post-transplant telomere length as well as the rate of post-transplant telomere attrition and outcomes following lung transplantation, including CLAD-free survival, for all recipients, regardless of native lung disease.
Methods
Study population
This was a single-centre prospective cohort study. All patients who underwent lung transplantation between June 1, 2014 and November 1, 2018 were eligible to participate. Recipients underwent solumedrol and basiliximab induction and were maintained on a combination of a calcineurin inhibitor (most commonly tacrolimus), a cell cycle inhibitor (most commonly mycophenolate mofetil), and prednisone. Recipients who developed leukopenia, defined as white blood cell count <3.0 cells·µL−1 first had a reduction in the dose of their cell cycle inhibitor or cessation of antiviral prophylaxis (where appropriative). Recipients who developed absolute neutrophil count <1000 cells·µL−1 were treated with granulocyte colony-stimulating factor (G-CSF).
Patients who provided written informed consent had blood collected during the first post-transplant year at the time of routine surveillance outpatient bronchoscopies (typically 1, 3, 6, and 12 months). Patients who did not provide informed consent were not eligible to participate. The Institutional Review Board approved this study.
Telomere length measurement
Relative telomere length was measured from recipient peripheral blood using a high throughput monoplex real-time quantitative (qPCR) assay [16]. qPCR was chosen to measure telomere length, in part, because it treats telomere length as a continuous variable (as opposed to an absolute cut-off such as <10th percentile of telomere length for age). This allows for detection of clinically relevant observations that may not be apparent in a cohort where telomere length is treated as a categorical variable.
The qPCR assay calculates the ratio between a recipient's telomeric repeat copy number (T) and a single copy reference gene (36B4) (S) by subtracting the average 36B4 threshold cycle value from the average telomere threshold cycle value (see Supplemental Material for further details). Subtracting the T/S ratio of a reference sample, consisting of a pooled genomic DNA sample, from the recipient's T/S ratio allows calculation of the relative T/S ratio. In this way, the relative T/S is defined in relation to a population reference curve. Final measurements are exponentiated to assure normality [17]. So, for example, a patient with an exponentiated telomere length of 1.2 would have a telomere length 20% longer than the pooled population reference sample. A telomere length of 0.8 would be 20% shorter than the pooled reference sample.
Our primary focus was on recipients with available early post-transplant samples. Early was defined as within the first 100 days post-transplant, with this time point chosen to avoid confounding by the effects of post-transplant immunosuppression, which is associated with telomere attrition, and to avoid enriching the cohort for cases in which outcomes (for example, CMV viraemia) occurred before telomere length measurement [18–20]. Recipients with early post-transplant samples were divided into those with long (third tertile) and short (first tertile) telomeres as previously described [21, 22]. For recipients with two or more samples available, change in telomere length over time was calculated. Recipients were then divided into those with the highest tertile telomere loss over time versus recipients in the other tertiles.
Study outcomes
The primary outcome was CLAD-free survival following transplant. CLAD was defined according to international consensus guidelines [23]. Secondary outcomes included time to: 1) leukopenia requiring cessation of immunosuppression or antiviral medication; 2) leukopenia requiring G-CSF; 3) chronic kidney disease (CKD) stage 3B or higher; 4) liver injury, defined as alanine aminotransferase or aspartate aminotransferase greater than three times the upper limit of normal (129 and 183 U·L−1, respectively); 5) CMV viraemia >1000 copies·mL−1 with or without end-organ involvement; 6) acute cellular rejection (ACR) of any grade. Additional outcomes included ACR score (defined as the sum of the A grades on each biopsy divided by the total number of biopsies) and airway rejection (BCR) score (defined as the sum of the B grades on each biopsy divided by the total number of biopsies). Recipients for whom the secondary outcome occurred before telomere length was measured were excluded from that particular analysis.
Statistical analysis
Selected demographic and clinical variables were analysed using descriptive statistics. For our primary outcome analysis, we used a Cox proportional hazard model to assess the relationship between early post-transplant telomere length and CLAD-free survival. For the purposes of this analysis, we treated telomere length as a continuous variable (defined as the percentage that the patient's telomere length was above or below the pooled reference sample in 5% increments) and separately, as a categorical variable (longest and shortest tertile of telomere length). We included other covariates associated with CLAD-free survival in an adjusted model, including age, native lung disease, lung allocation score (LAS), bilateral transplant status, and ACR score. We also used unadjusted Cox proportional hazard models to assess the relationship between early post-transplant telomere length and various secondary outcomes. The proportional hazard assumptions were confirmed using Schoenfeld residuals.
In a sensitivity analysis, we included the first telomere length available for all patients (not just those taken in the first 100 days) and used Cox proportional hazard models to assess hazard of subsequent CLAD-free survival (from the time of last telomere length measurement). Finally, for patients with two or more samples, we calculated absolute change in telomere length and change in telomere length over time. We then compared subsequent CLAD-free survival (from the time of last telomere length measurement) between those in the bottom tertile of telomere attrition (fastest telomere loss) and other recipients using a Cox proportional hazard model. All analyses were performed using Stata version 15 (Stata Corp, College Station, TX, USA).
Results
Study cohort
There were 215 patients who underwent lung transplant during the study enrolment period, 98 (45.6%) of whom consented to participate and had at least one blood sample collected that was of sufficient quality for telomere length analysis (figure 1). There were no significant differences in age, LAS at transplant, or native lung disease between those who did and did not consent. Characteristics of the included recipients are listed in table 1. The median number of telomere length measurements per recipient was 2 (interquartile range (IQR)=1–3). The median follow-up time was 2.2 years (IQR=1.3–2.7 years). During the follow-up time 17 (24.6%) recipients developed CLAD stage 1 or higher and 4 (5.8%) died. Additional outcome events are listed in table 2.
FIGURE 1.
Study cohort. There were 37 recipients who had both an early telomere length measurement and two or more telomere lengths measured post-transplant.
TABLE 1.
Study cohort characteristics
| Patients n | 69 |
| Age years | 57.9 (50.1–64.5) |
| Female | 23 (33.3) |
| Native lung disease | |
| Interstitial lung disease | 32 (46.4) |
| Chronic obstructive pulmonary disease | 18 (26.1) |
| Cystic fibrosis | 13 (18.8) |
| Other | 6 (8.6) |
| Lung allocation score at the time of transplantation | 37.3 (33.5–48.5) |
| Wait time days | 181 (38–416) |
| Longest ischaemic time min | 266 (214–317) |
| Cardiopulmonary bypass | 62 (89.9) |
| Cytomegalovirus donor positive, recipient negative | 27 (39.1) |
| Bilateral transplant | 57 (82.6) |
| Mechanical ventilation >5 d post-transplant | 8 (11.6) |
| Length of index hospitalisation days | 15 (13–20) |
| Follow-up time years | 2.2 (1.3–2.7) |
Data are presented as median (interquartile range) or n (%), unless otherwise stated.
TABLE 2.
Study cohort outcomes
| Patients n | 69 |
| Leukopenia requiring adjustment of immunosuppression or antivirals | 32 (46.4) |
| Leukopenia requiring G-CSF | 17 (24.6) |
| Chronic kidney disease stage 3B or higher | 27 (39.1) |
| CMV viraemia or end-organ damage | 11 (15.9) |
| ACR (any) | 31 (44.9) |
| ACR score | 0 (0–0.50) |
| BCR score | 0 (0–0) |
| Chronic lung allograft dysfunction (stage 1 or higher) | 17 (24.6) |
| Died | 4 (5.8) |
Data are presented as median (interquartile range) or n (%), unless otherwise stated. ACR: acute cellular rejection; BCR: airway rejection; CMV: cytomegalovirus; G-CSF: granulocyte colony-stimulating factor
Early post-transplant telomere length and CLAD-free survival
Among included recipients, 56 (81.1%) had telomere length measured within the first 100 days post-transplant (median time to measurement 43 days (IQR=33–78 days)) (figure 1). The median recipient telomere length was 0.87 (IQR=0.76–0.97) or 14% shorter than the reference pool. Recipients in the shortest tertile of telomere length (68.4% of whom had ILD) had a mean relative telomere length=0.68 (range 0.38–0.78) (table S1). Those in the longest tertile of telomere length had a mean relative telomere length=1.06 (range 0.94–1.31). Age and early post-transplant telomere length were strongly and inversely correlated (r=−0.57, p<0.001). Correlation was noted for both non-ILD (r=−0.72, p<0.001) and ILD patients (r=−0.35, p=0.07).
Early telomere length, treated as a continuous variable, was associated with decreased CLAD-free survival on unadjusted analysis (hazard ratio (HR)=1.24, 95% CI=1.07–1.44, p=0.004) (table 3). By way of interpretation, this indicates that a 5% decrease in telomere length compared to the population reference pool was associated with a 24% worse hazard of CLAD-free survival. This relationship persisted after adjusting for age, native lung disease, LAS score, bilateral transplant status, and ACR score (HR=1.24, 95% CI=1.04–1.48 p=0.02). There was improved adjusted CLAD-free survival in recipients in the longest tertile of telomere length compared to the other tertiles (HR=0.08, 95% CI=0.007–0.944, p=0.04). Finally, when restricting our analysis to cases of definitive stage 1 or higher CLAD, as characterised in the new CLAD consensus guidelines, early post-transplant telomere length was associated with worse definitive CLAD-free survival on unadjusted (HR=1.20, 95% CI=1.02–1.40, p=0.02) and adjusted analysis (HR=1.21, 95% CI=1.01–1.44, p=0.03) (Supplemental Table 2) [24].
TABLE 3.
Relationship between early post-transplant telomere length# and time to specified post-transplant outcomes (n=56)
| Hazard ratio | 95% CI | p-value | |
| Leukopenia requiring cessation of immunosuppression or antiviral prophylaxis medications | |||
| Telomere length¶ | 1.09 | 0.98–1.22 | 0.13 |
| Shortest telomeres+ | 1.67 | 0.76–3.69 | 0.20 |
| Longest telomeres§ | 0.60 | 0.24–1.50 | 0.27 |
| Leukopenia requiring granulocyte colony-stimulating factor | |||
| Telomere length | 1.17 | 1.01–1.35 | 0.03 |
| Shortest telomeres | 2.97 | 1.03–8.55 | 0.04 |
| Longest telomeres | 0.55 | 0.15–1.97 | 0.36 |
| Chronic kidney disease stage 3B or higher | |||
| Telomere length | 1.02 | 0.92–1.13 | 0.68 |
| Shortest telomeres | 0.61 | 0.24–1.54 | 0.30 |
| Longest telomeres | 0.86 | 0.37–2.02 | 0.74 |
| Cytomegalovirus viraemia or end-organ damage | |||
| Telomere length | 1.13 | 0.93–1.37 | 0.19 |
| Shortest telomeres | 2.43 | 0.70–8.41 | 0.16 |
| Longest telomeres | 0.45 | 0.09–2.13 | 0.32 |
| Acute cellular rejection (any) | |||
| Telomere length | 1.00 | 0.90–1.11 | 0.96 |
| Shortest telomeres | 0.75 | 0.31–1.83 | 0.54 |
| Longest telomeres | 1.15 | 0.50–2.64 | 0.74 |
| Chronic lung allograft dysfunction-free survival (unadjusted) | |||
| Telomere length | 1.24 | 1.07–1.44 | 0.004 |
| Shortest telomeres | 2.34 | 0.75–7.28 | 0.14 |
| Longest telomeres | 0.15 | 0.02–1.16 | 0.07 |
| Chronic lung allograft dysfunction-free survival (adjusted for age, native lung disease, lung allocation score, bilateral transplant status, and acute cellular rejection score) | |||
| Telomere length | 1.24 | 1.04–1.48 | 0.02 |
| Shortest telomeres | 1.79 | 0.50–6.43 | 0.37 |
| Longest telomeres | 0.08 | 0.007–0.944 | 0.04 |
#: As measured within the first 100 days of transplant; ¶: Based on the percent above or below the telomere length of the reference sample; +: Defined as shortest tertile of telomere length (mean relative telomere length 0.68, overall range 0.38–0.78); §: Defined as longest tertile of telomere length (mean relative telomere length 1.06, overall range 0.94–1.31).
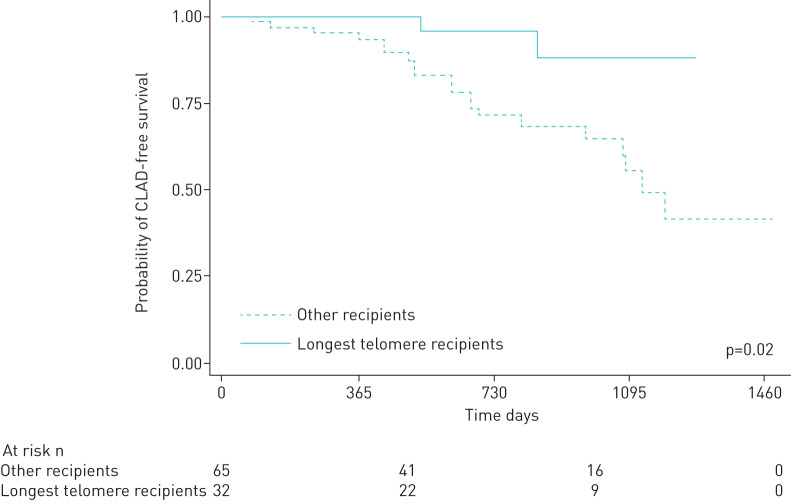
In the sensitivity analysis, the relationship between telomere length and subsequent CLAD-free survival persisted when including all recipients, regardless of time in the first year that telomere length was measured (HR=1.15, 95% CI=1.02–1.29, p=0.02). Among all recipients, those with longer telomere length had improved CLAD-free survival (HR=0.18, 95% CI=0.04–0.79, p=0.02) (figure 2).
FIGURE 2.
Relationship between chronic lung allograft dysfunction-free survival following telomere length measurement in recipients in the longest tertile of telomere length compared to other recipients (hazard ratio=0.18, 95% confidence interval=0.04–0.79, p=0.02).
Early post-transplant telomere length and other outcomes
Early post-transplant telomere length, treated as a continuous variable, was associated with leukopenia requiring G-CSF (HR=1.17, 95% CI=1.03–8.55, p=0.03). By way of interpretation, this indicates that a 5% decrease in telomere length compared to the population reference pool was associated with a 17% increase in hazard for leukopenia requiring G-CSF. This was particularly true for recipients in the shortest tertile of telomere length (HR=2.97, 95% CI=1.02–8.57, p=0.04). Including age as a covariate in this analysis did not significantly alter the relationship between shortest telomeres and leukopenia requiring G-CSF (HR=3.17, 95% CI=1.00–10.1, p=0.05). Telomere length (treated as a continuous or categorical variable) was not associated with CKD 3B or higher, CMV viraemia, or ACR. Among the 26 CMV-mismatched recipients, those with the shortest tertile of telomere length had an increased hazard for viraemia (HR=4.04, 95% CI=1.05–15.5, p=0.04).
There were insufficient numbers of patients started on dialysis in the cohort to calculate the relationship between telomere length and time to dialysis. There were also insufficient numbers of patients with significantly elevated transaminases to calculate the relationship between telomere length and transaminitis. There was no relationship between ACR score (r=−0.04, p=0.75) or BCR score (−0.15, p=0.25) and telomere length treated as a continuous variable. There was no relationship between ACR score (p=0.89) or BCR score (p=0.48) and shortest telomere length. There was no relationship between ACR score (p=0.41) or BCR score (p=0.30) and longest telomere length.
Change in telomere length over time
There were 50 patients who had two or more telomere length measurements available post-transplant (median measurements=2, IQR 2–3). One patient developed CLAD before the second telomere length was measured and was excluded from the attrition analysis. The median change in telomere length per post-transplant month was −0.003 (or a 0.3% decline in relative telomere length) (IQR=−0.014 to 0.005). The median change in telomere length in the tertile of the most rapid decliners was 2.5% per month (IQR=1.6% to 3.4%). Early post-transplant length was strongly correlated with subsequent post-transplant attrition, such that recipients with longer telomeres had more rapid attrition (r=−0.38, p=0.02). The two patients with the largest increase in telomere length were both being treated for a Pseudomonas aeruginosa pulmonary infection at the time that the second sample was acquired.
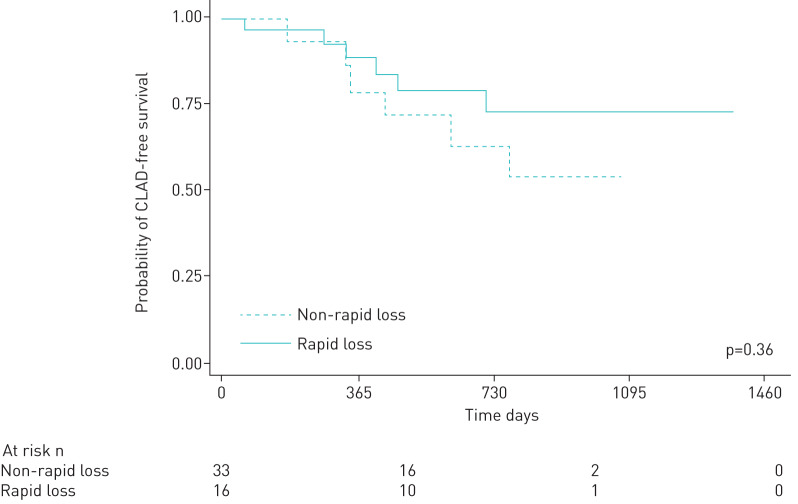
Patients with more rapid loss in telomere length (defined as the highest tertile of loss of telomere length over time post-transplant) did not have worse subsequent CLAD-free survival on unadjusted (HR 1.70, 95% CI=0.55–5.28, p=0.36) or adjusted analysis (HR=1.38, 95% CI=0.27–7.01, p=0.70) (figure 3).
FIGURE 3.
Relationship between chronic lung allograft dysfunction-free survival in patients with and without more rapid rates of telomere loss in the first post-transplant year (unadjusted hazard ratio=1.70, 95% confidence interval=0.55–5.28, p=0.36) or adjusted analysis (hazard ratio=1.38, 95% confidence interval=0.27–7.01, p=0.70).
Discussion
Although previous studies suggest that pre-lung transplant telomere length is associated with worse post-transplant outcomes, particularly decreased CLAD-free survival, the relationship between post-transplant telomere length and transplant-related outcomes has not been well-characterised, particularly across native lung disease groups [11–14]. In this single-centre prospective cohort study, we found that early post-transplant length was associated with clinically significant leukopenia and decreased CLAD-free survival, independently of age or underlying lung disease.
As in other nontransplant cohort studies, telomere length was strongly correlated with patient age [21, 25]. In this context, the median telomere length of our cohort (0.86) was not dramatically lower than what would be expected given the median age (58 years). Because previous studies on telomeres in transplant patients have used different measures of telomere length (e.g. flow cytometry fluorescence in situ hybridisation) [12], emphasised telomere-related mutations rather than telomere length [14], or focused on donor rather than recipient telomere length [26], it is difficult to directly compare our study population with existing literature. The fact that telomere length was associated with significant leukopenia, however, suggests that measurements in our cohort are an accurate reflection of clinically meaningful bone-marrow reserve.
The relationship between telomere length and/or telomere-related mutations and CLAD has been explored previously [11–14]. Our study expands on this literature in several ways. First, we did not restrict our analysis to those with very short telomeres (or short telomere syndromes). By treating telomere length as a continuous variable, we were able to demonstrate that telomere length is associated with decreased CLAD-free survival beyond recipients with short telomeres or telomere-related mutations [14]. Second, we included recipients with any native lung disease, not just ILD. This expands the applicability of telomere-related research in lung transplant recipients. Third, we assessed telomere length in the early post-transplant period rather than based on pre-transplant samples, suggesting that post-transplant telomere length may also serve as a marker for decreased CLAD-free survival. Unfortunately, we did not routinely measure telomere length pre-transplant so we do not know to what extent pre- and post-transplant telomere length correlate or whether our findings would have been similar using pre-transplant telomere length alone.
There are several possible explanations for the observed association between early post-transplant telomere length and CLAD-free survival. Although recipients with shorter telomere length were not at increased risk for leukopenia requiring cessation of immunosuppression, there was increased need for G-CSF in this population. This degree of leukopenia may have predisposed these individuals to infections, including respiratory viral infections that, in turn, contributed to the development of CLAD [27, 28]. Alternatively, individuals with short telomeres may have reduced or impaired capacity to populate their allograft with recipient-derived stem cells [29]. Identification of differences in recipient cell populations in allografts of short and long telomere recipients could help better assess this hypothesis. Reassuringly, an association between early post-transplant telomere length and CLAD-free survival was also observed when considering only cases of definitive CLAD, as defined in new consensus guidelines [24]. Future studies should, however, collect data on total lung capacity to allow for better characterisation of restrictive CLAD, as also recommended in the guidelines.
Similarly to Popescu et al. [30], we found that CMV-mismatched recipients with shorter telomere length had an increased hazard of CMV viraemia. Early case series of short telomere recipients also found a higher than expected incidence of transaminitis and kidney injury, which we did not observe [13, 15]. Finally, we found no relationship between early telomere length and ACR or ACR score. This contrasts with a previous study in which we found that shorter recipient telomere length (as measured in explanted lungs) was associated with reduced risk of ACR [31]. Differences in tissue assessed (explant versus peripheral blood), sample size, or event rate may account for the different findings in this study.
There are limited data for comparison of expected telomere attrition in the transplant population. Telomere shortening was more significant in our cohort than in other nontransplant populations, where telomere attrition is typically measured over years or decades rather than months [32]. Similarly to large nontransplant cohorts, however, approximately 20% of recipients showed a significant increase in telomere length over time [33]. This may be related to epigenetic regulation with secondary effects on telomere maintenance. Alternatively, acute infection or inflammation may result in mobilisation of quiescent haematopoietic cells and proliferation of peripheral blood mononuclear cells of longer telomere length, causing a transient elevation in average telomere length [34, 35]. As with other studies, telomere attrition was directly related to starting telomere length, which could explain why the rate of telomere attrition during the first year was not associated with CLAD-free survival [34]. Alternatively, the more rapid attrition in the longer telomere population may have represented regression to the mean. We note that the relatively small size of the telomere attrition group and the relatively short interval follow-up indicates that we were only powered to detect large effects on CLAD-free survival. It may be that a larger cohort or assessment over a longer period would identify either telomere stability or significant ongoing loss as a better marker of subsequent risk.
As with all other cohort studies involving telomere length and lung transplant outcomes, we do not know whether the development of specific management protocols for individuals with short telomeres could improve post-transplant CLAD and/or survival. Such protocols might include lower initial immunosuppression doses, particularly cell cycle inhibitors, more frequent laboratory monitoring for developing leukopenia, and extended duration of CMV prophylaxis in mismatched recipients. Although research on the relative impact of mechanistic target of rapamycin (mTOR) versus calcineurin inhibitors on telomere shortening is ongoing, there are some data that mTOR inhibitors may result in less shortening [19, 20]. We believe, however, that it would be premature to consider the clinical use of potential telomere lengthening therapies such as danazol or lithium outside of research trials [36, 37].
Limitations
As with other reports on telomere length and lung transplant outcomes, this was a single-centre study with a relatively small sample size and limited follow-up time, particularly given that the incidence of CLAD increases with time. Because we only enrolled patients who could provide informed consent at the time of outpatient bronchoscopies, we did not capture early deaths, recipients with prolonged index hospitalisation who did not have transbronchial biopsies, or those who were unable or unwilling to provide consent. In addition, while our chosen approach to telomere measurements (real-time PCR) allows us to treat telomere length continuously, there have been concerns about its interlaboratory reliability, particularly with regard to differences in temperature storage, DNA concentration, and purification [38, 39]. While interlaboratory differences may limit between-study comparisons, there is no evidence that there is dramatic intra-laboratory variability, particularly when samples are handled using the same methodological approach in an experienced core facility with appropriate controls [40–42]. Consensus on the appropriate telomere length assay (including the use of more sophisticated techniques such as telomere shortest length assay, which can identify the telomere length of all chromosomes) would be beneficial for future research. Finally, we did not have data on additional transplant outcomes such as primary graft dysfunction, antibody-mediated rejection, malignancy, or major bacterial or fungal infections that may be relevant to telomere length.
Conclusions
Early post-transplant telomere length is associated with decreased CLAD-free survival and clinically significant leukopenia in lung transplant recipients, regardless of native lung disease.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00003-2020_supp (108.2KB, pdf)
Acknowledgements
Additional support for this work was provided by the Brigham and Women's Hospital Department of Pathology Precision Medicine Initiative and by the Dana-Farber/Harvard Cancer Center Genotyping and Genetics for Population Sciences Core.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: A.M. Courtwright reports grants from the National Institutes of Health during the conduct of the study.
Conflict of interest: A.M. Lamattina has nothing to disclose.
Conflict of interest: M. Takahashi has nothing to disclose.
Conflict of interest: A.J. Trindade has nothing to disclose.
Conflict of interest: G.M. Hunninghake reports personal fees from Gerson Lehrman Group, Boehringer Ingelheim and Mitsubishi Chemical, outside the submitted work.
Conflict of interest: I.O. Rosas has nothing to disclose.
Conflict of interest: S. Agarwal reports personal fees from Telomere Diagnostics and Elixirgen outside the submitted work, and has a patent (WO2017066712A2) pending.
Conflict of interest: B.A. Raby reports personal fees for advisory board consultancy from Merck and Teva, personal fees for editorial services from UpToDate, Inc., outside the submitted work.
Conflict of interest: H.J. Goldberg has nothing to disclose.
Conflict of interest: S. El-Chemaly has nothing to disclose.
Support statement: Financial support for this study was provided by the National Institutes of Health (R01 HL130275) and John M. Kent Memorial Fund. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.MacNeil DE, Bensoussan HJ, Autexier C. Telomerase regulation from beginning to the end. Genet 2016; 7: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armanios MY, Chen JJ, Cogan JD, et al. . Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med 2007; 356: 1317–1326. doi: 10.1056/NEJMoa066157 [DOI] [PubMed] [Google Scholar]
- 3.Alder JK, Chen JJ, Lancaster L, et al. . Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci 2008; 105: 13051–13056. doi: 10.1073/pnas.0804280105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronkhite JT, Xing C, Raghu G, et al. . Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Resp Crit Care Med 2008; 178: 729–737. doi: 10.1164/rccm.200804-550OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. . Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci 2007; 104: 7552–7557. doi: 10.1073/pnas.0701009104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courtwright AM, El-Chemaly S. Telomeres in interstitial lung disease: the short and the long of it. Ann Am Thorac Soc 2019; 16: 175–181. doi: 10.1513/AnnalsATS.201808-508CME [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkarni HS, Cherikh WS, Chambers DC, et al. . Bronchiolitis obliterans syndrome–free survival after lung transplantation: an International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J Heart Lung Transplant 2019; 38: 5–16. doi: 10.1016/j.healun.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tissot A, Danger R, Claustre J, et al. . Early identification of chronic lung allograft dysfunction: the need of biomarkers. Front Immunol 2019; 10: 1681. doi: 10.3389/fimmu.2019.01681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameli P, Bargagli E, Fossi A, et al. . Exhaled nitric oxide and carbon monoxide in lung transplanted patients. Resp Med 2015; 109: 1224–1229. doi: 10.1016/j.rmed.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 10.Horie M, Levy L, Houbois C, et al. . Lung density analysis using quantitative chest CT for early prediction of chronic lung allograft dysfunction. Transplantation 2019; 103: 2645–2653. doi: 10.1097/TP.0000000000002771 [DOI] [PubMed] [Google Scholar]
- 11.Borie R, Kannengiesser C, Hirschi S, et al. . Groupe d'Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P). Severe hematologic complications after lung transplantation in patients with telomerase complex mutations. J Heart Lung Transplant 2015; 34: 538–546. doi: 10.1016/j.healun.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 12.Newton CA, Kozlitina J, Lines JR, et al. . Telomere length in patients with pulmonary fibrosis associated with chronic lung allograft dysfunction and post-lung transplantation survival. J Heart Lung Transplant 2017; 36: 845–853. doi: 10.1016/j.healun.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokman S, Singer JP, Devine MS, et al. . Clinical outcomes of lung transplant recipients with telomerase mutations. J Heart Lung Transplant 2015; 34: 1318–1324. doi: 10.1016/j.healun.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swaminathan AC, Neely ML, Frankel CW, et al. . Lung transplant outcomes in pulmonary fibrosis patients with telomere-related gene variants. Chest 2019; 156: 477–485. doi: 10.1016/j.chest.2019.03.030 [DOI] [PubMed] [Google Scholar]
- 15.Silhan LL, Shah PD, Chambers DC, et al. . Lung transplantation in telomerase mutation carriers with pulmonary fibrosis. Eur Respir J 2014; 44: 178–187. doi: 10.1183/09031936.00060014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong JY, De Vivo I, Lin X, et al. . The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS ONE 2014; 9: e87348. doi: 10.1371/journal.pone.0087348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crous-Bou M, Fung TT, Prescott J, et al. . Mediterranean diet and telomere length in Nurses’ Health Study: population based cohort study. BMJ 2014; 349: g6674. doi: 10.1136/bmj.g6674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crepin T, Carron C, Roubiou C, et al. . ATG-induced accelerated immune senescence: clinical implications in renal transplant recipients. Am J Transplant 2015; 15: 1028–1038. doi: 10.1111/ajt.13092 [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Gehrig PA, Whang YE, et al. . Rapamycin inhibits telomerase activity by decreasing the hTERT mRNA level in endometrial cancer cells. Mol Cancer Ther 2003; 2: 789–795. [PubMed] [Google Scholar]
- 20.Koppelstaetter C, Kern G, Leierer G, et al. . Effect of cyclosporine, tacrolimus and sirolimus on cellular senescence in renal epithelial cells. Toxicol In Vitro 2018; 48: 86–92. doi: 10.1016/j.tiv.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Gadalla SM, Wang T, Haagenson M, et al. . Association between donor leukocyte telomere length and survival after unrelated allogeneic hematopoietic cell transplantation for severe aplastic anemia. JAMA 2015; 313: 594–602. doi: 10.1001/jama.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouilette SW, Moore JS, McMahon AD, et al. . West of Scotland Coronary Prevention Study Group. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 2007; 369: 107–114. doi: 10.1016/S0140-6736(07)60071-3 [DOI] [PubMed] [Google Scholar]
- 23.Verleden GM, Raghu G, Meyer KC, et al. . A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant 2014; 33: 127–133. doi: 10.1016/j.healun.2013.10.022 [DOI] [PubMed] [Google Scholar]
- 24.Verleden GM, Glanville AR, Lease ED, et al. . Chronic lung allograft dysfunction: definition, diagnostic criteria and approaches to treatment. A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019; 38: 493–503. doi: 10.1016/j.healun.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 25.Müezzinler A, Zaineddin AK, Brenner H. A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 2013; 12: 509–519. doi: 10.1016/j.arr.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 26.Faust HE, Golden JA, Rajalingam R, et al. . Short lung transplant donor telomere length is associated with decreased CLAD-free survival. Thorax 2017; 72: 1052–1054. doi: 10.1136/thoraxjnl-2016-209897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen S, Janicki-Deverts D, Turner RB, et al. . Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA 2013; 309: 699–705. doi: 10.1001/jama.2013.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peghin M, Hirsch HH, Len Ó, et al. . Epidemiology and immediate indirect effects of respiratory viruses in lung transplant recipients: a 5-year prospective study. Am J Transplant 2017; 17: 1304–1312. doi: 10.1111/ajt.14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer H, Rampling D, Aurora P, et al. . Transbronchial biopsies provide longitudinal evidence for epithelial chimerism in children following sex mismatched lung transplantation. Thorax 2005; 60: 60–62. doi: 10.1136/thx.2004.029678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popescu I, Mannem H, Winters SA, et al. . Impaired cytomegalovirus immunity in idiopathic pulmonary fibrosis lung transplant recipients with short telomeres. Am J Resp Crit Care Med 2019; 199: 362–376. doi: 10.1164/rccm.201805-0825OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courtwright AM, Fried S, Villalba JA, et al. . Association of donor and recipient telomere length with clinical outcomes following lung transplantation. PLoS ONE 2016; 11: e0162409. doi: 10.1371/journal.pone.0162409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haapanen MJ, Perälä MM, Salonen MK, et al. . Telomere length and frailty: the Helsinki birth cohort study. J Am Med Dir Assoc 2018; 19: 658–662. doi: 10.1016/j.jamda.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 33.Nordfjäll K, Svenson U, Norrback KF, et al. . The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet 2009; 5: e1000375. doi: 10.1371/journal.pgen.1000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aviv A, Chen W, Gardner JP, et al. . Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol 2008; 169: 323–329. doi: 10.1093/aje/kwn338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svenson U, Nordfjäll K, Baird D, et al. . Blood cell telomere length is a dynamic feature. PLoS ONE 2011; 6: e21485. doi: 10.1371/journal.pone.0021485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsley DM, Dumitriu B, Liu D, et al. . Danazol treatment for telomere diseases. N Engl J Med 2016; 374: 1922–1931. doi: 10.1056/NEJMoa1515319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinsson L, Wei Y, Xu D, et al. . Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatry 2013; 3: e261. doi: 10.1038/tp.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin-Ruiz CM, Baird D, Roger L, et al. . Reproducibility of telomere length assessment: an international collaborative study. Int J Epidemiol 2014; 44: 1673–1683. doi: 10.1093/ije/dyu191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagnall CL, Hicks B, Teshome K, et al. . Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLoS ONE 2017; 12: e0184098. doi: 10.1371/journal.pone.0184098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vivo I, Prescott J, Wong JY, et al. . A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Prev Biomarkers 2009; 18: 1152–1156. doi: 10.1158/1055-9965.EPI-08-0998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grodstein F, Van Oijen M, Irizarry MC, et al. . Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the nurses’ health study. PloS ONE 2008; 3: e1590. doi: 10.1371/journal.pone.0001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prescott J, Karlson EW, Orr EH, et al. . A prospective study investigating prediagnostic leukocyte telomere length and risk of developing rheumatoid arthritis in women. J Rheumatol 2016; 43: 282–288. doi: 10.3899/jrheum.150184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00003-2020_supp (108.2KB, pdf)