Abstract
Introduction
Residence in a disadvantaged neighborhood associates with adverse health exposures and outcomes, and may increase risk for cognitive impairment and dementia. Utilization of a publicly available, geocoded disadvantage metric could facilitate efficient integration of social determinants of health into models of cognitive aging.
Methods
Using the validated Area Deprivation Index and two cognitive aging cohorts, we quantified Census block‐level poverty, education, housing, and employment characteristics for the neighborhoods of 2119 older adults. We assessed relationships between neighborhood disadvantage and cognitive performance in domains sensitive to age‐related change.
Results
Participants in the most disadvantaged neighborhoods (n = 156) were younger, more often female, and less often college‐educated or white than those in less disadvantaged neighborhoods (n = 1963). Disadvantaged neighborhood residence associated with poorer performance on tests of executive function, verbal learning, and memory.
Discussion
This geospatial metric of neighborhood disadvantage may be valuable for exploring socially rooted risk mechanisms, and prioritizing high‐risk communities for research recruitment and intervention.
Keywords: cognition, cognitive aging, dementia, disparities, neighborhood disadvantage, social determinants
1. INTRODUCTION
Alzheimer's disease and related dementias (ADRD) disproportionately impact economically disadvantaged, rural, and ethnoracial minority communities. 1 , 2 At a population level, these communities experience disproportionately high exposure to adverse living, learning, and working conditions—fundamental contextual factors known as social determinants of health (SDOH). 3 , 4
When assessed for discrete geographic areas, neighborhood‐level disadvantage encompasses SDOH including poverty, housing quality, and employment opportunities. Neighborhood disadvantage is a modifiable, policy‐actionable factor that may impact cognitive health independently of and through risk factors such as chronic stress and reduced access to educational opportunities, healthy food, and medical care. 5 , 6 Because it drives various and synchronous risk mechanisms, neighborhood‐level disadvantage is both a fundamental cause of disparities and a priority target for efficient, far‐reaching interventions. 7
A growing body of work exposes robust ties between upstream individual‐level SDOH for cognitive impairment and ADRD risk in later life 8 , 9 and highlights a need for expanded focus and action. The few studies exploring neighborhood‐level contextual disadvantage and cognitive risk have often relied upon resource‐intensive neighborhood ratings by research staff. 10 Two European studies reported that objective, geocoded neighborhood disadvantage also associates strongly with poorer global cognitive function. 11 , 12 Our objective was to investigate the utility of an efficient, publicly available U.S. Census–based neighborhood disadvantage metric, the Area Deprivation Index (ADI). The ADI is available for customized local‐level mapping and free download for every neighborhood in the United States through the National Institutes of Health (NIH)–funded Neighborhood Atlas. 7 , 13 Given the openness and availability of these neighborhood data, the ADI shows excellent potential as a harmonizable exposure assessment tool that facilitates SDOH modeling. In a first step to evaluating the usability of this new tool in ADRD research, we assessed associations between block group–level neighborhood disadvantage and cognitive function across several specific domains in a community‐based cognitive aging cohort.
2. METHODS
2.1. Data sources and sample
Participants (N = 2119) were drawn from the Wisconsin Registry for Alzheimer's Prevention (WRAP) study (n = 1501) 14 and the Wisconsin Alzheimer's Disease Research Center (ADRC) clinical core (n = 618). Participants included in this study sample were cognitively unimpaired at their most recent WRAP or ADRC visit (no diagnosis of mild cognitive impairment [MCI] or dementia by a clinical consensus committee) and had a documented address and full cognitive visit data from 2009 or after (within 5 years of available ADI metrics).
The longitudinal WRAP study and ADRC clinical core are cohorts of middle‐aged and older adults enriched for a parental history of AD. Parental history of dementia due to AD is validated through the review of parental medical or autopsy records, 14 or through completion of a Dementia Questionnaire by the study participant. The majority of participants are recruited through community outreach and word‐of‐mouth. WRAP and ADRC participants undergo study evaluations on an annual or biennial basis, including neuropsychological testing, clinical measurements, and comprehensive health history. 14
This study was approved by the institutional review board of the University of Wisconsin, with participant informed consent obtained and documented prior to all study procedures.
2.2. ADI construction and linkage
We used standard techniques to geocode all subjects according to their most recently reported address. The census block group (henceforth referred to as “neighborhood” as per previous research) of each geocode was linked to its ADI state decile score. 5 The ADI is a factor‐based index that uses 17 U.S. Census–based poverty, education, housing quality, and employment indicators to characterize and rank the socioeconomic contextual disadvantage of a particular neighborhood. 5 , 7 The full list of individual ADI indicators is available in an open‐access publication. 5 Employing the latest American Community Survey and Census data, we calculated and validated neighborhood‐level quantifications of neighborhood disadvantage for the full United States. The ADI is freely available through the Neighborhood Atlas, developed by our team and promoted for use by the National Institute on Aging (NIA). 7 , 13
2.3. Cognitive function assessment
Key cognitive outcome variables included test performance data from the WRAP or ADRC visit date closest to the time point of address documentation for all participants. We utilized scores from four cognitive tests representing four cognitive domains sensitive to age‐related change: verbal learning, delayed recall, processing speed, and executive function. Verbal learning was represented by the summed number of recalled words on Learning Trials 1‐5 of the Rey Auditory Verbal Learning Test (RAVLT), while delayed recall was represented by the number of words on the RAVLT Delayed Recall Trial. 15 Processing speed was represented by time to completion on the Trail Making Test A (Trails A), while another key aspect of executive function (speeded set switching) was represented by Trail Making Test B (Trails B). 16
2.4. Analyses
Cognitive outcomes were examined by neighborhood disadvantage state decile, with a binary variable for the ADI comparing the 20% most disadvantaged neighborhoods (Deciles 9 and 10) to those in the 80% least disadvantaged neighborhoods (Deciles 1 to 8). This binary categorization is consistent with previous studies showing that negative health outcomes primarily affect those in the highest deciles of disadvantage [5]. Multivariable linear regression was performed using SAS 9.4, to evaluate the association between living within disadvantaged neighborhood deciles and cognitive outcomes of interest, with adjustment for self‐reported age at visit, sex, race, college education (presence or absence of bachelor's degree attainment), parental history of dementia, and cohort (WRAP/ADRC).
RESEARCH IN CONTEXT
Systematic review: Literature searches using PubMed and Google Scholar utilized combinations of keywords such as “neighborhood,” “disadvantage,” “health,” “cognitive function,” “Alzheimer's,” and “dementia.” Review of results suggested that contextual neighborhood disadvantage was consistently associated with poor health outcomes. There was strong evidence for direct associations between staff‐rated or self‐perceived neighborhood quality and cognitive health; the search did not identify U.S.‐based studies using geocoded metrics to assess cognitive risk associated with neighborhood‐level disadvantage.
Interpretation: Using a validated metric of neighborhood disadvantage, we found that disadvantage at the U.S. Census block group level associated with poorer test performance across multiple cognitive domains in a cohort of older adults. These associations were robust to adjustment for multiple demographic covariates.
Future directions: Future studies should explore brain‐based mechanisms that link neighborhood disadvantage to cognitive function and impairment, and should assess these pathways in diverse cohorts to clarify the role of neighborhood disadvantage in well‐established but underexplained cognitive health disparities.
3. RESULTS
3.1. Sample characteristics
Participants living in the 20% most disadvantaged neighborhoods tended to be younger, more often female, and less often college educated or white compared to those residing within other neighborhoods. Parental history of dementia did not differ by neighborhood disadvantage (Table 1).
TABLE 1.
Characteristics overall, and stratified by those residing within the least disadvantaged 80% of neighborhoods versus within the most disadvantaged 20% of neighborhoods by ADIa
| Characteristics | Overall (N = 2119) | Least disadvantaged 80% (n = 1963) | Most disadvantaged 20% (n = 152) | P |
|---|---|---|---|---|
| Age in years, M (SD) | 63.7 (8.4) | 63.8 (8.3) | 61.8 (8.7) | .003 |
| Male gender, N (%) | 651 (30.7%) | 615 (31.3%) | 36 (23.8%) | .032 |
| Bachelors degree, N (%) | 1309 (61.8%) | 1242 (63.3%) | 67 (43.2%) | <.001 |
| Primary race, N (%) | <.001 | |||
| White | 1814 (85.6%) | 1742 (88.7%) | 72 (46.2%) | |
| African American | 228 (10.8%) | 155 (7.9%) | 73 (46.8%) | |
| American Indian/Alaska Native | 36 (1.7%) | 33 (1.7%) | 3 (1.9%) | |
| Asian/Pacific Islander | 5 (0.2%) | 5 (0.3%) | 0 (0.0%) | |
| Other | 36 (1.7%) | 28 (1.4%) | 8 (5.1%) | |
| Parental history of dementia, N (%) | 1418 (66.9%) | 1312 (68.0%) | 106 (30.3%) | .776 |
ADI, Area Deprivation Index.
3.2. Cognition
Residing within the 20% most disadvantaged neighborhoods, as compared to less disadvantaged neighborhoods, associated with fewer recalled words at a P < .01 level on tests of verbal learning (β = −2.29; 95% confidence interval [CI] −3.78 to 0.79) and delayed recall (β = −0.91; 95% CI −1.41 to 0.40), slower completion of an executive function task (β = 10.23; 95% CI 5.08 to 15.39) in fully adjusted models. No association with processing speed was observed (β = 0.83; 95% CI −0.64 to 2.30). These relationships can be seen in Figure 1.
FIGURE 1.
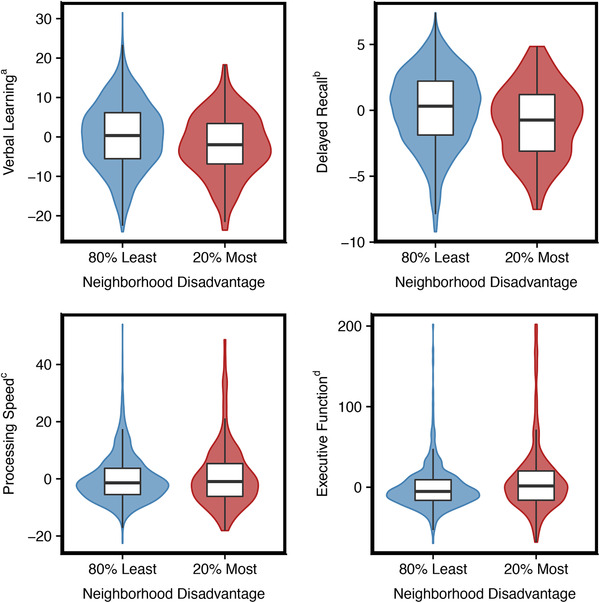
Cognitive function factor score box plots by neighborhood disadvantage. aRey Auditory Verbal Learning Test (RAVLT) 1‐5, bRAVLT delayed, cTrail A (seconds), dTrail B (seconds); ADI, Area Deprivation Index. For RAVLT outcomes, higher scores indicate better cognitive function. For trailmaking outcomes, higher scores indicate poorer cognitive function. Outcomes adjusted for age, gender, race, education, parental history of dementia, and study cohort. Red is the 20% most disadvantaged group. Blue is the 80% least disadvantaged group. Color width is the density of observation points at that unit of measure
4. DISCUSSION
These early cross‐sectional data suggest that neighborhood disadvantage associates with poorer cognitive function across multiple domains in middle‐aged and older adults. The results are consistent with and expand a small body of work examining objective area–level deprivation and global function in European cohorts 11 , 12 and self‐perceived neighborhood quality, episodic memory, and semantic fluency in a U.S. population–based cohort. 17 If cross‐sectional findings extend to accelerated declines in longitudinal analyses and replication in larger samples, establishing neighborhood disadvantage as a modifiable risk exposure has crucial institutional and policy implications. Neighborhood context can be efficiently measured across cohorts, targeted, and improved.
The findings also indicate that geocoded neighborhood disadvantage holds potential as a practical marker for research initiatives aiming to benefit communities at increased risk for cognitive dysfunction and ADRD. The ADI metric used in this study is a novel, accessible tool that can be leveraged by institutions and investigators to incorporate a key SDOH measure into cognitive aging research. It can be used to prioritize high‐risk neighborhoods for outreach, community stakeholder input, recruitment, and general study design in dementia research. By prioritizing those neighborhoods at the highest risk, we can potentially better define the factors that contribute to cognitive decline and vulnerability to the Alzheimer's clinical syndrome. Future studies exploring the ADI's relationship to neuroimaging‐ and fluid‐based ADRD biomarkers will illuminate mechanisms linking social context to brain health. Given its outstanding harmonizability when utilized across cohorts, this work is expected to facilitate the development and subsequent evaluation of new therapeutic measures.
Limitations of this preliminary study serve to clarify crucial next steps. First, there are inference constraints inherent to the cross‐sectional analysis, including our inability to rule out reverse causation. By excluding enrollees who developed MCI or dementia, we sought to reduce the influence of residential moves orchestrated to accommodate disability‐related economic stress or pending loss of independence. It is also worth noting that growing evidence suggests that social disparities in dementia risk are driven not by accelerated decline but by lower peak cognitive health, which places aging adults closer to impairment thresholds. 18
In addition, replication in well‐powered racial minority and population‐based cohorts will be key to understanding the role for neighborhood context in cognitive health disparities. As indicated by the small proportion (7.4%) of study participants residing in the state's 20% most disadvantaged neighborhoods, selection into many community‐based cognitive aging cohorts associates with high educational attainment and economic advantage. Small numbers of ethnoracial minority participants in our sample, and particularly within the most disadvantaged neighborhood deciles, prevented the examination of potential interrelationships between neighborhood disadvantage, cognition, and race, and ethnicity. Furthermore, methodological challenges inherent to measuring racial and education‐based cognitive aging disparities, such as test bias, are also plausible in neighborhood‐level analyses. Future ADI studies must explore the importance and the nuances of neighborhood context across diverse population strata.
The mechanisms of health disparities are multi‐factorial, but social determinants of health including neighborhood disadvantage plausibly root and underpin many of those pathways. 4 , 19 The present study is a first step in investigating the ADI as a neighborhood‐level contextual SDOH that can predict cognitive health and risk. As a metric, the ADI is both inherently generalizable and practically applicable, disseminated freely online through the Neighborhood Atlas. 7 , 20 Although it is employed across the nation in health delivery, policy, and outcomes research, this is the first time the ADI has been employed within ADRD‐focused research. This work suggests that integration of the ADI into ADRD research recruitment and data protocols can improve our ability to explore and understand the interactions between modifiable social and biological processes, and can inform the design of targeted, tailored interventions that reduce cognitive health and ADRD disparities.
FINANCIAL DISCLOSURES
This study was funded by National Institute on Aging Awards (RF1AG057784 [PI Kind, MPI Bendlin]; R01AG027161, R01AG021155 [PI Johnson]; P30AG062715 [PI Asthana]; R01AG054059 [PI Gleason]), National Institutes of Health‐National Institute on Minority Health and Health Disparities Award (R01MD010243 [PI Kind]), the UW Institute for Clinical and Translational Research grant (1UL1RR025011), and an Alzheimer's Association Research Fellowship Award (AARF‐18‐562958 [PI Zuelsdorff]). This material is the result of work also supported with the resources and the use of facilities at the William S Middleton Memorial Veterans Hospital Geriatric Research, Education and Clinical Center in Madison, WI (GRECC‐Manuscript # 002‐2019) and the University of Wisconsin Department of Medicine Health Services and Care Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Department of Veterans Affairs.
Zuelsdorff M, Larson JL, Hunt JFV, et al. The area deprivation index: a novel tool for harmonizable risk assessment in Alzheimer's disease research. Alzheimer's Dement. 2020;6:e12039 10.1002/trc2.12039
Contributor Information
Megan Zuelsdorff, Email: mlzuelsd@wisc.edu.
Amy J. H. Kind, Email: ajk@medicine.wisc.edu.
REFERENCES
- 1. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12(3):216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Russ TC, Batty GD, Hearnshaw GF, Fenton C, Starr JM. Geographical variation in dementia: systematic review with meta‐analysis. Int J Epidemiol. 2012;41(4):1012‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thornton RL, Glover CM, Cene CW, Glik DC, Henderson JA, Williams DR. Evaluating strategies for reducing health disparities by addressing the social determinants of health. Health Aff. 2016;35(8):1416‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Office of disease prevention and health promotion. Healthy people 2020: social determinants of health 2018 Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health.
- 5. Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30‐day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laraia BA, Karter AJ, Warton EM, Schillinger D, Moffet HH, Adler N. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE). Social Sci Med. 2012;74(7):1082‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kind AJH, Buckingham WR. Making neighborhood‐disadvantage metrics accessible—the neighborhood Atlas. N Engl J Med. 2018;378(26):2456‐2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement. 2018;4:510‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sisco S, Gross AL, Shih RA, et al. The role of early‐life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):557‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarke PJ, Weuve J, Barnes L, Evans DA, Mendes de Leon CF. Cognitive decline and the neighborhood environment. Ann Epidemiol. 2015;25(11):849‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, Melzer D. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2008;56(2):191‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCann A, McNulty H, Rigby J, et al. Effect of area‐level socioeconomic deprivation on risk of cognitive dysfunction in older adults. J Am Geriatr Soc. 2018;66(7):1269‐1275. [DOI] [PubMed] [Google Scholar]
- 13. Barr R. The neighborhood. 2019. Available at: https://www.nia.nih.gov/research/blog/2019/11/neighborhood-atlas-free-social-determinants-health-data-all.
- 14. Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin Registry for Alzheimer's Prevention: a review of findings and current directions. Alzheimers Dement. 2018;10:130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services; 1996. [Google Scholar]
- 16. Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. Oxford: Oxford University Press; 2004. [Google Scholar]
- 17. Zaheed AB, Sharifian N, Kraal AZ, Sol K, Hence A, Zahodne LB. Unique effects of perceived neighborhood physical disorder and social cohesion on episodic memory and semantic fluency. Arch Clin Neuropsychol. 2019;34(8):1346‐1355. [DOI] [PubMed] [Google Scholar]
- 18. Karlamangla AS, Miller‐Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170(3):331‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hill CV, Perez‐Stable EJ, Anderson NA, Bernard MA. The National Institute on Aging Health Disparities Research Framework. Ethn Dis. 2015;25(3):245‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. University of Wisconsin School of Medicine and public health. the neighborhood atlas 2018. Available at: https://www.neighborhoodatlas.medicine.wisc.edu.


