Abstract
Introduction
It has been reported that environmental factors such as hypoxia could contribute to the pathogenesis of Alzheimer's disease (AD). Therapeutics like hyperbaric oxygen treatment, which improves tissue oxygen supply and ameliorates hypoxic conditions in the brain, may be an alternative therapy for AD and amnestic mild cognitive impairment (aMCI). The present work aims to investigate the potential therapeutic effect of hyperbaric oxygen treatment for AD and aMCI.
Methods
We recruited 42 AD, 11 aMCI, and 30 control AD patients in this study. AD and aMCI patients were treated with 40 minutes of hyperbaric oxygen once a day for 20 days and assessed by neuropsychiatric assessments including Mini‐Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Activities of Daily Living (ADL) scale before and at 1‐, 3‐, and 6‐month follow‐up after treatment. Control AD patients who were not given hyperbaric oxygen treatment had similar clinical profile as hyperbaric oxygen treated AD. We examined 10 of the AD/aMCI patients with fluorodeoxyglucose positron emission tomography.
Results
In self‐comparison study, one course of hyperbaric oxygen treatment significantly improved the cognitive function assessed by MMSE and MoCA in AD patients after 1‐month follow‐up; such treatment also significantly improved MMSE score at 3‐month follow‐up and MoCA score at 1‐ and 3‐month follow‐up in aMCI patients. The ADL scale was significantly improved in AD patients after 1‐ and 3‐month follow‐up. Compared to the control AD patients, the MMSE and MoCA in hyperbaric oxygen treated AD patients were significantly improved after 1‐month follow‐up. Hyperbaric oxygen treatment also ameliorated the reduced brain glucose metabolism in some of the AD and aMCI patients.
Conclusion
Based on previous studies and our recent findings, we propose that hyperbaric oxygen treatment may be a promising alternative therapy for AD and aMCI.
Keywords: Alzheimer's disease, hyperbaric oxygen treatment, hypoxia, mild cognitive impairment
1. INTRODUCTION
Alzheimer's disease (AD) is the most common form of dementia in the elderly. It is generally considered that AD arises through the interactions between genetic and non‐genetic factors. 1 Genic factors are dominantly inherited mutations in amyloid beta (Aβ) precursor protein, presenilin 1, and presenilin 2; and there are several risk genes, including apolipoprotein E, which may contribute to the disease pathogenesis. 2 Non‐genetic factors such as brain trauma, cerebrovascular diseases, type 2 diabetes, intellectual activity, sleep disorders, and hypoxia are thought to play an important role in sporadic AD. 3 , 4 Results from animal studies have indicated that hypoxia affects many aspects of AD‐associated pathophysiology including Aβ and tau neuropathy, neuroinflammation, autophagy dysfunction, oxidative stress, and mitochondrial abnormality. 5 , 6 , 7 , 8 In AD patients, comorbidities such as stroke, cardiovascular diseases, obstructive sleep apnea syndrome, and chronic obstructive pulmonary disease (COPD) may cause acute and chronic hypoxic conditions. 8 , 9 , 10 A prospective study showed that women with sleep‐disordered breathing were more likely to develop mild cognitive impairment or dementia. 11 Several studies have reported that high‐altitude hypoxia exposure may cause deleterious effects on cognitive performance. 12 , 13 High altitude decreases brain oxygen saturation, reduces neural activity, induces immune response, and impairs cognitive functions. 14 These evidences imply that hypoxia may be an important culprit for cognitive decline. B hypoxia contributes to the pathogenesis of AD, anti‐hypoxia treatment may be helpful to ameliorate the disease. Hyperbaric oxygen treatment is a well‐established therapy used to treat neurological conditions by enhancing oxygen delivery to brain tissues and protect neurons. 15 , 16
According to the epidemiological data in 2005, 24.2 million people worldwide suffer from dementia with 4.6 million new cases per year. By 2040, there will be >81 million people suffering from dementia, 70% of which are AD patients. 17 , 18 As the population ages, AD will undoubtedly bring significant social and economic burdens to the world. However, there is still no cure or effective treatment to reverse the pathological process of AD. Currently there are only a few anti‐AD drugs in clinical use, such as cholinesterase inhibitors (ChEIs; donepezil, rivastigmine, and galantamine), and N‐Methyl‐D‐aspartic acid (NMDA) receptor antagonist (memantine). These drugs have a limited therapeutic effect on symptoms and poorly affect the progression of the disease. 19 Despite great efforts to develop new anti‐AD drugs aiming to stop the progression of AD, the therapeutic interventions predominantly targeting Aβ pathology have limited success. 20 Other novel therapeutics, including anti‐tau and anti‐inflammation interventions have not successfully yielded any good results. 19 , 21 Recent studies have indicated that dementia and stroke share some of the pathological risk factors resulting from small vessel degeneration; and dementia could be reduced through stroke prevention by using anticoagulation of atrial fibrillation and quit of smoking. 22 , 23 Microvascular flow impairment leading to tissue hypoxia has been demonstrated in AD and amnestic mild cognitive impairment (aMCI), and the degree of hypoxia correlates with the extent of cognitive decline. 23 Moreover, the hypoxia condition may cause energy failure resulting in dyshomeostasis of cytosol calcium ion concentration. The breakdown of the calcium ion signaling system is one of the common mechanisms of neurodegeneration. 23 Thus, hyperbaric oxygen treatment, which improves tissue oxygen supply and ameliorates hypoxic conditions, may be a potential therapeutic strategy for AD and aMCI.
In the present study, we recruited 42 AD and 11 aMCI patients for hyperbaric oxygen treatment and 30 AD patients without hyperbaric oxygen treatment as control. All the participants were tested with a battery of neuropsychiatric assessments before and at 1‐, 3‐, and 6‐month follow‐up after treatment. We aimed to investigate the potential therapeutic effect of hyperbaric oxygen treatment in patients with AD and aMCI.
2. METHODS
2.1. Patients
There were 42 AD and 11 aMCI patients enrolled in this hyperbaric oxygen study. All participants were from the First Afflicted Hospital of Dalian Medical University during 2015 to 2018. Another 30 AD patients who had similar clinical profile (age, sex, disease duration, and history of medication) but not undertaking hyperbaric oxygen treatment were recruited as control. The diagnosis of AD was made according to the criteria of the Diagnostic Statistical Manual of Mental Disorders IV (DSM IV) and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association. 24 , 25 , 26 And the diagnosis of aMCI was referred to the DSM IV and Petersen 2005 diagnostic criteria. 26 , 27 , 28 Patients with the following conditions were excluded: gait imbalance, extrapyramidal signs, seizures, autonomic failures, severe psychiatric conditions (major depression, psychosis, bipolar disorders), toxic exposure, conditions that affect intelligence (severe anemia, thyroid disorder, and vitamin B12 deficiency) and conditions that affect brain oxygen metabolism (cerebral vascular ischemic disease, anemia, and un‐controlled hypertension and diabetes). In addition, the participants were ruled out with any of the following contraindications of hyperbaric oxygen treatment: pneumothorax, rib fracture, chest trauma, cavitary pulmonary tuberculosis, resistant hypertension, severe pneumonia, asthma, pulmonary heart disease, and COPD. AD and control patients in this study were contemporarily maintained with their medications including memantine (20 mg/d) and/or rivastigmine (6 to 12 mg/d) regularly. Patients with aMCI were not treated with any cognitive medications.
The study of hyperbaric oxygen treatment in AD and aMCI was approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University before the study started. All enrolled patients and their legal guardians have been informed of the study procedure and the hazards that may result from the treatment, and they have agreed and signed the informed consent.
The demographic characteristics of patients enrolled in this study were summarized in Table S1 in supporting information. There were 42 AD patients (13 male/29 female). The mean age of AD patients was 70.17 ± 8.95 years old, and the mean disease course was 4.14 ± 2.31 years. All patients completed the 20‐day hyperbaric oxygen treatment and 1‐ and 3‐month follow‐up. Two of the AD patients were dropped at the 6‐month follow‐up: one died of cardiovascular disease, and the other one left the original resident district and moved to another city. There were 11 aMCI patients enrolled in this study (7 male/4 female). The mean age of those patients was 65.36 ± 9.50 years old, and the mean disease course was 1.73 ± 0.91 years. All 11 aMCI participants completed the whole study. There were 30 control AD (12 male/18 female). The mean age of those patients was 69.87 ± 7.79 years old, and the mean disease course was 3.93 ± 2.60 years. There were no differences between hyperbaric oxygen treated AD and control AD in age, sex, age of onset, education level, disease course, and Mini‐Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores at baseline level.
RESEARCH IN CONTEXT
Systematic review: Hypoxia is one of the important environmental factors that could contribute to the pathogenesis of Alzheimer's disease (AD). Currently approved drug treatments for AD have a limited therapeutic effect on symptoms and poorly affect the progression of the disease. Non‐pharmacological multi‐targeted interventions like hyperbaric oxygen treatment targeting hypoxia may have potential in the future treatment of AD.
Interpretation: We recruited 42 AD and 11 amnestic mild cognitive impairment (aMCI) patients for hyperbaric oxygen treatment and 30 AD patients without hyperbaric oxygen treatment as control and performed a 6‐month follow‐up study. We found that hyperbaric oxygen treatment could improve cognitive functions and ameliorate the reduced brain glucose metabolism in AD and aMCI patients.
Future directions: Multi‐center studies with larger numbers of participants with or without medications, multiple courses of treatments, and longer time of follow‐up should be applied in future studies.
2.2. Neuropsychiatric assessments
Neuropsychiatric assessments used in the study were MMSE, MoCA, and Activities of Daily Living (ADL) scale. 29 , 30 , 31 , 32 After the clinical history collection, physical examination, laboratory tests, and neuroimaging, the patients were examined with the neuropsychiatric assessments before the hyperbaric oxygen treatment and re‐examined at 1‐, 3‐, and 6‐month follow‐up. The MMSE assesses the cognitive functions of orientation, registration, attention and calculation, recall, and language. The MoCA includes six sub‐items of visuospatial abilities and executive functions, naming, memory, attention and calculation, language and abstract reasoning, and orientation to time and place. The ADL scale evaluates independence of daily activities (bathing, dressing, toileting, transferring, eating, and the use of incontinence materials) with 20 questions.
2.3. Hyperbaric oxygen treatment
All 42 AD and 11 aMCI participants undertook one course of hyperbaric oxygen treatment once a day for 20 days. Each treatment included 20‐minute pure oxygen inhalation (O2 = 99.9%, oxygen supply pressure 0.4 to 0.7 MPa, oxygen flow 10 to 15 L/h), 15 minutes interval, and then another 20‐minute pure oxygen inhalation (O2 = 99.9%, oxygen supply pressure 0.4 to 0.7 MPa, oxygen flow 10 to 15 L/h) under the pressure of 0.22 MPa (0.12 MPa on pressure gauge) in hyperbaric oxygen chamber (Medical Oxygen Chamber, SY3200‐8500, Yantai Hongyuan Oxygen Industrial Inc., Shandong, China).
2.4. Fluorodeoxyglucose positron emission tomography
Six of the AD patients and four of the aMCI patients underwent fluorodeoxyglucose positron emission tomography (FDG‐P) before and at 1 month after hyperbaric oxygen treatment. The 18F‐FDG (radiochemical purity >95%) was synthesized by the Eclipse RD cyclotron and the FDG synthesizer Explora FDG4 (Siemens AG, Germany). All patients fasted for at least 6 hours, and the body weight and serum glucose level were determined before the injection. The fasting blood glucose <9 mmol/L was acceptable. Then a dose of 0.15 mCi/kg of FDG was injected via a peripheral cannula after resting for 30 to 40 minutes. After injection, the patients were instructed to stay awake, keep eyes open, and not vocalize in a quiet and darkened room for 40 minutes. The images were then acquired with Siemens Biograph 64 HD P/CT (Siemens AG, Germany). All images were compared with the template and analyzed with NeuroQ™ Brain Imaging Analysis (Syntermed Inc., Atlanta, GA, USA).
2.5. Data analysis
Data were analyzed by SPSS 21.0 software (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA, USA). The results were considered to be statistically significant when P value was <0.05.
3. RESULTS
3.1. Cognitive assessments of AD and aMCI patients before and after hyperbaric oxygen treatment
3.1.1. MMSE score before and after treatment
Compared to the baseline level, the MMSE score was significantly improved at 1‐month follow‐up in AD patients (Table 1) and at 3‐month follow‐up in aMCI patients after hyperbaric oxygen treatment (Table 1). Compared to control AD, the hyperbaric oxygen treated AD patients got significant improvement in MMSE score at 1‐month follow‐up.
TABLE 1.
Self‐comparison of MMSE scores in AD and aMCI patients before and after hyperbaric oxygen treatment
| AD | aMCI | |||
|---|---|---|---|---|
| Time | Mean ± SD | P value | Mean ± SD | P value |
| Baseline | 15.19 ± 7.55 | – | 27.00 ± 2.19 | – |
| 1‐month follow‐up | 16.43 ± 8.21 | 0.005 * | 28.00 ± 2.28 | 0.070 |
| 3‐month follow‐up | 15.38 ± 8.05 | 0.750 | 28.45 ± 1.97 | 0.026 * |
| 6‐month follow‐up | 13.95 ± 7.63 | 0.056 | 27.82 ± 2.34 | 0.136 |
Data of MMSE scores in AD patients were analyzed by paired t test; data of MMSE scores in aMCI patients were analyzed by Wilcoxon signed‐rank test.
P < 0.05. Sample size: n = 42 in AD patients (n = 40 at 6‐month follow‐up); n = 11 in aMCI patients. The P values of 1‐, 3‐, and 6‐month follow‐up were compared with the baseline level.
Abbreviations: AD, Alzheimer's disease: aMCI, amnestic mild cognitive impairment; MMSE, Mini‐Mental State Examination; SD, standard deviation
3.1.2. MoCA score before and after treatment
The MoCA score was improved significantly at 1‐month follow‐up in AD patients and at 1‐month and 6‐month follow‐up in aMCI patients after hyperbaric oxygen treatment compared to the baseline level (Table 2). The six subitems of MoCA scores are summarized in Table 2. The visuospatial abilities and executive functions were significantly improved at 1‐month follow‐up and the language and abstract reasoning were significantly improved at 1‐, 3‐, and 6‐month follow‐up in AD patients after hyperbaric oxygen treatment compared to baseline level (Table 2). In aMCI patients, the language and abstract reasoning were improved at 1‐month follow‐up after hyperbaric oxygen treatment. Memory function of aMCI patients was ameliorated at 1‐, 3‐, and 6‐month follow‐up after hyperbaric oxygen treatment compared to baseline level (Table 2). Compared to control AD, the hyperbaric oxygen treated AD patients got significantly higher MoCA score at 1‐month follow‐up (Figure 1).
TABLE 2.
Self‐comparison of MoCA scores in AD and aMCI patients before and after hyperbaric oxygen treatment
| AD | aMCI | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1‐month follow‐up | 3‐month follow‐up | 6‐month follow‐up | Baseline | 1‐month follow‐up | 3‐month follow‐up | 6‐month follow‐up | |
| MoCA scores | 10.86 ± 6.70 | 12.71 ± 7.39 | 11.38 ± 6.64 | 10.53 ± 6.69 | 21.91 ± 3.27 | 24.73 ± 4.31 | 24.64 ± 5.07 | 25.18 ± 4.24 |
| P value | – | 0.000 * | 0.280 | 0.799 | – | 0.007 * | 0.059 | 0.019 * |
| Visuospatial abilities and executive functions | 1.45 ± 1.43 | 1.74 ± 1.36 | 1.60 ± 1.42 | 1.65 ± 1.44 | 3.82 ± 0.87 | 4.00 ± 1.00 | 4.18 ± 0.98 | 4.36 ± 0.67 |
| P value | – | 0.032 * | 0.275 | 0.094 | – | 0.414 | 0.340 | 0.058 |
| Naming | 1.86 ± 1.14 | 2.00 ± 1.04 | 1.83 ± 1.15 | 1.75 ± 1.08 | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | 2.91 ± 0.30 |
| P value | – | 0.326 | 0.837 | 0.572 | – | 1.00 | 1.00 | 0.317 |
| Memory | 0.48 ± 1.17 | 0.64 ± 1.34 | 0.48 ± 1.23 | 0.50 ± 1.20 | 1.00 ± 1.26 | 2.82 ± 2.09 | 2.73 ± 2.24 | 2.73 ± 2.05 |
| P value | – | 0.190 | 0.952 | 1.000 | – | 0.017 * | 0.024 * | 0.020 * |
| Attention and calculation | 3.00 ± 2.11 | 3.10 ± 1.96 | 3.21 ± 1.84 | 2.80 ± 1.90 | 5.64 ± 0.50 | 5.73 ± 0.47 | 5.64 ± 0.92 | 5.91 ± 0.30 |
| P value | – | 0.511 | 0.202 | 0.533 | – | 0.564 | 1.00 | 0.083 |
| Language and abstract reasoning | 1.81 ± 1.69 | 2.26 ± 1.86 | 1.50 ± 1.64 | 1.35 ± 1.59 | 3.33 ± 0.81 | 4.09 ± 0.30 | 3.73 ± 1.68 | 3.73 ± 1.74 |
| P value | – | 0.014 * | 0.040 * | 0.039 * | – | 0.033 * | 0.314 | 0.473 |
| Orientation to time and place | 2.40 ± 1.77 | 2.79 ± 1.96 | 2.36 ± 1.91 | 2.35 ± 1.73 | 5.00 ± 1.81 | 5.09 ± 1.22 | 5.36 ± 0.81 | 5.36 ± 1.21 |
| P value | – | 0.052 | 0.721 | 0.985 | – | 0.783 | 0.206 | 0.389 |
Data of MoCA scores in AD patients were analyzed by paired t test; data of MoCA scores in aMCI patients were analyzed by Wilcoxon signed‐rank test; data of the six subitems of MoCA scores in AD and aMCI patients were analyzed by Wilcoxon signed‐rank test.
P < 0.05. Sample size: n = 42 in AD patients (n = 40 at 6‐month follow‐up); n = 11 in aMCI patients. The P values of 1‐, 3‐, and 6‐month follow‐up were compared with the baseline level.
Abbreviations: AD, Alzheimer's disease: aMCI, amnestic mild cognitive impairment; MOCA, Montreal Cognitive Assessment
FIGURE 1.
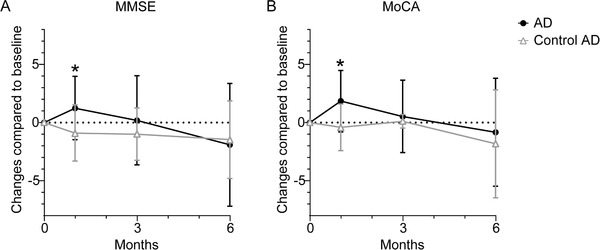
Neuropsychiatric assessments at 1‐, 3‐, and 6‐month follow‐up in hyperbaric oxygen treated Alzheimer's disease (AD) and control AD patients. A and B, Changes of Mini‐Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scores at 1‐, 3‐, and 6‐month follow‐up in AD after hyperbaric oxygen treatment versus control AD compared to baseline, respectively. Data were presented as mean ± standard deviation. Data of MMSE score in 1‐ and 6‐month follow‐up and MoCA score in 1‐, 3‐, and 6‐month follow‐up were analyzed by Mann‐Whitney test; data of MMSE score in 3 months was analyzed by t test. Sample size: n = 10 in 1‐month follow‐up, n = 9 in 3‐month follow‐up, and n = 11 in 6‐month follow‐up in control group; n = 42 in 1‐ and 3‐month follow‐up, n = 40 in 6‐month follow‐up in AD. * P < 0.05
3.2. ADL in AD and aMCI patients before and after hyperbaric oxygen treatment
Compared to the baseline level, the daily living activities of AD patients were significantly improved at 1‐month and 3‐month follow‐up after hyperbaric oxygen treatment (Table 3). There were no significant differences in ADL scores of aMCI patients after the hyperbaric oxygen treatment (Table 3).
TABLE 3.
Self‐comparison of ADL scores in AD and aMCI patients before and after hyperbaric oxygen treatment
| AD | aMCI | |||
|---|---|---|---|---|
| Time | Mean ± SD | P value | Mean ± SD | P value |
| Baseline | 38.19 ± 13.90 | – | 21.00 ± 1.26 | – |
| 1‐month follow‐up | 36.40 ± 13.89 | 0.008 * | 21.00 ± 1.41 | 1.000 |
| 3‐month follow‐up | 35.07 ± 14.14 | 0.004 * | 21.18 ± 1.78 | 0.655 |
| 6‐month follow‐up | 38.12 ± 14.21 | 0.678 | 20.73 ± 0.90 | 0.180 |
Data of ADL scale in AD and aMCI patients were analyzed by Wilcoxon signed‐rank test.
P < 0.05. Sample size: n = 42 in AD patients (n = 40 at 6‐month follow‐up); n = 11 in aMCI patients. The P values of 1‐, 3‐, and 6‐month follow‐up were compared with the baseline level.
Abbreviations: AD, Alzheimer's disease: ADL, Activities of Daily Living; aMCI, amnestic mild cognitive impairment; SD, standard deviation
3.3. MMSE, MoCA, and ADL scores in different stages, medications, and sexes in AD patients after hyperbaric oxygen treatment
The difference of MMSE, MoCA, and ADL scores in disease stages, medications, and sexes AD patients was further analyzed. As shown in Table S2 in supporting information, the MMSE score was increased significantly at 1‐month follow‐up after hyperbaric oxygen treatment in mild AD patients, the MoCA score was improved significantly at 1‐month follow‐up after hyperbaric oxygen treatment in mild and moderate AD patients, and the ADL score was increased significantly at 1‐month follow‐up after hyperbaric oxygen treatment in moderate AD patients. The difference of MMSE, MoCA, and ADL scores in AD patients with medications is shown in Table S3 in supporting information. The MMSE score was increased significantly at 1‐month follow‐up after hyperbaric oxygen treatment in AD patients maintained with ChEIs. And the MoCA score was improved significantly at 1‐month follow‐up after hyperbaric oxygen treatment in AD patients with combined medications (ChEIs + NMDA receptor antagonist). The ADL score was improved significantly at 1‐ and 3‐month follow‐up after hyperbaric oxygen treatment in AD patients with ChEIs. The difference of MMSE, MoCA, and ADL scores in AD patients male and female is shown in Table S4 in supporting information. The MMSE and MoCA scores were improved significantly at 1‐month follow‐up after hyperbaric oxygen treatment in both sexes. And the ADL score was increased significantly at 1‐ and 3‐month follow‐up after hyperbaric oxygen treatment in female AD patients.
3.4. Fluorodeoxyglucose positron emission tomography imaging before and after hyperbaric oxygen treatment
The representative images of AD and aMCI patients are shown in Figure 2. Compared to the images at the baseline, as the disease, AD patients showed a reduction of glucose metabolism in brain regions including right medial frontal gyrus, right posterior cingulate cortex, parietotemporal cortex, superior lateral temporal cortex, and left inferior frontal gyrus, but the hyperbaric oxygen treatment ameliorated the reduced glucose metabolism in brain regions including left medial frontal gyrus, right middle frontal gyrus, right inferior parietal lobule, left anterior cingulate gyrus, right associative visual cortex, left Broca area, and left inferior lateral anterior temporal cortex. Moreover, aMCI patients showed more brain regions with improved glucose metabolism including middle frontal gyrus, right inferior parietal lobule, right anterior cingulate gyrus, right Broca region, left parietotemporal cortex, superior lateral temporal cortex, left inferior frontal gyrus, right inferior lateral anterior temporal cortex, anterior medial temporal cortex, and left inferior lateral posterior temporal cortex after the hyperbaric oxygen treatment.
FIGURE 2.
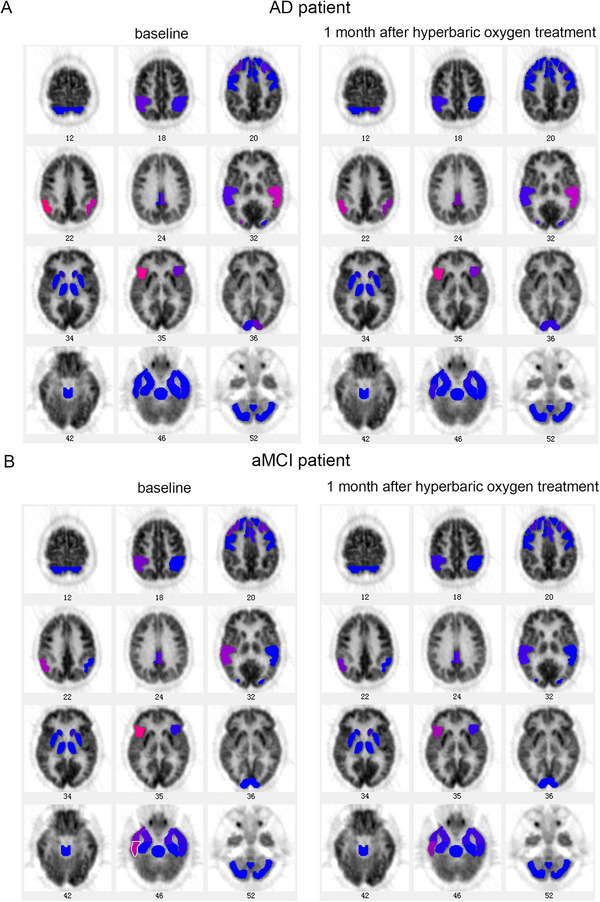
Representative images of fluorodeoxyglucose positron emission tomography. A, images of Alzheimer's disease (AD) patients before and 1 month after hyperbaric oxygen treatment; (B) images of amnestic mild cognitive impairment (aMCI) before and 1 month after hyperbaric oxygen treatment
4. DISCUSSION
Increasing evidence has shown that non‐pharmacological interventions have promising roles in the treatment of AD. 33 Non‐pharmacological therapies, such as transcranial magnetic stimulation, transcranial direct current stimulation, light therapy, regular and long‐term exercise, acupuncture, musical interventions, aromatherapy, and vagus nerve stimulation might have beneficial effects on cognitive and non‐cognitive functions. 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 In the present study, we applied one course of 20‐day hyperbaric oxygen treatment on AD and aMCI patients, showing that hyperbaric oxygen treatment could improve cognitive function at 1‐month follow‐up in AD patients. However, these improvements were not found 3 or 6 months after treatment, indicating that one course of hyperbaric oxygen treatment may temporarily ameliorate cognitive impairment in AD patients, but the effect was not permanent. In addition, the ADL score was improved in all 1‐, 3‐, and 6‐month follow‐up, implying that hyperbaric oxygen treatment could improve the patients’ daily living abilities with a longer benefit. Compared to control, one course of hyperbaric oxygen treatment significantly improved cognition in AD patients at 1‐month follow‐up, but the improvement was decreased with time. In aMCI patients, the hyperbaric oxygen treatment enhanced the cognitive function with longer beneficial effect. The results of MMSE, MoCA, and ADL scores in different stages of AD patients indicate that one course of hyperbaric oxygen treatment could improve cognitive impairment in AD patients with early stages. Compared to the currently approved drug donepezil that improved cognition by 0.7 in MMSE score up to 24 weeks, 42 in our study, one course of hyperbaric oxygen treatment can significantly enhance AD patients’ functions by 1.24 for at least 1 month, indicating that such treatment is a promising alternative therapy for AD, and it is possible that multiple courses of such therapy might have significantly longer benefits to AD patients. The images showed that hyperbaric oxygen treatment improved the glucose metabolism of AD patients in medial frontal gyrus associated with executive function, 43 and Broca area related to language processing, 44 which were consistent with the results of the MoCA scores. Thus, hyperbaric oxygen treatment may ameliorate cognitive function, delay the disease progress, and improve daily living activities in patients with AD in a limited duration. More interestingly, the MMSE scores in aMCI patients were significantly improved at 3‐month follow‐up, and the MoCA scores were significantly improved at 1‐month and 6‐month follow‐up. Images also showed improvement of glucose metabolism in brain regions associated with language function in aMCI patients, such as Broca area, inferior frontal gyrus, and superior lateral temporal cortex, and in brain regions linked to memory, such as anterior medial temporal lobe. The improvement of glucose metabolism seemed to be greater in aMCI patients than that in AD patients. The therapeutic effects in aMCI patients indicate that hyperbaric oxygen treatment might be a preventive strategy by blocking the conversion of aMCI to AD.
Hyperbaric oxygen treatment is a safe and routinely used medical procedure. Common complications of hyperbaric oxygen treatment include headache, claustrophobia, reversible myopia, and seizure. Most of those complications are mild and reversible when the treatment is stopped. 45 Some severe complications, such as oxygen toxicity and irreversible nuclear cataracts, are extremely rare. 46 , 47 In our study, only two patients experienced ear discomfort occasionally and the symptom was relieved when the procedure ended. Thus, hyperbaric oxygen treatment under 1.2 standard atmospheres and <1 hour per day is safe.
Aβ and tau pathologies are the two main hypotheses in AD onset and progression. Our previous study reported that chronic hypoxia can increase Aβ deposition and cause learning and memory deficits in AD mice. 5 Moreover, we found that chronic hypoxia can enhance the γ‐secretase activity to promote Aβ production through the downregulation of the DNA methyltransferase 3b to reduce the methylation at CpG sites in promoter region of genes of γ‐secretase components, indicating that chronic hypoxia can aggravate AD pathology through epigenetic modifications. 5 On the other hand, chronic hypoxia may upregulate protein kinases, including GSK‐3β and CDK5, and suppress phosphatase, PP2A, leading to an enhanced tau phosphorylation. 48 , 49 Because hypoxia is involved in AD pathogenesis, hyperbaric oxygen treatment targeting hypoxia may be helpful to delay or ameliorate the progression of AD. Studies have shown that hyperbaric oxygen treatment can improve cognitive behavioral performance, reduce Aβ load and tau hyperphosphorylation, and alleviate neuroinflammation in AD mouse models. 50 Studies have also shown that hyperbaric oxygen decreased lipid peroxidation, inhibited leucocyte activation, and restored the functional blood‐brain barrier. 16 Hyperbaric oxygen treatment might be a multi‐targeted non‐pharmacological intervention for AD. The underlying mechanisms need further investigation.
Hyperbaric oxygen treatment was occasionally studied in vascular dementia, showing that such therapy could improve the cognitive function and enhance the activities of daily living assessed by MMSE and ADL, respectively. 51 , 52 There was only a case report that hyperbaric oxygen treatment could reverse the patient's symptomatic decline and increase global brain metabolism assessed by. 53
In the present study, considering the ethic issue, approved drug treatment was maintained in hyperbaric oxygen treated AD and control AD. It is not known if the therapeutic effect of hyperbaric oxygen treatment is directly acting on brain functions or indirectly influencing the efficacy of the medications undertaken by AD patients. Multiple‐center studies with larger numbers of participants with or without medications, multiple courses of treatments, and longer time follow‐up, should be applied in the future study. Moreover, more measurements of AD progression in addition to neuropsychiatric assessments, such as Aβ levels and neuroimaging, should be performed. Furthermore, earlier application of hyperbaric oxygen treatment in aMCI or in the early stage AD is mostly needed to validate if the procedure can block the conversion from aMCI to AD and reduce the cognitive decline in AD.
CONFLICTS OF INTEREST
All the authors have no conflicts of interest to declare.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
This work was supported in part by funding from the National Natural Sciences Foundation of China (NSFC 81430021 and 81771521).
Chen J, Zhang F, Zhao L, et al. Hyperbaric oxygen ameliorates cognitive impairment in patients with Alzheimer's disease and amnestic mild cognitive impairment. Alzheimer's Dement. 2020;6:e12030 10.1002/trc2.12030
Jianwen Chen, Feng Zhang and Li Zhao: These authors contributed equally to this work.
Contributor Information
Chunbo Dong, Email: dcb101@sina.com.
Weidong Le, Email: wdle@sibs.ac.cn.
REFERENCES
- 1. Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. [DOI] [PubMed] [Google Scholar]
- 2. Karch CM, Cruchaga C, Goate AM. Alzheimer's disease genetics: from the bench to the clinic. Neuron. 2014;83:11‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88:640‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang F, Niu L, Li S, Le W. Pathological impacts of chronic hypoxia on Alzheimer's disease. ACS Chem Neurosci. 2019;10:902‐909. [DOI] [PubMed] [Google Scholar]
- 5. Liu H, Qiu H, Yang J, Ni J, Le W. Chronic hypoxia facilitates Alzheimer's disease through demethylation of gamma‐secretase by downregulating DNA methyltransferase 3b. Alzheimers Dement. 2016;12:130‐143. [DOI] [PubMed] [Google Scholar]
- 6. Liu H, Qiu H, Xiao Q, Le W. Chronic hypoxia‐induced autophagy aggravates the neuropathology of Alzheimer's disease through AMPK‐mTOR signaling in the APPSwe/PS1dE9 mouse model. J Alzheimers Dis. 2015;48:1019‐1032. [DOI] [PubMed] [Google Scholar]
- 7. Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Abeta generation by altering beta‐ and gamma‐cleavage of APP. Neurobiol Aging. 2009;30:1091‐1098. [DOI] [PubMed] [Google Scholar]
- 8. Zhang F, Zhong R, Qi H, et al. Impacts of acute hypoxia on Alzheimer's disease‐like pathologies in APP(swe)/PS1(dE9) mice and their wild type littermates. Front Neurosci. 2018;12:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daulatzai MA. Death by a thousand cuts in Alzheimer's disease: hypoxia–the prodrome. Neurotox Res. 2013;24:216‐243. [DOI] [PubMed] [Google Scholar]
- 10. Lin PJ, Zhong Y, Fillit HM, Cohen JT, Neumann PJ. Hospitalizations for ambulatory care sensitive conditions and unplanned readmissions among medicare beneficiaries with Alzheimer's disease. Alzheimers Dement. 2017;13:1174‐1178. [DOI] [PubMed] [Google Scholar]
- 11. Yaffe K, Laffan AM, Harrison SL, et al. Sleep‐disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma VK, Das SK, Dhar P, et al. Domain specific changes in cognition at high altitude and its correlation with hyperhomocysteinemia. PLoS One. 2014;9:e101448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rimoldi SF, Rexhaj E, Duplain H, et al. Acute and chronic altitude‐induced cognitive dysfunction in children and adolescents. J Pediatr. 2016;169:238‐243. [DOI] [PubMed] [Google Scholar]
- 14. Hu SL, Xiong W, Dai ZQ, Zhao HL, Feng H. Cognitive changes during prolonged stay at high altitude and its correlation with C‐reactive protein. PLoS One. 2016;11:e0146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang CC, Ho CH, Chen YC, et al. Hyperbaric oxygen therapy is associated with lower short‐ and long‐term mortality in patients with carbon monoxide poisoning. Chest. 2017;152:943‐953. [DOI] [PubMed] [Google Scholar]
- 16. Bennett MH, Weibel S, Wasiak J, Schnabel A, French C, Kranke P. Hyperbaric oxygen therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014(11):CD004954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112‐2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu LW. Alzheimer's disease: early diagnosis and treatment. Hong Kong Med J.. 2012;18:228‐237. [PubMed] [Google Scholar]
- 19. Graham WV, Bonito‐Oliva A, Sakmar TP. Update on Alzheimer's disease therapy and prevention strategies. Annu Rev Med. 2017;68:413‐430. [DOI] [PubMed] [Google Scholar]
- 20. Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer's disease: focus on disease modifying drugs. Br J Clin Pharmacol. 2012;73:504‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pedersen JT, Sigurdsson EM. Tau immunotherapy for Alzheimer's disease. Trends Mol Med. 2015;21:394‐402. [DOI] [PubMed] [Google Scholar]
- 22. Khachaturian ZS, Kuller LH, Khachaturian AS. Strategic goals and roadmap for dementia prevention by stroke prevention. Alzheimers Dement. 2019;15:865‐869. [DOI] [PubMed] [Google Scholar]
- 23. Hachinski V, Einhaupl K, Ganten D, et al. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimers Dement. 2019;15:961‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sachdev PS, Blacker D, Blazer DG, et al. Classifying neurocognitive disorders: the DSM‐5 approach. Nat Rev Neurol. 2014;10:634‐642. [DOI] [PubMed] [Google Scholar]
- 25. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS‐ADRDA criteria. Lancet Neurol. 2007;6:734‐746. [DOI] [PubMed] [Google Scholar]
- 26. Frota NAF, Nitrini R, Damasceno BP, et al. Criteria for the diagnosis of Alzheimer's disease: recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Dement Neuropsychol. 2011;5:146‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Portet F, Ousset PJ, Visser PJ, et al. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry. 2006;77:714‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183‐194. [DOI] [PubMed] [Google Scholar]
- 29. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 30. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695‐699. [DOI] [PubMed] [Google Scholar]
- 31. Velayudhan L, Ryu SH, Raczek M, et al. Review of brief cognitive tests for patients with suspected dementia. Int Psychogeriatr. 2014;26:1247‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sikkes SA, de Lange‐de Klerk ES, Pijnenburg YA, Scheltens P, Uitdehaag BM. A systematic review of instrumental activities of daily living scales in dementia: room for improvement. J Neurol Neurosurg Psychiatry. 2009;80:7‐12. [DOI] [PubMed] [Google Scholar]
- 33. Bahar‐Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer's or vascular type: a review. Alzheimers Res Ther. 2013;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Revesz D, Rydenhag B, Ben‐Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr. 2016;18:97‐104. [DOI] [PubMed] [Google Scholar]
- 35. Wang Z, Liang P, Zhao Z, et al. Acupuncture modulates resting state hippocampal functional connectivity in Alzheimer disease. PLoS One. 2014;9:e91160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jimbo D, Kimura Y, Taniguchi M, Inoue M, Urakami K. Effect of aromatherapy on patients with Alzheimer's disease. Psychogeriatrics. 2009;9:173‐179. [DOI] [PubMed] [Google Scholar]
- 37. Ohman H, Savikko N, Strandberg TE, et al. Effects of exercise on cognition: the finnish Alzheimer disease exercise trial: a randomized, controlled trial. J Am Geriatr Soc. 2016;64:731‐738. [DOI] [PubMed] [Google Scholar]
- 38. Samson S, Clement S, Narme P, Schiaratura L, Ehrle N. Efficacy of musical interventions in dementia: methodological requirements of nonpharmacological trials. Ann N Y Acad Sci. 2015;1337:249‐255. [DOI] [PubMed] [Google Scholar]
- 39. Cantone M, Di Pino G, Capone F, et al. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin Neurophysiol. 2014;125:1509‐1532. [DOI] [PubMed] [Google Scholar]
- 40. Riemersma‐van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642‐2655. [DOI] [PubMed] [Google Scholar]
- 41. Manenti R, Cotelli M, Robertson IH, Miniussi C. Transcranial brain stimulation studies of episodic memory in young adults, elderly adults and individuals with memory dysfunction: a review. Brain Stimul. 2012;5:103‐109. [DOI] [PubMed] [Google Scholar]
- 42. Tariot PN, Cummings JL, Katz IR, et al. A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting. J Am Geriatr Soc. 2001;49:1590‐1599. [PubMed] [Google Scholar]
- 43. Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no‐go decisions based on “what,” “when,” and “where” related information: an fMRI study. J Cogn Neurosci. 2005;17:981‐993. [DOI] [PubMed] [Google Scholar]
- 44. Flinker A, Korzeniewska A, Shestyuk AY, et al. Redefining the role of Broca's area in speech. Proc Natl Acad Sci U S A. 2015;112:2871‐2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dauwe PB, Pulikkottil BJ, Lavery L, Stuzin JM, Rohrich RJ. Does hyperbaric oxygen therapy work in facilitating acute wound healing: a systematic review. Plast Reconstr Surg. 2014;133:208e‐215e. [DOI] [PubMed] [Google Scholar]
- 46. McMonnies CW. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin Exp Optom. 2015;98:122‐125. [DOI] [PubMed] [Google Scholar]
- 47. Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68:113‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao L, Tian S, Gao H, Xu Y. Hypoxia increases Abeta‐induced tau phosphorylation by calpain and promotes behavioral consequences in AD transgenic mice. J Mol Neurosci. 2013;51:138‐147. [DOI] [PubMed] [Google Scholar]
- 49. Zhang F, Chen J, Zhao L, Dong C. Candidate biomarkers of multiple system atrophy in cerebrospinal fluid. Rev Neurosci. 2014;25:653‐662. [DOI] [PubMed] [Google Scholar]
- 50. Shapira R, Solomon B, Efrati S, Frenkel D, Ashery U. Hyperbaric oxygen therapy ameliorates pathophysiology of 3xTg‐AD mouse model by attenuating neuroinflammation. Neurobiol Aging. 2018;62:105‐119. [DOI] [PubMed] [Google Scholar]
- 51. Xu Y, Wang Q, Qu Z, Yang J, Zhang X, Zhao Y. Protective effect of hyperbaric oxygen therapy on cognitive function in patients with vascular dementia. Cell Transplant. 2019;28:1071‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiao Y, Wang J, Jiang S, Luo H. Hyperbaric oxygen therapy for vascular dementia. Cochrane Database Syst Rev. 2012(7):CD009425. [DOI] [PubMed] [Google Scholar]
- 53. Harch PG, Fogarty EF. Hyperbaric oxygen therapy for Alzheimer's dementia with positron emission tomography imaging: a case report. Med Gas Res. 2018;8:181‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


