Abstract
Chromone derivatives possess a spectrum of biological activities. Chromone has been recognized as a privileged structure for new drug invention and development. Substitution pattern of chromone scaffold determines different type of biological activities. The type, number and position of substituents connected to the chromone core play a vital role in determining pharmacological activities. In the present review, we have discussed new chromone derivatives as anticancer, anti-diabetic, antimicrobial, anti-inflammatory, antioxidant and as anti-Alzheimer agents. This review deals with the chromone derivatives prepared by combining chromone molecule with various natural and synthetic pharmacophores and pharmacological activities presented by them. The main aim is to highlight the diversified pharmacological activities exhibited by chromone hybrid molecules during the last eight to ten years.
Keywords: chromones; anticancer; antimicrobial; anti-Alzheimer; anti-inflammatory; antioxidant, anti-diabetic
1. INTRODUCTION
Chromone is a heterocyclic compound containing oxygen as heteroatom and has a benzo-γ-pyrone skeleton (Fig. 1, compound 1). Chromone is a natural molecule present in the diet of human and animals and shows less toxicity to mammalian cells [1]. Chromone containing natural and synthetic molecules displayed interesting biological activities [2]. Medicinal properties exhibited by chromone derivatives are antibacterial, antifungal, antioxidant, antimalarial, neuroprotective and HIV inhibitory potential [3–8]. Chromone derivatives have also shown promising anticancer and antiviral potential [9–13]. Anti-inflammatory, antiallergenic and antiulcer are other properties displayed by chromone derived molecules [14–16]. Chromone is treated as an attractive source for the synthesis of new drugs due to its valuable activities and low toxicity [17]. Chromone is considered as a single molecule which can combine with different types of receptors [18]. Modifications of chromone scaffold have been performed at benzene or pyrone ring by attachment of different substituents. 3-Formylchromone (Fig. 1, compound 2) is a frequently used precursor for the synthesis of chromone derivatives and can be prepared easily by the Vilsmeir – Haack reaction [19].
Fig. 1.
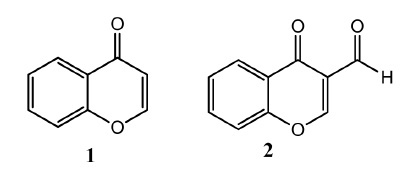
Structural formulas of (1) chromone and (2) 3-formyl chromone.
2. CHEMISTRY AND PHARMACOLOGY OF NEW DRUGS BASED ON CHROMONE SCAFFOLD
Chromone derived molecules have been discussed with respect to chemical structures, pharmacological activities and structure – activity relationship (SAR) in following sections.
2.1. Chromone Derivatives as Anticancer Agents
Cancer is a serious problem all around the world due to high mortality and there is demand to discover new leads as anticancer agents [20]. A number of anticancer drugs have been discovered from natural sources [21]. Chromone derived molecules have displayed excellent anticancer activities. Amin and co-workers synthesized new molecules by merging benzofuran and 5H- furo[3,2g]chrome-5-one and attached different heterocyclic rings through sulfonamide group. These compounds displayed in vitro activity against breast cancer cell line (MCF-7) ranging from 0.004 – 0.87 μM. Compound 3 (Fig. 2) presented prominent in vitro activity (IC50 = 0.056 ± 0.0027 μM) against MCF-7 cell line in comparison to standard drug (doxorubicin, IC50 = 0.62 0.0316 μM) and proved less toxicity to normal cell line (IC50 = 23 ± 1.02 μM). These derivatives also showed p38α MAPK (mitogen-activated protein kinase) inhibition activity. MAPK controls many biological functions such as cell growth, differentiation and inflammation [22]. Molecular docking studies showed that compound 3 formed four hydrogen bonds with K-53, M-109 and G-170 amino acids of MAPK [23]. Singh, et al. attached indole, pyrimidine, pyrazole with chromone to produce new derivatives. It was observed that introduction of 2,6-dichlorophenyl, 2,6-dichlorobenzoyl group along with indolinone produce notable activities. Compound 4 manifested prominent in vitro antitumor activity with 50 – 90% growth inhibition of all tumor cell lines and showed an average GI50 value of 3.2 μM. Compound 4 was more potent against leukemia (RPMI-8226 GI50 = 1.2 μM, SR GI50 = 1.4 μM) cell line, colon (HCT-15, GI50 = 0.6 μM), prostate (PC-3, GI50 = 1.3 μM), CNS (U251, GI50 = 1.4 μM) and melanoma cancer cell lines (LOX-IMVI, GI50 = 1.5 μM) [24]. Synthesis of chromone and sulfonamide comprising molecules was carried out by Awadallah, et al. in which two molecules were linked to each other by a large heterocyclic ring or by small linker groups such as methine amine or alkyl amine. Compound separated by small linker group dispensed greater activity. Upon in vitro evaluation, Compound 5 emerged as the most active against breast (MCF-7, IC50 = 0.72 μM) and lung (A-549, IC50 = 0.50 μM) cancer cell lines as compared to doxorubicin (MCF-7 IC50 = 33.13 ± 2.90 μM, A-549 IC50 = 26.81 ± 2.50 μM). Compound 5 presented selectivity for isoforms IX and XII of the human carbonic anhydrase (hCA). This compound induced apoptosis in both types of cancer cell. It was also observed that compounds having free sulfonamide group presented higher activity. When the sulfonamide group was attached with heterocyclic scaffold such as pyridine, pyrimidine, and isoxazole, less active derivatives were obtained [25]. Chen, et al. attached chromone molecule to 1-alkyl-1H-imidazole-2-yl via dienone as linker group. The nitrogen-containing heterocycles were used as bioisostere for phenols in the natural compound while the dienone linker was used as substitute for dienone in curcumin. Compound 6 presented excellent activity against prostate cancer (PC-3, IC50 = 1.8 ± 0.3 μM and LNCaP IC50 = 1.0 ± 0.2 μM) cell lines. The nitrogen atom of imidazole carries ethyl group. Replacement of ethyl group by longer chain has no significant influence on anticancer activity. Therefore they are excellent molecules for future investigations [26]. Dolatkhah, et al. used the three-component reaction involving chromone-3-carboxaldehyde, alkyl acetoacetate, urea or thiourea to produce 4H-chromone-1,2,3,4-tetrahydropyrimdine-5-carboxylates using MCM-41-SO3H nanoparticles as catalyst. The catalyst can be recycled and reused. Compound 7 presented prominent activity against leukemia cell line upon evaluation by microculture tetrazolium test (MTT) assay. This compound showed no toxicity to normal cell line human foreskin fibroblast (Hu02). Compound 5 showed high affinity (binding energy = -10.10 kcal/mol) with Ab1-kinase enzyme by Autodock-4 program [27]. Nam, et al. developed chromone derived analogues of lavendustin. Upon anticancer evaluation, compounds 8 (IC50 = 6.01 2.7 μM) and 9 (IC50 = 9.92 ± 3.6 μM) showed prominent activities against A-549 cell line. Compound 8 (IC50 = 6.89 2.6 μM) and 9 (IC50 = 7.86 ± 2.2 μM) also showed activity against HCT-15 cell lines. In compound 8, replacement of 4-methoxybenzyl with benzyl or phenethyl decreased the activity. In compound 9, replacement of 4-nitrobenzyl with 4-methoxybenzyl or benzyl group produced less active compounds against HCT-15 cell line [28].
Fig. 2.
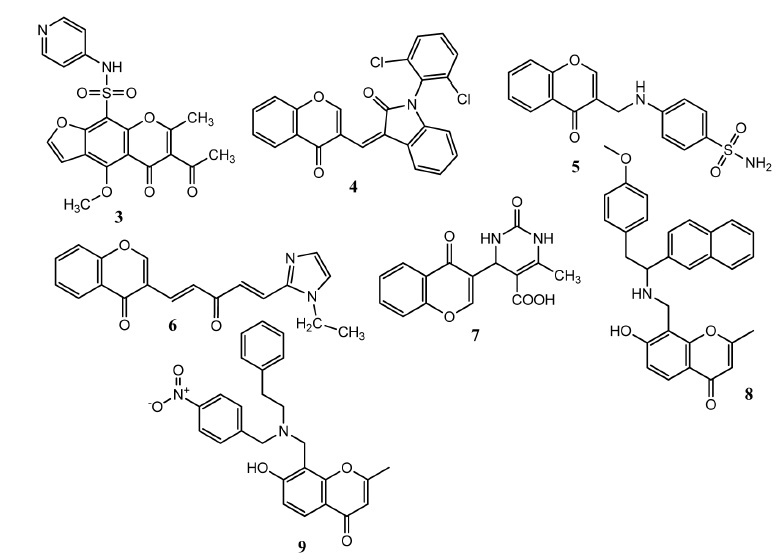
Chromone derived molecules 3 – 9 as anticancer agents.
Bhatia and co-workers synthesized chalcone-chromenone derived molecules. Upon in vitro evaluation, compound 10 (Fig. 3) showed prominent activity (87% growth inhibition) against colon cancer cell line (HCT-116) as compared to fluorouracil (67% inhibition). Compound 10 carries two halogen atoms each on the chromone core and chalcone fragment [29]. Ozen, et al. conjugated chromone molecule with 5-membered heterocyclic scaffolds thiazolidindiones, imidazolidindiones and thiohydantoins to produce new hybrid molecules. Compound 11 showed prominent activity against liver (Huh-7 IC50 = 5.2 μM) and breast cancer (MCF-7, IC50 = 4.9 μM) cell lines. This compound contains unsubstituted chromone while ethyl group is attached with thiohydantoin. When the chromone ring is substituted with methyl group, resultant compound showed less activity. Compound 11 did not show apoptosis but only reduced the replication of cells [30]. Singh and co-workers synthesized chromenopyridine derivatives based on the activity of chromones and pyridones. Derivatives bearing electron withdrawing groups at positions # 5, # 6 and # 7 of chromone scaffold showed better activity. Compound 12 appeared as the most potent against PC-3 (IC50 = 2.4 ± 3.4 μM), MCF-7 (IC50 = 10.7 ± 2.5 μM) and Hela (IC50 = 7.0 ± 3.5 μM) cancer cell lines. This compound contains allyl group attached to pyridone and showed better activity as compared to unsubstituted compounds [31]. Obreque-balbua and coworkers synthesized chromone derivatives as ABCC1 modulators. An amino or carboxamide group was introduced at position # 2. ABCC1 overexpression is detected in different types of cancers. Compound 13 was the most effective (IC50 = 11.3 ± 1.8 μM) in reducing the ABCC1 mediated resistance in comparison to reversan (IC50 = 4.3 ± 0.2 μM). Derivative 13 showed selectivity for ABCC1 over ABCG2 and ABCB1. It consists of chromone molecule connected to benzo[1, 2, 5]oxadiazole via piperazine linker group. Insertion of carbonyl group between chromone and piperazine decreased the activity [32]. Abdelhafez at al. synthesized benzofuran derivatives as vascular endothelial growth factor (VEGF) inhibitor. Bromovisnagin (14) was an intermediate product and consist of chromone core in its structure. Bromovisnagin (14) showed prominent anticancer (IC50 = 3.67 × 10 –7 – 7.65 × 10 –13 μM) potential as compared to other products in this series. Compound 14 was the most potent agent against MCF-7 (IC50 = 3.67 × 10 –7) as compared to standard drug epirubicin (IC50 = 2.22 × 10 –9). Docking studies of compound 14 with VEGFR2 (vascular endothelial growth factor receptor) kinase enzyme showed hydrogen bonding between furan oxygen and amino group of D1046. Methoxy group showed interaction with NH moiety of K868 [33]. Chand and colleagues synthesized substituted chromone, 4-oxo-4H-1-benzopyran derivatives as anticancer agents. Compounds having acrylate group at position # 3 demonstrated prominent activity. Derivatives having amide and acid group at this position proved less active. Compound 15 was the most active (50 – 60% inhibition) agent against colon (HT-29), breast (SK-OV-3) and ovarian (MDA-468) cell lines as compared to doxorubicin (80% inhibition). Compound 15 did not show in vitro Src kinase inhibitory activity (IC50 < 300 μM) as compared to staurosporin (IC50 = 0.6 μM) [34]. Han and colleagues synthesized succinimide derivatives by C-H functionalization of chromone, naphthoquinone and xanthone scaffolds with maleimide scaffold. Chromone derivatives (16a, b, c) showed only minute activity (IC50 <50 μM) versus MCF cell line [35]. EL-Garah, et al. synthesized chromone carboxamide derivatives. Compound 17 presented prominent activity (IC50 = 0.9 μM) against MCF-7 cancer cell line in comparison to tamoxifen (IC50 = 0.39 ± 0.01 μM). Structure activity relationship (SAR) studies highlighted that attachment of fluorine atom at position # 6 of chromone core produced more active derivatives. Replacement of fluorine atom by chloro and methyl groups produced less active derivatives. The presence of propyl side chain at the amide group also increased the activity. Compound 18 also presented in-vitro anti-inflammatory activity (79.9 ± 6.6% inhibition) as lipoxygenase (LOX) inhibitor. Derivatives having hydrophilic group showed better anti-inflammatory activity [36]. Ali, et al. synthesized chromone annulated phosphorous heterocycles as anticancer agents. Phosphorous reagents used in the synthesis were phosphorous halides, phosphorous sulfides and phosphonic acid. Anticancer activity was evaluated by using crystal violet blue assay. Compounds 19 and 20 exhibited prominent activities against HepG-2 (IC50 = 1.61 μg/mL, IC50 = 2.49 μg/mL) and HCT-116 (IC50 = 1.72 μg/mL, IC50 = 1.56 μg/mL) as compared to doxorubicin (Hep-G-2 IC50 = 0.467 μg/mL, HCT-116 IC50 = 0.468 μg/mL). The thiophosphoryl (P=S) bond was described as major cause of cytotoxicity of these compounds as compared to phosphoryl group (P=O) [37].
Fig. 3.
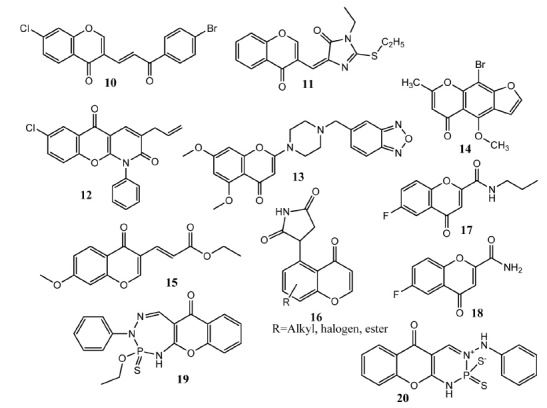
Chromone derivatives 10 – 20 as anticancer agents.
Sun, et al. carried out synthesis of thiopyrano[4,3-d]pyrimidne derivatives having chromone molecules. Compound 21 displayed significant inhibition of mTOR (IC50 = 1.10 ± 0.10 μM) and p13kα (IC50 = 0.92 ± 0.12 μM)kinases. Compound 21 (Fig. 4) displayed marvelous in vitro activity against MCF-7 (IC50 = 11.8 ± 6.9 μM), Hela (IC50 = 10.3 ± 0.58 μM) and HepG2 (IC50 = 8.77 ± 0.83 μM) cancer cell lines as evaluated by MTT assay. Docking investigation with mTOR and p13kα kinases highlighted that morpholine, chromone and hydrazinyl groups are essential for antitumor activities. Substitution on the chromone nucleus manifested significant influence on activity. Introduction of carboxylic group on the chromone core proved more active as compared to methyl, ethyl and hydroxyl group [38]. Abu-Aisheh, et al. synthesized amidrazone derivatives having flavone scaffold. Upon evaluation as anticancer agents by MTT assay, compounds 22 (IC50 = 1.42 ± 0.13 μM) and 23 (IC50 = 2.92 ± 0.94 μM) showed prominent in vitro activity versus breast cancer cell line (T47D) as compared to doxorubicin (IC50 = 0.33 ± 0.05 μM). Replacement of piperazine ring by piperidine produced less active agents against breast cancer. Derivatives having phenyl group at position # 2 presented higher activity as compared to methyl group. These compounds can be good candidates as anticancer agents [39]. Singh, et al. synthesized chromone and isoxazolidine based compounds by regioselective and stereoselective 1,3-dipolar cycloaddition reaction. Compound 24 (IC50 = 0.7 μM) exhibited prominent in vitro activity against A549 cell line in comparison to paclitaxel (IC50 = 0.4 μM). This compound bears disubstituted isoxazolidine scaffold. This compound also induced apoptosis in HL-60 cancer cell line [40]. Satyajit Singh and co-workers synthesized chromano piperidine fused isoxazolidine molecules. Derivatives having electron withdrawing groups at the chromone core presented superior activity. Compounds having aromatic ring attached with isoxazolidine presented better activity. Compound 25 showed prominent in vitro cytotoxicity against COLO (IC50 = 12.6 μM) as compared to fluorouracil (IC50 = 21 μM). Compound 25 also exhibited moderate activity against IMR-32 (IC50 = 55.2 μM) as compared to adriamycin (IC50 = 1.7 μM). Compound 26 (IC50 = 10.7 μM) presented prominent activity against neuroblastoma (IMR-32) cell line[41]. Zwergel and co-workers synthesized chromone and coumarin based benzofuran derivatives. Biological evaluation on human leukemia (k562) cell line was carried out. Compounds 27 and 28 exhibited prominent activities having 72% and 63% cell survival respectively by using 50 μM concentrations. Compound 27 showed 24% apoptosis. Substitution on chromone nucleus does not have significant influence on activity. Replacement of benzofuran by naphthofuranone produced more active compound towards apoptosis [42]. Zhu, et al. designed and synthesized thienopyrimidine and chromone derived compounds as mTOR/P13Kα inhibitors [43]. Compound 29 (97.2 ± 0.4% inhibition) presented prominent activity against mTOR/P13Kα kinase. Derivative 29 also manifested notable in vitro activity versus H460 (IC50 = 1.2 ± 0.3 μM) and PC-3 (IC50 = 0.85 ± 0.04 μM) cell lines in comparison to positive control (IC50 = 9.52 ± 0.29 μM, IC50 = 16.27 ± 0.54 μM against H-460 and PC-3). Chromone molecule having carboxylic acid or nitro group was found to be optimum for the activity in this series. Docking studies revealed that chromone molecule is vital for activity. Carboxylic group of chromone interacted via hydrogen bonding with TRP-2239 and ARG-2348. Morpholine group formed hydrogen bonding with VAL-2240 [44].
Fig. 4.
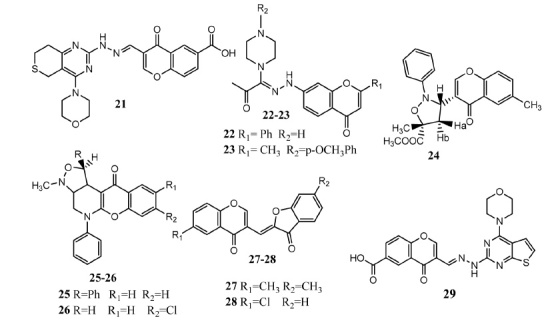
Chromone derivatives 21 – 29 as anticancer agents.
2.2. Chromone Derivatives as Antidiabetic Agents
Diabetes Mellitus is caused by the elevated level of glucose in the blood due to improper production of insulin. It is a very common problem all around the world [45]. Currently available anti-diabetic drugs are linked with various side effects [46]. Anti-diabetic properties of chromone derivatives are described in this section. Ceylan-unlusoy, et al. attached chromone scaffold with 2,4-imidazolidindione and 2,4-thiazolidindione. Attachment of heterocyclic rings at position # 3 produced more active derivatives as compared to position # 2. Compounds 30 (142.7 ± 17.60%) and 31 (155.4 ± 35.16%) (Fig. 5) showed prominent in vitro insulinotropic potential with dose of 1 μg/mL as compared to standard drug glibenclamide (138 ± 13.99%). Incorporation of methyl and ethyl group in the heterocyclic ring showed comparable activity to unsubstituted derivatives [47]. Later on, Ceylan-unlusoy, et al. synthesized chromone derivatives having imidazolidindione, 2,4-thiazolidindione and 2-thioxoimidazolidine-4-one heterocyclic cores. Derivatives having substituted 2,4-thiazolidinione ring demonstrated better activity and the most active agent carries methyl group at heterocyclic nitrogen. Therefore lipophilic groups at the heterocyclic nitrogen were found significant for increasing the activity. Compound 32 (dose = 0.001 μg/mL) was the most prominent which increased the release of insulin (120.6 ± 13.53%) as compared to glibenclamide (145.7 ± 7.74%) [48]. Wang, et al. linked chromone to the benzene sulfonamide through hydrazone linkage to produce new α-glucosidase inhibitors. The α-glucosidase enzyme is involved in the hydrolysis of carbohydrates and releases glucose. Compound 33 showed excellent in vitro inhibition (IC50 = 20.1 ± 0.19 μM) of this enzyme in comparison to acarbose (IC50 = 817.38 ± 6.27 μM). Compound 33 carries a free sulfonamide group attached to the phenylhydrazone. Replacement of phenylhydrazone by benzohydrazide and benzene ring by thiophine ring produced less active molecules. Compound 33 displayed non-competitive inhibition as determined by Lineweaver-Burk plot. In molecular docking studies, hydrogen bonding was observed between the drug molecule and Asp-214 and Arg-439 of enzyme [49]. One year later, Wang, et al. synthesized hybrid molecules comprising chromone-isatin as α-glucosidase inhibitors. These molecules exhibited excellent in vitro blockage of this enzyme and compound 34 (IC50 = 3.18 ± 0.12 μM) appeared as the most potent in this series. Substitution on the chromone core was very important where introduction of hydroxy group significantly enhances the activity. Molecular docking studies also showed interaction (binding energy = -8.8 kcal/mole) of this compound with the glucosidase enzyme. 4-Bromophenyl moiety showed hydrophobic interactions with Phe-157 and Phe-177, Ala-278 and Phe-300. Benzopyrone formed ð-ð interaction with Phe-300. Hydroxyl group formed hydrogen bonding with Gln-350 [50]. Alpha-amylase is an enzyme involved in the hydrolysis of carbohydrates. Salar, et al. connected chromone to hydrazinyl thiazole to produce the new hybrid molecules. Upon in vitro evaluation as the α-amylase inhibitors, Compound 35 showed prominent inhibition (IC50 = 2.826 ± 0.06 μM) of this enzyme in comparison to acarbose (IC50 = 1.9 ± 0.07 μM). Compound 35 showed a docking score of -7.1717 and π-π interactions with TRP-59 of α-amylase. Compound 35 also exhibited DPPH (2,2-diphenyl-1-picrylhydrazyl) (IC50 = 1.13 ± 0.15 μM) and
Fig. 5.
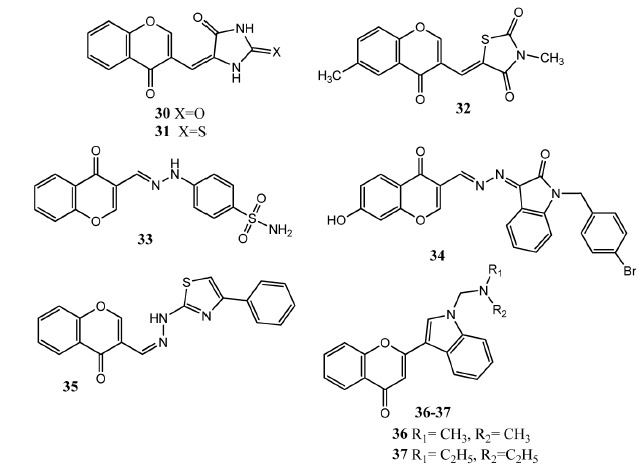
Chromone derivatives 30 – 37 as antidiabetic agents.
ABTS (2,2/-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (IC50 = 1.083 ± 0.15 μM) radical scavenging activities. Compound 35 bears unsubstituted phenyl ring with thiazole. Replacement of phenyl group with biphenyl core also produced more active compounds. Incorporation of hydroxyl group at the aromatic ring decreased the activity. Dichloro-substituted derivatives at the aromatic ring produced more active compounds as compared to mono-substituted compounds. Attachment of methyl group with chromone core also produced less active derivatives [51]. Parthiban, et al. synthesized quercetin based chromone derivatives as alpha-amylase inhibitors. Various modifications in the chromone core of quercitin were carried out such as introduction of furan and indole ring. Compounds 36 (IC50 = 13 ± 0.16 μM) and 37 (IC50 = 12 ± 0.14 μM) presented prominent in vitro activities as alpha amylase inhibitor as compared to quercitin (IC50 = 22 ± 0.01 μM). In these derivatives chromone core is attached with indole ring. Derivatives having aliphatic side chains at the indole nitrogen presented higher activity as compared to aromatic rings. Molecular docking studies showed that chromone core forms hydrophobic interaction with Trp58, Tyr62 and Leu165. Diethylamino group of compound 37 interacted via hydrogen bonding as well as hydrophobic interaction with Glu233 and Ile235. These derivatives have the potential for further investigations as anti-diabetic agents [52].
2.3. Antimicrobial Activity of Chromone Derived Molecules
Parasites such as bacteria, viruses, fungi, protozoa, trypanosomes, etc. cause various diseases in human beings and are responsible for high mortality and morbidity [53]. Chromone derivatives have also displayed antimicrobial activities. Bathini and co-workers synthesized chromone-2,3-dihydroquinazolin-4-one molecules in which dihydroquinazolinone was attached at position # 2. Compounds 38 and 39 (Fig. 6) were most active against Staphylococcus aureus (S. aureus) (MIC = 62.5 μg/mL) and Escherichia coli (E. coli) (MIC = 62.5 μg/mL) in comparison to ciprofloxacin (MIC = 25 – 50 μg/mL). Compound 40 presented antifungal activity (MFC = 200 μg/mL) against Candida albicans (C. albicans) as compared to griseofulvin (MFC = 500 μg/mL). Derivatives bearing halogen atoms at the chromone core displayed excellent activities [54]. Coa, et al. synthesized quinoline-chromone hybrid molecules as anti-leishmanial and antitrypanosomal agents. Although compound 41 demonstrated noticeable activities against Trypanosoma cruzi (T. cruzi, EC50 = 4.09 ± 0.24 μM), Leishmania panamensis (L. panamensis, EC50 = 6.11 ± 0.26 μM) respectively but it was masked by greater cytotoxicity (LC50 = 5.4 ± 0.9 μM) for mammalian U937 cells [55]. After one year, antiprotozoal hybrid molecules of chromones, quinoline and imidazole with furanchalcone were developed by Garcia, et al. Among these derivatives, chromone derived compounds did not show promising activity and also proved cytotoxic. Compound 43 displayed moderate in vitro activity against T. cruzi (EC50 = 25.58 ± 1.34 μM, SI = 1.07) as compared to standard drug benznidazole (EC50 = 40.3 ± 6.92 μM, SI = 17). Compounds 42 (EC50 = 44.02 ± 4.90 μM, SI = 0.69) and 43 (EC50 = 46 ± 4.77 μM, SI = 0.59) demonstrated moderate activity against L. panamensis as compared to standard drug meglumine antimonate (EC50 = 25.68 ± 5.74 μM). Compounds containing alkyl chain of 8 or 9 carbon atoms between chromone and furanchalcone presented higher activity [56]. Otero, et al. synthesized hybrid molecules of triclosan with chromone, coumarin and chalcone. Among the chromone derivatives, compound 44 (EC50 = 2.7 ± 0.4 μM, SI = 2.4) was found to be most active as compared to meglumine antimonate (EC50 = 6.3 ± 0.9 μM, SI = 78.7) upon in vitro evaluation. Compound 44 also presented in vitro cytotoxicity (LC50 = 6.4 ± 0.8 μM) for mammalian cell line U397. Therefore the increased anti-leishmanial activity was shrouded by the corresponding increase in the cytotoxicity. SAR studies showed that increase in chain length between two pharmacophores produced less active compounds [57]. Lerdsirisuk, et al. evaluated some chromone derivatives as anti-plasmodial agents which were previously recognized as protease inhibitors. These derivatives are substituted at position # 2 and position # 3. Compound 45 exhibited prominent activity (IC50 = 0.95 μM) against Plasmodium falciparum K1 (multidrug- resistant strain) as compared to standard drug primaquine (IC50 = 2.41 ± 13 μM). In molecular docking studies against plasmesin II (an aspartic protease involved in hemoglobin degradation), compound 45 exhibited binding free energy of –13.24 kcal/mol. 2-Nitrophenyl and 4-nitrobenzoyl and 4-nitrophenyl groups are directed towards hydrophobic pocket comprising Ile32, Phe111, Ile123 and Val78, Ile290, Leu292, Ile300. Hydroxyl groups interacted via hydrogen bonding with the carbonyl oxygen of Asn76 [58].
Fig. 6.
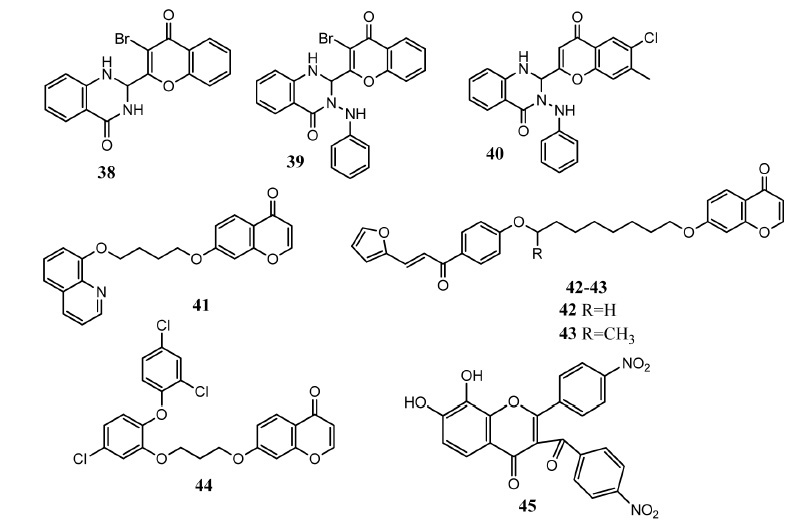
Chromone derivatives 38 – 45 as antimicrobial agents.
Nalla, et al. used click chemistry technique to attach chromone to the 1,4-disubstituted [1, 2, 3]triazole [59]. New hybrid molecules showed in vitro antimycobacterial activity and compound 46 (Fig. 7) emerged as the most potent (MIC = 1.56 μg/mL) as compared to rifampicin (MIC = 0.24 μg/mL). In molecular docking investigations, compound 46 (binding energy = –11.123 kcal/mol) showed interaction with enoyl-acyl carrier protein reductase enzyme, of Mycobacterium tuberculosis (M. tuberculosis). Compound 46 presented interaction with Asp-64, Trp-222 and Try-158 of the enzyme. Substituents at triazole ring played a crucial role in the activity and aromatic group presented less activity as compared to aliphatic substituents. The most active compound carries a long aliphatic chain attached with triazole scaffold. Further investigation revealed that this compound follows the Lipinski rule of five and possesses drug-like properties [60, 61]. Singh, et al. synthesized 3-furano-chromone derivatives. Upon investigation as in vitro anti-tubercular agent, these compounds (47 – 50) demonstrated moderate activity (10 – 12 μg/mL) against sensitive H37Rv as compared to ethambutol (2 μg/mL) and streptomycin (2 μg/mL). It was assumed that these compounds act on the lipoprotein part of the cell wall of Mycobacterium tuberculosis [62]. Cano, et al. synthesized fluorine-containing chromone and tetrazole hybrid molecules by Ugi-azide reaction [63] These derivatives displayed moderate antimicrobial activity as is evident by compound 51 (MIC = 20 μg/mL) activity versus Pseudomonas aeruginosa (P. aeruginosa) as compared to cefotaxime (MIC = 0.5 μg/mL). Compound 52 also displayed low antiamoebic activity (IC50 = 61.7 μg/mL) as compared to metronidazole (IC50 = 1.5 μg/mL). Compound 53 exhibited antifungal activity against Sporothrix schenckii (S. schenckii) at the concentration of 6.25 μg/μL. Introduction of halogen atoms at the chromone core produced more active derivatives as compared to nonhalogenated derivatives [64]. Reddy, et al. produced the fused hybrid molecules by reacting 2-amino chromone, benzaldehyde, 1,4-dimedone to produce chromeno-tetrahydroquinoline. Antibacterial activity was tested against P. aeruginosa, S. aureus, E. coli and Bacillus subtilis (B. subtilis). In these derivatives, tetrahydropyridine is embedded in dimedone and chromone scaffolds. Agar well diffusion method was used for assessing antibacterial activity. Compounds 54, 55 and 56 were most active derivatives (ZOI = 22 – 37 mm) as compared to streptomycin (ZOI = 25 – 40 mm) against these bacteria. Derivatives bearing small alkyl chains demonstrated better activity. Introduction of cyclic rings e.g. cyclopropyl, cyclobutyl and cyclopentyl at this position produced less active derivatives [65]. Pouramiri, et al. synthesized 1-aryl-3-amino-N-benzyl-1H-benzo[f]chromeno-2-carboxamide derived compounds as antibacterial agents. Compound 57 (MIC = 1.56 mg/mL), 58 (MIC = 1.56 mg/mL) and 59 (MIC = 0.78 mg/mL) presented prominent activity against P. aeruginosa. Compounds 57 (MIC = 3.125 mg/mL) and 59 (MIC = 1.56 mg/mL) presented excellent antifungal activity against C. albicans [66]. Tiwari and co-workers synthesized chromone and pyrimidine hybrid molecules by applying green chemistry procedure. Upon in vitro antibacterial evaluation, compounds 60 (MIC100 = 14 μg/mL) and 61 (MIC100 = 16 μg/mL) manifested activity against E. coli1411 as compared to cycloserine (MIC100 = 16 μg/mL). Compound 62 showed outstanding antifungal activity against Aspergillus flavus (A. flavus, MIC = 15 μg/mL), Aspergillus niger (A. niger MIC = 16 μg/mL) and Cryptococcus neoformans (C. neoformans MIC = 15 μg/mL) as compared to standard drug miconazole (MIC100 = 12 μg/mL) respectively. Compound 62 carries electron-donating group at the chromone core and proved more active as compared to other derivatives having fluorine and methyl groups. Compound 62 possesses a sulfur atom and hydrazine groups at the pyrimidine ring. Compound 62 showed a binding score of -7.24 and it formed hydrogen bonding interaction with Tyr-173 and Hem-500 of lanosterol 14-alpha demethylase enzyme of C. albicans. Pharmacokinetic analysis proved that these derivatives possess decent drug-like properties [67]. Li, et al. attached chromone with pyrazole heterocyclic scaffold through a flexible chain to produce nematocidal agents. Two pharmacophores were linked through amide and alkyl ester groups. Upon in vivo evaluation, derivatives 63 and 64 showed excellent activity (100%) against Meliodogyne incognita by using concentrations 10 mg/mL and 25 mg/mL. Standard drug tioxazafen exhibited 100% inhibition at 25 mg/mL and 92.9% at 10 mg/mL. SAR studies revealed that incorporation of methyl, ethyl and phenyl groups in the linker chain produced less active derivatives. Attachment of trifluoromethyl group at position # 3 of pyrazole scaffold produced more active derivatives as compared to bromide, cyanide and methyl groups [68].
Fig. 7.
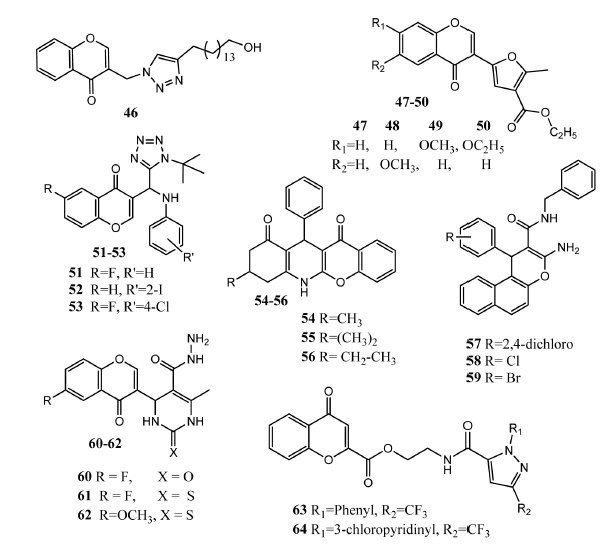
Chromone derivatives 46 – 64 as antimicrobial agents.
Siddiqui, et al. synthesized prazolyl pyranopyridines derivatives by the reaction of 3-acetoacetyl benzopyranopyridone with different hydrazine derivatives. Benzopyranopyridones contain condensed chromone ring and are the intermediate products in this series. Among these derivatives, compound 65 (Fig. 8) manifested prominent activity against Salmonella typhi (S. typhi, ZOI = 12 mm), Salmonella dysenteriae (S. dysenteriae, ZOI = 19 mm) and Klebsiella pneumoniae (K. pneumoniae, ZOI = 18 mm) as compared to chloramphenicol (ZOI = 16 mm, 23 mm, 23 mm against these bacteria) [69]. Batula, et al. synthesized aryl isoxazole and 6-fluorochromone carboxylate derivatives in which both these scaffolds were linked by ester linkage. The introduction of aryl groups at the isoxazole core enhanced the antibacterial activity. Compound 68 showed activity against A. niger (MIC = 18.75 μg/mL) and C. albicans (MIC = 18.75 μg/mL) as compared to amphotericin B (MIC = 1.562, 6.25 μg/mL respectively against these microbes). Introduction of halogen-substituted phenyl rings significantly increased antifungal activity. Compounds 66 and 67 were most active against B. subtilis having MIC value of 9.375 μg/mL for each compound as compared to streptomycin (MIC = 6.25 μg/mL) [70]. Hessein and co-workers synthesized furochromone, furocoumarin and benzofuran derivatives having sulfonamide group. Upon in vitro evaluation, compound 69 exhibited antimicrobial potential against E. coli (ZOI = 20 mm) and Aspergillus ochraceus (ZOI = 19 mm). Compound 70 (ZOI = 19 mm) was most prominent against E.coli as compared to chloramphenicol (ZOI = 38 mm). Attachment of aminophenyl and biphenyl ring to the sulfonamide group significantly improved the antibacterial activity. Addition of benzotriazole or biphenyl acetamide group did not improve the antimicrobial activity [71]. Aggarwal, et al. synthesized 1,2,4-dithiazolyl derivatives as antimicrobial agents. Compound 71 showed prominent activity against B. subtilis (MIC = 0.78 μg/mL), S. cerevisiae (MIC = 6.25 μg/mL) and C. albicans (MIC = 3.12 μg/mL) as compared to fluconazole (MIC = 1.9 μg/mL, 3.9 μg/mL versus S. cerevisiae, C. albicans). Compound 72 presented excellent activity against E. coli (MIC = 1.56 μg/mL) and B. subtilis (MIC = 1.56 μg/mL) as compared to gentamicin (MIC = 0.9 μg/mL and 0.75 μg/mL against E. coli and B. subtilis). SAR investigation declared that substitution of the phenyl ring at 1,2,4-dithiazolyl ring is very important for antimicrobial activity and addition of the halogen atom at para-position significantly improves the activity. Addition of electron-withdrawing group e.g. chlorine and bromine at position # 6 and # 7 of chromone scaffold also enhances activity while attachment of electron-donating group e.g. methyl lowers the activity [72]. Gadhave, et al. synthesized fluorine containing pyrazolone derivatives having chromone molecule by a knoevenagel condensation reaction [73]. Compound 75 (Fig. 9) showed prominent activity against Streptococcus pyogenes (MIC = 62.5 μg/mL) in comparison to ampicillin (MIC = 100 μg/mL). Compounds 76 was most active against S. aureus having MIC value of 62.5 μg/mL. Compound 77 was most active against P. aeruginosa (MIC = 62.5 μg/mL) in comparison to ampicillin (MIC = 250 μg/mL). It was found that addition of propyl and trifluoromethyl group at the pyrazole ring produced potent derivatives against gram-positive bacteria [74]. Knoevenagel-cope condensation reaction was also used by Bari, et al. to attach chromone to isatin using thiazolidone as linker. Compounds 76 (ZOI = 47 mm) and 77 (ZOI = 40 mm) exhibited prominent activity against S. aureus in comparison to ciprofloxacin (ZOI = 45 mm). Derivatives in this series proved more potent against gram positive bacteria. Compound 78 was the most active antifungal agent against A. niger (ZOI = 22 mm) and A. flavus (ZOI = 25 mm) as compared to amphotericin B (ZOI =35 mm, 30 mm against these microbes) [75]. El-Ziaty, et al. attached furanone with chromone scaffold at position # 3. Compound 79 showed moderate activity versus E.coli (ZOI=13 mm) and S. aureus (ZOI = 15 mm) in comparison to amoxicillin (ZOI = 21 – 25 mm). Compound 79 also exhibited antifungal activity against C. albicans (ZOI = 10 mm) as compared to amphotericin B (ZOI = 21 mm) [76]. Nastasa, et al. conjugated chromone with 2,4-thiazolidindione through methylene group. Thiazolidindione ring was further attached with different aromatic cores. Compounds 80 and 81 presented prominent antibacterial (ZOI = 18 – 22 mm) and antifungal activity (ZOI = 18 – 22 mm) as compared to standard drug gentamicin (ZOI = 18 – 22 mm). Different concentrations were used for testing antibacterial activity and no direct relationship was observed between concentration and antibacterial activity [77].
Fig. 8.
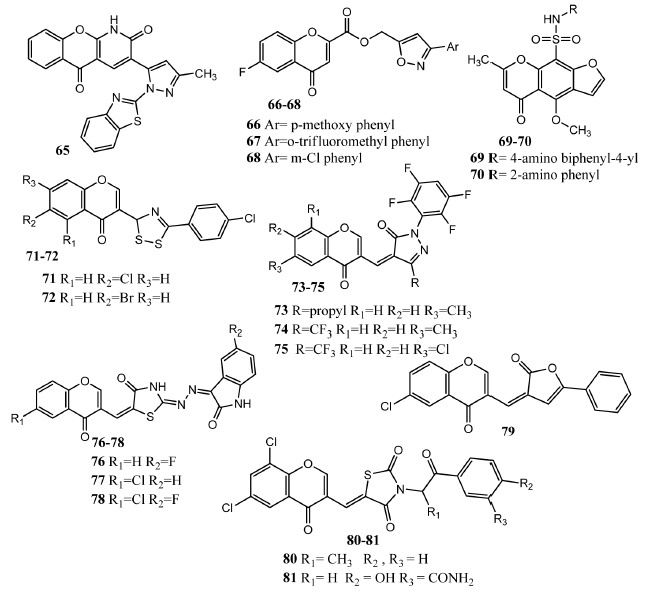
Chromones 65 – 81 as antimicrobial agents.
Fig. 9.
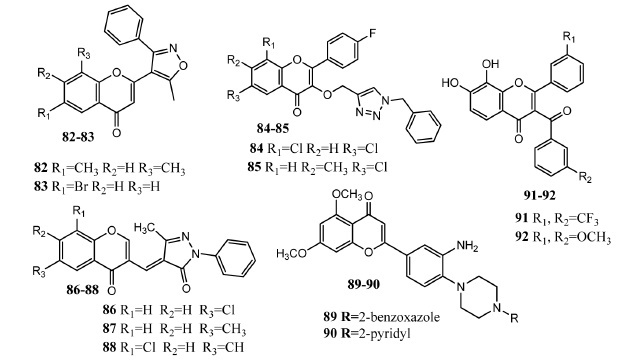
Chromone derivatives 82 – 90 as antimicrobial agents.
Badhade, et al. synthesized chromone and isoxazole conjugates without any linker group. Upon in vitro evaluation as antibacterial agents, these derivatives exhibited excellent activity. Compounds 82 and 83 (Fig. 9) exhibited notable activities against B. subtilis and S. aureus presenting MIC values of 10 μg/mL equivalent to standard drug. Incorporation of halogen and alkyl groups in the chromone ring produced more active compounds [78]. Chromone scaffold was attached with 1,2,3-triazole by Dofe and co-workers to produce new derivatives. Compounds 84 and 85 exhibited prominent antifungal activities (MIC = 12.5 μg/mL) against C. albicans for both compounds as compared to miconazole (MIC = 12.5 μg/mL). Derivatives 84 and 85 showed prominent antibacterial activity (MIC = 25 μg/mL) against B. subtilis. Compound 84 bears dichloro substituted chromone skeleton while 85 consists of methyl and chlorine substituted chromone ring. Docking studies of compounds 84 and 85 with KASIII (enzyme) of E. coli showed scores of -4.99221 and -4.73574 respectively. Docking studies of compounds 84 and 85 with KASIII of S. aureus showed scores of -4.86242 and -4.82797 respectively [79]. Gholap synthesized chromone and pyrazole conjugates by the reaction of methylene group of pyrazolone with chromone-3-carbaldehyde. Compounds 86, 87 and 88 demonstrated antimicrobial activities in this series. Compound 86 exhibited prominent activity against S. aureus (zone of inhibition, ZOI = 50 mm) and E. coli (ZOI = 40 mm) in comparison to chloramphenicol (ZOI = 44 and 38 mm against S. aureus and E. coli, respectively). Derivatives 87 and 88 were most potent against S. albus (ZOI = 45 mm) and E. coli (ZOI = 36 mm) as compared to standard drugs [80]. Less work has been reported about the antiviral activity of chromone hybrid molecules. Kim, et al. attached 3-amino-4-piperazine phenyl to the chromone core to produce new antiviral agents. Upon evaluation as antiviral agents, compound 89 was most active (EC50 = 1.5 μM) against hepatitis C virus but showed lower selectivity (SI = 2.6). Compound 90 presented moderate anti-SARS (severe acute respiratory syndrome) activity (EC50 = 34 μM) and selectivity (SI = 2.9) [81]. Ungwitayatorn, et al. synthesized 2,3-disubstituted chromone derivatives as anti-HIV agents. Compound 91 (IC50 = 0.34 μM, 93.16% inhibition) and 92 (IC50 = 0.65 μM, 92.27% inhibition) exhibited prominent anti-HIV activity as compared to standard drug pepstatin A (91.07 ± 1.53%). Molecular docking studies with HIV-1 protease showed that 3’-trifluoromethyl phenyl group of compound 91 interacted with hydrophobic pocket (Leu23, Val32, Val82). 3-Trifluoromethyl benzoyl group also formed hydrophobic interactions with binding site comprising Val32, Ile50, Pro81 and Val82. Hydroxyl groups of chromone scaffold interacted via hydrogen bonding with the carbonyl oxygen of Asp25 and Asp25’ [8].
2.4. Chromone Derivatives as Anti-Inflammatory Agents
The process of inflammation is related to various diseases. Nonsteroidal anti-inflammatory agents are frequently used as remedy of inflammation but are associated with the major adverse effect of gastrointestinal ulceration [82]. Therefore the development of new anti-inflammatory drugs having good safety profiles is necessary. Chromone derivatives have also presented anti-inflammatory activities. Shaveta, et al. conjugated chromone with oxindole to produce new hybrid molecules. Compounds 93 (IC50 = 0.029 μM, SI = 46) and 94 (IC50 = 0.020 μM, SI = 337) (Fig. 10) were found to be most active in vitro inhibitors of COX-2 and also demonstrated selectivity in comparison to celecoxib (IC50 = 0.04 μM, SI = 375). Selectivity index of compound 94 was found equivalent to celecoxib. Compound 93 also presented analgesic activity upon in vivo evaluation. Docking interaction of compound 93 showed that amino and carbonyl groups of indole, as well as carbonyl oxygen of chromone, interacted with COX-2 enzyme through hydrogen bonding[83]. Venkateswararao, et al. designed and synthesized chromenone based compounds as inhibitors of interleukin-5 (IL-5). These derivatives carry cyclohexyl methoxy group at position # 5. Upon in vitro evaluation, compounds 95 (IC50 = 4.0 μM) and 96 (IC50 = 6.5 μM) displayed prominent activities as IL-5 inhibitors. Compounds having a hydroxyl group in the propyl chain and electron-donating functionalities at position # 4 of ring B demonstrated prominent activity. Saturation of chromone to chromane ring decreased the IL-5 inhibitory activity. Substitution of hydroxyl group in the propyl chain by keto group produced less active compounds [84]. Hatnapure, et al. synthesized chrysin containing flavonoids. Aryl group at position # 2 was replaced by n-substituted piperazine ring to produce new molecules. Derivatives in this series exhibited anti-inflammatory as well as antimicrobial activity. Compound 97 exhibited prominent inhibition of IL-6 (93% inhibition) and TNFα (87% inhibition) as compared to standard drug dexamethasone (71 and 84% inhibition of TNFα and IL-6 respectively). In compound 97, piperazine and chromone scaffolds are separated by methylene group and piperazine is further attached to a pyrimidine. Replacement of pyrimidine ring by pyridine, p-methoxyphenyl ring produced equipotent derivatives. Substitution of pyrimidine ring by methyl, ethyl and acetyl groups produced less active derivatives [85].
Fig. 10.
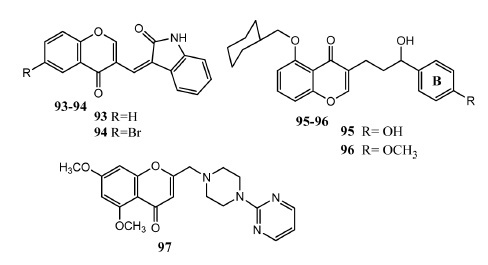
Chromone derivatives 93 – 97 as anti-inflammatory agents.
2.5. Chromone Derived Molecules for Treating Alzheimer’s and Other Neuronal Disorders
Alzheimer’s disease (AD) is a neurological ailment mostly among the elder people and is indicated by memory loss and dementia [86]. Chromone core has been attached with various other pharmacophores to produce new multifunctional agents. Natural compounds have potential to inhibit some toxicities of Alzheimer disease [87]. Liu, et al. attached chromone-2-carboxamide with alkylbenzylamines as anti-Alzheimer agents. Compound 98 (Fig. 11) displayed excellent in vitro acetylcholinesterase (AChE) inhibition (IC50 = 0.07 ± 0.01 μM, SI = 735.7) in comparison to donepezil (IC50 = 0.015 ± 0.002 μM, SI = 1380.0). Compound 98 showed competitive as well as non-competitive inhibition. This compound also presented in vitro self-induced (59.2 ± 1.6%) and Cu+2 induced (48.3 ± 1.7%) beta-amyloid (Aβ) aggregation inhibition in comparison to curcumin (self induced 43.1 ± 1.1%, Cu+2 induced 58.0 ± 2.3%). Derivatives having n-ethyl group showed better activity as compared to n-methyl group. Increase in the chain length of methylene linker also increased the activity. The compounds in this series showed weak inhibition (IC50 > 20 μM) of butyrylcholinesterase (BuChE). Molecular docking studies showed that chromone molecule binds with peripheral anionic site (PAS) by π-π interaction with Phe-288 and Phe-290, Phe-331 and Tyr-334 of TcAChe. Carbonyl group of chromone interacted with Tyr-121 through hydrogen bonding. Benzene ring formed π interaction with Trp-84 in the catalytic anionic site (CAS) [88]. Parveen, et al. synthesized hybrid molecules comprising chromone and chromane scaffolds. Both pharmacophores were attached to each other through Knoevenagel condensation reaction. Compound 99 was the prominent in vitro inhibitor (IC50 = 0.27 ± 0.3 μM) of AChE as compared to tacrine (IC50 = 0.19 ± 0.03 μM). Molecular modeling investigation of this derivative also showed interaction with the active site of AChE. The presence of amino group at position # 2 of chromone ring forms additional hydrogen bonding with Tyr-510 and Gly-523 of AChE. Benzene ring of chroman fragment showed π-cation interaction with Arg-522. The increased activity was also linked to the extra amino functionality in the chromone ring [18]. Hybrid molecules of 4-oxo-4H-chromene and tacrine were synthesized by Fernandez-Bachiller and colleagues in which cholinesterase inhibitory property of tacrine and antioxidant property of chromone were combined. Alkylene diamine was used as linker between these two pharmacophores and a chain of ten carbon atoms was found optimal for best activity. Hybrid proved more potent than parent drug tacrine but showed inhibition of AChE and BuChE enzymes. Compound 100 showed the most active in vitro inhibition of AChE (IC50 = 0.035 ± 0.001 nM) in comparison to standard drug tacrine (IC50 = 40 ± 2 nM). Compound 101 was the most active blocker of BuChE (IC50 = 0.038 ± 0.4 nM) as compared to tacrine (IC50 = 10 ± 0.4 nM). Compound 102 presented more selectivity for AChE (IC50 = 0.090 ± 0.003) than BuChE (IC50 = 95 ± 4). These compound also showed good penetration ability across BBB [89]. Li, et al. attached chromone to the pharmacophoric part of clioquinol to produce multifunctional ligands. These molecules were linked together through Schiff bases. Attachment of different substituent at chromone or phenyl core produced more active compounds as compared to unsubstituted derivatives. Compound 103 presented an effective inhibition of MAO-A (IC50 = 5.12 μM) and MAO-B (IC50 = 0.816 μM) in comparison to standard drug iproniazide (MAO-A IC50 = 6.46 ± 0.5 μM, MAO-B IC50 = 7.98 ± 0.37 μM). Compound 103 possesses potential anti-Alzheimer activity as it showed antioxidant activity (ORAC = 3.62), capacity to penetrate BBB and moderate inhibition (75.1% at the dose of 20 μM) of Aβ aggregation. Attachment of methyl group at the position # 6 of chromone produced more active derivatives. Replacement of phenyl ring by naphthalene or pyridine ring produced less active derivatives. Docking studies with MAO revealed that chromone part of 103 formed π-π interaction with Phe-208 while the carbonyl group interacted with Gln-215. 2-Amino-4-methyl phenol group occupied the hydrophobic pocket formed by Leu-171, Ile-198 and Ile-199 [90]. Mackhaeva, et al. synthesized 2-vinyl chromone derivatives as selective butyryl cholinesterase (BuChE) inhibitors. Derivatives having n-benzyl and n-vinyl methoxy carbonyl substituted compounds presented more selectivity for BuChE. In this series, compound 104 appeared as the most potent inhibitor (IC50 = 2.27 ± 0.18 μM) of BuChE in comparison to tacrine (IC50 = 0.0290 ± 0.0002 μM). Compound 104 carry bromine atom at position # 6 of chromone core. Introduction of ethoxy group at position # 8 of chromone core produced less active derivatives. Molecular docking studies showed that methoxy carbonyl group interacted with anionic site formed by Gly116, Gly117 and Ala199. The n-benzyl group interacted with Tyr440 and Trp430 and showed more affinity (binding free energy = 1.5 kcal/mol) as compared to n-methyl substituent at this position [91]. Cagide and co-workers synthesized chromone-2-phenyl carboxamide derived compounds as adenosine A1 (hA1) receptor ligands. Different substituents were attached at the exocyclic phenyl ring. Compound 105 presented prominent activity at hA1 (ki=0.219 μM) receptor and this compound also exhibited selectivity for this receptor as compared to hA3. It bears nitro group at ortho position of phenyl ring. Shifting of nitro group at meta or para position produced less active derivatives. Authors explained that receptor sites of hA1 (lined by Asn170, Glu170, Glu172 and Thr270) and hA3 (Ser73, Gln167, Val169, Leu264) are different from each other. These derivatives also follow Lipinski rule of five and possess drug like properties [92]. Ali Abid, et al. synthesized sulfonyl hydrazone derivatives of 3-formyl chromones as monoamino oxidase (MAO) inhibitors. These compounds showed capacity to inhibit MAO-A and MAO-B enzymes simultaneously. Compound 106 was the most active (IC50 = 0.33 ± 0.01 μM) in vitro MAO-A inhibitor in comparison to clorgyline (IC50 = 0.004 ± 0.0003 μM). It bears chlorine atom at position # 6 of chromone skeleton. Replacement of chlorine by methyl group produced less active derivatives. Compound 107 was the most active (IC50 = 1.12 ± 0.02 μM) MAO-B inhibitor as compared to deprenyl (IC50 = 0.0196 ± 0.001 μM). It carries fluorine atom at position # 6 and replacement of fluorine atom by chlorine or bromine atom produced less active derivatives. Molecular docking studies of compound 107 with MAO-B showed that sulfonamide oxygen interacted by hydrogen bonding with Tyr60. Oxygen atoms of chromone ring and carbonyl group interacted through hydrogen bonding with Gln206 and Tyr435. Derivatives in this series exhibited good pharmacokinetic properties such as oral bioavailability [93].
Fig. 11.
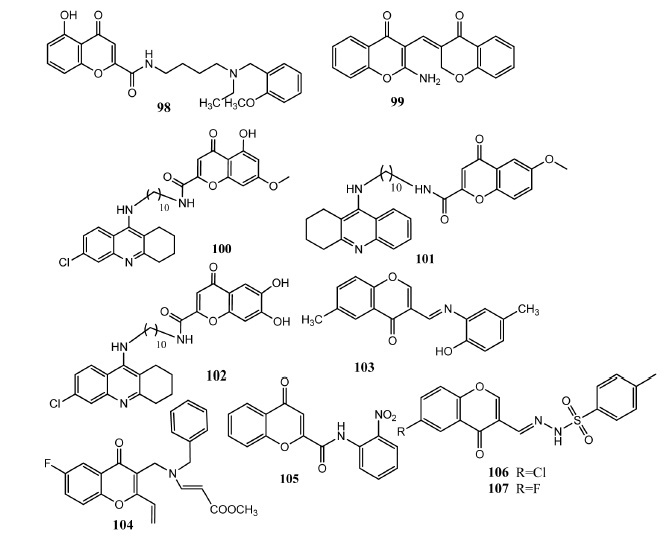
Chromone derived molecules 98 – 107 as anti-Alzheimer agents.
2.6. Antioxidant Agents Having Chromone Scaffold
Reactive oxygen species produce oxidative damage to various biomolecules. Naturally occurring compounds have also displayed antioxidant activities [94]. Chromone derivatives also play significant role as antioxidants and free radical scavengers [95]. Berczynski, et al. (2013) evaluated chromone conjugated derivatives of 2,4-thiazolidinedione, 2,4-imidazolidinedione, 2-thioxo-imidazolidine-4-one as antioxidant agents. Derivatives having 2-thioxo-imidazolidine scaffold exhibited prominent activity against OH·, DPPH· free radicals. Compound 108 (Fig. 12) appeared as the most active in vitro free radical scavenger (IC50 = 0.353 ± 0.04 mmol/L) in comparison to standard drug tiron (IC50 = 0.451 ± 0.065 mmol/L). In compound 108, 2, 3-double of chromone is in conjugation with the 4-carbonyl group and this is the reason for increased antioxidant activity [96]. Berczynskai, et al. (2013) evaluated chromone molecules having 2,4-thiazolidinedione and 2,4-imidazolidinedione heterocyclic rings for antioxidant activities. Heterocyclic rings were further attached with substituted aromatic rings. In DPPH (1,1-diphenyl-2-picryl-hydrazyl) radical scavenging assay, compounds 109 (IC50 = 194 ± 3.5 μmol/L) and 110 (IC50 = 364 ± 3.3 μmol/L) presented prominent activity as compared to vitamin C (IC50 = 346 ± 28 μmol/L). By using the DMPO-OH (5,5-dimethyl-1-pyrroline-1-oxide) spin adduct method, 109 (41.7% inhibition) and 110 (35.1% inhibition) also exhibited free radical scavenging activity [97]. Takao and colleagues synthesized 3-styryl chromone derivatives by konevenagel reaction as α-glucosidase inhibitor and as antioxidant agents. Compounds 111 (EC50 = 17 μM) and 112 (EC50 = 23 μM) showed prominent in vitro activities as DPPH scavenger as compared to ascorbic acid (EC50 = 23 μM). Compounds 111 (IC50 = 16 μM) and 112 (IC50 = 10 μM) also exhibited inhibition of α-glucosidase enzyme. Both compounds contain catechol ring attached to the chromone scaffold. SAR studies showed that incorporation of methyl group and halogen atoms on the ring B did not increase the activity but the incorporation of hydroxyl group produced active molecules. Dihydroxy derivatives showed better activity as compared to monohydoxy derivatives. [98]. Proenca, et al. attached chromone with 3,4-dihydroxy phenyl core via 1,3-diene system. Compound 113 (IC50 = 125 ± 13 μM) and 114 (IC50 = 121 ± 9 μM) showed prominent activity as scavenger of hydrogen peroxide (H2O2) as compared to standard drug quercetin (IC50 = 1338 ± 42 μM). Compound 114 also exhibited effective scavenging of HOCl (IC50 = 17 ± 3 μM), ROO· and ONOO–·(IC50 = 0.29 ± 0.02 μM). Incorporation of hydroxyl groups both at position # 5 and # 7 of chromone scaffold was important for the antioxidant activity. No prominent difference in the activity was observed for these hydroxyl groups [99]. Soengas, et al. synthesized chromone-3-pyrazoly derivatives as free radical scavenger and alpha glucosidase inhibitor. Compound 115 appeared as the most effective antioxidant agent (IC50 = 23 μM) as compared to standard drug α-tocopherol (IC50 = 23 μM). It possesses catechol group in its structure and it proved more active as compared to monohydroxyl derivatives. Addition of hydroxyl group in the chromone ring had no significant influence on the activity [100]. Rao, et al. synthesized styryl chromone derivatives in which styryl group was attached at position # 2 of chromone core. Antioxidant activity was monitored as scavenger of superoxide free radical. Antioxidant activity was dependent on the number of hydroxyl groups, and compound 116 emerged as the most active agent (IC50 = 234 μM) in comparison to vitamin C (IC50 = 852 μM). Compound 116 also showed excellent antibacterial activity against Xanthomonas campestris (ZOI = 11.3 mm) and Agarobacterium tumafaceins (ZOI = 11 mm) as compared to streptomycin (ZOI = 12 – 15 mm). Chromone derivatives having hydroxyl group also presented better antimicrobial activity. Replacement of hydroxyl group with methoxy group decreased antibacterial activity and increased antifungal activity [101]. Demetgül and co-workers linked chromone with chitosan to produce the chromone-chitosan Schiff base. Upon in vitro evaluation as antioxidant agent by DPPH radical scavenging assay, compound 117 emerged as the most potent (IC50 = 0.88 mg/mL) antioxidant agent as compared to chitosan alone. The increased antioxidant activity was related to the phenolic group present in chromone [102].
Fig. 12.
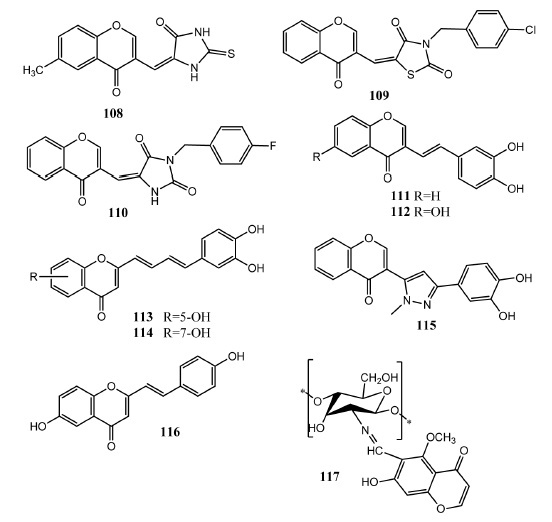
Chromone derivatives 108 – 117 as antioxidant agents.
Thus, chromone is a promising scaffold for the synthesis of new therapeutic agents, and hybrid molecules presented in this review can be used as lead for future drug discovery.
References
- 1.J. R. S. Hoult, M. A. Moroney, and M. Payα, Actions of Flavonoids and Coumarins on Lipoxygenase and Cyclooxygenase, Academic Press (1994), pp. 443 – 454. [DOI] [PubMed]
- 2.Bhat AS, Whetstone JL, Brueggemeier RW. Tetrahedron Lett. 1999;40(13):2469–2472. [Google Scholar]
- 3.Al Nakib T, Bezjak V, Meegan M, et al. Eur. J. Med. Chem. 1990;25(5):455–462. [Google Scholar]
- 4.Isaka M, Sappan M, Auncharoen P, et al. Phytochem. Lett. 2010;3(3):152–155. [Google Scholar]
- 5.Jovanovic SV, Steenken S, Tosic M, et al. J. Am. Chem. Soc. 1994;116(11):4846–4851. [Google Scholar]
- 6.Kidwai M, Saxena S, Khan MKR, et al. Bioorg. Med. Chem. Lett. 2005;15(19):4295–4298. doi: 10.1016/j.bmcl.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Larget R, Lockhart B, Renard P, et al. Bioorg. Med. Chem. Lett. 2000;10(8):835–838. doi: 10.1016/s0960-894x(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 8.Ungwitayatorn J, Wiwat C, Samee W, et al. JMoSt. 2011;1001(1–3):152–161. [Google Scholar]
- 9.Desideri N, Mastromarino P, Conti C. Antiviral Chem. Chemother. 2003;14(4):195–203. doi: 10.1177/095632020301400404. [DOI] [PubMed] [Google Scholar]
- 10.Houghton PJ, Woldemariam TZ, Khan AI, et al. Antiviral Res. 1994;25(3):235–244. doi: 10.1016/0166-3542(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Ding Y, Miao Y, et al. Eur. J. Med. Chem. 2009;44(9):3687–3696. doi: 10.1016/j.ejmech.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Mazzei M, Balbi A, Sottofattori E, et al. Eur. J. Med. Chem. 1993;28(9):669–674. [Google Scholar]
- 13.Nam DH, Lee KY, Moon CS, et al. Eur. J. Med. Chem. 2010;45(9):4288–4292. doi: 10.1016/j.ejmech.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Chung S-T, Huang W-H, Huang C-K, et al. Res. Chem. Intermed. 2016;42(2):1195–1215. [Google Scholar]
- 15.Kirkiacharian S, Tongo HG, Bastide J, et al. Eur. J. Med. Chem. 1989;24(5):541–546. [Google Scholar]
- 16.Parmar NS, Tariq M, Ageel AM. Res. Commun. Chem. Pathol. Pharmacol. 1987;58(1):15–25. [PubMed] [Google Scholar]
- 17.Horton DA, Bourne GT, Smythe ML. Chem. Rev. 2003;103(3):893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 18.M. Parveen, A. M. Malla, Z. Yaseen, et al., J. Photochem. Photobiol. B: Biol., 130(179 – 187 (2014). [DOI] [PubMed]
- 19.Nohara A, Umetani T, Sanno Y. Tetrahedron Lett. 1973;14(22):1995–1998. [Google Scholar]
- 20.Byrappa S, Raj MH, Kungyal T, et al. Eur. J. Med. Chem. 2017;126:218–224. doi: 10.1016/j.ejmech.2016.09.094. [DOI] [PubMed] [Google Scholar]
- 21.Newman DJ, Cragg GM, Snader KM. J. Nat. Prod. 2003;66(7):1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 22.Koul HK, Pal M, Koul S. Genes.Cancer. 2013;4(9–10):342–359. doi: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin KM, Syam YM, Anwar MM, et al. Bioorg. Chem. 2018;76:487–500. doi: 10.1016/j.bioorg.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Singh P, Kaur M, Holzer W. Eur. J. Med. Chem. 2010;45(11):4968–4982. doi: 10.1016/j.ejmech.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Awadallah FM, El-Waei TA, Hanna MM, et al. Eur. J. Med. Chem. 2015;96:425–435. doi: 10.1016/j.ejmech.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q-H, Yu K, Zhang X, et al. Bioorg. Med. Chem. Lett. 2015;25(20):4553–4556. doi: 10.1016/j.bmcl.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 27.Dolatkhah Z, Javanshir S, Sadr AS, et al. J. Chem. Inf. Model. 2017;57(6):1246–1257. doi: 10.1021/acs.jcim.6b00138. [DOI] [PubMed] [Google Scholar]
- 28.Nam DH, Lee KY, Moon CS, et al. Eur. J. Med. Chem. 2010;45(9):4288–4292. doi: 10.1016/j.ejmech.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 29.R. K. Bhatia, L. Singh, R. Garg, et al., [Please refine].
- 30.Ozen C, Ceylan-Unlusoy M, Ozturk M, et al. Med. Chem. Res. 2018;27(1):153–160. [Google Scholar]
- 31.B. Singh, V. Sharma, G. Singh, et al., Int. J. Med. Chem., 2013 (2013).
- 32.Obreque-Balboa JE, Sun Q, Bernhardt G, et al. Eur. J. Med. Chem. 2016;109:124–133. doi: 10.1016/j.ejmech.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Abdelhafez OM, Amin KM, Ali HI, et al. RSC Adv. 2014;4(23):11569–11579. [Google Scholar]
- 34.Chand K, Tiwari RK, Kumar S, et al. J. Heterocycl. Chem. 2015;52(2):562–572. [Google Scholar]
- 35.Han SH, Kim S, De U, et al. J. Org. Chem. 2016;81(24):12416–12425. doi: 10.1021/acs.joc.6b02577. [DOI] [PubMed] [Google Scholar]
- 36.Bousejra-ElGarah F, Lajoie B, Souchard J-P, et al. Med. Chem. Res. 2016;25(11):2547–2556. [Google Scholar]
- 37.Ali TE, Ibrahim MA, El-Edfawy SM. Phosphorus, Sulfur, Silicon Relat. Elem. 2017;192(7):819–826. [Google Scholar]
- 38.Sun C, Chen C, Xu S, et al. Biorg. Med. Chem. 2016;24(16):3862–3869. doi: 10.1016/j.bmc.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 39.M. N. Abu-Aisheh, M. S. Mustafa, M. M. El-Abadelah, et al., Eur. J. Med. Chem., 54(65 – 74 (2012). [DOI] [PubMed]
- 40.Singh G, Kaur A, Sharma V, et al. Med. Chem. Comm. 2013;4(6):972–978. [Google Scholar]
- 41.Singh S, Chopra A, Singh G, et al. J. Pharm. Res. 2013;7(4):337–341. [Google Scholar]
- 42.Zwergel C, Valente S, Salvato A, et al. Med. Chem. Comm. 2013;4(12):1571–1579. [Google Scholar]
- 43.Rodon J, Dienstmann R, Serra V, et al. Nature Rev. Clin. Oncol. 2013;10(3):143. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 44.Zhu W, Chen C, Sun C, et al. Eur. J. Med. Chem. 2015;93:64–73. doi: 10.1016/j.ejmech.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization (2017); http: //www.who.int/mediacentre/factsheets/fs138/en/.
- 46.Zhang BB, Moller DE. Curr. Opin. Chem. Biol. 2000;4(4):461–467. doi: 10.1016/s1367-5931(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 47.Ceylan-Ünlüsoy M, Verspohl EJ, Ertan R. J. Enzyme Inhib. Med. Chem. 2010;25(6):784–789. doi: 10.3109/14756360903357544. [DOI] [PubMed] [Google Scholar]
- 48.Unlusoy MC, Kazak C, Bayro O, et al. J. Enzyme Inhib. Med. Chem. 2013;28(6):1205–1210. doi: 10.3109/14756366.2012.723207. [DOI] [PubMed] [Google Scholar]
- 49.Wang G, Chen M, Wang J, et al. Bioorg. Med. Chem. Lett. 2017;27(13):2957–2961. doi: 10.1016/j.bmcl.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Wang G, Chen M, Qiu J, et al. Bioorg. Med. Chem. Lett. 2018;28(2):113–116. doi: 10.1016/j.bmcl.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 51.Salar U, Khan KM, Chigurupati S, et al. Sci. Rep. 2017;7(1):16980. doi: 10.1038/s41598-017-17261-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valentina P, Ilango K, Chander S, et al. Bioorg. Chem. 2017;74:158–165. doi: 10.1016/j.bioorg.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 53.Morens DM, Folkers GK, Fauci AS. Nature. 2004;430(6996):242. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.P. K. Bathini, H. Yerrabelly, and J. R. Yerrabelly, Org. Chem., (Part 3), 212 – 228 (2018).
- 55.Coa JC, García E, Carda M, et al. Med. Chem. Res. 2017;26(7):1405–1414. [Google Scholar]
- 56.García E, Coa JC, Otero E, et al. Med. Chem. Res. 2018;27(2):497–511. [Google Scholar]
- 57.Otero E, Vergara S, Robledo SM, et al. Molecules. 2014;19(9):13251–13266. doi: 10.3390/molecules190913251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lerdsirisuk P, Maicheen C, Ungwitayatorn J. Bioorg. Chem. 2014;57:142–147. doi: 10.1016/j.bioorg.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Kolb HC, Finn M, Sharpless KB. Angew. Chem. Int. Ed. 2001;40(11):2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 60.Lipinski CA, Lombardo F, Dominy BW, et al. Adv. Drug Del. Rev. 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 61.Nalla V, Shaikh A, Bapat S, et al. Royal Soc. Open Sci. 2018;5(4):171750. doi: 10.1098/rsos.171750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh A, Bimal D, Kumar R, et al. Synth. Commun. 2018;48(18):2339–2346. [Google Scholar]
- 63.Bräse S, Gil C, Knepper K, et al. Angew. Chem. Int. Ed. 2005;44(33):5188–5240. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- 64.P. A. Cano, A. Islas-Jαcome, Á. Rangel-Serrano, et al., Molecules20(7), 12436 – 12449 (2015). [DOI] [PMC free article] [PubMed]
- 65.Reddy MU, Reddy MS, Chakravartula V. Asian. J. Res. Chem. 2018;11(1):55–60. [Google Scholar]
- 66.Pouramiri B, Kermani ET, Khaleghi M. J, Iran. Chem. Soc. 2017;14(11):2331–2337. [Google Scholar]
- 67.Tiwari SV, Seijas JA, Vazquez-Tato MP, et al. Molecules. 2018;23(2):440. doi: 10.3390/molecules23020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li W, Li J, Shen H, et al. Chin. Chem. Lett. 2018;29(6):911–914. [Google Scholar]
- 69.Siddiqui ZN, Praveen S, Farooq F. Chem. Papers. 2010;64(6):818–824. [Google Scholar]
- 70.Battula K, Narsimha S, Nagavelli VR, et al. J. Serb. Chem. Soc. 2016;81:88–88. [Google Scholar]
- 71.Hessein SA, El-Sharief MA, Abbas SY, et al. Croat. Chem. Acta. 2016;89(1):91–100. [Google Scholar]
- 72.N. Aggarwal, V. Sharma, H. Kaur, et al., Int. J. Med. Chem,.2013 ((2013). [DOI] [PMC free article] [PubMed]
- 73.Knoevenagel E. Ber. Deutsch. Chem. Gesellschaft. 1898;31(3):2596–2619. [Google Scholar]
- 74.A. Gadhave, S. Kuchekar, and B. Karale, J. Chem,.2013 ((2012).
- 75.Bari A, Ali S, Kadi A, et al. Chem. Heterocycl. Comp. 2014;49(12):1723–1731. [Google Scholar]
- 76.El-Ziaty AK, Abou-Elmagd WS, Ramadan SK, et al. Synth. Commun. 2017;47(5):471–480. [Google Scholar]
- 77.Nastasã CM, Duma M, Pîrnãu A, et al. Cluj. Med. 2016;89(1):122. doi: 10.15386/cjmed-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Badadhe P, Patil L, Bhagat S, et al. J. Heterocycl. Chem. 2013;50(5):999–1004. [Google Scholar]
- 79.Dofe VS, Sarkate AP, Lokwani DK, et al. Res. Chem. Intermed. 2017;43(1):15–28. [Google Scholar]
- 80.Gholap SS. Heterocycl. Lett. 2012;2(4):461–466. [Google Scholar]
- 81.Kim MK, Yoon H, Barnard DL, et al. Chem. Pharm. Bull. 2013;61(4):486–488. doi: 10.1248/cpb.c12-01050. [DOI] [PubMed] [Google Scholar]
- 82.Cash JM, Klippel JH. New Engl. J. Med. 1994;330(19):1368–1375. doi: 10.1056/NEJM199405123301908. [DOI] [PubMed] [Google Scholar]
- 83.Shaveta A, Singh M. Kaur, et al. Eur. J. Med. Chem. 2014;77:185–192. doi: 10.1016/j.ejmech.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 84.Venkateswararao E, Sharma VK, Lee K-C, et al. Bioorg. Med. Chem. 2013;21(9):2543–2550. doi: 10.1016/j.bmc.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 85.Hatnapure GD, Keche AP, Rodge AH, et al. Bioorg. Med. Chem. Lett. 2012;22(20):6385–6390. doi: 10.1016/j.bmcl.2012.08.071. [DOI] [PubMed] [Google Scholar]
- 86.Townsend M. J. Alzheimer’s Dis. 2011;24(s2):43–52. doi: 10.3233/JAD-2011-110020. [DOI] [PubMed] [Google Scholar]
- 87.Kim H, Park B-S, Lee K-G, et al. J. Agric. Food Chem. 2005;53(22):8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 88.Liu Q, Qiang X, Li Y, et al. Bioorgan. Med. Chem. 2015;23(5):911–923. doi: 10.1016/j.bmc.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 89.M. I. Fernaìndez-Bachiller, C. n. Peìrez, L. Monjas, et al., J. Med. Chem., 55(3), 1303 – 1317 (2012). [DOI] [PubMed]
- 90.Li F, Wu J-J, Wang J, et al. Bioorg. Med. Chem. 2017;25(14):3815–3826. doi: 10.1016/j.bmc.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 91.Makhaeva GF, Boltneva NP, Lushchekina SV, et al. Bioorg. Med. Chem. 2018;26(16):4716–4725. doi: 10.1016/j.bmc.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Cagide F, Reis J, Gaspar A, et al. RSC Adv. 2016;6(52):46972–46976. [Google Scholar]
- 93.Abid SMA, Younus HA, Al-Rashida M, et al. Bioorg. Chem. 2017;75:291–302. doi: 10.1016/j.bioorg.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Valko M, Leibfritz D, Moncol J, et al. The Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Yadav P, Parshad B, Manchanda P, et al. Curr. Top. Med. Chem. 2014;14(22):2552–2575. doi: 10.2174/1568026614666141203141317. [DOI] [PubMed] [Google Scholar]
- 96.Berczyñski P, Duchnik E, Kruk I, et al. Luminescence. 2014;29(4):367–373. doi: 10.1002/bio.2554. [DOI] [PubMed] [Google Scholar]
- 97.P. Berczyñski, A. K3adna, I. Kruk, et al., J. Flu., 23(6), 1319 – 1327 (2013). [DOI] [PubMed]
- 98.Takao K, Ishikawa R, Sugita Y. Chem. Pharm. Bull. 2014;62(8):810–815. doi: 10.1248/cpb.c14-00351. [DOI] [PubMed] [Google Scholar]
- 99.Proença C, Albuquerque HM, Ribeiro D, et al. Eur. J. Med. Chem. 2016;115:381–392. doi: 10.1016/j.ejmech.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 100.R. G. Soengas, V. L. Silva, D. Ide, et al., Tetrahedron, 7 2(23), 3198 – 3203 (2016).
- 101.Rao M, Ujwala B, Priyadarshini P, et al. Der. Pharma. Chemica. 2016;8(7):1–6. [Google Scholar]
- 102.Demetgül C, Beyazit N. Carbohydr. Polym. 2018;181:812–817. doi: 10.1016/j.carbpol.2017.11.074. [DOI] [PubMed] [Google Scholar]


