Abstract
Characterizing the effects of obstructive sleep apnea (OSA) on the aging brain could be key in our understanding of neurodegeneration in this population. Our objective was to assess white matter properties in newly diagnosed and untreated adults with mild to severe OSA. Sixty‐five adults aged 55 to 85 were recruited and divided into three groups: control (apnea‐hypopnea index ≤5/hr; n = 18; 65.2 ± 7.2 years old), mild (>5 to ≤15 hr; n = 27; 64.2 ± 5.3 years old) and moderate to severe OSA (>15/hr; n = 20; 65.2 ± 5.5 years old). Diffusion tensor imaging metrics (fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity, and mean diffusivity) were compared between groups with Tract‐Based Spatial Statistics within the white matter skeleton created by the technique. Groups were also compared for white matter hyperintensities volume and the free‐water (FW) fraction. Compared with controls, mild OSA participants showed widespread areas of lower diffusivity (p < .05 corrected) and lower FW fraction (p < .05). Participants with moderate to severe OSA showed lower AD in the corpus callosum compared with controls (p < .05 corrected). No between‐group differences were observed for FA or white matter hyperintensities. Lower white matter diffusivity metrics is especially marked in mild OSA, suggesting that even the milder form may lead to detrimental outcomes. In moderate to severe OSA, competing pathological responses might have led to partial normalization of diffusion metrics.
Keywords: aging, diffusion tensor imaging, hypoxia, magnetic resonance imaging, sleep and neurodegenerative disorders, sleep‐disordered breathing, white matter
1. INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep disorder characterized by repetitive upper airway collapse during sleep that causes intermittent hypoxemia, repeated arousals, and sleep fragmentation (Malhotra & White, 2002). Recent meta‐analyses concluded that OSA is a risk factor for cognitive decline and incident dementia (Leng, McEvoy, Allen, & Yaffe, 2017; Shi et al., 2018; Zhu & Zhao, 2018). These epidemiological findings have given rise to several mechanistic hypotheses linking OSA to neurodegeneration: OSA is associated with amyloid‐β generation and altered clearance, tau hyperphosphorylation, inflammation, oxidative stress, and metabolic and vascular deregulation (Baril, Carrier, et al., 2018; Baril, Gagnon, et al., 2018; Gosselin, Baril, Osorio, Kaminska, & Carrier, 2019; Polsek et al., 2018; Rosenzweig et al., 2015). In addition, OSA in late middle‐aged and older adults could precipitate the progression toward dementia by creating microstructural cerebral changes, especially white matter damage. The interest for this topic came from evidence that white matter is especially sensitive to processes present in OSA such as hypoxia (Goldberg & Ransom, 2003), and that white matter microstructure alterations are predictive of cognitive decline (Selnes et al., 2013).
Diffusion tensor imaging (DTI) analysis technique is used for sequences acquired with diffusion magnetic resonance imaging (MRI). It allows investigating regional brain white matter microstructure in vivo by measuring water diffusion. This technique takes advantage of the highly oriented structure of myelinated axons in the white matter that limits the diffusion of water molecules: how water molecules diffuse provides information about the cellular microstructure that restricts their movement and about water displacements between intracellular and extracellular spaces. DTI assesses white matter microstructure by computing, in each voxel, the magnitude of diffusion in perpendicular axes, that is, the diffusion tensor. Four metrics are computed based on the diffusion tensor: fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD). FA is a relative anisotropy index computed with all directions: water molecules that diffuse in an anisotropic fashion have a preferential direction, which is the case in cerebral white matter. Diffusivity metrics correspond to the magnitude of water diffusion in specific directions, where AD is the diffusion magnitude along axons, RD is perpendicular to axons, and MD is the average diffusivity in all directions. DTI has been extensively used in the context of dementia: Alzheimer's disease and mild cognitive impairment are associated with widespread decreases in FA and increases in MD (Sexton, Kalu, Filippini, Mackay, & Ebmeier, 2011), as well as increased RD and AD (Sun et al., 2014). Low FA is generally considered as a marker of poor white matter integrity while higher diffusivities (MD, RD, and AD) represent processes that allow water to diffuse more freely in the extracellular space. These pathological processes may include axonal injuries, demyelination, Wallerian degeneration, and vasogenic edema (extracellular edema) (Pitkonen et al., 2012; Winklewski et al., 2018; Zhang, Aggarwal, & Mori, 2012).
A more heterogeneous portrait was found in middle‐aged adults with OSA (results are summarized in Table 1) (Castronovo et al., 2014; Chen et al., 2015; Koo et al., 2019; Kumar et al., 2012; Kumar et al., 2014; Lee et al., 2019; Macey et al., 2008; Macey et al., 2012; Zhang et al., 2019). In fact, while decreased FA suggesting poor white matter integrity was repeatedly found in middle‐aged individuals with OSA, as would be predicted by ongoing neurodegenerative processes (Sexton et al., 2011), lower diffusivities (MD, RD, and AD) were reported more consistently than higher diffusivities. Interestingly, lower diffusivities can be observed with various acute pathologic processes that restrict water movement inside cells, such as reactive gliosis, beaded or fragmented axons, and cytotoxic edema (intracellular swelling) (Caverzasi et al., 2014; Pitkonen et al., 2012; Winklewski et al., 2018; Zhang et al., 2012). Yet, no DTI study has investigated white matter diffusion properties specifically in late middle‐aged and older adults with OSA, a population at higher risk of presenting ongoing neurodegenerative processes. Moreover, it remains unknown whether individuals with mild OSA (apnea‐hypopnea index [AHI] between 5 and 15 events/hr) show white matter diffusion property changes. The prevalence of mild OSA is high in late middle‐aged and older adults at around 30% up to 71% (Tufik, Santos‐Silva, Taddei, & Bittencourt, 2010). However, there is still no clear evidence as to whether mild OSA leads to adverse outcomes, especially regarding brain health (Chowdhuri et al., 2016). Answering this question could change decisions regarding treatment. Moreover, OSA of a milder severity tends to worsen over time when left untreated (Sahlman, Pukkila, Seppa, & Tuomilehto, 2007). Thus, mild OSA may represent a temporal window during which an efficient treatment could prevent neurodegeneration.
Table 1.
Water diffusivity changes found in previous studies by comparing DTI metrics between OSA and control groups
| Groups, sex | AHI (events/hr) | Age (yr/old) | FA | MD | RD | AD | Interpretation by the authors |
|---|---|---|---|---|---|---|---|
| Macey et al. (2008) | ↓ | n/a | n/a | n/a | Extensive altered white matter diffusion properties in OSA were observed and could potentially reflect damage from hypoxia, oxidative stress, and inflammation. | ||
|
41 OSA, 7 W Recruited through a sleep disorders laboratory |
≥15; 35.7 ± 18.1 | 46.3 ± 8.9 | |||||
| 69 controls, 25 W | n/a | 47.5 ± 8.8 | |||||
| Macey, Kumar, Yan‐Go, Woo, and Harper (2012) | ↓ | n/a | n/a | n/a | An OSA by sex interaction was observed. Women with OSA showed lower FA compared to controls, while this difference was not found in men. This could be representative of different psychological symptomatologies such as depression and anxiety, and/or cardiovascular symptoms in women with OSA specifically. | ||
| 20 M OSA | ≥15; 25.5 ± 2.9 | 48.9 ± 1.7 | |||||
|
10 W OSA Recruited through a sleep disorders laboratory |
≥15; 22.5 ± 4.1 | 52.6 ± 2.4 | |||||
| 30 M controls | n/a | 49.2 ± 1.4 | |||||
| 20 W controls | n/a | 50.3 ± 1.7 | |||||
| Kumar et al. (2012) | n/a | ↓ | n/a | n/a | Reduced global and regional MD with OSA in multiple brain regions suggests acute tissue injury and cytotoxic edema, probably as a result of hypoxia and cardiovascular changes. | ||
|
23 OSA, 3 W Recruited through a sleep disorders laboratory |
≥15; 34.9 ± 24.1 | 44.4 ± 9.3 | |||||
| 23 controls, 3 W | n/a | 45.3 ± 11.0 | |||||
| Kumar et al. (2014) | n/a | n/a | ↓ | ↓ | Reduced global and widespread regional RD and AD in OSA may indicate acute axonal and myelin injury, respectively, that could be the result of axonal and myelin swelling. | ||
|
23 OSA, 3 W Recruited through a sleep disorders laboratory |
≥15; 34.9 ± 24.1 | 44.4 ± 9.3 | |||||
| 23 controls, 3 W | n/a | 45.3 ± 11.0 | |||||
| Castronovo et al. (2014) | ↓ | ↓ | n/a | n/a | Before treatment, OSA subjects had lower FA and MD that were concomitant with cognitive impairments, mood alterations, and sleepiness. After 3‐month and 1‐year treatments, a near complete reversal of white matter altered integrity was progressively shown. This reversal was present with recovery to normal levels of cognitive functions. | ||
| 13 M OSA, before and after 3‐month and 1‐year CPAP treatments | ≥30; 61.4 ± 9.8 | 43.2 ± 7.6 | |||||
| 15 M controls | <5; 1.6 ± 1.5 | 42.2 ± 6.6 | |||||
| Chen et al. (2015) | ↓ | Ø | ↑ | Ø | Altered white matter diffusion properties that suggested demyelination in OSA were correlated with leukocyte early apoptosis that is a marker of systemic inflammation. | ||
|
20 OSA, 2 W Recruited through a sleep center |
≥30; 58.9 ± 14.5 | 38.6 ± 9.9 | |||||
| 14 controls, 3 W | <5; 2.9 ± 1.3 | 38.2 ± 9.9 | |||||
| Lee et al. (2019) | ↓ | ↑ | ↑ | Ø | White matter diffusion properties in OSA suggested reduced axonal number and density and/or altered organization. The tracts with increased FA could account for altered structural brain network properties. | ||
|
135 OSA, 96 W Recruited in a prospective community‐based cohort |
≥5; 12.5 ± 10.0 (76% mild OSA) | 59.0 ± 5.9 | |||||
| 165 controls, 119 W | <5; 1.9 ± 1.5 | 58.0 ± 6.0 | |||||
| Koo, Kim, Kim, Seong, and Joo (2019) | ↓ | ↑ | n/a | n/a | Localized white matter FA reductions in the uncinate fasciculus correlated with a lower performance to a working memory task, suggesting that white matter alterations may underlie cognitive function in OSA. | ||
|
38 M OSA Recruited through a sleep clinic |
≥30; 56.8 ± 26.2 | 45.0 ± 6.6 | |||||
| 41 M controls | <5; 2.3 ± 2.2 | 37.2 ± 10.7 | |||||
|
(B. Zhang et al., 2019) 20 OSA, 16W |
↓ | ↑ | ↑ | Ø | The authors investigated white matter microstructure of the corpus callosum, and found that in the anterior corpus callosum, alterations were associated with worse prospective memory and sustained attention in OSA. | ||
|
Recruited through a sleep disorders hospital |
≥15; 49.0 ± 22.5 |
43.1 ± 10.5 |
|||||
|
24 controls, 15W |
n/a |
40.7 ± 10.0 |
|||||
Abbreviations: AD, axial diffusivity; AHI, apnea–hypopnea index; CPAP, continuous positive airway pressure treatment; DTI, diffusion tensor imaging; FA, fractional anisotropy; MD, mean diffusivity; M, men; OSA, obstructive sleep apnea; n/a, nonapplicable or nonavailable; RD, radial diffusivity; W, women; Ø, no between‐group differences.
In the present study, we aimed to characterize white matter diffusion properties in late middle‐aged and older adults in the context of different OSA severity levels. We evaluated a group of mild and a group of moderate to severe OSA adults aged 55 and older and compared them to nonapneic participants. Our hypotheses were that (a) both mild and moderate to severe OSA groups will show altered white matter diffusion properties when compared with controls; and (b) white matter will be more affected in the moderate to severe OSA group than the mild OSA one. We combined multiple neuroimaging techniques and analyses in order to clarify the white matter portrait in our sample, as well as potentially better hypothesize underlying pathophysiological processes. We used free‐water (FW) imaging, a novel technique using diffusion MRI that estimates water that diffuses freely, which is associated with edema (Pasternak, Sochen, Gur, Intrator, & Assaf, 2009). The FW fraction is altered in normal aging, in the early stages of neurodegeneration, and in dementia and cerebrovascular diseases (Chad, Pasternak, Salat, & Chen, 2018; Ji et al., 2017; Montal et al., 2018; Vipin et al., 2019). Additionally, we evaluated the presence of white matter hyperintensities, which represent white matter small‐vessel ischemic injuries (Brickman, Muraskin, & Zimmerman, 2009) and could thus clarify the nature of white matter alterations in OSA.
2. MATERIALS AND METHODS
2.1. Sample and protocol
Between 2012 and 2017, we recruited 65 subjects aged between 55 and 85 years old. A few participants were recruited from an OSA clinic waiting list (N = 9) in the Pulmonology Department of the Hôpital du Sacré‐Coeur de Montréal in the Centre integré universitaire de santé et de services sociaux du Nord de l'île‐de‐Montréal. People were approached based on their suitability to the inclusion/exclusion criteria and preliminary estimation of OSA severity measured with a home Embletta evaluation. Thus, participants from the waiting list were recruited independently of their reason for consultation (i.e., personal complaint of suspected OSA or medical advice). The rest of the sample was recruited through newspaper ads (N = 56), which asked for people interested to participate in a study on OSA and brain health. Our recruitment methods allowed us to obtain a sample with a wide range of OSA severity from non‐OSA controls to severe OSA.
Parts of the sample were included in previous papers by our group (Baril et al., 2015; Baril et al., 2017; Baril, Carrier, et al., 2018; Baril, Gagnon, et al., 2018; Gagnon et al., 2018; Gagnon et al., 2019; Gosselin et al., 2016). Exclusion criteria were as follows: neurological or psychiatric diseases, cerebrovascular diseases, dementia, history of traumatic brain injury, pulmonary diseases, sleep disorders other than OSA, being treated for OSA, morbid obesity (body mass index [BMI] >40), the usage of medication that influence sleep, breathing or brain functioning (e.g., benzodiazepines, anticonvulsants, sedatives, antidepressants, and antipsychotics), and drug and alcohol abuse. The research protocol was approved by Ethics' Committees of the Centre integré universitaire de santé et de services sociaux du Nord de l'île‐de‐Montréal and the Functional Neuroimaging Unit of the Institut Universitaire de Gériatrie de Montréal. Each participant gave written informed consent before starting the study according to the declaration of Helsinki.
2.2. Polysomnographic recording
We have extensively described our full‐night in‐laboratory polysomnography protocol in previous studies (Baril et al., 2015; Baril et al., 2017; Gosselin et al., 2016). This recording included the following measurements: thoraco‐abdominal strain gauges, oronasal canula and thermal sensors, transcutaneous finger pulse oximeter, EEG, electrooculograms, chin and anterior tibialis EMG, and ECG. An experienced medical electrophysiology technologist performed sleep and respiratory scoring based on the American Academy of Sleep Medicine guidelines (Berry et al., 2012; Iber, Ancoli‐Israel, Chesson, & Quan, 2007). The AHI was computed as follows: apneas+hypopneas/sleep duration in hours. Participants were divided into three groups: a control group (AHI ≤ 5/hr), a mild OSA group (AHI > 5 to ≤15/hr), and moderate to severe OSA (AHI > 15/hr). This classification was based on published standard recommendations (American Academy of Sleep Medicine Task Force, 1999). Apneas were defined as an airflow reduction ≥90% lasting >10 s. Hypopneas were defined as an airflow reduction ≥30% lasting >10 s accompanied by either an oxygen desaturation ≥3% or arousal, as recommended in 2012 (Berry et al., 2012). It should be noted that criteria recommended for the scoring of hypopneas were modified over time, which can affect comparisons of OSA severity levels with previous studies (Duce, Milosavljevic, & Hukins, 2015).
2.3. Questionnaires
All subjects completed the Index of Vascular Burden (Villeneuve, Belleville, Massoud, Bocti, & Gauthier, 2009). This index is the summation of self‐reported cardiovascular conditions and risk factors, including hypertension, hyperlipidemia, diabetes, and coronary diseases. The Epworth Sleepiness Scale (Johns, 1991), the Beck Depression Inventory‐II (Beck, Steer, Ball, & Ranieri, 1996), and the Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, 1988) were used to characterize groups. The Montreal Cognitive Assessment was used to characterize global cognitive performance (Nasreddine et al., 2005).
2.4. Genotyping
BDNF rs6265 Val66Met and ApoE4 alleles were genotyped to ensure that carriers did not differ between groups, and thus, did not influence white matter analyses. In fact, the ApoE4 is the strongest genetic risk factor for Alzheimer's disease (Poirier, 2005), and the Val66Met allele of the BNDF gene has been associated with both dementia and altered white matter microstructure (Rezaei, Asgari Mobarake, Saberi, Keshavarz, & Leili, 2016; Tost et al., 2013). Our group published the detailed genotyping protocol previously (Gosselin et al., 2016).
2.5. MRI acquisition
All subjects were tested using a 3 Tesla MRI system (Magnetom Trio, Siemens Healthcare, USA) with a 32‐channel head coil. First, a three‐dimensional T1‐weighted Turbo Flash multi‐echo Magnetization‐prepared rapid gradient‐echo was acquired with the following parameters: repetition time = 2,530 ms/root mean square of four echo times = 1.64 ms, 3.50 ms, 5.36 ms, 7.22 ms; matrix size = 256 × 256; field of view = 256 × 256 mm; voxel size = 1.0 mm isotropic; flip angle = 7°; and 176 sagittal orientations, pixel bandwidth = 651 Hz/Px. In addition, a T2‐weighted sequence (repetition time = 6,100 ms; echo time = 98 ms; 35 slices; matrix size = 448 × 358 mm; field of view = 220 mm; voxel size = 0.49 × 0.49 × 3.0 mm; flip angle = 120°; pixel bandwidth = 223 Hz/Px) and a fluid‐attenuated inversion recovery (FLAIR) sequence (repetition time = 9,000 ms; echo time = 89 ms; 35 slices; matrix size = 256 × 174 mm; field of view = 220 mm; voxel size = 0.43 × 0.43 × 3.0 mm; flip angle = 130°; pixel bandwidth = 283 Hz/Px) were acquired.
Afterward, a pulsed spin echo diffusion‐weighted imaging sequence (echo‐planar imaging) was acquired for all subjects. A reference image without diffusion was acquired (b = 0 s/mm2). Sixty‐four uniformly distributed directions were acquired with a b value of 1000s/mm2 with the following parameters: repetition time = 9,100 ms; echo time = 89 ms; 72 slices; matrix size = 120 × 120 mm; field of view = 240 × 240 mm; voxel size = 2.0 mm isotropic; flip angle = 90°; pixel bandwidth = 1,667 Hz/Px.
2.6. Tract‐based spatial statistics (TBSS) and DTI processing
Diffusion images were preprocessed with the Toolkit for Analysis in Diffusion MRI described online (www.unf-montreal.ca/toad/html/en/; Functional Neuroimaging Unit, Institut Universitaire de Gériatrie de Montréal, Montreal, Canada). This automated pipeline includes several steps: eddy‐current and motion correction; upsampling to anatomical image resolution; registration of diffusion‐weighted images to the anatomical image and atlas registration; MRtrix 3.0 tensor reconstruction with an iteratively reweighted linear least square estimator; and the extraction of FA, MD, RD, and AD.
After diffusion images were preprocessed, the TBSS technique was applied (Smith et al., 2006) as used previously by our group (Sanchez et al., 2019). Briefly, in order to reduce the impact of misaligned white matter tracts when comparing different participants, the TBSS technique uses FA values to create a mean skeleton of white matter tracts, which represents the center of white matter tracts of the group. Then, individual values of the center of the white matter tracts of each participant are projected onto the mean skeleton for statistical analyses. All TBSS steps were performed with FSL such as it is described online (www.fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS/UserGuide; Analysis Group, FMRIB, Oxford, UK). FA images were registered, transformed nonlinearly, and aligned to the FRMB58_FA standard space image. Images also underwent an affine transformation to the Montreal Neurological Institute (MNI) 152 space. A mean FA skeleton of all subjects was then created and a threshold of 0.3 was chosen in order to maximize the elimination of voxel containing gray matter and cerebrospinal fluid (CSF). The thresholded mean FA skeleton determined which voxels were investigated. Individual FA data of all subjects were then projected onto the threshold mean FA skeleton to perform statistical analyses. All these steps were also applied to MD, RD, and AD images of all subjects, resulting in mean skeleton images and individual projected images for each metric.
2.7. FW imaging
We used the FW imaging model described by (Pasternak et al., 2009). This model uses diffusion‐weighted MRI data to estimate both the tissue and isotropic FW compartments in each voxel, as implemented in AMICO (Daducci et al., 2015). This model computes for every subject an FW fraction map with every voxel ranging from 0 to 1. Values approaching 1 correspond to voxel composed of water that diffuses almost completely freely. Values approaching 0 correspond to minimal FW diffusion in the extracellular space. The average FW fraction brain value was extracted for each subject and adjusted in statistical models for the volume of CSF. CSF volume was obtained using FreeSurfer image analysis suite version 5.3.0, which has been extensively described online (www.surfer.nmr.mgh.harvard.edu/; Laboratory for Computational Neuroimaging, Center for Biomedical Imaging, Charlestown, MA, USA). By adding CSF as a covariate, FW fraction was investigated without the confounding effect of the CSF in order to evaluate FW fraction of the brain tissue.
2.8. White matter hyperintensities quantification
To measure the volume of white matter hyperintensities, we used the lesion growth algorithm into the lesion segmentation toolbox version 2.0.15 (www.statistical-modelling.de/lst.html; Morphometry Group and Department of Statistics, Munich, Germany; Structural Brain Mapping Group, Jena, Germany) implemented in SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12/; Wellcome Trust Centre for Neuroimaging, London, UK) and MATLAB 9.1 (www.mathworks.com/; MathWorks, Natick, MA, USA). TI‐weighted and FLAIR MRI images were coregistered together. A binary lesion map was constructed for hyperintense areas on FLAIR images using a threshold of 0.1 (but 0.2 for 9 participants). As suggested in the guidelines of the lesion segmentation toolbox, these thresholds were chosen visually by assessing the efficiency of several thresholds between 0.05 and 1.0 to correctly identify white matter hyperintensities for each subject. A threshold of 0.2 was chosen for participants with a less defined contrast between white matter hyperintensities and normal appearing white matter. A neuroradiologist visually identified white matter hyperintensities. The total volume of white matter hyperintensities was then obtained for each subject in ml.
2.9. Statistical analyses
2.9.1. Descriptive statistics
Statistics were performed with SPSS 19 (IBM SPSS Statistics, New York, USA), except for DTI analyses that were performed within FSL. To assess group differences for demographic and sleep data, one‐way ANOVAs or chi‐square tests were performed between control, mild OSA, and moderate to severe OSA groups.
2.9.2. DTI analyses
To assess group differences on white matter, FA, MD, RD, and AD, voxel‐wise two‐group t tests were performed between all groups using the command “randomize” within FSL. These analyses were adjusted for multiple comparisons at p < .05 with family‐wise error (FWE) corrected for multiple comparisons using the Monte Carlo (10,000 conditional permutations) and the threshold‐free cluster enhancement methods implemented into FSL. These statistical tests were therefore performed on every voxel of the white matter skeleton. DTI analyses were adjusted statistically for BMI.
2.9.3. Complementary neuroimaging techniques
The brain average FW fraction and the white matter hyperintensities volume were compared between the three groups with two‐tailed ANCOVAs adjusted for BMI. The p‐threshold was set at p < .05. For FW imaging, volume of CSF was added as a covariate.
3. RESULTS
3.1. Subject characteristics
Table 2 presents demographic and sleep variables of control, mild OSA, and moderate to severe OSA groups. There was no between‐group difference for self‐reported sleepiness, depression and anxiety symptoms, global cognitive performance, and the proportion of BDNF Met and ApoE4 carriers. The moderate to severe OSA group was composed of more men and had a higher BMI than controls and those with mild OSA. Twelve participants from the moderate to severe OSA groups (60%) had severe OSA (AHI > 30), and only four of those had an AHI > 40.
Table 2.
Demographic and sleep variables of control, mild OSA, and moderate to severe OSA groups
| Variables | Control [1] | Mild OSA [2] | Moderate to severe OSA [3] | F or X 2 | Post hoc tests |
|---|---|---|---|---|---|
| Apnea–hypopnea index criteria for groups | ≤5 | >5 to ≤15 | >15 | n/a | |
| Number of subjects | 18 | 27 | 20 | n/a | |
| Demographic and clinical variables | |||||
| Sex (#; %men) | 11; 61.1% | 18; 66.7% | 19; 95.0% | 6.9* | 1, 2 < 3 |
| Age (years) | 65.2 (7.2) | 64.2 (5.3) | 65.2 (5.5) | 0.2 | |
| Education (years) | 16.6 (3.7) | 14.6 (2.7) | 15.9 (3.0) | 2.3 | |
| Body mass index (kg/m2) | 25.0 (3.1) | 26.4 (3.4) | 28.6 (2.5) | 6.5** | 1, 2 < 3 |
| Vascular burden index | 0.8 (0.9) | 1.0 (1.1) | 1.0 (0.9) | 0.3 | |
| Vascular burden index ≥2 | 4; 22.2% | 9; 33.3% | 5; 25.0% | 0.8 | |
| ApoE4 allele carriers (#, %) | 5; 27.8% | 6; 23.1% | 4; 20.0% | 0.3 | |
| BDNF Met allele carriers (#, %) | 5; 27.8% | 6; 23.1% | 4; 20.0% | 0.3 | |
| Epworth Sleepiness Scale | 7.7 (5.4) | 7.4 (4.9) | 9.4 (4.0) | 1.1 | |
| Beck Depression Inventory II | 4.7 (4.3) | 5.6 (4.9) | 8.6 (5.8) | 3.1 | |
| Beck Anxiety Inventory | 4.3 (5.3) | 3.4 (3.2) | 4.3 (4.8) | 0.4 | |
| Montreal cognitive assessment | 27.9 (2.3) | 27.5 (2.2) | 27.2 (2.8) | 0.2 | |
| Polysomnographic variables | |||||
| Total sleep time (min) | 355.6 (70.5) | 349.7 (66.3) | 372.0 (41.9) | 0.8 | |
| Sleep efficiency (%) | 79.2 (11.6) | 78.1 (12.2) | 82.3 (9.1) | 0.8 | |
| Awakenings (#) | 32.0 (17.7) | 35.9 (13.8) | 54.0 (26.4) | 7.3** | 1, 2 < 3 |
| Micro‐arousal index (events/hr) | 11.6 (4.0) | 13.2 (5.3) | 19.7 (8.2) | 10.2*** | 1, 2 < 3 |
| Stages transitions (#) | 210.3 (72.5) | 208.3 (46.6) | 292.0 (76.0) | 11.5*** | 1, 2 < 3 |
| Apnea–hypopnea index (events/hr) | 2.3 (1.8) | 9.1 (1.6) | 33.9 (12.8) | 102.5*** | 1 < 2 < 3 |
| Apnea index (events/hr) | 1.0 (1.4) | 2.8 (2.4) | 23.3 (4.6) | 46.4*** | 1, 2 < 3 |
| Hypopnea index (events/hr) | 1.3 (1.3) | 6.3 (2.2) | 10.6 (5.2) | 38.1*** | 1 < 2 < 3 |
| Mean O2 saturation (%) | 95.1 (0.8) | 94.7 (1.3) | 94.1 (0.9) | 5.0* | 1 > 3 |
| Minimal O2 saturation (%) | 89.6 (3.3) | 88.6 (2.8) | 83.2 (5.2) | 16.5*** | 1, 2 > 3 |
| Sleep time with O2 saturation < 90% (min) | 0.4 (0.9) | 1.7 (5.6) | 11.1 (14.5) | 8.9*** | 1, 2 < 3 |
| O2 saturation drops >3% index (events/hr) | 0.8 (0.8) | 3.0 (2.2) | 17.2 (11.2) | 39.9*** | 1, 2 < 3 |
Note: Results are presented with mean (SD). ANOVAs were performed, except for proportion differences that were assessed with chi‐square tests.
Abbreviations: ApoE4, apolipoprotein; BDNF, brain‐derived neurotrophic factor; OSA, obstructive sleep apnea; n/a, nonapplicable.
*p < .05; **p < .01; ***p < .001.
3.2. Lower diffusivities in OSA groups
Participants with mild OSA showed widespread areas of lower MD compared with controls. The affected white matter tracts included projection fibers (corona radiata, internal capsule, cerebral peduncle, and the posterior thalamic radiation), the corpus callosum, and major association fibers such as the superior longitudinal fasciculus in the bilateral frontal, temporal, parietal, and occipital lobes. These areas of lower MD are detailed in Table 3, Panel (a) and Figure 1a.
Table 3.
Reduced diffusivities in mild and moderate to severe OSA groups compared with controls
| Cluster size | T | Peak MNI coordinates | Fiber type and white matter tracts (lobes or regions) | ||
|---|---|---|---|---|---|
| Panel (a) Reduced MD in mild OSA compared with controls | |||||
|
16,151 209 78 |
5.26 4.32 3.21 |
31 −30 −30 |
−46 −30 −18 |
16 2 9 |
Projections
|
Association
| |||||
Commissural
| |||||
| Panel (b) Reduced RD in mild OSA compared with controls | |||||
|
595 2,919 37 21 |
4.91 4.45 4.04 4.05 |
18 −22 −28 −30 |
50 −82 −20 −30 |
−4 11 −7 2 |
Projection
|
Association
| |||||
Commissural
| |||||
| Panel (c) Reduced AD in mild OSA compared with controls | |||||
| 17,376 | 5.52 | −28 | −47 | 19 | Projection
|
Association
| |||||
Commissural
| |||||
| Panel (d) Reduced AD in moderate to severe OSA compared with controls | |||||
| 2,178 | 4.47 | −5 | 1 | 26 | Commissural
|
Note: Group comparisons were adjusted for body mass index (BMI). Family‐wise error (FWE)‐corrected p < .05.
Abbreviations: AD, axial diffusivity; MD, mean diffusivity; MNI, Montreal Neurological Institute; OSA, obstructive sleep apnea; RD, radial diffusivity.
Figure 1.
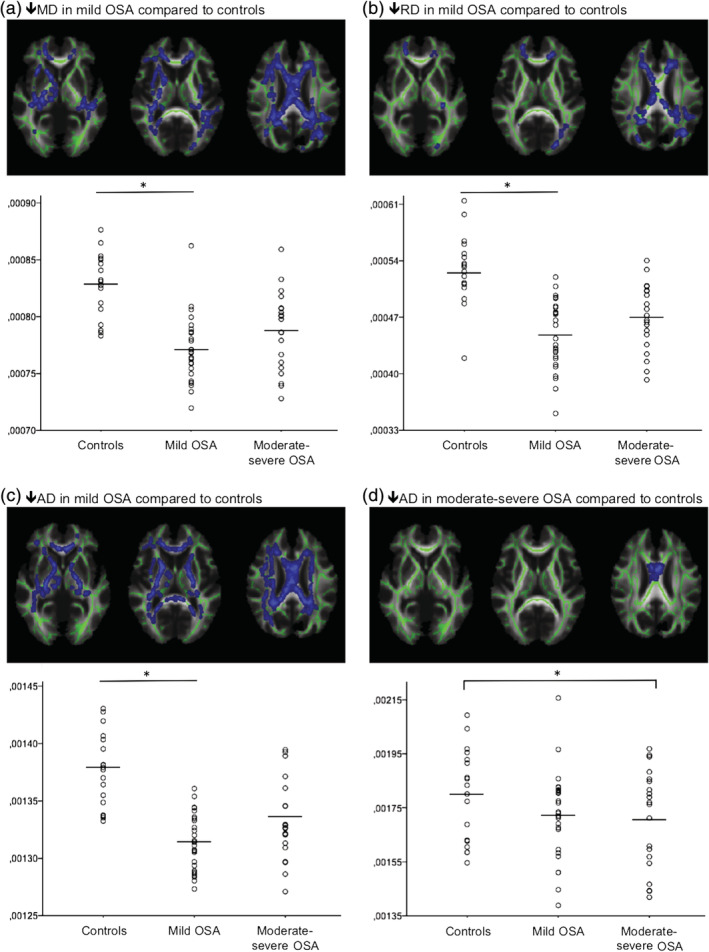
Reduced white matter water diffusivities in OSA revealed by TBSS DTI analysis. Reduced (a) MD, (b) RD, and (c) AD values in mild OSA were observed compared with controls in multiple white matter tracts, including multiple projection and association fibers as well as within commissural fibers. Reduced (d) AD value in moderate to severe OSA compared with controls was observed in the corpus callosum. Average values of clusters showing significant group differences were extracted for each participant and are presented by groups. The moderate to severe OSA group had intermediate levels of diffusivity between controls and mild OSA, which led to nonsignificant group differences in most white matter tracts for (a) MD, (b) RD, and (c) AD. Group differences were considered significant at FWE‐corrected p < .05, and BMI was included as a covariate. Green lines represent the mean white matter skeleton over which statistical analyses were performed. AD, axial diffusivity; BMI, body mass index; DTI, diffusion tensor imaging; FWE, family‐wise error; MD, mean diffusivity; OSA, obstructive sleep apnea; RD, radial diffusivity; TBSS, tract‐based spatial statistics
The mild OSA group had lower white matter RD compared with controls in projection fibers (corona radiata, internal capsule, and the posterior thalamic radiation), the corpus callosum, and major association fibers such as the superior longitudinal fasciculus in the bilateral parietal and left temporal and occipital lobes. These areas of lower RD are shown in Table 3, Panel (b) and Figure 1b.
The mild OSA group had lower AD compared with controls in projection fibers (corona radiata, internal capsule, cerebral peduncle, and the posterior thalamic radiation), the corpus callosum, and major association fibers such as the superior longitudinal fasciculus in the bilateral frontal and temporal lobes and right parietal lobe. These areas of lower AD are shown in Table 3, Panel (c) and Figure 1c.
Moreover, the moderate to severe OSA group had significantly lower AD compared with controls in the body of the corpus callosum, which is shown in Table 3, Panel (d) and Figure 1d.
No between‐group differences were observed for FA.
3.3. Reduced FW fraction in mild OSA individuals
When comparing the average brain FW fraction between groups (see Figure 2a), the ANCOVA revealed a significant group difference, adjusted for BMI and CSF volume. The mild OSA group had lower FW fraction compared with controls (Figure 2b, p = .035), which suggests that more water is restricted by brain tissue and less water can freely diffuse in the extracellular space. No group difference was found for the moderate to severe OSA group. A lower FW fraction correlated with a lower average AD in the clusters observed between the mild OSA and controls (p = .005), adjusted for CSF volume, age, and BMI.
Figure 2.
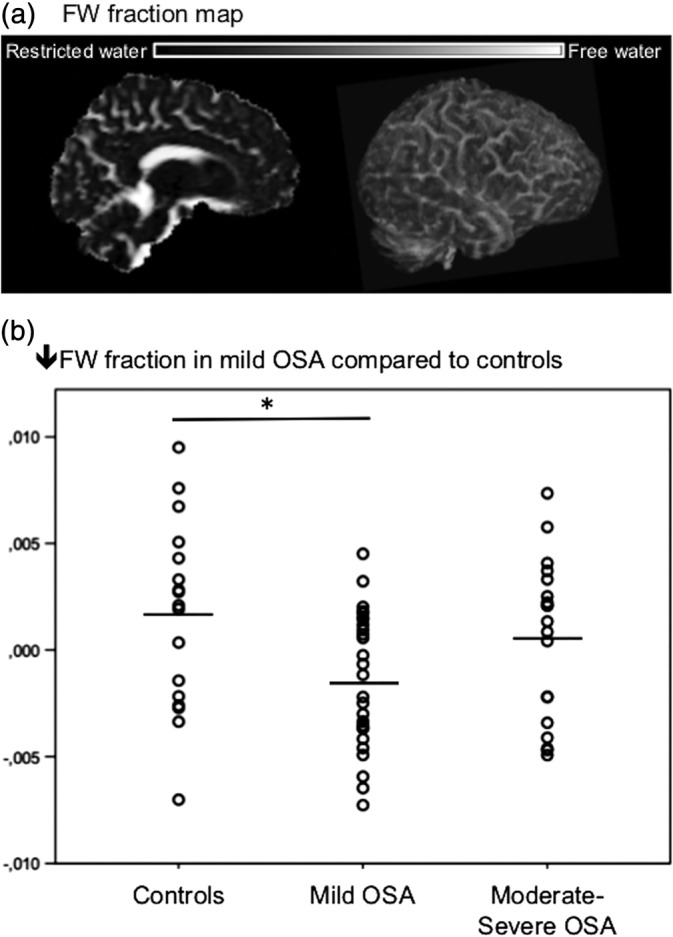
Reduced FW fraction in mild OSA. (a) For each subject, the average FW fraction was extracted with a value ranging from 0 (restricted water) to 1 (water that diffuses freely). (b) The average‐fitted FW fraction value for each subject is presented by groups. The mild OSA group showed significantly lower FW fraction compared with controls at p < .05, adjusted for BMI and CSF volume. BMI, body mass index; CSF, cerebrospinal fluid; FW, free‐water; OSA, obstructive sleep apnea
3.4. No higher white matter hyperintensities volume in OSA
No group difference was observed for white matter hyperintensities volume (p = .17) and number of white matter hypersintensities (p = .11): controls (3.1 ± 2.7 ml, 18.18 ± 7.38 lesions); mild OSA (1.9 ± 1.9 ml, 14.26 ± 5.69 lesions); moderate to severe OSA (2.15 ± 1.42 ml, 15.84 ± 7.40 lesions).
3.5. Sensitivity analyses
The following sensitivity analyses were determined post hoc and performed on the significant DTI results and the FW fraction. These sensitivity analyses were designed to clarify the nature of the relation between lower diffusivities and FW fraction with OSA severity, as well as to investigate whether our results are still present when we account for demographic and clinical characteristics of our sample. For DTI results, the average value of clusters showing significant group differences was extracted for each participant and used for sensitivity analyses.
3.5.1. Curve estimation between reduced diffusivities and FW and OSA severity
Because results of lower diffusivities and FW fraction were more extensive in mild OSA compared with controls than in moderate to severe OSA, it is possible to suspect a U‐shape relationship with OSA severity. We performed curve estimations between the average significant diffusion values and the FW fraction with the AHI. The logarithm of the AHI was used since it was skewed to the left. For the averaged MD, RD and AD in clusters showing group differences between mild OSA group and controls, both linear and quadratic functions showed a significant fit (Figure 3a–c, p < .005). This suggests that the white matter of moderate to severe OSA shows great heterogeneity, with part of the group featuring lower diffusivities seems to support a linear relationship while the other part featuring higher diffusivities seems to support a U‐shape relationship. However, both the linear and quadratic functions were nonsignificant in the corpus callosum subregion that showed a group difference between moderate to severe OSA individuals compared with controls (Figure 3d).
Figure 3.
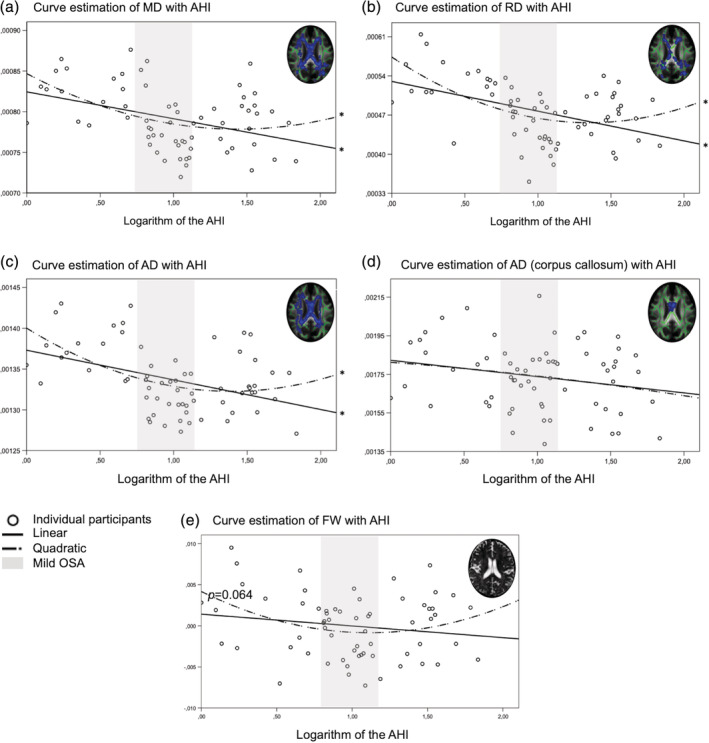
Distribution of white matter water diffusivities and FW fraction in relation with OSA severity. The average value of clusters showing significant group differences was extracted and estimated against the logarithm of the AHI. Both a linear and a quadratic fit were observed for (a) MD, (b) RD, and (c) AD. This was not the case for (d) AD specifically in the corpus callosum. For the (e) FW fraction fitted for CSF volume, a trend was observed for the quadratic fit (p = .064). Overall, these curve estimations showed that both linear and curvilinear relations are significant for MD, RD, and AD with OSA severity, suggesting a high level of heterogeneity in the moderate to severe OSA group. AHI values corresponding to the mild OSA group are showed in gray. AD, axial diffusivity; AHI, apnea–hypopnea index; CSF, cerebrospinal fluid; FW, free‐water; MD, mean diffusivity; OSA, obstructive sleep apnea; RD, radial diffusivity
Curve estimation revealed a trend for a quadratic fit between FW fraction and the logarithm of the AHI (Figure 3e, p = .064), suggesting a U‐shape relationship between OSA severity and FW.
3.5.2. Correlations between reduced diffusivities and FW with sleep parameters in mild OSA
Because the mild OSA group showed extensive white matter changes compared with controls, we investigated what sleep parameters might be related to these results. We performed correlations between multiple sleep parameters and the average diffusion values in significant clusters and the FW fraction in the subsample of individuals with an AHI ≤ 15. Reduced RD and AD were correlated with more oxygen saturation drops over 3% (p = .023 and p = .020, respectively), independently of age and BMI. Lower FW fraction was correlated with a lower minimal oxygen saturation during the night (p = .044), independently of age, BMI, and CSF volume. No correlation was observed for any variable related to sleep quality and fragmentation.
3.5.3. Reduced diffusivities and FW in specific OSA subsamples
In order to clarify whether our findings between groups are affected by the characteristics of our sample, further analyses were performed within subsamples according to demographic or clinical variables (e.g., age, sex, cognitive status, cardiovascular risk, genetics) to assess whether our effects are maintained. ANOVAs between controls, mild OSA, and moderate to severe OSA groups were performed on average significant diffusion values and FW fraction.
Removing ApoE4 allele carriers, BDNF Met allele carriers, those under 65 years old or those with excessive sleepiness (Epworth Sleepiness Scale ≥10) did not change the results. Although removing women did not weaken our results, it actually revealed a group difference between OSA groups, where lower MD was observed in mild OSA compared with moderate to severe OSA groups. Removing those with multiple cardiovascular risk factors and diseases (Vascular Burden Index ≥2) did not change the results, but revealed lower FW fraction in mild OSA individuals compared with moderate to severe OSA. Removing those with cognitive impairment (Montreal Cognitive Assessment <26) or those over 65 years old removed the lower FW fraction effect observed in mild OSA individuals as compared to controls.
4. DISCUSSION
In this study, we aimed to better understand how different levels of OSA severities in late middle‐aged and older adults are associated with brain microstructure, and more specifically with white matter diffusion properties. Our results showed that, rather than presenting the standard pattern of higher diffusivities and lower FA observed with neurodegeneration (Sexton et al., 2011; Sun et al., 2014), individuals with mild OSA compared with controls had widespread areas of lower diffusivity metrics (MD, RD, AD) along the skeleton in the center of white matter in projection, association, and commissural fibers, but not brainstem tracts. In the mild OSA group, this white matter diffusion pattern was combined with significant lower FW fraction without changes in white matter hyperintensities volume. A similar pattern of lower diffusivities was previously reported in middle‐aged individuals with moderate to severe OSA individuals (summarized in Table 1). More specifically, one group reported lower MD, RD, and AD (Kumar et al., 2012; Kumar et al., 2014). Another group also reported lower MD (Castronovo et al., 2014). We did not find lower FA in OSA contrary to what was reported by others (summarized in Table 1). Because FA is derived from diffusivity in both perpendicular and parallel orientations, lower FA is generally associated with low AD, high RD, or both concomitantly. In this study, the concomitant lower AD and RD suggest lower diffusivities in all directions, which may have resulted in unchanged FA in our participants with mild OSA.
A different pattern of white matter diffusivity properties was observed in our participants with moderate to severe OSA. In fact, they did not show this pattern of general diffusivity lowering compared to controls: they only showed lower AD limited to the corpus callosum. This region, especially the anterior part that is myelinated later during development, has been hypothesized to be particularly vulnerable to OSA (Zhang et al., 2019). Altered white matter microstructure in the corpus callosum may be associated with cognition and mood symptomatology during neurodegenerative processes (Di Paola et al., 2015).
The impacts of mild OSA are currently a source of debate in the literature (Brown, 2007; Chowdhuri et al., 2016; Littner, 2007). The present study adds to the literature by showing that altered white matter diffusion properties can be observed in late middle‐aged and older adults with mild OSA.
4.1. Potential mechanisms underlying reduced diffusivities and FW in mild OSA
A clear understanding of what DTI metrics measure is necessary to infer underlying pathophysiological processes in OSA, and that insight comes mostly from animal or human studies with histopathology measurements. Reduced RD reflects pathological processes such as the separation of myelin sheaths (Shereen et al., 2011). Reduced AD is observed with beaded or fragmented axons (Winklewski et al., 2018). In addition to early axonal and myelin damage, decreased RD and AD, as well as MD, can be observed with processes that limit water movement, including reactive gliosis and inflammatory cellular infiltration, and cytotoxic edema (increased intracellular water) (Anderova et al., 2011; Boretius et al., 2012; Winklewski et al., 2018; Zhang et al., 2012). Thus, all these processes could underlie lower white matter diffusivities observed in our OSA groups, especially in those with mild OSA. Reduced FW fraction in mild OSA supports the hypothesis that intracellular cytotoxic edema and/or reactive gliosis are ongoing. Indeed, lower extracellular water fraction could be explained both by the presence of more water inside the cells (cytotoxic edema) and increased cell density (reactive gliosis).
Hypoxemia in OSA may explain the presence of these pathological processes. Although we did not observe group differences between mild OSA and controls for hypoxemia measures, lower diffusivities and FW fraction correlated with more oxygen desaturation in those with an AHI ≤ 15. The acute stage following cerebral hypoxia/ischemia in animal models is characterized by lower diffusivities (MD, RD, AD) as well as histologic evidence of cytotoxic edema, lower extracellular water fraction, and reactive gliosis (Anderova et al., 2011; Pitkonen et al., 2012; Shereen et al., 2011). Interestingly, cytotoxic edema, increased total water content, and reactive gliosis were also observed in animal models of OSA (Aviles‐Reyes et al., 2010; Baronio et al., 2013). Cerebral swelling was reported in the case of edema due to hypoxia in altitude (Kallenberg et al., 2007; Sagoo et al., 2017). Moreover, in a previous study by our group, we found that nocturnal hypoxia values were associated with multiple regions of gray matter hypertrophy and thickening that we attributed to edema and/or reactive gliosis (Baril et al., 2017). Overall, OSA‐related hypoxia could cause cytotoxic edema and reactive gliosis that can be captured in vivo through a pattern of lower white matter diffusivities as well as lower FW fraction.
4.2. Potential biphasic relationship between OSA severity and white matter diffusion properties
Our study also found that altered white matter diffusion in moderate to severe OSA cases compared with controls was considerably less extensive than what was observed in mild OSA. Moderate to severe OSA individuals were especially heterogeneous in their white matter properties, and we hypothesize that this level of OSA severity may lead to diverse pathological responses that blurred white matter diffusion properties. In fact, cytotoxic edema, reactive gliosis, vasogenic edema, and cellular loss may have been present concomitantly with different intensities across participants, and therefore could have led to a greater variability in the moderate to severe OSA group. Because some of these pathological responses are known to decrease water diffusivity (cytotoxic edema, reactive gliosis) while other potentially more severe responses are known to increase water diffusivity (vasogenic edema, cellular loss), their concomitant presence might explain a transient apparent normalization of DTI metrics. Consistently, in ischemic conditions, normal diffusivity was observed with a large array of competing pathological mechanisms (Li et al., 2002). Further studies are needed to better understand the underlying mechanisms explaining white matter changes in both mild OSA and moderate to severe OSA.
Interestingly, water diffusivity metrics are known as stage‐specific measures in neurodegenerative diseases or ischemic conditions (Ahlhelm, Schneider, Backens, Reith, & Hagen, 2002; Anderova et al., 2011; Pitkonen et al., 2012; Ryan et al., 2013), which decrease first in acute stages and increase later on in chronic stages. More specifically, this biphasic pattern was recently reported where early neurodegeneration was characterized by lower FW and MD, whereas the opposite was observed in later stages (Montal et al., 2018). As was previously suggested (Rosenzweig et al., 2015), a biphasic model of pathological processes may be present in OSA, although it remains speculative. Consistently, a previous study showed that OSA was associated with both increased and decreased cortical thickness in various brain regions in individuals at risk for dementia, suggesting heterogeneous underlying pathological mechanisms (Cross et al., 2018). A biphasic white matter diffusion properties pattern could explain how our results varied with different levels of OSA severity, where early and acute mechanisms could characterize mild OSA followed by cellular loss and potential neurodegeneration when the disease worsens. However, we did not find higher diffusivity in subjects with more severe OSA, but higher MD and RD in adults with more severe OSA than in the present study was reported previously (Chen et al., 2015; Koo et al., 2019; Zhang et al., 2019), suggesting that our moderate to severe group may not have been severe enough to lead to this biphasic pattern. Moreover, we did not observe more white matter hyperintensities in OSA groups. Previous studies reported either more white matter hyperintensities (Baik, Seo, Yoon, Kim, & Shin, 2015; Del Brutto, Mera, Zambrano, & Castillo, 2017; Kim et al., 2013; Song et al., 2017) or no changes in OSA (Castillo, Del Brutto, Andrade Mde, Zambrano, & Nader, 2015; Lutsey et al., 2016; Schulz et al., 2013). Increased diffusivity is reported in individuals with a high white matter hyperintensities load (Svard et al., 2017), which might explain why our sample characterized by lower white matter water diffusivities did not present more white matter hyperintensities. The absence of an abnormal hyperintensities burden suggested that our OSA groups did not present significant ischemic white matter lesions. Thus, white matter changes observed with DTI and FW may represent a reversible stage of cerebral pathological responses that may be reversed if OSA is treated. Interestingly, a lower diffusivity in OSA individuals was reported to be reversible after a 1‐year treatment (Castronovo et al., 2014). Moreover, OSA patients in our sample were mostly asymptomatic regarding sleepiness, mood, and cognition (see Table 1). It is possible that higher white matter diffusivities represent underlying pathological processes that also lead to observable symptomatology.
4.3. Limitations
DTI with TBSS is a sensitive technique but is less reliable in regions of crossing fibers. However, characteristics of our methods and results suggested that crossing fibers was not the source of our findings in mild and moderate to severe OSA. In fact, we found extremely large clusters of white matter diffusivity changes as big as 16,000 voxels, which is unlikely to be explained by crossing fiber artifacts. Second, especially in mild OSA, the presence of a lower FW supported our hypothesis and was consistent with DTI findings.
Our groups were not equal for sex: the moderate to severe OSA group was mostly composed of men. Because a previous study found a moderating effect of sex on DTI metrics (Macey et al., 2012), and because removing women seems to strengthen the U‐shape relationship for MD, further studies should evaluate how sex and OSA severity interact.
5. CONCLUSIONS
In this study, we sought to evaluate how OSA of different severity levels is associated with cerebral white matter. We found that mild OSA showed widespread areas of lower white matter diffusivity compared with control participants. In contrast, moderate to severe OSA was characterized by focal lower diffusivity parallel to the axons in the corpus callosum compared with controls. The novelty of our study was the investigation of both mild and moderate to severe OSA in middle‐aged and older adults, which led to a better understanding of how OSA affects brain health with aging. Our results add to previous findings showing that mild OSA may lead to adverse outcomes (Jahn, Gouveris, & Matthias, 2016; Luz et al., 2016). Strengths of our study include the combined use of multiple neuroimaging techniques, including FW imaging, a novel measurement that improves results interpretation.
Mild OSA worsens to moderate to severe OSA in about half of untreated individuals over 4 years (Sahlman et al., 2007). Longitudinal studies are needed to clarify whether abnormal white matter properties observed in mild nontreated OSA are evolving into increased diffusivities and white matter hyperintensities over time. Moreover, longitudinal studies are also necessary to evaluate whether white matter diffusivity changes are associated with potential neurodegeneration and cognitive decline. Since there are no current therapies able to cure dementia, the identification of modifiable risk factors, such as OSA, remains the most promising intervention to prevent or slow neurodegeneration.
CONFLICT OF INTEREST
Dr Montplaisir serves as a consultant for Merck Pharmaceutical, Jazz Pharmaceutical, UBC Canada, and Valeant Pharmaceutical. Dr Carrier is the principal investigator of the Canadian Sleep and Circadian Network CSCN, which received grants from RANA, Respironics, and Meck. These associations with commercial entities from J.C. and J.M. were not related and did not support the present work. All other authors report no disclosures.
ACKNOWLEDGMENTS
For their support for data acquisition, the authors wish to thank Hélène Blais, Caroline d'Aragon, Dr Dominique Petit, Joëlle Robert, Sarah‐Hélène Julien, Maxime Fortin, Marc‐André D. Gareau, and Dr Maria Tuineag. This study was funded by government granting agencies by grants to Dr Nadia Gosselin as a principal investigator: Canadian Institutes of Health Research (CIHR) and the Fonds de la recherche du Québec—Santé (FRQS).
Baril A‐A, Gagnon K, Descoteaux M, et al. Cerebral white matter diffusion properties and free‐water with obstructive sleep apnea severity in older adults. Hum Brain Mapp. 2020;41:2686–2701. 10.1002/hbm.24971
Funding information Canadian Institutes of Health Research; Fonds de Recherche du Québec ‐ Santé
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ahlhelm, F. , Schneider, G. , Backens, M. , Reith, W. , & Hagen, T. (2002). Time course of the apparent diffusion coefficient after cerebral infarction. European Radiology, 12(9), 2322–2329. 10.1007/s00330-001-1291-0 [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine Task Force . (1999). Sleep‐related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep, 22(5), 667–689. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10450601 [PubMed] [Google Scholar]
- Anderova, M. , Vorisek, I. , Pivonkova, H. , Benesova, J. , Vargova, L. , Cicanic, M. , … Sykova, E. (2011). Cell death/proliferation and alterations in glial morphology contribute to changes in diffusivity in the rat hippocampus after hypoxia‐ischemia. Journal of Cerebral Blood Flow and Metabolism, 31(3), 894–907. 10.1038/jcbfm.2010.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles‐Reyes, R. X. , Angelo, M. F. , Villarreal, A. , Rios, H. , Lazarowski, A. , & Ramos, A. J. (2010). Intermittent hypoxia during sleep induces reactive gliosis and limited neuronal death in rats: Implications for sleep apnea. Journal of Neurochemistry, 112(4), 854–869. 10.1111/j.1471-4159.2009.06535.x [DOI] [PubMed] [Google Scholar]
- Baik, I. , Seo, H. S. , Yoon, D. , Kim, S. H. , & Shin, C. (2015). Associations of sleep apnea, NRG1 polymorphisms, alcohol consumption, and cerebral white matter hyperintensities: Analysis with genome‐wide association data. Sleep, 38(7), 1137–1143. 10.5665/sleep.4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril, A. A. , Carrier, J. , Lafreniere, A. , Warby, S. , Poirier, J. , Osorio, R. S. , … Circadian, N. (2018). Biomarkers of dementia in obstructive sleep apnea. Sleep Medicine Reviews, 42, 139–148. 10.1016/j.smrv.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril, A. A. , Gagnon, K. , Arbour, C. , Soucy, J. P. , Montplaisir, J. , Gagnon, J. F. , & Gosselin, N. (2015). Regional cerebral blood flow during wakeful rest in older subjects with mild to severe obstructive sleep apnea. Sleep, 38(9), 1439–1449. 10.5665/sleep.4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril, A. A. , Gagnon, K. , Brayet, P. , Montplaisir, J. , Carrier, J. , Soucy, J. P. , … Gosselin, N. (2018). Obstructive sleep apnea during REM sleep and daytime cerebral functioning: A regional cerebral blood flow study using high‐resolution SPECT. Journal of Cerebral Blood Flow & Metabolism, 0271678X1881410 10.1177/0271678X18814106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril, A. A. , Gagnon, K. , Brayet, P. , Montplaisir, J. , De Beaumont, L. , Carrier, J. , … Gosselin, N. (2017). Gray matter hypertrophy and thickening with obstructive sleep apnea in middle‐aged and older adults. American Journal of Respiratory and Critical Care Medicine, 195(11), 1509–1518. 10.1164/rccm.201606-1271OC [DOI] [PubMed] [Google Scholar]
- Baronio, D. , Martinez, D. , Fiori, C. Z. , Bambini‐Junior, V. , Forgiarini, L. F. , Pase da Rosa, D. , … Cerski, M. R. (2013). Altered aquaporins in the brains of mice submitted to intermittent hypoxia model of sleep apnea. Respiratory Physiology & Neurobiology, 185(2), 217–221. 10.1016/j.resp.2012.10.012 [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Epstein, N. , Brown, G. , & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3204199 [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , Ball, R. , & Ranieri, W. (1996). Comparison of Beck depression inventories ‐IA and ‐II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588–597. 10.1207/s15327752jpa6703_13 [DOI] [PubMed] [Google Scholar]
- Berry, R. B. , Budhiraja, R. , Gottlieb, D. J. , Gozal, D. , Iber, C. , Kapur, V. K. , … American Academy of Sleep Medicine . (2012). Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine, 8(5), 597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boretius, S. , Escher, A. , Dallenga, T. , Wrzos, C. , Tammer, R. , Bruck, W. , … Stadelmann, C. (2012). Assessment of lesion pathology in a new animal model of MS by multiparametric MRI and DTI. NeuroImage, 59(3), 2678–2688. 10.1016/j.neuroimage.2011.08.051 [DOI] [PubMed] [Google Scholar]
- Brickman, A. M. , Muraskin, J. , & Zimmerman, M. E. (2009). Structural neuroimaging in Altheimer's disease: Do white matter hyperintensities matter? Dialogues in Clinical Neuroscience, 11(2), 181–190. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19585953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, L. K. (2007). Mild obstructive sleep apnea syndrome should be treated. Pro. Journal of Clinical Sleep Medicine, 3(3), 259–262. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17561591 [PMC free article] [PubMed] [Google Scholar]
- Castillo, P. R. , Del Brutto, O. H. , Andrade Mde, L. , Zambrano, M. , & Nader, J. A. (2015). The association of sleep‐disordered breathing with high cerebral pulsatility might not be related to diffuse small vessel disease. A pilot study. BMC Research Notes, 8, 500 10.1186/s13104-015-1481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castronovo, V. , Scifo, P. , Castellano, A. , Aloia, M. S. , Iadanza, A. , Marelli, S. , … Falini, A. (2014). White matter integrity in obstructive sleep apnea before and after treatment. Sleep, 37(9), 1465–1475. 10.5665/sleep.3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caverzasi, E. , Mandelli, M. L. , DeArmond, S. J. , Hess, C. P. , Vitali, P. , Papinutto, N. , … Henry, R. G. (2014). White matter involvement in sporadic Creutzfeldt‐Jakob disease. Brain, 137(Pt 12), 3339–3354. 10.1093/brain/awu298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad, J. A. , Pasternak, O. , Salat, D. H. , & Chen, J. J. (2018). Re‐examining age‐related differences in white matter microstructure with free‐water corrected diffusion tensor imaging. Neurobiology of Aging, 71, 161–170. 10.1016/j.neurobiolaging.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. L. , Lu, C. H. , Lin, H. C. , Chen, P. C. , Chou, K. H. , Lin, W. M. , … Lin, W. C. (2015). White matter damage and systemic inflammation in obstructive sleep apnea. Sleep, 38(3), 361–370. 10.5665/sleep.4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhuri, S. , Quan, S. F. , Almeida, F. , Ayappa, I. , Batool‐Anwar, S. , Budhiraja, R. , … Slyman, A., on behalf of the ATS Ad Hoc Committee on Mild Obstructive Sleep Apnea (2016). An official American Thoracic Society research statement: Impact of mild obstructive sleep apnea in adults. American Journal of Respiratory and Critical Care Medicine, 193(9), e37–e54. 10.1164/rccm.201602-0361ST [DOI] [PubMed] [Google Scholar]
- Cross, N. E. , Memarian, N. , Duffy, S. L. , Paquola, C. , LaMonica, H. , D'Rozario, A. , … Naismith, S. L. (2018). Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia. The European Respiratory Journal, 52(1), 1800740 10.1183/13993003.00740-2018 [DOI] [PubMed] [Google Scholar]
- Daducci, A. , Canales‐Rodriguez, E. J. , Zhang, H. , Dyrby, T. B. , Alexander, D. C. , & Thiran, J. P. (2015). Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. NeuroImage, 105, 32–44. 10.1016/j.neuroimage.2014.10.026 [DOI] [PubMed] [Google Scholar]
- Del Brutto, O. H. , Mera, R. M. , Zambrano, M. , & Castillo, P. R. (2017). Relationship between obstructive sleep apnea and neuroimaging signatures of cerebral small vessel disease in community‐dwelling older adults. The Atahualpa project. Sleep Medicine, 37, 10–12. 10.1016/j.sleep.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Di Paola, M. , Phillips, O. , Orfei, M. D. , Piras, F. , Cacciari, C. , Caltagirone, C. , & Spalletta, G. (2015). Corpus callosum structure is topographically correlated with the early course of cognition and depression in Alzheimer's disease. Journal of Alzheimer's Disease, 45(4), 1097–1108. 10.3233/JAD-142895 [DOI] [PubMed] [Google Scholar]
- Duce, B. , Milosavljevic, J. , & Hukins, C. (2015). The 2012 AASM respiratory event criteria increase the incidence of hypopneas in an adult sleep center population. Journal of Clinical Sleep Medicine, 11(12), 1425–1431. 10.5664/jcsm.5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon, K. , Baril, A. A. , Montplaisir, J. , Carrier, J. , Chami, S. , Gauthier, S. , … Gosselin, N. (2018). Detection of mild cognitive impairment in middle‐aged and older adults with obstructive sleep apnoea. The European Respiratory Journal, 52(5), 1801137 10.1183/13993003.01137-2018 [DOI] [PubMed] [Google Scholar]
- Gagnon, K. , Baril, A. A. , Montplaisir, J. , Carrier, J. , De Beaumont, L. , D'Aragon, C. , … Gosselin, N. (2019). Disconnection between self‐reported and objective cognitive impairment in obstructive sleep apnea. Journal of Clinical Sleep Medicine, 15(3), 409–415. 10.5664/jcsm.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, M. P. , & Ransom, B. R. (2003). New light on white matter. Stroke, 34(2), 330–332. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12574526 [DOI] [PubMed] [Google Scholar]
- Gosselin, N. , Baril, A. A. , Osorio, R. S. , Kaminska, M. , & Carrier, J. (2019). Obstructive sleep apnea and the risk of cognitive decline in older adults. American Journal of Respiratory and Critical Care Medicine, 199(2), 142–148. 10.1164/rccm.201801-0204PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin, N. , De Beaumont, L. , Gagnon, K. , Baril, A. A. , Mongrain, V. , Blais, H. , … Carrier, J. (2016). BDNF Val66Met polymorphism interacts with sleep consolidation to predict ability to create new declarative memories. The Journal of Neuroscience, 36(32), 8390–8398. 10.1523/JNEUROSCI.4432-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber, C. , Ancoli‐Israel, S. , Chesson, A. L. J. , & Quan, S. F. (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications (1st ed.). Westchester: American Academy of Sleep Medicine. [Google Scholar]
- Jahn, C. , Gouveris, H. , & Matthias, C. (2016). Systemic inflammation in patients with compromised upper airway anatomy and primary snoring or mild obstructive sleep apnea. European Archives of Oto‐Rhino‐Laryngology, 273(10), 3429–3433. 10.1007/s00405-016-4103-5 [DOI] [PubMed] [Google Scholar]
- Ji, F. , Pasternak, O. , Liu, S. , Loke, Y. M. , Choo, B. L. , Hilal, S. , … Zhou, J. (2017). Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer's disease with and without cerebrovascular disease. Alzheimer's Research & Therapy, 9(1), 63 10.1186/s13195-017-0292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep, 14(6), 540–545. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1798888 [DOI] [PubMed] [Google Scholar]
- Kallenberg, K. , Bailey, D. M. , Christ, S. , Mohr, A. , Roukens, R. , Menold, E. , … Knauth, M. (2007). Magnetic resonance imaging evidence of cytotoxic cerebral edema in acute mountain sickness. Journal of Cerebral Blood Flow and Metabolism, 27(5), 1064–1071. 10.1038/sj.jcbfm.9600404 [DOI] [PubMed] [Google Scholar]
- Kim, H. , Yun, C. H. , Thomas, R. J. , Lee, S. H. , Seo, H. S. , Cho, E. R. , … Shin, C. (2013). Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle‐aged and older general population. Sleep, 36(5), 709–715B. 10.5665/sleep.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo, D. L. , Kim, H. R. , Kim, H. , Seong, J. K. , & Joo, E. Y. (2019). White matter tract‐specific alterations in male patients with untreated obstructive sleep apnea are associated with worse cognitive function. Sleep, zsz247. 10.1093/sleep/zsz247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R. , Chavez, A. S. , Macey, P. M. , Woo, M. A. , Yan‐Go, F. L. , & Harper, R. M. (2012). Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. Journal of Neuroscience Research, 90(10), 2043–2052. 10.1002/jnr.23083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, R. , Pham, T. T. , Macey, P. M. , Woo, M. A. , Yan‐Go, F. L. , & Harper, R. M. (2014). Abnormal myelin and axonal integrity in recently diagnosed patients with obstructive sleep apnea. Sleep, 37(4), 723–732. 10.5665/sleep.3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. H. , Yun, C. H. , Min, A. , Hwang, Y. H. , Lee, S. K. , Kim, D. Y. , … Shin, C. (2019). Altered structural brain network resulting from white matter injury in obstructive sleep apnea. Sleep, zsz120. 10.1093/sleep/zsz120 [DOI] [PubMed] [Google Scholar]
- Leng, Y. , McEvoy, C. T. , Allen, I. E. , & Yaffe, K. (2017). Association of sleep‐disordered breathing with cognitive function and risk of cognitive impairment: A systematic review and meta‐analysis. JAMA Neurology, 74(10), 1237–1245. 10.1001/jamaneurol.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Liu, K. F. , Silva, M. D. , Meng, X. , Gerriets, T. , Helmer, K. G. , … Fisher, M. (2002). Acute postischemic renormalization of the apparent diffusion coefficient of water is not associated with reversal of astrocytic swelling and neuronal shrinkage in rats. American Journal of Neuroradiology, 23(2), 180–188. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11847039 [PMC free article] [PubMed] [Google Scholar]
- Littner, M. R. (2007). Mild obstructive sleep apnea syndrome should not be treated. Journal of Clinical Sleep Medicine, 3(3), 263–264. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17561592 [PMC free article] [PubMed] [Google Scholar]
- Lutsey, P. L. , Norby, F. L. , Gottesman, R. F. , Mosley, T. , MacLehose, R. F. , Punjabi, N. M. , … Alonso, A. (2016). Sleep apnea, sleep duration and brain MRI markers of cerebral vascular disease and Alzheimer's disease: The atherosclerosis risk in communities study (ARIC). PLoS One, 11(7), e0158758 10.1371/journal.pone.0158758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz, G. P. , Guimaraes, T. M. , Weaver, T. E. , Nery, L. E. , E Silva, L. O. , Badke, L. , … Bittencourt, L. (2016). Impaired sustained attention and lapses are present in patients with mild obstructive sleep apnea. Sleep & Breathing, 20(2), 681–687. 10.1007/s11325-015-1279-7 [DOI] [PubMed] [Google Scholar]
- Macey, P. M. , Kumar, R. , Woo, M. A. , Valladares, E. M. , Yan‐Go, F. L. , & Harper, R. M. (2008). Brain structural changes in obstructive sleep apnea. Sleep, 31(7), 967–977. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18652092 [PMC free article] [PubMed] [Google Scholar]
- Macey, P. M. , Kumar, R. , Yan‐Go, F. L. , Woo, M. A. , & Harper, R. M. (2012). Sex differences in white matter alterations accompanying obstructive sleep apnea. Sleep, 35(12), 1603–1613. 10.5665/sleep.2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, A. , & White, D. P. (2002). Obstructive sleep apnoea. Lancet, 360(9328), 237–245. 10.1016/S0140-6736(02)09464-3 [DOI] [PubMed] [Google Scholar]
- Montal, V. , Vilaplana, E. , Alcolea, D. , Pegueroles, J. , Pasternak, O. , Gonzalez‐Ortiz, S. , … Fortea, J. (2018). Cortical microstructural changes along the Alzheimer's disease continuum. Alzheimers Dement, 14(3), 340–351. 10.1016/j.jalz.2017.09.013 [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips, N. A. , Bedirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , … Chertkow, H. (2005). The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Pasternak, O. , Sochen, N. , Gur, Y. , Intrator, N. , & Assaf, Y. (2009). Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine, 62(3), 717–730. 10.1002/mrm.22055 [DOI] [PubMed] [Google Scholar]
- Pitkonen, M. , Abo‐Ramadan, U. , Marinkovic, I. , Pedrono, E. , Hasan, K. M. , Strbian, D. , … Tatlisumak, T. (2012). Long‐term evolution of diffusion tensor indices after temporary experimental ischemic stroke in rats. Brain Research, 1445, 103–110. 10.1016/j.brainres.2012.01.043 [DOI] [PubMed] [Google Scholar]
- Poirier, J. (2005). Apolipoprotein E, cholesterol transport and synthesis in sporadic Alzheimer's disease. Neurobiology of Aging, 26(3), 355–361. 10.1016/j.neurobiolaging.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Polsek, D. , Gildeh, N. , Cash, D. , Winsky‐Sommerer, R. , Williams, S. C. R. , Turkheimer, F. , … Rosenzweig, I. (2018). Obstructive sleep apnoea and Alzheimer's disease: In search of shared pathomechanisms. Neuroscience and Biobehavioral Reviews, 86, 142–149. 10.1016/j.neubiorev.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei, S. , Asgari Mobarake, K. , Saberi, A. , Keshavarz, P. , & Leili, E. K. (2016). Brain‐derived neurotrophic factor (BDNF) Val66Met polymorphism and post‐stroke dementia: A hospital‐based study from northern Iran. Neurological Sciences, 37(6), 935–942. 10.1007/s10072-016-2520-2 [DOI] [PubMed] [Google Scholar]
- Rosenzweig, I. , Glasser, M. , Polsek, D. , Leschziner, G. D. , Williams, S. C. , & Morrell, M. J. (2015). Sleep apnoea and the brain: A complex relationship. The Lancet Respiratory Medicine, 3(5), 404–414. 10.1016/S2213-2600(15)00090-9 [DOI] [PubMed] [Google Scholar]
- Ryan, N. S. , Keihaninejad, S. , Shakespeare, T. J. , Lehmann, M. , Crutch, S. J. , Malone, I. B. , … Fox, N. C. (2013). Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer's disease. Brain, 136(Pt 5), 1399–1414. 10.1093/brain/awt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagoo, R. S. , Hutchinson, C. E. , Wright, A. , Handford, C. , Parsons, H. , Sherwood, V. , … Expedition, S. (2017). Magnetic resonance investigation into the mechanisms involved in the development of high‐altitude cerebral edema. Journal of Cerebral Blood Flow and Metabolism, 37(1), 319–331. 10.1177/0271678X15625350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlman, J. , Pukkila, M. , Seppa, J. , & Tuomilehto, H. (2007). Evolution of mild obstructive sleep apnea after different treatments. Laryngoscope, 117(6), 1107–1111. 10.1097/MLG.0b013e3180514d08 [DOI] [PubMed] [Google Scholar]
- Sanchez, E. , El‐Khatib, H. , Arbour, C. , Bedetti, C. , Blais, H. , Marcotte, K. , … Gosselin, N. (2019). Brain white matter damage and its association with neuronal synchrony during sleep. Brain, 142(3), 674–687. 10.1093/brain/awy348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, U. G. , Mason, R. H. , Craig, S. E. , Howard, S. , Nicoll, D. J. , Kohler, M. , … Stradling, J. R. (2013). Leukoaraiosis on MRI in patients with minimally symptomatic obstructive sleep apnoea. Cerebrovascular Diseases, 35(4), 363–369. 10.1159/000348845 [DOI] [PubMed] [Google Scholar]
- Selnes, P. , Aarsland, D. , Bjornerud, A. , Gjerstad, L. , Wallin, A. , Hessen, E. , … Fladby, T. (2013). Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. Journal of Alzheimer's Disease, 33(3), 723–736. 10.3233/JAD-2012-121603 [DOI] [PubMed] [Google Scholar]
- Sexton, C. E. , Kalu, U. G. , Filippini, N. , Mackay, C. E. , & Ebmeier, K. P. (2011). A meta‐analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neurobiology of Aging, 32(12), 2322.e5–2322.e8. 10.1016/j.neurobiolaging.2010.05.019 [DOI] [PubMed] [Google Scholar]
- Shereen, A. , Nemkul, N. , Yang, D. , Adhami, F. , Dunn, R. S. , Hazen, M. L. , … Kuan, C. Y. (2011). Ex vivo diffusion tensor imaging and neuropathological correlation in a murine model of hypoxia‐ischemia‐induced thrombotic stroke. Journal of Cerebral Blood Flow and Metabolism, 31(4), 1155–1169. 10.1038/jcbfm.2010.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. , Chen, S. J. , Ma, M. Y. , Bao, Y. P. , Han, Y. , Wang, Y. M. , … Lu, L. (2018). Sleep disturbances increase the risk of dementia: A systematic review and meta‐analysis. Sleep Medicine Reviews, 40, 4–16. 10.1016/j.smrv.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Johansen‐Berg, H. , Rueckert, D. , Nichols, T. E. , Mackay, C. E. , … Behrens, T. E. (2006). Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage, 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Song, T. J. , Park, J. H. , Choi, K. H. , Kim, J. H. , Choi, Y. , Chang, Y. , … Lee, H. W. (2017). Is obstructive sleep apnea associated with the presence of intracranial cerebral atherosclerosis? Sleep & Breathing, 21(3), 639–646. 10.1007/s11325-016-1450-9 [DOI] [PubMed] [Google Scholar]
- Sun, X. , Salat, D. , Upchurch, K. , Deason, R. , Kowall, N. , Budson, A. , & Alzheimer's Disease Neuroimaging Initiative . (2014). Destruction of white matter integrity in patients with mild cognitive impairment and Alzheimer disease. Journal of Investigative Medicine, 62(7), 927–933. 10.1097/JIM.0000000000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svard, D. , Nilsson, M. , Lampinen, B. , Latt, J. , Sundgren, P. C. , Stomrud, E. , … van Westen, D. (2017). The effect of white matter hyperintensities on statistical analysis of diffusion tensor imaging in cognitively healthy elderly and prodromal Alzheimer's disease. PLoS One, 12(9), e0185239 10.1371/journal.pone.0185239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost, H. , Alam, T. , Geramita, M. , Rebsch, C. , Kolachana, B. , Dickinson, D. , … Marenco, S. (2013). Effects of the BDNF Val66Met polymorphism on white matter microstructure in healthy adults. Neuropsychopharmacology, 38(3), 525–532. 10.1038/npp.2012.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufik, S. , Santos‐Silva, R. , Taddei, J. A. , & Bittencourt, L. R. (2010). Obstructive sleep apnea syndrome in the Sao Paulo epidemiologic sleep study. Sleep Medicine, 11(5), 441–446. 10.1016/j.sleep.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Villeneuve, S. , Belleville, S. , Massoud, F. , Bocti, C. , & Gauthier, S. (2009). Impact of vascular risk factors and diseases on cognition in persons with mild cognitive impairment. Dementia and Geriatric Cognitive Disorders, 27(4), 375–381. 10.1159/000209965 [DOI] [PubMed] [Google Scholar]
- Vipin, A. , Ng, K. K. , Ji, F. , Shim, H. Y. , Lim, J. K. W. , Pasternak, O. , … Alzheimer's Disease Neuroimaging Initiative . (2019). Amyloid burden accelerates white matter degradation in cognitively normal elderly individuals. Human Brain Mapping, 40(7), 2065–2075. 10.1002/hbm.24507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklewski, P. J. , Sabisz, A. , Naumczyk, P. , Jodzio, K. , Szurowska, E. , & Szarmach, A. (2018). Understanding the physiopathology behind axial and radial diffusivity changes—What do we know? Frontiers in Neurology, 9, 92 10.3389/fneur.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Zhu, D. M. , Zhao, W. , Zhang, Y. , Yang, Y. , Zhang, C. , … Yu, Y. (2019). Selective microstructural integrity impairments of the anterior corpus callosum are associated with cognitive deficits in obstructive sleep apnea. Brain and Behavior: A Cognitive Neuroscience Perspective, 9(12), e01482 10.1002/brb3.1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Aggarwal, M. , & Mori, S. (2012). Structural insights into the rodent CNS via diffusion tensor imaging. Trends in Neurosciences, 35(7), 412–421. 10.1016/j.tins.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , & Zhao, Y. (2018). Sleep‐disordered breathing and the risk of cognitive decline: A meta‐analysis of 19,940 participants. Sleep & Breathing, 22(1), 165–173. 10.1007/s11325-017-1562-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.


