Abstract
Consistent findings postulate disturbed glutamatergic function (more specifically a hypofunction of the ionotropic NMDA receptors) as an important pathophysiologic mechanism in schizophrenia. However, the role of the metabotropic glutamatergic receptors type 5 (mGluR5) in this disease remains unclear. In this study, we investigated their significance (using [11C]ABP688) for psychopathology and cognition in male patients with chronic schizophrenia and healthy controls. In the patient group, lower mGluR5 binding potential (BPND) values in the left temporal cortex and caudate were associated with higher general symptom levels (negative and depressive symptoms), lower levels of global functioning and worse cognitive performance. At the same time, in both groups, mGluR5 BPND were significantly lower in smokers (F [27,1] = 15.500; p = .001), but without significant differences between the groups. Our findings provide support for the concept that the impaired function of mGluR5 underlies the symptoms of schizophrenia. They further supply a new perspective on the complex relationship between tobacco addiction and schizophrenia by identifying glutamatergic neurotransmission—in particularly mGluR5—as a possible connection to a shared vulnerability.
Keywords: chronic schizophrenia, cognition, mGluR5 receptor, negative symptoms, positron emission tomography
1. INTRODUCTION
More than 100 years after the implementation of the schizophrenia concept, this severe illness remains one of the most mysterious and costliest mental disorders in terms of human suffering and social expenditure, with a lifetime prevalence of about 1% worldwide (van Os & Kapur, 2009). Numerous genetic and environmental factors contribute to the development of schizophrenia (Howes, McCutcheon, Owen, & Murray, 2017). On a neurobiological level, a coherent body of evidence attributes a prominent role to the dysfunctions of dopaminergic neurotransmission, although pharmacological and other studies show that dopaminergic dysfunction is unlikely to explain the full range associated to the disorder (Winton‐Brown, Fusar‐Poli, Ungless, & Howes, 2014).
Complementary evidence from pharmacology, physiology, and brain imaging suggest that glutamatergic dysfunction might contribute to the biological processes underlying some of the clinical features in schizophrenia (Moghaddam & Javitt, 2012), and this has been further supported by multiple animal, post‐mortem and genetic studies (Egerton & Stone, 2012; Hammond, Shan, Meador‐Woodruff, & McCullumsmith, 2014; Javitt, 2010). These investigations implicate a complex deregulation of glutamatergic neurotransmission, not solely limited to a simple deficit in glutamatergic neurotransmission but rather mainly associated with aberrant glutamate receptor function and localization (Hammond et al., 2014).
In particular, there are consistent findings which imply that schizophrenia is primarily associated with the hypofunction of N‐methyl‐d‐aspartate glutamate receptors (NMDA‐R), leading to a hypostimulation of gamma‐amino‐butyric‐acid (GABA) inhibitory interneurons and disinhibited glutamate release (Egerton & Stone, 2012). The evidence for this conclusion originates from the psychosis model experimentally induced by noncompetitive NMDA‐R antagonists, such as phencyclidine (PCP) and ketamine (Abi‐Saab, D'Souza, Moghaddam, & Krystal, 1998). However, in vivo investigation of NMDA‐R is difficult due to the high nonspecific binding and poor signal of the available NMDA‐R radioligands (Waterhouse, 2003). To date, there has only been one published SPECT study of NMDA‐R binding in schizophrenia patients that confirms a relative deficit in NMDA‐R activity in the left hippocampus in unmedicated subjects (Pilowsky et al., 2006).
New Positron emission tomography (PET) radiotracers allow the investigation of another type of receptor closely associated with NMDA‐R—the metabotropic glutamatergic receptors type 5 (mGluR5). The mGluR5 receptors are G‐protein coupled receptors abundantly expressed on cortical, hippocampal and striatal regions in the human brain and are highly implicated in the pathophysiology of schizophrenia (Matosin, Fernandez‐Enright, Lum, & Newell, 2017). The mGluR5 receptors share a structural and functional link with the NMDA‐R (Newell & Matosin, 2014) and jointly modulate glutamatergic signaling (Newell, 2013). The activation of mGluR5 results in an enhanced functionality of the NMDA‐R (Niswender & Conn, 2010). In this connection, it has been shown that a mGluR5 agonist effectively reverses the (pro‐psychotic) behavioral effects of ketamine (Chan, Chiu, Sou, & Chen, 2008), indicating, once again, that mGluR5 plays an important role in the regulation of NMDA‐mediated neurotransmission in schizophrenia‐related processes.
To date, a genetic linkage study (Volk, Eggan, & Lewis, 2010) and, more significantly, studies from animal experiments have provided conclusive evidence for mGlu5 receptors playing a significant role in the pathogenesis of schizophrenia. An investigation has demonstrated deficiencies in NMDA‐dependent plasticity and learning behaviors in mGluR5 knockout mice (Lu et al., 1997). Furthermore, mGluR5 knockout animals exhibit deficits in prepulse inhibition, a measure of sensorimotor gating, which is impaired in schizophrenia patients and can be reversed by antipsychotic agents (Chen, Stoker, & Markou, 2010). Moreover, mGluR5 antagonists have been shown to induce locomotor hyperactivity, prepulse inhibition (PPI) deficits, and learning impairments (Campbell et al., 2004; Homayoun, Stefani, Adams, Tamagan, & Moghaddam, 2004; Kinney et al., 2003; Pietraszek et al., 2005). On the other hand, it has been shown that mGluR5 activation ameliorated cognitive deficits in several animal models (Balschun, Zuschratter, & Wetzel, 2006). It has also been demonstrated that mGluR5 plays an important role in the induction of long‐lasting forms of synaptic plasticity, including long‐term depression (LTD) and long‐term potentiation (LTP) of transmission at multiple glutamatergic synapses and also induces long‐lasting changes in neuronal excitability (Kullmann & Lamsa, 2008). Overall, the animal studies referred to here emphasized that a decreased availability (or/and function) in mGluR5 may be involved in the symptomatology of schizophrenia.
Taken together these findings have directed attention toward a possible therapeutic modification of the symptoms of schizophrenia via mGluR5. Indeed, positive allosteric modulators (PAM) of mGluR5 receptors revealed some promising effects. These drugs act by binding to the allosteric site on the mGluR5 to potentiate its activation and subsequently the activation of NMDA‐R by glutamate (Chan et al., 2008; Niswender & Conn, 2010). Thereby, they show some utility as novel antipsychotic and cognition‐enhancing drugs with efficacy against all three symptom‐domains (positive, negative, and cognitive; Maksymetz, Moran, & Conn, 2017). In addition, several recent animal studies have confirmed the pro‐cognitive effects of PAM (Golubeva, Moloney, O'Connor, Dinan, & Cryan, 2016).
However, the success of new mGluR5‐based therapies depends on a deeper understanding of the role of mGluR5 in schizophrenia and other brain disorders.
The recently developed radiotracer [11C]ABP688 (3‐(6‐methyl‐pyridine‐2‐ylethynyl)‐cyclohex‐2‐enone‐O‐[11C]‐methyloxime) has opened up new possibilities to evaluate mGluR5 in PET studies as it binds to the allosteric site of the mGluR5 and is not able to bind glutamate (Ametamey et al., 2007). Previous investigations have shown gender differences (Smart et al., 2019) and a sufficient test–retest reliability for this radiotracer (Smart et al., 2018) and confirmed its ability to detect physiological changes in endogenous glutamate levels (DeLorenzo, Kumar, Mann, & Parsey, 2011).
[11C]ABP688 has previously been used to investigate several psychiatric illnesses and repeated intraindividual measures using [11C]ABP688 have been able to show a reduction in mGluR5 availability following the administration of ketamine in patients with depression and healthy volunteers (DeLorenzo et al., 2015; Esterlis et al., 2018). Furthermore, an association between lower mGluR5 availability and related functional connectivity alterations has been reported in drug‐naïve young adults with major depression without comorbidity (Kim et al., 2019). Moreover, altered mGluR5 availability was shown in the temporal lobe and amygdala in patients with an alcohol disorder (Akkus et al., 2018) and obsessive–compulsive disorder (Akkus et al., 2014).
Only a small number of studies investigated the role of mGluR5 receptors in schizophrenia. Akkus et al. (2017) did not find differences in mGluR5 distribution volume ratio (DVR) between controls and patients but found a correlation between DVR in the medial orbitofrontal cortex and the use of antipsychotics. A highly significant global reduction in mGluR5 density has been reported in smokers and also in ex‐smokers and is thought to be a possible result of an adaptation to a chronic increase in glutamate, induced by chronic nicotine administration (Akkus et al., 2013; Akkus et al., 2016; Hulka et al., 2014). Bearing in mind the very high prevalence of smoking (more than 60%) in subjects with schizophrenia compared to the general population (de Leon & Diaz, 2005; Sagud et al., 2009), the possible mediating role of mGluR5 as a linkage between schizophrenia and smoking (tobacco misuse or addiction) requires a closer investigation. Indeed, patients with schizophrenia and comorbid nicotine dependence are more likely to have an earlier age of onset of the disease, higher symptom severity as expressed by the Positive and Negative Syndrome Scale (PANSS; Schwartz et al., 2005) and a lower school performance (Riala et al., 2005) than nonsmoking patients. However, it is still unclear whether the neurobiology of schizophrenia makes patients more vulnerable to the addiction or whether nicotine is used with the aim to “recover” cognitive deficits (De Luca et al., 2004).
The aim of our exploratory study was to investigate the role of mGluR5 receptors for psychopathology and cognition in patients suffering from schizophrenia. Taking into consideration the association between NMDA‐R and mGluR5 receptors, we hypothesized that a higher availability of mGluR5 receptors will be advantageous for better cognition and less pronounced psychopathology in schizophrenia patients due to the modulatory effects on NMDA‐R. Furthermore, we expected a general reduction of mGluR5 receptor binding in schizophrenia patients compared to healthy volunteers. In accordance with previous studies, we also expected a strong reduction in receptor binding due to smoking status in both groups.
2. MATERIALS AND METHODS
2.1. Participants
The sample consists of 15 male patients diagnosed with schizophrenia and 15 healthy male volunteers matched for age, smoking status, gender, and education. Both groups had an average age of 40.20 ± 10.40 and 41.60 ± 10.80 years, respectively. Subjects were instructed not to drink coffee or alcohol and not to take any medicine within 24 hr before the PET measurement (except their daily medication). Smokers were not allowed to smoke for 2 hr prior to injection of the tracer.
All patients were treated with second‐generation antipsychotics (mean chlorpromazine equivalents (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010): 277.8 ± 195.1 mg/day (ranging between 94.3 and 683.8 mg/day); mean olanzapine equivalents according to the classical mean doses method (Leucht et al., 2015) 17.2 ± 9.8 mg (ranging between 5 and 37.15 mg). Ten patients were treated mono‐therapeutically, while five received two antipsychotics: Amisulpride and clozapine (3), aripiprazole and clozapine (1), or quetiapine and sertindole (1).
The study was approved by the Ethics Committee of the Medical Faculty at the RWTH Aachen University and the German Federal Office for Radiation Protection (Bundesamt für Strahlenschutz). Patients were recruited from Uniklinik RWTH Aachen and complied to the diagnostic criteria according to DSM‐IV (First, Spitzer, Gibbon, & Williams, 2002). Informed consent was obtained for all subjects.
2.2. General psychopathology and functioning assessment
The general psychopathology in patients suffering from schizophrenia was assessed using the PANSS (Kay, Fiszbein, & Opler, 1987). The analysis was performed according to the traditional three‐factorial structure. For a more precise assessment of the negative symptoms, we used the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1982). The scale consists of five subscales that evaluate five different domains of negative symptoms: Affective flattening, alogia, avolition‐apathy, anhedonia‐asociality, and attentional impairment. Each domain is split into observable behavioral symptoms and a global level. These are rated on a scale from 0 to 5. Higher scores describe more pronounced symptoms. For the assessment of depressive symptoms, we used the Hamilton Depression Scale (HAMD) with 21 items (Hamilton, 1960). The general functioning level was assessed using the Global Assessment of Functioning (GAF; Piersma & Boes, 1997), constructed as an overall (global) measure of how patients are doing. GAF rates psychological, social, and occupational functioning, covering the range from positive mental health to severe psychopathology (Aas, 2011). The 100‐point scales of GAF are divided into intervals, each with 10 points (for example 31–40 and 51–60). The 10‐points intervals have anchor points (verbal instructions) describing symptoms and functioning that are relevant for scoring. The anchor points for interval 1–10 describe the most severely ill, and the anchor points for interval 91–100 describe the healthiest (Aas, 2010). We used the German version of the scale (American Psychological Association, 2003). The assessment of social and occupational functioning using the Global Functioning: Social (GF: Social; Auther, Smith, & Cornblatt, 2006) and the Global Functioning: Role (GF: Role; Niendam, Bearden, Johnson, & Cannon, 2006) scales. The scales have been partially derived from Social and Occupational Functioning Assessment Scale (SOFAS) from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) and the GAF, as it appears in the Scale of Prodromal Symptoms (SOPS; Cornblatt et al., 2007). They represent parallel (one targeting social, the other role) well‐anchored scales that take age and phase of illness into account. The functioning level of the patients is rated during a structured interview, ranging between 1 (“extreme social isolation”/“extreme role dysfunction”) and 10 (“superior social/interpersonal functioning”/“superior role functioning”).
2.3. Neurocognitive tests
The neuropsychological test battery consisted of several tests, chosen according to the MATRICS Consensus Cognitive Battery (Nuechterlein et al., 2008). The focus in our examination was on three cognitive domains, recently shown to explain about 86% of the variance for the composed cognitive score in stable patients with schizophrenia (Georgiades et al., 2017): Speed of processing (Trail Making Test, Part A, the coding task from the Wechsler Intelligence Scale [WAIS‐IV]), working memory (Letter‐Number Span test and Corsi block‐tapping task) and visual learning (Rey Visual Design Learning Test).
2.3.1. Trail‐making test
The Trail‐Making Test (TMT) is a popular test because of its high sensitivity to the presence of cognitive impairment (Kortte, Horner, & Windham, 2002). The test is divided into two parts. In Part A, the participant is required to draw a line connecting 25 numbers consecutively as quickly as possible. In Part B, the participant must draw a line alternating between numbers and letters in consecutive order. Performance is assessed by the time taken to complete each trial correctly. The TMT reflects a combination of several cognitive functions, measuring complex visual scanning with a motor component, motor speed, and agility. Part B is particularly sensitive to cognitive flexibility and executive function. The difference score TMT(B)–TMT(A) and the ratio score TMT(B)/TMT(A) are reported to be more indicative for executive control abilities (Sanchez‐Cubillo et al., 2009) and an index of cognitive flexibility, relatively independent of manual dexterity (Vazzana et al., 2010).
2.3.2. Number‐symbol coding task
The number‐symbol coding task is a subtest of the revised Wechsler Intelligence Scale (WAIS‐IV; Lawrence, Saklofske, Coalson, & Raiford, 2010). Participants are asked to pair symbols to numbers and write them into blank squares as quickly as possible, referring to a digit symbol key outlined at the top of the examination sheet. Scoring is based on the number of correct substitutions made in 120 s. It primarily quantifies the speed of processing but also quantifies short‐term visual memory, learning ability, cognitive flexibility, attention, concentration, and motivation.
2.3.3. Letter‐number span
In the Letter–Number Span (LNS; Gold, Carpenter, Randolph, Goldberg, & Weinberger, 1997), the tester verbally presents increasingly longer sequences of intermixed numbers and letters at a rate of one per second. After each sequence, the participant is asked to repeat the numbers in ascending order first, and then the letters in alphabetical order. The letter–number sequences range from two up to a maximum length of seven stimuli. Four trials are presented for each length of the sequence. The test is discontinued when the subject fails four consecutive trials of the same length. One point is scored for each correctly repeated sequence (maximum total score is 24 points). This test measures the performance of the working memory.
2.3.4. Corsi block‐tapping task
The Corsi block‐tapping task (Corsi, 1973) is a simple and powerful test used for the assessment of deficits in immediate nonverbal memory and for clarifying theoretical conceptions of visuospatial memory (Berch, Krikorian, & Huha, 1998). The original Corsi apparatus consisted of a set of nine identical blocks (3 × 3 × 3 cm) irregularly positioned on a wooden board (23 × 28 cm). The experimenter points to a series of blocks at a rate of one block per second. Subsequently, the participant is required to point to the same blocks in their order of presentation. The length of the block sequences increases until recall is no longer correct (Vandierendonck, Kemps, Fastame, & Szmalec, 2004). The final test scores represent a sum of correct recalls performed forward and backward.
2.3.5. Rey visual design learning test
The Rey visual design learning test (RVDLT; Strauss, Sherman, & Spreen, 2006) is an instrument that assesses immediate memory span, new learning, and recognition memory for nonverbal material (Wilhelm, 2004). Fifteen cards with geometric figures are presented over five learning trials, with immediate recall tested after each presentation. The total score is the number of correctly memorized items reproduced by means of drawings in the trials and a delayed recall test. In the recognition test, subjects are asked to pick the target items from a set of 30 items, which contains 15 targets and 15 nontargets.
2.3.6. Z‐composite score
For additional analysis, we calculated the summarized z‐scores for all cognitive parameters. Here, the subscores from all of the tests were transformed such that higher values consistently corresponded to better cognitive performance. These transformed measures were then converted to standardized scores by setting the sample mean of each measure to zero and the standard deviation to 1. A composite z‐score was then computed as the mean score of the sum of the several standardized scores.
2.4. Positron emission tomography
Radiosynthesis of [11C]ABP688 was performed according to the method already reported (Elmenhorst et al., 2016). The average molar activity at the injection time was 101.70 ± 45.33 MBq/nmol. All subjects were measured using a 3 T hybrid MR‐BrainPET insert system (Herzog et al., 2011). Our bolus plus infusion protocol was optimized based on a previous publication that reported a K bolus = 53 min and a steady‐state after 40 min (Burger et al., 2010). In our protocol, the bolus injection (50% of total activity), followed by 65 min of infusion with 92 ml/hr infusion rate, was applied after positioning the subject in the scanner. The average total injected activity per subject was 525 ± 55 MBq. A distribution equilibrium was observed after 30 min (also reported by other researchers; Akkus et al., 2014; Deschwanden et al., 2011). Starting simultaneously with the bolus injection, the PET data were acquired in list mode for 65 min. The image reconstruction was performed with 3D‐OP‐OSEM (van Velden et al., 2008; 2 subsets, 32 iterations), with an isotropic voxel of 1.25 mm into a volume consisting of 153 transverse slices of 256 × 256 pixels, and a frame scheme of 10 × 60 s; 11 × 300 s. The images were corrected for attenuation (Rota Kops, Herzog, & Shah, 2014), random and scattered coincidences, and dead time. Postprocessing was performed first by filtering with a 2.5 mm 3D Gaussian filter and then by performing motion correction with frame‐by‐frame realignment. Herby, the reference image was obtained, on average, at 5 min postinjection (Scheins et al., 2019 2019).
PMOD software version 3.9 was used to define the volumes of interest (VOIs) with T1 MPRAGE images serving as the anatomical reference. All images were normalized to the MNI space, and the Hammers atlas (Hammers et al., 2003) was used for activity concentration analysis. The maximum probability operation was applied to generate the VOIs related to the gray matter cortex (GM) in all brain temporofrontal regions except in basal ganglia regions. The cerebellum GM has been chosen as the most appropriate reference region for human brain studies on mGluR5 (Akkus et al., 2017; Akkus et al., 2018; Smart et al., 2018). In all cases, the nondisplaceable binding potential (BPND) of [11C]ABP688 was estimated as:
where C region is the specifically bound radioligand concentration and C ref is the nondisplaceable ligand concentration in the cerebellar GM reference tissue. BPND values were calculated in an average of 5 min frame after 30 min p.i during the equilibrium plateau.
2.5. Statistical analysis
Statistical analyses were performed using the Statistical Package 25.0 (IBM SPSS Inc., Chicago, IL). We compared the neurocognitive performance between the groups using Student's t‐tests. The relationship between the mGluR5 receptor binding (expressed as BPND values) and psychopathology, as well as neurocognitive performance (in case of neurocognitive performance separately for each group), was exploratory examined by calculating the Spearman's rank correlation coefficient between scores reached in the neurocognitive tests and BPND in seven observed brain regions. We calculated r‐squared values (r 2) to evaluate the goodness‐of‐fits and to determine what fraction of the variability of the independent variable is explained by particularly independent variables. In all tests, values of p < .05 were considered statistically significant. All tests were two‐tailed. Missing values were not replaced. For the analysis of the group effects, BPND values were applied in general linear models, including all analyzed brain regions as a repeated variable, groups and smoking status as factors (between‐subject effects) and age as a covariate. Corrections for multiple comparisons using the Bonferroni method were applied and updated p values were reported.
3. RESULTS
3.1. General psychopathology
The general sociodemographic properties are given in Table 1. The psychopathological properties of the patient sample, as well as separately between subgroups with different smoking statuses, are given in Table 2. The scores were compared between the subgroups using the nonparametric Wilcoxon test. The “smokers” subgroup had higher scores in all tests rating the general psychopathology (PANSS, SANS, and HAMD).
Table 1.
Baseline demographics and clinical characteristics of patients and healthy volunteers
| Patients (N = 15) | Healthy controls (N = 15) | |
|---|---|---|
| Age | 40.33 ± 11.07 | 41.67 ± 11.15 |
| Ethnic background | ||
| Caucasian | 12 (80%) | 15 (100%) |
| African | 1 (6.7%) | 0 (0%) |
| Arabian | 2 (13.3%) | 0 (0%) |
| Years of education | 11.00 ± 1.46 | 11.73 ± 1.49 |
| Estimated IQ (WST) | 103.53 ± 12.81 | 111.93 ± 25.64 |
| BMI | 28.75 ± 4.13 | 24.03 ± 7.44 |
| Smoking status | ||
| Yes | 9 (60%) | 8 (53.3%) |
| No | 6 (40%) | 7 (46.7%) |
| Mean illness duration (years) | 19.00 ± 11.02 | – |
| Diagnosis (ICD‐10) | ||
| F20.0 | 11 (73.3%) | – |
| F20.3 | 1 (6.7%) | – |
| F20.6 | 2 (13.3%) | – |
| F20.9 | 1 (6.7%) | – |
Note: Data are given as mean ± SD, or n/N (%).
Abbreviations: BMI, body‐mass index; F20.0, Paranoid Schizophrenia; F20.3, Undifferentiated Schizophrenia; F20.6, Simple Schizophrenia; F20.9, Schizophrenia, Unspecified; WST, Wortschatztest (vocabulary test; Klaus‐Helmut et al.1992).
Table 2.
Baseline clinical assessment scores in the patient group
| Patients (N = 15) | Smokers (N = 9) | Nonsmokers (N = 7) | Smokers versus nonsmokers (p values) | |
|---|---|---|---|---|
| Basic assessment scores | ||||
| PANSS | 68.20 ± 9.38 | 72.0 ± 10.30 | 62.50 ± 3.30 | .057 |
| PANSS positive | 15.60 ± 2.35 | 16.80 ± 2.70 | 14.83 ± 1.60 | .388 |
| PANSS negative | 17.07 ± 4.43 | 19.00 ± 4.90 | 14.20 ± 0.40 | .026 |
| PANSS general score | 35.53 ± 4.24 | 36.90 ± 4.90 | 33.50 ± 1.90 | .181 |
| SANS: Total score | 17.60 ± 19.43 | 26.70 ± 20.30 | 4.00 ± 5.10 | .008* |
| SANS: Affective flattening | 2.73 ± 4.83 | 4.40 ± 5.70 | 0.20 ± 0.40 | .145 |
| SANS: Alogia | 1.13 ± 1.92 | 1.90 ± 2.20 | 0.00 ± 0.00 | .088 |
| SANS: Avolition | 3.53 ± 3.58 | 5.30 ± 3.60 | 0.80 ± 0.80 | .088 |
| SANS: Anhedonia | 6.93 ± 6.68 | 10.00 ± 6.50 | 2.30 ± 3.80 | .026 |
| SANS: Attention impairment | 3.37 ± 4.37 | 5.00 ± 4.90 | 0.70 ± 1.20 | .026 |
| HAMD | 5.80 ± 6.61 | 9.10 ± 6.70 | 0.80 ± 1.20 | <.001* |
| GAF | 64.67 ± 17.12 | 54.30 ± 11.90 | 80.20 ± 10.50 | .002* |
| GF: Social | 7.43 ± 1.60 | 6.40 ± 0.90 | 8.80 ± 1.20 | .003* |
| GF: Role | 5.79 ± 2.89 | 3.80 ± 1.90 | 8.50 ± 0.80 | .001* |
Notes: Scores were further depicted for smokers and nonsmokers separately and compared between the subgroups using the nonparametric Wilcoxon test. *Significant results after applying Bonferroni correction (p < .0083). Values are given as mean ± SD.
Abbreviations: GAF, Global Functioning Scale; GF: Role, Global Functioning: Role; GF: Social, Global Functioning: Social; HAMD, Hamilton Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; SHAPS, Snaith–Hamilton Pleasure Scale.
In particular, the comparison between the subgroups regarding the SANS total score (p = .008) and the HAMD score (p < .001) remained significant after Bonferroni correction (corrected p‐value for six different psychopathological tests: p < .0083). Both test procedures for the assessment of global functioning (GAF and GF) revealed significantly higher values in nonsmoker than smoker subjects in terms of higher functioning levels (GAF: p = .002, GF social: p = .003, and GF Role: p = .001).
3.2. BPND analysis
No significant differences in BPND were observed between healthy controls and schizophrenia patients, when considering all of the observed brain regions (F [27,1] = 0.285; p = .598; effect ηp 2 = 0.010). Age as a covariate was not significant in the BPND general linear model analysis (p > .05). The BPND did not correlate with the medication intake (chlorpromazine equivalents) and illness duration in any region. However, we observed, as others (Akkus et al., 2013; Akkus et al., 2016; Akkus et al., 2017), a significant difference between smokers and nonsmokers F (27,1) = 15.500; p = .001; effect ηp 2 = 0.365, and a reduction of up to 50% in BPND values in smokers, according to the observed brain region (Figure 1a,b). Figure 2 shows the BPND comparison between groups, but this was not found to be significant. The BPND values between the groups and according to smoking status are presented in Table 3.
Figure 1.
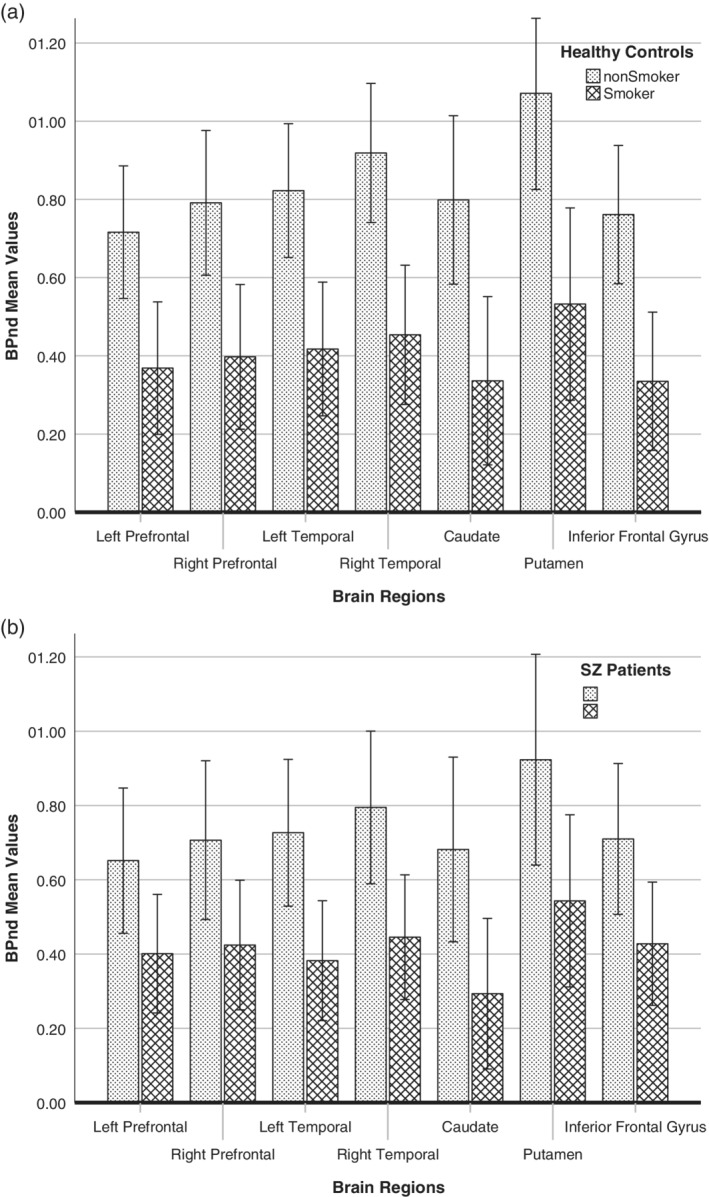
(a) Mean BPND values in seven regions of the brain in healthy controls according to smoking status, and (b) Mean BPND values in seven regions of the brain in schizophrenia patients according to smoking status (error bars are ±2 SEM)
Figure 2.
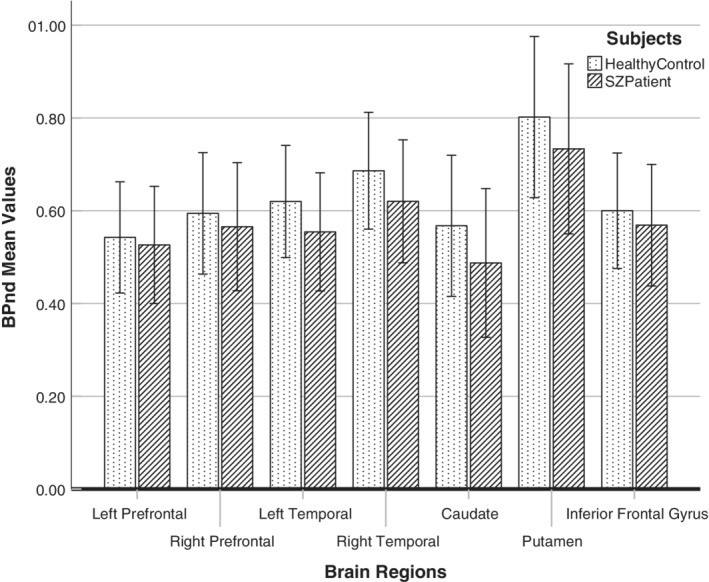
Comparison between mean BPND values in seven regions of the brain for healthy controls and patients with schizophrenia (error bars are ±2 SEM)
Table 3.
BPND values, comparison between smokers and nonsmokers subgroups and comparison between smoker/nonsmoker considering both groups (evaluated by repeated measures general linear models including all regions in the analysis with groups and smoking status as factors)
| Patients | Healthy controls | |||||
|---|---|---|---|---|---|---|
| Smokers (N = 9) | Nonsmokers (N = 6) | Smokers/nonsmokers (N = 15) | Smokers (N = 8) | Nonsmokers (N = 7) | Smokers/nonsmokers (N = 15) | |
| Prefrontal left | 0.40 ± 0.08 | 0.65 ± 0.09 | 0.62 ± 0.09 | 0.37 ± 0.06 | 0.72 ± 0.11 | 0.51 ± 0.13 |
| Prefrontal right | 0.42 ± 0.08 | 0.71 ± 0.09 | 0.59 ± 0.09 | 0.40 ± 0.06 | 0.79 ± 0.12 | 0.51 ± 0.13 |
| Temporal left | 0.38 ± 0.07 | 0.73 ± 0.08 | 0.52 ± 0.08 | 0.42 ± 0.07 | 0.82 ± 0.12 | 0.51 ± 0.14 |
| Temporal right | 0.44 ± 0.07 | 0.79 ± 0.08 | 0.56 ± 0.08 | 0.45 ± 0.07 | 0.92 ± 0.12 | 0.49 ± 0.14 |
| Caudate | 0.29 ± 0.11 | 0.68 ± 0.07 | 0.43 ± 0.11 | 0.34 ± 0.08 | 0.80 ± 0.13 | 0.42 ± 0.15 |
| Putamen | 0.54 ± 0.11 | 0.92 ± 0.09 | 0.58 ± 0.12 | 0.53 ± 0.09 | 1.07 ± 0.17 | 0.50 ± 0.12 |
| Inferior frontal gyrus | 0.43 ± 0.08 | 0.71 ± 0.09 | 0.60 ± 0.09 | 0.42 ± 0.06 | 0.78 ± 0.12 | 0.54 ± 0.13 |
Note: Nondisplaceable binding potential (BPND) values are represented by mean ± SEM.
3.3. Relationship between general psychopathology and mGluR5 receptor binding
The relationship between general psychopathology and mGluR5 receptor binding was examined by calculating a Spearman's rank correlation coefficient between scores reached in the tests for psychopathological assessment and BPND in the seven observed brain regions (prefrontal cortex right and left; temporal cortex right and left; caudate; putamen and inferior frontal gyrus). These regions were selected based on our previous literature review of schizophrenia research (Corti et al., 2011; Fatemi, Folsom, Rooney, & Thuras, 2013; Gupta et al., 2005; Ohnuma, Augood, Arai, McKenna, & Emson, 1998; Volk et al., 2010) relating to frequently involved regions and were also based on studies which used [11C]ABP688 to show higher uptake (mGluR5 receptor density) in the selected regions (Akkus et al., 2017; Ametamey et al., 2007). The correlations are given in Table 4.
Table 4.
Relationship between the general psychopathology and mGluR5 receptor binding in SZ patients examined by calculating Spearman's rank correlation coefficient between scores reached in the tests for psychopathological assessment and BPND values in the seven observed brain regions
| Left prefrontal gyrus | Right prefrontal gyrus | Left temporal | Right temporal | Caudate | Putamen | Inferior frontal gyrus | ||
|---|---|---|---|---|---|---|---|---|
| Subjects | N | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| PANSS positive | r | −.488 | −.494 | −.431 | −.469 | −.057 | −.492 | −.522 |
| p | .065 | .061 | .109 | .078 | .025 | .062 | .046 | |
| PANSS negative | r | −.473 | −.535 | −.714* | −.610 | −.679* | −.539 | −.639 |
| p | .075 | .04 | .003* | .016 | .005* | .038 | .010 | |
| PANSS general | r | −.638 | −.679* | −.665* | −.596 | −.803* | −.612 | −.665* |
| p | .01 | .005* | .007* | .019 | .000* | .015 | .007* | |
| PANSS total | r | −.632 | −.668* | −.734* | −.669* | −.793* | −.621 | −.727* |
| p | .011 | .007* | .002* | .006* | .000* | .013 | .002* | |
| SANS: Affective flattening | r | −.612 | −.608 | −.669* | −.563 | −.631 | −.500 | −.660* |
| p | .015 | .016 | .006* | .029 | .012 | .058 | .007* | |
| SANS: Alogia | r | −.486 | −.541 | −.690* | −.561 | −.708* | −.475 | −.618 |
| p | .066 | .037 | .004* | .029 | .003* | .074 | .014 | |
| SANS: Avolition‐apathy | r | −.644 | −.687* | −.792* | −.714* | −.762* | −.687* | −.773* |
| p | .01 | .005* | .000* | .003* | .001* | .005* | .001* | |
| SANS: Anhedonia | r | −.208 | −.293 | −.482 | −.315 | −.533 | −.177 | −.278 |
| p | .457 | .289 | .069 | .252 | .041 | .529 | .315 | |
| SANS: Attentional impairment | r | −.343 | −.426 | −.549 | −.384 | −.600 | −.343 | −.413 |
| p | .210 | .114 | .034 | .158 | .018 | .210 | .126 | |
| SANS: Global score | r | −.411 | −.503 | −.620 | −.463 | −.715* | −.401 | −.498 |
| p | .128 | .056 | .014 | .082 | .003* | .138 | .059 | |
| HAMD | r | −.369 | −.428 | −.590 | −.455 | −.663* | −.430 | −.456 |
| p | .175 | .111 | .020 | .089 | .007* | .110 | .088 | |
| GAF | r | .547 | .606 | .707* | .596 | .799* | .553 | .606 |
| p | .035 | .017 | .003* | .019 | .000* | .033 | .017 | |
| GF:SOCIAL | r | .493 | .582 | .691* | .641 | .705* | .497 | .525 |
| p | .073 | .029 | .006* | .013 | .005* | .070 | .054 | |
| GAF:GLOBAL | r | .478 | .561 | .719* | .660 | .711* | .510 | .568 |
| p | .084 | .037 | .004* | .01 | .004* | .062 | .034 |
Note: *Significant correlations after applying Bonferroni correction (p < .0083).
Abbreviations: GAF, Global Functioning Scale; GF: Role, Global Functioning; GF: Social, Global Functioning: Social; HAMD, Hamilton Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms.
The PANSS total score correlated significantly with BPND in almost all the observed regions (except the left prefrontal gyrus and putamen, with respect to the Bonferroni corrected significance level 0.0083). Figure 3a shows the caudate as an example. The PANSS general score correlated significantly with BPND in the right prefrontal gyrus, left temporal, caudate and the inferior frontal gyrus (r values between −0.665 and −0.803; p ≤ .0083). The PANSS negative score correlated significantly with BPND in the left temporal and caudate (r values −.714 and −.679, respectively; p ≤ .0083 both). The PANSS positive score did not correlate significantly with BPND. The SANS global score only correlated significantly with BPND in the caudate (Figure 3b).
Figure 3.
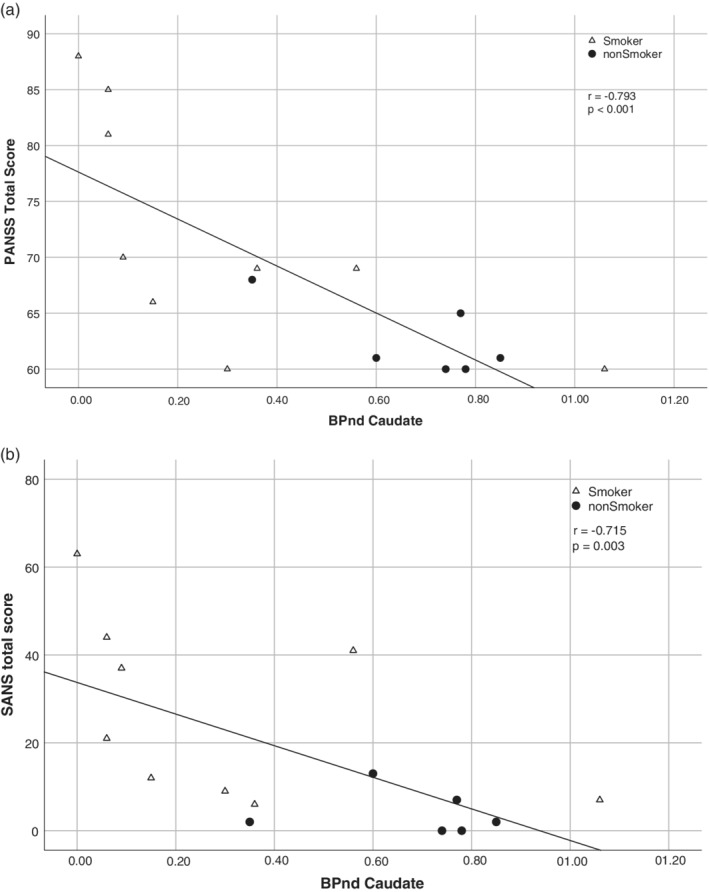
Association between the (a) PANSS total score and mGluR5 receptor binding, and the (b) SANS total score and mGluR5 receptor binding in the caudate in schizophrenia patients
With respect to the single subscales, the SANS‐affective flattening subscale correlated with BPND in the left temporal and inferior frontal gyrus (r values: −.669 and −.660, respectively). Furthermore, the SANS‐Alogia subscale correlated significantly with BPND in the left temporal and caudate regions (r values −.690 and −.708, respectively). The SANS Avolition/apathy subscale correlated significantly with BPND in almost of all regions (r values between −.687 and −.792), except in the left prefrontal gyrus. The SANS‐Anhedonia subscale did not correlate with the analyzed regions, as was also noticed for the SANS attentional impairment subscale (p > .0083 for all). The HAMD score correlated significantly with BPND in the caudate (r = −.660). The GAF correlated significantly with BPND in the left temporal (r = .710) and caudate (r = −.799; Figure 4a). The GF‐social functioning score showed also significant correlations with BPND values in the left temporal and caudate (r values: .690 and .705, respectively). Finally, a similar high correlation can be found with the GF‐global functioning score in the left temporal (Figure 4b) and caudate (r values: .719 and .711, respectively).
Figure 4.
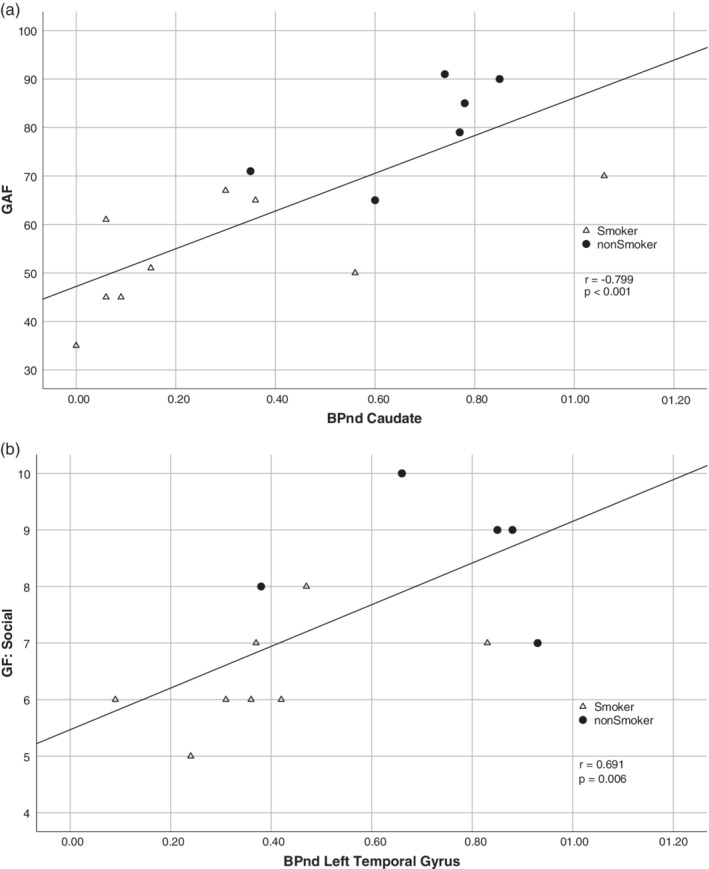
Association between (a) Global Functioning Scale (GAF) and mGluR5 receptor binding in the caudate, and (b) Global Functioning: Social Scale (GF: Social) and mGluR5 receptor binding in the left temporal cortex in schizophrenia patients
3.4. Neurocognitive performance
All results relating to the neurocognitive tests are shown in Table 5. After applying the Bonferroni correction, all correlations with a p value ≤.0083 were considered significant.
Table 5.
Results of the neurocognitive tests performed by schizophrenia patients and healthy controls and comparison between the groups (Student's t‐test)
| Patients | Healthy controls | Patients versus healthy controls (t‐test) p values | |
|---|---|---|---|
| TMT(A) (s) | 39.35 ± 15.74 | 28.80 ± 9.61 | .039 |
| TMT(B) (s) | 97.06 ± 45.42 | 61.75 ± 25.81 | .022 |
| TMT(B)–TMT(A) | 59.19 ± 35.43 | 32.37 ± 21.07 | .031 |
| TMT(B)/TMT(A) | 2.62 ± 0.94 | 2.16 ± 0.67 | .172 |
| CT (sum score) | 59.67 ± 17.34 | 68.50 ± 14.75 | .150 |
| LNS (sum score) | 18.60 ± 2.23 | 20.93 ± 1.77 | .005* |
| LNS (longest sequence) | 4.80 ± 0.94 | 5.64 ± 0.75 | .013 |
| CBT—forward | 7.93 ± 1.28 | 9.00 ± 2.04 | .100 |
| CBT—backward | 8.13 ± 3.89 | 8.50 ± 1.61 | .750 |
| RVDLT valid recognitions | 59.53 ± 20.33 | 81.21 ± 13.63 | .002* |
| RVDLT—errors | 10.00 ± 6.19 | 7.14 ± 6.73 | .240 |
| z‐composite score | −0.34 ± 0.70 | 0.36 ± 0.45 | .003* |
Notes: Values are given as mean ± SD. *Significant results after Bonferroni correction (p < .0083).
Abbreviations: CBT, Corsi block‐tapping task; CT, Number‐symbol coding task; LNS, Letter–Number Span; RVDLT, Rey Visual Design Learning Test; TMT(A), Trail‐Making Test (A); TMT(B), Trail‐Making Test (B).
3.5. Performance speed, mental flexibility, and executive functions
Schizophrenia patients (SZ) in both TMT subtests required a longer time to perform the tasks than healthy controls (HC). However, the results were not significant after Bonferroni correction (p > .0083 for all). In the coding task, there were no significant differences between SZ and HC (p = .15).
3.6. Working memory
In the letter‐number span (LNS), the SZ patients reached a significantly lower sum score than the healthy controls (p = .005), but not a significant maximal sequence length (p = .013). The performance of the patients in the Corsi block‐tapping task (forward and backward) was not significantly different from the HC (p = .10 and p = .75, respectively).
3.7. Visual memory
In the RVDLT, patients performed worse than the healthy controls, showing a significantly lower number of valid recognitions (p = .002), but a nonsignificant number of errors (p = .240).
3.8. Composite z‐score
Using the procedure described in the methods section, we calculated composite z‐scores for all cognitive tasks and found them to be significantly lower in patients (p = .003).
3.9. Role of smoking for neurocognitive performance
The results of the neurocognitive tests are depicted separately for smoking status in Table 6. The comparison between the subgroups was performed using the nonparametric Wilcoxon test. In the patient group, smokers and nonsmoker subjects differed significantly only in the coding task (p = .003). No significant differences were found for any of the tasks in the healthy controls group (p > .0083).
Table 6.
Results of the neurocognitive tests performed by schizophrenia patients and healthy controls, separately depicted for smokers and nonsmokers
| Patients | Healthy controls | |||||
|---|---|---|---|---|---|---|
| Smoker (N = 9) | Nonsmoker (N = 6) | Smoker versus nonsmoker (Wilcoxon‐test; p‐value) | Smoker (N = 8) | Nonsmoker (N = 7) | Smoker versus nonsmoker (Wilcoxon‐test; p‐value) | |
| TMT(A) (s) | 47.04 ± 14.9 | 27.80 ± 8.44 | .026 | 34.56 ± 9.13 | 30.07 ± 6.33 | .038 |
| TMT(B) (s) | 121.00 ± 42.95 | 65.15 ± 25.35 | .029 | 78.60 ± 23.46 | 49.70 ± 21.17 | .073 |
| TMT(B)–TMT(A) | 75.59 ± 35.75 | 37.33 ± 21.68 | .059 | 40.40 ± 22.61 | 26.64 ± 19.51 | .343 |
| TMT(B)/TMT(A) | 2.76 ± 0.98 | 2.43 ± 0.95 | .491 | 2.09 ± 0.53 | 2.20 ± 0.79 | .900 |
| CT (sum score) | 50.33 ± 13.03 | 73.67 ± 13.35 | .003* | 63.57 ± 9.98 | 73.43 ± 17.74 | .165 |
| LNS (sum score) | 18.00 ± 2.00 | 19.50 ± 2.43 | .272 | 20.57 ± 1.98 | 21.29 ± 1.60 | .456 |
| LNS (longest sequence) | 4.56 ± 0.89 | 5.17 ± 0.98 | .272 | 5.43 ± 0.79 | 5.86 ± 0.69 | .259 |
| CBT—forwards | 7.56 ± 1.01 | 8.50 ± 1.52 | .181 | 9.14 ± 2.27 | 8.86 ± 1.95 | .902 |
| CBT—backwards | 8.00 ± 4.87 | 8.30 ± 2.1 | .388 | 8.86 ± 0.90 | 8.14 ± 2.11 | .259 |
| RVDLT valid recognitions | 55.67 ± 20.89 | 65.33 ± 19.78 | .388 | 79.57 ± 11.99 | 82.86 ± 15.89 | .710 |
| RVDLT—errors | 10.11 ± 7.01 | 9.83 ± 5.35 | .955 | 7.43 ± 6.19 | 6.86 ± 7.73 | .710 |
| z‐composite score | −0.64 ± 0.64 | 0.10 ± 0.56 | .036 | 0.22 ± 0.38 | 0.51 ± 0.49 | .165 |
Notes: In addition, a comparison between smokers and nonsmokers (Wilcoxon test). Values are given as mean ± SD, and *significant result after Bonferroni correction (p < .0083).
Abbreviations: CBT, Corsi block‐tapping task; CT, Number‐symbol coding task; LNS, Letter–Number Span (LNS); RVDLT, Rey Visual Design Learning Test; TMT(A), Trail‐Making Test (A); TMT(B), Trail‐Making Test (B).
3.10. Relationship between neurocognitive performance and mGluR5 BPND values
The relationship between the neurocognitive performance and mGluR5 binding was examined separately for each group by calculating a Spearman's rank correlation coefficient between the scores reached in the neurocognitive tests and BPND values in all observed brain regions. All correlations for the patient group are shown in Table 7.
Table 7.
Relationship between the neurocognitive performance and mGluR5 receptor binding exploratory examined by calculating Spearman's rank correlation coefficient between scores reached in the neurocognitive tests and BPND for the seven observed brain regions in the patient group
| Left prefrontal gyrus | Right prefrontal Gyrus | Left temporal | Right temporal | Caudate | Putamen | Inferior frontal gyrus | ||
|---|---|---|---|---|---|---|---|---|
| Subjects | N | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| TMT(A) (s) | r | −.408 | −.445 | −.544 | −.423 | −.571 | −.449 | −.486 |
| p | .131 | .096 | .036 | .117 | .026 | .093 | .066 | |
| CT | r | .531 | .550 | .750* | .615 | .665* | .517 | .576 |
| p | .042 | .033 | .001* | .015 | .007* | .049 | .025 | |
| LNS (sum score) | r | −.051 | −.114 | .082 | .028 | −.022 | −.069 | −.035 |
| p | .857 | .685 | .772 | .921 | .939 | .807 | .903 | |
| LNS (longest sequence) | r | −.090 | −.145 | .016 | −.081 | .039 | −.125 | −.065 |
| p | .749 | .607 | .955 | .773 | .891 | .658 | .817 | |
| CBT—forward | r | .164 | .190 | .311 | .216 | .222 | .170 | .221 |
| p | .559 | .497 | .259 | .439 | .426 | .545 | .429 | |
| CBT—backward | r | .283 | .322 | .245 | .191 | .524 | .255 | .330 |
| p | .307 | .242 | .378 | .495 | .045 | .359 | .230 | |
| RVDLT valid recognitions | r | .193 | .209 | .164 | .079 | .363 | .241 | .220 |
| p | .490 | .454 | .560 | .780 | .183 | .386 | .430 | |
| RVDLT—errors | r | −.048 | −.074 | .026 | .084 | −.204 | −.097 | −.015 |
| p | .866 | .794 | .927 | .765 | .466 | .731 | .957 | |
| Z‐composite score | r | .288 | .307 | .433 | .300 | .500 | .321 | .322 |
| p | .298 | .265 | .107 | .277 | .057 | .243 | .242 | |
| TMT(B) (s) | r | −.203 | −.279 | −.392 | −.398 | −.341 | −.385 | .304 |
| p | .487 | .334 | .166 | .158 | .233 | .175 | .291 | |
| TMT(B)–TMT(A) | r | −.161 | −.231 | −.315 | −.343 | −.271 | −.358 | −.264 |
| p | .583 | .427 | .273 | .230 | .349 | .208 | .361 | |
| TMT(B)/TMT(A) | r | .242 | .169 | .103 | .075 | .084 | .055 | .123 |
| p | .404 | .563 | .725 | .799 | .776 | .852 | .674 |
Note: *Significant correlations after Bonferroni correction (p < .0083).
Abbreviations: CBT, Corsi block‐tapping task; CT, Number‐symbol coding task; LNS, Letter–Number Span (LNS); RVDLT, Rey Visual Design Learning Test; TMT(A), Trail‐Making Test (A); TMT(B), Trail‐Making Test (B).
After applying Bonferroni correction, only correlations between the coding task performance and the caudate (Figure 5a), and the left temporal (Figure 5b) remained significant (r values: .665 and .750, respective; p < .0083 for both). No significant correlations were found in the HC group.
Figure 5.
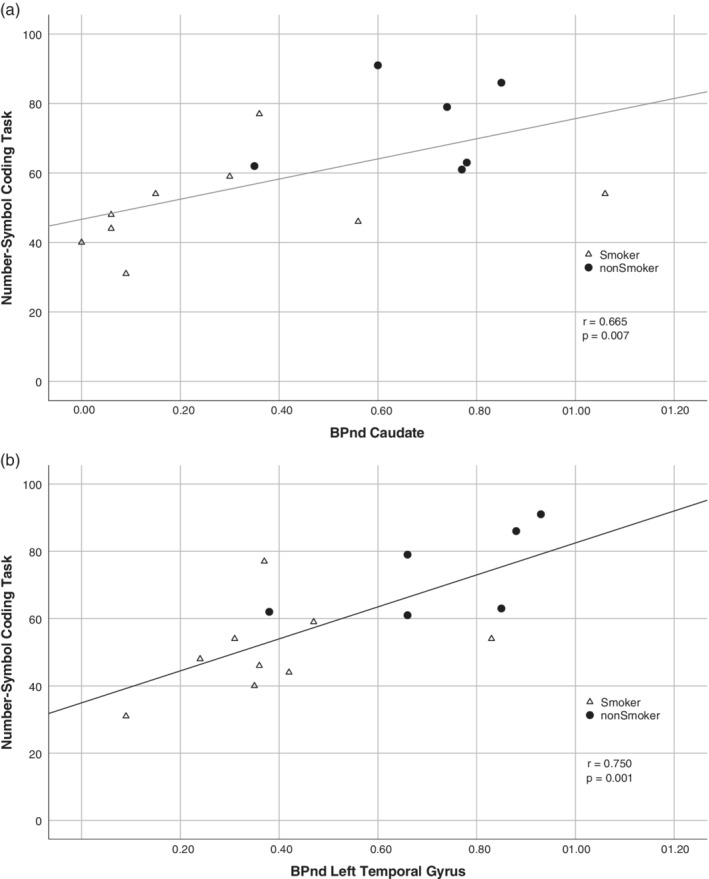
Association between the neurocognitive performance in (a) Number‐Symbol Coding Task and mGluR5 receptor binding in caudate, and (b) Number‐Symbol Coding Task and mGluR5 receptor binding in the left temporal cortex in schizophrenia patients
4. DISCUSSION
This exploratory PET study aimed to contribute to a better understanding of the role of glutamatergic neurotransmission in psychopathology and cognitive impairments in schizophrenia. Such associations have been indicated in previous animal studies, but up to now, they have not been sufficiently proven in patients suffering from schizophrenia. Thus, we tested for correlations between the mGluR5 receptor BPND and psychopathology and cognitive performance in selected striatal and frontotemporal cortex regions using [11C]ABP688 in two groups: One comprising 15 male schizophrenia patients (which all were chronically ill and on stable medication with second‐generation antipsychotics) and one comprising 15 demographically matched healthy volunteers.
Our study revealed the following four main findings.
First, higher mGluR5 BPND values were associated with lower general symptom levels (in particular negative and depressive symptoms as measured with the PANSS, SANS and HAMD scores), as well as with higher levels of global functioning scores (GAF, GF).
Second, patients with higher mGluR5 BPND values showed better cognitive performance. Such an association was not found in healthy controls.
Third, no significant differences were found in mGluR5 BPND between healthy subjects and patients with schizophrenia. The numerically higher values in healthy controls were not statistically significant. Smoking status showed a strong effect in both groups, suggesting a close association between tobacco/nicotine use and the glutamatergic system that is particularly pronounced in schizophrenia.
Finally, in the patient group, being an active smoker was a common denominator for individuals with more pronounced negative symptoms, worse global functioning, poorer cognitive performance as well as lower mGluR5 BPND. This finding raises the question as to whether glutamatergic dysfunction, particularly associated with a downregulation of mGluR5, may be a link between disease severity and comorbid nicotine addiction in schizophrenia.
Our results are in line with the current conception of the glutamate hypothesis of schizophrenia, which emphasizes the main role of the aberrant glutamate receptors in this disease (Hammond et al., 2014). Up to now, the most consistent findings have been reported for NMDA‐R, suggesting that NMDA‐R dysfunction represents a final common pathway leading from pathogenesis to symptoms in schizophrenia (Javitt, 2010). Comparatively, relatively little is known about the involvement of mGluR5 receptors in the pathophysiology of schizophrenia. A considerable number of studies have emphasized the apparently significant role of mGluR5 signaling in schizophrenia and its interaction with abnormal NMDA‐R activity in this disease (Wang et al., 2018). Furthermore, the importance of mGluR5 for schizophrenia has been confirmed in genetic (Kordi‐Tamandani, Dahmardeh, & Torkamanzehi, 2013; The Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium, 2015) and animal studies (as already outlined in the introduction). Additionally, Wang et al. (2018) recently provided the first direct evidence for mGluR5 hypoactivity in dorsolateral prefrontal cortex tissue taken post‐mortem from schizophrenia patients, although several previous investigations could not find altered mGluR5 levels postmortem in schizophrenia (Matosin, Frank, Deng, Huang, & Newell, 2013) or psychotic depression (Matosin et al., 2014). Furthermore, the concept of mGluR5 dysregulation in schizophrenia is supported by several other investigations, including various disturbed mechanisms underlying transcription and translation of mGluR5, as well as alterations to mGluR5 trafficking, distributions, phosphorylation, and protein–protein interactions (Matosin et al., 2017). If it is accepted that mGluR5 hypofunction is an underlying factor in the symptomatology of schizophrenia, higher mGluR5 receptor availability may mitigate the symptoms. Our analysis supports this concept as it reveals lower general symptom levels (in particular, negative and depressive symptoms as measured with PANSS, SANS, and HAMD scores) and higher levels of global functioning associated with higher mGluR5 BPND.
In our study, higher mGluR5 receptor binding was beneficial in terms of lower SANS and HAMD scores. In line with this finding, Thiebes et al. (2017) reported a significant increase in negative symptoms after antagonizing NMDA‐R with ketamine in healthy volunteers. This was also tightly associated with deficits in mismatch negativity (MMN), which is known to be robustly reduced in schizophrenia (Salisbury, Shenton, Griggs, Bonner‐Jackson, & McCarley, 2002). Thus, further investigation of ways to increase mGluR5 associated glutamatergic functionality (and consecutively the NMDA‐receptor activity) may be a promising approach for the treatment of negative symptoms in schizophrenia.
Our second major finding suggests the involvement of mGluR5 receptors in cognition in schizophrenia patients. Generally, numerous previous investigations have indicated that glutamatergic synapses in the brain, responsible for fast excitatory neurotransmission, play a critical role in a broad range of cognitive functions. Thereby, the majority of studies support the key role of NMDA‐R in cognition (Dauvermann, Lee, & Dawson, 2017). Nevertheless, pharmacological studies using NMDA‐R modulators, such as glycine, d‐serine, or d‐cycloserine, in order to improve cognitive performance in humans, have been less persuasive up to now (Iwata et al., 2015). Thus, during recent years, the focus of interest for some drug development studies has moved to the mGluR5 as a target of positive allosteric modulators (PAM), as referred to in the introduction. In terms of this connection, Matosin et al. (2013) reported unaltered allosteric binding site and mGluR5 protein levels in schizophrenia pathology, suggesting that the binding potential of mGluR5 PAMs will not be affected in patients with schizophrenia, qualifying them as suitable therapeutic agents. Our finding of an association between cognition and the mGluR5 supports this approach and encourages the idea of further development of such agents in order to target cognitive impairments in schizophrenia.
Interestingly, the association between higher mGluR5 receptor binding and better cognitive performance primarily concerned the coding task performance and the mGluR5 receptor binding in the left temporal cortex and caudate (bilateral). Generally, a slower speed of information processing is considered to be one of the central features of cognitive impairment in schizophrenia (Rodriguez‐Sanchez et al., 2007). Several investigations support the contention that global cognitive dysfunction in schizophrenia may be determined, to some extent, by impaired processing speed (Andersen et al., 2013). Indeed, impairments in coding tasks are often significantly more pronounced than impairments in measures from memory, attention, reasoning, and problem solving, and working memory domains in schizophrenia (Andersen et al., 2013). Coding task performance deficits have usually been associated with altered function in the parietal, temporal, and frontal regions (Turken et al., 2008). In particular, a strong correlation has been reported between impaired cognition and volume decrement or structural abnormalities in some temporal lobe subregions in schizophrenia (Roalf et al., 2017). Furthermore, it has also been shown that the speed of processing is associated with frontotemporal white matter abnormalities (Rigucci et al., 2013). In our study, higher mGluR5 receptor binding in the left (but not in the right) temporal region was beneficial for speed of information processing. This asymmetry is in accordance with the long‐standing notion that schizophrenia is characterized by a disruption of the normal pattern of emerging cerebral asymmetry, which primarily affects development in the left hemisphere and leads to more prominent left hemisphere dysfunction (Crow et al., 1989). Thus, our findings emphasize the importance of glutamatergic neurotransmission in the left temporal region for information processing in schizophrenia.
Furthermore, the speed of information processing was associated with mGluR5 BPND in the caudate. These results complement previous studies describing the importance of striatal dopaminergic neurotransmission for cognition in schizophrenia. Reeves et al. (2005) reported a significant association between striatal D2‐dopamine receptor availability and spatial planning. In our own previous work, we were also able to demonstrate an association between better executive functional and higher striatal D2‐dopamine receptor availability in schizophrenia patients but not in healthy volunteers (Veselinovic et al., 2013). With the current results, we provide additional confirmation of the importance of striatal neurotransmission for cognition in schizophrenia patients, thereby showing that both the dopaminergic system and also the glutamatergic system seem to be involved in the underlying processes.
However, it is not possible to speculate about the actual mechanisms behind the potentially pro‐cognitive effects conveyed by the mGluR5 receptors based on our data. Although some previous investigators attributed such an effect to the interaction of the mGluR5 receptor with the NMDA‐R, the role of other independent mechanisms is also conceivable (Conn, Lindsley, & Jones, 2009). Several earlier investigations have indicated that mGluR5 form homomers and heteromers with other receptors at the plasma membrane, in a similar way to other G protein‐coupled receptors. Some evidence emphasizes an abnormal function of oligomers, consisting of mGluR5, dopamine D2 (D2R) and adenosine A2A receptors (A2AR‐D2R‐mGluR5; Cabello et al., 2009), as well as D2R‐NMDA hetero‐receptor complexes as major contributors to schizophrenia symptoms (Borroto‐Escuela et al., 2017). According to these findings, altered receptor–receptor interactions in iso‐receptors and hetero‐receptor complexes, as well as changes in their balance with each other, may be a basis for altered neuromodulation and dysfunction of the brain circuits, resulting in deficits in learning, memory, emotions, and motivation in depression, addiction, and schizophrenia (Borroto‐Escuela et al., 2017). Consequently, these hetero‐receptors may provide targets for a new therapeutic approach for some neuropsychiatric disorders (Fuxe et al., 2003; Popoli et al., 2001).
In our study, we were unable to demonstrate a significant difference regarding the mGluR5 availability between schizophrenia patients and healthy volunteers. We observed a trend of lower BPND in all analyzed regions in the patient group, although without a statistical significance. To the best of our knowledge, the only previous similar in vivo study using the same radioligand, also reported an absence of difference in mGluR5 DVR between patients and controls (Akkus et al., 2017). Furthermore, in this study, smoking status was associated with marked global reductions in mGluR5 availability in both groups. In our study, we confirm these findings. Acknowledging the very high prevalence of smoking (more than 60%) in subjects with schizophrenia (Chapman, Ragg, & McGeechan, 2009) and their greater difficulties in quitting smoking (Wing, Wass, Soh, & George, 2012), a more precise understanding of the common neurobiological basis of schizophrenia and nicotine dependence is of particular importance. Several investigations have emphasized the potential usage of tobacco by schizophrenia patients for self‐medication purposes in order to alleviate specific symptoms (Lucatch, Lowe, Clark, Kozak, & George, 2018), particularly negative symptoms and dysfunctional reward circuits (Peechatka, Whitton, Farmer, Pizzagalli, & Janes, 2015). However, investigations in this field have yielded mixed findings. Several authors have reported comparable or even worse cognition within chronic smokers with schizophrenia (Hickling et al., 2018). Moreover, studies that carefully controlled for the time since the last cigarette have found that smoking produces cognitive deficits in schizophrenia, particularly in working memory, visual learning, and attention (Reed, Harris, & Olincy, 2016). Thus, opposing to the earlier self‐medication hypothesis, those investigations indicate a bidirectional relationship between smoking and psychosis wherein cigarette smoking may be causally related to a risk of psychosis, possibly via a shared genetic liability (Quigley & MacCabe, 2019). In our study, smoking habit was a common denominator for more pronounced negative and depressive symptoms, poorer functioning level, lower cognitive performance, and lower mGluR5 receptor availability. This finding emphasizes a possible mediating role of glutamate, and in particular mGluR5, as a link between schizophrenia and smoking.
Several methodological limitations should be kept in mind when interpreting our results. One of the main limitations of the study may be the joint consideration of smokers and nonsmokers as one group during the investigation of the associations between BPND and psychopathology and cognition. However, the role of smoking for all observed parameters has been discussed extensively. Despite this possible drawback, this integrative approach highlights the potential common basis of symptom severity and nicotine addiction, which could be anchored in glutamatergic dysfunctions.
A second potential limitation of the study is that the relatively small sample size prohibits a broader generalization of our findings. This is a common problem in PET studies due to the high requirements for patients suitable for inclusion. A particular challenge for studies using [11C]ABP688 is the necessity to account for the smoking status as a specific influence factor and to ensure a perfect matching regarding this confounder. However, as the effect of smoking is so grave and has already been replicated across studies, an increase in sample size would probably not change our results significantly.
A third potential limitation of our study relates to the illness duration of the patients included in the sample (from 8 to 32 years), and thus different illness stages. However, one pre‐request for inclusion in the study was a pre‐existing clinical attribution as a chronic patient. In particular, first‐episode patients were not included. Furthermore, our analysis revealed an absence of significant correlations between the BPND and illness duration in all regions. In addition, in order to reduce the sample heterogeneity, only male subjects were included and patients with comorbid consumption of illegal drugs (incl. cannabis) were excluded. One more variability factor previously described for PET studies using [11C]ABP688—the daytime variability (DeLorenzo et al., 2017)—was eliminated by scheduling all PET data acquisitions at the same time of the day (late morning) for all subjects in this study. Thus, our study design ensured the elimination of several confounding factors (gender diversity, cannabis influence, study design based on mGluR5/glutamate system variations) that were not considered in some other previous studies investigating the glutamatergic system in schizophrenia.
Fourth, although all patients in our study were treated with second‐generation antipsychotics in common doses, we did not control for the type and duration of previous medication. Due to the lack of a central/lifetime medical register, this information was not available in a reliable form in the majority of the cases. Nevertheless, we could not find any association between the medication (e.g., CPZ equivalents) and mGluR5 BPND.
5. CONCLUSION
Our study revealed no significant differences (10% on average in all evaluated brain regions in nonsmokers subjects) between schizophrenia patients and healthy controls regarding mGluR5 receptor availability. However, low mGluR5 receptor availability was identified as a common factor associated with more pronounced negative and depressive symptoms, lower levels of global functioning and poorer cognitive performance in a sample of 15 male, chronically ill, stable schizophrenia patients. The findings were strongly associated with the smoking status of the subjects. The causal direction of this linkage remains unclear and requires further investigation.
In particular, future mGluR5 imaging studies should, ideally, include unmedicated, nonsmoking patients from the first onset of the disease. However, despite some possible limitations, our study provides a new perspective on the complex relationship between tobacco smoking and schizophrenia and points toward the possible role of glutamatergic neurotransmission—particularly the metabotropic glutamate receptors type 5—in this interrelation. Further investigation may be a promising approach for a better understanding of the common aspects of inclination toward tobacco smoking and psychosis.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
C.R.B.: PET bolus‐infusion protocol optimization, data acquisition, PET imaging and plasma data analysis, image processing (BPND), statistical design and analysis, manuscript writing (52%), correction, and revision. T.V.: Psychopathology scores and BPND correlation analysis, statistical analysis, manuscript writing (48%), correction and revision. R.R.: Data acquisition and revision of the manuscript. J.M.: PET bolus‐infusion study design, data acquisition, and revision of the manuscript. L.O.: Volunteer screening and data acquisition. A.R.: Volunteer screening and revision of the manuscript. S.R.: Data acquisition and revision of the manuscript. K.H.: Study design and revision of the manuscript. W.K.: Study design and revision of the manuscript. C.W.: Study design and revision of the manuscript. E.R.K.: MR‐PET attenuation correction, revision, and correction of the manuscript. L.T.: MR‐PET hardware and data acquisition. J.S.: PET reconstruction and head motion correction methodology. F.B.: MR‐PET hardware integration and study design. B.N.: Revision of the manuscript. J.E.: Revision and corrections of the manuscript. H.H.: PET study design discussions, revision, and corrections of the manuscript. K.‐J.L.: Revision of the manuscript. N.J.S.: MR‐PET hardware and revision of the manuscript. C.L.: Study design and setup, data analysis revision, manuscript corrections, and revision. I.N.: Study design and setup, approval ethics and BfS, and revision of the manuscript.
ACKNOWLEDGMENTS
The authors thank Magdalene Vögeling, Cornelia Frey, Silke Frensch, and Suzanne Schaden for their technical assistance in data acquisition, blood sampling, and metabolite correction. The authors would like to thank Markus Lang and Stefan Stüsgen for the [11C]ABP688 production and Dr Andreas Matusch for the implementation of the metabolite correction methods and support in the equilibrium analysis. Finally, the authors would like to thank Claire Rick for proofreading the manuscript. This work was in part supported by the EU‐FP7 funded project TRIMAGE (No. 602621). C.R.B. thanks DAAD for financial support (No. 57299294). These data are part of the Dr rer. Medic thesis at the Medical Faculty of the RWTH Aachen University, Germany of Cláudia Régio Brambilla, and Dr med. Thesis at the Medical Faculty of the RWTH Aachen University, Germany of Andrej Ruch.
Régio Brambilla C, Veselinović T, Rajkumar R, et al. mGluR5 receptor availability is associated with lower levels of negative symptoms and better cognition in male patients with chronic schizophrenia. Hum Brain Mapp. 2020;41:2762–2781. 10.1002/hbm.24976
Cláudia R. Brambilla and Tanja Veselinović contributed equally to this study.
Christoph Lerche and Irene Neuner shared equally senior authorship.
Funding information Deutscher Akademischer Austauschdienst, Grant/Award Number: 57299294; Seventh Framework Programme, Grant/Award Number: 602621
DATA AVAILABILITY STATEMENT
Raw data were generated at Forschungszentrum Jülich and RWTH Uniklinik Aachen. Derived data supporting the findings of this study are available from the corresponding and first authors C.R.B and T.V. on request.
REFERENCES
- Aas, I. H. (2010). Global assessment of functioning (GAF): Properties and frontier of current knowledge. Annals of General Psychiatry, 9, 20 10.1186/1744-859X-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aas, I. H. (2011). Guidelines for rating global assessment of functioning (GAF). Annals of General Psychiatry, 10, 2 10.1186/1744-859X-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi‐Saab, W. M. , D'Souza, D. C. , Moghaddam, B. , & Krystal, J. H. (1998). The NMDA antagonist model for schizophrenia: Promise and pitfalls. Pharmacopsychiatry, 31(Suppl 2), 104–109. 10.1055/s-2007-979354 [DOI] [PubMed] [Google Scholar]
- Akkus, F. , Ametamey, S. M. , Treyer, V. , Burger, C. , Johayem, A. , Umbricht, D. , … Hasler, G. (2013). Marked global reduction in mGluR5 receptor binding in smokers and ex‐smokers determined by [11C]ABP688 positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America, 110(2), 737–742. 10.1073/pnas.1210984110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus, F. , Mihov, Y. , Treyer, V. , Ametamey, S. M. , Johayem, A. , Senn, S. , … Hasler, G. (2018). Metabotropic glutamate receptor 5 binding in male patients with alcohol use disorder. Translational Psychiatry, 8(1), 17 10.1038/s41398-017-0066-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus, F. , Terbeck, S. , Ametamey, S. M. , Rufer, M. , Treyer, V. , Burger, C. , … Hasler, G. (2014). Metabotropic glutamate receptor 5 binding in patients with obsessive‐compulsive disorder. The International Journal of Neuropsychopharmacology, 17(12), 1915–1922. 10.1017/S1461145714000716 [DOI] [PubMed] [Google Scholar]
- Akkus, F. , Treyer, V. , Ametamey, S. M. , Johayem, A. , Buck, A. , & Hasler, G. (2017). Metabotropic glutamate receptor 5 neuroimaging in schizophrenia. Schizophrenia Research, 183, 95–101. 10.1016/j.schres.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Akkus, F. , Treyer, V. , Johayem, A. , Ametamey, S. M. , Mancilla, B. G. , Sovago, J. , … Hasler, G. (2016). Association of Long‐Term Nicotine Abstinence with Normal Metabotropic Glutamate Receptor‐5 binding. Biological Psychiatry, 79(6), 474–480. 10.1016/j.biopsych.2015.02.027 [DOI] [PubMed] [Google Scholar]
- American Psychological Association . (2003). Diagnostisches und Statistisches Manual psychischer Störungen—Textrevision (DSM‐IV‐TR). Göttingen: Hogrefe. [Google Scholar]
- Ametamey, S. M. , Treyer, V. , Streffer, J. , Wyss, M. T. , Schmidt, M. , Blagoev, M. , … Buck, A. (2007). Human PET studies of metabotropic glutamate receptor subtype 5 with 11C‐ABP688. Journal of Nuclear Medicine, 48(2), 247–252. [PubMed] [Google Scholar]
- Andersen, R. , Fagerlund, B. , Rasmussen, H. , Ebdrup, B. H. , Aggernaes, B. , Gade, A. , … Glenthoj, B. (2013). The influence of impaired processing speed on cognition in first‐episode antipsychotic‐naive schizophrenic patients. European Psychiatry, 28(6), 332–339. 10.1016/j.eurpsy.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Andreasen, N. C. (1982). Negative symptoms in schizophrenia. Definition and reliability. Archives of General Psychiatry, 39(7), 784–788. [DOI] [PubMed] [Google Scholar]
- Andreasen, N. C. , Pressler, M. , Nopoulos, P. , Miller, D. , & Ho, B. C. (2010). Antipsychotic dose equivalents and dose‐years: A standardized method for comparing exposure to different drugs. Biological Psychiatry, 67(3), 255–262. 10.1016/j.biopsych.2009.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auther, A. , Smith, C. , & Cornblatt, B. (2006). Global functioning: Social scale (GF: Social). Glen Oaks, NY: Zucker‐Hillside Hospital. [Google Scholar]
- Balschun, D. , Zuschratter, W. , & Wetzel, W. (2006). Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience, 142(3), 691–702. 10.1016/j.neuroscience.2006.06.043 [DOI] [PubMed] [Google Scholar]
- Berch, D. B. , Krikorian, R. , & Huha, E. M. (1998). The Corsi block‐tapping task: Methodological and theoretical considerations. Brain and Cognition, 38(3), 317–338. 10.1006/brcg.1998.1039 [DOI] [PubMed] [Google Scholar]
- Borroto‐Escuela, D. O. , Carlsson, J. , Ambrogini, P. , Narvaez, M. , Wydra, K. , Tarakanov, A. O. , … Fuxe, K. (2017). Understanding the role of GPCR Heteroreceptor complexes in modulating the brain networks in health and disease. Frontiers in Cellular Neuroscience, 11, 37 10.3389/fncel.2017.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, C. , Deschwanden, A. , Ametamey, S. , Johayem, A. , Mancosu, B. , Wyss, M. , … Buck, A. (2010). Evaluation of a bolus/infusion protocol for 11C‐ABP688, a PET tracer for mGluR5. Nuclear Medicine and Biology, 37(7), 845–851. 10.1016/j.nucmedbio.2010.04.107 [DOI] [PubMed] [Google Scholar]
- Cabello, N. , Gandia, J. , Bertarelli, D. C. , Watanabe, M. , Lluis, C. , Franco, R. , … Ciruela, F. (2009). Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher‐order oligomers in living cells. Journal of Neurochemistry, 109(5), 1497–1507. 10.1111/j.1471-4159.2009.06078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, U. C. , Lalwani, K. , Hernandez, L. , Kinney, G. G. , Conn, P. J. , & Bristow, L. J. (2004). The mGluR5 antagonist 2‐methyl‐6‐(phenylethynyl)‐pyridine (MPEP) potentiates PCP‐induced cognitive deficits in rats. Psychopharmacology, 175(3), 310–318. 10.1007/s00213-004-1827-5 [DOI] [PubMed] [Google Scholar]
- Chan, M. H. , Chiu, P. H. , Sou, J. H. , & Chen, H. H. (2008). Attenuation of ketamine‐evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology, 198(1), 141–148. 10.1007/s00213-008-1103-1 [DOI] [PubMed] [Google Scholar]
- Chapman, S. , Ragg, M. , & McGeechan, K. (2009). Citation bias in reported smoking prevalence in people with schizophrenia. The Australian and New Zealand Journal of Psychiatry, 43(3), 277–282. 10.1080/00048670802653372 [DOI] [PubMed] [Google Scholar]
- Chen, H. H. , Stoker, A. , & Markou, A. (2010). The glutamatergic compounds sarcosine and N‐acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology, 209(4), 343–350. 10.1007/s00213-010-1802-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn, P. J. , Lindsley, C. W. , & Jones, C. K. (2009). Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends in Pharmacological Sciences, 30(1), 25–31. 10.1016/j.tips.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt, B. A. , Auther, A. M. , Niendam, T. , Smith, C. W. , Zinberg, J. , Bearden, C. E. , & Cannon, T. D. (2007). Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophrenia Bulletin, 33(3), 688–702. 10.1093/schbul/sbm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi, P. M. (1973). Human memory and the medial temporal region of the brain [PhD thesis]. Ann Arbor, MI: Department of Psychology, ProQuest Information & Learning. [Google Scholar]
- Corti, C. , Xuereb, J. H. , Crepaldi, L. , Corsi, M. , Michielin, F. , & Ferraguti, F. (2011). Altered levels of glutamatergic receptors and Na+/K+ ATPase‐alpha1 in the prefrontal cortex of subjects with schizophrenia. Schizophrenia Research, 128(1–3), 7–14. 10.1016/j.schres.2011.01.021 [DOI] [PubMed] [Google Scholar]
- Crow, T. J. , Ball, J. , Bloom, S. R. , Brown, R. , Bruton, C. J. , Colter, N. , … Roberts, G. W. (1989). Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Archives of General Psychiatry, 46(12), 1145–1150. [DOI] [PubMed] [Google Scholar]
- Dauvermann, M. R. , Lee, G. , & Dawson, N. (2017). Glutamatergic regulation of cognition and functional brain connectivity: Insights from pharmacological, genetic and translational schizophrenia research. British Journal of Pharmacology, 174(19), 3136–3160. 10.1111/bph.13919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon, J. , & Diaz, F. J. (2005). A meta‐analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research, 76(2–3), 135–157. 10.1016/j.schres.2005.02.010 [DOI] [PubMed] [Google Scholar]
- De Luca, V. , Wong, A. H. C. , Muller, D. J. , Wong, G. W. H. , Tyndale, R. F. , & Kennedy, J. L. (2004). Evidence of association between smoking and α7 nicotinic receptor subunit gene in schizophrenia patients. Neuropsychopharmacology, 29(8), 1522–1526. 10.1038/sj.npp.1300466 [DOI] [PubMed] [Google Scholar]
- DeLorenzo, C. , DellaGioia, N. , Bloch, M. , Sanacora, G. , Nabulsi, N. , Abdallah, C. , … Esterlis, I. (2015). In vivo ketamine‐induced changes in [(1)(1)C]ABP688 binding to metabotropic glutamate receptor subtype 5. Biological Psychiatry, 77(3), 266–275. 10.1016/j.biopsych.2014.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo, C. , Gallezot, J. D. , Gardus, J. , Yang, J. , Planeta, B. , Nabulsi, N. , … Esterlis, I. (2017). In vivo variation in same‐day estimates of metabotropic glutamate receptor subtype 5 binding using [(11)C]ABP688 and [(18)F]FPEB. Journal of Cerebral Blood Flow and Metabolism, 37(8), 2716–2727. 10.1177/0271678X16673646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo, C. , Kumar, J. S. , Mann, J. J. , & Parsey, R. V. (2011). In vivo variation in metabotropic glutamate receptor subtype 5 binding using positron emission tomography and [11C]ABP688. Journal of Cerebral Blood Flow and Metabolism, 31(11), 2169–2180. 10.1038/jcbfm.2011.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschwanden, A. , Karolewicz, B. , Feyissa, A. M. , Treyer, V. , Ametamey, S. M. , Johayem, A. , … Hasler, G. (2011). Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. The American Journal of Psychiatry, 168(7), 727–734. 10.1176/appi.ajp.2011.09111607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton, A. , & Stone, J. M. (2012). The glutamate hypothesis of schizophrenia: Neuroimaging and drug development. Current Pharmaceutical Biotechnology, 13(8), 1500–1512. [DOI] [PubMed] [Google Scholar]
- Elmenhorst, D. , Mertens, K. , Kroll, T. , Oskamp, A. , Ermert, J. , Elmenhorst, E. M. , … Bauer, A. (2016). Circadian variation of metabotropic glutamate receptor 5 availability in the rat brain. Journal of Sleep Research, 25(6), 754–761. 10.1111/jsr.12432 [DOI] [PubMed] [Google Scholar]
- Esterlis, I. , DellaGioia, N. , Pietrzak, R. H. , Matuskey, D. , Nabulsi, N. , Abdallah, C. G. , … DeLorenzo, C. (2018). Ketamine‐induced reduction in mGluR5 availability is associated with an antidepressant response: An [(11)C]ABP688 and PET imaging study in depression. Molecular Psychiatry, 23(4), 824–832. 10.1038/mp.2017.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi, S. H. , Folsom, T. D. , Rooney, R. J. , & Thuras, P. D. (2013). mRNA and protein expression for novel GABAA receptors theta and rho2 are altered in schizophrenia and mood disorders; relevance to FMRP‐mGluR5 signaling pathway. Translational Psychiatry, 3, e271 10.1038/tp.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Gibbon, M. , & Williams, J. B. (2002). Structured clinical interview for DSM‐IV‐TR axis I disorders, research version, patient edition. New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fuxe, K. , Agnati, L. F. , Jacobsen, K. , Hillion, J. , Canals, M. , Torvinen, M. , … Ferre, S. (2003). Receptor heteromerization in adenosine A2A receptor signaling: Relevance for striatal function and Parkinson's disease. Neurology, 61(11 Suppl 6), S19–S23. 10.1212/01.wnl.0000095206.44418.5c [DOI] [PubMed] [Google Scholar]
- Georgiades, A. , Davis, V. G. , Atkins, A. S. , Khan, A. , Walker, T. W. , Loebel, A. , … Keefe, R. S. E. (2017). Psychometric characteristics of the MATRICS consensus cognitive battery in a large pooled cohort of stable schizophrenia patients. Schizophrenia Research, 190, 172–179. 10.1016/j.schres.2017.03.040 [DOI] [PubMed] [Google Scholar]
- Gold, J. M. , Carpenter, C. , Randolph, C. , Goldberg, T. E. , & Weinberger, D. R. (1997). Auditory working memory and Wisconsin card sorting test performance in schizophrenia. Archives of General Psychiatry, 54(2), 159–165. [DOI] [PubMed] [Google Scholar]
- Golubeva, A. V. , Moloney, R. D. , O'Connor, R. M. , Dinan, T. G. , & Cryan, J. F. (2016). Metabotropic glutamate receptors in central nervous system diseases. Current Drug Targets, 17(5), 538–616. [DOI] [PubMed] [Google Scholar]
- Gupta, D. S. , McCullumsmith, R. E. , Beneyto, M. , Haroutunian, V. , Davis, K. L. , & Meador‐Woodruff, J. H. (2005). Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse, 57(3), 123–131. 10.1002/syn.20164 [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers, A. , Allom, R. , Koepp, M. J. , Free, S. L. , Myers, R. , Lemieux, L. , … Duncan, J. S. (2003). Three‐dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Human Brain Mapping, 19(4), 224–247. 10.1002/hbm.10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, J. , Shan, D. , Meador‐Woodruff, J. , & McCullumsmith, R. (2014). Evidence of glutamatergic dysfunction in the pathophysiology of schizophrenia In Popoli M., Diamond D., & Sanacora G. (Eds.), Synaptic stress and pathogenesis of neuropsychiatric disorders (pp. 265–294). New York: Springer. [Google Scholar]
- Herzog, H. , Langen, K. J. , Weirich, C. , Rota Kops, E. , Kaffanke, J. , Tellmann, L. , … Shah, N. J. (2011). High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin, 50(2), 74–82. 10.3413/Nukmed-0347-10-09 [DOI] [PubMed] [Google Scholar]
- Hickling, L. M. , Perez‐Iglesias, R. , Ortiz‐Garcia de la Foz, V. , Balanza‐Martinez, V. , McGuire, P. , Crespo‐Facorro, B. , & Ayesa‐Arriola, R. (2018). Tobacco smoking and its association with cognition in first episode psychosis patients. Schizophrenia Research, 192, 269–273. 10.1016/j.schres.2017.04.018 [DOI] [PubMed] [Google Scholar]
- Homayoun, H. , Stefani, M. R. , Adams, B. W. , Tamagan, G. D. , & Moghaddam, B. (2004). Functional interaction between NMDA and mGlu5 receptors: Effects on working memory, instrumental learning, motor Behaviors, and dopamine release. Neuropsychopharmacology, 29(7), 1259–1269. 10.1038/sj.npp.1300417 [DOI] [PubMed] [Google Scholar]
- Howes, O. D. , McCutcheon, R. , Owen, M. J. , & Murray, R. M. (2017). The role of genes, stress, and dopamine in the development of schizophrenia. Biological Psychiatry, 81(1), 9–20. 10.1016/j.biopsych.2016.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka, L. M. , Treyer, V. , Scheidegger, M. , Preller, K. H. , Vonmoos, M. , Baumgartner, M. R. , … Quednow, B. B. (2014). Smoking but not cocaine use is associated with lower cerebral metabotropic glutamate receptor 5 density in humans. Molecular Psychiatry, 19(5), 625–632. 10.1038/mp.2013.51 [DOI] [PubMed] [Google Scholar]
- Iwata, Y. , Nakajima, S. , Suzuki, T. , Keefe, R. S. , Plitman, E. , Chung, J. K. , … Uchida, H. (2015). Effects of glutamate positive modulators on cognitive deficits in schizophrenia: A systematic review and meta‐analysis of double‐blind randomized controlled trials. Molecular Psychiatry, 20(10), 1151–1160. 10.1038/mp.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt, D. C. (2010). Glutamatergic theories of schizophrenia. The Israel Journal of Psychiatry and Related Sciences, 47(1), 4–16. [PubMed] [Google Scholar]
- Kay, S. R. , Fiszbein, A. , & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Kim, J. H. , Joo, Y. H. , Son, Y. D. , Kim, J. H. , Kim, Y. K. , Kim, H. K. , … Ido, T. (2019). In vivo metabotropic glutamate receptor 5 availability‐associated functional connectivity alterations in drug‐naive young adults with major depression. European Neuropsychopharmacology, 29(2), 278–290. 10.1016/j.euroneuro.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Kinney, G. G. , Burno, M. , Campbell, U. C. , Hernandez, L. M. , Rodriguez, D. , Bristow, L. J. , & Conn, P. J. (2003). Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. The Journal of Pharmacology and Experimental Therapeutics, 306(1), 116–123. 10.1124/jpet.103.048702 [DOI] [PubMed] [Google Scholar]
- Kordi‐Tamandani, D. M. , Dahmardeh, N. , & Torkamanzehi, A. (2013). Evaluation of hypermethylation and expression pattern of GMR2, GMR5, GMR8, and GRIA3 in patients with schizophrenia. Gene, 515(1), 163–166. 10.1016/j.gene.2012.10.075 [DOI] [PubMed] [Google Scholar]
- Kortte, K. B. , Horner, M. D. , & Windham, W. K. (2002). The trail making test, part B: Cognitive flexibility or ability to maintain set? Applied Neuropsychology, 9(2), 106–109. 10.1207/S15324826AN0902_5 [DOI] [PubMed] [Google Scholar]
- Kullmann, D. M. , & Lamsa, K. (2008). Roles of distinct glutamate receptors in induction of anti‐Hebbian long‐term potentiation. The Journal of Physiology, 586(6), 1481–1486. 10.1113/jphysiol.2007.148064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, G. , Saklofske, D. , Coalson, D. , & Raiford, S. (2010). WAIS‐IV clinical use and interpretation. San Diego, CA: Academic Press. [Google Scholar]
- Leucht, S. , Samara, M. , Heres, S. , Patel, M. X. , Furukawa, T. , Cipriani, A. , … Davis, J. M. (2015). Dose equivalents for second‐generation antipsychotic drugs: The classical mean dose method. Schizophrenia Bulletin, 41(6), 1397–1402. 10.1093/schbul/sbv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y.‐M. , Jia, Z. , Janus, C. , Henderson, J. T. , Gerlai, R. , Wojtowicz, J. M. , & Roder, J. C. (1997). Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long‐term potentiation (LTP) but normal CA3 LTP. The Journal of Neuroscience, 17(13), 5196–5205. 10.1523/jneurosci.17-13-05196.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucatch, A. M. , Lowe, D. J. E. , Clark, R. C. , Kozak, K. , & George, T. P. (2018). Neurobiological determinants of tobacco smoking in schizophrenia. Frontiers in Psychiatry, 9, 672 10.3389/fpsyt.2018.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymetz, J. , Moran, S. P. , & Conn, P. J. (2017). Targeting metabotropic glutamate receptors for novel treatments of schizophrenia. Molecular Brain, 10(1), 15 10.1186/s13041-017-0293-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosin, N. , Fernandez‐Enright, F. , Frank, E. , Deng, C. , Wong, J. , Huang, X. F. , & Newell, K. A. (2014). Metabotropic glutamate receptor mGluR2/3 and mGluR5 binding in the anterior cingulate cortex in psychotic and nonpsychotic depression, bipolar disorder and schizophrenia: Implications for novel mGluR‐based therapeutics. Journal of Psychiatry & Neuroscience, 39(6), 407–416. 10.1503/jpn.130242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matosin, N. , Fernandez‐Enright, F. , Lum, J. S. , & Newell, K. A. (2017). Shifting towards a model of mGluR5 dysregulation in schizophrenia: Consequences for future schizophrenia treatment. Neuropharmacology, 115, 73–91. 10.1016/j.neuropharm.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Matosin, N. , Frank, E. , Deng, C. , Huang, X. F. , & Newell, K. A. (2013). Metabotropic glutamate receptor 5 binding and protein expression in schizophrenia and following antipsychotic drug treatment. Schizophrenia Research, 146(1–3), 170–176. 10.1016/j.schres.2013.01.018 [DOI] [PubMed] [Google Scholar]
- Moghaddam, B. , & Javitt, D. (2012). From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology, 37(1), 4–15. 10.1038/npp.2011.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium . (2015). Psychiatric genome‐wide association study analyses implicate neuronal, immune and histone pathways. Nature Neuroscience, 18(2), 199–209. 10.1038/nn.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell, K. A. (2013). Metabotropic glutamate receptor 5 in schizophrenia: Emerging evidence for the development of antipsychotic drugs. Future Medicinal Chemistry, 5(13), 1471–1474. 10.4155/fmc.13.137 [DOI] [PubMed] [Google Scholar]
- Newell, K. A. , & Matosin, N. (2014). Rethinking metabotropic glutamate receptor 5 pathological findings in psychiatric disorders: Implications for the future of novel therapeutics. BMC Psychiatry, 14, 23 10.1186/1471-244X-14-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam, T. , Bearden, C. , Johnson, J. , & Cannon, T. (2006). Global functioning: Role scale (GF: Role). Los Angeles, CA: University of California, Los Angeles. [Google Scholar]
- Niswender, C. M. , & Conn, P. J. (2010). Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annual Review of Pharmacology and Toxicology, 50, 295–322. 10.1146/annurev.pharmtox.011008.145533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein, K. H. , Green, M. F. , Kern, R. S. , Baade, L. E. , Barch, D. M. , Cohen, J. D. , … Marder, S. R. (2008). The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. The American Journal of Psychiatry, 165(2), 203–213. 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- Ohnuma, T. , Augood, S. J. , Arai, H. , McKenna, P. J. , & Emson, P. C. (1998). Expression of the human excitatory amino acid transporter 2 and metabotropic glutamate receptors 3 and 5 in the prefrontal cortex from normal individuals and patients with schizophrenia. Brain Research. Molecular Brain Research, 56(1–2), 207–217. [DOI] [PubMed] [Google Scholar]
- Peechatka, A. L. , Whitton, A. E. , Farmer, S. L. , Pizzagalli, D. A. , & Janes, A. C. (2015). Cigarette craving is associated with blunted reward processing in nicotine‐dependent smokers. Drug and Alcohol Dependence, 155, 202–207. 10.1016/j.drugalcdep.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma, H. L. , & Boes, J. L. (1997). The GAF and psychiatric outcome: A descriptive report. Community Mental Health Journal, 33(1), 35–41. [DOI] [PubMed] [Google Scholar]
- Pietraszek, M. , Gravius, A. , Schafer, D. , Weil, T. , Trifanova, D. , & Danysz, W. (2005). mGluR5, but not mGluR1, antagonist modifies MK‐801‐induced locomotor activity and deficit of prepulse inhibition. Neuropharmacology, 49(1), 73–85. 10.1016/j.neuropharm.2005.01.027 [DOI] [PubMed] [Google Scholar]
- Pilowsky, L. S. , Bressan, R. A. , Stone, J. M. , Erlandsson, K. , Mulligan, R. S. , Krystal, J. H. , & Ell, P. J. (2006). First in vivo evidence of an NMDA receptor deficit in medication‐free schizophrenic patients. Molecular Psychiatry, 11(2), 118–119. 10.1038/sj.mp.4001751 [DOI] [PubMed] [Google Scholar]
- Popoli, P. , Pezzola, A. , Torvinen, M. , Reggio, R. , Pintor, A. , Scarchilli, L. , … Ferre, S. (2001). The selective mGlu(5) receptor agonist CHPG inhibits quinpirole‐induced turning in 6‐hydroxydopamine‐lesioned rats and modulates the binding characteristics of dopamine D(2) receptors in the rat striatum: Interactions with adenosine a(2a) receptors. Neuropsychopharmacology, 25(4), 505–513. 10.1016/S0893-133X(01)00256-1 [DOI] [PubMed] [Google Scholar]
- Quigley, H. , & MacCabe, J. H. (2019). The relationship between nicotine and psychosis. Therapeutic Advances in Psychopharmacology, 9, 2045125319859969 10.1177/2045125319859969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, A. C. , Harris, J. G. , & Olincy, A. (2016). Schizophrenia, smoking status, and performance on the matrics cognitive consensus battery. Psychiatry Research, 246, 1–8. 10.1016/j.psychres.2016.08.062 [DOI] [PubMed] [Google Scholar]
- Reeves, S. J. , Grasby, P. M. , Howard, R. J. , Bantick, R. A. , Asselin, M. C. , & Mehta, M. A. (2005). A positron emission tomography (PET) investigation of the role of striatal dopamine (D2) receptor availability in spatial cognition. NeuroImage, 28(1), 216–226. 10.1016/j.neuroimage.2005.05.034 [DOI] [PubMed] [Google Scholar]
- Riala, K. , Hakko, H. , Isohanni, M. , Jokelainen, J. , Weiser, M. , & Rasanen, P. (2005). Poor premorbid school performance is associated with later cigarette smoking among schizophrenia patients. Psychiatry Research, 137(1–2), 137–141. 10.1016/j.psychres.2005.07.014 [DOI] [PubMed] [Google Scholar]
- Rigucci, S. , Rossi‐Espagnet, C. , Ferracuti, S. , De Carolis, A. , Corigliano, V. , Carducci, F. , … Comparelli, A. (2013). Anatomical substrates of cognitive and clinical dimensions in first episode schizophrenia. Acta Psychiatrica Scandinavica, 128(4), 261–270. 10.1111/acps.12051 [DOI] [PubMed] [Google Scholar]
- Roalf, D. R. , Quarmley, M. , Calkins, M. E. , Satterthwaite, T. D. , Ruparel, K. , Elliott, M. A. , … Turetsky, B. I. (2017). Temporal lobe volume decrements in psychosis Spectrum youths. Schizophrenia Bulletin, 43(3), 601–610. 10.1093/schbul/sbw112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Sanchez, J. M. , Crespo‐Facorro, B. , Gonzalez‐Blanch, C. , Perez‐Iglesias, R. , Vazquez‐Barquero, J. L. , & PAFIP Group Study . (2007). Cognitive dysfunction in first‐episode psychosis: The processing speed hypothesis. The British Journal of Psychiatry. Supplement, 51, s107–s110. 10.1192/bjp.191.51.s107 [DOI] [PubMed] [Google Scholar]
- Rota Kops, E. , Herzog, H. , & Shah, N. J. (2014). Comparison template‐based with CT‐based attenuation correction for hybrid MR/PET scanners. EJNMMI Physics, 1(Suppl 1), A47 10.1186/2197-7364-1-S1-A47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagud, M. , Mihaljevic‐Peles, A. , Muck‐Seler, D. , Pivac, N. , Vuksan‐Cusa, B. , Brataljenovic, T. , & Jakovljevic, M. (2009). Smoking and schizophrenia. Psychiatria Danubina, 21(3), 371–375. [PubMed] [Google Scholar]
- Salisbury, D. F. , Shenton, M. E. , Griggs, C. B. , Bonner‐Jackson, A. , & McCarley, R. W. (2002). Mismatch negativity in chronic schizophrenia and first‐episode schizophrenia. Archives of General Psychiatry, 59(8), 686–694. 10.1001/archpsyc.59.8.686 [DOI] [PubMed] [Google Scholar]
- Sanchez‐Cubillo, I. , Perianez, J. A. , Adrover‐Roig, D. , Rodriguez‐Sanchez, J. M. , Rios‐Lago, M. , Tirapu, J. , & Barcelo, F. (2009). Construct validity of the trail making test: Role of task‐switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society, 15(3), 438–450. 10.1017/S1355617709090626 [DOI] [PubMed] [Google Scholar]
- Scheins, J. , Brambilla, C. R. , Mauler, J. , Rota Kops, E. , Tellmann, L. , Lerche, C. W. , & Shah, N. J. (2019). Image‐based Motion Correction for the Siemens Hybrid MR/BrainPET Scanner Paper presented at the 57. Jahrestagung der Deutschen Gesellschaft für Nuklearmedizin 2019, Bremen, Germany. Retrieved from https://www.nuklearmedizin.de/jahrestagungen/abstr_online2019/abstract_detail.php?navId=227&aId=149.
- Schwartz, K. , Iancu, I. , Stryjer, R. , Chelben, J. , Kotler, M. , Weizman, A. , & Rehavi, M. (2005). Reduced platelet vesicular monoamine transporter density in smoking schizophrenia patients. European Neuropsychopharmacology, 15(5), 557–561. 10.1016/j.euroneuro.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Smart, K. , Cox, S. M. L. , Nagano‐Saito, A. , Rosa‐Neto, P. , Leyton, M. , & Benkelfat, C. (2018). Test‐retest variability of [(11) C]ABP688 estimates of metabotropic glutamate receptor subtype 5 availability in humans. Synapse, 72(9), e22041 10.1002/syn.22041 [DOI] [PubMed] [Google Scholar]
- Smart, K. , Cox, S. M. L. , Scala, S. G. , Tippler, M. , Jaworska, N. , Boivin, M. , … Leyton, M. (2019). Sex differences in [(11)C]ABP688 binding: A positron emission tomography study of mGlu5 receptors. European Journal of Nuclear Medicine and Molecular Imaging, 46(5), 1179–1183. 10.1007/s00259-018-4252-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, E. , Sherman, E. M. , & Spreen, O. (2006). A compendium of neuropsychological tests: Administration, norms, and commentary. Washington, DC: American Chemical Society. [Google Scholar]
- Thiebes, S. , Leicht, G. , Curic, S. , Steinmann, S. , Polomac, N. , Andreou, C. , … Mulert, C. (2017). Glutamatergic deficit and schizophrenia‐like negative symptoms: New evidence from ketamine‐induced mismatch negativity alterations in healthy male humans. Journal of Psychiatry & Neuroscience, 42(4), 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken, A. , Whitfield‐Gabrieli, S. , Bammer, R. , Baldo, J. V. , Dronkers, N. F. , & Gabrieli, J. D. (2008). Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. NeuroImage, 42(2), 1032–1044. 10.1016/j.neuroimage.2008.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os, J. , & Kapur, S. (2009). Schizophrenia. Lancet, 374(9690), 635–645. 10.1016/S0140-6736(09)60995-8 [DOI] [PubMed] [Google Scholar]
- van Velden, F. H. , Kloet, R. W. , van Berckel, B. N. , Wolfensberger, S. P. , Lammertsma, A. A. , & Boellaard, R. (2008). Comparison of 3D‐OP‐OSEM and 3D‐FBP reconstruction algorithms for high‐resolution research Tomograph studies: Effects of randoms estimation methods. Physics in Medicine and Biology, 53(12), 3217–3230. 10.1088/0031-9155/53/12/010 [DOI] [PubMed] [Google Scholar]
- Vandierendonck, A. , Kemps, E. , Fastame, M. C. , & Szmalec, A. (2004). Working memory components of the Corsi blocks task. British Journal of Psychology, 95(Pt 1), 57–79. 10.1348/000712604322779460 [DOI] [PubMed] [Google Scholar]
- Vazzana, R. , Bandinelli, S. , Lauretani, F. , Volpato, S. , Lauretani, F. , Di Iorio, A. , … Ferrucci, L. (2010). Trail making test predicts physical impairment and mortality in older persons. Journal of the American Geriatrics Society, 58(4), 719–723. 10.1111/j.1532-5415.2010.02780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselinovic, T. , Schorn, H. , Vernaleken, I. B. , Hiemke, C. , Zernig, G. , Gur, R. , & Grunder, G. (2013). Effects of antipsychotic treatment on cognition in healthy subjects. Journal of Psychopharmacology, 27(4), 374–385. 10.1177/0269881112466183 [DOI] [PubMed] [Google Scholar]
- Volk, D. W. , Eggan, S. M. , & Lewis, D. A. (2010). Alterations in metabotropic glutamate receptor 1alpha and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia. The American Journal of Psychiatry, 167(12), 1489–1498. 10.1176/appi.ajp.2010.10030318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. Y. , MacDonald, M. L. , Borgmann‐Winter, K. E. , Banerjee, A. , Sleiman, P. , Tom, A. , … Hahn, C. G. (2018). mGluR5 hypofunction is integral to glutamatergic dysregulation in schizophrenia. Molecular Psychiatry. 10.1038/s41380-018-0234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, R. N. (2003). Imaging the PCP site of the NMDA ion channel. Nuclear Medicine and Biology, 30(8), 869–878. [DOI] [PubMed] [Google Scholar]
- Wilhelm, P. W. (2004). Reliability and validity of the Rey visual design learning test in primary school children. Journal of Clinical and Experimental Neuropsychology, 26(7), 981–994. 10.1080/13803390490511076 [DOI] [PubMed] [Google Scholar]
- Wing, V. C. , Wass, C. E. , Soh, D. W. , & George, T. P. (2012). A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Annals of the New York Academy of Sciences, 1248, 89–106. 10.1111/j.1749-6632.2011.06261.x [DOI] [PubMed] [Google Scholar]
- Winton‐Brown, T. T. , Fusar‐Poli, P. , Ungless, M. A. , & Howes, O. D. (2014). Dopaminergic basis of salience dysregulation in psychosis. Trends in Neurosciences, 37(2), 85–94. 10.1016/j.tins.2013.11.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at Forschungszentrum Jülich and RWTH Uniklinik Aachen. Derived data supporting the findings of this study are available from the corresponding and first authors C.R.B and T.V. on request.


