Abstract
Background
Liver is a vital organ that plays a major role in the elimination of xenobiotics from the body. Diseases that affect the liver become major health problems and challenge health-care professionals as well as the pharmaceutical industry. Since the conventional treatment of liver diseases is associated with a wide range of adverse effects, botanical agents are commonly used. Among these agents, Clutia abyssinica is the most widely used herb in Ethiopian traditional medicine.
Objective
To evaluate the hepatoprotective activity of the crude 80% methanol extract and solvent fractions of Clutia abyssinica leaves in mice.
Methods
The leaves of Clutia abyssinica were extracted by cold maceration using 80% methanol as a solvent, and the solvent fractions were obtained in liquid–liquid extraction with chloroform, n-butanol and distilled water. Male mice were treated with the vehicles (distilled water or 2% Tween 80), three different doses (100, 200 and 400 mg/kg) of the crude 80% methanol extract and three solvent fractions, the standard drug (silymarin 100 mg/kg), and the hepatotoxicant carbon tetrachloride (CCl4). Then, the levels of biomarkers of liver injury – such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) – and liver function such as total protein, albumin, and bilirubin were measured. Evaluation of the change in body weight and liver weight, histopathologic examination and in vitro antioxidant assay against CCl4-induced hepatotoxicity were also carried out.
Results
The 80% methanol extract decreased the absolute and relative weight of the liver of mice at the doses of 200 and 400 mg/kg (p<0.01 and p<0.001, respectively). It also suppressed the plasma levels of AST, ALT and ALP (p<0.001) in the aforementioned doses. Among fractions, the n-butanol fraction showed maximum hepatoprotective activity in its dose of 200 and 400 mg/kg (p<0.001, in all cases). Likewise, the chloroform fraction (400 mg/kg) reduced to a similar extent (p<0.001 in all cases). In stark contrast, the aqueous fraction failed to affect the levels of all biomarkers of hepatocyte injury. The crude methanol extract and n-butanol fraction were able to return the normal hepatic architecture of hepatocytes and scavenge free radicals in the 1,1-diphenylpicrylhydrazyl (DPPH) assay.
Conclusion
Clutia abyssinica is endowed with hepatoprotective activity, probably mediated via its antioxidant activity. Thus, Clutia abyssinica can be taken as one candidate for the development of hepatoprotective agents because of its good safety profile.
Keywords: antioxidant, CCl4, Clutia abyssinica, hepatoprotective, mice
Background
Clutia abyssinica commonly called “large or smooth-fruited lightning-bush” in English,1,2 “Fiyele-Feji” in Amharic3 is a shrub that belongs to the family Euphorbiaceae.3 Geographically, it is distributed from Congo east to Eritrea and Somalia and through eastern Africa; south to Zambia, Angola, Mozambique and South Africa.4 Traditionally, all parts of Clutia abyssinica have many medicinal uses against a variety of diseases. The roots are used for the treatment of several diseases such as; vomiting,3 roundworm infestation, difficulty of urination, stomachache,5 colic pain in infants,6 and erectile dysfunction in adults.2
The leaves are used to treat ectoparasite infestation, dysentery,3 gastritis, hypertension,7 herpes zoster, superficial fungal infections (tinea capitis and ringworm), and internal parasite infections,8,9 anthrax,9 and they are also used orally for the treatment of liver diseases, hepatitis/jaundice.10,11 Both the roots and leaves are used in the treatment of venereal and skin diseases, chest problems, cancer and fertility in humans.12
Some scientific studies revealed its in vivo antimalarial,13 anti-trypanosomal,14 analgesic,15 anti-inflammatory,16 antipyretic,17 diuretic18 and in vitro antioxidant and anti-proliferative19 activities. The medicinal value of Clutia abyssinica may be due to its constituents that produce a definite physiological action. Phytochemical analysis of the root extract showed that this plant is found to contain alkaloids, saponins, anthraquinones, phenolics, tannins, terpenoids, flavonoids12 and a complex mixture of 5-methyl coumarins.20 But it was not found to contain glycosides.12
Materials and Methods
Chemicals and Reagents
Chemicals and reagents used were distilled water (EPHARM, Ethiopia), 2% Tween80 (Oxford Lab Fine Chem LLP, India), absolute methanol (SIGMA-ALDRICH, Germany), n-butanol (SIGMA-ALDRICH, Germany), chloroform (SIGMA-ALDRICH, Germany), CCl4 (Oxford Lab Fine Chem LLP, India), 10% formalin (Novochem Engineering, India), ether (Puyer BioPharma Ltd., P.R. China), normal saline (EPHARM, Ethiopia), liquid paraffin (Oxford Lab Fine Chem LLP, India), paraffin wax (Oxford Lab Fine Chem LLP, India), hematoxylin (Santa Cruz Biotechnology, Inc., USA), eosin (Santa Cruz Biotechnology, Inc., USA), xylene (scienTEST - bioKEMIX GmbH, Germany), 2,2-diphenyl-1-picrylhydrazyl [DPPH] (Chemos GmbH & Co. KG, Germany), the standard drug silymarin (Silybon-140, Micro Lab Limited, India), assay kits for liver chemistry (HUMANA, Germany) and other chemicals and reagents for phytochemical tests. All reagents used were of analytical grade.
Plant Material
The leaves of Clutia abyssinica were collected in January 2019 from its natural habitat around Debre Markos about 301 km northwest of Addis Ababa. Identification of the plant specimen was done by a taxonomist at the National Herbarium, Department of Biology, Addis Ababa University and a voucher specimen (BG02/2011) was deposited for future reference. The collected leaves were gently washed with tap water to remove dirt and dried under shade for a period of 2 weeks. Then, they were pulverized into coarse powder by using mortar and pestle.
Experimental Animals
Healthy Swiss albino mice of either sex (female for acute toxicity and male for the main study) with the age of 6–8 weeks and having a weight range of 28–36 g inbred in the animal house of the Department of Pharmacy, Wollo University were used for the experiment. The mice were housed in polypropylene cages (6 mice per cage) under standard environmental conditions and 12 h-12 h light-dark cycle. The animals were allowed free access to tap water and laboratory pellet and acclimatized to laboratory conditions for 1 week before the experiment.
Preparation of Plant Extracts
Three hundred grams of the coarse powder was extracted by cold maceration using 80% methanol as a solvent. The extract was filtered by using muslin cloth and Whatman® grade 1 filter paper and the marc was re-extracted for the second and third time by adding another fresh solvent. The fluid extracts were combined and concentrated in a rotary evaporator (Buchi, Rotavapor R-210/215, Switzerland) under reduced pressure at 40°C. The concentrated filtrate was then frozen in a refrigerator and dried in a lyophilizer (Lyophilizer, OPR-FDU-5012, Korea) and the percent yield of the dried leaf extract was 19.8 (%w/w). Two-third of the crude 80% methanol extract (39.6 g) was then successively fractionated using chloroform, n-butanol, and distilled water. First, the crude extract was suspended in 200 mL of warm water and the suspension was shaken in a separatory funnel by adding 50 mL chloroform three times in a separated process. The chloroform fraction was obtained in three separately performed fractionations. Second, the aqueous residue was shaken with 50 mL n-butanol three times separately and n-butanol fraction was obtained. The aqueous fraction was obtained as a residue after the filtration of the n-butanol fraction. Then, the three solvent fractions, chloroform, n-butanol and aqueous, were concentrated on a rotary evaporator, water bath, and lyophilizer, respectively. Finally, the crude 80% methanol extract, chloroform, and n-butanol fractions were stored in a deep freezer (−20°C), whereas the aqueous fraction was stored in a desiccator until used for the experiment.
Acute Oral Toxicity Test
Acute oral toxicity test for the crude 80% methanol extract and the three solvent fractions was performed according to the organization for economic co-operation and development guidelines.21 All mice were fasted (3–4 h) before and 1–2 h after administration of the 80% methanol extract and the solvent fractions. First, a sighting study was performed to determine the starting dose. For this female mice were used and each mouse was given 2000 mg/kg of the 80% methanol extract and solvent fractions as a single dose by oral gavage. Since no death was observed within 24 h, additional four mice were used for each of the 80% methanol extract and solvent fractions and administered at the same dose mentioned above. Then, mice were observed continuously for 4 h with 30 min interval and then for 14 consecutive days with an interval of 24 h for the general signs and symptoms of toxicity (diarrhea, weight loss, tremor, lethargy, and paralysis), food and water intake, and mortality.
Grouping and Dosing of Animals
Mice were randomly assigned into 16 groups (two negative controls, positive control, toxicant control, and 12 test groups) comprising of six animals per group for hepatoprotective activity test. Negative controls were treated with the vehicles used for reconstitution (2% Tween 80 in water (2%TW80) for 80% methanol extract, chloroform and n-butanol fraction, and distilled water (DW) for aqueous fraction) 1mL/kg orally for 6 days. Positive controls were administered with standard drug, silymarin 100 mg/kg for 6 days and CCl4 (50% CCl4 dissolved in liquid paraffin in 1:1, 2 mL/kg i.p.) on the 4th day. Toxicant controls were given CCl4 (2 mL/kg, i.p.) on the 4th day and vehicle (1 mL/kg of 2%TW80) thereafter. Among the test groups (group 5, 6 and 7) were administered with 100 mg/kg (Me100), 200 mg/kg (Me200) and 400 mg/kg (Me400) doses of 80% methanol leaf extract for 6 days. Test groups (group 8, 9 and 10) were treated with 100 mg/kg (CF100), 200 mg/kg (CF200) and 400 mg/kg (CF400) doses of chloroform fraction for 6 days. Test groups (group 11, 12 and 13) were treated with 100 mg/kg (BF100), 200 mg/kg (BF200) and 400 mg/kg (BF400) doses of n-butanol fraction for 6 days. Test groups (group 14, 15 and 16) were received 100 mg/kg (AF100), 200 mg/kg (AF200) and 400 mg/kg (AF400) doses of aqueous fraction for 6 days. All test groups were given CCl4 (2 mL/kg) on the 4th-day via intraperitoneal injection. Doses were determined using data from the acute toxicity test. Route of administration for the test sample, standard drug and vehicles was orally by using oral gavage and the volume administered was 1mL/100 g.
Hepatoprotective Activity
Hepatoprotective activity was determined following the method used by Sintayehu et al.22 All mice were subjected to fasting for a period of 3–4 h and weighed individually and their weight was recorded as initial weight at the beginning of the experiment. Animals were divided into groups comprising of six animals per group and dosed as described above in the “grouping and dosing of animals” section.
On the seventh day, mice were weighed and sacrificed under light ether anesthesia. Blood sample was collected in heparinized sterile centrifuge tubes from each mouse by cardiac puncture and tubes were placed in a centrifuge and undergone separation at 3000 rpm for 10 min. Clear serum was obtained and subjected to analysis for liver function tests; the levels of albumin, total protein and total bilirubin, and liver chemistry such as; the levels of ALT, AST, and ALP by using the automatic analyzer and commercial assay kits. In addition, the liver of each mouse was harvested, weighed and examined for gross and microscopic pathology.
The hepatoprotective activity of the extract and fractions can be calculated as percentage protection which is; % protection = 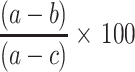 where a is the mean value of the marker produced by hepatotoxin; b is the mean value of the marker produced by toxin plus test material, and c is the mean value produced by the vehicle control.22
where a is the mean value of the marker produced by hepatotoxin; b is the mean value of the marker produced by toxin plus test material, and c is the mean value produced by the vehicle control.22
Histopathological Examination
The liver of each mouse was excised and washed with normal saline and fixed in 10% formalin. Formalin-fixed tissue was washed in tap water. Then, it was dehydrated in serial ethanol and cleared in xylene. Finally, xylene cleared tissue was embedded in paraffin wax. Sections of 4–5 microns thickness were made from the paraffin-embedded block and stained with hematoxylin and eosin for histopathological observations. Microscopic slides were prepared and examined under a microscope. Images were captured using Olympus DP12 CCD camera at an original magnification of 10x (Olympus DP12 Microsystems Digital Imaging Olympus, Japan).
Phytochemical Screening
The qualitative phytochemical investigation of the 80% methanol extract and the solvent fractions (chloroform, n-butanol and aqueous) were carried out using standardized tests to identify the presence of secondary metabolites like polyphenols, tannins, saponins, flavonoids, terpenoids, steroids, alkaloids and cardiac glycosides as follows.
Test for Tannins: about 0.25 g of 80% methanol extract and each fraction was boiled in 10 mL of water separately in a test tube and then filtered. In each sample, a few drops of 10% ferric chloride were added and observed for the formation of precipitate or color change. A bluish-black or brownish-green precipitate indicates the presence of tannins.23
Test for Saponins: to 0.25 g of 80% methanol extract and each fraction, 5 mL of distilled water was added. Then, the mixture was shaken vigorously for 2 min and observed for the formation of a stable persistent froth, which indicates the presence of saponins.23
Test for Terpenoids: about 0.25 g of 80% methanol extract and each fraction was added in different test tubes. Two mL of chloroform was added in each test tube. Then, 3 mL of concentrated sulfuric acid was carefully added in each of them to form a layer. A reddish-brown coloration at the interface indicates the presence of terpenoids.23
Test for Flavonoids: about 0.5 g of 80% methanol extract and each fraction was added in different test tubes. Then, 10 mL of distilled water was added in each test tube and boiled for 5 min. The mixture was filtered while hot and allowed to cool. Few drops of 20% sodium hydroxide solution were added to 1 mL of the cooled filtrate. A change to yellow color, which on the addition of hydrochloric acid changed to colorless solution indicates the presence of flavonoids.24,25
Test for Cardiac glycosides: about 0.5 g of 80% methanol extract and each fraction was dissolved in distilled water in different test tubes. In 5 mL of each solution, 2 mL of glacial acetic acid containing one drop of ferric chloride solution was added. This was underplayed with 1 mL of concentrated sulfuric acid. The formation of the brown ring at the interface indicates the presence of cardiac glycosides.26
Test for Alkaloids: about 5 mL of 5% hydrochloric acid was added to 0.5 g of 80% methanol extract and each fraction, and heated on a water bath. When cooled, few drops of Dragendroff’s reagent (potassium bismuth iodide) were added. The appearance of the reddish-brown precipitate indicates the presence of alkaloids.23,25
Test for Polyphenols: to 2 mL of filtered solutions of 80% methanol extract and each fraction, three to four drops of 1% FeCl3 solution were added. The formation of bluish-black color indicates the presence of phenols.23,26
Test for Steroids: one mL of 80% methanol extract and each fraction was dissolved in 10 mL of chloroform and an equal volume of concentrated sulfuric acid was added by sides of the test tube. The upper layer turns red and the sulfuric acid layer showed yellow with green fluorescence, which indicates the presence of steroids.27
DPPH Scavenging Activity
The free radical scavenging capacity of the extract and solvent fractions was determined by using DPPH.28 About 25 mg of DPPH was dissolved in a liter of methanol to prepare the stock solution. On a separate process, 500 mg of the crude extract and solvent fractions were dissolved in methanol in a way to prepare serial dilution by taking the minimum concentration of the working solution. Finally, 3.9 mL of DPPH solution was mixed with 0.1 mL of the sample to prepare the working solution. Then, UV spectrophotometer was set at zero by using methanol (blank solution) to cancel the solvent effect. Freshly prepared DPPH solution was taken in test tubes and the extract and fractions were added followed by serial dilutions, and after 30 min, the absorbance was read at 517 nm UV range using a spectrophotometer. Ascorbic acid was used as a reference standard and dissolved in methanol to make the stock solution with the same concentration.
Percent scavenging of the DPPH-free radical was measured by using the following equation:
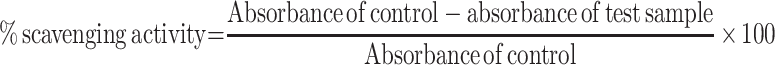 |
.29
Statistical Analysis
The results of the study are expressed as the mean ± standard error of the mean (S.E.M). Statistical analysis of the data was performed with one-way analysis of variance (ANOVA) followed by Tukey post hoc multiple comparison test. Significant differences were set at p values lower than 0.05.
Ethical Consideration
The mice were handled as per the international animal care and welfare,30 and the national institute of health guidelines for the care and use of laboratory animals.31 Besides, ethical clearance was obtained from the ethical review committee of the college of medicine and health sciences, Wollo University.
Results
Percent Yield of the 80% Methanol Crude Extract and Solvent Fractions
The leaves of Clutia abyssinica were extracted with 80% methanol by using cold maceration technique and further fractionated by using solvents of different polarities (chloroform, n-butanol and distilled water). The crude extract and solvent fractions were investigated for their hepatoprotective activity. The percentage yields of the extract and solvent fractions are given in Table 1. The percent yield of 80% methanol crude extract of the leaves of the plant was found to be 19.8%.
Table 1.
Percentage Yields of 80% Methanol Crude Extract and Solvent Fractions of the Leaves of Clutia abyssinica
| Extractive Solvent Used | Percent Yield of Extract (%w/w) |
|---|---|
| 80% Methanol | 19.8% |
| n-Butanol | 26.4% |
| Chloroform | 11.8% |
| Distilled water | 37.5% |
Acute Oral Toxicity Test
The acute oral toxicity test of 80% methanol leaf extract and solvent fractions of Clutia abyssinica indicated that neither the 80% methanol extract nor the solvent fractions caused gross behavioral changes and mortality within 24 h as well as in the next 14 days, indicating that the median lethal oral dose of the methanol extract and fractions were greater than 2000 mg/kg in mice.
Hepatoprotective Activity
Effect on Body Weight, Change in Body Weight and Absolute and Relative Liver Weight
80% Methanol Extract
The effect of 80% methanol extract on body weight, changes in body weight, absolute and relative liver weight of mice is shown in Table 2.
Table 2.
Effect of 80% Methanol Extract of the Leaves of Clutia abyssinica on Body Weight, Change in Body Weight and Absolute and Relative Liver Weight of Mice Administered with CCl4
| Group | Body Weight (g) | Change in Body Weight | Liver Weight (g) | Relative Liver Weight (%) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| 2%TW80 | 32.50 ± 1.09 | 34.62 ± 0.95 | 2.12 ± 0.52 | 2.40 ± 0.07 | 6.94 ± 0.14 |
| CCl4 | 32.50 ± 0.89 | 29.34 ± 0.56a** | −3.16 ± 0.66a*** | 3.56 ± 0.21a** | 12.16 ± 0.76a*** |
| Silymarin + CCl4 | 32.00 ± 1.03 | 34.00 ± 0.89b** | 2.00 ± 0.37b*** | 2.62 ± 0.19b* | 7.70 ± 0.52b*** |
| Me100 + CCl4 | 31.67 ± 0.92 | 29.19 ± 0.41a**,c**,d**,e*** | −2.48 ± 0.59a***,c***,d**,e*** | 3.28 ± 0.30 | 11.24 ± 1.02a**,c*,e** |
| Me200 + CCl4 | 32.50 ± 1.34 | 34.48 ± 1.31b** | 1.98 ± 0.46b*** | 2.86 ± 0.20 | 8.35 ± 0.67b** |
| Me400 + CCl4 | 32.83 ± 1.02 | 34.87 ± 0.78b*** | 2.04 ± 1.21b*** | 2.52 ± 0.23b* | 7.25 ± 0.69b*** |
Notes: Each value represents mean ± S.E.M; n=6; aAgainst 2%TW80; bAgainst CCl4; cAgainst standard (silymarin); dAgainst Me200 + CCl4; eAgainst Me400 + CCl4; *p < 0.05, **p < 0.01, ***p < 0.001.
Abreviations: Me100, 80% methanol extract 100 mg/kg; Me200, 80% methanol extract 200 mg/kg; Me400, 80% methanol extract 400 mg/kg; silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2mL/kg; 2%TW80, 2% Tween 80 in water.
Me100 administered mice did not show increment in the change in body weight, whereas, Me200 and Me400 treated mice produced a significant increase in change in body weight (p<0.001), as compared to CCl4 treated mice. The absolute and relative weights of the liver of mice treated with Me200 and Me400 decreased significantly (p<0.01 and p<0.001), respectively, as compared to the toxicant-treated group. In contrast, the change in body weight of mice treated with CCl4 significantly (p<0.001) decreased, as compared to control and the absolute and relative liver weights of mice treated with CCl4 significantly increased (p<0.001) as compared to control.
Solvent Fractions
The effect of solvent fractions of 80% methanol extract on body weight, changes in body weight, absolute and relative liver weight of mice is shown in Table 3. The n-butanol fraction showed a significant increment in the change in body weight of mice at the dose of 200 and 400 mg/kg (p<0.001). On the other hand, BF100 did not produce any detectable difference in change in body weight, absolute and relative liver weight of mice compared to toxicant control. By contrast, BF200 and BF400 significantly decreased the relative liver weight of mice (p<0.001).
Table 3.
Effect of Solvent Fractions of Clutia abyssinica Leaf Extract on Body Weight, Change in Body Weight and Absolute and Relative Liver Weight of Mice Administered with CCl4
| Group | Body Weight (g) | Change in Body Weight | Liver Weight (g) | Relative Liver Weight (%) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| DW | 32.33 ± 1.17 | 34.41 ± 0.63 | 2.08 ± 0.61 | 2.46 ± 0.19 | 7.14 ± 0.52 |
| 2%TW80 | 32.50 ± 1.09 | 34.62 ± 0.87 | 2.12 ± 0.52 | 2.40 ± 0.07 | 6.94 ± 0.14 |
| CCl4 | 32.50 ± 0.89 | 29.34 ± 0.56a** | −3.16 ± 0.66a*** | 3.56 ± 0.21a** | 12.16 ± 0.76a*** |
| Silymarin + CCl4 | 32.00 ± 1.03 | 34.00 ± 0.89b* | 2.00 ± 0.37b*** | 2.62 ± 0.19b* | 7.70 ± 0.52b*** |
| BF100 + CCl4 | 33.00 ± 0.82 | 30.80 ± 0.84d* | −2.20 ± 0.37a***,c**,d**,e** | 3.36 ± 0.14a* | 10.89 ± 0.22a***,c**,d*,e* |
| BF200 + CCl4 | 33.05 ± 1.01 | 34.92 ± 0.90b** | 1.87 ± 0.48b*** | 2.91 ± 0.27 | 8.34 ± 0.76b*** |
| BF400 + CCl4 | 31.33 ± 0.67 | 33.27 ± 1.41 | 1.94 ± 1.20b*** | 2.78 ± 0.24 | 8.37 ± 0.66b*** |
| CF100 + CCl4 | 31.67 ± 1.02 | 29.37 ± 0.98a**,c* | −2.30 ± 0.32a***,c*** | 3.47 ± 0.29a* | 11.89 ± 1.09a**,c** |
| CF200 + CCl4 | 30.83 ± 0.79 | 28.61 ± 0.81a***,c** | −2.22 ± 0.16a***,c*** | 3.62 ± 0.32a* | 12.63 ± 0.98a***,c** |
| CF400 + CCl4 | 32.67 ± 0.99 | 31.52 ± 1.19 | −1.15 ± 0.30a***,b*,c* | 2.98 ± 0.28 | 9.41 ± 0.72 |
| AF100 + CCl4 | 32.00 ± 1.55 | 29.66 ± 1.70a* | −2.34 ± 0.4a***,c*** | 3.58 ± 0.34a* | 12.35 ± 1.58a**,c** |
| AF200 + CCl4 | 32.77 ± 1.20 | 30.29 ± 1.15 | −2.47 ± 0.33a***,c*** | 3.43 ± 0.25a* | 11.38 ± 0.83a* |
| AF400 + CCl4 | 32.83 ± 1.42 | 30.31 ± 1.32 | −2.52 ± 0.22a***,c*** | 3.65 ± 0.26a**,c* | 12.04 ± 0.68a**,c* |
Notes: Each value represents mean ± S.E.M; n=6; aAgainst negative control; bAgainst CCl4; cAgainst standard (silymarin); dAgainst 200 mg/kg; eAgainst 400 mg/kg; *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: BF, n-butanol fraction; CF, chloroform fraction; AF, aqueous fraction; silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2mL/kg; 2%TW80, 2% Tween 80 in water; DW, distilled water.
Besides, Table 3 also demonstrated the effect of chloroform and aqueous fractions on change in body weight, absolute and relative weight of liver of mice injected with CCl4. Mice pre-and post-treated with CF400 showed an increased change in body weight (p<0.05), whereas the other two doses of chloroform fraction and all doses of an aqueous fraction did not produce any detectable difference compared to toxicant controls.
Effect on Serum Biochemical Markers of Liver Injury
80% Methanol Extract
The hepatotoxic agent, CCl4, caused significant liver damage as indicated by an increase in the level of liver chemistry biomarkers such as; AST, ALT and ALP and a decrease in liver function biomarkers; total protein, albumin and bilirubin (Table 4). CCl4 induced elevation in AST by 146.8% (p<0.001), ALT by 135.7% (p<0.001), and ALP by 135% (p<0.001) (Table 4). Mice pre- and post-treated with crude 80% methanol extract of the leaves of Clutia abyssinica at 200 mg/kg and 400 mg/kg doses significantly reduced levels of AST, ALT and ALP (p<0.001) when compared with CCl4 administered controls. As compared to the lower doses, the higher one (400 mg/kg) demonstrated a better hepatoprotective activity. The decline in liver chemistry biomarkers in descending order was ALP (92%) > ALT (91.7%) > AST (90.4%) for 400 mg/kg. For the 200 mg/kg ALP and AST were decreased by 79.3%, whereas, ALT by 75.7%.
Table 4.
Effect of 80% Methanol Extract of the Leaves of Clutia abyssinica on Liver Function and Liver Chemistry of Mice Administered with CCl4
| Group | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Total Protein (g/dL) | Albumin (mg/dL) | Bilirubin (mg/dL) |
|---|---|---|---|---|---|---|
| 2%TW80 | 85.22 ± 2.53 | 94.02 ± 2.62 | 150.43 ± 3.61 | 5.11 ± 0.47 | 2.35 ± 0.14 | 1.18 ± 0.22 |
| CCl4 | 210.38 ± 8.17a*** | 221.57 ± 7.40a*** | 353.61 ± 8.48a*** | 3.21 ± 0.29a** | 1.28 ± 0.18a* | 3.83 ± 0.41a*** |
| Silymarin + CCl4 | 90.95 ± 2.85 (95.4)b*** | 96.29 ± 3.29 (98.2)b*** | 154.87 ± 4.32 (97.8)b*** | 5.08 ± 0.18b* | 2.32 ± 0.27b* | 1.21 ± 0.21b*** |
| Me100 + CCl4 | 198.82 ± 5.21 (9.2)a***,c***,d***,e*** | 210.71 ± 3.88 (8.5)a***,c***,d***,e*** | 336.03 ± 4.80 (8.7)a***,c***,d***,e*** | 3.78 ± 0.37 | 1.68 ± 0.23 | 3.79 ± 0.32a***,c***,d***,e*** |
| Me200 + CCl4 | 115.63 ± 4.29 (75.7)a**,b***,c* | 120.46 ± 2.36 (79.3)a**,b***,c** | 192.53 ± 3.69 (79.3)a***,b***,c***,e* | 4.86 ± 0.31b* | 2.23 ± 0.22 | 1.26 ± 0.34b*** |
| Me400 + CCl4 | 97.25 ± 3.23 (90.4)b*** | 104.57 ± 3.26 (91.7)b*** | 166.78 ± 4.16 (92)b***,d* | 5.06 ± 0.44b* | 2.28 ± 0.28b* | 1.23 ± 0.19b*** |
Notes: Each value represents mean ± S.E.M; n=6; aAgainst 2%TW80; bAgainst CCl4; cAgainst standard (silymarin); dAgainst Me200 + CCl4; eAgainst Me400 + CCl4; *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: Me100, 80% methanol extract 100 mg/kg; Me200, 80% methanol extract 200 mg/kg; Me400, 80% methanol extract 400 mg/kg; silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2mL/kg; 2%TW80, 2% Tween 80 in water.
Similarly, the synthetic and detoxification capability of the liver had increased significantly in 80% methanol extract pre-and post-treated mice (Table 4). Me200 and Me400 administered mice showed a significantly increased level of total protein (p<0.05), and a significantly decreased level of bilirubin (p<0.001). Me100 did not show a significant change in liver biochemical and function markers as compared to CCl4 administered controls.
Solvent Fractions
The fractions prepared produced differential effects on biomarkers of liver damage. Whilst the aqueous fraction failed to alter any of the biomarkers, the n-butanol and chloroform fractions produced a consistent reduction (Table 5).
Table 5.
Effect of Solvent Fractions of Clutia abyssinica Leaf Extract on Liver Function and Liver Chemistry of Mice Administered with CCl4
| Group | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | Total Protein (g/dL) | Albumin (mg/dL) | Bilirubin (mg/dL) |
|---|---|---|---|---|---|---|
| DW | 84.43 ± 3.45 | 93.62 ± 2.54 | 148.84 ± 2.66 | 5.17 ± 0.32 | 2.40 ± 0.18 | 1.14 ± 0.20 |
| 2%TW80 | 85.22 ± 2.53 | 94.02 ± 2.62 | 150.43 ± 3.61 | 5.11 ± 0.47 | 2.35 ± 0.14 | 1.18 ± 0.22 |
| CCl4 | 210.38 ± 8.17a*** | 221.57 ± 7.40a*** | 353.61 ± 8.48a*** | 3.21 ± 0.29a** | 1.28 ± 0.18a* | 3.83 ± 0.41a*** |
| Silymarin + CCl4 | 90.95 ± 2.85 (95.4)b*** | 96.29 ± 3.29 (98.2)b*** | 154.87 ± 4.32 (97.8)b*** | 5.08 ± 0.18b* | 2.32 ± 0.27b* | 1.21 ± 0.21b*** |
| BF100 + CCl4 | 202.74 ± 5.19 (6.1)a***,c***,d***,e*** | 214.53 ± 4.36 (5.5)a***,c***,d***,e*** | 342.54 ± 8.12 (5.4)a***,c***,d***,e*** | 3.69 ± 0.38 | 1.63 ± 0.36 | 3.86 ± 0.32a***,c***,d***,e*** |
| BF200 + CCl4 | 121.42 ± 4.17 (71.1)a***,b***,c** | 125.23 ± 3.96 (75.5)a***,b***,c*** | 200.82 ± 4.48 (75.2)a***,b***,c***,e* | 4.46 ± 0.50 | 2.14 ± 0.14 | 1.58 ± 0.28b*** |
| BF400 + CCl4 | 111.38 ± 2.75 (79.1)a**,b***,c* | 108.61 ± 2.57 (88.6)b*** | 172.63 ± 3.11 (89.1)b***,d* | 4.96 ± 0.48b* | 2.30 ± 0.37 | 1.22 ± 0.30b*** |
| CF100 + CCl4 | 207.06 ± 6.83 (2.7)a***,c***,e*** | 223.62 ± 5.46 (−1.6)a***,c***,e*** | 356.22 ± 7.14 (−1.3)a***,c***,e*** | 3.37 ± 0.42a*,c* | 1.52 ± 0.48 | 3.74 ± 0.51a***,c***,e** |
| CF200 + CCl4 | 197.87 ± 4.60 (10)a***,c***,e*** | 213.14 ± 4.51 (6.6)a***,c***,e*** | 340.34 ± 5.63 (6.5)a***,c***,e*** | 3.41 ± 0.45a* | 1.66 ± 0.47 | 3.43 ± 0.48a**,c**,e* |
| CF400 + CCl4 | 127.18 ± 3.25 (66.5)a***,b***,c***,d*** | 133.28 ± 3.47 (69.2)a***,b***,c***,d*** | 213.63 ± 3.92 (68.9)a***,b***,c***,d*** | 4.72 ± 0.43 | 2.18 ± 0.44 | 1.61 ± 0.19b**,d* |
| AF100 + CCl4 | 211.93 ± 4.53 (−1.2)a***,c*** | 222.47 ± 4.76 (−0.7)a***,c*** | 355.54 ± 7.93 (−0.9)a***,c*** | 3.18 ± 0.39a**,c** | 1.38 ± 0.34 | 3.77 ± 0.63a***,c***,e** |
| AF200 + CCl4 | 208.68 ± 4.12 (1.3)a***,c*** | 214.75 ± 3.87 (5.3)a***,c*** | 346.98 ± 6.43 (3.2)a***,c*** | 3.31 ± 0.29a**,c** | 1.42 ± 0.28 | 3.68 ± 0.42a***,c***,e** |
| AF400 + CCl4 | 202.19 ± 7.41 (6.5)a***,c*** | 207.37 ± 7.76 (11.1)a***,c*** | 343.83 ± 7.53 (4.8)a***,c*** | 3.25 ± 0.38a**,c** | 1.26 ± 0.32 |
|
Notes: Each value represents mean ± S.E.M; n=6; aAgainst negative control; bAgainst CCl4; cAgainst standard (silymarin); dAgainst 200 mg/kg; eAgainst 400 mg/kg; *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: BF, n-butanol fraction; CF, chloroform fraction; AF, aqueous fraction; silymarin, silymarin 100 mg/kg; CCl4, carbon tetrachloride 2mL/kg; 2%TW80, 2% Tween 80 in water; DW, distilled water.
Even though n-butanol fraction did not produce any detectable change in all biomarkers of liver injury at its lowest dose, it produced a significant decrease in serum ALP, ALT and AST levels by 89.1%, 88.6% and 79.1% in 400 mg/kg and 68.9, 69.2 and 66.5 in 200 mg/kg doses, respectively (p<0.001). In stark contrast to the n-butanol fraction, the chloroform fraction failed to produce any significant change in all biomarkers of liver damage at 100 mg/kg and 200 mg/kg doses. The higher dose, however, was able to significantly decrease ALT by 69.2%, ALP by 68.9% and AST by 66.5% (p<0.001) (Table 5). As it is presented in Table 5, the aqueous fraction failed to produce any significant change in all biomarkers of liver damage at all doses employed.
Radical Scavenging Activity
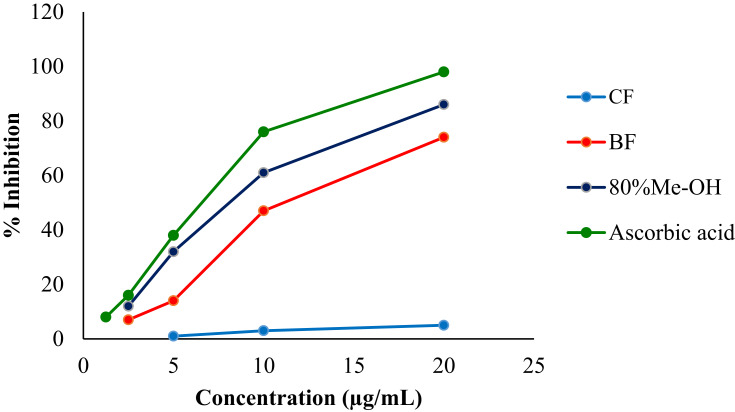
The 80% methanol extract of the leaves of Clutia abyssinica and its n-butanol and chloroform fractions were evaluated for DPPH scavenging activity and the data are presented in Figure 1. It was observed that both the crude hydromethanolic extract and n-butanol fraction showed a concentration-dependent inhibition of free radicals. The maximum percentage inhibition of DPPH by the crude methanol extract and n-butanol fraction were 86% and 74%, respectively, whist for the standard was 98% at a concentration of 20 μg/mL. The IC50 value for the crude 80% methanol extract, n-butanol fraction and ascorbic acid were calculated to be 9.9 μg/mL, 13.1 μg/mL and 8.3 μg/mL, respectively, whereas the chloroform fraction had a calculated IC50 value of 194.5 μg/mL (Figure 1).
Figure 1.
DPPH Scavenging activity of 80% methanol extract, n-butanol and chloroform fractions of the leaves of Clutia abyssinica.
Abbreviations: Ascorbic acid, standard antioxidant; BF, n-butanol fraction; CF, chloroform fraction; 80%Me-OH, 80% methanol crude extract.
Phytochemical Screening
The crude 80% methanol extract and solvent fractions of Clutia abyssinica leaves were tested for the composition of medicinally active compounds. The 80% methanol crude extract and n-butanol fraction were found to be positive for alkaloids, flavonoids, polyphenols, saponins, tannins, and terpenoids (Table 6). As it is presented in Table 6, alkaloids, flavonoids, polyphenols, saponins, and tannins were detected in chloroform fraction, whereas polyphenols, saponins, tannins, and terpenoids were only found in the aqueous fraction.
Table 6.
Phytochemical Screening of 80% Methanol Extract and Solvent Fractions of Clutia abyssinica Leaves
| Metabolites | 80% Methanol Extract | Solvent Fractions | ||
|---|---|---|---|---|
| Chloroform Fraction | n-Butanol Fraction | Aqueous Fraction | ||
| Alkaloids | + | + | + | – |
| Cardiac glycosides | – | – | – | – |
| Flavonoids | + | + | + | – |
| Polyphenols | + | + | + | + |
| Saponins | + | + | + | + |
| Steroids | – | – | – | – |
| Tannins | + | + | + | + |
| Terpenoids | + | – | + | + |
Notes: + = presence, − = absence.
The Effect of Crude Extract and Solvent Fractions on Histology of Liver
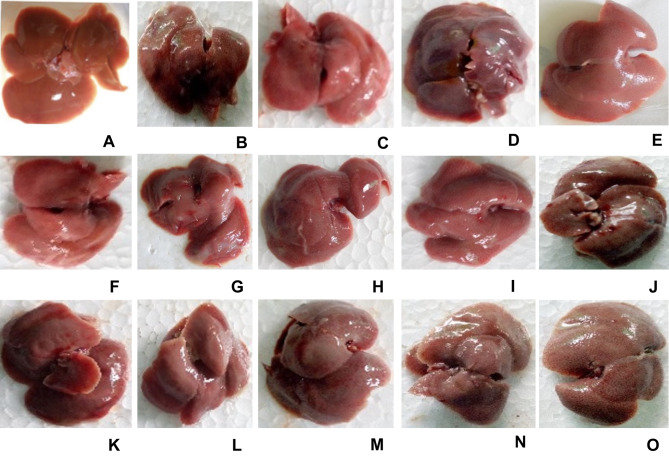
The morphological examination of mice liver tissue showed the visible darkened nodules, gross, and irregular surface suggesting the severe hepatocellular damage in CCl4-treated mice as compared to negative controls (Figure 2). As it is presented in Figure 2, pre- and post-treatment with crude 80% methanol extract and n-butanol fraction at the doses of 200 and 400 mg/kg, as well as silymarin at 100 mg/kg protected the liver from CCl4-induced damage.
Figure 2.
Photograph of liver of mice treated with 80% methanol extract and solvent fractions of Clutia abyssinica leaf.
Notes: (A) Negative control (received 2%TW80 only), (B) toxicant control (administered with CCl4 only), (C) positive control (silymarin + CCl4), (D) M100 + CCl4, (E) M200 + CCl4, (F) M400 + CCl4, (G) BF100 + CCl4, (H) BF200 + CCl4, (I) BF400 + CCl4, (J) CF100 + CCl4, (K) CF200 + CCl4, (L) CF400 + CCl4, (M) AF100 + CCl4, (N) AF200 + CCl4, (O) AF400 + CCl4.
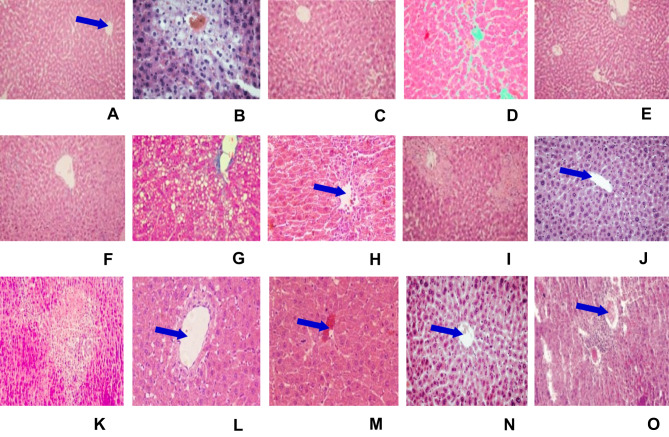
The hepatoprotective effect of Clutia abyssinica leaf extract and solvent fractions in CCl4-induced liver damage were further confirmed by histopathological examinations. Liver cells disarrangement, necrosis, hyperplasia, infiltration, and inflammation were observed in CCl4 administered mice (Figure 3B). Whilst mice pre- and post-treated with 80% methanol extract and n-butanol fraction showed signs of protection in the doses of 200 mg/kg and 400 mg/kg, which was shown as reduction/absence of inflammatory cells, vascular congestion, cellular degeneration, necrosis, and vacuoles (Figure 3E, F, H, and I, respectively). In contrast, the lowest doses of 80% methanol extract, n-butanol fraction and all employed doses of aqueous fraction were failed to protect the liver of mice from CCl4-induced injury (Figure 3D, G, M–O, respectively). The chloroform fraction showed a protective effect at the dose of 400 mg/kg (Figure 3L) but failed to protect CCl4-induced liver damage in the other doses administered (Figure 3J and K). Normal histological structures were observed in the liver of mice treated with silymarin (100 mg/kg) and 2%TW80 (Figure 3A and C, respectively).
Figure 3.
Microphotograph of hematoxylin and eosin (H & E)-stained sections of liver. Micrometer glass slides were prepared in 50 µm intervals.
Notes: (A) Negative control (received 2%TW80 only), (B) toxicant control (administered with CCl4 only), (C) positive control (silymarin + CCl4), (D) M100 + CCl4, (E) M200 + CCl4, (F) M400 + CCl4, (G) BF100 + CCl4, (H) BF200 + CCl4, (I) BF400 + CCl4, (J) CF100 + CCl4, (K) CF200 + CCl4, (L) CF400 + CCl4, (M) AF100 + CCl4, (N) AF200 + CCl4, (O) AF400 + CCl4. The blue arrow indicates the central vein. Microphotograph of H & E-stained section of liver from a negative control mouse showing normal histological structure (A, ×10). CCl4-treated mouse liver showing necrosis, infiltration, vacuolization, and disarrangement of hepatocytes (B, ×10). The liver section of the mouse administered crude leaf extract showing nearly normal appearance of hepatocytes (F, ×10), whereas, mice pre- and post-treated with n-butanol fraction showed mild degree of liver damage and inflammatory cell, protection from hepatocyte degradation and centrilobular necrosis (H and I, ×10). Silymarin-treated mouse liver showing normal appearance of hepatocytes (C,×10).
Discussion
The liver has an indispensable role in life via its endowed metabolic and detoxification capability.32 As it is exposed to several endogenous and xenobiotic agents, a myriad of intermediate and end products are produced and can cause hepatocellular death and constitute the principal causes of liver disease.33
To ensure the survival of an individual and maintain the function of the liver, the conventional treatment focus on symptom management and liver transplantation in severe cases of liver disease.34 But, there are no drugs currently in use to increase the detoxification power of the organ. Therefore, testing and the use of botanical hepatoprotective agents are substantially increasing. So it would be highly imperative to demonstrate the effectiveness of the plant extracts in the presence of chemical-induced hepatotoxicity. CCl4, a potent hepatotoxic agent, is the most widely used criterion for evaluating the hepatoprotective activity of plant extracts.35 Mice were treated with Clutia abyssinica leaf extract and different solvent fractions pre- and post-administration of CCl4. Several studies indicate that CCl4 can produce centrizonal hemorrhagic hepatic necrosis in human and experimental animals.36 Thus, in this study, mice administered with CCl4 resulted in the increased liver weight of mice via the development of infiltration, vacuolization, and inflammation in the liver (Figure 3B). Hence, the weights of the mice were decreased and their liver weights were increased (Table 2). On the other hand, mice pre- and post-treated with 80% methanol extract and n-butanol fraction showed no significant difference in body weight, both absolute and relative liver weight of mice as compared to the negative control (Table 2). CCl4-induced hepatotoxicity is used to evaluate the hepatoprotective potential of plant extracts in several animal models. CCl4 is reductively bioactivated by cytochrome P450 2E1 into highly unstable reactive free radicals; trichloromethyl radical and trichloromethyl peroxyl radical.37,38 These may cause cellular damage via peroxidation of membrane lipids and covalently bind with other macromolecules within hepatocytes. Membrane damage results in the release of both cytosolic and endoplasmic enzymes, which show the presence of damage in liver structure and function.39 These are manifested as elevation in the levels of AST, ALT, and ALP.40 So, measuring the levels of these biomarkers of liver damage can reveal the hepatoprotective activity of the plant extract and solvent fractions.41
In the present study, the 80% methanol extract showed a reduction in the levels of AST, ALT, and ALP in a dose-dependent manner. The 80% methanol extract did not produce a visible effect in all biomarkers of hepatic injury in its lower dose, but medium and higher doses were able to produce a significant reduction in the levels of AST, ALT, and ALP (Table 4). This could probably suggest that the lower dose might be below the minimum effective dose, which cannot elicit a significant reduction in liver enzyme levels and the other two doses might be large enough to cause a significant reduction. The percent reduction of biomarkers of liver injury showed that 200 mg/kg and 400 mg/kg of the hydromethanolic extract exerted a nearly similar effect as that of the standard (Table 4). Pre- and post-treatment with 80% methanol extract at the two doses (200 mg/kg and 400 mg/kg) except for the dose of 100 mg/kg, largely modulated the severity of CCl4-induced liver damage. Enzyme levels’ return to near-normal levels in 80% methanol pre- and post-treated mice shows that 80% methanol extract can stabilize liver cell membranes and prevent the leakage of enzymes. Preventing the production of free radicals and neutralizing them as well as the protection potential of this plant against hepatotoxins can be other probable reasons for the healing effect of Clutia abyssinica leaf extract.
Liver is the main site of protein synthesis, especially albumin and detoxification of bilirubin as well.32 In this study, the levels of total protein, albumin, and bilirubin were used to assess liver synthetic and detoxification capability. Increased levels of total protein and albumin and decreased levels of bilirubin were observed in 200 mg/kg and 400 mg/kg methanol extract pre-and post-treated mice (Table 4). This indicates that methanol extract can prevent the decline in the protein synthesis capacity of the liver and increase the detoxification power of the liver probably through stabilizing endoplasmic reticulum and resynthesizing protein or through neutralizing reactive free radicals by scavenger compounds and regeneration of liver architecture.42
To concentrate or separate the active principles, fractionation of the crude 80% methanol extract was done by using solvents of different polarities. This study showed that the aqueous fraction did not show any detectable change in the biomarkers of liver injury at all doses employed (Table 5). This suggests that most of the polar components of the leaf of the plant might be devoid of any hepatoprotective activity. In contrast, the chloroform fraction produced a significant reduction in serum biomarkers of liver injury at 400 mg/kg. This is possibly due to the increased concentration of active components in the larger dose and could indicate that less polar components of the plant might have hepatoprotective activity with increasing concentration. Although BF100 was unable to produce a detectable change in the levels of all biomarkers, BF200 and BF400 produced a significant reduction in the levels of AST, ALT, and ALP with increased dose. This could be surmised from the percent reduction in the levels of ALP, ALT and AST for the two doses, where BF400 displayed a better activity than B200 (89.1%, 88.6% and 79.1% vs 68.9%, 69.2%, and 66.5%), respectively (Table 5). It is also of note that CF400 produced a reduction in the levels of biomarkers, which were lower than that of B200 and B400. This collectively suggests that ingredients of the plant responsible for hepatoprotective effect probably are semi-polar and better fractionated by n-butanol than the other solvents used. These biochemical effects of the crude 80% methanol extract and n-butanol fraction of the leaves of Clutia abyssinica were supported by the results of histopathological examination, as evidenced by a decrease in the incidence and severity of histopathological hepatic lesions, necrosis, infiltration, and vacuolization (Figure 3F and I, respectively).
The active principle(s) responsible for the hepatoprotective activity of the 80% methanol extract and solvent fractions of Clutia abyssinica is/are, so far, not known, so it is not identified which compounds are exactly responsible for the antioxidant and hepatoprotective activities. Previous studies showed that alkaloids and flavonoids were found to have antioxidant activity.43,44 Preliminary phytochemical analysis was done on the 80% methanol crude extract and solvent fractions revealed a variety of secondary metabolites that appeared to be differentially distributed across the extract and fractions (Table 6). It is reasonable to suggest that the phytochemicals shown in Table 6 may act individually or synergistically to produce the observed hepatoprotective activity of Clutia abyssinica. Possibly, flavonoids and alkaloids present in the crude leaf extract and n-butanol fraction exerted hepatoprotective effect by their free radical scavenging activity, prevention of lipid peroxidation and damage to cells as such an action has been suggested for some other plants.45 Besides, alkaloids and flavonoids are known as natural antioxidants by their free radical scavenging activity.46–48
As the liver is continuously exposed to oxidative stress, the release of free radicals is the main hepatotoxicity mechanism of toxicants. In oxidative stress, the balance between the formation of reactive oxygen species and the amount of antioxidants is disturbed. Oxidative stress causes damage to cell components, such as proteins, lipids and nucleic acids.49–51 To confirm the antioxidant activity of the plant extract, in vitro DPPH radical scavenging assay was carried out. In this free radical scavenging assay, 80% methanol extract and n-butanol fraction of the leaves of Clutia abyssinica were observed to inhibit with the maximum value of 86% and 74% at the concentration of 20 µg/mL (Figure 1). The crude methanol extract of Clutia abyssinica had a calculated IC50 value of 9.9 μg/mL, which is nearly similar to the calculated IC50 value of the known antioxidant, ascorbic acid, ie 8.3 μg/mL. As it is explained for other plants,45,52,53 the 80% methanol extract and n-butanol fraction might act via their free radical scavenging, neutralization of free radicals and inhibition of necrosis via several pathways.
To sum up, this study provided further evidence that the 80% methanol extract, as well as the n-butanol fraction, possessed a comparable hepatoprotective activity with that of the standard drug. Results obtained from the solvent fractions revealed that there was a dose-dependent reduction in all biomarkers of liver injury in pre- and post-treatment of n-butanol fraction. Therefore, this data seems to indicate that the hepatoprotective effect of the plant is distributed to semi-polar bioactive principles contained in the n-butanol fraction. Even though the hepatoprotective mechanism of the plant extract is yet not elucidated, the observed antioxidant activity is one of the anticipated mechanisms. Above all, the 80% methanol extract and solvent fractions of the leaves of Clutia abyssinica would be rewarded as safe based on the results of acute oral toxicity study. Moreover, isolation and characterization of novel antioxidants will be done in future studies by using HPLC/LC-MS techniques.
Conclusion
The results of serum biochemical markers and histopathological studies in the crude 80% methanol extract and n-butanol fraction pre- and post-treated group support the hepatoprotective effect and provide evidence for the traditional use of Clutia abyssinica for treatment of liver disorders. The larger doses of both the crude 80% methanol leaf extract and n-butanol fraction produced a remarkable hepatoprotective activity, which was comparable to silymarin. The presence of natural antioxidants in the 80% methanol extract and n-butanol fraction may explain the observed hepatoprotective and in vitro antioxidant activities. These suggest that synergy created between the antioxidant activity and intrinsic protective effects of the plant extract underlie attenuation of CCl4-induced liver injury.
Acknowledgment
Wollo University is greatly acknowledged.
Abbreviations
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CCl4, carbon tetrachloride.
Ethics Approval and Consent to Participate
The study was approved by the Ethical Review Board of College of Medicine and Health Sciences of Wollo University.
Author Contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bijekar SR, Gayatri M. Ethanomedicinal properties of Euphorbiaceae family-a comprehensive review. Int J Phytomed. 2014;6(2):144–156. [Google Scholar]
- 2.Kipkore W, Wanjohi B, Rono H, Kigen G. A study of the medicinal plants used by the Marakwet Community in Kenya. J Ethnobiol Ethnomed. 2014;10(1):22. doi: 10.1186/1746-4269-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche District, Central Ethiopia. Curr Res J Biol Sci. 2014;6(4):154–167. doi: 10.19026/crjbs.6.5515 [DOI] [Google Scholar]
- 4.Matu EN. Clutia abyssinica Jaub. & Spach In: Schmelzer GH, Gurib-Fakim A, editors. PROTA (Plant Resources of Tropical Africa/Ressources Végétales De l’Afrique Tropicale). Wageningen, Netherlands; 2008. Available from: https://uses.plantnet-project.org/en/Clutia_abyssinica_(PROTA). Accessed January 2020. [Google Scholar]
- 5.de Boer HJ, Kool A, Broberg A, Mziray WR, Hedberg I, Levenfors JJ. Anti-fungal and anti-bacterial activity of some herbal remedies from Tanzania. J Ethnopharmacol. 2005;96(3):461–469. doi: 10.1016/j.jep.2004.09.035 [DOI] [PubMed] [Google Scholar]
- 6.Kigen G, Some F, Kibosia J. Ethnomedicinal plants traditionally used by the keiyo community in Elgeyo Marakwet County, Kenya. J Biodivers Biopros Dev. 2014;1:3. [Google Scholar]
- 7.Andemariam SW. Legislative Regulation of Traditional Medicinal Knowledge in Eritrea via-a-vis Eritrea’s Commitments under the Convention on Biological Diversity: issues and Alternatives. Law Env’t Dev J. 2010;6:130. [Google Scholar]
- 8.Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):65. doi: 10.1186/1746-4269-9-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teklay A. Traditional medicinal plants for ethnoveterinary medicine used in Kilte Awulaelo district, Tigray region, Northern Ethiopia. Adv Med Plant Res. 2015;3(4):137–150. [Google Scholar]
- 10.Mekuanent T, Zebene A, Solomon Z. Ethnobotanical study of medicinal plants in Chilga district, Northwestern Ethiopia. J Nat Remedies. 2015;15(2):88–112. doi: 10.18311/jnr/2015/476 [DOI] [Google Scholar]
- 11.Mukazayire M-J, Minani V, Ruffo CK, Bizuru E, Stévigny C, Duez P. Traditional phytotherapy remedies used in Southern Rwanda for the treatment of liver diseases. J Ethnopharmacol. 2011;138(2):415–431. doi: 10.1016/j.jep.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 12.Pascaline J, Charles M, Lukhoba C, George O. Phytochemical constituents of some medicinal plants used by the Nandis of South Nandi district, Kenya. J Anim Plant Sci. 2011;9(3):1201–1210. [Google Scholar]
- 13.Muthaura C, Rukunga G, Chhabra S, et al. Antimalarial activity of some plants traditionally used in Meru district of Kenya. Phytother Res. 2007;21(9):860–867. doi: 10.1002/ptr.2170 [DOI] [PubMed] [Google Scholar]
- 14.Feyera T, Terefe G, Shibeshi W. Evaluation of in vivo antitrypanosomal activity of crude extracts of Artemisia abyssinica against a Trypanosoma congolense isolate. BMC Complement Altern Med. 2014;14(1):117. doi: 10.1186/1472-6882-14-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koech S, Ouko R, Michael N, Ireri M, Ngugi M, Njagi N. Analgesic activity of dichloromethanolic root extract of Clutia abyssinica in swiss albino mice. Nat Prod Chem Res. 2017;5(255):2. [Google Scholar]
- 16.Koech S, Maoga J, Sindani A, Ireri M, Mwonjoria J. Anti-inflammatory activity of dichloromethanolic root extract of Clutia abyssinica in Swiss Albino Mice. J Pharmacogn Nat Prod. 2017;3(132):615–634. [Google Scholar]
- 17.Koech S, Sindani A, Maoga J, Ouko R, Njagi N. Anti-pyretic potential of dichloromethanolic root extract of Clutia abyssinica in Wistar Albino Rats. Med Aromat Plants. 2017;6(281):2167–0412.1000281. [Google Scholar]
- 18.Tegegne A, Mishra B, Geta M. Evaluation of in vivo diuretic activity of methanolic extracts of Clutia Abyssinica (Euphorbiaceae) Roots in Wistar Albino Rats. Int Ann Med. 2017;1:9. doi: 10.24087/IAM.2017.1.8.215 [DOI] [Google Scholar]
- 19.Tauchen J, Doskocil I, Caffi C, et al. In vitro antioxidant and anti-proliferative activity of Ethiopian medicinal plant extracts. Ind Crops Prod. 2015;74:671–679. doi: 10.1016/j.indcrop.2015.05.068 [DOI] [Google Scholar]
- 20.Waigh RD, Zerihun BM, Maitland DJ. Ten 5-methylcoumarins from Clutia abyssinica. Phytochemistry. 1991;30(1):333–335. doi: 10.1016/0031-9422(91)84149-M [DOI] [Google Scholar]
- 21.Guideline OO. 425: acute oral toxicity—up-and-down procedure. OECD Guidelines Test Chem. 2001;2:12–16. [Google Scholar]
- 22.Sintayehu B, Bucar F, Veeresham C, Asres K. Hepatoprotective and free radical scavenging activities of extracts and a major compound isolated from the leaves of Cineraria abyssinica Sch. Bip. exA. Rich. J Pharmacogn. 2012;4(29):40–46. doi: 10.5530/pj.2012.29.6 [DOI] [Google Scholar]
- 23.Esatu H, Alemayehu I, Haile E, et al. Phenolic glycosides from roots of Clerodendrum myricoides. Am J Essent Oils Nat Prod. 2015;3(1):01–6. [Google Scholar]
- 24.Hossain MA, AL-Raqmi KAS, AL-Mijizy ZH, Weli AM, Al-Riyami Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3(9):705–710. doi: 10.1016/S2221-1691(13)60142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106. [Google Scholar]
- 26.Rohini MV, Padmini E. Preliminary phytochemical screening of selected medicinal plants of polyherbal formulation. J Pharmacogn Phytochem. 2016;5(5):277. [Google Scholar]
- 27.Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J. 2017;2017:1–7. doi: 10.1155/2017/5873648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahu RK, Kar M, Routray R. DPPH free radical scavenging activity of some leafy vegetables used by tribals of odisha, India. J Med Plants. 2013;1(4):21–27. [Google Scholar]
- 29.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199. doi: 10.1038/1811199a0 [DOI] [Google Scholar]
- 30.Vogel HG, Vogel WH. Drug Discovery and Evaluation: Pharmacological Assays. Springer Science & Business Media; 2013. [Google Scholar]
- 31.National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2010. Available from: https://grants.nih.gov/grants/…/guide-for-the-care-and-use-of-laboratory-animals.pdf. Accessed January 2020. [Google Scholar]
- 32.Muriel P. Liver Pathophysiology: Therapies and Antioxidants. Academic Press; 2017. [Google Scholar]
- 33.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349(5):474–485. doi: 10.1056/NEJMra021844 [DOI] [PubMed] [Google Scholar]
- 34.Lampertico P, Agarwal K, Berg T EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 35.Pritchard MT, Apte U. Models to Study Liver Regeneration. Liver Regeneration. Elsevier; 2015:15–40. [Google Scholar]
- 36.De S, Suresh R, Babu AMSS, Aneela S. In-vivo hepatoprotective activity of methanolic extracts of Sphaeranthus amaranthoides and Oldenlandia umbellate. J Pharmacogn. 2017;9(1). [Google Scholar]
- 37.Jeong HG. Inhibition of cytochrome P450 2E1 expression by oleanolic acid: hepatoprotective effects against carbon tetrachloride-induced hepatic injury. Toxicol Lett. 1999;105(3):215–222. doi: 10.1016/S0378-4274(99)00004-1 [DOI] [PubMed] [Google Scholar]
- 38.Connor HD, Thurman R, Galizi M, Mason R. The formation of a novel free radical metabolite from CCl4 in the perfused rat liver and in vivo. J Biol Chem. 1986;261(10):4542–4548. [PubMed] [Google Scholar]
- 39.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134(6):1641–1654. doi: 10.1053/j.gastro.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug‐induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59(2):661–670. doi: 10.1002/hep.26709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanafy A, Aldawsari HM, Badr JM, Ibrahim AK, Abdel-Hady -SE-S. Evaluation of hepatoprotective activity of Adansonia digitata extract on acetaminophen-induced hepatotoxicity in Rats. Evid Based Complement Alternat Med. 2016;2016:1–7. doi: 10.1155/2016/4579149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elaut G, Henkens T, Papeleu P, et al. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr Drug Metab. 2006;7(6):629–660. doi: 10.2174/138920006778017759 [DOI] [PubMed] [Google Scholar]
- 43.Singab ANB, Youssef DT, Noaman E, Kotb S. Hepatoprotective effect of flavonol glycosides rich fraction from egyptianVicia calcarata desf. against CCI 4-induced liver damage in rats. Arch Pharm Res. 2005;28(7):791–798. doi: 10.1007/BF02977344 [DOI] [PubMed] [Google Scholar]
- 44.Pietta P-G. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035–1042. doi: 10.1021/np9904509 [DOI] [PubMed] [Google Scholar]
- 45.Cheedella HK, Alluri R, Ghanta KM. Hepatoprotective and antioxidant effect of Ecbolium viride (Forssk.) Alston roots against paracetamol-induced hepatotoxicity in albino wistar rats. J Pharm Res. 2013;7(6):496–501. doi: 10.1016/j.jopr.2013.06.001 [DOI] [Google Scholar]
- 46.Mohammadi-Motamed S, Shahidi-Motlagh S, Bagherzadeh H, Azad Forouz S, Tafazoli H. Evaluation of antioxidant activity of Ruta graveolens L. extract on inhibition of lipid peroxidation and DPPH radicals and the effects of some external factors on plant extract’s potency. Res J Pharmacogn. 2014;1(1):45–50. [Google Scholar]
- 47.Medpilwar M, Maru D, Upadhyay M, Lavania N, Vernekar M, Harmalkar MN. In-vitro antioxidant and anti-lipid peroxidation activity of ethanolic extracts of bougainvillea shubhra, bougainvillea peruviana and bougainvillea bhuttiana golden glow: a comparative study. J Nat Remedies. 2015;15(1):43–48. doi: 10.18311/jnr/2015/475 [DOI] [Google Scholar]
- 48.Brunetti C, Di Ferdinando M, Fini A, Pollastri S, Tattini M. Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci. 2013;14(2):3540–3555. doi: 10.3390/ijms14023540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spahis S, Delvin E, Borys J-M, Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid Redox Signal. 2017;26(10):519–541. doi: 10.1089/ars.2016.6776 [DOI] [PubMed] [Google Scholar]
- 50.Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082. doi: 10.3748/wjg.v20.i25.8082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7(7–8):1040–1052. doi: 10.1089/ars.2005.7.1040 [DOI] [PubMed] [Google Scholar]
- 52.Taleb A, Ahmad KA, Ihsan AU, et al. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed Pharmacother. 2018;102:689–698. doi: 10.1016/j.biopha.2018.03.140 [DOI] [PubMed] [Google Scholar]
- 53.Vargas-Mendoza N, Madrigal-Santillán E, Morales-González Á, et al. Hepatoprotective effect of silymarin. World J Hepatol. 2014;6(3):144. doi: 10.4254/wjh.v6.i3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]