Highlights
-
•
Chloramphenicol resistant strains of Neisseria meningitidis are present in three countries across Southeast Asia.
-
•
These strains are all closely related to each other and to resistant strains previously observed in Vietnam and France.
-
•
This lineage has acquired other antimicrobial resistance genes during its spread.
Keywords: Neisseria meningitidis, Genomics, Antimicrobial resistance
Abstract
Objectives
Invasive disease caused by Neisseria meningitidis is a significant health concern globally, but our knowledge of the prevailing serogroups, antimicrobial susceptibility patterns, and genetics of N. meningitidis in Southeast Asia is limited. Chloramphenicol resistance in N. meningitidis has rarely been reported, but was first described in isolates from Vietnam in 1998. We aimed to characterise eight chloramphenicol resistant meningococcal isolates collected between 2007 and 2018 from diagnostic microbiology laboratories in Cambodia, Thailand and the Lao People's Democratic Republic (Laos).
Methods
Whole-genome sequencing was used to generate genome sequences from 18 meningococcal isolates including the eight chloramphenicol resistant isolates. We identified antimicrobial resistance genes present in these strains, and examined the phylogenetic relationships between strains.
Results
The eight resistant strains all contain the same chloramphenicol resistance gene first described in 1998, and are closely related to each other. Strains resistant to penicillin, tetracycline, and ciprofloxacin were also observed, including a chloramphenicol-resistant strain which has acquired penicillin and ciprofloxacin resistance.
Conclusions
This study suggests that chloramphenicol-resistant N. meningitidis is more widespread than previously thought, and that the previously-identified resistant lineage is now found in multiple countries in Southeast Asia.
Introduction
Invasive disease caused by Neisseria meningitidis is an important global health concern, being associated with significant rates of mortality and long-term sequelae (Vyse et al., 2013). The burden of meningococcal disease in Southeast Asia is poorly described, with few data on prevailing serogroups and antimicrobial susceptibility patterns (Vyse et al., 2011). The Mahidol-Oxford Tropical Health Network (MORU) includes diagnostic microbiology laboratories in Thailand, Laos and Cambodia, where N. meningitidis is an infrequently isolated pathogen. Detection of a chloramphenicol-resistant strain of N. meningitidis at our laboratory site in Laos in November 2017 prompted a review of published reports of chloramphenicol resistance in N. meningitidis and review of chloramphenicol susceptibility amongst meningococcal isolates in our laboratory network. Although no longer standard therapy for meningococcal meningitis, chloramphenicol is commonly used in beta-lactam intolerant patients and is recommended as an alternative agent in international guidelines for treatment of meningococcal meningitis (Tunkel et al., 2004, McGill et al., 2016), and therefore resistance to this agent remains an issue of clinical importance.
Chloramphenicol resistance in N. meningitidis has rarely been reported, first being described in 1998 in 11 isolates from Vietnam and one isolate from France, due to the presence of a chloramphenicol transferase gene (catP) possibly derived from a Clostridium perfringens transposable element (Galimand et al., 1998). Two further isolates with the same catP insertion were later described in Australia (Shultz et al., 2003) amongst 1382 isolates. More recently, chloramphenicol resistance was reported in one of 2888 isolates in Brazil (Gorla et al., 2018), and a further highly resistant isolate was found in Vietnam in 2014 (Tran et al., 2019). Despite historical use of single-dose oily chloramphenicol for epidemic management of meningococcal meningitis (Fuller et al., 2003), we could find no reports of chloramphenicol resistance from Africa, although susceptibility information on African isolates is limited (Hedberg et al., 2009).
We identified chloramphenicol resistance in eight of 25 isolates of N. meningitidis in our network laboratories over an 11 year time period. Given the seemingly high frequency of chloramphenicol resistance amongst our N. meningitidis isolates, we aimed to characterise the genetic basis of this using whole genome sequencing. In addition, due to the scarcity of meningococcal data from Southeast Asia, we report serogroup data and phenotypic and genotypic susceptibilities to other routinely tested antimicrobial agents in 18 of these isolates that were available for sequencing.
Methods
Study sites
Clinical isolates from three laboratories were included. The Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Laos provides diagnostic microbiology services to Mahosot Hospital as well as other hospitals in Vientiane and other provincial sites. The Microbiology Laboratory of the Shoklo Malaria Research Unit (SMRU), Mae Sot, Thailand supports healthcare provision to marginalised populations on the Thailand-Myanmar border. The Cambodia-Oxford Medical Research Unit (COMRU) provides diagnostic microbiology services to the Angkor Hospital for Children, a paediatric teaching hospital in Siem Reap, Cambodia. All laboratories routinely store clinically significant bacterial isolates at -80 °C.
Sample selection, organism identification and phenotypic antimicrobial susceptibility testing
Laboratory records were reviewed for all clinical isolates of N. meningitidis up until 31st March 2018, with data available from year 2000 in LOMWRU, 2007 in SMRU and from 2013 in COMRU. A total of thirty-four isolates were identified (9 from LOMWRU, 18 from SMRU, 7 from COMRU), of which 25 had susceptibility data for chloramphenicol and were stored and viable. N. meningitidis isolates were originally identified by API NH (bioMérieux, France) and had undergone antimicrobial susceptibility testing (AST) by disk diffusion for chloramphenicol, ciprofloxacin and ceftriaxone, and Etest (bioMérieux, France) for penicillin, according to Clinical and Laboratory Standards Institute (CLSI) standards and breakpoints. Before genotypic characterisation, isolates were subcultured on to chocolate agar from storage at −80 °C and underwent confirmation of identity by MALDI-TOF (Vitek MS, bioMérieux) and AST repeated by Etest (bioMérieux, France) according to manufacturer's instructions and CLSI 2018 standards (Wayne, PA, Clinical and Laboratory Standards Institute 2018). Two isolates failed to confirm as N. meningitidis, and five were removed as duplicate isolates from patients in whom N. meningitidis was isolated from multiple sites, leaving 18 isolates for analysis.
Patient-related metadata
Demographic data, clinical syndrome, antibiotic treatment and status on discharge were obtained from laboratory records and patient charts where available.
Genotypic characterization
Sequencing was performed at the MORU laboratories in Bangkok, Thailand. Libraries were made from extracted DNA using the Illumina Nextera XT library preparation kit (Illumina, San Diego, CA, USA) and quantified on an Agilent BioAnalyser (Agilent Technologies, Santa Clara, CA, USA). The pooled libraries were sequenced on one run of an Illumina MiSeq sequencer using the v2 reagent kit to give 250 bp paired-end reads. The genome assembly of chloramphenicol-resistant strain DuyDNT from Vietnam was obtained from Genbank under accession RPSF00000000.
Spades v3.11.1 (Bankevich et al., 2012) was used to assemble genomes from whole-genome sequencing. Abricate v0.8.10 (Seemann, 2018a) was used to look for acquired resistance genes using the bundled ResFinder database (updated 2018-10-02) (Zankari et al., 2012). BLAST was used to search for specific antibiotic resistance genes. Prokka 1.13.3 was used to annotate the assembled genomes.
In silico serogrouping of the isolates was performed by retrieving the sequences of the genes in the capsule synthesis region of the capsule that are unique to each serogroup (csb, csc, csw and csy) for the B, C, W and Y serogroups respectively, and determining their presence or absence using BLAST to search the genome assemblies.
Snippy v4.3.6 (Seemann, 2018b) was used to call SNPs against the MC58 reference genome from raw sequence data and from the assembled DuyDNT genome. Initial phylogenetic trees were generated from the core SNP fasta files using IQ-TREE (Nguyen et al., 2015) under the GTR + F + ASC + R3model which was the best fit using BIC implemented in ModelFinder (Kalyaanamoorthy et al., 2017). The initial tree was used as input to Gubbins v2.3.1 (Croucher et al., 2015) to generate a phylogeny accounting for recombination.
Results
Phenotypic characteristics and patient-related data
Eighteen isolates of N. meningitidis (11 from Thailand, 5 from Cambodia, 2 from Laos) from 18 patients were included in the analysis (Table 1). Eight of 18 (44.4%) meningococcal isolates were resistant to chloramphenicol. Four of the resistant isolates were collected from Thailand, and two each from Cambodia and Laos. All isolates were susceptible to ceftriaxone, but 10/18 (55.5%) isolates had reduced susceptibility to penicillin, including one isolate which was resistant according to CLSI criteria (Table 2). Phenotypic ciprofloxacin resistance was detected in two isolates.
Table 1.
Clinical information on the samples.
| Patient | Sample ID | Unit | Specimen date | Sample type | Age (years) | Sex | Diagnosis | Antibiotic therapy | Status at discharge |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NM01 | SMRU | 25/7/07 | CSF | 7 | M | Meningitis | Unknown | Unknown |
| 2 | NM03 | SMRU | 25/11/09 | BC | 1 | F | Sepsis | Ceftriaxone | Well |
| 3 | NM04 | SMRU | 15/11/10 | BC | <1 | M | Meningitis | Ceftriaxone | Well |
| 4 | NM06 | SMRU | 19/6/11 | BC | 0.2 | M | Meningitis | Unknown | Unknown |
| 5 | NM07 | SMRU | 4/10/11 | BC | 0.5 | M | Meningitis | Ceftriaxone | Well |
| 6 | NM09 | SMRU | 7/1/12 | BC | 0.2 | M | Meningitis | Ceftriaxone | Well |
| 7 | NM11 | SMRU | 18/1/12 | BC | 3 | M | Meningitis | Ceftriaxone | Well |
| 8 | NM12 | SMRU | 28/12/12 | BC | 0.3 | F | Sepsis | Ceftriaxone | Well |
| 9 | NM13 | SMRU | 13/5/13 | BC | 0.6 | M | Sepsis | Ceftriaxone | Well |
| 10 | NM14 | SMRU | 15/2/14 | BC | 0.3 | F | Sepsis | Ceftriaxone | Well |
| 11 | NM15 | SMRU | 19/12/14 | BC | 0.3 | M | Sepsis | Unknown | Well |
| 12 | NM16 | LOMWRU | 10/11/17 | CSF | 60 | F | Meningitis | Ceftriaxone | Well |
| 13 | NM18 | COMRU | 13/10/13 | BC | 0.14 | F | Meningitis/Sepsis | Ceftriaxone | Well |
| 14 | NM19 | COMRU | 25/4/14 | BC | 1.84 | F | Sepsis | Ceftriaxone | Well |
| 15 | NM20 | COMRU | 31/3/15 | BC | 0.91 | F | Pneumonia/Sepsis | Ceftriaxone | Well |
| 16 | NM21 | COMRU | 24/7/16 | BC | 0.07 | M | Meningitis/Sepsis | Unknown | Well |
| 17 | NM23 | COMRU | 22/8/17 | BC | 0.33 | F | Meningitis | Ceftriaxone | Well |
| 18 | NM25 | LOMWRU | 21/3/18 | BC | 0.25 | M | Meningitis | Ceftriaxone | Well |
SMRU, Shoklo Malaria Research Unit, Mae Sot, Thailand; LOMWRU, Lao-Oxford-Mahosot Hospital Wellcome Trust Research Unit, Vientiane, Laos; COMRU, Cambodia Oxford Medical Research Unit, Siem Reap, Cambodia.
CSF, cerebrospinal fluid; BC, blood culture.
Table 2.
Antibiotic resistance and genetic data on the samples. Minimum inhibitory concentration for each antibiotic is shown in brackets.
| Sample ID | Phenotypic antibiotic susceptibility |
Genotypic antibiotic resistance | PEN phenotype/genotype discordance | Serogroup | MLST | Clonal complex | |||
|---|---|---|---|---|---|---|---|---|---|
| CHL | CIP | CRO | PEN | ||||||
| NM01 | R (128) | S (0.004) | S (<0.016) | I (0.125) | CHL, PEN | B | 1487 | ||
| NM03 | S (1) | S (0.004) | S (<0.016) | I (0.25) | PEN | B | 14488 | ST-41/44 complex | |
| NM04 | S (2) | S (0.004) | S (<0.016) | S (0.064) | B | 14489 | |||
| NM06 | S (2) | S (0.004) | S (<0.016) | S (0.064) | B | 32 | ST-32 complex | ||
| NM07 | S (1) | R (0.125) | S (<0.016) | I (0.25) | PEN, CIP, TET | C | 3256 | ||
| NM09 | S (2) | S (0.004) | S (<0.016) | S (0.064) | B | 5604 | |||
| NM11 | R 128) | S (0.004) | S (<0.016) | S (0.064) | CHL | B | 14496 | ||
| NM12 | R (128) | S (0.004) | S (<0.016) | R (0.5) | CHL, PEN | B | 1576 | ||
| NM13 | R (128) | S (0.004) | S (<0.016) | S (0.064) | CHL | B | 1576 | ||
| NM14 | S (2) | S (0.004) | S (<0.016) | I (0.25) | PEN | B | 1145 | ST-41/44 complex | |
| NM15 | S (1) | S (0.008) | S (<0.016) | I (0.25) | PEN | B | 41 | ST-41/44 complex | |
| NM16 | R (32) | S 0.032 | S (0.002) | I (0.125) | CHL | Yes | B | 1576 | |
| NM18 | R (32) | R (0.25) | S (0.016) | I (0.125) | CHL, CIP, PEN | B | 1576 | ||
| NM19 | S (1) | S (0.008) | S (0.016) | S (0.032) | C | 14503 | ST-4821 complex | ||
| NM20 | R (32) | S (0.016) | S (0.016) | S (0.064) | CHL, PEN | Yes | B | 11005 | |
| NM21 | S (2) | S (0.016) | S (0.016) | S (0.016) | B | 12811 | |||
| NM23 | S (2) | S (0.016) | S (0.016) | I (0.125) | PEN | B | 14507 | ||
| NM25 | R (32) | S (0.004) | S (0.002) | I (0.25) | CHL, PEN | B | 1576 | ||
CHL, chloramphenicol; CIP, ciprofloxacin; CRO, ceftriaxone; PEN, penicillin; TET, tetracycline.
S, susceptible; I, intermediate; R, resistant; MIC, minimum inhibitory concentration.
Despite COMRU being the only site with a purely paediatric population, 17/18 patients were children under 8 years old, with a median age of approximately 4 months (one patient <1 year old with no recorded date of birth was attributed the age of 1 year for the purposes of the calculation). Most patients presented with meningitis and/or sepsis, all patients with known antibiotic treatment (14/18) were treated with ceftriaxone, and all of the 16 patients with known outcome were discharged alive following clinical improvement.
Genotypic characterization
All 18 isolates underwent sequencing. 0.4–2.4 million sequence reads were obtained from each isolate, giving 42-233X coverage of the genome. The genomes were assembled in 133-248 contigs, with a total length of 2,124,885–2,217,307 bp and n50 between 40.4 and 82.5 kb.
We determined the serogroups of the isolates from the genome assemblies by looking for the presence of the unique capsule synthesis genes for the B, C, W and Y serogroups which commonly cause invasive disease (Harrison et al., 2013). Two isolates showed the presence of the csc gene, indicating they are serogroup C, while the other 16 isolates, including all the resistant isolates, had the csb gene, indicating they are serogroup B. No matches for the serogroup W or Y genes could be found.
We determined the sequence types of the isolates in our sample set (Table 1). Of the chloramphenicol resistant isolates, five were ST-1576, one was ST-11005, and two isolates had novel sequence types (assigned as ST-14487 and ST-14496). ST-1576 and ST-11005 differ only at the gdh locus, and the two novel sequence types also differ from ST-1576 at only one locus. None of the chloramphenicol susceptible isolates were from ST-1576 or ST-11005, but they were from multiple sequence types, including four isolates with novel sequence types (assigned as ST-14488, ST-14489, ST-14503, and ST-14507). Two isolates from Thailand were ST-41 and ST-1145, and one was from ST-32. These sequence types are part of the ST-41/44 and ST-32 hypervirulent clonal complexes commonly seen in invasive disease (Racloz and Luiz, 2010). The novel ST-14488 sequence type was also part of the ST-41/44 complex. The ST-14503 isolate belonged to the ST-4821 clonal complex previously associated with an outbreak in China (Peng et al., 2008). The other isolates could not be assigned to a clonal complex.
By searching for the presence of antimicrobial resistance genes in the isolates, we identified a 623 bp catP chloramphenicol transferase gene with the same flanking sequences in all eight chloramphenicol resistant isolates. It had previously been shown that a catP gene was present in the highly chloramphenicol-resistant DuyDNT isolated in Vietnam in 2014, and the same catP gene and flanking regions also present in this isolate. By alignment of the region surrounding the catP gene to the MC58 reference genome, it was determined that the chloramphenicol resistance gene was found inserted between genes NMB1351 and NMB1352. The catP gene was identical to the catP gene previously reported in France (Galimand et al., 1998) and a comparison of the sequences surrounding the insertion show that it was inserted in the same position in the genome (Supplementary Figure 1). The insertion is 986 bp identical to the Tn4451 transposon from C. perfringens, but contains only the catP gene and no complete flanking genes.
Ten of our isolates had reduced susceptibility or resistance to penicillin, including two of the chloramphenicol-resistant isolates (NM18 and NM25). We extracted the penA gene sequence to look for the five amino acid changes known to confer reduced susceptibility to penicillin (Taha et al., 2007). Nine isolates had all five amino acid changes, while the other nine isolates had none of the five changes. Concordance between the phenotypic and genotypic susceptibility was not complete – one isolate (NM16) with reduced susceptibility phenotypically did not display the amino acid changes, while one isolate (NM20) with the penA changes did not show phenotypic reduced susceptibility (Table 2). The isolate determined to be resistant to penicillin had the same penA allele as an isolate with only reduced susceptibility. The blaROB-1 gene is also known to confer penicillin resistance in N. meningitidis (Tsang et al., 2019), but this gene was not present in any of our strains.
Isolate NM07 from Thailand was found to contain the tetracycline resistance gene tetB. NM07 and NM18, carry the gyrA Thr91 → Ile mutation known to cause ciprofloxacin resistance (Enríquez et al., 2008, Skoczynska et al., 2008) and are both phenotypically resistant to ciprofloxacin.
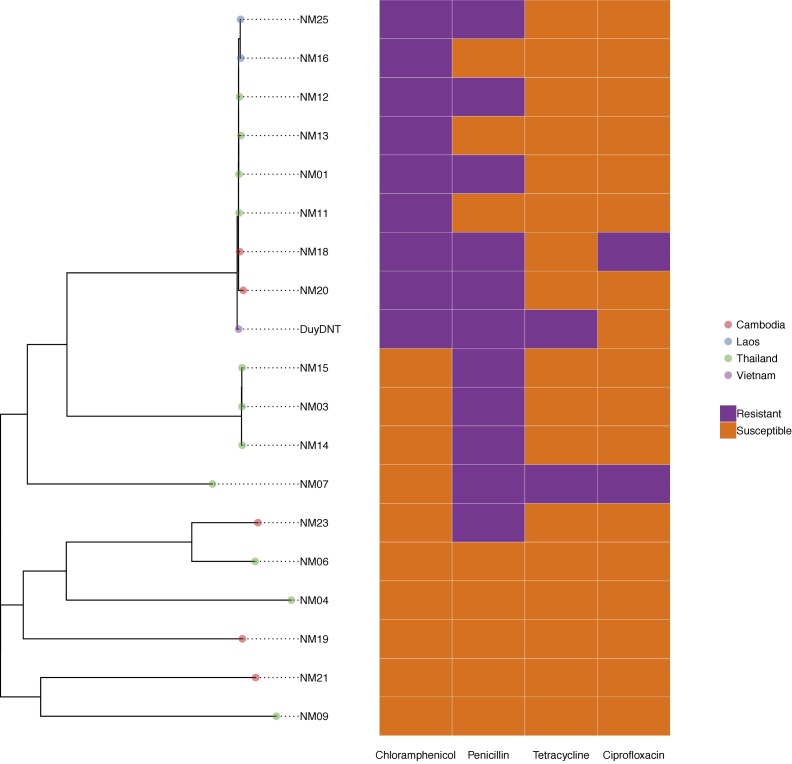
We generated a phylogeny of the 18 genomes from this study and the previously-reported DuyDNT chloramphenicol-resistant isolate (Figure 1) after removing recombinant SNPs, as N. meningitidis is highly recombinogenic (Feil et al., 1999). This shows that the chloramphenicol-resistant isolates from all four countries are in a single clade, with the isolates from each country clustered together within this clade.
Figure 1.
A phylogeny of the 19 Neisseria meningitidis strains showing country of origin and resistance genotypes. The phylogeny was generated by Gubbins using RAxML and the scale bar indicates the number of SNPs.
Discussion
We describe phenotypic and genotypic characteristics of 18 isolates of N. meningitidis from three countries in Southeast Asia isolated over an 11 year period. Most strikingly, chloramphenicol resistance, although rarely reported in the literature, was detected in a higher proportion (44.4%) of isolates compared to studies from other regions reporting on susceptibilities of large numbers of isolates, suggesting this is proportionately more common in Southeast Asia. The identical catP insertion across the isolates in this study and the previously reported isolates from Vietnam and Australia suggests that chloramphenicol resistance in N. meningitidis was acquired once in the ST-1576 lineage, and that lineage has now spread across Southeast Asia.
A high proportion of our isolates (55.5%) displayed reduced susceptibility to penicillin, 9/10 of which had genetic markers known to confer reduced penicillin susceptibility. However, a penicillin-susceptible isolate also carried these genetic markers, although the MIC in this isolate was close to the intermediate breakpoint. There may also be further genetic determinants of resistance in these strains, as we have limited knowledge of N. meningitidis genomes in Southeast Asia.
We also identified isolates resistant to multiple antibiotics including an isolate resistant to chloramphenicol and ciprofloxacin with reduced susceptibility to penicillin, and the DuyDNT isolate is susceptible to ciprofloxacin but carries a tetracycline resistance gene. The first reported chloramphenicol resistant N. meningitidis isolates were susceptible to penicillin, tetracycline, and quinolones (Galimand et al., 1998), suggesting that this lineage has acquired resistance to multiple antibiotics during its spread.
Our isolates were predominantly serogroup B, with only two patients with strains from serogroup C. This is consistent with previous reports of serogroup B isolates causing sporadic disease in Thailand (Pancharoen et al., 2000, Borrow et al., 2017) while very little information is available about common serogroups in Laos and Cambodia.
Five of the isolates we identified come from hypervirulent clonal complexes commonly implicated in invasive disease, including the ST-32 and ST-41/44 clonal complexes involved in multiple epidemics (Harrison et al., 2009), and the ST-4821 clonal complex first identified in China in 2003 (Peng et al., 2008). The remaining thirteen isolates could not be assigned to one of these lineages, and many of these isolates were from novel sequence types. This suggests predominantly sporadic infection arising from the nasopharyngeal flora rather than an ongoing serogroup B epidemic.
There are several limitations of this study. The study involved only three sites and a relatively small number of isolates, reflecting the relative rarity of invasive meningococcal disease in our populations, giving only a snapshot of the epidemiology across the region. Furthermore, only culture-positive isolates were included, which may have underestimated the true number of cases and selected for more resistant isolates given the seemingly high prevalence of pre-hospital antibiotic treatment regionally (Khennavong et al., 2011), although relatively few additional cases were identified by routine CSF PCR for N. meningitidis in our laboratories. Nevertheless, our results suggest that resistance to chloramphenicol is relatively common in meningococcal isolates from Southeast Asia. This remains clinically important given the role of chloramphenicol as a common second-line agent for treatment of meningococcal meningitis. N. meningitidis which is non-susceptible to third-generation cephalosporins (Deghmane et al., 2017) has been recently reported in France, with a penA allele which may have been acquired from Neisseria gonorrhoeae, raising the possibility that recombination with extensively drug-resistant N. gonorrhoeae could result in the spread of further drug resistance in N. meningitidis. Our phenotypic and genotypic characterisation of isolates from three countries in Southeast Asia will add to the limited published data of the epidemiology of meningococcal disease in Southeast Asia, and aid future surveillance of drug-resistant isolates.
Authors’ contribution
DD and PT conceived the project. EMB and TPC performed data analysis and drafted the manuscript. JT, WW, VC, SS, JH, and CL performed laboratory experiments. EMB, TPC, JH, CL, PT and DD edited the final manuscript. All authors reviewed and approved the final manuscript.
Data availability
Raw sequence data has been deposited in the European Nucleotide Archive as project PRJEB30968. Novel sequence types and isolates have been deposited in PubMLST.
Funding
This work was funded by a Wellcome Trust grant (106698) to the Thailand Major Overseas Programme. The funders had no role in study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Ethical approval
Ethical approval was not required.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This publication made use of the PubMLST website (https://pubmlst.org/) developed by Keith Jolley (Jolley and Maiden, 2010) and sited at the University of Oxford. The development of that website was funded by the Wellcome Trust. We acknowledge the laboratory staff who assisted with this project, including Jiraporn Tangmanakit and Pattaraporn Hinfonthong at SMRU. We also thank Dr Manivanh Vongsouvath, Mahosot Microbiology Laboratory Director, Professor Paul Newton, Director of LOMWRU, and the staff of Mahosot Hospital and Lao Ministry of Health for their help and support. This work was funded by a Wellcome Trust grant (106698) to the Thailand Major Overseas Programme.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2020.03.081.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow R., Alarcón P., Carlos J., Caugant D.A., Christensen H., Debbag R. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–328. doi: 10.1080/14760584.2017.1258308. [DOI] [PubMed] [Google Scholar]
- Croucher N.J., Page A.J., Connor T.R., Delaney A.J., Keane J.A., Bentley S.D. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43(3):e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deghmane A.-E., Hong E., Taha M.-K. Emergence of meningococci with reduced susceptibility to third-generation cephalosporins. J Antimicrob Chemother. 2017;72(1):95–98. doi: 10.1093/jac/dkw400. [DOI] [PubMed] [Google Scholar]
- Enríquez R., Abad R., Salcedo C., Pérez S., Vázquez J.A. Fluoroquinolone resistance in Neisseria meningitidis in Spain. J Antimicrob Chemother. 2008;61(2):286–290. doi: 10.1093/jac/dkm452. [DOI] [PubMed] [Google Scholar]
- Feil E.J., Maiden M.C., Achtman M., Spratt B.G. The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol Biol Evol. 1999;16(11):1496–1502. doi: 10.1093/oxfordjournals.molbev.a026061. [DOI] [PubMed] [Google Scholar]
- Fuller D.G., Duke T., Shann F., Curtis N. Antibiotic treatment for bacterial meningitis in children in developing countries. Ann Trop Paediatr. 2003;23(4):233–253. doi: 10.1179/027249303225007752. [DOI] [PubMed] [Google Scholar]
- Galimand M., Gerbaud G., Guibourdenche M., Riou J.Y., Courvalin P. High-level chloramphenicol resistance in Neisseria meningitidis. N Engl J Med. 1998;339(13):868–874. doi: 10.1056/NEJM199809243391302. [DOI] [PubMed] [Google Scholar]
- Gorla M.C., Pinhata J.M.W., Dias U.J., de Moraes C., Lemos A.P. Surveillance of antimicrobial resistance in Neisseria meningitidis strains isolated from invasive cases in Brazil from 2009 to 2016. J Med Microbiol. 2018;67(6):750–756. doi: 10.1099/jmm.0.000743. [DOI] [PubMed] [Google Scholar]
- Harrison L.H., Trotter C.L., Ramsay M.E. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl. 2):B51–B63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- Harrison O.B., Claus H., Jiang Y., Bennett J.S., Bratcher H.B., Jolley K.A. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19(4):566–573. doi: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedberg S.T., Fredlund H., Nicolas P., Caugant D.A., Olcén P., Unemo M. Antibiotic susceptibility and characteristics of Neisseria meningitidis isolates from the African meningitis belt, 2000 to 2006: phenotypic and genotypic perspectives. Antimicrob Agents Chemother. 2009;53(4):1561–1566. doi: 10.1128/AAC.00994-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolley K.A., Maiden M.C.J. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S., ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017;14(6):587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khennavong M., Davone V., Vongsouvath M., Phetsouvanh R., Silisouk J., Rattana O. Urine antibiotic activity in patients presenting to hospitals in Laos: implications for worsening antibiotic resistance. Am J Trop Med Hyg. 2011;85(2):295–302. doi: 10.4269/ajtmh.2011.11-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill F., Heyderman R.S., Michael B.D., Defres S., Beeching N.J., Borrow R. The UK joint specialist societies guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent adults. J Infect. 2016;72(4):405–438. doi: 10.1016/j.jinf.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancharoen C., Hongsiriwon S., Swasdichai K., Puthanakit T., Tangsathapornpong A., Lolekha S. Epidemiology of invasive meningococcal disease in 13 government hospitals in Thailand, 1994–1999. Southeast Asian J Trop Med Public Health. 2000;31(4):708–711. [PubMed] [Google Scholar]
- Peng J., Yang L., Yang F., Yang J., Yan Y., Nie H. Characterization of ST-4821 complex, a unique Neisseria meningitidis clone. Genomics. 2008;91(1):78–87. doi: 10.1016/j.ygeno.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Racloz V.N., Luiz S.J.D. The elusive meningococcal meningitis serogroup: a systematic review of serogroup B epidemiology. BMC Infect Dis. 2010;10:175. doi: 10.1186/1471-2334-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann T. Abricate [Internet]. Github. Available from: https://github.com/tseemann/abricate [cited 19 December 2018].
- Seemann T. Snippy [Internet]. Github. Available from: https://github.com/tseemann/snippy [cited 11 December 2018].
- Shultz T.R., Tapsall J.W., White P.A., Ryan C.S., Lyras D., Rood J.I. Chloramphenicol-resistant Neisseria meningitidis containing catP isolated in Australia. J Antimicrob Chemother. 2003;52(5):856–859. doi: 10.1093/jac/dkg452. [DOI] [PubMed] [Google Scholar]
- Skoczynska A., Alonso J.-M., Taha M.-K. Ciprofloxacin resistance in Neisseria meningitidis, France. Emerg Infect Dis. 2008;14(8):1322–1323. doi: 10.3201/eid1408.080040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M.-K., Vázquez J.A., Hong E., Bennett D.E., Bertrand S., Bukovski S. Target gene sequencing to characterize the penicillin G susceptibility of Neisseria meningitidis. Antimicrob Agents Chemother. 2007;51(8):2784–2792. doi: 10.1128/AAC.00412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.X., Le T.T., Trieu L.P., Austin C.M., Van Quyen D., Nguyen H.M. Whole-genome sequencing and characterization of an antibiotic resistant Neisseria meningitidis B isolate from a military unit in Vietnam. Ann Clin Microbiol Antimicrob. 2019;18(1):16. doi: 10.1186/s12941-019-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang R.S.W., Ahmad T., Jamieson F.B., Tyrrell G.J. WGS analysis of a penicillin-resistant Neisseria meningitidis strain containing a chromosomal ROB-1 β-lactamase gene. J Antimicrob Chemother. 2019;74(1):22–28. doi: 10.1093/jac/dky391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunkel A.R., Hartman B.J., Kaplan S.L., Kaufman B.A., Roos K.L., Scheld W.M. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- Vyse A., Anonychuk A., Jäkel A., Wieffer H., Nadel S. The burden and impact of severe and long-term sequelae of meningococcal disease. Expert Rev Anti Infect Ther. 2013;11(6):597–604. doi: 10.1586/eri.13.42. [DOI] [PubMed] [Google Scholar]
- Vyse A., Wolter J.M., Chen J., Ng T., Soriano-Gabarro M. Meningococcal disease in Asia: an under-recognized public health burden. Epidemiol Infect. 2011;139(7):967–985. doi: 10.1017/S0950268811000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne, PA, Clinical and Laboratory Standards Institute, CLSI . 28th ed. 2018. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. [Google Scholar]
- Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67(11):2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequence data has been deposited in the European Nucleotide Archive as project PRJEB30968. Novel sequence types and isolates have been deposited in PubMLST.