Highlights
-
•
Neuro-immune crosstalk occurs in distinct anatomical niches in the intestine.
-
•
Neuro-immune cell niches maintain gut homeostasis and modulate inflammation.
-
•
Neuron-macrophage crosstalk in the muscularis is crucial for neuronal survival and peristalsis.
-
•
Mast cell mediators activate and sensitize nerve terminals, leading to aberrant pain perception.
-
•
Neurons modulate ILC function during infection and inflammation.
Abstract
Intestinal homeostasis relies on the reciprocal crosstalk between enteric neurons and immune cells, which together form neuro-immune units that occupy distinct anatomical niches within the gut. Here we will review the recent advances in our understanding of neuro-immune crosstalk within the gut, with focus on macrophages, mast cells and innate lymphoid cells. In particular, we will discuss the role of neuron-immune cell crosstalk in homeostasis, and how aberrant communication may underlie disease in the gastro-intestinal tract.
Current Opinion in Neurobiology 2020, 62:68–75
This review comes from a themed issue on Brain, gut and immune system interactions
Edited by Isaac Chiu and Asya Rolls
For a complete overview see the Issue and the Editorial
Available online 18th December 2019
https://doi.org/10.1016/j.conb.2019.11.020
0959-4388/© 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Intestinal homeostasis relies on the coordinated crosstalk between various cellular networks. An excellent example is provided by the nervous and immune system, which communicate with each other via a plethora of neuronal and immunological signals. These neuro-immune interactions appear to be essential to rapidly sense and respond to multiple environmental cues in the intestine both in health and disease. Indeed, recent studies have emphasized that neuro-immune cell units are located in strategic anatomical positions to maintain intestinal homeostasis [1]. This review will focus on the exciting advances in recent years unraveling the crosstalk between the nervous system and immune cells, in particular macrophages, mast cells and innate lymphoid cells.
Overview of the autonomic nervous system
The gastro-intestinal (GI) tract is innervated by components of the peripheral nervous system, which together with the central nervous system form the autonomic system. The peripheral nervous system consists of the sympathetic nervous system, of which the main neurotransmitter is norepinephrine, and the parasympathetic nervous system, whose primary neurotransmitter is acetylcholine. Together the sympathetic and parasympathetic (i.e. vagus nerve) nervous system provide the extrinsic neural control of vital gut functions including regulation of smooth muscle contractility, blood flow and fluid secretion [2]. Besides this efferent brain-to-gut communication, peripheral information is conveyed to the central nervous system through vagal afferent neurons of the nodose ganglion and via nociceptive and visceroceptive splanchnic and pelvic afferents originating from the thoracolumbar and lumbosacral dorsal root ganglia, respectively [3]. In general, vagal afferents provide information about physiological processes including hunger and satiety, whilst spinal afferents inform the brain about pain and discomfort. Indeed, splanchnic afferents express voltage-gated sodium channels such as Nav1.8 and Nav1.9, purinoceptors and transient receptor potential (TRP) channels involved in pain perception, whilst vagal afferents are activated by hunger/satiety-related peptide hormones (e.g. cholecystokinin and leptin) released by enteroendocrine cells. Of note, both types of afferents also express receptors for pro-inflammatory mediators released by immune cells. Upon their activation, the afferent neurons will transmit information to the brain to modulate tissue inflammation accordingly [4,5].
The GI tract itself is also under control of the intrinsic enteric nervous system (ENS), which consists of an extensive network of interconnected neurons and glia arranged in the myenteric and submucosal plexus, capable of functioning autonomously from the central nervous system. The ENS is equipped with sensory (i.e. primary afferent), motor and interneurons of which submucosal neurons control fluid secretion and transport, nutrient absorption and blood flow, whereas myenteric neurons mainly coordinate gut muscle contractility. Although most enteric neurons are of cholinergic origin, other types of neurons communicate via the release of neurotransmitters and neuropeptides such as nitric oxide (NO), adenosine triphosphate (ATP), vasoactive intestinal peptide (VIP) and calcitonin gene-related peptide (CGRP). Interestingly, each part of the autonomic nervous system has recently been identified as a crucial gatekeeper of immune homeostasis and modulator of intestinal inflammation [2].
Neuro-immune cell crosstalk in the gut
Compared to other organs, the GI tract is noteworthy in that it is continuously exposed to an external milieu consisting of commensals, pathogens and dietary food antigens. Gut immune cells are thus challenged with the complex task of remaining tolerant towards trillions of commensal bacteria, while being simultaneously poised to defend our body against invading pathogens. To this end, a plethora of innate and adaptive immune cells are strategically positioned in the gut wall to rapidly sense, react and adapt to the ever-changing conditions of the intestine [6,7].
The fact that different immune cells of the gut express neurotransmitter and neuropeptide receptors clearly suggests that neuron-derived signals may modulate immune cell function. Reciprocally, enteric neurons express cytokine receptors enabling them to respond to inflammatory signals. This reciprocal repertoire suggests the existence of a neuro-immune crosstalk contributing to gut homeostasis. In line, neurons and immune cells truly share anatomical niches and interact functionally, forming neuro-immune units in distinct regions of the gut [7,8].
Neuron-macrophage crosstalk
Macrophages (Mφs) represent an integral part of the gut innate immune system and are devoted to constantly surveil their environment to rapidly recognize and phagocytose pathogens and debris. Gut resident Mφs constitute a heterogeneous population of CX3CR1+ cells strategically positioned within the different intestinal layers, reaching the highest density in the lamina propria compartment. Here, Mφ are mostly located close to the intestinal epithelium, where they phagocytose bacterial antigens and secrete mediators driving epithelial cell renewal [6]. Deeper within the mucosa, Mφs can also be found at the crypt base of villi and closely associated to blood vessels and neurons of the submucosal plexus. Secluded from luminal signals, a substantial number of intestinal Mφs are located in the muscularis externa, that is, muscularis Mφ (mMφ), in close proximity to the myenteric plexus. In addition, a lower number of mMφs are also found within the circular and longitudinal muscle layers and within the serosal layer of the gut [6,9].
Mφ phenotype depends on the niche, in which Mφ reside. Accordingly, the genetic signature of lamina propria Mφ (LPMφ) differs from that of their muscularis counterparts. Gabanyi and colleagues showed that LpMφ possessed a rather pro-inflammatory gene signature, most likely imposed by luminal and epithelial signals [9]. In contrast, mMφ exhibited a more tissue-protective phenotype with the expression of genes such as Arg1 and Cd163. Enteric neurons in the myenteric plexus closely communicate with mMφ and contribute to their survival and maintenance through the secretion of the growth factor colony stimulating factor 1 (CSF-1). Of note, CSF-1 release by enteric neurons is mediated by cues derived from microbial commensals. Conversely, mMφ are crucial for neuronal survival and function [9]. Indeed, Muller and colleagues showed that release of bone morphogenic protein type 2 (BMP2) by mMφ maintains peristalsis through activation of the BMP2 receptor expressed by enteric neurons [10]. In addition, we recently showed that depletion of a subset of long-lived mMφ led to a reduction of over 50% of myenteric neurons, impaired intestinal contractility and prolonged intestinal transit [11]. Recent studies suggest that mMφ may also play a role in shaping ENS connectivity, as the constitutive absence of mMφ in Csf1op/op mice was shown to cause disorganization of the architecture of the myenteric plexus and increased neuronal density [10]. This is in line with recent data showing phagocytosis of neuronal debris by mMφ during steady-state, suggesting that mMφ actively shape ENS architecture in development and adulthood [12] (Figure 1).
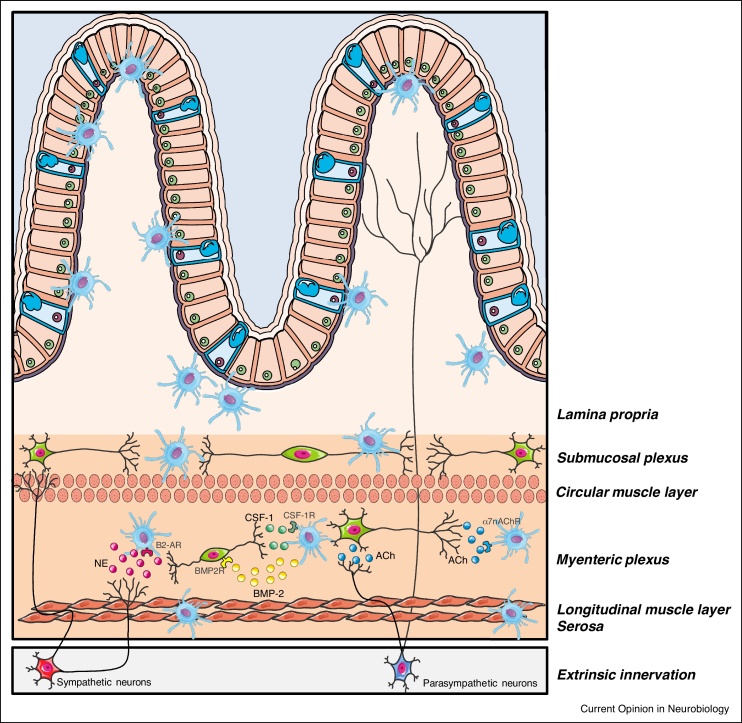
Figure 1.
Neuron-macrophage crosstalk in the gut.
During homeostasis, enteric neurons maintain the muscularis macrophage (mMφ) population through the production of colony stimulating factor-1 (CSF-1), a growth factor necessary for Mφ survival. Reciprocally, mMφ directly control neuronal survival via the release of bone morphogenic protein type 2 (BMP2). During inflammation, cholinergic enteric neurons dampen mMφ activation via the interaction of acetylcholine (ACh) and α7 nicotinic acetylcholine receptor (α7 nAChR). In addition, in the context of bacterial infection extrinsic sympathetic fibers release noradrenaline (NE) promoting a tissue-protective phenotype in mMφ expressing β2 adrenergic receptor (β2-AR). Other abbreviations: CSF-1R, colony stimulating factor-1 receptor; BMP2R, bone morphogenic protein type 2 receptor.
Neuron-Mφ crosstalk in the muscularis externa has previously been extensively described in the context of inflammation. In a model of sterile intestinal inflammation, our group demonstrated that vagus nerve stimulation reduced intestinal inflammation by activation of cholinergic enteric neurons in close proximity to mMφ expressing the α7 nicotonic acetylcholine receptor (α7nAChR) [13,14]. This mMφ-dampening effect is most likely mediated by enteric neurons, since vagal efferents only synapse with cholinergic myenteric neurons but not directly with mMφ [15]. In line, activation of cholinergic enteric neurons by treatment with the 5-HT4 receptor agonist prucalopride reduced the inflammatory response and improved clinical recovery in a model of surgery-induced inflammation, a finding that was confirmed in patients undergoing abdominal surgery [14]. Furthermore, in a model of Salmonella-induced intestinal inflammation, the tissue-protective phenotype of mMφ was upregulated, an effect likely mediated by interaction between extrinsic sympathetic fibers and mMφ expressing the β2-adrenergic receptor (β2-AR) (Figure 1) [9]. Taken together, these findings clearly show reciprocal regulation and support between mMφ and intestinal innervation.
Although neuron-Mφ interaction in the muscularis externa is quite evident, it remains unclear whether a similar crosstalk exists within the intestinal mucosa. Indeed, while the mucosal compartment of the gut is highly innervated, evidence indicating direct vagal and sympathetic communication with LPMφ is lacking. In line with these observations, LPMφ express rather low levels of most nAChR and adrenoceptors, indirectly arguing against a modulatory effect of vagal and sympathetic fibers on these immune cells [9]. However, sympathetic fibers are present around the epithelial crypts, and as this region is well-vascularized, norepinephrine may act as a chemoattractant to recruit circulating immune cells into the mucosa and guiding them to sympathetic terminals [16]. In line, Asano et al. recently described a unique CD169+ subpopulation of LPMφ-enriched near the crypt base of the villi close to lymphoid tissue [17]. These crypt-associated LPMφ expand during experimental colitis and contribute to disease severity via their ability to attract monocytes via release of the CCR2/CCR3/CCR5 ligand CCL8 [17]. In addition, similarly as to what was observed in the myenteric plexus, depletion of long-lived Mφ leads to loss of enteric neurons in the submucosal plexus, with consequent impairment of intestinal secretion [11]. These findings support the hypothesis of neuron-Mφ crosstalk within the lamina propria; however, further investigation is warranted to fully elucidate the mechanisms involved.
Neuron-mast cell crosstalk
Mast cells constitute a heterogeneous immune cell population that acts as sentinels at the mucosal surface to provide a first line of defense against invading pathogens. Moreover, mast cells are involved in a diverse range of homeostatic processes such as tissue repair and remodeling. Therefore, mast cells possess a wide array of pattern recognition receptors (i.e. Toll-like receptors), Fc and Mas-related G-protein-coupled (MRG) receptors. In addition, mast cells sense cell stress and tissue damage via an array of receptors such as cytokine, alarmin and purigenic receptors. Upon activation, mast cells rapidly release their preformed granule-stored mediators containing histamine, serotonin (5-HT), heparin and various proteases (i.e. chymase, tryptase, etc.) followed by the release of de novo synthesized lipid mediators including leukotrienes, prostaglandins and cytokines [18,19].
In the intestine, the largest number of mast cells can be found in the mucosal and submucosal layers, while they are rarely present in the muscular and serosal layers. Extrinsic afferent nerve endings as well as enteric neurons lie in close contact to the mucosal mast cells and about 70% of mucosal mast cells directly interact with nerve fibers, while another 20% are located within 2 μm. The close spatial association between mast cells and nerves has suggested the existence of a bidirectional and functional neuro-mast cell axis. Indeed, neuronal stimulation of mast cells is a key component of neurogenic inflammation, in which neuronal secretion of neuropeptides including CGRP, VIP and substance P (SP) causes mast cell degranulation. The released mast cell mediators not only strengthen the inflammatory response, but in addition create a positive-feedback loop via activation of the nociceptors, driving neurogenic inflammation [20]. Furthermore, Zheng and colleagues showed that psychological stress evokes the release of the neuropeptide SP by nervous terminals. Release of SP acts on intestinal immune cells. Specifically, eosinophils were found to respond to SP by producing corticotrophin releasing hormone (CRH). In turn, CRH triggers mast cell degranulation via CRF1 receptor, causing impaired intestinal epithelial barrier function, which is typically observed during stress (Figure 2) [21, 22, 23].
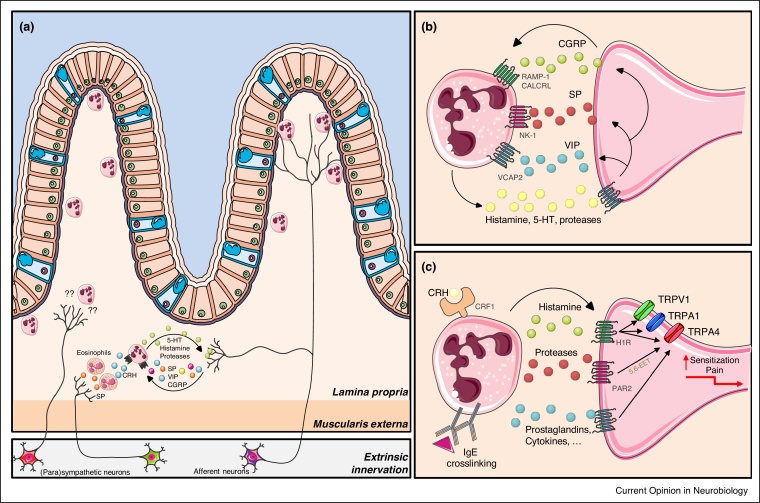
Figure 2.
Neuron-mast cell crosstalk in the intestine.
((a) and (b)) Neuronal stimulation of mast cells via the neuropeptides vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP) and substance P (SP) cause mast cell degranulation driving neurogenic inflammation. In the context of psychological stress, SP is also released by nervous terminals, specifically acting on eosinophils. In turn, these immune cells produce corticotrophin releasing hormone (CRH) triggering mast cell degranulation via the CRF1 receptor. ((a) and (c)) Mast cell activation in response to IgE crosslinking or stress triggers the release of mast cell mediators including histamine, serotonin (5-HT), prostaglandins and pro-inflammatory cytokines. In particular, histamine and mast cell tryptase activate afferent neurons through their interaction with the histamine receptor 1 (H1R) and protease-activated receptor 2 (PAR2), respectively. This leads to the activation and sensitization of nociceptors by potentiating transient receptor potential (TRP) vanilloid 1 (TRPV1), TRPV4 and TRP ankyrin (TRPA)1 leading to an aberrant pain response. Also 5-HT increases afferent neuron discharge and sensitizes TRPV1 and TRPV4 channels. Other abbreviations: RAMP1, receptor activity modifying protein 1; CALCRL, Calcitonin receptor-like; NK1, Neurokinin 1 receptor, VCAP, vasoactive intestinal peptide; 5,6-epoxyeicosatrienoic acid (5,6-EET).
The most well-studied mast cell mediators are histamine and 5-HT. Histamine has been repeatedly linked to pain symptoms in irritable bowel syndrome (IBS), as it has been shown that this mediator directly activates afferent neurons via interaction with histamine receptor 1 (H1R) [24]. In addition, histamine sensitizes TRP vanilloid 1 (TRPV1), TRPV4, TRP ankyrin (TRPA)1 and Nav1.8 channels, leading to increased neuronal discharge and aberrant pain perception. If persistent, this increased nerve excitability can contribute to abdominal pain and IBS [21,25, 26, 27]. Another mast cell mediator associated to abdominal pain severity in IBS patients is 5-HT [28]. 5-HT increases afferent neuron excitability and similar to histamine, sensitizes TRPV1 and TRPV4 channels [28, 29, 30]. Other mast cell mediators, such as proteases, have also been shown to be increased in IBS patients [25]. For instance, mast cell tryptase cleaves within the extracellular domain of the protease-activated receptor 2 (PAR2) on nerve terminals, leading to intracellular synthesis of the TRPV4 agonist 5,6-epoxyeicosatrienoic acid (5,6-EET), a polyunsaturated fatty acid metabolite, activation and sensitization of nociceptors by potentiating TRPV1, TRPV4 and TRPA1 and aberrant pain perception (Figure 2) [31, 32, 33, 34].
Opposed to afferent nociceptors, only limited evidence indicates sympathetic and cholinergic modulation of mast cell function. Parasympathetic regulation of mast cells is suggested by nAChR and muscarinic AChR expression on mast cells, the close anatomical proximity between mast cells and cholinergic nerve fibers and the fact that nAChR agonists attenuate mast cell responses. In contrast, sympathetic control of mast cells is indicated by their expression of β2-AR and the evidence that β2-AR agonists inhibit the release of histamine and other inflammatory mediators [35]. However, additional studies are required to fully comprehend the mechanisms involved.
Neuron-innate lymphoid cell crosstalk
Innate lymphoid cells (ILC) are immune cells belonging to the lymphoid lineage and are essential players in acute innate immune responses to infection and tissue remodeling, in addition to regulating adaptive immunity and resolving inflammation. They are relatively rare cell types but are abundantly present at barrier surfaces such as the intestinal mucosa, where they have been implicated in maintaining barrier integrity. Unlike other types of immune cells, ILCs do not recognize-specific antigen-associated or pathogen-associated molecular patterns but are rather alerted to incoming threats by host-derived signals including alarmins, lipid mediators and neuropeptides [1]. Recently, several studies have suggested neural regulation of ILCs. Group 2 ILCs (ILC2), which play a key role in anti-helminth immunity, lie close to submucosal cholinergic neurons that express the neuropeptide neuromedin U (NMU), and were recently shown to selectively express the NMU receptor 1. Acting in concert with the epithelial-derived alarmins IL-33 and IL-25, NMU promotes ILC2 proliferation and release of type 2 cytokines favoring worm expulsion [36,37]. In contrast, sympathetic fibers attenuate ILC2 responses, serving as a negative regulator to maintain the balance between promotion of host-protective ILC2 responses and prevention of chronic type 2 inflammation [38]. The neuropeptide CGRP acts as another negative regulator of ILC2 responses and proliferation, but does selectively promote IL-5 production [39]. ILC2s also respond to VIP signaling leading to increased IL-5 production via activation of the VIP receptor 2 (VPAC2). As IL-5 is central to regulation of eosinophil accumulation, this implicates that ILC2 play a critical role in regulating metabolic cycling (Figure 3) [40].
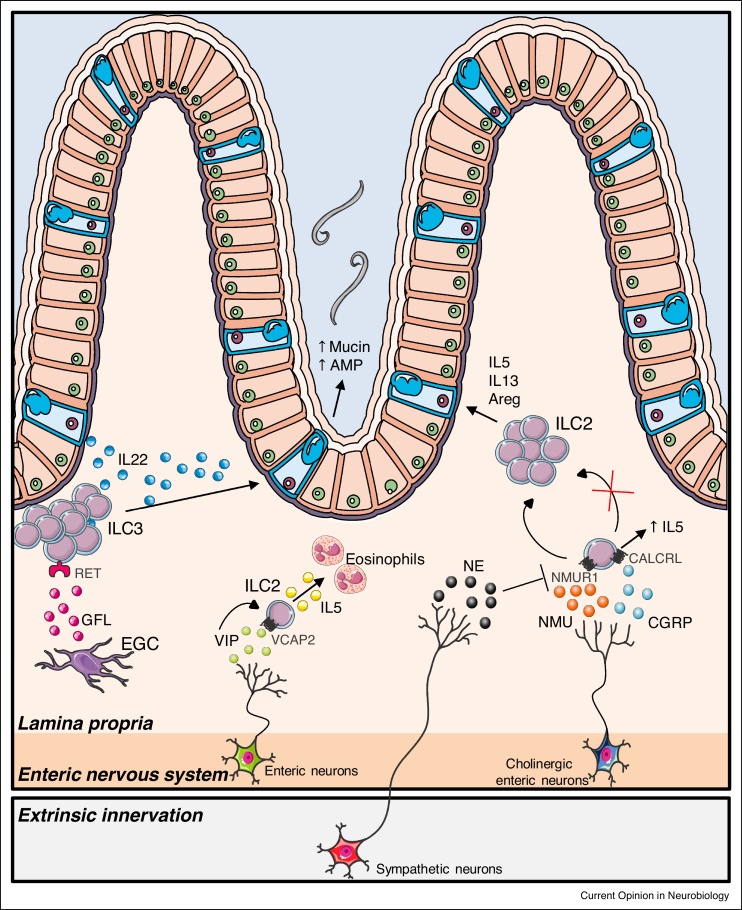
Figure 3.
Neuron-innate lymphoid cell (ILC) crosstalk in the gut.
Cholinergic neurons sense and respond to parasitic infection via the release of neuromedin U (NMU). This neuropeptide induces the activation of NMUR1-expressing group 2 ILCs (ILC2s) contributing to ILC2 proliferation and production of type 2 cytokines favoring worm expulsion. In contrast, β2-adrenergic-receptor-mediated signaling inhibits ILC2 activity in intestinal infection models. The neuropeptide calcitonin gene-related peptide (CGRP) released by cholinergic enteric neurons also antagonizes ILC2 responses, but does promote IL-5 production. In addition, ILC2s respond to vasoactive intestinal peptide (VIP) signaling, and activation of the VIP receptor 2 (VPAC2) leads to increased IL-5 production and eosinophil level regulation. Group 3 ILCs (ILC3s), in contrast, are controlled by enteric glial cells (EGC). EGCs sense microbial-derived and host-derived cues and subsequently release glial cell-derived neurotrophic factor family ligands (GFL). In turn, these neurotrophic molecules activate RET-expressing ILC3s, leading to the production of the tissue-protective cytokine interleukin-22 (IL-22). Other abbreviations: CALCRL, Calcitonin receptor-like.
Group 3 ILCs (ILC3) have been shown to interact with enteric glia via neurotrophic factors such as glial-derived neurotrophic factor (GDNF) family ligands (GFL). Enteric glial cells, historically described as the structural support of neurons, integrate microbial-derived and host-derived cues to produce neurotrophic factors. In turn, these neurotrophic molecules activate neighboring ILC3s expressing the neuroregulatory receptor RET, leading to the production of the tissue-protective cytokine interleukin-22 (IL-22). Specific deletion of RET in ILC3 decreased IL-22 release causing blunted epithelial reactivity and increased susceptibility to intestinal infection and inflammation. Thus, glial cells facilitate the maintenance of gut homeostasis via orchestrated release of neurotrophic factors affecting ILC3 (Figure 3) [41].
Conclusions
The recent years have seen a rapid expansion in our knowledge regarding the existence and function of neuro-immune units in the gut; however, many questions remain unanswered. In particular, untangling the cell types and mediators involved in neuro-immune signaling has proven to be difficult due to the complexity, and diversity, of the intestinal mucosa. The advent of single-cell technology and potent bioinformatics tools may provide a crucial advantage in unravelling this complex crosstalk, and may shed light on the molecular mechanisms involved. Further studies should focus on pinpointing these molecular mechanisms, understanding how they support homeostasis, and how they may underlie intestinal pathology. Indeed, neuro-immune interaction may provide an attractive therapeutic target not only in diseases characterized by abdominal pain, but also chronic neurodegenerative disorders of the GI tract.
Funding
This work was supported by the European Research Council (ERC) Advanced Grant (NEUMACS-Zkd-833816) to GEB. NS is supported by a postdoctoral research fellowship of FWO. MFV is supported by a PhD fellowship of FWO.
Conflict of interest statement
Nothing declared.
References
- 1.Veiga-Fernandes H., Artis D. Neuronal-immune system cross-talk in homeostasis. Science. 2018;359:1465–1466. doi: 10.1126/science.aap9598. [DOI] [PubMed] [Google Scholar]
- 2.Yoo B.B., Mazmanian S.K. The enteric network: interactions between the immune and nervous systems of the gut. Immunity. 2017;46:910–926. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benarroch E.E. Autonomic nervous system and neuroimmune interactions: new insights and clinical implications. Neurology. 2019;92:377–385. doi: 10.1212/WNL.0000000000006942. [DOI] [PubMed] [Google Scholar]
- 5.Brierley S.M., Hibberd T.J., Spencer N.J. Spinal afferent innervation of the colon and rectum. Front Cell Neurosci. 2018;12:467. doi: 10.3389/fncel.2018.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Schepper S., Stakenborg N., Matteoli G., Verheijden S., Boeckxstaens G.E. Muscularis macrophages: key players in intestinal homeostasis and disease. Cell Immunol. 2018;330:142–150. doi: 10.1016/j.cellimm.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godinho-Silva C., Cardoso F., Veiga-Fernandes H. Neuro-immune cell units: a new paradigm in physiology. Annu Rev Immunol. 2019;37:19–46. doi: 10.1146/annurev-immunol-042718-041812. [DOI] [PubMed] [Google Scholar]
- 8.Chesne J., Cardoso V., Veiga-Fernandes H. Neuro-immune regulation of mucosal physiology. Mucosal Immunol. 2019;12:10–20. doi: 10.1038/s41385-018-0063-y. [DOI] [PubMed] [Google Scholar]
- 9.Gabanyi I., Muller P.A., Feighery L., Oliveira T.Y., Costa-Pinto F.A., Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller P.A., Koscso B., Rajani G.M., Stevanovic K., Berres M.L., Hashimoto D., Mortha A., Leboeuf M., Li X.M., Mucida D. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Schepper S., Verheijden S., Aguilera-Lizarraga J., Viola M.F., Boesmans W., Stakenborg N., Voytyuk I., Schmidt I., Boeckx B., Dierckx de Casterle I. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175:400–415.e3. doi: 10.1016/j.cell.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni S., Micci M.A., Leser J., Shin C., Tang S.C., Fu Y.Y., Liu L., Li Q., Saha M., Li C. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matteoli G., Gomez-Pinilla P.J., Nemethova A., Di Giovangiulio M., Cailotto C., van Bree S.H., Michel K., Tracey K.J., Schemann M., Boesmans W. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2013;63:938–948. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 14.Stakenborg N., Labeeuw E., Gomez-Pinilla P.J., De Schepper S., Aerts R., Goverse G., Farro G., Appeltans I., Meroni E., Stakenborg M. Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut. 2019;68:1406–1416. doi: 10.1136/gutjnl-2018-317263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cailotto C., Costes L.M., van der Vliet J., van Bree S.H., van Heerikhuize J.J., Buijs R.M., Boeckxstaens G.E. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol Motil. 2012;24:191–200. doi: 10.1111/j.1365-2982.2011.01824.x. e193. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan D.P., Bui T., Muller W.A., Butin-Israeli V., Sumagin R. In vivo imaging reveals unique neutrophil transendothelial migration patterns in inflamed intestines. Mucosal Immunol. 2018;11:1571–1581. doi: 10.1038/s41385-018-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asano K., Takahashi N., Ushiki M., Monya M., Aihara F., Kuboki E., Moriyama S., Iida M., Kitamura H., Qiu C.H. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun. 2015;6:7802. doi: 10.1038/ncomms8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsythe P. Mast cells in neuroimmune interactions. Trends Neurosci. 2019;42:43–55. doi: 10.1016/j.tins.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Dudeck A., Koberle M., Goldmann O., Meyer N., Dudeck J., Lemmens S., Rohde M., Roldan N.G., Dietze-Schwonberg K., Orinska Z. Mast cells as protectors of health. J Allergy Clin Immunol. 2018;144(4S):S4–S18. doi: 10.1016/j.jaci.2018.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Buhner S., Barki N., Greiter W., Giesbertz P., Demir I.E., Ceyhan G.O., Zeller F., Daniel H., Schemann M. Calcium imaging of nerve-mast cell signaling in the human intestine. Front Physiol. 2017;8:971. doi: 10.3389/fphys.2017.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wouters M.M., Vicario M., Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65:155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 22.van Diest S.A., Stanisor O.I., Boeckxstaens G.E., de Jonge W.J., van den Wijngaard R.M. Relevance of mast cell-nerve interactions in intestinal nociception. Biochim Biophys Acta. 2012;1822:74–84. doi: 10.1016/j.bbadis.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Zheng P.Y., Feng B.S., Oluwole C., Struiksma S., Chen X., Li P., Tang S.G., Yang P.C. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. 2009;58:1473–1479. doi: 10.1136/gut.2009.181701. [DOI] [PubMed] [Google Scholar]
- 24.Kim B.M., Lee S.H., Shim W.S., Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett. 2004;361:159–162. doi: 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Cenac N., Andrews C.N., Holzhausen M., Chapman K., Cottrell G., Andrade-Gordon P., Steinhoff M., Barbara G., Beck P., Bunnett N.W. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wouters M.M., Balemans D., Van Wanrooy S., Dooley J., Cibert-Goton V., Alpizar Y.A., Valdez-Morales E.E., Nasser Y., Van Veldhoven P.P., Vanbrabant W. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology. 2016;150:875–887.e9. doi: 10.1053/j.gastro.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Balemans D., Aguilera-Lizarraga J., Florens M.V., Jain P., Denadai-Souza A., Viola M.F., Alpizar Y.A., Van Der Merwe S., Vanden Berghe P., Talavera K. Histamine-mediated potentiation of transient receptor potential (TRP) ankyrin 1 and TRP vanilloid 4 signaling in submucosal neurons in patients with irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2019;316:G338–G349. doi: 10.1152/ajpgi.00116.2018. [DOI] [PubMed] [Google Scholar]
- 28.Salzer I., Gantumur E., Yousuf A., Boehm S. Control of sensory neuron excitability by serotonin involves 5HT2C receptors and Ca(2+)-activated chloride channels. Neuropharmacology. 2016;110:277–286. doi: 10.1016/j.neuropharm.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cenac N., Altier C., Motta J.P., d’Aldebert E., Galeano S., Zamponi G.W., Vergnolle N. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut. 2010;59:481–488. doi: 10.1136/gut.2009.192567. [DOI] [PubMed] [Google Scholar]
- 30.Takayama Y., Uta D., Furue H., Tominaga M. Pain-enhancing mechanism through interaction between TRPV1 and anoctamin 1 in sensory neurons. Proc Natl Acad Sci U S A. 2015;112:5213–5218. doi: 10.1073/pnas.1421507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai Y., Moriyama T., Higashi T., Togashi K., Kobayashi K., Yamanaka H., Tominaga M., Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai Y., Wang S., Tominaga M., Yamamoto S., Fukuoka T., Higashi T., Kobayashi K., Obata K., Yamanaka H., Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grant A.D., Cottrell G.S., Amadesi S., Trevisani M., Nicoletti P., Materazzi S., Altier C., Cenac N., Zamponi G.W., Bautista-Cruz F. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cenac N., Bautzova T., Le Faouder P., Veldhuis N.A., Poole D.P., Rolland C., Bertrand J., Liedtke W., Dubourdeau M., Bertrand-Michel J. Quantification and potential functions of endogenous agonists of transient receptor potential channels in patients with irritable bowel syndrome. Gastroenterology. 2015;149:433–444.e7. doi: 10.1053/j.gastro.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Veiga-Fernandes H., Mucida D. Neuro-immune interactions at barrier surfaces. Cell. 2016;165:801–811. doi: 10.1016/j.cell.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardoso V., Chesne J., Ribeiro H., Garcia-Cassani B., Carvalho T., Bouchery T., Shah K., Barbosa-Morais N.L., Harris N., Veiga-Fernandes H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549:277–281. doi: 10.1038/nature23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klose C.S.N., Mahlakoiv T., Moeller J.B., Rankin L.C., Flamar A.L., Kabata H., Monticelli L.A., Moriyama S., Putzel G.G., Rakhilin N. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549:282–286. doi: 10.1038/nature23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriyama S., Brestoff J.R., Flamar A.L., Moeller J.B., Klose C.S.N., Rankin L.C., Yudanin N.A., Monticelli L.A., Putzel G.G., Rodewald H.R. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359:1056–1061. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- 39.Xu H., Ding J., Porter C.B.M., Wallrapp A., Tabaka M., Ma S., Fu S., Guo X., Riesenfeld S.J., Su C. Transcriptional atlas of intestinal immune cells reveals that neuropeptide alpha-CGRP modulates group 2 innate lymphoid cell responses. Immunity. 2019;51:696–708.e9. doi: 10.1016/j.immuni.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nussbaum J.C., Van Dyken S.J., von Moltke J., Cheng L.E., Mohapatra A., Molofsky A.B., Thornton E.E., Krummel M.F., Chawla A., Liang H.E. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibiza S., Garcia-Cassani B., Ribeiro H., Carvalho T., Almeida L., Marques R., Misic A.M., Bartow-McKenney C., Larson D.M., Pavan W.J. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]