Highlights
-
•
Japanese encephalitis virus (JEV) remains a leading cause of neurological infection in Asia.
-
•
A systematic review identified 20,212 published human cases of laboratory-confirmed JEV infections from 205 studies.
-
•
15,167 (75%) of cases were confirmed with the lowest confidence diagnostic test, i.e., level 3 or 4, or level 4.
-
•
Only 109 (53%) of the studies reported contemporaneous testing for dengue-specific antibodies.
-
•
A fundamental pre-requisite for the control of JE is lacking — that of a simple and specific diagnostic procedure that can be adapted for point-of-care tests and readily used throughout JE endemic regions of the world.
Keywords: Flaviviruses, Neurological infection, Diagnostics
Abstract
Objective
Japanese encephalitis virus infection (JE) remains a leading cause of neurological disease in Asia, mainly involving individuals living in remote areas with limited access to treatment centers and diagnostic facilities. Laboratory confirmation is fundamental for the justification and implementation of vaccination programs. We reviewed the literature on historical developments and current diagnostic capability worldwide, to identify knowledge gaps and instill urgency to address them.
Methods
Searches were performed in Web of Science and PubMed using the term 'Japanese encephalitis' up to 13th October 2019. Studies reporting laboratory-confirmed symptomatic JE cases in humans were included, and data on details of diagnostic tests were extracted. A JE case was classified according to confirmatory levels (Fischer et al., 2008; Campbell et al., 2011; Pearce et al., 2018; Heffelfinger et al., 2017), where level 1 represented the highest level of confidence.
Findings
20,212 published JE cases were identified from 205 studies. 15,167 (75%) of these positive cases were confirmed with the lowest-confidence diagnostic tests (level 3 or 4, or level 4). Only 109 (53%) of the studies reported contemporaneous testing for dengue-specific antibodies.
Conclusion
A fundamental pre-requisite for the control of JEV is lacking — that of a simple and specific diagnostic procedure that can be adapted for point-of-care tests and readily used throughout JE-endemic regions of the world.
Introduction
The mosquito-borne flavivirus Japanese encephalitis virus (JEV) accounts for an estimated 68,000 cases of Japanese encephalitis and 709,000 disability-adjusted life years annually (Fischer et al., 2008, Campbell et al., 2011). Japanese encephalitis virus (JEV) primarily affects children in rural areas when JEV-infected mosquitoes feed on humans rather than their primary amplifying hosts, pigs, or reservoir hosts, i.e., aquatic birds (Pearce et al., 2018). Sustained efforts from international agencies have supported the introduction of immunization programs into routine health control schedules in countries with endemic JEV transmission, Table 1 (Heffelfinger et al., 2017). The evidence suggests that vaccination has had an impact on JE incidence (Heffelfinger et al., 2017, Impoinvil et al., 2013, Yang et al., 2016, Ozawa et al., 2017, Upreti et al., 2017, Yu et al., 2018, Muniaraj and Rajamannar, 2019). However, JEV remains a leading cause of neurological infection in endemic countries, and the Joint World Health Organisation (WHO)/United Nations Children's Fund (UNICEF) surveillance data do not substantiate the improvements cited in the past ten years, with sustained numbers of reported patients over this period, see Figure 1.
Table 1.
Country-specific data on the introduction of Japanese encephalitis virus vaccine in JEV endemic countries. Data adapted from the CDC report by Heffelfinger et al. 2017 and updated with WHO surveillance data (Heffelfinger et al., 2017, World Health Organization, 2017a, World Health Organization, 2019a).
| Country | WHO region | Vaccine in schedule (2019) | JE immunization program | Year introduced subnationally | Year introduced nationally | Scheduled age (months) for vaccine | Vaccine used in immunisation program |
|---|---|---|---|---|---|---|---|
| Australia | WPRO | Yes | Risk areas: outer islands of Torres Straits | n/a | n/a | 12 | Live-recombinant |
| Bangladesh | SEARO | No | None | n/a | n/a | – | – |
| Bhutan | SEARO | No | None | n/a | n/a | – | – |
| Brunei Darussalam | WPRO | No | None | n/a | n/a | – | – |
| Cambodia | WPRO | Yes | National | 2009 | 2015 | 9 | Live-attenuated |
| People’s Republic of China | WPRO | Yes | National; excluding Qinghai, Tibet, Xinjiang, and Hong Kong which do not have endemic transmission | 2003* | 2008 | 8 | Live-attenuated |
| DPR of Korea | SEARO | No | None; JEV vaccination campaign in 2016 | n/a | n/a | – | – |
| India | SEARO | Yes | Subnational | 2007 | n/a | 9–11 | Live-attenuated |
| Indonesia | SEARO | Yes | Subnational: Bali | 2018 | n/a | – | – |
| Japan | WPRO | Yes | National | <2002 | <2007 | 6 | Inactivated vero cell derived |
| Lao PDR | WPRO | Yes | National | 2013 | 2015 | 9–11 | Live-attenuated |
| Malaysia | WPRO | Yes | Subnational: Sarawak and Sabah | 2002 | n/a | 9 | Live-recombinant |
| Myanmar | SEARO | Yes | National | n/a | 2018 | – | – |
| Nepal | SEARO | Yes | National | 2007 | 2017 | 12 | Live-attenuated |
| Pakistan | EMRO | No | None | n/a | n/a | – | – |
| Papua New Guinea | WPRO | No | None | n/a | n/a | – | – |
| Philippines | WPRO | Yes | Subnational: Regions I-III, and the Cordillera Administrative Region | 2018 | n/a | – | – |
| Republic of Korea | WPRO | Yes | National | n/a | <2002 | 12 | Live-attenuated, Live-recombinant, Inactivated vero cell and mouse brain derived |
| Russian Federation | EURO | No | None | n/a | n/a | – | – |
| Singapore | WPRO | No | None | n/a | n/a | – | – |
| Sri Lanka | SEARO | Yes | National | 2001 | 2011 | 12 | Live-attenuated |
| Republic of China | WPRO | Yes | National | 1963 | 1968 | 15 | Inactivated mouse brain derived |
| Thailand | SEARO | Yes | National | n/a | <2002 | 12 | Live-attenuated and Live-recombinant |
| Timor-Leste | SEARO | No | None | n/a | n/a | – | – |
| Vietnam | WPRO | Yes | National | <2002 | 2015 | 12 | Inactivated mouse brain derived |
According to official WHO data, although it is acknowledged that the People’s Republic of China has performed widespread vaccination since 1971 (Gao et al., 2014). WPRO = Western Pacific Regional Office; SEARO = South-East Asia Regional Office.
Figure 1.
Number of JE cases reported annually over the last decade based on WHO/UNICEF surveillance (World Health Organization, 2019b). Data include probable* and laboratory-confirmed cases reported by JEV endemic countries. *WHO definition of a probable case (Solomon et al., 2008a) = A case that meets the clinical case definition for acute encephalitis syndrome (AES) that occurs in close geographical and temporal relationship to a laboratory-confirmed case of JE, in the context of an outbreak. Note that these data represent only reported cases, and are not considered to be an accurate representation of global JE incidence. The weaknesses of these data are discussed in the main text.
JE cases reported to WHO/UNICEF have significant limitations. For example, increased awareness of the disease and access to laboratory capacity may contribute to increased case reporting. Conversely, surveillance data are likely to represent only a small proportion of patients (M’Ikanatha et al., 2013). This is particularly relevant for JE, occurring predominantly in rural areas lacking diagnostic capacity (Vaughn and Hoke, 1992). There are no rapid or point-of-care tests for JE in clinical use (Sengvilaipaseuth et al., 2017), and the WHO recommended standard diagnostic assay is an ELISA test that requires trained professionals, appropriate resources, and several hours for the results of the tests to be obtained (Western WHOROft, 2014). In a survey performed by WHO/UNICEF in 2017, 21 countries responded, of which 11 met the minimum surveillance standards (World Health Organization, 2018, World Health Organization, 2017b). Equally, there are problems of specificity of the most widely used diagnostic test, JE MAC-ELISA (Dubot-Peres et al., 2015). This is an increasing issue, with increasing endemicity of other flaviviruses and vaccination coverage.
The reasons for the persistence of JE as a public health problem are complex and multifactorial. A fundamental principle that must be kept in mind is that JE is a zoonotic infection; human immunization will never eradicate it in the natural environment and therefore sustained vaccination coverage is necessary. However, in countries that do have vaccination programs, they are not necessarily uniformly implemented nationwide, and in some areas, vaccine coverage is sub-optimal. While there are many reasons for inadequate coverage, this remains a neglected aspect of JE vaccination programs (Murhekar et al., 2017). Furthermore, immunization strategies are constrained by the absence of adequate diagnostic capacity to investigate the burden of disease, the impact of vaccination (Tandale et al., 2018), and the dynamic epidemiology of JE. For example, in common with other emerging arboviruses (Gould et al., 2017), JE has the propensity to emerge and become established in new geographical regions (Mackenzie et al., 2004). In recent years there have been increasingly frequent reports of cases in peri-urban and urban areas (Gould et al., 2017), as well as new regions such as Rajasthan, India.
Moreover, evidence for autochthonous transmission of JEV in Angola was recently reported (Simon-Loriere et al., 2017a). JEV RNA has also recently been detected in birds in Italy (Preziuso et al., 2018). This most likely represents the globally increased movement, via transportation, of animals and goods. Increased pig farming in urban areas of Asian countries also impacts on virus amplification. Finally, the live attenuated vaccine in widespread use is based on JEV genotype 3, even though in recent decades, there has been large-scale genotype displacement to genotype 1 (Wei et al., 2019), and evidence of detection of genotype 5 (Cao et al., 2016).
Accordingly, we performed a comprehensive review of the evolution of current diagnostic tests for JE. We tackled this by performing a systematic review of published laboratory-confirmed symptomatic cases of JE in humans, and extracted data on the laboratory procedures employed. We also appraised novel tests either under development or conceptually applicable for future diagnostic purposes. Data analysis informed our discussion on future perspectives for research.
Methods
Searches were performed in the Web of Science and PubMed using the text word term 'Japanese encephalitis' up to 13th October 2019. The abstracts were reviewed, and a full text was obtained for those potentially containing information on human cases of JE in the English language. The full-text articles were then reviewed for those reporting symptomatic human cases of laboratory-confirmed JE. The search was limited to JE cases confirmed during the acute illness or hospitalization rather than seroprevalence, with geographic information at least to the country of onset of illness, and temporal information at least to the year of diagnosis. Data were extracted on details of the diagnostic confirmation of JE cases. A JE case was classified according to the confirmatory level (Fischer et al., 2008, Campbell et al., 2011, Pearce et al., 2018, Heffelfinger et al., 2017) developed from existing WHO and CDC criteria, where 1 provides the highest level of confidence based on the diagnostic test used, as illustrated in Table 2.
Table 2.
Diagnostic criteria used to assess JE laboratory-confirmed patients.*
| Level 1 | JEV RNA detected in any specimen by RT-PCR. |
| Virus isolation by inoculation of any specimen in cell culture or animal with characteristic cytopathic effect and confirmation by detection of JEV RNA or virus antigen. | |
| JEV virus antigen detected from brain tissue or CSF by immunofluorescence or immunohistochemistry | |
| Level 2 | Seroconversion or ≥ 4x rise in anti-JEV Ab by seroneutralization or detection of neutralizing antibody in CSF; Samples should be tested alongside other endemic flaviviruses (e.g., dengue viruses) |
| Level 3 | Anti-JEV IgM detected in CSF; Samples should be tested alongside other endemic flaviviruses (e.g., dengue viruses) |
| Seroconversion or 4x rise in anti-JEV Ab HI, CF, IFA, or seroconversion by ELISA; Samples should be tested alongside other endemic flaviviruses (e.g., dengue viruses). | |
| Level 4 | Anti-JEV IgM detected in serum in one sample (acute/convalescent), or seroneutralisation tested in one sample or single high titer HI/CF/IFA; Samples must be tested alongside other endemic flaviviruses (e.g., dengue viruses) |
RT-PCR = reverse transcription-polymerase chain reaction; RNA = ribonucleic acid; CSF = cerebrospinal fluid; Ab = antibody; ELISA = enzyme-linked immunosorbent assay; HI = haemagglutination inhibition; CF = complement fixation test; IFA = indirect immunofluorescence assay.
Confirmation of JE is categorized into levels 1-4 based on existing WHO and CDC criteria, such that level 1 provides the highest level of confidence.
Results
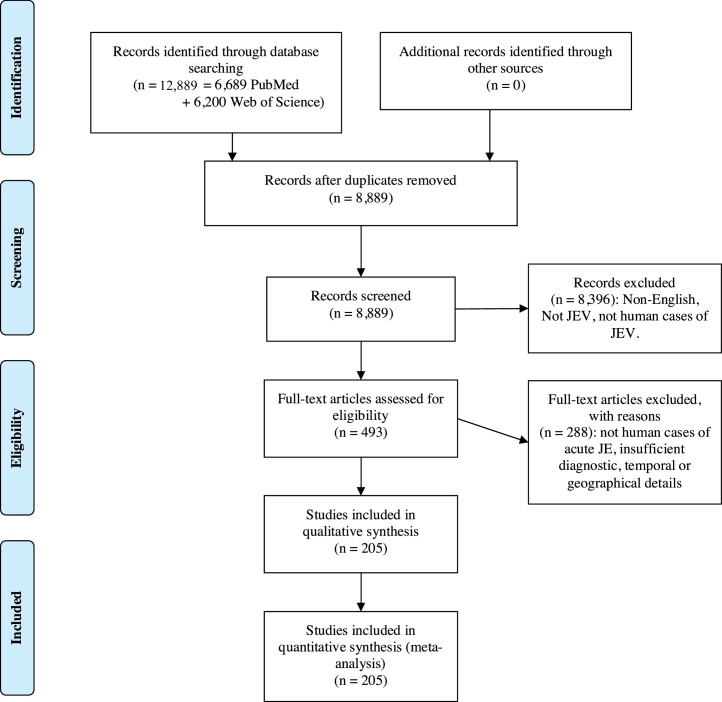
This review identified 205 studies in 22 countries in which a total of 20,212 JE patients were confirmed by laboratory tests; see Figure 2 for the PRISMA flow diagram. Patients were predominantly diagnosed in Asia, with a suggested case of autochthonous transmission diagnosed in Angola. The studies incorporated a variety of methods for the diagnostic tests, including conventional and novel approaches, as summarised in Table 3. The data do not provide evidence of change in the certainty of diagnosis through time, see Table 4.
Figure 2.
PRISMA flow diagram.
Table 3.
Diagnostic methods used for evidence of Japanese encephalitis virus infection.
| Diagnostic method | Confirmatory level | Advantage | Disadvantage |
|---|---|---|---|
|
Virus detection: Virus Isolation: Inoculation of patient samples into animals Inoculation of patient samples onto primary chick or duck embryo cells, and cell lines including Vero, LLCMK, C6/36, MRC and AP61 Inoculation of patient samples into mosquitoes Virus antigen detection: Reverse passive haemagglutination Immunofluorescence microscopy Staphylococcal coagglutination tests using polyclonal or monoclonal antibodies Monoclonal antibody/immunogold/silver-staining (M-IGSS) ELISA to detect a viral protein (NS1) |
Level 1 | Direct detection of the virus or viral protein and high specificity Viral isolation provides molecular epidemiological data |
Low sensitivity, laborious and viral isolation requires biosafety 3 laboratory capacity |
|
Molecular detection: Conventional RT-PCR Real-time RT-PCR Nested PCR Specific vs. pan-flavivirus Multiplex PCR Next-generation sequencing |
Level 1 | Direct detection of the viral genome, provides high specificity and additional molecular epidemiological data | Low sensitivity |
|
Antibody detection: Seroneutralization IgM antibody capture ELISA Avidin biotin system Biotin-labeled immunosorbent assay Nitrocellulose membrane-based immunoglobulin M capture dot enzyme immunoassay Haemagglutination inhibition +/- sucrose gradient density centrifugation and 2-mercaptoethanol treatment (2-ME) to detect IgM Complement fixation test Single radial hemolysis |
Level 2 for seroconversion demonstrated by neutralization Level 3 for detection of IgM in CSF and for seroconversion or ≥4x rise in Ab titer; Level 4 for detection of Ab detection in a single sample |
Good sensitivity Good specificity for primary infection Commercial kit available Good sensitivity |
Cross-reaction with other flaviviruses Requires paired samples Laborious Difficult to interpret in secondary infection Limited specificity Cross reaction with other flaviviruses Requires paired samples Difficult to interpret in secondary infection |
Table 4.
Temporal changes in JE diagnostic confirmatory level. Percentage of symptomatic human JE cases reported in the English-language literature in blocks of five years that were confirmed by laboratory testing Level 1-4*.
 |
A total of 20,212 laboratory-confirmed JE cases were identified. Data are reported according to the year of publication. Inclusion criteria also required geographical (country) and temporal (year) data. Confirmatory levels of JE diagnosis detailed in Table 2; level 1 provides the highest level of confidence, level 3 or 4 refers to cases that were reported as IgM detected in CSF and/or serum.
Overview of JEV diagnostic testing
The first isolation of JEV was in 1934 when Hayashi demonstrated that a filterable agent inoculated into monkeys produced encephalitis (Hayashi, 1933). The experiment was performed using homogenized brain obtained at post-mortem from a fatally-infected child who presented with encephalitis in Tokyo during the 1924 epidemic. Early studies relied on clinicopathological correlates in infected humans, when compared with those observed following animal inoculation of post-mortem samples. Subsequently, hamster, porcine, and human cell culture systems were developed, which revealed cytopathic effects when inoculated with JEV-infectious specimens (Miyake, 1964, GC-Y et al., 1965). As these procedures improved, mosquitoes and mosquito-cell cultures were added to the resources for isolation and identification of JEV (Gajanana et al., 1996, Johnson et al., 1985). Cerebrospinal fluid (CSF) and other body fluids were also included for analysis (Kumar et al., 1990, Kumar et al., 1994). Subsequently, JEV antigen detection procedures including complement fixation, immunofluorescence microscopy of cells in CSF, reverse passive haemagglutination, and staphylococcal coagglutination (Mathur et al., 1982, Ravi et al., 1989a, Zhang et al., 1989, Mathur et al., 1990) were added to the list of diagnostic tests. Nonetheless, assays involving direct virus detection are minimally useful for the diagnosis of JE as the level of viremia is usually low, and the virus is detectable only briefly early in the infection (Kumagai and Kurokochi, 1950).
In the mid-twentieth century, investigation of the antigenic properties of JEV soon led to the development of serological assays, including complement fixation (Casals and Palacios, 1941a), inhibition of haemagglutination (Clarke and Casals, 1958), and virus neutralization tests (Sabin, 1947, Sever, 1962). Early reports of human infection in 1947 used a seroneutralization technique in which a patient sample was mixed with virus and inoculated into mice (Sabin, 1947, Sabin et al., 1947a, Kuttner and Ts’un, 1936). In 1941, Casals and Palacios published a report on the application of the complement fixation technique (Casals and Palacios, 1941b). The method was used for many years, although it was insensitive, particularly during the acute illness (Rose, 1992). In 1958, Clarke and Casals published a report on the application of the haemagglutination inhibition test (HI) (Clarke and Casals, 1958). The principle exploits the fact that JEV envelope protein agglutinates erythrocytes. Anti-JEV antibodies, developed following infection, bind to JEV protein and thus prevent erythrocyte agglutination, hence the term haemagglutination-inhibition. This remained the method of choice for JE diagnosis, by serological methods, for many years (Okuno et al., 1975, Ding et al., 2007, Barzaga, 1989, Thisyakorn and Nimmannitya, 1985) and was subsequently adapted as a more convenient microtiter method (Gajanana et al., 1996, Sever, 1962, Gunakasem et al., 1981). However, limitations in the sensitivity and specificity of the assay were recognized by Clarke and Casals (Clarke and Casals, 1958). Moreover, the test relies on the combined paired results obtained from acute and convalescent serum samples, thus taking weeks for confirmation (Burke et al., 1987). Other serological methods, such as single-radial hemolysis (Chan et al., 1985, Duca et al., 1979, George and Pavri, 1986), were also introduced. However, inadequacies were readily acknowledged, and it was accepted practice to perform these tests in parallel with others, thus increasing the workload and extending the time for results to be obtained (Buescher et al., 1959, Cardosa et al., 1991).
The plaque-reduction neutralization test (PRNT) was subsequently developed as the gold-standard for JE diagnosis, using paired acute and convalescent sera and comparison with other endemic flaviviruses, and it remains so today (Hills et al., 2009). The demonstration of increasing anti-JEV neutralizing antibody titer in the convalescent serum and the absence, or at least fourfold lower titer for neutralizing antibodies against control-related flaviviruses, provides a robust diagnosis. However, this is laborious, time-consuming, and requires high-level containment facilities for safe manipulation of infectious JEV in cell culture. Mainly for these reasons, the anti-JEV IgM capture ELISA (JEV MAC-ELISA) was developed during the 1980s and has been incorporated as the WHO standard procedure for JE diagnosis (Burke et al., 1982, World Health Organization, 2007). Although commercial JEV MAC ELISAs are manufactured, they may be hard to access in endemic countries (for example, there is no supplier in Laos), relatively expensive, and require costly ELISA readers and significant technical training. The performance of the kit requires specialized laboratory equipment, and thus are by no means point-of-care tests. In addition, field studies suggest that the sensitivity is 50-70% (Robinson et al., 2010), and concerns have been raised regarding the diagnostic specificity (Dubot-Peres et al., 2015). In the last two decades, there has been increased availability of molecular testing, providing crucial data on molecular epidemiology. As well, the aforementioned low and brief viremia limits the role of screening for JEV RNA for diagnostic purposes. Similarly, advances in techniques such as near-atomic resolution cryo-electron microscopy contribute to our understanding of the detailed viral structure, but not to the routine detection of human infection (Figure 3).
Figure 3.
Chronological representation of discoveries related to the detection of Japanese encephalitis virus infection (Miyake, 1964, Sever, 1962, Kuttner and Ts’un, 1936, Casals and Palacios, 1941b, Rose, 1992, Okuno et al., 1975, Casals and Palacios, 1941c, Ravi et al., 1989b).
Specific findings of JEV diagnostics review
Studies reporting on the use of seroneutralization, IgM ELISA and RT-PCR are discussed below, since these assays are, at present, the ones most widely incorporated into clinical diagnostics.
Seroneutralization assays: Thirty-two studies identifying evidence of JE using neutralization assays (see Table 5) are included, of which 15 clearly performed tests on acute and convalescent sera, indicating seroconversion (Cardosa et al., 1991, Peiris et al., 1992, CDC, 2005, CDC, 2011, Anga et al., 2010, Anukumar et al., 2014a, Hennessy et al., 1996, Hossain et al., 2010, Langevin et al., 2012a, Lee et al., 2012, Li et al., 2016, Olsen et al., 2010, Ompusunggu et al., 2008, Saito et al., 1999a, Solomon et al., 2008b, Sunwoo et al., 2016, Touch et al., 2009a). These were largely PRNT (14 articles) and/or a microtiter modification in a 96-well plate (4 articles). Three studies performed focus-reduction seroneutralization tests (FRNT), a high-throughput modification of the PRNT involving 96-well plates and an immunocalorimetric assay for end-point determination. Other studies did not describe their methods in detail or cite references to support them. Eleven reported JEV strain used (Sabin (1947); Cardosa et al., 1991; Li et al. (2016); Saito et al. (2015); Benenson et al., 1975a; Ravi et al., 2009; Desai et al. (1997a); Anukumar et al., 2014b; Kyaw et al., 2019; Sabin et al., 1947b; Borah et al., 2011b): they were all genotype 3 viruses, except for one that reported the use of genotype 1 and 3 strains to enable neutralization-based genotype differentiation (Saito et al., 2015). Five studies indicated JEV inoculating dose: the end-point was identified by visual inspection of the cytopathic effect (CPE), staining, or immunofluorescence. All reports appeared to use two-fold dilutions of serum samples, between 1:10 to 1:640 or higher. In terms of quality control, three studies detailed other viruses used, and the use of replicates. Five studies (Sabin (1947); Cardosa et al., 1991; Ravi et al., 2009; Anukumar et al., 2014b; Sabin et al., 1947b) included the use of other flaviviruses such as dengue viruses, West Nile virus, or yellow fever virus.
Table 5.
Details of seroneutralization testing.
| Reference | Country sampling | Country testing | Technique | JEV Strain | Other viruses tested | Cells | Virus Dose | End-point | Samples tested | Algorithm for seroneutralisation |
|---|---|---|---|---|---|---|---|---|---|---|
| Sabin et al. (1947a) | Republic of Korea | Japan | Mice inoculation | G3 (Nakayama), human brain, Tokyo, Japan, 1935 (EF571853) | NR | Intracerebral and intraperitoneal inoculation in mice | NR | NR | Acute and f/up serum, and CSF | All samples |
| Sabin et al. (1947b) | China | Japan | Mice inoculation | G3 (Nakayama), human brain, Tokyo, Japan, 1935 (EF571853) | NR | Intracerebral and intraperitoneal | NR | NR | Acute and f/up serum, and CSF | All samples |
| Sabin (1947) | Japan | Japan | Mice inoculation | G3 (Nakayama), human brain, Tokyo, Japan, 1935 (EF571853) | NR | Intracerebral and intraperitoneal | NR | NR | Acute and f/up serum, and CSF | All samples |
| Edelman and Pariyanonda (1973) | Vietnam | Vietnam | PRNT | NR | DENV | NR | NR | NR | NR | NR |
| Benenson et al. (1975b) | Thailand | Thailand | PRNT | G3 strain (Nakayama), human brain, Tokyo, Japan, 1935 (EF571853) | DENV 4 | LLC-MK2 cells | 50-100 PFU | NR | Acute and f/up serum | NR |
| Hoke et al. (1988) | Thailand | Thailand | NR | NR | NR | NR | NR | NR | NR | NR |
| Cardosa et al. (1991) | Malaysia | Malaysia | PRNT | G3 strain (Nakayama), human brain, Tokyo, Japan, 1935 (EF571853) | DENV 2 (16681 strain) | Aedes albopictus C6/36 cells | NR | 50%* | Acute and f/up serum | Confirmatory testing after positive MAC-ELISA |
| Peiris et al. (1992) | Sri Lanka | Sri Lanka | Microtitre VNT | NR | NR | Porcine stable (PS) kidney cells | NR | 80%* | Serum (NR if acute and/or f/up) | NR |
| Wittesjo et al. (1995) | Indonesia | Sweden | PRNT | NR | NR | NR | NR | 80%* | Acute and f/up serum | NR |
| Hennessy et al. (1996) | China | U.S.A. | PRNT | NR | NR | NR | NR | NR | CSF, Acute and f/up serum | All samples |
| Desai et al. (1997b) | India | India | Microtitre VNT | G3 (P20778/P20), human brain, Vellore, India, 1958 (AF080251) | NR | Porcine stable (PS) kidney cells | 100 TCID 50 | 100%* | CSF | All samples |
| Saito et al. (1999c) | Japan | Japan | FRNT | NR | YFV | BHK-21 cells | NR | 50%* | CSF, Acute and f/up serum | All samples |
| Tiroumourougane et al. (2003) | India | India | NR | NR | NR | NR | NR | NR | NR | NR |
| Cutfield et al. (2005) | China | New Zealand | NR | NR | NR | NR | NR | NR | Acute and f/up serum | NR |
| CDC (2005) | Thailand | U.S.A. | NR | NR | NR | NR | NR | NR | Acute and f/up serum | NR |
| Ompusunggu et al. (2008) | Indonesia | Indonesia | PRNT | NR | NR | NR | NR | NR | Serum (NR if acute and/or convalescent) | NR |
| Lehtinen et al. (2008) | Thailand | Finland | PRNT | NR | DENV 2 | NR | NR | NR | Acute and f/up serum | NR |
| Ravi et al. (2009) | India | India | PRNT | ChimeriVax™-JEV | ChimeriVax™-DENV 2 | Vero cells | NR | NR | CSF | Confirmatory testing after positive or equivocal MAC-ELISA |
| Touch et al. (2009b) | Cambodia | Cambodia | PRNT | NR | NR | Vero cells | NR | NR | NR | NR |
| Anga et al. (2010) | Papua New Guinea | Australia | PRNT | NR | NR | NR | NR | NR | NR | NR |
| Hossain et al. (2010) | Bangladesh | U.S.A. | PRNT | NR | NR | NR | NR | 90%* | NR | NR |
| CDC (2011) | U.S.A. (Travellers from the Philippines and Thailand) | U.S.A. | NR | NR | NR | NR | NR | NR | CSF | NR |
| Borah et al. (2011b) | India | India | Microtitre VNT | G3 strain (P20778/P20), human brain, Vellore, India, 1958 (AF080251) | NR | BHK-21 cells | 100 TCID50 in 50 μL | 50%* | Acute and f/up serum | Patients with paired serum available after MAC-ELISA tested |
| Lee et al. (2012) | Republic of Korea | Republic of Korea | NR | Not reported | NR | NR | NR | NR | Acute and convalescent serum | Confirmatory testing after positive MAC-ELISA/HI/IIF. |
| Langevin et al. (2012b) | Canada (Traveller from Thailand) | Canada | NR | NR | WNV and DENV | NR | NR | NR | CSF, Acute and convalescent serum | All samples |
| Hills et al. (2014) | China, Taiwan, Republic of Korea | U.S.A. | NR | NR | NR | NR | NR | NR | Acute and f/up serum | NR |
| Anukumar et al. (2014b) | India | India | Microtitre VNT | G3 (P3), human brain, Bankura, India, 1973 (AB379813/Z34095) | WNV | Porcine stable (PS) kidney cells | 100 TCID50 | 50%* | Acute serum | All acute serum |
| Rayamajhi et al. (2015) | Nepal | U.S. | PRNT | NR | DENV, WNV, and Powassan viruses. | NR | NR | NR | NR | Confirmatory testing after positive or equivocal MAC-ELISA |
| Saito et al. (2015) | Laos | Japan | FRNT | Nakayama (a pathogenic and vaccine strain, Tokyo, Japan, human brain, 1935, G3), Beijing-1 (a pathogenic and vaccine strain, Beijing, China, human brain, 1949, G3), P19-Br (an isolate, Chiang Mai, Thailand, human brain, 1982, G1), LaVS56 (an isolate, Vientiane, Lao PDR, swine sera, 1993, G1), and LaVS145 (an isolate, Vientiane, Lao PDR, swine sera, 1993, G1) | DENV 1 (Hawaiian), 2 (New Guinea B), 3 (H-87), and 4 (H-241) and WNV | BHK-21 cells | NR | 50%* | Acute and f/up serum | All samples |
| Li et al. (2016) | China | China | PRNT | G3 strain (733913), human brain, Beijing, China, 1949 (AY243805/AY243844) | NR | BHK-21 cells | 100 PFUs | 90%* | Acute and f/up serum | All serum |
| Sunwoo et al. (2016) | Republic of Korea | Republic of Korea | NR | NR | NR | NR | NR | NR | NR | NR |
| Kyaw et al. (2019) | Myanmar | Myanmar | FRNT and PRNT | G3 strain (JaOrS982), mosquitos, Japan, 1982 (NC_001437) | DENV 1-4 | NR | NR | NR | CSF | NR |
G1 and 3 = genotype 1 and 3; NR = not reported; CSF = cerebrospinal fluid; PRNT = plaque reduction neutralization test; DENV = Dengue virus; VNT = viral neutralization test; FRNT = focus reduction neutralization test; TCID = median tissue culture infectious dose.
titer required to reduce dengue viral plaques/focus/CPE by 50%, 80%, or 90%. MAC-ELISA = IgM antibody capture enzyme-linked immunosorbent assay, HI = haemagglutination assay, IIF = Indirect immunofluorescence assay.
Studies followed different algorithms for including neutralization in patient testing, but it was primarily performed to confirm equivocal cases in other serological tests. Acute and/or follow-up serum and/or CSF were tested. For studies that did report individual results, confirmation was rarely achieved as there was either insufficient serum, failure to detect a four-fold rise of antibody titer in the convalescent serum, or cross-reactivity was detected with related viruses included as controls in the tests.
IgM ELISA: One hundred and sixty-three (80%) studies reported the results of tests using IgM MAC-ELISA methods. Notably, 115 of these studies tested both CSF and serum samples and presented results for the different body fluids separately. One hundred and twenty-two (74%) reported the method, of which 66 (40%) used in-house methods, and 33 (20%) used commercial kits. The primary in-house methods involved those described by Burke et al. (1982), Innis et al. (1989), the National Institute of Virology, Pune (Prasad et al., 1993). Commercial kits were purchased from PanBio (Touch et al., 2009b), Venture Technologies (Cardosa et al., 2002), XCyton Diagnostics Ltd. (Borthakur et al., 2013), and Shanghai B&C Biological Technology Co. Ltd. (Feng et al., 2013). There was minimal reporting of quality control measures such as control specimens and repeat testing of positives; 46 (28%) reported following the manufacturer’s instructions. In total, 7,584 JE patients (38%) were diagnosed by MAC-ELISA in serum and/or CSF, i.e., results for the different body fluids were not reported separately, with 3,668 (18%) positive in CSF alone. Ninety-one (56%) studies using MAC-ELISA also reported testing for dengue virus infection to confirm specificity for JEV, i.e., they were not cross-reactive with dengue viruses.
Molecular tests: Forty-one studies (25%) reported the use of reverse transcription-polymerase chain reaction tests (RT-PCR), of which 13 (68%) described the methods used or cited corresponding references. These targeted various regions of JEV genome, including the capsid (C), pre-membrane (prM), envelope (E), non-structural (NS) proteins NS2A, NS3, NS5 and the untranslated regions (UTR). These studies reported the use of the conventional RT-PCR standard test, with nested or hemi-nested techniques, and real-time techniques (RT-qPCR) using hydrolysis probes or SYBR green. The studies reported the use of conventional RT-PCR either standard, with nested or hemi-nested techniques, and the real-time techniques (RT-qPCR) using hydrolysis probes or SYBR green. In total, 332 (1.7%) patients were positive when tested by RT-PCR.
Other tests: Fifty-eight (28%) studies reported the isolation of JEV in vivo or in vitro. Forty-two (20%) performed HI, 14 (7%) performed complement fixation, and seven (4%) performed indirect immunofluorescence assays. Two (1%) studies diagnosed cases by next-generation sequencing.
Discussion
This review reveals that current JE diagnostic techniques are confined mainly to those with a low confidence level, i.e., anti-JEV IgM detected in serum samples, or in which reported results do not differentiate between detection of anti-JEV IgM in CSF and serum. There is no doubt that the introduction of IgM ELISA testing in serum samples represents real progress. However, we are now much better informed about the limitations of relying on this method of detection. We also acknowledge as a limitation that the studies included in this review were performed in different settings, with different constraints, financial limitations, and resources available. Nonetheless, we highlight the need for both an improvement in the accuracy of routine laboratory diagnostics, and also the development of point-of-care tests to confirm cases in JE endemic areas that frequently have no laboratory capacity. Below, we discuss the existing assays in more detail.
Seroneutralization tests: Seroneutralization is considered the gold standard for the diagnosis of infections due to pathogenic viruses such as JEV, but in the published literature cited here, it was only performed in approximately 16% (32/205) of studies as laboratory confirmation. This is probably because performing seroneutralization is technically demanding and requires sufficient volumes of serum/CSF to enable the inclusion of control viruses and duplicates of each titration. Since JEV is a human pathogen with high individual risk, seroneutralization has to be performed in a biosafety level 3 laboratory, placing additional burdens on time, cost, and qualified personnel. Another potential complication may arise when sera from patients who have previously been exposed to JEV-related flaviviruses may contain higher titers against the closely related flaviviruses than against JEV (“doctrine of original antigenic sin”) (Francis, 1960). For example, the titers of anti-YFV neutralizing antibodies were higher than anti-JEV neutralizing antibodies in JE patients who had previously received the yellow fever vaccine (Saito et al., 1999c).
Similarly, in a study testing for West Nile virus and JEV, 18 patients’ data remained equivocal due to high levels of antigenic cross-reactivity between these viruses (Anukumar et al., 2014b). The neutralization test may only be strictly applicable as the gold-standard for vaccine efficacy studies when a baseline serum sample is compared with a convalescent sample taken at a fixed interval 1-3 months later. To confirm acute JEV, neutralization is an imperfect gold standard. Severe constraints on being able to arrange for sample testing by neutralization, and the results being interpretable without cross-reactive positivity due to other flaviviruses (which is relatively rare in JEV endemic areas), impede 'neutralization confirmation.' The neutralization titers obtained may be affected by the particular strain of challenge virus utilized (Ferguson et al., 2008). A final issue with the neutralization test is the inability to detect non-neutralizing antibodies, thus potentially reducing the analytical sensitivity (Johnson et al., 2016). Therefore, the practicalities of PRNT and diagnostic yield when testing field samples can be low, although the specificity is potentially high.
IgM ELISA: Anti-JEV IgM detection by MAC-ELISA is the WHO recommended standard diagnostic test, and 80% (163/205) of studies reported the use of a MAC-ELISA. It is recognized that JEV diagnosis by testing CSF provides considerably more reliable confirmation than the use of serum (Granerod et al., 2010a). However, obtaining acute and convalescent CSF and serum samples can be difficult, particularly in rural Asia where access is logistically tricky, and personnel and appropriate facilities are limited. Only 91 (56%) studies reported contemporaneous testing for anti-dengue-specific antibodies. There are issues in the accurate full reporting of results, both for the breakdown of which patients were diagnosed by testing CSF and/or serum, and contemporaneous testing for dengue-specific antibodies.
Reverse-transcription polymerase chain reaction assays (RT-PCR) for the detection of JEV RNA:
Diagnosis by the detection of the viral genome by DNA amplification generated by RT-PCR is a valuable addition to diagnostic procedures for RNA viruses. The test has high analytical sensitivity, is very specific, and can provide additional information that can be exploited to understand the molecular epidemiology of the detected virus. Nonetheless, JE cases are rarely confirmed (1.7% in this review) using RT-PCR technology, although this will undoubtedly increase in usage as point-of-care and automated methods are developed. Poor reporting of the techniques used in many publications hinders our ability to make comparisons of the efficacy of different methods (see Bharucha et al. (2018a). There does appear to be higher analytical sensitivity in studies that used nested and hemi-nested techniques as compared to those using single RT-PCR; however, these techniques are notoriously prone to contamination, causing false-positive results. It is also recognized that the sensitivity of nucleic acid (and protein) detection will continue to increase as technology improves (Bai et al., 2018, Zang et al., 2019). Evidence to suggest that this will be the case arises from the high cycle threshold (Ct) of patients that are confirmed, and the fact that blood donor transmission has been seen in WNV patients who were negative by RT-qPCR tests (Dodd et al., 2015). The recent detection of JEV RNA in throat swabs of JE patients suggests that this non-invasive procedure may marginally improve diagnostic yield (Bharucha et al., 2018b). There have now been two cases of JE, confirmed by RT-PCR, that were first identified by metagenomic next-generation sequencing (mNGS) (Simon-Loriere et al., 2017b, Mai et al., 2017); the first detection of JEV RNA in human urine, and JEV detection in serum from an African patient with a co-YFV infection. The latter was not confirmed by an orthogonal method and remains questionable. Nonetheless, unbiased mNGS technology (see below), application, and reporting will continue to improve, and could potentially detect JEV in new locations (Brown et al., 2018).
Requirements of a new test for the detection of JEV infection
CNS infections are challenging syndromes to diagnose and treat, even in the most highly resourced centers (Kelly et al., 2012, Solomon et al., 2012). It is estimated that they may be caused by >100 different pathogens, including novel and emerging pathogens (Granerod et al., 2010b). Current approaches to diagnosis in routine clinical practice involves targeted strategies, suggesting that some (potentially treatable) infectious aetiologies are missed (Brown et al., 2018). Clinical diagnosis is rarely absolute, and confirmation requires access to appropriate laboratory facilities and personnel, lacking in many areas worldwide (Wilson et al., 2018). While the analysis of brain biopsy material is the gold standard, it is not possible in most cases. Aetiological diagnosis usually involves an invasive lumbar puncture (LP) to obtain CSF, which in turn requires the appropriate clinical skills, infrastructure, and patient acceptability. Diagnostic assays are frequently difficult to interpret, may demonstrate poor accuracy and poor discrimination between previous vaccination or non-neurological JEV infection (Granerod et al., 2010a). Targeted research is needed to raise the bar for both the improvement in laboratory diagnostics as well as the development of point-of-care tests (John et al., 2015).
In clinical and epidemiological situations, the detection of JEV RNA can provide an invaluable indication of infection. The sensitivity of this test is, to a large extent, limited by a combination of the short period of viremia, the relatively low concentration of virus in CSF, and the fragility of RNA. The introduction of highly sensitive point-of-care tests that may be used to analyze multiple body fluids in parallel would partly resolve these challenges. Recently, there have been significant developments in highly sensitive molecular point-of-care tests for flaviviruses, such as reverse transcription loop-mediated isothermal amplification (RT-LAMP), reverse transcription recombinase polymerase amplification (RT-RPA), nucleic acid sequence-based amplification (NASBA), transcription-mediated amplification (TMA), helicase-dependent amplification (HDA), and nicking enzyme amplification reaction (NEAR) (Calvert et al., 2017, Ganguli et al., 2017, Kurosaki et al., 2017, Mauk et al., 2017, Priye et al., 2017, Yaren et al., 2018, Castro et al., 2018, Guo et al., 2018, Kim et al., 2018a, Kumar et al., 2018, Lopez-Jimena et al., 2018, Lamb et al., 2018, Sabalza et al., 2018, Song et al., 2018, Zhao and Feng, 2019, Abd et al., 2017, Chan et al., 2018, Tan et al., 2018, Vasileva Wand et al., 2018, Saa et al., 2018). Microfluidics, chips, paper-based devices, and biosensors are also being developed (Onyango et al., 2017, Adegoke et al., 2017, Afsahi et al., 2018, Ariffin et al., 2018, Wasik et al., 2017).
For the time being, we will need to rely on serology for diagnostic confirmation. During the past three years, with the international focus on emerging flaviviruses following the chikungunya virus and theZika virus global epidemics, there have been intensified efforts to reduce cost, increase throughput, and improve specificity. These include the analysis of IgA (Amaro et al., 2019, Warnecke et al., 2019, Colonetti et al., 2018, Nascimento et al., 2018, Rockstroh et al., 2017, Zhang et al., 2017, Huang et al., 2017, Balmaseda et al., 2008, Balmaseda et al., 2003, Yap et al., 2011) and IgG subclasses (Nascimento et al., 2018), antibody avidity (Amaro et al., 2019, de Vasconcelos et al., 2018, Ronnberg et al., 2017, Tsai et al., 2018, Shen et al., 2017), incorporation of blocking agents, IgG depletion (Calvert et al., 2018) and production of specific monoclonal antibodies for identification of specific viral epitopes (Zhu et al., 2018, Lebani et al., 2017, Piyasena et al., 2017, Kim et al., 2018b, Frietze et al., 2017). This recent work highlights the inherent challenges of serological techniques for JE identification. As Lindsey et al. describe, antigenic cross-reactivity between related viruses can make it virtually impossible to distinguish the cause of the infection (Lindsey et al., 2018). For example, cross-reactive IgM class antibodies may not be stimulated during a related secondary flavivirus infection. On the other hand, IgA may be produced during a secondary flavivirus infection, and a laboratory-defined ‘seroconversion’ might be detected following a secondary flavivirus infection by a related flavivirus.
Evidence suggests that the secreted viral JEV non-structural protein 1 (NS1) is present at very low concentrations in serum or CSF, unlike in dengue (Li et al., 2012, Kumar et al., 2011). A novel alternative approach would be to analyze the host response, using transcriptomics or proteomics. However, questions of specificity and how these would be translated into point-of-care tests will require detailed investigation and the development of innovative methodologies.
In summary, while the diagnosis of JE has been possible for many years, it still requires specialized high-containment laboratories and appropriately trained scientists. Therefore, it cannot be reliably carried out in many resource-limited regions where JEV is endemic/epidemic. A fundamental pre-requisite in the public health strategy for the control of JE is lacking, that of a reliable and simple diagnostic procedure that can be adapted for point-of-care tests, and readily available for use throughout JEV endemic regions of the world. Improved diagnostic capabilities throughout JEV affected areas will not only benefit individual patients (through accurate diagnosis) but lead to higher quality surveillance data and a better understanding of the distribution of JE risk, enabling improved targeting and evaluation of interventions. The lack of diagnostic capabilities for JE is a barrier to understanding the actual disease burden and the impact of public health strategies.
Ethical approval
Ethical approval was not required for this study.
Conflict of interest statement
None of the authors have any conflict of interest to report.
References
Acknowledgment
The authors acknowledge Joshua Longbottom and Kirsten Duda for helping to assemble the study reference library. The work was supported by the University of Oxford and the Medical Research Council [grant number MR/N013468/1]. It was also supported by the Oxford Glycobiology endowment, the Institute of Research for Development (IRD), Aix-Marseille University, the Wellcome Trust of Great Britain that supports the work of LOMWRU.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.03.039.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abd E., Wahed A., Sanabani S.S., Faye O., Pessoa R., Patriota J.V. Rapid molecular detection of Zika virus in acute-phase urine samples using the recombinase polymerase amplification assay. PLoS Curr. 2017;9 doi: 10.1371/currents.outbreaks.a7f1db2c7d66c3fc0ea0a774305d319e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adegoke O., Morita M., Kato T., Ito M., Suzuki T., Park E.Y. Localized surface plasmon resonance-mediated fluorescence signals in plasmonic nanoparticle-quantum dot hybrids for ultrasensitive Zika virus RNA detection via hairpin hybridization assays. Biosens Bioelectron. 2017;94:513–522. doi: 10.1016/j.bios.2017.03.046. [DOI] [PubMed] [Google Scholar]
- Afsahi S., Lerner M.B., Goldstein J.M., Lee J., Tang X., Bagarozzi D.A., Jr. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens Bioelectron. 2018;100:85–88. doi: 10.1016/j.bios.2017.08.051. [DOI] [PubMed] [Google Scholar]
- Amaro F., Sanchez-Seco M.P., Vazquez A., Alves M.J., Ze-Ze L., Luz M.T. The application and interpretation of IgG Avidity and IgA ELISA Tests to Characterize Zika virus infections. Viruses. 2019;11(2) doi: 10.3390/v11020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anga G., Barnabas R., Kaminiel O., Tefuarani N., Vince J., Ripa P. The aetiology, clinical presentations and outcome of febrile encephalopathy in children in Papua New Guinea. Ann Trop Paediatr. 2010;30(2):109–118. doi: 10.1179/146532810X12703902243818. [DOI] [PubMed] [Google Scholar]
- Anukumar B., Sapkal G.N., Tandale B.V., Balasubramanian R., Gangale D. West Nile Encephalitis outbreak in Kerala, India, 2011. J Clin Virol. 2014;61(1):152–155. doi: 10.1016/j.jcv.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Anukumar B., Sapkal G.N., Tandale B.V., Balasubramanian R., Gangale D. West Nile encephalitis outbreak in Kerala, India, 2011. J Clin Virol. 2014;61(1):152–155. doi: 10.1016/j.jcv.2014.06.003. [DOI] [PubMed] [Google Scholar]
- Ariffin E.Y., Tan L.L., Abd Karim N.H., Yook Heng L. Optical DNA Biosensor Based on Square-Planar Ethyl Piperidine Substituted Nickel(II) Salphen Complex for Dengue Virus Detection. Sensors (Basel, Switzerland) 2018;18(4) doi: 10.3390/s18041173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Xiao Y., Suo Y., Shen Y., Shao Y., Zhang D. Enhancement of PCR Sensitivity and Yield Using Thiol-modified Primers. Sci Rep. 2018;8(1):14858. doi: 10.1038/s41598-018-33223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmaseda A., Guzman M.G., Hammond S., Robleto G., Flores C., Tellez Y. Diagnosis of dengue virus infection by detection of specific immunoglobulin M (IgM) and IgA antibodies in serum and saliva. Clin Diagn Lab Immunol. 2003;10(2):317–322. doi: 10.1128/CDLI.10.2.317-322.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmaseda A., Saborio S., Tellez Y., Mercado J.C., Pérez L., Hammond S.N. Evaluation of immunological markers in serum, filter-paper blood spots, and saliva for dengue diagnosis and epidemiological studies. J Clin Virol. 2008;43:287–291. doi: 10.1016/j.jcv.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Barzaga N.G. A review of Japanese Encephalitis cases in the Philippines (1972-1985) Southeast Asian J Trop Med Public Health. 1989;20(4):587–592. [PubMed] [Google Scholar]
- Benenson M.W., Top F.H., Jr., Gresso W., Ames C.W., Altstatt L.B. The virulence to man of Japanese encephalitis virus in Thailand. Am J Trop Med Hyg. 1975;24(6 Pt 1):974–980. doi: 10.4269/ajtmh.1975.24.974. [DOI] [PubMed] [Google Scholar]
- Benenson M.W., Top F.H., Gresso W., Ames C.W., Altstatt L.B. Virulence to man of Japanese Encephalitis-virus in Thailand. Am J Trop Med Hyg. 1975;24(6):974–980. doi: 10.4269/ajtmh.1975.24.974. [DOI] [PubMed] [Google Scholar]
- Bharucha T., Sengvilaipaseuth O., Vongsouvath M., Vongsouvath M., Davong V., Panyanouvong P. Development of an improved RT-qPCR Assay for detection of Japanese encephalitis virus (JEV) RNA including a systematic review and comprehensive comparison with published methods. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha T., Sengvilaipaseuth O., Seephonelee M., Vongsouvath M., Vongsouvath M., Rattanavong S. Detection of Japanese Encephalitis virus RNA in Human Throat Samples in Laos – a pilot study. Sci Rep. 2018;8 doi: 10.1038/s41598-018-26333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah J., Dutta P., Khan S.A., Mahanta J. A comparison of clinical features of Japanese encephalitis virus infection in the adult and pediatric age group with Acute Encephalitis Syndrome. J Clin Virol. 2011;52(1):45–49. doi: 10.1016/j.jcv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Borah J., Dutta P., Khan S.A., Mahanta J. A comparison of clinical features of Japanese encephalitis virus infection in the adult and pediatric age group with Acute Encephalitis Syndrome. J Clin Virol. 2011;52(1):45–49. doi: 10.1016/j.jcv.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Borthakur A., Das N., Bora B. Data from the World Health Organization (WHO) National Network Laboratory for Japanese Encephalitis. J Glob Infect Dis. 2013;5(2):76–79. doi: 10.4103/0974-777X.112294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.R., Bharucha T., Breuer J. Encephalitis diagnosis using metagenomics: application of next generation sequencing for undiagnosed cases. J Infect. 2018;76(3):225–240. doi: 10.1016/j.jinf.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher E.L., Scherer W.F., Grossberg S.E., Chanock R.M., Van Philpot B. Immunologic studies of Japanese Encephalitis virus in Japan. I Antibody Responses Following Overt Infect Man. 1959;83(6):582–593. [PubMed] [Google Scholar]
- Burke D.S., Nisalak A., Ussery M.A. Antibody capture immunoassay detection of japanese Encephalitis virus immunoglobulin m and g antibodies in cerebrospinal fluid. J Clin Microbiol. 1982;16(6):1034–1042. doi: 10.1128/jcm.16.6.1034-1042.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D.S., Nisalak A., Gentry M.K. Detection of flavivirus antibodies in human serum by epitope-blocking immunoassay. J Med Virol. 1987;23(2):165–173. doi: 10.1002/jmv.1890230209. [DOI] [PubMed] [Google Scholar]
- Calvert A.E., Biggerstaff B.J., Tanner N.A., Lauterbach M., Lanciotti R.S. Rapid colorimetric detection of Zika virus from serum and urine specimens by reverse transcription loop-mediated isothermal amplification (RT-LAMP) PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0185340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert A.E., Boroughs K.L., Laven J., Stovall J.L., Luy B.E., Kosoy O.I. Incorporation of IgG depletion in a neutralization assay facilitates differential diagnosis of Zika and Dengue in secondary flavivirus infection cases. J Clin Microbiol. 2018;56(6) doi: 10.1128/JCM.00234-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G., Hills S., Fischer M., Jacobson J., Hoke C., Hombach J. Estimated global incidence of Japanese Encephalitis. Bull World Health Organ. 2011;89:766–774. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Fu S., Gao X., Li M., Cui S., Li X. Low protective efficacy of the current Japanese Encephalitis vaccine against the emerging genotype 5 Japanese Encephalitis virus. PLoS Negl Trop Dis. 2016;10(5) doi: 10.1371/journal.pntd.0004686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardosa M.J., Choo B.H., Zuraini I. A serological study of Japanese Encephalitis virus infections in northern Peninsular Malaysia. Southeast Asian J Trop Med Public Health. 1991;22(3):341–346. [PubMed] [Google Scholar]
- Cardosa M.J., Wang S.M., Sum M.S., Tio P.H. Antibodies against prM protein distinguish between previous infection with dengue and Japanese encephalitis viruses. BMC Microbiol. 2002;2:9. doi: 10.1186/1471-2180-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals J., Palacios R. Diagnosis of epidemic Encephalitis by complement-fixation test. Science. 1941;94(2440):330. doi: 10.1126/science.94.2440.330. [DOI] [PubMed] [Google Scholar]
- Casals J., Palacios R. The complement fixation test in the diagnosis of virus infections of the central nervous system. J Exp Med. 1941;74(5):409–426. doi: 10.1084/jem.74.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals J., Palacios R. Diagnosis of epidemic Encephalitis by complement-fixation test. Science. 1941;94(2440):330. doi: 10.1126/science.94.2440.330. [DOI] [PubMed] [Google Scholar]
- Castro T., Sabalza M., Barber C., Abrams W., Da Costa A.C., De Padua Milagres F.A. Rapid diagnosis of Zika virus through saliva and urine by Loop-mediated isothermal amplification (LAMP) J Oral Microbiol. 2018;10(1) doi: 10.1080/20002297.2018.1510712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Japanese Encephalitis in a U.S. traveler returning from Thailand, 2004. MMWR Morb Mortal Wkly Rep. 2005;54(5):123–125. [PubMed] [Google Scholar]
- CDC Japanese Encephalitis in two children–United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(9):276–278. [PubMed] [Google Scholar]
- Chan Y.C., Tan H.C., Tan S.H., Balachandran K. The use of the single radial haemolysis technique in the serological diagnosis of dengue and Japanese Encephalitis virus infections. Bull World Health Organ. 1985;63(6):1043–1053. [PMC free article] [PubMed] [Google Scholar]
- Chan K., Wong P.Y., Parikh C., Wong S. Moving toward rapid and low-cost point-of-care molecular diagnostics with a repurposed 3D printer and RPA. Anal Biochem. 2018;545:4–12. doi: 10.1016/j.ab.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke D.H., Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Colonetti T., Rocha B.V.E., Grande A.J., Alexandre M.C.M., Dondossola E.R., Madeira K. Accuracy of immunoglobulin M and immunoglobulin A of saliva in early diagnosis of dengue: Systematic Review and Meta-analysis. Anais da Academia Brasileira de Ciencias. 2018;90(3):3147–3154. doi: 10.1590/0001-3765201820170989. [DOI] [PubMed] [Google Scholar]
- Cutfield N.J., Anderson N.E., Brickell K., Hueston L., Pikholz C., Roxburgh R. Japanese encephalitis acquired during travel in China. Int Med J. 2005;35(8):497–498. doi: 10.1111/j.1445-5994.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- de Vasconcelos Z.F.M., Azevedo R.C., Thompson N., Gomes L., Guida L., Moreira M.E.L. Challenges for molecular and serological ZIKV infection confirmation. Childs Nerv Syst. 2018;34(1):79–84. doi: 10.1007/s00381-017-3641-5. [DOI] [PubMed] [Google Scholar]
- Desai A., Shankar S.K., Jayakumar P.N., Chandramuki A., Gourie-Devi M., Ravikumar B.V. Co-existence of cerebral cysticercosis with Japanese encephalitis: a prognostic modulator. Epidemiol Infect. 1997;118(2):165–171. doi: 10.1017/s0950268896007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Shankar S.K., Jayakumar P.N., Chandramuki A., GourieDevi M., Ravikumar B.V. Co-existence of cerebral cysticercosis with Japanese encephalitis: a prognostic modulator. Epidemiol Infect. 1997;118(2):165–171. doi: 10.1017/s0950268896007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Hong Z., Zhao S.J., Clemens J.D., Zhou B., Wang B. Long-term disability from acute childhood Japanese Encephalitis in Shanghai, China. Am J Trop Med Hyg. 2007;77(3):528–533. [PubMed] [Google Scholar]
- Dodd R.Y., Foster G.A., Stramer S.L. Keeping blood transfusion safe from West Nile Virus: American Red Cross Experience, 2003 to 2012. Transfusion Med Rev. 2015;29(3):153–161. doi: 10.1016/j.tmrv.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Dubot-Peres A., Sengvilaipaseuth O., Chanthongthip A., Newton P.N., de Lamballerie X. How many patients with anti-JEV IgM in cerebrospinal fluid really have Japanese Encephalitis? Lancet Infect Dis. 2015;15(12):1376–1377. doi: 10.1016/S1473-3099(15)00405-3. [DOI] [PubMed] [Google Scholar]
- Duca M., Duca E., Ionescu L., Abdalla H. Single radial haemolysis for the assay of antibodies to some haemagglutinating arboviruses. Bull World Health Organ. 1979;57(6):937–942. [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Pariyanonda A. Human immunoglobulin M antibody in the sero-diagnosis of Japanese encephalitis virus infections. Am J Epidemiol. 1973;98(1):29–38. doi: 10.1093/oxfordjournals.aje.a121529. [DOI] [PubMed] [Google Scholar]
- Feng Y., Fu S., Zhang H., Petersen L.R., Zhang B., Gao X. High incidence of Japanese encephalitis, southern China. Emerg Infect Dis. 2013;19(4):672–673. doi: 10.3201/eid1904.120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M., Johnes S., Li L., Heath A., Barrett A. Effect of genomic variation in the challenge virus on the neutralization titres of recipients of inactivated JE vaccines – report of a collaborative study on PRNT50 assays for Japanese encephalitis virus (JE) antibodies. Biologicals. 2008;36(2):111–116. doi: 10.1016/j.biologicals.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Fischer M., Hills S., Staples E., Johnson B., Yaich M., Solomon T. 2008. Japanese Encephalitis prevention and control: advances, challenges, and new initiatives. Emerging infections 8: American Society of Microbiology. [Google Scholar]
- Francis T.J. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104:572–578. [Google Scholar]
- Frietze K.M., Pascale J.M., Moreno B., Chackerian B., Peabody D.S. Pathogen-specific deep sequence-coupled biopanning: a method for surveying human antibody responses. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajanana A., Samuel P.P., Thenmozhi V., Rajendran R. An appraisal of some recent diagnostic assays for Japanese Encephalitis. Southeast Asian J Trop Med Public Health. 1996;27(4):673–679. [PubMed] [Google Scholar]
- Ganguli A., Ornob A., Yu H., Damhorst G.L., Chen W., Sun F. Hands-free smartphone-based diagnostics for simultaneous detection of Zika, Chikungunya, and Dengue at point-of-care. Biomed Microdevices. 2017;19(4):73. doi: 10.1007/s10544-017-0209-9. [DOI] [PubMed] [Google Scholar]
- Gao X., Li X., Li M., Fu S., Wang H., Lu Z. Vaccine strategies for the control and prevention of Japanese encephalitis in Mainland China, 1951-2011. PLoS Negl Trop Dis. 2014;8(8):e3015. doi: 10.1371/journal.pntd.0003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GC-Y Lee, Grayston J.T., Kenny G.E. Growth of Japanese Encephalitis virus in cell culture. J Infect Dis. 1965;115(4):321–329. doi: 10.1093/infdis/115.4.321. [DOI] [PubMed] [Google Scholar]
- George S., Pavri K. Identification of flaviviruses by the single-radial-haemolysis test. Indian J Med Res. 1986;84:565–570. [PubMed] [Google Scholar]
- Gould E., Pettersson J., Higgs S., Charrel R., de Lamballerie X. Emerging arboviruses: why today? One Health. 2017;4(Supplement C):1–13. doi: 10.1016/j.onehlt.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granerod J., Cunningham R., Zuckerman M., Mutton K., Davies N.W., Walsh A.L. Causality in acute encephalitis: defining aetiologies. Epidemiol Infect. 2010;138(6):783–800. doi: 10.1017/S0950268810000725. [DOI] [PubMed] [Google Scholar]
- Granerod J., Ambrose H.E., Davies N.W., Clewley J.P., Walsh A.L., Morgan D. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- Gunakasem P., Chantrasri C., Simasathien P., Chaiyanun S., Jatanasen S., Pariyanonth A. Surveillance of Japanese Encephalitis cases in Thailand. Southeast Asian J Trop Med Public Health. 1981;12(3):333–337. [PubMed] [Google Scholar]
- Guo X.G., Zhou Y.Z., Li Q., Wang W., Wen J.Z., Zheng L. Rapid and reliable diagnostic method to detect Zika virus by real-time fluorescence reverse transcription loop-mediated isothermal amplification. AMB Express. 2018;8(1):60. doi: 10.1186/s13568-018-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.H., Yu W.F., Cai J., Qian D., Zhao T.X., Xu Z.Z. 1989. A rapid method for detection of flavivirus antigens: Staphylococcal co-agglutination test using monoclonal antibodies to Japanese Encephalitis virus; pp. 24–31. [PubMed] [Google Scholar]
- Hayashi M. Übertragung des Virus von Encephalitis epidemica japonica auf Affen. Psychiatry Clin Neurosci. 1933;1(1):419–465. [Google Scholar]
- Heffelfinger J.D., Li X., Batmunkh N., Grabovac V., Diorditsa S., Liyanage J.B. Japanese Encephalitis surveillance and immunization – Asia and Western Pacific Regions, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(22):579–583. doi: 10.15585/mmwr.mm6622a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy S., Liu Z., Tsai T.F., Strom B.L., Wan C.M., Liu H.L. Effectiveness of live-attenuated Japanese Encephalitis vaccine (SA14-14-2): a case-control study. Lancet. 1996;347(9015):1583–1586. doi: 10.1016/s0140-6736(96)91075-2. [DOI] [PubMed] [Google Scholar]
- Hills S., Dabbagh A., Jacobson J., Marfin A., Featherstone D., Hombach J. Evidence and rationale for the World Health Organization recommended standards for Japanese Encephalitis surveillance. BMC Infect Dis. 2009;9:214. doi: 10.1186/1471-2334-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills S.L., Stoltey J., Martinez D., Kim P.Y., Sheriff H., Zangeneh A. A case series of three US adults with Japanese encephalitis, 2010-2012. J Travel Med. 2014;21(5):310–313. doi: 10.1111/jtm.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke C.H., Nisalak A., Sangawhipa N., Jatanasen S., Laorakapongse T., Innis B.L. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319(10):608–614. doi: 10.1056/NEJM198809083191004. [DOI] [PubMed] [Google Scholar]
- Hossain M.J., Gurley E.S., Montgomery S., Petersen L., Sejvar J., Fischer M. Hospital-based surveillance for Japanese Encephalitis at four sites in Bangladesh, 2003-2005. Am J Trop Med Hyg. 2010;82(2):344–349. doi: 10.4269/ajtmh.2010.09-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.H., Chang Y.H., Lin C.Y., Wang W.H., Kuan H.C., Hsieh Y.J. Shared IgG Infection Signatures vs. Hemorrhage-Restricted IgA clusters in human Dengue: a phenotype of differential class-switch via TGFbeta1. Front Immunol. 2017;8:1726. doi: 10.3389/fimmu.2017.01726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impoinvil D.E., Ooi M.H., Diggle P.J., Caminade C., Cardosa M.J., Morse A.P. The effect of vaccination coverage and climate on Japanese Encephalitis in Sarawak, Malaysia. PLoS Negl Trop Dis. 2013;7(8):e2334. doi: 10.1371/journal.pntd.0002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis B.L., Nisalak a, Nimmannitya S., Kusalerdchariya S., Chongswasdi V., Suntayakorn S. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- John C.C., Carabin H., Montano S.M., Bangirana P., Zunt J.R., Peterson P.K. Global research priorities for infections that affect the nervous system. Nature. 2015;527(7578):S178–S186. doi: 10.1038/nature16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.T., Burke D.S., Elwell M., Leake C.J., Nisalak A., Hoke C.H. Japanese Encephalitis: immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann Neurol. 1985;18(5):567–573. doi: 10.1002/ana.410180510. [DOI] [PubMed] [Google Scholar]
- Johnson B.W., Goodman C.H., Jee Y., Featherstone D.A. Differential diagnosis of Japanese Encephalitis virus infections with the Inbios JE Detect and DEN Detect MAC-ELISA Kits. Am J Trop Med Hyg. 2016;94(4):820–828. doi: 10.4269/ajtmh.15-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C., Sohal A., Michael B.D., Riordan A., Solomon T., Kneen R. Suboptimal management of central nervous system infections in children: a multi-centre retrospective study. BMC Pediatrics. 2012;12:145. doi: 10.1186/1471-2431-12-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.G., Baek S.H., Kim S., Kim H.I., Lee S.W., Phan L.M.T. Rapid discriminative detection of dengue viruses via loop mediated isothermal amplification. Talanta. 2018;190:391–396. doi: 10.1016/j.talanta.2018.08.019. [DOI] [PubMed] [Google Scholar]
- Kim D.T.H., Bao D.T., Park H., Ngoc N.M., Yeo S.J. Development of a novel peptide aptamer-based immunoassay to detect Zika virus in serum and urine. Theranostics. 2018;8(13):3629–3642. doi: 10.7150/thno.25955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K., Kurokochi Y. New method of diagnosis of Japanese B encephalitis. Jpn Med J. 1950;3(4):237–242. doi: 10.7883/yoken1948.3.237. [DOI] [PubMed] [Google Scholar]
- Kumar R., Mathur A., Kumar A., Sethi G.D., Sharma S., Chaturvedi U.C. Virological investigations of acute encephalopathy in India. Arch Dis Child. 1990;65(11):1227–1230. doi: 10.1136/adc.65.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Selvan A.S., Sharma S., Mathur A., Misra P.K., Singh G.K. Clinical predictors of Japanese Encephalitis. Neuroepidemiology. 1994;13(3):97–102. doi: 10.1159/000110365. [DOI] [PubMed] [Google Scholar]
- Kumar J.S., Parida M., Rao P.L. Monoclonal antibody-based antigen capture immunoassay for detection of circulating non-structural protein NS1: Implications for early diagnosis of japanese encephalitis virus infection. J Med Virol. 2011;83(6):1063–1070. doi: 10.1002/jmv.22097. [DOI] [PubMed] [Google Scholar]
- Kumar J.S., Saxena D., Parida M., Rathinam S. Evaluation of real-time reverse-transcription loop-mediated isothermal amplification assay for clinical diagnosis of West Nile virus in patients. Indian J Med Res. 2018;147(3):293–298. doi: 10.4103/0971-5916.234607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki Y., Martins D.B.G., Kimura M., Catena A.D.S., Borba M., Mattos S.D.S. Development and evaluation of a rapid molecular diagnostic test for Zika virus infection by reverse transcription loop-mediated isothermal amplification. Sci Rep. 2017;7(1):13503. doi: 10.1038/s41598-017-13836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttner A.G., Ts’un T. Encephalitis in North China. Results obtained with neutralization tests. J Clin Invest. 1936;15(5):525–530. doi: 10.1172/JCI100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw A.K., Ngwe Tun M.M., Nabeshima T., Buerano C.C., Ando T., Inoue S. Japanese Encephalitis- and Dengue-Associated acute encephalitis syndrome cases in Myanmar. Am J Trop Med Hyg. 2019;100(3):643–646. doi: 10.4269/ajtmh.18-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb L.E., Bartolone S.N., Tree M.O., Conway M.J., Rossignol J., Smith C.P. Rapid detection of Zika Virus in urine samples and infected mosquitos by reverse transcription-loop-mediated isothermal amplification. Sci Rep. 2018;8(1):3803. doi: 10.1038/s41598-018-22102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin S., Libman M., Drebot M.A., Laverdiere M. A case of Japanese Encephalitis virus infection acquired during a trip in Thailand. J Travel Med. 2012;19(2):127–129. doi: 10.1111/j.1708-8305.2011.00582.x. [DOI] [PubMed] [Google Scholar]
- Langevin S., Libman M., Drebot M.A., Laverdiere M. A case of Japanese encephalitis virus infection acquired during a trip in Thailand. J Travel Med. 2012;19(2):127–129. doi: 10.1111/j.1708-8305.2011.00582.x. [DOI] [PubMed] [Google Scholar]
- Lebani K., Jones M.L., Watterson D., Ranzoni A., Traves R.J., Young P.R. Isolation of serotype-specific antibodies against dengue virus non-structural protein 1 using phage display and application in a multiplexed serotyping assay. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W., Choe Y.J., Kim J.H., Song K.M., Cho H., Bae G.R. Epidemiology of Japanese encephalitis in South Korea, 2007-2010. Int J Infect Dis. 2012;16(6):e448–52. doi: 10.1016/j.ijid.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Lehtinen V.A., Huhtamo E., Siikamaki H., Vapalahti O. Japanese encephalitis in a Finnish traveler on a two-week holiday in Thailand. J Clin Virol. 2008;43(1):93–95. doi: 10.1016/j.jcv.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Li Y.Z., Counor D., Lu P., Liang G.D., Vu T.Q., Phan T.N. A specific and sensitive antigen capture assay for NS1 protein quantitation in Japanese encephalitis virus infection. J Virol Methods. 2012;179(1):8–16. doi: 10.1016/j.jviromet.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Li J.W., Gao X.Y., Wu Y., Fu S.H., Tan X.J., Cao Y.X. A Centralized Report on Pediatric Japanese Encephalitis cases from Beijing Children’s Hospital, 2013. Biomed Environ Sci. 2016;29(12):902–908. doi: 10.3967/bes2016.121. [DOI] [PubMed] [Google Scholar]
- Lindsey N.P., Staples J.E., Powell K., Rabe I.B., Fischer M., Powers A.M. Ability to serologically confirm recent Zika virus infection in areas with varying past incidence of dengue virus infection in the United States and U.S. Territories in 2016. J Clin Microbiol. 2018;56(1) doi: 10.1128/JCM.01115-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Jimena B., Bekaert M., Bakheit M., Frischmann S., Patel P., Simon-Loriere E. Development and validation of four one-step real-time RT-LAMP assays for specific detection of each dengue virus serotype. PLoS Negl Trop Dis. 2018;12(5) doi: 10.1371/journal.pntd.0006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M’Ikanatha N.M., Lynfield R., Van Beneden C.A., de Valk H. John Wiley & Sons, Incorporated; Hoboken, United Kingdom: 2013. Infectious disease surveillance. [Google Scholar]
- Mackenzie J.S., Gubler D.J., Petersen L.R. Emerging flaviviruses: the spread and resurgence of Japanese Encephalitis, West Nile and dengue viruses. Nat Med. 2004;10 doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- Mai N.T.H., Phu N.H., Nhu L.N.T., Hong N.T.T., Hanh N.H.H., Nguyet L.A. Central nervous system infection diagnosis by next-generation sequencing: a glimpse into the future? Open Forum Infect Dis. 2017;4(2) doi: 10.1093/ofid/ofx046. (no pagination)(ofx046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A., Chaturvedi U.C., Tandon H.O., Agarwal A.K., Mathur G.P., Nag D. Japanese Encephalitis epidemic in Uttar Pradesh, India during 1978. Indian J Med Res. 1982;75:161–169. [PubMed] [Google Scholar]
- Mathur A., Kumar R., Sharma S., Kulshreshtha R., Kumar A., Chaturvedi U.C. Rapid diagnosis of Japanese Encephalitis by immunofluorescent examination of cerebrospinal fluid. Indian J Med Res. 1990;91:1–4. [PubMed] [Google Scholar]
- Mauk M.G., Song J., Bau H.H., Liu C. Point-of-care molecular test for Zika infection. Clin Lab Int. 2017;41:25–27. [PMC free article] [PubMed] [Google Scholar]
- Miyake M. The pathology of Japanese Encephalitis. A review. Bull World Health Organ. 1964;30:153–160. [PMC free article] [PubMed] [Google Scholar]
- Muniaraj M., Rajamannar V. Impact of SA 14-14-2 vaccination on the occurrence of Japanese Encephalitis in India. Hum Vaccin Immunother. 2019;15(4):834–840. doi: 10.1080/21645515.2018.1564435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar M.V., Oak C., Ranjan P., Kanagasabai K., Shinde S., Pandey A.K. Coverage & missed opportunity for Japanese Encephalitis vaccine, Gorakhpur division, Uttar Pradesh, India, 2015: implications for Japanese Encephalitis control. Indian J Med Res. 2017;145(1):63–69. doi: 10.4103/ijmr.IJMR_712_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento E.J.M., Huleatt J.W., Cordeiro M.T., Castanha P.M.S., George J.K., Grebe E. Development of antibody biomarkers of long term and recent dengue virus infections. J Virol Methods. 2018;257:62–68. doi: 10.1016/j.jviromet.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Okuno T., Tseng P.T., Hsu S.T., Huang C.T., Kuo C.C. Japanese Encephalitis surveillance in China (Province of Taiwan) during 1968-1971. I. Geographical and seasonal features of case outbreaks. Jpn J Med Sci Biol. 1975;28(5–6):235–253. doi: 10.7883/yoken1952.28.235. [DOI] [PubMed] [Google Scholar]
- Olsen S.J., Supawat K., Campbell A.P., Anantapreecha S., Liamsuwan S., Tunlayadechanont S. Japanese Encephalitis virus remains an important cause of encephalitis in Thailand. Int J Infect Dis. 2010;14(10):e888–92. doi: 10.1016/j.ijid.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Ompusunggu S., Hills S.L., Maha M.S., Moniaga V.A., Susilarini N.K., Widjaya A. Confirmation of Japanese encephalitis as an endemic human disease through sentinel surveillance in Indonesia. Am J Trop Med Hyg. 2008;79(6):963–970. [PubMed] [Google Scholar]
- Onyango C.O., Loparev V., Lidechi S., Bhullar V., Schmid D.S., Radford K. Evaluation of a TaqMan array card for detection of central nervous system infections. J Clin Microbiol. 2017;55(7):2035–2044. doi: 10.1128/JCM.02469-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Clark S., Portnoy A., Grewal S., Stack M.L., Sinha A. Estimated economic impact of vaccinations in 73 low- and middle-income countries, 2001-2020. Bull World Health Organ. 2017;95(9):629–638. doi: 10.2471/BLT.16.178475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce J.C., Learoyd T.P., Langendorf B.J., Logan J.G. Japanese Encephalitis: the vectors, ecology and potential for expansion. J Travel Med. 2018;25(suppl_1):S16–S26. doi: 10.1093/jtm/tay009. [DOI] [PubMed] [Google Scholar]
- Peiris J.S., Amerasinghe F.P., Amerasinghe F.P.H., Ratnayake C.B., Karunaratne S.H., Tsai T.F. Japanese Encephalitis in Sri Lanka–the study of an epidemic: vector incrimination, porcine infection and human disease. Trans R Soc Trop Med Hyg. 1992;86(3):307–313. doi: 10.1016/0035-9203(92)90325-7. [DOI] [PubMed] [Google Scholar]
- Piyasena T.B.H., Setoh Y.X., Hobson-Peters J., Prow N.A., Bielefeldt-Ohmann H., Khromykh A.A. Differential diagnosis of flavivirus infections in horses using viral envelope protein domain III antigens in enzyme-linked immunosorbent assay. Vector Borne Zoonotic Dis. 2017;17(12):825–835. doi: 10.1089/vbz.2017.2172. [DOI] [PubMed] [Google Scholar]
- Prasad S.R., Kumar V., Marwaha R.K., Batra K.L., Rath R.K., Pal S.R. An epidemic of encephalitis in Haryana: serological evidence of Japanese encephalitis in a few patients. Indian Pediatr. 1993;30(7):905–910. [PubMed] [Google Scholar]
- Preziuso S., Mari S., Mariotti F., Rossi G. Detection of Japanese Encephalitis virus in bone marrow of healthy young wild birds collected in 1997-2000 in Central Italy. Zoonoses Public Health. 2018;65(7):798–804. doi: 10.1111/zph.12501. [DOI] [PubMed] [Google Scholar]
- Priye A., Bird S.W., Light Y.K., Ball C.S., Negrete O.A., Meagher R.J. A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci Rep. 2017;7:44778. doi: 10.1038/srep44778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi V., Premkumar S., Chandramuki A., Kimura-Kuroda J. A reverse passive haemagglutination test for detection of Japanese Encephalitis virus antigens in cerebrospinal fluid. J Virol Methods. 1989;23(3):291–298. doi: 10.1016/0166-0934(89)90161-4. [DOI] [PubMed] [Google Scholar]
- Ravi V., Premkumar S., Chandramuki A., Kimura-Kuroda J. A reverse passive haemagglutination test for detection of Japanese Encephalitis virus antigens in cerebrospinal fluid. J Virol Methods. 1989;23(3):291–298. doi: 10.1016/0166-0934(89)90161-4. [DOI] [PubMed] [Google Scholar]
- Ravi V., Robinson J.S., Russell B.J., Desai A., Ramamurty N., Featherstone D. Evaluation of IgM antibody capture enzyme-linked immunosorbent assay kits for detection of IgM against Japanese encephalitis virus in cerebrospinal fluid samples. Am J Trop Med Hyg. 2009;81(6):1144–1150. doi: 10.4269/ajtmh.2009.09-0144. [DOI] [PubMed] [Google Scholar]
- Rayamajhi A., Nightingale S., Bhatta N.K., Singh R., Kneen R., Ledger E. A preliminary randomized double blind placebo-controlled trial of intravenous immunoglobulin for Japanese encephalitis in Nepal. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J.S., Featherstone D., Vasanthapuram R., Biggerstaff B.J., Desai A., Ramamurty N. Evaluation of three commercially available Japanese Encephalitis virus IgM enzyme-linked immunosorbent assays. Am J Trop Med Hyg. 2010;83(5):1146–1155. doi: 10.4269/ajtmh.2010.10-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockstroh A., Moges B., Barzon L., Sinigaglia A., Palu G., Kumbukgolla W. Specific detection of dengue and Zika virus antibodies using envelope proteins with mutations in the conserved fusion loop. Emerg Microbes Infect. 2017;6(11):e99. doi: 10.1038/emi.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnberg B., Gustafsson A., Vapalahti O., Emmerich P., Lundkvist A., Schmidt-Chanasit J. Compensating for cross-reactions using avidity and computation in a suspension multiplex immunoassay for serotyping of Zika versus other flavivirus infections. Med Microbiol Immunol. 2017;206(5):383–401. doi: 10.1007/s00430-017-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N.R. American Society for Microbiology; 1992. Microbiology ASf. Manual of Clinical Laboratory Immunology. [Google Scholar]
- Saa P., Proctor M., Foster G., Krysztof D., Winton C., Linnen J.M. Investigational Testing for Zika Virus among U.S. Blood Donors. N Engl J Med. 2018;378(19):1778–1788. doi: 10.1056/NEJMoa1714977. [DOI] [PubMed] [Google Scholar]
- Sabalza M., Yasmin R., Barber C.A., Castro T., Malamud D., Kim B.J. Detection of Zika virus using reverse-transcription LAMP coupled with reverse dot blot analysis in saliva. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0192398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A.B. Epidemic Encephalitis in military personnel; isolation of Japanese B virus on Okinawa in 1945, serologic diagnosis, clinical manifestations, epidemiologic aspects and use of mouse brain vaccine. J Am Med Assoc. 1947;133(5):281–293. doi: 10.1001/jama.1947.02880050001001. [DOI] [PubMed] [Google Scholar]
- Sabin A.B., Schlesinger R.W., Ginder D.R., Matumoto M. Japanese B Encephalitis in American soldiers in Korea. A J Hyg. 1947;46(3) doi: 10.1093/oxfordjournals.aje.a119174. 356-&. [DOI] [PubMed] [Google Scholar]
- Sabin A.B., Schlesinger R.W., Ginder D.R. Clinically apparent and inapparent infection with Japanese B encephalitis virus in Shanghai and Tientsin. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, NY) 1947;65(2):183–187. doi: 10.3181/00379727-65-15903. [DOI] [PubMed] [Google Scholar]
- Saito M., Sunagawa T., Makino Y., Tadano M., Hasegawa H., Kanemura K. Three Japanese encephalitis cases in Okinawa, Japan, 1991. Southeast Asian J Trop Med Public Health. 1999;30(2):277–279. [PubMed] [Google Scholar]
- Saito M., Sunagawa T., Makino Y., Tadano M., Hasegawa H., Kanemura K. Three Japanese encephalitis cases in Okinawa, Japan, 1991. Southeast Asian J Trop Med Public Health. 1999;30(2):277–279. [PubMed] [Google Scholar]
- Saito M., Sunagawa T., Makino Y., Tadano M., Hasegawa H., Kanemura K. Three Japanese encephalitis cases in Okinawa, Japan, 1991. Southeast Asian J Trop Med Public Health. 1999;30(2):277–279. [PubMed] [Google Scholar]
- Saito M., Soukaloun D., Phongsavath K., Phommasack B., Makino Y. Epidemiological study of Japanese encephalitis virus in Vientiane, Lao PDR, in 1990s. Sci World J. 2015;2015 doi: 10.1155/2015/235934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengvilaipaseuth O., Castonguay-Vanier J., Chanthongthip A., Phonemixay O., Thongpaseuth S., Vongsouvath M. Poor performance of two rapid immunochromatographic assays for anti-Japanese Encephalitis virus immunoglobulin M detection in cerebrospinal fluid and serum from patients with suspected Japanese Encephalitis virus infection in Laos. Trans R Soc Trop Med Hyg. 2017;111(8):373–377. doi: 10.1093/trstmh/trx067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever J.L. Application of a microtechnique to viral serological investigations. J Immunol. 1962;88:320–329. [PubMed] [Google Scholar]
- Shen W.F., Galula J.U., Chang G.J., Wu H.C., King C.C., Chao D.Y. Improving dengue viral antigens detection in dengue patient serum specimens using a low pH glycine buffer treatment. J Microbiol Immunol Infect. 2017;50(2):167–174. doi: 10.1016/j.jmii.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Simon-Loriere E., Faye O., Prot M., Casademont I., Fall G., Fernandez-Garcia M.D. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. N Engl J Med. 2017;376(15):1483–1485. doi: 10.1056/NEJMc1701600. [DOI] [PubMed] [Google Scholar]
- Simon-Loriere E., Faye O., Prot M., Casademont I., Fall G., Fernandez-Garcia M.D. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. N Engl J Med. 2017;376(15):1483–1485. doi: 10.1056/NEJMc1701600. [DOI] [PubMed] [Google Scholar]
- Solomon T., Thi T.T., Lewthwaite P., Mong H.O., Kneen R., Nguyen M.D. A cohort study to assess the new WHO Japanese Encephalitis surveillance standards. Bull World Health Organ. 2008;86:178–186. doi: 10.2471/BLT.07.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T., Thao T.T., Lewthwaite P., Ooi M.H., Kneen R., Dung N.M. A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bull World Health Organ. 2008;86(3):178–186. doi: 10.2471/BLT.07.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T., Michael B.D., Smith P.E., Sanderson F., Davies N.W., Hart I.J. Management of suspected viral encephalitis in adults–association of British Neurologists and British Infection Association National Guidelines. J Infect. 2012;64(4):347–373. doi: 10.1016/j.jinf.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Song J., Pandian V., Mauk M.G., Bau H.H., Cherry S., Tisi L.C. Smartphone-Based Mobile Detection Platform for Molecular Diagnostics and Spatiotemporal Disease Mapping. Anal Chem. 2018;90(7):4823–4831. doi: 10.1021/acs.analchem.8b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunwoo J.S., Jung K.H., Lee S.T., Lee S.T.K., Chu K. Reemergence of Japanese Encephalitis in South Korea, 2010-2015. Emerging Infect Dis. 2016;22(10):1841–1843. doi: 10.3201/eid2210.160288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K.K., Azizan N.S., Yaacob C.N., Che Mat Seri N.A.A., Samsudin N.I., Teoh B.T. Operational utility of the reverse-transcription recombinase polymerase amplification for detection of dengue virus. BMC Infect Dis. 2018;18(1):169. doi: 10.1186/s12879-018-3065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandale B.V., Khan S.A., Kushwaha K.P., Rahman H., Gore M.M., Sapkal G.N. Effectiveness of Japanese Encephalitis SA 14-14-2 live attenuated vaccine among Indian children: Retrospective 1:4 matched case-control study. J Infect Public Health. 2018;11(5):713–719. doi: 10.1016/j.jiph.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Team RDC . 2010. R: A language and environment for statistical computing.. R Foundation for Statistical Computing [Internet] [Google Scholar]
- Thisyakorn U., Nimmannitya S. Japanese Encephalitis in Thai children, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 1985;16(1):93–97. [PubMed] [Google Scholar]
- Tiroumourougane S.V., Raghava P., Srinivasan S., Badrinath Management parameters affecting the outcome of Japanese encephalitis. J Trop Pediat. 2003;49(3):153–156. doi: 10.1093/tropej/49.3.153. [DOI] [PubMed] [Google Scholar]
- Touch S., Hills S., Sokhal B., Samnang C., Sovann L., Khieu V. Epidemiology and burden of disease from Japanese encephalitis in Cambodia: results from two years of sentinel surveillance. Trop Med Int Health. 2009;14(11):1365–1373. doi: 10.1111/j.1365-3156.2009.02380.x. [DOI] [PubMed] [Google Scholar]
- Touch S., Hills S., Sokhal B., Samnang C., Sovann L., Khieu V. Epidemiology and burden of disease from Japanese encephalitis in Cambodia: results from two years of sentinel surveillance. Trop Med Int Health: TM IH. 2009;14:1365–1373. doi: 10.1111/j.1365-3156.2009.02380.x. [DOI] [PubMed] [Google Scholar]
- Tsai W.Y., Youn H.H., Tyson J., Brites C., Tsai J.J., Pedroso C. Use of Urea Wash ELISA to Distinguish Zika and Dengue Virus Infections. Emerging Infect Dis. 2018;24(7):1355–1359. doi: 10.3201/eid2407.171170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upreti S.R., Lindsey N.P., Bohara R., Choudhary G.R., Shakya S., Gautam M. Updated estimation of the impact of a Japanese Encephalitis immunization program with live, attenuated SA 14-14-2 vaccine in Nepal. PLoS Negl Trop Dis. 2017;11(9) doi: 10.1371/journal.pntd.0005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileva Wand N.I., Bonney L.C., Watson R.J., Graham V., Hewson R. Point-of-care diagnostic assay for the detection of Zika virus using the recombinase polymerase amplification method. J Gen Virol. 2018;99(8):1012–1026. doi: 10.1099/jgv.0.001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn D.W., Hoke C.H. The epidemiology of Japanese Encephalitis:prospects for prevention. Epidemiolog Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- Warnecke J.M., Lattwein E., Saschenbrecker S., Stocker W., Schlumberger W., Steinhagen K. Added value of IgA antibodies against Zika virus non-structural protein 1 in the diagnosis of acute Zika virus infections. J Virol Methods. 2019;267:8–15. doi: 10.1016/j.jviromet.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Wasik D., Mulchandani A., Yates M.V. A heparin-functionalized carbon nanotube-based affinity biosensor for dengue virus. Biosens Bioelectron. 2017;91:811–816. doi: 10.1016/j.bios.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Wei J., Wang X., Zhang J., Guo S., Pang L., Shi K. Partial cross-protection between Japanese Encephalitis virus genotype I and III in mice. PLoS Negl Trop Dis. 2019;13(8) doi: 10.1371/journal.pntd.0007601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western WHOROft, editor. Training Report Fourth Hands-on Training Workshop on the Laboratory Diagnosis of Japanese Encephalitis. Osong; 2014. [Google Scholar]
- Wilson M.L., Fleming K.A., Kuti M.A., Looi L.M., Lago N., Ru K. Access to pathology and laboratory medicine services: a crucial gap. The Lancet. 2018;391(10133):1927–1938. doi: 10.1016/S0140-6736(18)30458-6. [DOI] [PubMed] [Google Scholar]
- Wittesjo B., Eitrem R., Niklasson B., Vene S., Mangiafico J.A. Japanese encephalitis after a 10-day holiday in Bali. Lancet. 1995;345(8953):856. doi: 10.1016/s0140-6736(95)92990-8. [DOI] [PubMed] [Google Scholar]
- World Health Organization G . 2007. Manual for the Laboratory Diagnosis of Japanese Encephalitis Virus Infection. [Google Scholar]
- World Health Organization G . 2017. Immunization, vaccines and biologicals. Data, statistics and graphics. [updated 25/9/18] [Google Scholar]
- World Health Organization G . 2017. JRF Supplementary Questionnaire on Surveillance. [Google Scholar]
- World Health Organization G . second edition. 2018. Surveillance standards for vaccine-preventable diseases. Chapter 10: Japanese Encephalitis. [Google Scholar]
- World Health Organization G . 2019. Immunization, Vaccines and Biologicals. Data, statistics and graphics. Immunization coverage or administered doses. Official country reported coverage estimates time series.https://www.who.int/immunization/monitoring_surveillance/data/en/ [Available from]: [Google Scholar]
- World Health Organization G . 2019. Immunization, Vaccines and Biologicals. Data, statistics and graphics. Disease incidence. Reported incidence time series is available in html and in excel.https://www.who.int/immunization/monitoring_surveillance/data/en/ [updated 15/07/2019. Available from]: [Google Scholar]
- Yang Y., Liang N., Tan Y., Xie Z. Epidemiological trends and characteristics of Japanese Encephalitis changed based on the vaccination program between 1960 and 2013 in Guangxi Zhuang Autonomous Region, Southern China. Int J Infect Dis. 2016;45:135–138. doi: 10.1016/j.ijid.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Yap G., Sil B.K., Ng L.C. Use of saliva for early dengue diagnosis. PLoS Negl Trop Dis. 2011;5(5):e1046. doi: 10.1371/journal.pntd.0001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaren O., Alto B.W., Bradley K.M., Moussatche P., Glushakova L., Benner S.A. Multiplexed Isothermal amplification based diagnostic platform to detect Zika, Chikungunya, and Dengue 1. J Visualized Exp: JoVE. 2018;(133) doi: 10.3791/57051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Lee L.A., Liu Y., Scherpbier R.W., Wen N., Zhang G. Vaccine-preventable disease control in the People’s Republic of China: 1949–2016. Vaccine. 2018;36(52):8131–8137. doi: 10.1016/j.vaccine.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang F., Su Z., Zhou L., Konduru K., Kaplan G., Chou S.Y. Ultrasensitive Ebola virus antigen sensing via 3D nanoantenna arrays. Adv Mater (Deerfield Beach, Fla) 2019 doi: 10.1002/adma.201902331. [DOI] [PubMed] [Google Scholar]
- Zhang B., Pinsky B.A., Ananta J.S., Zhao S., Arulkumar S., Wan H. Diagnosis of Zika virus infection on a nanotechnology platform. Nat Med. 2017;23(5):548–550. doi: 10.1038/nm.4302. [DOI] [PubMed] [Google Scholar]
- Zhao J., Feng R. Sensitive and rapid detection of Zika virus by loop-mediated isothermal amplification. Virus Genes. 2019;55(1):43–50. doi: 10.1007/s11262-018-1612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., He J., Chen W., Ho H.P., Kong S.K., Wang C. Development of peptide-based chemiluminescence enzyme immunoassay (CLEIA) for diagnosis of dengue virus infection in human. Anal Biochem. 2018;556:112–118. doi: 10.1016/j.ab.2018.06.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.