Abstract
EFLamide (EFLa) is a neuropeptide known for a long time from crustaceans, chelicerates and myriapods. Recently, EFLa-encoding genes were identified in the genomes of apterygote hexapods including basal insect species. In pterygote insects, however, evidence of EFLa was limited to partial sequences in the bed bug (Cimex), migratory locust and a few phasmid species. Here we present identification of a full length EFLa-encoding transcript in the linden bug, Pyrrhocoris apterus (Heteroptera). We created complete null mutants allowing unambiguous anatomical location of this peptide in the central nervous system. Only 2–3 EFLa-expressing cells are located very close to each other near to the surface of the lateral protocerebrum with dense neuronal arborization. Homozygous null EFLa mutants are fully viable and do not have any visible defect in development, reproduction, lifespan, diapause induction or circadian rhythmicity. Phylogenetic analysis revealed that EFLa-encoding transcripts are produced by alternative splicing of a gene that also produces Prohormone-4. However, this Proh-4/EFLa connection is found only in Hemiptera and Locusta, whereas EFLa-encoding transcripts in apterygote hexapods, chelicerates and crustaceans are clearly distinct from Proh-4 genes. The exact mechanism leading to the fused Proh-4/EFLa transcript is not yet determined, and might be a result of canonical cis-splicing, cis-splicing of adjacent genes (cis-SAG), or trans-splicing.
Keywords: EFLamide, Null mutant, CRISPR/Cas9, TRH, Alternative splicing, In silico peptide prediction
Graphical abstract

Highlights
-
•
The first EFLa/TRH-like mutants in arthropods were engineered and characterized.
-
•
EFLa nulls are fully viable with no effect on development, aging or fertility.
-
•
EFLa is expressed in 4–6 brain cells in the lateral protocerebrum.
-
•
EFLa neurons densely project to proto- and deutocerebrum.
-
•
EFLa in Heteroptera and Locusta originates from alternative splicing of Proh-4.
1. Introduction
Neuropeptides are the most diverse and largest class of neuronally-secreted signaling molecules. These peptides affect a plethora of biological processes, ranging from development to physiology and behavior, acting as neurotransmitters, neuromodulators or neurohormones.
Despite remarkable sequence diversity, all neuropeptides share the following properties: (i) they are derived from larger preprohormone proteins that contain an N-terminally positioned signal peptide that targets the precursor protein for secretion (Douglass et al., 1984). (ii) They are derived from preprohormones following cleavage at dibasic sites recognized by convertases (Veenstra, 2000), and (iii) they often are subject to post-translational modifications, with the most common modification being conversion of a C-terminal glycine to an amide group (Eipper et al., 1992).
The neuropeptide signaling is known in Bilateralia and occurs even in cnidarians (Jekely, 2013; Elphick et al., 2018). Many neuropeptide signaling pathways are conserved across taxa, however, the minimal sequence similarity preserved in already short peptide sequences frequently prevents unambiguous detection of any relationship. Often, the evolution of neuropeptide receptors helps to reveal relationships between the peptide ligands (Mirabeau and Joly, 2013).
The neuropeptide toolkit of arthropods, one of the richest and most diverse groups of organisms, is remarkably diverse. However, functional research is strongly biased towards holometabola and particularly to Drosophila melanogaster, an amazing model organism with unprecedented experimental tools. Importantly, holometabola have lost a significant number of neuropeptide signaling pathways (Hansen et al., 2010; Veenstra, 2014). Thus, comparative research on basal insect groups became rewarding for uncovering the ancestral neuropeptide toolkit and it is also the crucial first step for planning functional experiments. In recent years, remarkable progress of next generation sequencing paved the way for in silico discoveries of neuropeptides from genome drafts and transcriptomes of insects (Veenstra, 2019; Tanaka et al., 2014; Predel et al., 2018), non-pterygote hexapods (Derst et al., 2016), chelicerates (Veenstra et al., 2012) and crustaceans (Veenstra, 2016). In some cases, even several new putative insect neuropeptides were discovered in one species (Liessem et al., 2018).
Remarkable progress of receptor deorphanization in recent years further shed light on evolution of neuropeptide signaling. Identification of a receptor often revealed relatedness that cannot be deduced from the sequence of ligands. One such example includes EFLamides (EFLa), neuropeptides originally predicted in silico from the genome of the mite Tetranychus urticae (Veenstra et al., 2012), which later were found to be orthologs of thyrotropin-releasing hormone (TRH) (Bauknecht and Jékely, 2015; Van Sinay et al., 2017). A recent study further confirmed that the EFLa receptor (EFLaR) from a polyneopteran insect species, Locusta migratoria, is activated by a physiologically relevant concentration of Locusta EFLa (Veenstra and Šimo, 2020).
Our study was performed on the linden bug, Pyrrhocoris apterus, a heteropteran species that has been used in research for more than 5 decades (reviewed by Socha, 1993). Early experiments provided the remarkable discovery of the paper factor, a Juvenile hormone (JH)-mimicking compound produced in North American trees (Slama and Williams, 1966). The role of JH was further revealed in the context of development (Konopova et al., 2011), reproduction (Smykal et al., 2014) and circadian clock gene expression (Dolezel et al., 2008; Bajgar et al., 2013a,b). Although some neuropeptides were described and characterized in P. apterus, including two adipokinetic hormones (Kodrik et al., 2000, 2002), adipokinetic hormone receptor (Ibrahim et al., 2017), and three insulin-like peptides (Smykal et al., 2020), a detailed neuropeptide inventory is not yet available for this species. During our analysis of a P. apterus transcriptome a new neuropeptide candidate, TVGTEFLamide (EFLa), was identified. Our goal was to test if this new candidate fulfils criteria to be considered as a putative neuropeptide, to pinpoint where it is expressed, and (ideally) identify its role in P. apterus biology. Therefore, we have created complete EFLa null mutants in P. apterus, the first EFLa/TRH-like mutants in arthropods, and analyzed their development, lifespan, reproduction and circadian phenotypes. In addition, we performed detailed phylogenetic comparison of EFLa-coding genes in arthropods, mapped the presence of EFLa and its receptor on insect phylogeny, and discovered an interesting connection between EFLa and Prohormone-4 (Proh-4) in several insect species.
2. Materials and methods
2.1. Insect rearing
Laboratory strain Oldrichovec (Pivarciova et al., 2016), which has been kept in the laboratory for more than 55 generations since 2010 and phenotypically corresponds to wild type bugs, was used in all experiments, including gene editing and subsequent backcrosses. For simplicity, it is abbreviated as wild type (wt) throughout this study. P. apterus were maintained in the laboratory at 25 °C under a diapause preventing long day photoperiod consisting of 18 h light and 6 h dark phase (LD 18:6). If the ability to diapause was tested, bugs were reared from early developmental stages at 25 °C under short day photoperiod (12 h light and 12 h dark phase, briefly SD 12:12).
2.2. Gene editing –EFLa null mutants
EFLa null mutants were engineered by CRISPR/Cas9 approach, where non-homologous-end-joining repair (NHEJ) mechanism resulted in a deletion removing sequence coding for the putatively active peptide. The detailed protocol including gRNA sequence, embryo injection and mutant detection is published elsewhere (Kotwica-Rolinska et al., 2019). Founder mutants were backcrossed to wt strain (identical to the strain where the mutations were induced), heterozygous offspring were identified by PCR and used again in subsequent backcross to wt to remove any off-target mutations. Heterozygotes resulting from the 6th backcross were mated together and resulting homozygotes were used to establish a clean mutant line. Heterozygous and homozygous bugs were identified by PCR. Seven mutant lines were originally established and up to three of them were further phenotypically characterized.
2.3. Duration of development
Homozygous mutants of EFla08, EFla011 and EFLa016 (numbers reflect order during the screening process) were single self-crossed to obtain homozygous eggs or back-crossed to wt, to obtain heterozygous eggs. Single crosses of wt bugs were used for controls. All developmental events were recorded daily. When a clutch of eggs was laid, parents were transferred to a new Petri dish. For egg development, duration is determined for the entire clutch, not for individual eggs. Afterwards, exuviae were counted and removed daily. For presentation, the number of all individuals of particular developmental stage of the same genotype was set as 100% and the daily percentage of newly emerged bugs was plotted.
2.4. Duration of oviposition cycles
The mutants were prepared identically to experiment 2.3. Adult virgin females of wt (controls), heterozygotes and homozygotes of EFLa011 and EFLa016 lines were put separately into Petri dishes within 24 h after adult ecdysis and then egg-laying was recorded daily. When a clutch of eggs was laid, the date was recorded and eggs were removed. Recording was carried out until the fifth consecutive oviposition cycle.
2.5. Lifespan
The mutants were prepared identically to experiment 2.3. Female virgin bugs of wt (controls), heterozygotes and homozygotes of EFla011 and EFLa016 lines were collected at the day of adult ecdysis and kept individually in Petri dishes (diameter 70 mm). Petri dishes were kept at 25 °C under LD 18:6 on the same shelf in the same incubator to ensure as identical conditions as possible and all mutants and controls were reared and analyzed in parallel (±one week). The number of dead females was controlled daily.
2.6. Diapause phenotype
Homozygous EFla011mutants were back-crossed to wild type. Heterozygotes resulting from this backcross were self-mated and their offspring was reared from the second instar in diapause-promoting conditions (SD 12:12 and 25 °C). Adult females were kept individually to detect any egg laying. Females were dissected two weeks after adult ecdysis and the ovarian morphology was determined. Females with small ovaries without eggs and vitellogenic follicles were scored as diapausing, whereas females laying eggs or having large ovaries were considered as reproductive (see Smykal et al., 2014 for ovarian morphology images). The genotype of females was determined by PCR afterwards.
2.7. Locomotor activity and circadian rhythmicity
Homozygous EFla011mutants were back-crossed to wild type. Heterozygotes resulting from this backcross were self-mated and resulting male offspring at 3–5 days after adult ecdysis were individually transferred to test tubes (diameter 25 mm, length 150 mm), equipped with a water reservoir on one side and a peeled linden seed (Tilia cordata) wrapped in textile mesh attached to the other side. Tubes were placed in LAM25 monitors (Trikinetics, Waltham, MA, USA) horizontally with infrared beams crossing the tube in the middle. Locomotor activity was recorded in 5 min bins. Bugs were entrained for five days to the photo-regime LD 18:6 and then released to constant dark at 25 °C for 12 days. The genotype of males was determined by PCR after the experiment.
The daily profile of locomotor activity was analyzed as previously (Kotwica-Rolinska et al., 2017) in ActogramJ software (Schmid et al., 2011). Briefly, activity of all individuals of a particular genotype was averaged, smoothed (Gaussian smooth 3) and displayed using Graphpad7 (Prism) software.
Lomb-Scargle periodogram in ActogramJ was used to determine the rhythmicity of bugs and the length of the free running period (τ) in constant conditions and double-plotted actograms were further controlled by eye. Three categories were defined: (1) rhythmic males: periodogram peak crossed the significance threshold and PN value calculated by ActogramJ software was >35. (2) complex males: periodogram peak crossed the significance line and PN values were >35 but more than one periodicity value was found. (3) arrhythmic males: periodogram peak did not cross the significance line or periodogram peak crossed the significance line but PN value was <35 (as described in Pivarciova et al., 2016; Kaniewska et al., 2020).
2.8. Statistical analysis
Statistical analysis was performed in the GraphPad software (Prism). One-way ANOVA was used to analyze differences in the development, locomotor activity and τ. Two-way ANOVA was used to analyze the effect of the EFLa mutation on the duration of oviposition cycles. Survival was analyzed by the Mantel–Cox log-rank test.
2.9. In silico data mining
EFLa preprohormone mRNA was identified manually in the P. apterus transcriptome. First, a library of all putative proteins was built from the transcriptome (brain, fat body, gut), including interior open reading frames. Then, signal P was used to determine proteins containing signal peptide. This dataset of putatively exported proteins was prospected for candidate biologically active peptides containing EFLG motif followed by a cleavage site (KR, RR). The search for EFLa prohormone orthologs was done in GenBank using BLAST-P and T-BLAST-N algorithms in non-redundant protein sequences, reference RNA sequences, and in Transcriptome Shotgun Assemblies (TSA) with various taxonomic limits to optimize the search. Similarly, Proh-4 encoding transcripts were identified using various query sequences. Additional EFLa preprohormone sequences were retrieved from supplementary material of Ders at al. (2016). The nucleotide and protein sequences were aligned in Geneious 11 (Biometters).
Signal-P 5.0 (http://www.cbs.dtu.dk/services/SignalP/) (Almagro Armenteros et al., 2019) and NeuroPred (http://stagbeetle.animal.uiuc.edu/cgi-bin/neuropred.py) (Southey et al., 2006) were used to predict preprohormone processing and identify expected peptides. These peptides were aligned either in Geneious or manually and the alignment was used to plot the consensus as a Logo (http://weblogo.berkeley.edu/logo.cgi).
To retrieve receptors of EFLa and ETH, respectively, TSAs, non-redundant protein sequences, whole genome shotgun sequences (wgs) and genomes available in GenBank were analyzed using BLAST-P and T-BLAST-N algorithms. Retrieved sequences were aligned as proteins to known receptors (MAFFT, Geneious 11) and their relationship to a particular G protein-coupled receptor (GPCR) group was further determined using phylogenetic analysis (Fast tree, Geneious 11).
2.10. cDNA cloning and RNA interference (RNAi)
EFLa/Proh-4 transcript fragment was amplified with gene-specific primers (Fw: 5′-CCCGCCGGACACCAGAGA-3′ and Rev: 5′-AGTCCTCGTCGTAGCCGTCAAGAC-3′), 329 bp product was cloned into pGEM-T-easy plasmid (Promega) and verified by sequencing. PCR was used to amplify the insert and replace SP6 with T7 promoter. Double-stranded RNA (dsRNA) was prepared from PCR template using the T7 RNA polymerase with the MEGAscript kit (Thermo Fisher Scientific) and injected into P. apterus adults as described previously (Bajgar et al., 2013a). Adults received 3 μl of dsRNA at a concentration of 2–4 μg/μl in Ringer's solution; control animals were injected with heterologous dsRNA derived from bacterial β-galactosidase (lacZ) gene or with the Ringer's solution alone.
2.11. mRNA quantification
Analyzed tissues (brain, gut, fat body) were dissected in RNAse-free Ringer's solution and total RNA was isolated with Trizol reagent, residual genomic DNA removed with Turbo DNAse and 1 μg of total RNA was used for cDNA synthesis using SuperScript III reverse transcriptase (all from Thermo Fisher Scientific). Relative transcript levels were measured by reverse transcription quantitative PCR (RT qPCR) using qPCR 2_ SYBR Master Mix (Top Bio) and a C1000 Thermal Cycler (Bio-Rad). EFLa and Proh-4 common forward primer (5′-GACGGTGCCTGCATCTCCAT-3′) was combined either with EFLa-specific reverse (5′-AGTTCCGACGGTCCTCTTCAAA-3′), or with Proh-4-specific reverse (5′-CCAGCGGGGCAAGAGCATC-3′) primer. Both measured transcripts were normalized to relative levels of ribosomal protein 49 (rp49) mRNA (Dolezel et al., 2007). Primer efficiency was evaluated by an RT qPCR method on a six-point standard curve prepared separately from purified PCR products for every gene tested.
2.12. EFLa antibody & whole mount immunohistochemistry
Polyclonal antibody against putative P. apterus EFLa was produced in rat (Moravian Biotech, Czech Republic). Immunization was done with TVGTEFLa peptide, amino-terminally coupled to keyhole limpet haemocyanine (KLH) via glutaraldehyde. Brains were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4. Afterwards, brains were subjected to several washes in 0.3% TX-100 in PBS (PBST) and incubated for 1 h in blocking solution consisting of 5% normal goat serum (NGS) in PBST. Next, brains were incubated overnight at 4 °C with primary rat anti-EFLa antiserum diluted 1:1000 in 5% NGS in PBST. Brains were washed several times with PBST and incubated overnight at 4 °C with fluorescent secondary goat anti-rat AlexaFluor 488 antibody (ThermoFisher Scientific) diluted 1:1000 in the blocking solution. Brains were then washed several times in PBST and mounted in Vectashield mounting medium (Vector Laboratories). The samples were imaged under Laser Scanning Confocal Microscope FluoView FV1000 (Olympus) using objective UPLSAPO 10× or UPLSAPO 20xO, correction of brightness in depth. All confocal images were processed and analyzed by ImageJ software (NIH). 3D models were reconstructed by stitching of the particular frames in the software XuvStitch (XuvTools). The compound image was then analyzed in the software Imaris (Bitplane) by using modules: Easy 3D, Surpass - Surfaces, Surpass - Ortho Slicer and Animation and scanned under Series (Olympus). The specificity of the antibody was tested on P. apterus EFLa null mutants generated by CRISPR/Cas9.
3. Results
3.1. EFLa gene and transcripts in P. apterus
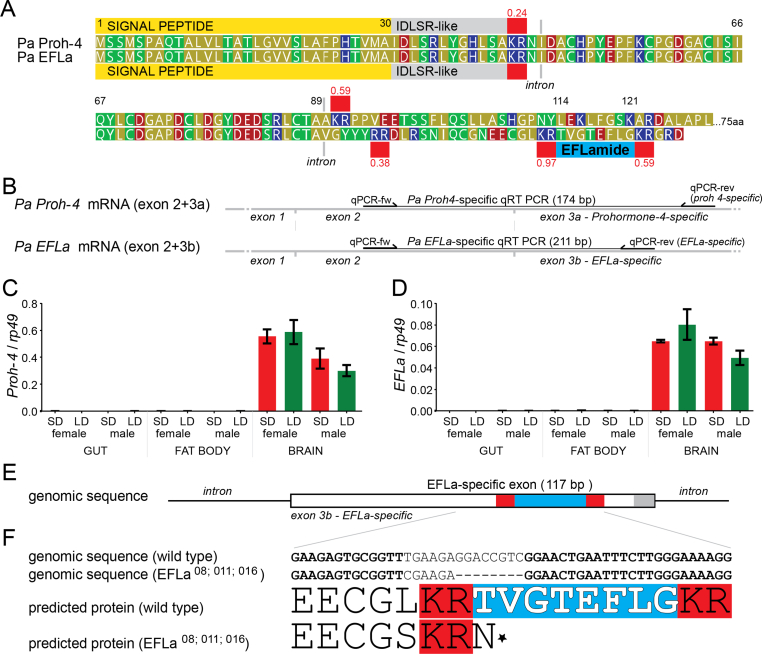
During exploration of P. apterus transcriptome, a putative new neuropeptide precursor was identified. The following sequence criteria indicated that the peptide might be biologically relevant: (i) the preprohormone starts with a clearly predicted signal peptide, (ii) the putative neuropeptide, TVGTEFLG, is surrounded by convertase cleavage sites, and (iii) the last amino acid of the peptide, glycine, could serve as amidation signal, a hallmark of many neuropeptides (Fig. 1A). Therefore, the peptide was named EFLa to indicate its connection with other EFLa-type neuropeptides. Closer in silico analysis discovered transcripts identical with the EFLa mRNA in the first nucleotides coding for the signal peptide and the predicted IDLSRF-like neuropeptide, but distinct at the 3′ end, where EFLa is encoded. IDLSRF-like peptide is highly conserved amino acid motif characteristic for Prohormone-4 (Proh-4) found first in the honey bee (Hummon et al., 2006). BLAST search in GenBank and detailed protein alignments revealed high similarity of EFLa transcripts with Proh-4 in P. apterus, suggesting that EFLa-coding exon is fused downstream of the first two Proh-4 exons. Indeed, a physical presence of both transcripts in brain tissue was confirmed by PCR on brain cDNA followed by Sanger sequencing and independently with different pair of primers by RT qPCR (Fig. 1B, C,D).
Fig. 1.
EFLa is a neuropeptide which results from alternative splicing of the Prohormone-4 gene in P. apterus. (A) Alignment of Proh-4 and EFLa preprohormone protein sequences. Signal peptide is shown in yellow (SignalP 5.0 prediction), convertase cleavage sites are highlighted in red with values indicating NeuroPred cleavage prediction scores. Both preprohormones contain a 12 aa peptide (IDLSRLYGHLSA) located between the signal peptide and first cleavage site. The EFLa preprohormone contains an 8 aa peptide (TVGTEFLG) motif surrounded by two cleavage sites. (B) Detailed scheme of mRNA indicates the position of intron-exon boundaries and transcript-specific reverse primers used (C,D) to confirm brain-specific expression of Proh-4 and EFLa (graphs show mean ± SEM). LD - long day, SD - short day. In both transcripts, the expression was significantly different in brains when compared to other tissues (One-way ANOVA, p < 0.001), but the differences between sexes or photoperiods were not significant (p > 0.05). (E) 117 bp long exon 3 codes for EFLa peptide, its non-coding untranslated terminal region (UTR) is shown in gray. (E,F) Detail of the genomic sequence in wild type and EFLa null mutants, where 8 bp deletion results in a stop codon (shown as asterisk). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Since typical neurohormones and neuropeptides are preferentially expressed in the Central Nervous System (CNS), we quantified EFLa and Proh-4 by RT qPCR with universal forward and transcript-specific reverse primers. The minimal expression levels in gut and fat body contrasted with the clear expression in brains of both sexes. The amount of Proh-4 was approximately 10-times the level of EFLa (Fig. 1C and D).
To further shed some light on the role of this putative neuropeptide, null mutants were engineered in P. apterus (for technical details see Kotwica-Rolinska et al., 2019). Briefly, Cas9 was guided to EFLa-specific exon 3b (Fig. 1E) to cleave right upstream of the peptide-coding sequence. Deletion of eight nucleotides resulted in a frameshift and premature stop codon that removed the entire amino acid motif of EFLa (Fig. 1F). Three mutants with identical deletions were retrieved and separately backcrossed to wild type strain for up to eight generations. Two or three of the mutant strains were used in further experiments to clarify the effect of the mutation and distinguish it from the genetic background.
3.2. EFLa is expressed in 2–3 cells of each brain hemisphere
Polyclonal antibodies raised against the TVGTEFLa peptide labeled approximately twenty neuronal cell bodies in the wild type adult brain. Majority of these cells were labeled also in the EFL011 and EFL016 mutants (all EFLa mutants are homozygous viable), indicating that the antibodies cross-react with other antigens. However, intensive staining detected in a bilateral cluster of two to three neurons located in the lateral protocerebrum were not observed in the mutants indicating their EFLa-specificity (Fig. 2A–C). The EFLa-specific cell bodies were located very close to each other near to the surface of the lateral protocerebrum. Neuronal projections rising from those cells run to the medial protocerebrum where they intensively ramify. This ramification gives off a dense neuronal arborization, which projects in several directions (Fig. 2G): (1) anteriorly to the midline of the ipsilateral proto- and deutocerebrum, (2) posteriorly around the central body to the contralateral hemispheres, (3) laterally to the dorsal protocerebrum and to the frontoventral deutocerebrum. These lateral branches turn to the posterior brain passing ventrally of the EFLa specific neurons and merging with a neuronal network in the posterior brain.
Fig. 2.
Localisation of immunoreactivity with anti EFLa antibody in the brain of P. apterus, with red arrowheads labelling EFLa-expressing cell bodies (A–C) Dorsal view of the whole brain of wild type, EFLa011, and EFLa016 mutants, respectively. (D) Detailed image of EFLa positive neurons and their neuronal projections. (E, F) Comparable EFLa immunoreactivity was observed in control bugs injected with double strand RNA (ds) for lacZ (E), and with dsEFLa (F). Red arrows indicate the EFLa-specific signal in a bilateral cluster of neurons in the lateral protocerebrum. (G) Extracted EFLa-specific immunoreactivity in a 3D reconstruction of the brain. (H) The efficiency of gene silencing by dsRNA was confirmed by RT qPCR, yet the IHC signal was strong in EFLa-specific cell bodies of RNAi animals (I), and only marginally reduced in their arborizations (J) (One-way ANOVA p > 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In parallel, RNA interference was used to silence expression of EFLa. Although the knockdown was reasonably efficient, resulting in ~90% reduction in mRNA level that persisted low for 3, 7 and 10 days (Fig. 2H), EFLa immunoreactivity in cell bodies of bona-fide EFLa neurons was not affected even 10 days after EFLa dsRNA injection (Fig. 2F and I), and the immunoreactivity was only marginally reduced in arborizations (Fig. 2F and J). Cumulatively this suggests that EFLa might be a stable peptide with a long half-live.
3.3. EFLa null-mutants are fully viable with no obvious developmental defects
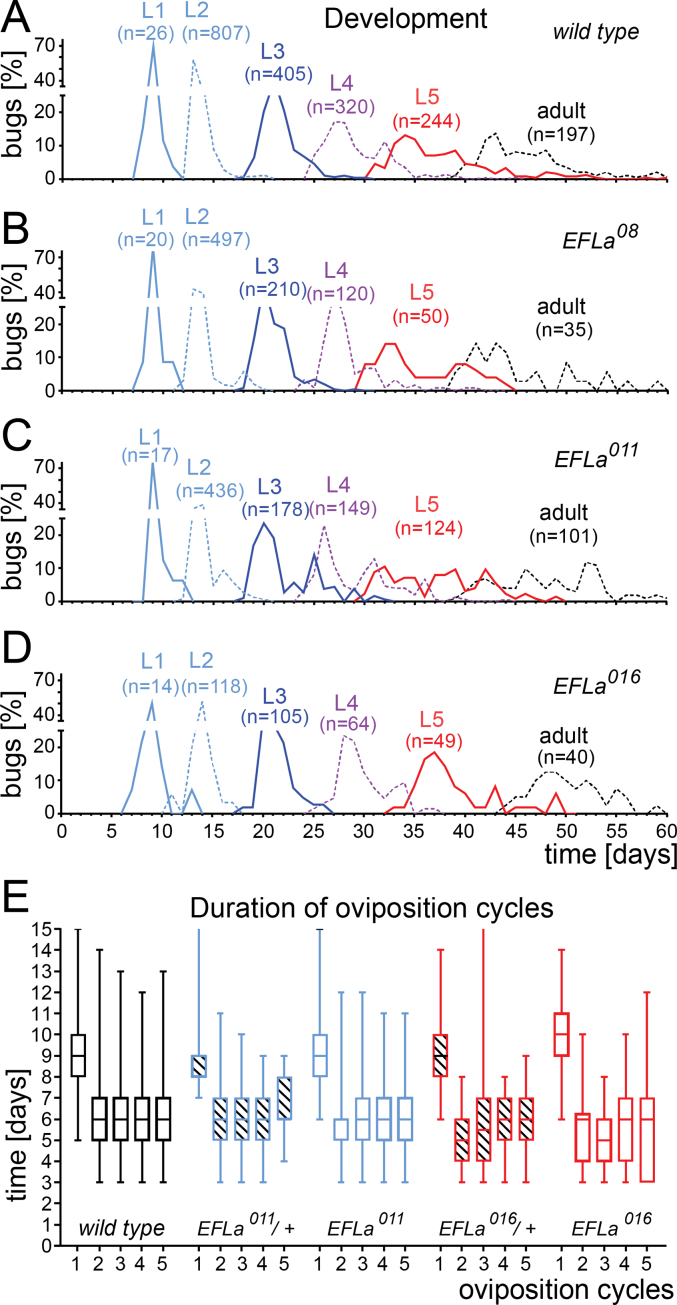
We have used all assays available in our lab for P. apterus to investigate the physiological roles of EFLa. The development from the egg to the adult takes 40–50 days at 25 °C in wild type animals. Comparable duration of all instars and the entire development was observed in all three mutants (Fig. 3A–D) and also in heterozygotes (data not shown).
Fig. 3.
Comparison of development in wild-type and EFLa-mutant P. apterus. (A–D) Timing of transition to a new instar is shown on x-axis. Time ‘0’ corresponds to egg laying. Transition to L1 indicating egg development is determined for the entire clutch, not for individual eggs. Sum of all individuals of particular developmental stage of the same genotype was set as 100% and the daily percentage of newly emerged bugs is plotted. (E) Duration of first five oviposition cycles shown as mean, 25–75 percentile (box), and min-max (whiskers) is not significantly different between tested genotypes (Two-way ANOVA p > 0.05).
P. apterus wild type females lay the first batch of eggs approximately on the ninth day after adult eclosion and afterwards are able to lay eggs in several oviposition cycles separated by six days when kept at 25 °C with a diapause-preventing long photoperiod. Comparable duration was determined for the EFLa011 and EFLa016 homozygotes and heterozygotes (Fig. 3E). To further characterize possible roles of EFLa, lifespan was compared between wild types and EFLa011 and EFLa016 homozygotes and heterozygotes. The survival of virgin females at reproduction-promoting conditions of LD and 25 °C is not affected by loss of EFLa (Fig. 4A and B).
Fig. 4.
Comparison of lifespan of adult reproductive virgin wild-type and EFLa-mutant P. apterus females, determined in LD regime and 25 °C for homozygotes (A) and heterozygotes (B). y-axis indicates percent survival, the actual n is shown above the graph for all genotypes. Survival is not significantly affected by mutation of EFLa gene (Mantel–Cox log-rank test).
3.4. Circadian clock and photoperiodic time measurement are not influenced by EFLa
Adult P. apterus bugs are active mostly during the long day (LD) with a relatively broad activity peak. Both homozygous mutants show comparable timing of activity rise and duration through the photophase of the long photoperiod (Fig. 5A). The average locomotor activity differs between EFLa011 and EFLa016, but is not different from the wild type (Fig. 5B). Therefore, these differences seem to be resulting from the genetic background and are not caused by the lack of EFLa.
Fig. 5.
Locomotor activity, circadian clock and diapause in wild-type and EFLa-mutants. (A) Locomotor activity in long day (LD) regime (gray background corresponds to dark) shown as mean ± SEM. (B) The average activity in LD is not different between wt and mutants, although mutants differ between each other (One-way ANOVA). (C) Photoperiodic induction of diapause is identical in wt and EFLa011 homozygotes and heterozygotes, respectively. (D) The free running period and (E) percent rhythmicity determined from 10 days in constant dark (DD). (F) The average activity in DD is not different between tested genotypes. Different small letter above categories indicate statistical difference p < 0.05 (One-way ANOVA).
Then we tested the ability of P. apterus to detect short days (SD) and to communicate this information further downstream. Under short photoperiod, wild type bugs undergo reproductive arrest, diapause, which is characterized by small ovaries without eggs. Indeed, ~96% of wt females are diapausing in SD conditions and a comparable amount of EFLa011 homozygotes (94.4%) and heterozygotes (96%) enter diapause too (Fig. 5C).
The free running period of locomotor activity recorded under constant dark conditions (DD) differs between EFLa011 and EFLa016, but this difference can be clearly attributed to genetic background, because one mutant (EFLa011) produces a faster circadian clock than the wild type line, whereas the trend is completely the opposite in the second mutant (Fig. 5D). Neither the percent rhythmicity, nor the average activity in DD is influenced by loss of EFLa (Fig. 5E and F).
3.5. Evolution of EFLa in arthropods
The link between Proh-4 and EFLa observed in P. apterus prompted us to explore the evolution of EFLa-coding genes. Orthologous full length EFLa transcripts were identified in Lygus (Heteroptera), in Bemisia (Sternorrhyncha) and in Locusta (Polyneoptera). In all cases, the EFLa preprohormone also contained the highly conserved IDLSR-like peptide characteristic for Proh-4 (Fig. 6A). Indeed, Lygus, Bemisia and Locusta Proh-4 are identical to EFLa preprohormone in their first 85, 82 and 90 amino acids, respectively (Fig. 6B). Proh-4 sequences are highly conserved, which allowed us to unambiguously explore its possible co-evolution with EFLa. Clearly, EFLa prohormones of basal hexapods, Crustacea and Chelicerata are neither identical, nor even similar to Proh-4. Thus, the connection of EFLa with Proh-4 is an evolutionary novelty found only in Hemiptera and Locusta (Fig. 6A). Whether EFLa exists in holometabolan insects is unclear. However, our focused attempts to identify EFLa or even alternative splicing of Proh-4 were repeatedly unsuccessful in Holometabola, Psocodea and Thysanoptera.
Fig. 6.
Co-evolution of EFLa with Proh-4 is found in Locusta and Hemiptera but not in basal hexapods. (A) Simplified tree illustrates the phylogenetic relationship between analyzed species. The table shows number of TEFLa or SEFLa motifs predicted within each preprohormone. Proh-4 was identified in representative species (+) and its alternative fusion to EFLa transcript (Proh-4+EFLa) is either identified (yes) or excluded (no). The peptide sequence logo shows frequency of amino acids in predicted EFLa peptides from particular species and groups of species. Schematic depiction of EFLa preprohormones from each species indicates the presence and position of important sequence features. In Hemiptera and Locusta, 1 or 2 EFLa motifs are found in the preprohormone, which also encodes for IDLSR-like peptide. Multiple SEFLa repeats found in preprohormones in Chelicerata, Crustacea and wingless hexapods (Protura, Collembola, Diplura, Archaeognatha, and Zygenthoma) are never combined with IDLSR-like peptide. (B) Detail of protein alignment indicates shared preprohormone region between EFLa and Proh-4 in Hemiptera (from the N-terminus up to the arrow). Asterisk indicates the C-terminus of the protein. See supplement (Fig. S1) for alignment of all EFLa sequences with Proh-4.
There is one EFLa peptide encoded in Bemisia (SIGTEFLG) and Pyrrhocoris (TVGTEFLG) preprohormones that differ in two initial amino acids. In Lygus, two identical TVGTEFLG sequences are found and the same arrangement exists in the partial sequence from Lopidea (both species belong to heteropteran family Miridae) indicating that tandem EFLa organization is not an artifact of the transcriptome assembly. The organization of EFLa preprohormones in basal hexapods is more diverse. The number of EFLa motifs is varies and reaches up to 18 paracopies in several species (Fig. 6A, Derst et al., 2016). The lowest copy number might be as low as 4 in Tetranychus (Veenstra et al., 2012).
3.6. Presence of EFLa-receptor (EFLaR) and ligand in insects
Our inability to repeatedly find EFLa transcripts and genes in holometabolan insects, aphids, and some polyneopteran orders, prompted us to explore the distribution of EFLaR in major insect lineages. Although absence of a particular gene may reflect just an imperfect genome assembly, or, in the case of the transcriptomic analysis result from low expression of the gene in the sequenced tissue, repeated failure to identify EFLaR in multiple species belonging to one taxonomic group becomes informative. We also plotted the presence of the receptor for ecdysis triggering hormone (ETH), the closest relative of EFLaR.
Despite our systematic search in genomes, transcriptomes and proteomes of several holometabolan insects, we were not able to find EFLaR and EFLa (representative species are shown in Fig. 7; the only EFLaR sequence retrieved from Ragoletis clusters clearly with mite sequences, and is, therefore interpreted as a result of interspecific contamination of the sequenced material). Similarly to Holometabola, no EFLaR and EFLa was identified in Psocodea and Thysanoptera.
Fig. 7.
Phylogenetic distribution of EFLa-like receptor and its ligand in insects. Simplified insect phylogeny according to recent molecular data (Misof et al., 2014; Johnson et al., 2018; Wipfler et al., 2019) indicates insect groups with particular species where EFLa receptor, ligand and related ETH receptor were (+) or were not (empty box) identified. Presence indicates confidence that identified sequence is reliably assigned as a particular receptor homolog, however, even partial sequences were included, if the analysis was reliable. Thus, this scheme should be interpreted as an indication of where the EFLa receptor gene is present, but it does imply that the gene is also functional. See supplement for actual sequences. Sternor. – Sternorrhyncha. Auchenor. - Auchenorrhyncha.
A more complicated scenario was observed in Hemiptera, a group consisting of three orders: Sternorrhyncha, Auchenorrhyncha and Heteroptera (Johnson et al., 2018). In basal Sternorrhyncha, such as Bemisia and Diaphorina, both EFLaR and EFLa are found (Fig. 7). However, in the apical Sternorrhyncha, Aphids, we repeatedly failed to identify either the receptor or the ligand, although aphids belong to one of the most sequenced insect groups. A comparable situation were observed in Auchenorrhyncha, where ligand and receptor was identified in Cicadomorpha, but in a sister group containing planthoppers (Fulgoromorpha), only the receptor was identified.
In Polyneoptera, a monophyletic assembly represented here by Orthoptera, Blattodea and Phasmatodea, a picture analogous to situation observed previously in Hemiptera was found. Both receptor and ligand were identified in Locusta (Orthoptera) and Timema (Phasmatodea), but, despite our repeated effort, neither ligand nor receptor was retrieved from any Blattodea, including species with sequenced genomes and reasonably covered brain transcriptomes.
Altogether, available data suggest five possible independent losses of EFLa signaling in insects (Fig. 7): (i) Holometabola together with order Psocodea; (ii) order Thysanoptera, thrips; (iii) superfamily Aphidoidea, aphids; (iv) infraorder Fulgoromorpha, planthoppers; and (v) order Blattodea with cockroaches and termites.
4. Discussion
This study and concurrent research on locusts (Veenstra and Šimo, 2020) provide the first analysis of EFLa-type neuropeptide expression in insects, although genes encoding EFLa-type neuropeptides were previously identified in basal hexapods including insects such as the firebrat Thermobia domestica and Atelura formicaria (Derst et al., 2016), the bed bug Cimex lectularius, locust, and phasmids (Predel et al., 2018; Veenstra, 2019). Various EFLa-encoding genes and related FFamide, FLamide and FVamide genes have been identified in crustaceans, mollusks and annelids (Conzelmann et al., 2013; Veenstra, 2010, 2011, 2012).
To elucidate the function of EFLa, complete null mutants were engineered in P. apterus by CRISPR/Cas9 technology. One key aspect of any reverse-genetic experiment is to ensure that the desired mutation, such as the EFLa removal, is not accompanied by any unintentional changes in the genome, the off target mutations. This is usually solved by outcrossing the mutant line to wt strain. While this backcrossing can be efficiently done in D. melanogaster, the generation time of many emerging model organisms is significantly longer and thus protracts the CRISPR/Cas9 experiment, which might be a serious practical limitation for the research. In our case, 6 rounds of backcrosses were used. With subsequent amplification of the “cleaned” lines, the procedure took ~15 months to obtain a sufficient number of individuals for phenotypic characterization.
Up to three independently backcrossed EFLa mutant lines were used in experiments and compared to wt. Our data indicate that EFLa removal does not affect development duration and adult bugs are phenotypically normal, including no anatomical defect observed, their locomotion is comparable to wt, and their lifespan is not affected. EFLa mutants are able to discriminate between long and short photoperiods to enter reproductive diapause and their circadian clock is functional. Since the expression pattern is reasonably similar in P. apterus and Locusta, it is plausible to suggest that comparable modulatory roles of EFLa are shared between species, although, of course, details might differ. Interestingly, EFLa expression pattern in Locusta prompted Veenstra and Šimo (2020) to suggest “… that the ELFamide neurons exert a modulatory input onto the navigation system of the locust by simultaneous targeting several stages of the sky compass system in the locust brain.” Whether EFLa is involved in some sort of neuromodulation in P. apterus is unknown. Since P. apterus is not a migratory species, neither its navigation nor its sky compass has been studied, to our knowledge.
Obviously, a neuropeptide or neuromodulator might affect a plethora of biological phenomena, thus negative results observed in a limited number of assays do not indicate that EFLa has no function in P. apterus. However, even this eventuality is plausible, because EFLa and its receptor were identified only in some insects, whereas they are absent and thus most likely lost in others (see caveats below). Since unused ligand or receptors can slowly accumulate deleterious mutations, early steps in this process cannot be excluded in P. apterus. When we map the presence of EFLa ligands and its receptors on insect phylogeny, up to five possible loses of either both or only the ligand are suggested (Fig. 7). Nevertheless, it is problematic to prove loss of a gene in general and particularly if it codes for a short ligand, therefore, any negative data need to be interpreted carefully. Yet, if a gene is repeatedly missing in all representatives of a particular monophyletic lineage, its absence becomes meaningful. In the case of planthoppers, EFLaR is still present (Tanaka et al., 2014) and only the ligand is missing, which invites speculation that the early steps of signaling loss are captured. The second hemipteran group where EFLa signaling was independently lost, aphids, lacks both the receptor and ligand. Given the agricultural importance of planthoppers and aphids, reasonable number of transcriptomes and genomes from additional species is expected in future and will facilitate further confirmations. Moreover, the short ligand-coding sequences can be retrieved even from raw Illumina reads, thus the challenging process of genome assembly will not prevent either EFLa identification, or will further support its likely absence in planthoppers.
A similarly interesting situation is seen in Polyneoptera. This assemblage of insect orders includes Orthoptera, where activation of EFLa receptor by EFLa was functionally confirmed for Locusta (Veenstra and Šimo, 2020) and phasmids with only partially identified EFLa genes (Veenstra, 2019). However, in cockroaches and termites, representatives of the most apical polyneopteran order, Blattodea (Wipfler et al., 2019), neither ligand nor receptor is identified (Fig. 7). It is worth noting that polyneopteran genomes are one of the largest in animals, which does not prevent or even limit their sequencing, yet the assembly is often complicated. However, with remarkable interest in social insects, termite genomic and transcriptomic data are quickly growing and will clarify our hypothesis.
Our study points to another interesting and perhaps even provocative finding, the fusion of EFLa mRNA downstream of sequence originating from Proh-4. In fact, the connection of Proh-4 and EFLa was independently identified by Veenstra and Šimo (2020) in Locusta, however, interpreted as an artifact generated during the transcript assembly. At that time, their cautious approach was completely correct and even now we cannot entirely exclude the possibility that the fused Proh-4/EFLa encoding transcripts are artifacts. However, in the light of new data, the connection of EFLa and Proh-4 seems to be reasonably supported. Firstly, the physical presence of existing mRNA was confirmed in P. apterus by two independent PCR experiments, the first aiming at confirmation of transcript sequences, and the second addressing the expression level of both transcripts. Secondly, the fused Proh-4/EFLa encoding transcripts were found in four insect species (P. apterus, Lygus, Bemisia, Locusta) each of them coded by slightly different RNA sequence due to different codon usage, yet, the Proh-4 and EFLa fusion occurs in an identical position (coding for amino acids RLCTA, Fig. 6) in all four species. Nevertheless, the exact process of how EFLa-specific exon is connected to the Proh-4 sequence is unclear. The possible mechanism might involve canonical cis-splicing, cis-splicing of adjacent genes (cis-SAG), or even trans-splicing, a molecular mechanism relatively rare in insects (Kong et al., 2015), although even examples of trans-spliced neuropeptides were reported in mosquitoes (Robertson et al., 2007). The quality of available genome assemblies for P. apterus, Lygus, Bemisia, and Locusta does not allow us to clarify this issue at this point, but, with emerging sequencing technologies one can expect that this task will be unambiguously solved in near future.
Another standing question is dating when the connection of Proh-4 and EFLa originated during the insect evolution. Solving this issue also strongly depends on high quality transcriptomes and genomes. In chelicerates, crustaceans, basal hexapods and basal insects including early winged Ephemeroptera, well conserved Proh-4 do no overlap with EFLa-coding transcripts and these transcripts seem to code all essential prohormone properties, including the signal peptide, thus they are very likely full transcripts (Supplementary Fig. S1). Therefore, the most plausible still provisional estimation dates the connection of EFLa with Proh-4 to the common ancestor of Polyneoptera and Hemiptera, which is approximately 380 Mya according to the most recent phylogenies (Misof et al., 2014; Johnson et al., 2018).
Both our and the concurrent study by Veenstra and Šimo (2020) confirmed that the genes with two or even only one EFLa motif copy are expressed in insects. It will be very interesting to see the expression pattern and level of EFLa in basal hexapods, where genes with as many as 18 paracopies are frequently found. Despite the confirmation and identification of EFLa-positive neurons, the role of the peptide in insects is elusive. Although it might be possible that EFLa has lost entirely its function, it is equally conceivable that the neuropeptide has some specific role that is not obvious under normal rearing conditions.
Acknowledgement
We appreciate the help of Magdalena M. Kaniewska during developmental experiments and all members of our laboratory for a stimulating atmosphere. We thank Martina Hajdušková (www.biographix.cz) for the insect scheme used in the graphical abstract. We thank Vlastík Smýkal for critical reading of the manuscript and we also appreciate suggestions from anonymous reviewers of the paper. The work was supported from the European Research Council (ERC) under the European Union's Horizon 2020 Programme Grant Agreement 726049. J.P. and M.H. were supported by the Czech Science Foundation (GACR, grant# 15-23681S).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ibmb.2020.103376.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Almagro Armenteros J.J., Tsirigos K.D., Sonderby C.K., Petersen T.N., Winther O., Brunak S., von Heijne G., Nielsen H. Signalp 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019;37:420–423. doi: 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
- Bajgar A., Jindra M., Dolezel D. Autonomous regulation of the insect gut by circadian genes acting downstream of juvenile hormone signaling. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4416–4421. doi: 10.1073/pnas.1217060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar A., Dolezel D., Hodkova M. Endocrine regulation of non-circadian behavior of circadian genes in insect gut. J. Insect Physiol. 2013;59:881–886. doi: 10.1016/j.jinsphys.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Bauknecht P., Jékely G. Large-scale combinatorial deorphanization of Platynereis neuropeptide GPCRs. Cell Rep. 2015;12:684–693. doi: 10.1016/j.celrep.2015.06.052. [DOI] [PubMed] [Google Scholar]
- Conzelmann M., Williams E.A., Krug K., Franz-Wachtel M., Macek B., Jékely G. The neuropeptide complement of the marine annelid Platynereis dumerilii. BMC Genom. 2013;14:906. doi: 10.1186/1471-2164-14-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derst C., Dircksen H., Meusemann K., Zhou X., Liu S., Predel R. Evolution of neuropeptides in non-pterygote hexapods. BMC Evol. Biol. 2016;16:51. doi: 10.1186/s12862-016-0621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezel D., Sauman I., Kostal V., Hodkova M. Photoperiodic and food signals control expression pattern of the clock gene, period, in the linden bug, Pyrrhocoris apterus. J. Biol. Rhythm. 2007;22:335–342. doi: 10.1177/0748730407303624. [DOI] [PubMed] [Google Scholar]
- Dolezel D., Zdechovanova L., Sauman I., Hodkova M. Endocrine-dependent expression of circadian clock genes in insects. Cell. Mol. Life Sci. 2008;65:964–969. doi: 10.1007/s00018-008-7506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu. Rev. Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Eipper B.A., Stoffers D.A., Mains R.E. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu. Rev. Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Elphick M.R., Mirabeau O., Larhammar D. Evolution of neuropeptide signalling systems. J. Exp. Biol. 2018;221 doi: 10.1242/jeb.151092. jeb151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K.K., Stafflinger E., Schneider M., Hauser F., Cazzamali G., Williamson M., Kollmann M., Schachtner J., Grimmelikhuijzen C.J. Discovery of a novel insect neuropeptide signaling system closely related to the insect adipokinetic hormone and corazonin hormonal systems. J. Biol. Chem. 2010;285:10736–10747. doi: 10.1074/jbc.M109.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummon A.B., Richmond T.A., Verleyen P., Baggerman G., Huybrechts J., Ewing M.A., Vierstraete E., Rodriguez-Zas S.L., Schoofs L., Robinson G.E., Sweedler J.V. From the genome to the proteome: uncovering peptides in the Apis brain. Science. 2006;314:647–649. doi: 10.1126/science.1124128. [DOI] [PubMed] [Google Scholar]
- Ibrahim E.A.S., Hejníková M., Shaik H.A., Doležel D., Kodrík D. Adipokinetic hormone activities in insect body infected by entomopathogenic nematode. J. Insect Physiol. 2017;98:347–355. doi: 10.1016/j.jinsphys.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Jekely G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8702–8707. doi: 10.1073/pnas.1221833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.P., Dietrich C.H., Friedrich F., Beutel R.G., Wipfler B., Peters R.S., Allen J.M., Petersen M., Donath A., Walden K.K.O. Phylogenomics and the evolution of hemipteroid insects. Proc. Natl. Acad. Sci. U. S. A. 2018;115:12775–12780. doi: 10.1073/pnas.1815820115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniewska M.M., Vaneckova H., Dolezel D., Kotwica-Rolinska J. Light and temperature synchronizes locomotor activity in the linden bug, Pyrrhocoris apterus. Front. Physiol. 2020 doi: 10.3389/fphys.2020.00242. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodrik D., Socha R., Simek P., Zemek R., Goldsworthy G.J. A new member of the AKH/RPCH family that stimulates locomotory activity in the firebug, Pyrrhocoris apterus (Heteroptera) Insect Biochem. Mol. Biol. 2000;30:489–498. doi: 10.1016/S0965-1748(00)00025-4. [DOI] [PubMed] [Google Scholar]
- Kodrik D., Simek P., Lepsa L., Socha R. Identification of the cockroach neuropeptide Pea-CAH-II as a second adipokinetic hormone in the firebug Pyrrhocoris apterus. Peptides. 2002;23:585–587. doi: 10.1016/S0196-9781(01)00627-1. [DOI] [PubMed] [Google Scholar]
- Kong Y., Zhou H., Yu Y., Chen L., Hao P., Li X. The evolutionary landscape of intergenic trans-splicing events in insects. Nat. Commun. 2015;6:8734. doi: 10.1038/ncomms9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopova B., Smykal V., Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PloS One. 2011;6(12) doi: 10.1371/journal.pone.0028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwica-Rolinska J., Pivarciova L., Vaneckova H., Dolezel D. The role of circadian clock genes in the photoperiodic timer of the linden bug, Pyrrhocoris apterus, during the nymphal stage. Physiol. Entomol. 2017;42:266–273. doi: 10.1111/phen.12197. [DOI] [Google Scholar]
- Kotwica-Rolinska J., Chodakova L., Chvalova D., Kristofova L., Fenclova I., Provaznik J., Bertolutti M., Wu B.C., Dolezel D. CRISPR/Cas9 Genome editing introduction and optimization in the non-model insect Pyrrhocoris apterus. Front. Physiol. 2019;10:891. doi: 10.3389/fphys.2019.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liessem S., Ragionieri L., Neupert S., Buschges A., Predel R. Transcriptomic and neuropeptidomic analysis of the stick insect, Carausius morosus. J. Proteome Res. 2018;6:2192–2204. doi: 10.1021/acs.jproteome.8b00155. [DOI] [PubMed] [Google Scholar]
- Mirabeau O., Joly J.S. Molecular evolution of peptidergic signaling systems in 418 bilaterians. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E2028–E2037. doi: 10.1073/pnas.1219956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misof B. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014;346:763–767. doi: 10.1126/science.1257570. [DOI] [PubMed] [Google Scholar]
- Pivarciova L., Vaneckova H., Provaznik J., Wu B.C., Pivarci M., Peckova O., Bazalova O., Cada S., Kment P., Kotwica-Rolinska J., Dolezel D. Unexpected geographic variability of the free running period in the linden bug, Pyrrhocoris apterus. J. Biol. Rhythm. 2016;31:568–576. doi: 10.1177/0748730416671213. [DOI] [PubMed] [Google Scholar]
- Predel R., Neupert S., Derst C., Reinhardt K., Wegener C. Neuropeptidomics of the bed bug Cimex lectularius. J. Proteome Res. 2018;17:440–454. doi: 10.1021/acs.jproteome.7b00630. [DOI] [PubMed] [Google Scholar]
- Robertson H.M., Navik J.A., Walden K.K., Honegger H.W. The bursicon gene in mosquitoes: an unusual example of mRNA trans-splicing. Genetics. 2007;176:1351–1353. doi: 10.1534/genetics.107.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B., Helfrich-Forster C., Yoshii T. A new ImageJ plug-in "ActogramJ" for chronobiological analyses. J. Biol. Rhythm. 2011;26:464–467. doi: 10.1177/0748730411414264. [DOI] [PubMed] [Google Scholar]
- Slama K., Williams C.M. Paper factor as an inhibitor of embryonic development of european bug Pyrrhocoris apterus. Nature. 1966;210:329–330. doi: 10.1038/210329a0. [DOI] [PubMed] [Google Scholar]
- Smykal V., Bajgar A., Provaznik J., Fexova S., Buricova M., Takaki K., Hodkova M., Jindra M., Dolezel D. Juvenile hormone signaling during reproduction and development of the linden bug, Pyrrhocoris apterus. Insect Biochem. Mol. Biol. 2014;45 doi: 10.1016/j.ibmb.2013.12.003. 69e76. [DOI] [PubMed] [Google Scholar]
- Smýkal V., Pivarči M., Provazník J., Bazalová O., Jedlička P., Lukšan O., Horák A., Vaněčková H., Beneš V., Fiala I., Hanus R., Doležel D. Complex evolution of insect insulin receptors and homologous decoy receptors, and functional significance of their multiplicity. Mol. Biol. Evol. 2020 doi: 10.1093/molbev/msaa048. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socha R. Pyrrhocoris apterus (Heteroptera) - an experimental model species - a review. Eur. J. Entomol. 1993;90:241–286. [Google Scholar]
- Southey B.R., Amare A., Zimmerman T.A., Rodriguez-Zas S.L., Sweedler J.V. Neuropred: a tool to predict cleavage sites in neuropeptide precursors and provide the masses of the resulting peptides. Nucleic Acids Res. 2006;34:W267–W272. doi: 10.1093/nar/gkl161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Suetsugu Y., Yamamoto K., Noda H., Shinoda T. Transcriptome analysis of 460 neuropeptides and G-protein coupled receptors (GPCRs) for neuropeptides in the brown planthopper Nilaparvata lugens. Peptides. 2014;53:125–133. doi: 10.1016/j.peptides.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Van Sinay E., Mirabeau O., Depuydt G., Van Hiel M.B., Peymen K., Watteyne J., Zels S., Schoofs L., Beets I. Evolutionarily conserved TRH neuropeptide pathway regulates growth in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2017;114:E4065–E4074. doi: 10.1073/pnas.1617392114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra J.A. Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch. Insect Biochem. Physiol. 2000;43:49–63. doi: 10.1002/(SICI)1520-6327(200002)43:2<49::AID-ARCH1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Veenstra J.A. Neurohormones and neuropeptides encoded by the genome of Lottia gigantea, with reference to other mollusks and insects. Gen. Comp. Endocrinol. 2010;167:86–103. doi: 10.1016/j.ygcen.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Veenstra J.A. Neuropeptide evolution: neurohormones and neuropeptides predicted from the genomes of Capitella teleta and Helobdella robusta. Gen. Comp. Endocrinol. 2011;171:160–175. doi: 10.1016/j.ygcen.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Veenstra J.A. The contribution of the genomes of a termite and a locust to our understanding of insect neuropeptides and neurohormones. Front. Physiol. 2014;5:454. doi: 10.3389/fphys.2014.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra J.A. Similarities between decapod and insect neuropeptidomes. PeerJ. 2016;4 doi: 10.7717/peerj.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra J.A. Two Lys-vasopressin-like peptides, EFLamide, and other phasmid neuropeptides. Gen. Comp. Endocrinol. 2019;278:3–11. doi: 10.1016/j.ygcen.2018.04.027. [DOI] [PubMed] [Google Scholar]
- Veenstra J.A., Šimo L. The TRH-ortholog EFLamide in the migratory locust. Insect Biochem. Mol. Biol. 2020;116 doi: 10.1016/j.ibmb.2019.103281. [DOI] [PubMed] [Google Scholar]
- Veenstra J.A., Rombauts S., Grbić M. In silico cloning of genes encoding neuropeptides, neurohormones and their putative G-protein coupled receptors in a spider mite. Insect Biochem. Mol. Biol. 2012;42:277–295. doi: 10.1016/j.ibmb.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Wipfler B., Letsch H., Frandsen P.B., Kapli P., Mayer C., Bartel D., Buckley T.R., Donath A., Edgerly-Rooks J.S., Fujita M. Evolutionary history of Polyneoptera and its implications for our understanding of early winged insects. Proc. Natl. Acad. Sci. U. S. A. 2019;116:3024–3029. doi: 10.1073/pnas.1817794116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.