Abstract
Given the vast investments made in national immunization programmes (NIPs) and the significance of NIPs to public health, it is important to understand what influences the optimal performance of NIPs. It has been established that well-performing NIPs require enabling health systems. However, systematic evidence on how the performance of health systems impacts on NIPs is lacking, especially from sub-Saharan Africa. We conducted a qualitative systematic review to synthesize the available evidence on health systems constraints and facilitators of NIPs in sub-Saharan Africa, using human papillomavirus immunization programmes as a proxy. Fifty-four articles published between 2008 and 2018 were found to be eligible. Data extraction was guided by an analytical model on the interface between NIPs and health systems. A cross-cutting thematic analysis of the extracted data was performed. This systematic review provides evidence necessary for informing ongoing health systems strengthening initiatives in sub-Saharan Africa. There is evidence to suggest that NIPs in sub-Saharan Africa have surmounted significant health systems constraints and have achieved notable public health success. This success can be attributed to strong political endorsement for vaccines, clear governance structures and effective collaboration with global partners. Despite this, significant health systems constraints persist in service delivery, vaccine communication, community engagement, the capacity of the health workforce and sustainable financing. These constraints could derail further progress if not addressed through health systems strengthening efforts. There is a need to expand the research agenda to include the comprehensive evaluation of health systems constraints and facilitators of NIPs within sub-Saharan Africa.
Keywords: Africa, cervical cancer, health systems, human papillomavirus, HPV vaccine, immunization, national immunization programmes
Key Messages
National immunization programmes (NIPs) are embedded within health systems. However, the interactions between NIPs and health systems are poorly understood.
This systematic review provides the evidence of how NIPs and health systems interact by reporting on the health systems constraints and facilitators of NIPs in sub-Saharan Africa.
Strong political will, clear governance structures and effective collaboration with global partners have been major facilitators of NIPs in sub-Saharan Africa. Despite this, significant health systems constraints persist in service delivery, vaccine communication, community engagement, the capacity of the health workforce and sustainable financing.
The findings of this review have relevance for ongoing health systems strengthening initiatives in sub-Saharan Africa, especially where NIPs are concerned. By providing a better understanding of what works—and does not work—for NIPs, health systems strengthening initiatives could be better designed to adequately respond to the burden of vaccine-preventable diseases in sub-Saharan Africa.
Introduction
It has become increasingly apparent that some of the major challenges experienced in scaling up the performance of national immunization programmes (NIPs) in low- and middle- income countries (LMICs) are not necessarily programme specific, but rather challenges in wider health systems functioning (SAGE, 2016). This is in line with the notion that NIPs exist in a continuous interaction with the health systems that deliver them (World Health Organization Maximizing Positive Synergies Collaborative Group, 2009; Shen et al., 2014; SAGE, 2016). As such, major health systems constraints could have a significant impact on how NIPs perform. For example, financial, technical, logistical, political and socioeconomic constraints have been cited as negatively impacting on the overall performance of NIPs in LMICs (SAGE, 2016). Unfortunately, these health systems constraints appear to be even more prevalent in sub-Saharan Africa (SAGE, 2016).
In sub-Saharan Africa, NIPs have undergone steady advancements since the establishment of the Expanded Program on Immunization in 1974 (WHO, 2013a). Tremendous progress has been made in increasing access to lifesaving vaccines and reducing the burden of vaccine-preventable diseases in the region (Arevshatian et al., 2007; Machingaidze et al., 2013). Despite this, sub-Saharan Africa continues to lag in meeting global immunization targets (SAGE, 2016; Mihigo et al., 2017). Although substantial investments have been dedicated to NIPs in sub-Saharan Africa, this has not been enough to improve their performance to levels required to significantly reduce the disproportionate burden of vaccine-preventable diseases in the region. In this regard, global actors have recognized the harrowing state of health systems within sub-Saharan Africa as a major barrier to achieving further improvements in the performance of NIPs (SAGE, 2016). This has contributed to making health systems strengthening in sub-Saharan Africa a matter of global public health priority (SAGE, 2016). It has been argued, however, that while ongoing health systems strengthening efforts are absolutely vital, they have not been able to achieve their intended outcomes (Marchal et al., 2009; Chee et al., 2013). Some of the challenges faced have been attributed to the fact that health systems constraints are not adequately defined and as such interventions are often poorly designed and weakly targeted in the long term (Marchal et al., 2009; Goeman et al., 2010; Chee et al., 2013). Systematic evidence on how health systems constraints (and facilitators) impact on the performance of NIPs in sub-Saharan African could be very useful in better informing health systems strengthening efforts in the region. Unfortunately, such evidence is lacking.
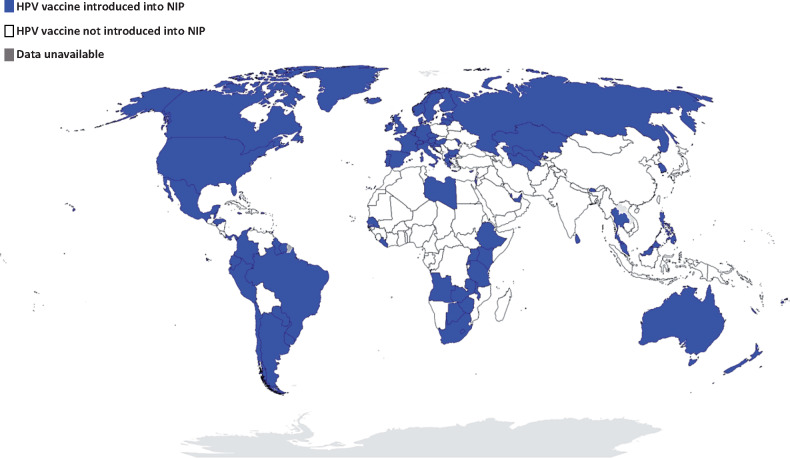
We report on a qualitative systematic review study that sought to determine how health systems constraints and facilitators impact on the performance of NIPs in sub-Saharan Africa. The NIPs serve as a platform for the delivery of several immunization services. These include routine childhood, adolescent and maternal immunization programmes, mass immunization campaigns, outbreak response or emergency vaccination services and introduction of new, improved or underused vaccines into existing NIPs. Ultimately, an in-depth review incorporating the mass of these services would not be appropriate. As such, a single immunization programme—the human papillomavirus (HPV) immunization programme—was selected as a proxy or tracer immunization programme for the purpose of this systematic review. Our interest in HPV immunization programmes stems from the fact that they present a unique challenge to health systems in sub-Saharan Africa compared with routine childhood immunization programmes. Foremost is the fact that HPV vaccines are not widely accessible through NIPs in most sub-Saharan African countries as compared with other regions of the world (see Figure 1 and Supplementary Table S1).
Figure 1.
Global progress in the implementation of nationwide HPV immunization programmes. Data from countries with planned or partial HPV vaccine introduction, or HPV vaccine demonstration projects, are not represented here [drawing on data from http://www.hpvcentre.net, WHO (2019), Gallagher et al. (2018), LaMontagne et al. (2017) and Herrero et al. (2015)].
Three HPV vaccines are currently licenced for use; the four-valent vaccine was licenced in 2006, the bivalent vaccine was licenced in 2007 and the nine-valent vaccine was licenced in 2014 (Herrero et al., 2015; WHO, 2017a). All three prophylactic HPV vaccines have been proven to be safe and effective against persistent HPV infection, which is implicated in a broad spectrum of cancers in both men and women (Lu et al., 2011; Herrero et al., 2015; Garland et al., 2016). The primary focus of this systematic review is HPV vaccination for the prevention of cervical cancer in women. All three HPV vaccines confer immune protection against the HPV oncogenic Types 16 and 18, which are implicated in 70% of all cervical cancers. Cervical cancer, in turn, accounts for 84% of all HPV-associated cancers (WHO, 2017a,b). Given the burden of the disease, the World Health Organization (WHO) recognizes the prevention of cervical cancer as priority and recommends that member states introduce the HPV vaccine into their NIPs (WHO, 2017b). The schedule recommended by WHO is two doses of the HPV vaccine, administered 6 months apart and prioritizing adolescent girls between the ages of 9 and 14 years, prior to sexual debut (WHO, 2017b).
As at December 2019, ∼112 countries and overseas territories had established nationwide HPV immunization programmes (Herrero et al., 2015; LaMontagne et al., 2017; Gallagher et al., 2018; WHO, 2018; 2019). Of this total, only 17 countries are in sub-Saharan Africa (LaMontagne et al., 2017; Gallagher et al., 2018). Another major challenge is the fact that HPV immunization coverage rates in sub-Saharan Africa are reported to be among the lowest (1.2% among 10–20-year-old females) in the world (Bruni et al., 2016). This implies that a substantial proportion of adolescent girls is excluded from the full benefits of the HPV vaccine. This is most concerning, considering that cervical cancer incidence rates in the region are among the highest (31.0 to >40 per 100 000 women) in the world (Louie et al., 2009; de Martel et al., 2017; Arbyn et al., 2020). In addition, cervical cancer is one of the most common causes of cancer-related deaths among women in sub-Saharan Africa (De Vuyst et al., 2013; Arbyn et al., 2020). Evidently, the status of HPV immunization programmes in sub-Saharan Africa is a matter of major public health concern. Expanding access to lifesaving HPV vaccines is a significant challenge for NIPs and health systems in the region (Denny et al., 2012; Sankaranarayanan et al., 2012; Wigle et al., 2013). There is an obvious need for intervention, informed by synthesized evidence on the health systems constraints and facilitators of HPV immunization programmes in sub-Saharan Africa.
Methods
A qualitative systematic review study was conducted to address the following research question: ‘How do health systems constraints and facilitators impact on the performance of HPV immunization programmes in sub-Saharan Africa?’
A search strategy was developed using key search terms and search term synonyms, which focused on three thematic areas: NIPs, health systems and HPV immunization programmes (full search strategy in Supplementary Table S2). The literature search was limited to studies conducted in sub-Saharan African countries. Using this search strategy, peer-reviewed and grey literature sources were sought through electronic databases such as PubMed, Web of Science, Scopus and EBSCOhost. In addition, supplementary searches for literature sources that may have been missed during the initial electronic search were conducted through Google Scholar as well as organizational websites like WHO (http://www.who.int/en/), GAVI, the Vaccine Alliance (https://www.gavi.org/) and the HPV Information Centre (http://www.hpvcentre.net/). The last date of the primary literature search was August 2018. Updated searches were performed until December 2018. However, this did not yield any additional literature sources relevant to this systematic review.
Only full texts of empirical studies conducted and published between 2008 and 2018 were considered eligible for this systematic review. This is because the past 10 years has been characterized by intensified global efforts to implement HPV immunization programmes, especially in sub-Saharan Africa (Sankaranarayanan et al., 2012; LaMontagne et al., 2017). Reviewing studies conducted within this period therefore enhanced the relevance of the review findings. Eligible studies also included those that used qualitative, quantitative and mixed method study designs. However, modelling studies, reviews, descriptive reports and commentaries reporting on secondary research findings were excluded from this review. In addition, studies published in languages other than English were excluded because of resource constraints. Finally, studies reporting on interventions other than HPV immunization programmes were excluded from the review.
After selection, full texts of eligible studies were critically appraised for the appropriateness of the methods used and the findings reported. Ethical considerations and rigour were also assessed. Evidence of reflexivity, where appropriate, was assessed throughout the texts including authors’ affiliations, research funding and declaration of potential conflict of interest. The quality appraisal was conducted with the assistance of published appraisal tools developed for multiple study designs by the Critical Appraisal Skills Programme (http://www.casp-uk.net/). These appraisal tools have been used previously in other qualitative systematic reviews (Walter et al., 2004; Kane et al., 2007; Chan, 2013).
The analytical approach for this systematic review was guided by an analytical model developed as part of a preliminary scoping review (see Figure 2). While there are several analytical and strategic frameworks proposed for assessing the performance of health systems, these frameworks differ in their starting points, operationalization, as well as process and outcome measures (Arah et al., 2003; Murray and Evans, 2003; Murray and Frenk, 2000; Kruk and Freedman, 2008; WHO, 2011; Mounier-Jack et al., 2014). It is also established that no single framework can holistically evaluate the interaction between disease control interventions and the health system (Mounier-Jack et al., 2014). It is for this reason that an initial scoping review was performed to gain an in-depth understanding of the interaction between NIPs and health systems. This scoping review resulted in an analytical model that integrated the eight components of NIPs as described by Shen et al. (2014) and the dimensions of the health system (WHO, 2007). Six cross-cutting themes are addressed in this analytical model, namely, (1) the governance and policy landscape, (2) the capacity of the health workforce, (3) the availability of potent vaccines, cold chain and logistics systems, (4) the quality of health service delivery, (5) the state of health information systems and community partnerships and (6) the availability of equitable and sustainable health financing. The reasoning behind this analytical model is that the interaction among the six cross-cutting themes—which are influenced by underlying contextual factors—will determine whether key NIP targets (like optimal immunization coverage and improved population health) are attained.
Figure 2.
An analytical model for in-depth assessment of the interface between NIPs and health systems.
Study findings related to health systems constraints and facilitators of HPV immunization programmes were extracted and organized according to the six themes of the model. During the first stage of the analysis process, full texts were imported into Rayyan, a web-based application for systematic reviews, where they were screened for eligibility (Ouzzani et al., 2016). Eligible studies were then coded using inductive and deductive approaches. The codes used were related to the types of health systems constraints and facilitators reported in the study findings. Relevant findings from each study were then extracted and recorded in a database. The extracted data set was then subjected to rigorous thematic analysis with careful consideration for prevailing contextual factors reported for the relevant countries.
Given that majority of sub-Saharan African countries is yet to implement nationwide HPV immunization programmes through their NIPs, we explored outcomes on health systems constraints to implementation. Where studies investigated potential or anticipated health systems constraints and facilitators to the performance of nationwide HPV immunization programmes should they be implemented, these outcomes were also considered. In countries with existing HPV immunization programmes (at the time the studies were conducted), either as part of NIPs or demonstration projects, we also explored findings related to health systems constraints and facilitators to the acceptance and uptake of the HPV vaccine.
Results
Characteristics of studies included in this review
The literature search yielded a total of 356 published records. Overall, 355 of these records were retrieved from electronic databases, while a search in Google Scholar yielded an additional record. No additional unique records were found through organizational websites. Of the total output, 54 full-text articles were found to be eligible for inclusion in the systematic review. Figure 3 shows the screening and selection process for this systematic review. A summary of the quality appraisal of the 54 studies selected for inclusion in this systematic review is provided in Supplementary Table S3. Overall, 52 of the 54 studies met all seven criteria assessed in the appraisal and so were classified as ‘high quality’. The two other studies were classified as low quality and moderate quality (the former not meeting rigour requirements, and the second judged to have inappropriate design, analysis and rigour aspects).
Figure 3.

Flowchart of the literature search and selection process. †Reasons for exclusion: study outcomes not relevant for this systematic review (n = 68); study design not relevant for this systematic review (n = 55); wrong study population (n = 13); duplicate records (n = 6); full-text unavailable (n = 4); and wrong study period (pre-2008) (n = 1).
The 54 full-text articles included in this review reported on studies conducted in 20 countries in sub-Saharan Africa, namely, Botswana, Burundi, Cameroon, Côte d’Ivoire, Gambia, Ghana, Kenya, Lesotho, Madagascar, Malawi, Mali, Mozambique, Niger, Nigeria, Rwanda, Senegal, South Africa, Tanzania, Uganda and Zambia (a summary of the data extraction table is provided in Supplementary Table S4). Most articles focused on South Africa (10), Nigeria (8), Kenya (6) and Uganda (6). Where articles reported on findings from multiple countries, only those findings relating to sub-Saharan African countries were extracted. The majority (31) of studies made use of qualitative study designs, while others adopted mixed method (10) or quantitative (10) study designs. A population-based intervention study and a project evaluation were also included in this review (see Table 1).
Table 1.
Characteristics of studies included in the systematic review
| No. | Author (year) | Title | Country | Study design | Availability of HPV vaccinea |
|---|---|---|---|---|---|
| 1 | Audu et al. (2014) | Awareness and perception of human papilloma virus vaccine among healthcare professionals in Nigeria | Nigeria | Cross-sectional, questionnaire based | Not available |
| 2 | Ayissi et al. (2012) | Awareness, acceptability and uptake of human papilloma virus vaccine among Cameroonian school-attending female adolescents | Cameroon | Cross-sectional, questionnaire based | Demonstration project |
| 3 | Bardají et al. (2018) | Awareness of cervical cancer and willingness to be vaccinated against human papillomavirus in Mozambican adolescent girls | Mozambique | Quantitative, cross-sectional | Demonstration project |
| 4 | Botha et al. (2015) | The Vaccine and Cervical Cancer Screen (VACCS) project: acceptance of human papillomavirus vaccination in a school-based programme in two provinces of South Africa | South Africa | Quantitative | Demonstration project |
| 5 | Botwright et al. (2017) | Experiences of operational costs of HPV vaccine delivery strategies in Gavi-supported demonstration projects | African countries | Quantitative | Demonstration project |
| 6 | Chigbu et al. (2017) | The impact of community health educators on uptake of cervical and breast cancer prevention services in Nigeria | Nigeria | Prospective population-based intervention | Demonstration project |
| 7 | Coleman et al. (2011) | HPV vaccine acceptability in Ghana, West Africa | Ghana | Qualitative, questionnaire based | Available in the private sector only |
| 8 | De Groot et al. (2017) | Knowledge, attitudes, practices and willingness to vaccinate in preparation for the introduction of HPV vaccines in Bamako, Mali | Mali | Qualitative, household survey | Demonstration project |
| 9 | DiAngi et al. (2011) | A cross-sectional study of HPV vaccine acceptability in Gaborone, Botswana | Botswana | Cross-sectional, survey | Available in the private sector only |
| 10 | Francis et al. (2011) | A qualitative analysis of South African women's knowledge, attitudes, and beliefs about HPV and cervical cancer prevention, vaccine awareness and acceptance, and maternal-child communication about sexual health | South Africa | Qualitative | Available in the private sector only |
| 11 | Francis et al. (2010) | Examining attitudes and knowledge about HPV and cervical cancer risk among female clinic attendees in Johannesburg, South Africa | South Africa | Quantitative | Not available |
| 12 | Friedman et al. (2014) | Preparing for human papillomavirus vaccine introduction in Kenya: implications from focus-group and interview discussions with caregivers and opinion leaders in Western Kenya | Kenya | Qualitative | Not available |
| 13 | Harries et al. (2009) | Preparing for HPV vaccination in South Africa: key challenges and opinions | South Africa | Qualitative | Available in the private sector only |
| 14 | Hoque (2015) | Acceptability of human papillomavirus vaccination among academics at the University of KwaZulu-Natal, South Africa | South Africa | Qualitative, cross-sectional | Available through NIP |
| 15 | Hoque (2016) | Factors influencing the recommendation of the human papillomavirus vaccine by South African doctors working in a tertiary hospital | South Africa | Quantitative, cross-sectional | Available through NIP |
| 16 | Hoque et al. (2013) | Human papillomavirus vaccination acceptability among female university students in South Africa | South Africa | Qualitative, cross-sectional | Available in the private sector only |
| 17 | Hutubessy et al. (2012) | A case study using the United Republic of Tanzania: costing nationwide HPV vaccine delivery using the WHO Cervical Cancer Prevention and Control Costing Tool | Tanzania | Quantitative | Not available |
| 18 | Kamya et al. (2017) | Evaluating global health partnerships: a case study of a Gavi HPV vaccine application process in Uganda | Uganda | Mixed-methods case study | Available through NIP |
| 19 | Katz et al. (2013) | A qualitative analysis of factors influencing HPV vaccine uptake in Soweto, South Africa among adolescents and their caregivers | South Africa | Qualitative | Available in the private sector only |
| 20 | Ladner et al. (2012) | Assessment of eight HPV vaccination programs implemented in lowest income countries | Lesotho and Cameroon (non-African countries included) | Mixed methods | Demonstration project |
| 21 | Ladner et al. (2014) | Performance of 21 HPV vaccination programs implemented in low and middle-income countries, 2009-2013 | African countries (non-African LMICs included) | Quantitative | Demonstration project |
| 22 | LaMontagne et al. (2011) | Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries | Uganda (non-African LMICs included) | Mixed methods, cross-sectional | Demonstration project |
| 23 | Levin et al. (2013) | Delivery cost of human papillomavirus vaccination of young adolescent girls in Peru, Uganda and Vietnam | Uganda (non-African LMICs included) | Mixed methods | Demonstration project |
| 24 | Mabeya et al. (2018) | Uptake of three doses of HPV vaccine by primary school girls in Eldoret, Kenya; a prospective cohort study in a malaria endemic setting | Kenya | Cross-sectional, questionnaire based | Demonstration project |
| 25 | MacPhail et al. (2013) | Using HPV vaccination for promotion of an adolescent package of care: opportunity and perspectives | South Africa | Qualitative, cross-sectional | Available in the private sector only |
| 26 | Makwe and Anorlu (2011) | Knowledge of and attitude toward human papillomavirus infection and vaccines among female nurses at a tertiary hospital in Nigeria | Nigeria | Qualitative, cross-sectional | Not available |
| 27 | Masika et al. (2015) | Knowledge on HPV vaccine and cervical cancer facilitates vaccine acceptability among school teachers in Kitui County, Kenya | Kenya | Mixed methods, cross-sectional | Demonstration project |
| 28 | Massey et al. (2017) | Human papillomavirus (HPV) awareness and vaccine receptivity among Senegalese adolescents | Senegal | Quantitative, questionnaire based | Demonstration project |
| 29 | Moodley et al. (2013) | High uptake of Gardasil vaccine among 9–12-year-old schoolgirls participating in an HPV vaccination demonstration project in KwaZulu-Natal province, South Africa | South Africa | Mixed methods | Available in the private sector only |
| 30 | Morhason-Bello et al. (2015) | Willingness of reproductive-aged women in a Nigerian community to accept human papillomavirus vaccination for their children | Nigeria | Quantitative, multistage household survey | Available in the private sector only |
| 31 | Msyamboza et al. (2017) | Implementation of a human papillomavirus vaccination demonstration project in Malawi: successes and challenges | Malawi | Mixed methods, cross-sectional | Demonstration project |
| 32 | Mugisha et al. (2015) | Feasibility of delivering HPV vaccine to girls aged 10 to 15 years in Uganda | Uganda | Qualitative | Demonstration project |
| 33 | Ndizeye et al. (2018) | Knowledge and practices of general practitioners at district hospitals towards cervical cancer prevention in Burundi, 2015: a cross-sectional study | Burundi | Descriptive, cross-sectional | Not available |
| 34 | Ngabo et al. (2015) | A cost comparison of introducing and delivering pneumococcal, rotavirus and human papillomavirus vaccines in Rwanda | Rwanda | Quantitative | Available through NIP |
| 35 | Odunyemi et al. (2018) | Effect of nursing intervention on mothers’ knowledge of cervical cancer and acceptance of human papillomavirus vaccination for their adolescent daughters in Abuja—Nigeria | Nigeria | Quasi-experimental study | Available in the private sector only |
| 36 | Ogembo et al. (2014) | Achieving high uptake of human papillomavirus vaccine in Cameroon: lessons learned in overcoming challenges | Cameroon | Project evaluation | Demonstration project |
| 37 | Okunade et al. (2017) | Knowledge and acceptability of human papillomavirus vaccination among women attending the gynaecological outpatient clinics of a university teaching hospital in Lagos, Nigeria | Nigeria | Descriptive, cross-sectional | Available in the private sector only |
| 38 | Poole et al. (2013) | A cross-sectional study to assess HPV knowledge and HPV vaccine acceptability in Mali | Mali | Qualitative, cross-sectional | Not available |
| 39 | Ports et al. (2013) | Barriers and facilitators to HPV vaccination: perspectives from Malawian women | Malawi | Qualitative | Not available |
| 40 | Quentin et al. (2012) | Costs of delivering human papillomavirus vaccination to schoolgirls in Mwanza Region, Tanzania | Tanzania | Mixed methods | Demonstration project |
| 41 | Remes et al. (2012) | A qualitative study of HPV vaccine acceptability among health workers, teachers, parents, female pupils and religious leaders in northwest Tanzania | Tanzania | Qualitative | Not available |
| 42 | Tchounga et al. (2014) | Cervical cancer prevention in reproductive health services: knowledge, attitudes and practices of midwives in Côte d’Ivoire, West Africa | Cote d’Ivoire | Qualitative, cross-sectional | Available in the private sector only |
| 43 | Torres-Rueda et al. (2016) | HPV vaccine introduction in Rwanda: impacts on the broader health system | Rwanda | Mixed methods | Available through NIP |
| 44 | Turiho et al. (2014) | Effect of school-based human papillomavirus (HPV) vaccination on adolescent girls’ knowledge and acceptability of the HPV vaccine in Ibanda district in Uganda | Uganda | Cross-sectional, mixed methods | Demonstration project |
| 45 | Turiho et al. (2017) | Perceptions of human papillomavirus vaccination of adolescent schoolgirls in western Uganda and their implications for acceptability of HPV vaccination: a qualitative study | Uganda | Qualitative | Demonstration project |
| 46 | Ugwu et al. (2013) | Acceptability of human papilloma virus vaccine and cervical cancer screening among female health-care workers in Enugu, Southeast Nigeria | Nigeria | Cross-sectional, questionnaire based | Available in the private sector only |
| 47 | Umeh et al. (2016) | Mothers’ willingness to pay for HPV vaccines in Anambra state, Nigeria: a cross sectional contingent valuation study | Nigeria | Cross-sectional, survey | Available in the private sector only |
| 48 | Urasa and Darj (2011) | Knowledge of cervical cancer and screening practices of nurses at a regional hospital in Tanzania | Tanzania | Descriptive, cross-sectional | Available in the private sector only |
| 49 | Venturas and Umeh (2017) | Health professional feedback on HPV vaccination roll-out in a developing country | Zambia | Qualitative | Demonstration project |
| 50 | Vermandere et al. (2015) | Implementation of an HPV vaccination program in Eldoret, Kenya: results from a qualitative assessment by key stakeholders | Kenya | Qualitative | Demonstration project |
| 51 | Vermandere et al. (2014) | Determinants of acceptance and subsequent uptake of the HPV vaccine in a cohort in Eldoret, Kenya | Kenya | Qualitative, longitudinal study | Demonstration project |
| 52 | Wamai et al. (2013) | Awareness, knowledge and beliefs about HPV, cervical cancer and HPV vaccines among nurses in Cameroon: an exploratory study | Cameroon | Qualitative, questionnaire based | Demonstration project |
| 53 | Watson-Jones et al. (2015) | Access and attitudes to HPV vaccination amongst hard-to-reach populations in Kenya | Kenya | Qualitative | Demonstration project |
| 54 | Watson-Jones et al. (2012) | Reasons for receiving or not receiving HPV vaccination in primary schoolgirls in Tanzania: a case control study | Tanzania | Qualitative | Demonstration project |
Availability of the HPV vaccine at the time the studies was conducted.
Most studies (33/54) were primarily concerned with assessing knowledge, awareness and acceptability of the HPV vaccine among key populations such as adolescents, parents and caregivers, health workers, teachers and religious leaders (see Table 1). The articles also reported on the availability of the HPV vaccine in the relevant countries at the time each study was conducted. This information tended to change in some countries overtime and presented a unique opportunity to assess the evolution of health systems constraints and facilitators to HPV immunization programmes in these countries. From the overall studies selected for inclusion, the HPV vaccine was reported to be available through the NIPs of South Africa, Rwanda and Uganda. In most countries where the studies were conducted, the HPV vaccine was only available through demonstration projects (see Table 1). Nine articles reported on studies conducted at a time when the HPV vaccine was not available, either through NIPs, demonstration projects or in private health facilities. These studies were mainly focused on determining the feasibility of implementing HPV immunization programmes in the relevant countries by assessing potential health systems constraints and facilitators.
Health systems constraints and facilitators of HPV immunization programmes in sub-Saharan Africa
We synthesized the available evidence on health systems constraints and facilitators of HPV immunization programmes in sub-Saharan Africa. We considered findings pertaining to health systems constraints and facilitators to (1) HPV vaccine introduction into NIPs, (2) HPV vaccine acceptance and uptake and (3) the overall performance of HPV immunization programmes (include existing nationwide programmes or demonstration projects and future HPV immunization programmes). The findings of this review have been organized and reported under six cross-cutting themes based on the interface between NIPs and health systems.
The governance and policy landscape
Where NIPs are concerned, governance functions include the decision- and policy-making processes that go into developing immunization programme-related standards and guidelines, overall programme organization, as well as training and supervision. These functions cut across global, national, sub-national and local or facility levels. Accordingly, this theme explored findings related to leadership, management and stewardship throughout the HPV vaccine policy- and decision-making cascade. The available evidence highlights the importance of clear governance and management structures, the involvement of political champions, the support of policy influencers (including governmental and non-governmental organizations) and the role of strong and inclusive partnerships to the optimal performance of HPV immunization programmes in sub-Saharan Africa (Harries et al., 2009; Ladner et al., 2014; Mugisha et al., 2015; Torres-Rueda et al., 2016; Kamya et al., 2017). For example, prior to the implementation of an HPV immunization programme in South Africa, Harries et al. (2009) sought to identify potential barriers and facilitators of such a programme. It was reported that strong collaboration between the national departments of health and education, as well as between the private and public health sectors, was necessary to support and sustain an HPV immunization programme (Harries et al., 2009). These findings were echoed in studies conducted in Rwanda and Uganda where the HPV vaccine had been introduced as part of NIPs (Torres-Rueda et al., 2016; Kamya et al., 2017). In addition, Kamya et al. (2017) recognized the role of the First Lady of Uganda as a champion for HPV vaccine introduction in the country. This level of endorsement ensured that the HPV immunization programme remained a priority in the national policy agenda. It was also reported that a diverse and inclusive network of stakeholders from governmental and non-governmental agencies, having past immunization partnership experience and assigned clear roles and responsibilities, was invaluable to a GAVI HPV vaccine application process in Uganda (Kamya et al., 2017). Apart from the national level, the role of governance structures and processes at the health facility level was also identified as critical to HPV immunization programmes. In a study that assessed how HPV vaccine introduction impacted on the Rwandan health system, it was reported that extensive planning and supervision at the health facility level contributed to the successful integration of the HPV vaccine into the NIP (Torres-Rueda et al., 2016).
The capacity of the health workforce
The size and competency of the health workforce emerged from the evidence base as priority issues where HPV immunization programmes were concerned. Most studies relating to health workers were conducted at a time when the HPV vaccine was not widely available to the general population, either because it was yet to be introduced in the country or was only available through the private health sector. In a study conducted by Remes et al. (2012), health workers identified staff shortages as a potential constraint to implementation of nationwide HPV immunization programme in Tanzania. The major health systems constraint identified in other studies, however, was the inadequate training of the existing workforce in sub-Saharan Africa. Several studies reported that health workers (including doctors, nurses and midwives) exhibited suboptimal knowledge on HPV infection, the pathogenesis and prevention of cervical cancer, the availability, safety and effectiveness of the HPV vaccine and the recommended HPV immunization schedule (Makwe and Anorlu, 2011; Urasa and Darj, 2011; Remes et al., 2012; Wamai et al., 2013; Audu et al., 2014; Tchounga et al., 2014; Hoque, 2016; Venturas and Umeh, 2017; Ndizeye et al., 2018). When the HPV vaccine was not widely available, the proportion of health workers with optimal knowledge about the safety and effectiveness of the HPV vaccine ranged from 13% among nurses in Nigeria (Makwe and Anorlu, 2011) to 55% among general practitioners in Burundi (Ndizeye et al., 2018). However, this increased to 78.9% when the HPV vaccine was available through demonstration projects (Wamai et al., 2013). Health workers’ limited knowledge about the safety of the HPV vaccine was reported to negatively impact on their acceptance of the vaccine for their adolescent daughters as well as their ability to recommend the vaccine to their clients (Makwe and Anorlu, 2011; Wamai et al., 2013; Audu et al., 2014; Hoque, 2016; Venturas and Umeh, 2017). In a study conducted in Cameroon where an HPV vaccine demonstration project was ongoing, 69.7% of nurses surveyed indicated that they often recommended the vaccine to their clients. However, 63.9% of these nurses remained concerned about the potential side effects of the vaccine (Wamai et al., 2013). Poor access to appropriate training was cited as the primary reason for the suboptimal level of knowledge about HPV infection, cervical cancer and HPV immunization, among health workers in sub-Saharan African countries (Urasa and Darj, 2011; Tchounga et al., 2014; Venturas and Umeh, 2017).
Where health workers received adequate training, it was reported that they were capable of providing sound recommendations about the HPV vaccine to clients, thereby positively influencing the acceptance and uptake of the vaccine (Katz et al., 2013; Chigbu et al., 2017; Odunyemi et al., 2018). Additional facilitators of HPV immunization programmes included the involvement of well-trained school health teams as well as community health workers who were reported to play a key role in health promotion and social mobilization for HPV immunization programmes (Moodley et al., 2013; Watson-Jones et al., 2015).
The availability of potent vaccines, cold chain and logistics systems
Research evidence on how the availability of the HPV vaccine and the capacity of cold chain and logistics systems (for vaccine delivery, transport and storage) impact on the performance of HPV immunization programmes in sub-Saharan Africa was rather limited. Studies conducted by Okunade et al. (2017) and Ugwu et al. (2013) addressed the limited availability of the HPV vaccine and how this served as a barrier to the acceptance and uptake of the vaccine in Nigeria. At the time both studies were conducted, the HPV vaccine was not widely available to the general population but was provided through the private health sector at a cost (Ugwu et al., 2013; Okunade et al., 2017). These findings are not surprising as it is well established that lack of access remains one of the key barriers to improved vaccination coverage within sub-Saharan Africa. With regard to cold chain and logistics systems, Torres-Rueda et al. (2016) reported that the Rwandan NIP conducted a cold chain inventory to access the capacity of the system prior to the introduction of the HPV vaccine. This formed part of the planning and training implemented in advance of introducing the HPV vaccine into the NIP.
The quality of health service delivery
There was much in the literature about the health systems constraints and facilitators to accessing health services that are safe, people centred, integrated and efficient and how this impacts on the performance of HPV immunization programmes in sub-Saharan Africa (see Table 2). Studies exploring the most efficient and effective HPV vaccine delivery models were well represented in the evidence base (LaMontagne et al., 2011; Hutubessy et al., 2012; Ladner et al., 2012; Quentin et al., 2012; Levin et al., 2013; Moodley et al., 2013; Ladner et al., 2014; Ogembo et al., 2014; Watson-Jones et al., 2015; Botwright et al., 2017; Msyamboza et al., 2017). Two main HPV vaccine delivery models, namely, the school-based and health facility-based vaccine delivery strategies were explored. Overall, the findings were consistent, suggesting that adopting a school-based vaccine delivery strategy where eligibility to receive the vaccine was based on school grade or class and not on the age of the recipient was the most effective model (LaMontagne et al., 2011; Ladner et al., 2012; Moodley et al., 2013; Ladner et al., 2014; Mugisha et al., 2015; Msyamboza et al., 2017). This is because the school-based strategy was reported to achieve higher HPV immunization coverage rates compared with the health facility-based strategy (LaMontagne et al., 2011; Ladner et al., 2012, 2014; Mugisha et al., 2015).
Table 2.
Summary of health systems constraints and facilitators of HPV immunization programmes in sub-Saharan Africa
| Theme (na) | Health systems constraints | Health systems facilitators |
|---|---|---|
| The governance and policy landscape (5) |
|
|
| The capacity of the health workforce (17) |
|
|
| The availability of potent vaccines, cold chain and logistics systems (3) |
|
|
| The quality of health service delivery (16) |
|
|
| The state of health information systems and community partnerships (33) |
|
|
| The availability of equitable and sustainable health financing (8) |
|
|
Numbers in parenthesis represent the number of articles that reported findings related to each theme. Some articles reported on findings pertaining to more than one theme. A detailed description of each of the studies used and the themes they relate to is presented in Supplementary Table S4.
Several challenges to delivering the HPV vaccine through the school-based strategy in sub-Saharan Africa have been documented, however, and these include absenteeism, high school dropout rates among girls and girls transferring out of the district at the time of the immunization programmes (Msyamboza et al., 2017). Physical barriers to accessing the HPV vaccine through schools have also been reported in some hard-to-reach communities in Kenya. These barriers have been associated with non-attendance or delayed enrolment in schools because of long distances (up to 10 km) and safety concerns about possibly encountering wildlife on the way to school (Watson-Jones et al., 2015). In areas like this, where road networks are poorly developed or unsafe, vaccinators have been reported to experience difficulty in accessing schools to provide HPV immunization (Masika et al., 2015). To mitigate these challenges, a mixed vaccine delivery model is highly recommended in the literature and involves coupling the school-based strategy with community outreach immunization campaigns. This mixed vaccine delivery model has been shown to be feasible in sub-Saharan Africa, expanding the reach of HPV immunization programmes, improving adherence to the immunization schedule and scaling up immunization coverage rates (Ladner et al., 2012; Ogembo et al., 2014; Msyamboza et al., 2017).
It was evident from the studies reviewed that the introduction of the HPV vaccine has exposed gaps in the development of adolescent and school health services in sub-Saharan Africa. This has been considered in the evidence base as a constraint to HPV immunization programmes because of the logistical challenges to providing health services to a previously underserved adolescent population and the greater resource requirements associated with creating new vaccine delivery infrastructure (Harries et al., 2009; Remes et al., 2012; Ngabo et al., 2015). A study conducted in South Africa by MacPhail et al. (2013) also identified gaps in the appropriate integration of the HPV immunization programme with other adolescent health services such as sex education, screening and preventive services, assistance with substance abuse and provision of other adolescent vaccines.
The state of health information systems and community partnerships
Three main dimensions were explored under this theme; (1) health information, education and communication about HPV infection, cervical cancer and HPV immunization; (2) community partnerships during HPV immunization programmes; and (3) the generation and use of surveillance and immunization data. After reviewing the evidence base, the state of health information systems and community partnerships emerged as the most researched theme where HPV immunization programmes in sub-Saharan Africa are concerned (see Table 2).
Several studies were conducted to assess the level of awareness about HPV infection, cervical cancer and HPV immunization among general populations in sub-Saharan Africa. Study participants included key stakeholders like adolescents, women of reproductive age, parents or caregivers, teachers, opinion leaders, religious leaders and university students and academics (Francis et al., 2010; Coleman et al., 2011; DiAngi et al., 2011; Francis et al., 2011; Ayissi et al., 2012; Remes et al., 2012; Watson-Jones et al., 2012; Hoque et al., 2013; MacPhail et al., 2013; Poole et al., 2013; Ports et al., 2013; Turiho et al., 2014; Vermandere et al., 2014; Botha et al., 2015; Hoque, 2015; Masika et al., 2015; Morhason-Bello et al., 2015; Vermandere et al., 2015; De Groot et al., 2017; Massey et al., 2017; Okunade et al., 2017; Turiho et al., 2017; Bardají et al., 2018). Majority of these studies found that the level of awareness among the general population was rather limited. With the exception of university academics, the proportion of the general population found to be aware of HPV infection, cervical cancer and the HPV vaccine was generally low and ranged from 8.6% to 36.5%, 61% to 87% and 0% to 40%, respectively (Francis et al., 2010; Coleman et al., 2011; DiAngi et al., 2011; Remes et al., 2012; Ports et al., 2013; Friedman et al., 2014; De Groot et al., 2017; Massey et al., 2017; Okunade et al., 2017). In comparison, the highest level of awareness about HPV (100%) and cervical cancer (96%) was recorded among university academics (Hoque, 2015).
The low level of awareness demonstrated among the general population was reported to fuel misconceptions about the safety and benefits of the HPV vaccine. Some misconceptions about the potential side effects of the vaccine included infertility and death, while some feared that introduction of the vaccine would promote promiscuity and early sexual debut among adolescent girls (Watson-Jones et al., 2012; Ogembo et al., 2014; Morhason-Bello et al., 2015). Other studies also reported that some participants believed that the HPV vaccine would prevent HIV and pregnancy, or even safeguard the fertility of adolescent girls (Vermandere et al., 2015; Turiho et al., 2017). Ultimately, low level of awareness was a major constraint to the acceptance and uptake of the HPV vaccine in sub-Saharan Africa (Vermandere et al., 2014). When intensive social mobilization and community sensitization interventions were implemented to provide culturally appropriate information about the risks of cervical cancer and the safety and effectiveness of the HPV vaccine (often as part of vaccine demonstration projects; see Figure 4), an increase in vaccine acceptance and uptake was reported (Coleman et al., 2011; DiAngi et al., 2011; Ayissi et al., 2012; Ports et al., 2013; Turiho et al., 2014; Botha et al., 2015; Hoque, 2015; Masika et al., 2015; Vermandere et al., 2015; De Groot et al., 2017; Bardají et al., 2018). Participants also identified health workers as their most trusted source of information about the HPV vaccine (Ayissi et al., 2012; Ports et al., 2013; Massey et al., 2017). Engaging key stakeholders like adolescents (both males and females), parents (including fathers) or caregivers, municipal and religious leaders and school teachers, at the onset of HPV immunization programmes, was considered as a major facilitator to vaccine acceptance and uptake (Ports et al., 2013; Ogembo et al., 2014; Masika et al., 2015; Vermandere et al., 2015).
Figure 4.
Summary of health systems constraints and facilitators of HPV immunization programmes in sub-Saharan Africa.
Very few studies reported on how the generation and use of surveillance and immunization data (including immunization coverage data) impacted on the performance of HPV immunization programmes in sub-Saharan Africa. Where studies explored this dimension, the evidence found was rather limited. For example, a study conducted to assess the capacity of school health teams to carry out future HPV immunization in schools within the KwaZulu-Natal province of South Africa found that the reliance on paper-based vaccine records would be a major constraint to real-time monitoring and evaluation of the immunization programme. To mitigate this challenge, the use of electronic data capturing methods was recommended (Moodley et al., 2013). In addition to this, Torres-Rueda et al. (2016) highlighted the importance of strengthening the surveillance of adverse events following immunization in Rwanda where a nationwide HPV immunization programme was already in existence (see Figure 4).
The availability of equitable and sustainable health financing
The final theme examined national health financing mechanisms in sub-Saharan Africa, including the availability of equitable and sustainable financing mechanisms for HPV immunization programmes. Research evidence on this theme was found to be limited. None of the studies included in the review addressed any dedicated financing mechanisms earmarked for the introduction and sustainable delivery of the HPV vaccine. Instead, the available evidence on financing was mainly focused on determining the economic and financial costs of social mobilization campaigns and HPV vaccine delivery models (Hutubessy et al., 2012; Quentin et al., 2012; Levin et al., 2013; Ngabo et al., 2015; Botwright et al., 2017).
With the exception of South Africa, HPV vaccine procurement costs were reported to be largely covered by external funding sources (from organizations like GAVI, Program for Appropriate Technology in Health and the Gardasil Access Program), in countries where the HPV immunization programme had been implemented as part of demonstration projects or NIPs (Ladner et al., 2014; Mugisha et al., 2015; Ngabo et al., 2015; Botwright et al., 2017). In most cases, national governments are expected to co-finance the cost of delivering the vaccine. However, this was a major constraint to implementing HPV immunization programmes, given that the financial cost of introducing and delivering the HPV vaccine was significantly higher than that of routine childhood vaccines (Levin et al., 2013; Ngabo et al., 2015). Reasons given for this included the fact that the infrastructure for delivering vaccines to adolescents was largely underdeveloped prior to the introduction of the HPV vaccine. As such financial costs were reported to be higher in countries that could not leverage existing immunization infrastructure and had greater resource requirements (Ngabo et al., 2015; Botwright et al., 2017).
It was reported that a substantial cost component was required to train health workers, cover per diems, organize effective social mobilization and community sensitization campaigns and deliver the vaccine through the school-based strategy (Hutubessy et al., 2012; Levin et al., 2013; Ngabo et al., 2015; Botwright et al., 2017). With regard to the school-based vaccine delivery strategy, adopting an age-based eligibility approach was found to be a constraint to HPV immunization programmes as it was less cost-effective compared with the grade-based approach (Quentin et al., 2012). However, delivering the HPV vaccine through health facilities incurred lower economic costs compared with the school-based strategy but achieved lower immunization coverage rates (Levin et al., 2013). To offset the cost of delivering the HPV vaccine, some countries were reported to charge a fee for immunization, while in other countries, the vaccine was only available through the private health sector at unaffordable costs (Ugwu et al., 2013; Ogembo et al., 2014; Umeh et al., 2016; Okunade et al., 2017; Odunyemi et al., 2018). In these countries, socioeconomic constraints to accessing the HPV vaccine negatively impacted on vaccine acceptance and uptake.
Discussion
There is growing consensus that the success of key health programmes in achieving improved population health is highly dependent on the performance of their health systems (Travis et al., 2004; Balabanova et al., 2010). In this regard, previous systematic reviews have assessed the health systems constraints and facilitators to the effective performance of several health programmes, such as those targeted at HIV anti-retroviral treatment, prevention of mother-to-child transmission of HIV and the prevention and control of breast cancer, as well as other chronic diseases (Colvin et al., 2014; Bowser et al., 2017; Watt et al., 2017). The primary intent of these reviews is to build up the evidence base required to inform health systems strengthening efforts. This has been spurred by the realization that strong health systems are able to withstand acute shocks or avoid them altogether, to provide health programmes with the support they require to run effectively (Travis et al., 2004; Balabanova et al., 2010; Hanvoravongchai et al., 2011; Wang et al., 2013). To date, no rigorous systematic reviews exist on the health systems constraints and facilitators of NIPs, especially from the sub-Saharan African region.
At the World Health Assembly held in May 2012, 194 member states endorsed the Global Vaccine Action Plan. One of the visions of the Global Vaccine Action Plan is to advance universal access to immunization between 2011 and 2020 (WHO, 2013b; WHO, 2015). To date, regional immunization efforts have been heavily focused on routine childhood immunization programmes, which have achieved remarkable public health success within the subcontinent (Arevshatian et al., 2007; Machingaidze et al., 2013). However, if sub-Saharan Africa is to have a chance of achieving the vision of the Global Vaccine Action Plan beyond 2020, countries will have to scale up the performance of existing immunization programmes, introduce and improve the uptake of underused vaccines and extend access to lifesaving vaccines to underserved and hard-to-reach populations (WHO, 2013b; Nanni et al., 2017; Bonner et al., 2018). Scaling up the HPV immunization programmes, which target the adolescent population, presents a unique opportunity to contribute to attaining the vision of the Global Vaccine Action Plan beyond 2020. In this regard, Nanni et al. (2017) advocate for ‘global consensus, political will, policies, global and country infrastructure and financing mechanisms’ to accelerate universal access to the HPV vaccine for adolescent populations in low- and middle-income regions like sub-Saharan Africa. To facilitate this, it is important to provide decision-makers with robust systematic evidence of what works (and what does not work) for HPV immunization programmes in sub-Saharan Africa, especially where health systems are concerned.
The findings of this systematic review are consistent with the recommendations made by Nanni et al. (2017). In sub-Saharan African countries like Uganda where national governments have strongly endorsed the HPV vaccine and have made concerted efforts through national policy to ensure that the vaccine is widely available to the population, HPV immunization programmes have been successfully implemented as part of NIPs and vaccine acceptance and uptake has significantly improved (Mugisha et al., 2015; Kamya et al., 2017). Global infrastructure for HPV immunization programmes has typically come in the form of external funding, and expert and technical support from organizations like GAVI, Program for Appropriate Technology in Health and the Gardasil Access Program (Ladner et al., 2014; Mugisha et al., 2015; Ngabo et al., 2015; Botwright et al., 2017). The support of these global partners has been instrumental in facilitating the introduction of HPV immunization programmes in sub-Saharan Africa, either through demonstration projects or NIPs (Oluwole and Kraemer, 2013; Ladner et al., 2014; Gallagher et al., 2017b, 2018). While these health systems facilitators (strong political endorsement, clear governance structures and partnerships with global partners) have achieved some progress for HPV immunization programmes in sub-Saharan Africa, the region continues to fall short in meeting global targets for implementing HPV immunization programmes. In addition to this, HPV immunization coverage rates in sub-Saharan Africa are recorded among the lowest in the world (Bruni et al., 2016; LaMontagne et al., 2017; Gallagher et al., 2017a, 2018). These persistent challenges may be the result of significant health systems constraints in sub-Saharan Africa, as has been suggested previously (Bello et al., 2011; Wigle et al., 2013; SAGE, 2016).
The most recurrent health systems constraint identified by this systematic review was the low level of knowledge and awareness on HPV infection, the risk of cervical cancer and the safety and effectiveness of the HPV vaccine, among general populations in sub-Saharan Africa. This finding is highly relevant given the well-established link between levels of knowledge, awareness and attitudes towards the HPV vaccine, and vaccine acceptance and uptake (Garcini et al., 2012; Kessels et al., 2012; Hopkins and Wood, 2013; Maseko et al., 2015). The gap identified in the level of knowledge and awareness among the general population is not unique to the sub-Saharan African context and has been reported in other parts of the world where HPV immunization programmes have long been in existence (Hendry et al., 2013; Cartmell et al., 2018). Nonetheless, this calls into question the effectiveness of existing HPV vaccine communication strategies in sub-Saharan Africa. Key stakeholders, including adolescents and their caregivers, depend on reliable communication to make informed decisions about lifesaving HPV vaccines and take ownership of their health (Bonner et al., 2018). Evidently, there is a need to strengthen health systems in sub-Saharan Africa by reinforcing the role of health promotion activities, especially where HPV immunization programmes are concerned. Where intensified social mobilization and community sensitization campaigns have been implemented, this was shown to increase the demand for the HPV vaccine. In addition to this, the importance of designing culturally appropriate communication strategies, which are also accessible to populations living in hard-to-reach communities, was emphasized in the literature (Friedman et al., 2014; Watson-Jones et al., 2015).
The limited capacity of health workers was identified as another major health systems constraint to the performance of HPV immunization programmes in sub-Saharan Africa. Overall, health workers demonstrated suboptimal levels of knowledge and awareness about HPV infection, the risk of cervical cancer and the safety and effectiveness of the HPV vaccine. These findings are consistent with those reported in other resource limited settings (Nganwai et al., 2008; Ali et al., 2010; Chawla et al., 2016). In contrast, reports from some high resource settings indicate high levels of knowledge and awareness about HPV infection, cervical cancer and the HPV vaccine, among health workers (Rosen et al., 2018). This is a matter of major public health concern in sub-Saharan Africa, given that cadres like nurses and community health workers are at the frontline of HPV immunization programmes and have been identified as the most trusted source of information on HPV immunization (Ayissi et al., 2012; Ports et al., 2013; Watson-Jones et al., 2015; Massey et al., 2017). There is a need, therefore, to reinforce the capacity of health workers in sub-Saharan Africa. This can be achieved through continued training and education as immunization programmes evolve, to ensure that health workers can provide sound and up-to-date vaccine recommendations to their clients.
Historically, adolescents have not been the ‘typical’ client base of most health systems. However, with the introduction of HPV immunization programmes, this notion is beginning to change (Ladner et al., 2012; MacPhail et al., 2013; Nanni et al., 2017). This systematic review has found that poorly developed adolescent and school health services present a significant challenge to effectively delivering HPV vaccines in sub-Saharan Africa. This is further compounded by weak country infrastructure, including underdeveloped and unsafe road networks, as well as geographically inaccessible schools and health facilities, which create physical barriers to reaching target populations with the HPV vaccine (Masika et al., 2015; Watson-Jones et al., 2015; Msyamboza et al., 2017). A review by Sankaranarayanan et al. (2012), which sought to address the infrastructure requirements for HPV immunization programmes in sub-Saharan Africa, found that, although leveraging the existing NIP resources (e.g. human resources and cold chain and logistics systems) could improve the performance of HPV immunization programmes, greater investments in infrastructure are still needed. This calls for intersectoral collaboration within national governments, involving relevant Ministries like Health, Education and Finance. Improving adolescent and school health services to support HPV immunization programmes and administration of other adolescent vaccines also deserves particular attention in the health policy agenda in sub-Saharan Africa (Nanni et al. 2017).
Finally, this review found that significant financial constraints impact on the introduction of HPV immunization programmes and the delivery of the vaccine to adolescents in sub-Saharan Africa. The HPV vaccine is more costly than other routine childhood vaccines. In addition, the HPV vaccine delivery models incur higher financial and economic costs when compared with routine childhood immunization programmes (Levin et al., 2013; Ngabo et al., 2015). These financial barriers are intensified when considered in light of unique sub-Saharan African contextual issues, such as the limited health budgets of some national governments and the high burden of diseases, which has given rise to multiple health programmes (such as those targeting malaria, HIV, and other vaccine-preventable diseases) competing for the limited resources (Bello et al., 2011). Altogether, these financial constraints significantly obstruct the widespread implementation of HPV immunization programmes in sub-Saharan Africa (Gallagher et al., 2018). While substantial investments have been made by global partners, co-financing commitments make HPV vaccine introduction unaffordable for some national governments in sub-Saharan Africa (Gallagher et al., 2018). Increasing national investments in HPV immunization programmes will require reforms in health policy, although this will have to be informed by country-specific evidence on the resource needs and the most effective and sustainable financing mechanisms to be adopted.
From 2020, other sub-Saharan African countries will be looking to introduce HPV immunization programmes nationwide. While this will be an impressive move towards expanding access to lifesaving HPV vaccines within the region, it is important that key decision-makers take into consideration the health systems constraints and facilitators presented in this review. Countries could draw on the health systems facilitators to well-performing HPV immunization programmes in developing country-specific standards and guidelines for their immunization programmes. The findings on health systems constraints also serve as a set of ‘lessons learned’ for HPV immunization programmes in sub-Saharan Africa so far and could be used to inform interventions to scale up the performance of existing immunization programmes or to guide the implementation of new HPV immunization programmes where necessary.
Although this systematic review assessed health systems constraints and facilitators to HPV immunization programmes, the findings have significant relevance for scaling up NIPs in general. The major health systems constraints and facilitators pertaining to service delivery, the health workforce, vaccine communication and community partnerships, as well as governance and policy, are applicable to almost all services delivered through NIPs. For example, barriers to accessing vaccines due to ineffective service delivery, staff shortages, lack of awareness about vaccines, weak engagement with key stakeholders, as well as poor governance and policy structures have been suggested to negatively impact on the performance of NIPs in most LMICs, and the findings of this review are in support of this (Shen et al., 2014; SAGE, 2016; Mihigo et al., 2017). What has not been sufficiently addressed in the existing literature is how to overcome these constraints and strengthen broader health systems to support optimal NIP performance. In addition to this, detailed considerations of the cost-effectiveness of strengthening the broader health system in the anticipation of scaling up NIP performance are scarce. This systematic review provides evidence on health systems facilitators, which can be leveraged in developing interventions to mitigate some of the system-wide barriers to scaling up NIPs. It is important to caution, however, that HPV immunization programmes have some unique characteristics when compared with other NIP services. First, HPV immunization programmes are targeted at the adolescent population while most NIP services have typically been focused on infants and children. Second, the vaccine delivery models for HPV immunization programmes are also different from those typically used in other NIP services. As such certain variations exist in the health systems functions needed to support HPV immunization programmes compared with routine childhood immunization services, as discussed previously. Despite this, NIPs are beginning to evolve towards a ‘life-course approach’ to immunization by expanding target age groups and extending immunization services to adolescents and adults who have been previously underserved (Mehta et al., 2014; Langley, 2015). In this regard, the findings of this systematic review could be useful for informing health systems strengthening initiatives to support the expanding scope of NIPs, especially in sub-Saharan Africa.
Limitations of this systematic review include the geographical restriction to sub-Saharan Africa. The findings and recommendations of the review may not be generalizable to parts of the world that do not share similar contexts with countries in sub-Saharan Africa. The language restriction applied during the search strategy is another limitation as relevant studies conducted and published from non-Anglophone countries may have been missed. While this could imply that evidence on fragile populations with the highest burden of disease and greatest need for intervention may have been excluded, it is important to caution that this is reflective of the broader field and scope of the literature. It is also worth noting that most studies on knowledge and awareness were based on self-reporting and might be subject to reporting bias. Interpretation of the findings of this systematic review may have been limited to the analytical approach used, which was guided by an analytical model developed as part of a preliminary scoping review. Finally, the use of HPV immunization programmes as a proxy for NIPs may not give a comprehensive assessment given some of the programmatic differences with other routine childhood immunization programmes. The generalizability of the findings of this systematic review for other NIP services may therefore require further investigation, adapting and using the six cross-cutting themes on the interface between NIPs and health systems.
Conclusion
This systematic review provides evidence of how NIPs and health systems interact by reporting on the health systems constraints and facilitators of NIPs in sub-Saharan Africa. In addition, the findings show how these system-wide constraints and facilitators impact on the performance of NIPs. There is evidence to suggest that NIPs in sub-Saharan Africa have surmounted significant health systems constraints and have achieved notable public health success. This success can be attributed to strong political endorsement for vaccines, clear governance structures and effective collaboration with global partners. Despite this, important health systems constraints persist and could derail further progress if not addressed through health systems strengthening efforts. Gaps in the evidence base pertaining to cold chain and logistics systems, data generation and use, as well as national financing mechanisms needed to sustain NIPs in sub-Saharan Africa were also identified. This calls for an expansion of the research agenda, not only to address these gaps, but also to continuously evaluate health systems constraints and facilitators of NIPs in sub-Saharan Africa. The findings of this review are relevant to ongoing health systems strengthening initiatives in sub-Saharan Africa. By enhancing our understanding of what works—‘and does not work’—for NIPs, health systems strengthening initiatives could be better designed to adequately respond to the burden of vaccine-preventable diseases in sub-Saharan Africa.
Supplementary data
Supplementary data are available at Health Policy and Planning online.
Conflict of interest statement. None declared.
Ethical approval. No ethical approval was required for this study.
Supplementary Material
References
- Ali SF, Ayub S, Manzoor NF. et al. 2010. Knowledge and awareness about cervical cancer and its prevention amongst interns and nursing staff in tertiary care hospitals in Karachi, Pakistan. PLoS One 5: e11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arah OA, Klazinga NS, Delnoij DMJ. et al. 2003. Conceptual frameworks for health systems performance: a quest for effectiveness, quality, and improvement. International Journal for Quality in Health Care 15: 377–98. [DOI] [PubMed] [Google Scholar]
- Arbyn M, Weiderpass E, Bruni L. et al. 2020. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health 8: e191–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevshatian L, Clements CJ, Lwanga SK. et al. 2007. An evaluation of infant immunization in Africa: is a transformation in progress? Bulletin of the World Health Organization 85: 449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audu BM, Bukar M, Ibrahim AI. et al. 2014. Awareness and perception of human papillomavirus vaccine among healthcare professionals in Nigeria. Journal of Obstetrics and Gynaecology 34: 714–7. [DOI] [PubMed] [Google Scholar]
- Ayissi C, Wamai R, Oduwo G. et al. 2012. Awareness, acceptability and uptake of human papillomavirus vaccine among Cameroonian school-attending female adolescents. Journal of Community Health 37: 1127–35. [DOI] [PubMed] [Google Scholar]
- Balabanova D, McKee M, Mills A. et al. 2010. What can global health institutions do to help strengthen health systems in low income countries? Health Research Policy and Systems 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardají A, Mindu C, Augusto OJ. et al. 2018. Awareness of cervical cancer and willingness to be vaccinated against human papillomavirus in Mozambican adolescent girls. Papillomavirus Research 5: 156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello FA, Enabor OO, Adewole IF.. 2011. Human papillomavirus vaccination for control of cervical cancer: a challenge for developing countries. African Journal of Reproductive Health 15: 25–30. [PubMed] [Google Scholar]
- Bonner K, Banura C, Basta NE.. 2018. HPV vaccination strategies targeting hard-to-reach populations: out-of-school girls in LMICs. Vaccine 36: 191–3. [DOI] [PubMed] [Google Scholar]
- Botha MH, van der Merwe FH, Snyman LC. et al. 2015. The vaccine and cervical cancer screen (VACCS) project: acceptance of human papillomavirus vaccination in a school-based programme in two provinces of South Africa. South African Medical Journal 105: 40–3. [DOI] [PubMed] [Google Scholar]
- Botwright S, Holroyd T, Nanda S. et al. 2017. Experiences of operational costs of HPV vaccine delivery strategies in Gavi-supported demonstration projects. PLoS One 12: e0182663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser D, Marqusee H, El Koussa M, Atun R.. 2017. Health system barriers and enablers to early access to breast cancer screening, detection, and diagnosis: a global analysis applied to the MENA region. Public Health 152: 58–74. [DOI] [PubMed] [Google Scholar]
- Bruni L, Diaz M, Barrionuevo-Rosas L. et al. 2016. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Global Health 4: e463. [DOI] [PubMed] [Google Scholar]
- Cartmell KB, Young-Pierce J, McGue S. et al. 2018. Barriers, facilitators, and potential strategies for increasing HPV vaccination: a statewide assessment to inform action. Papillomavirus Research 5: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Z. 2013. A systematic review of critical thinking in nursing education. Nurse Education Today 33: 236–40. [DOI] [PubMed] [Google Scholar]
- Chawla PC, Chawla A, Chaudhary S.. 2016. Knowledge, attitude & practice on human papillomavirus vaccination: a cross-sectional study among healthcare providers. The Indian Journal of Medical Research 144: 741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee G, Pielemeier N, Lion A. et al. 2013. Why differentiating between health system support and health system strengthening is needed. The International Journal of Health Planning and Management 28: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigbu CO, Onyebuchi AK, Onyeka TC. et al. 2017. The impact of community health educators on uptake of cervical and breast cancer prevention services in Nigeria. International Journal of Gynecology & Obstetrics 137: 319–24. [DOI] [PubMed] [Google Scholar]
- Coleman MA, Levison J, Sangi-Haghpeykar H.. 2011. HPV vaccine acceptability in Ghana, West Africa. Vaccine 29: 3945–50. [DOI] [PubMed] [Google Scholar]
- Colvin CJ, Konopka S, Chalker JC. et al. 2014. A systematic review of health system barriers and enablers for antiretroviral therapy (ART) for HIV-infected pregnant and postpartum women. PLoS One 9: e108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot AS, Tounkara K, Rochas M. et al. 2017. Knowledge, attitudes, practices and willingness to vaccinate in preparation for the introduction of HPV vaccines in Bamako, Mali. PLoS One 12: e0171631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martel C, Plummer M, Vignat J. et al. 2017. Worldwide burden of cancer attributable to HPV by site, country and HPV type. International Journal of Cancer 141: 664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst H, Alemany L, Lacey C. et al. 2013. The burden of human papillomavirus infections and related diseases in sub-Saharan Africa. Vaccine 31: F32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny LA, Sankaranarayanan R, De Vuyst H. et al. 2012. Recommendations for cervical cancer prevention in sub-Saharan Africa. Vaccine 31: F74. [DOI] [PubMed] [Google Scholar]
- DiAngi YT, Panozzo CA, Ramogola-Masire D. et al. 2011. A cross-sectional study of HPV vaccine acceptability in Gaborone, Botswana. PLoS One 6: e25481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SA, Battle-Fisher M, Liverpool J. et al. 2011. A qualitative analysis of South African women’s knowledge, attitudes, and beliefs about HPV and cervical cancer prevention, vaccine awareness and acceptance, and maternal-child communication about sexual health. Vaccine 29: 8760–5. [DOI] [PubMed] [Google Scholar]
- Francis SA, Nelson J, Liverpool J. et al. 2010. Examining attitudes and knowledge about HPV and cervical cancer risk among female clinic attendees in Johannesburg, South Africa. Vaccine 28: 8026–32. [DOI] [PubMed] [Google Scholar]
- Friedman AL, Oruko KO, Habel MA. et al. 2014. Preparing for human papillomavirus vaccine introduction in Kenya: implications from focus-group and interview discussions with caregivers and opinion leaders in Western Kenya. BMC Public Health 14: 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KE, Howard N, Kabakama S. et al. 2017a. Human papillomavirus (HPV) vaccine coverage achievements in low and middle-income countries 2007-2016. Papillomavirus Research 4: 72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KE, Howard N, Kabakama S. et al. 2017b. Lessons learnt from human papillomavirus (HPV) vaccination in 45 low- and middle-income countries. PLoS One 12: e0177773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher KE, LaMontagne DS, Watson-Jones D.. 2018. Status of HPV vaccine introduction and barriers to country uptake. Vaccine 36: 4761–7. [DOI] [PubMed] [Google Scholar]
- Garcini LM, Galvan T, Barnack-Tavlaris JL.. 2012. The study of human papillomavirus (HPV) vaccine uptake from a parental perspective: a systematic review of observational studies in the United States. Vaccine 30: 4588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SM, Kjaer SK, Muñoz N. et al. 2016. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clinical Infectious Diseases 63: 519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeman L, Galichet B, Porignon DG. et al. 2010. The response to flexibility: country intervention choices in the first four rounds of the GAVI health systems strengthening applications. Health Policy and Planning 25: 292–9. [DOI] [PubMed] [Google Scholar]
- Hanvoravongchai P, Mounier-Jack S, Oliveira Cruz V. et al. 2011. Impact of measles elimination activities on immunization services and health systems: findings from six countries. The Journal of Infectious Diseases 204: S82–9. [DOI] [PubMed] [Google Scholar]
- Harries J, Moodley J, Barone MA. et al. 2009. Preparing for HPV vaccination in South Africa: key challenges and opinions. Vaccine 27: 38–44. [DOI] [PubMed] [Google Scholar]
- Hendry M, Lewis R, Clements A. et al. 2013. “HPV? Never heard of it!”: a systematic review of girls’ and parents’ information needs, views and preferences about human papillomavirus vaccination. Vaccine 31: 5152–67. [DOI] [PubMed] [Google Scholar]
- Herrero R, González P, Markowitz LE.. 2015. Present status of human papillomavirus vaccine development and implementation. The Lancet. Oncology 16: e216. [DOI] [PubMed] [Google Scholar]
- Hopkins TG, Wood N.. 2013. Female human papillomavirus (HPV) vaccination: global uptake and the impact of attitudes. Vaccine 31: 1673–9. [DOI] [PubMed] [Google Scholar]
- Hoque ME. 2015. Acceptability of human papillomavirus vaccination among academics at the University of KwaZulu-Natal, South Africa. South African Family Practice 57: 318–21. [Google Scholar]
- Hoque ME. 2016. Factors influencing the recommendation of the human papillomavirus vaccine by South African doctors working in a tertiary hospital. African Health Sciences 16: 567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque ME, Ghuman S, Hal GV.. 2013. Human papillomavirus vaccination acceptability among female university students in South Africa. Asian Pacific Journal of Cancer Prevention 14: 4865–9. [DOI] [PubMed] [Google Scholar]
- Hutubessy R, Levin A, Wang S. et al. 2012. A case study using the United Republic of Tanzania: costing nationwide HPV vaccine delivery using the WHO Cervical Cancer Prevention and Control Costing Tool. BMC Medicine 10: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamya C, Shearer J, Asiimwe G. et al. 2017. Evaluating global health partnerships: a case study of a Gavi HPV vaccine application process in Uganda. International Journal of Health Policy and Management 6: 327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane GA, Wood VA, Barlow J.. 2007. Parenting programmes: a systematic review and synthesis of qualitative research. Child: Care, Health and Development 33: 784–93. [DOI] [PubMed] [Google Scholar]
- Katz IT, Nkala B, Dietrich J. et al. 2013. A qualitative analysis of factors influencing HPV vaccine uptake in Soweto, South Africa among adolescents and their caregivers. PLoS One 8: e72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels SJM, Marshall HS, Watson M. et al. 2012. Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine 30: 3546–56. [DOI] [PubMed] [Google Scholar]
- Kruk ME, Freedman LP.. 2008. Assessing health system performance in developing countries: a review of the literature. Health Policy 85: 263–76. [DOI] [PubMed] [Google Scholar]
- Ladner J, Besson M, Hampshire R. et al. 2012. Assessment of eight HPV vaccination programs implemented in lowest income countries. BMC Public Health 12: 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner J, Besson M, Rodrigues M. et al. 2014. Performance of 21 HPV vaccination programs implemented in low and middle-income countries, 2009-2013. BMC Public Health 14: 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne DS, Barge S, Le NT. et al. 2011. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bulletin of the World Health Organization 89: 821–30B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne DS, Bloem PJN, Brotherton JML. et al. 2017. Progress in HPV vaccination in low‐ and lower‐middle‐income countries. International Journal of Gynecology & Obstetrics 138: 7–14. [DOI] [PubMed] [Google Scholar]
- Langley JM. 2015. Adolescent immunization—protecting youth and preparing them for a healthy future. International Journal of Pediatrics and Adolescent Medicine 2: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin CE, Van Minh H, Odaga J. et al. 2013. Delivery cost of human papillomavirus vaccination of young adolescent girls in Peru, Uganda and Vietnam. Bulletin of the World Health Organization 91: 585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie KS, de Sanjose S, Mayaud P.. 2009. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. Tropical Medicine & International Health 14: 1287–302. [DOI] [PubMed] [Google Scholar]
- Lu B, Kumar A, Castellsague X. et al. 2011. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infectious Diseases 11: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabeya H, Menon S, Weyers S.. et al. 2018. Uptake of three doses of HPV vaccine by primary school girls in Eldoret, Kenya; a prospective cohort study in a maleria endemic setting. BMC Cancer 18:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machingaidze S, Wiysonge CS, Hussey GD.. 2013. Strengthening the expanded programme on immunization in Africa: looking beyond 2015. PLoS Medicine 10: e1001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhail C, Venables E, Rees H. et al. 2013. Using HPV vaccination for promotion of an adolescent package of care: opportunity and perspectives. BMC Public Health 13: 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makwe CC, Anorlu RI.. 2011. Knowledge of and attitude toward human papillomavirus infection and vaccines among female nurses at a tertiary hospital in Nigeria. International Journal of Women’s Health 3: 313–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal B, Cavalli A, Kegels G.. 2009. Global health actors claim to support health system strengthening—is this reality or rhetoric? PLoS Medicine 6: e1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maseko FC, Chirwa ML, Muula AS.. 2015. Cervical cancer control and prevention in Malawi: need for policy improvement. Pan African Medical Journal 22: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masika MM, Ogembo JG, Chabeda SV. et al. 2015. Knowledge on HPV vaccine and cervical cancer facilitates vaccine acceptability among school teachers in Kitui County, Kenya. PLoS One 10: e0135563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PM, Boansi RK, Gipson JD. et al. 2017. Human papillomavirus (HPV) awareness and vaccine receptivity among Senegalese adolescents. Tropical Medicine & International Health 22: 113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta B, Chawla S, Kumar V, Jindal H, Bhatt B.. 2014. Adult immunization: the need to address. Human Vaccines & Immunotherapeutics 10: 306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihigo R, Okeibunor J, Anya B. et al. 2017. Challenges of immunization in the African Region. Pan African Medical Journal 27: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley I, Tathiah N, Mubaiwa V. et al. 2013. High uptake of Gardasil vaccine among 9–12-year-old schoolgirls participating in an HPV vaccination demonstration project in KwaZulu-Natal, South Africa. South African Medical Journal 103: 318–21. [DOI] [PubMed] [Google Scholar]
- Morhason-Bello IO, Wallis S, Adedokun BO. et al. 2015. Willingness of reproductive-aged women in a Nigerian community to accept human papillomavirus vaccination for their children. Journal of Obstetrics and Gynaecology Research 41: 1621–9. [DOI] [PubMed] [Google Scholar]
- Mounier-Jack S, Burchett HED, Griffiths UK. et al. 2014. Measuring the health systems impact of disease control programmes: a critical reflection on the WHO building blocks framework. BMC Public Health 14: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msyamboza KP, Mwagomba BM, Valle M. et al. 2017. Implementation of a human papillomavirus vaccination demonstration project in Malawi: successes and challenges. BMC Public Health 17: 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugisha E, LaMontagne DS, Katahoire AR. et al. 2015. Feasibility of delivering HPV vaccine to girls aged 10 to 15 years in Uganda. African Health Sciences 15: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Evans D.. 2003. Health systems performance assessment: debates, methods and empiricism. http://apps.who.int/iris/handle/10665/42735, accessed 12 March 2020.
- Murray CJL, Frenk J.. 2000. A framework for assessing the performance of health systems. Bulletin of the World Health Organization 78: 717–31. [PMC free article] [PubMed] [Google Scholar]
- Nanni A, Meredith S, Gati S. et al. 2017. Strengthening global vaccine access for adolescents and adults. Vaccine 35: 6823–7. [DOI] [PubMed] [Google Scholar]
- Ndizeye Z, Vanden Broeck D, Vermandere H. et al. 2018. Knowledge and practices of general practitioners at district hospitals towards cervical cancer prevention in Burundi, 2015: a cross-sectional study. Globalization and Health 14: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngabo F, Levin A, Wang SA. et al. 2015. A cost comparison of introducing and delivering pneumococcal, rotavirus and human papillomavirus vaccines in Rwanda. Vaccine 33: 7357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nganwai P, Truadpon P, Inpa C. et al. 2008. Knowledge, attitudes and practices vis-a-vis cervical cancer among registered nurses at the Faculty of Medicine, Khon Kaen University, Thailand. Asian Pacific Journal of Cancer Prevention 9: 15–8. [PubMed] [Google Scholar]
- Odunyemi FT, Ndikom CM, Oluwatosin OA.. 2018. Effect of nursing intervention on mothers’ knowledge of cervical cancer and acceptance of human papillomavirus vaccination for their adolescent daughters in Abuja—Nigeria. Asia-Pacific Journal of Oncology Nursing 5: 223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogembo JG, Manga S, Nulah K. et al. 2014. Achieving high uptake of human papillomavirus vaccine in Cameroon: lessons learned in overcoming challenges. Vaccine 32: 4399–403. [DOI] [PubMed] [Google Scholar]
- Okunade KS, Sunmonu O, Osanyin GE. et al. 2017. Knowledge and acceptability of human papillomavirus vaccination among women attending the gynaecological outpatient clinics of a university teaching hospital in Lagos, Nigeria. Journal of Tropical Medicine 2017: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwole D, Kraemer J; Pink Ribbon Red Ribbon. 2013. Innovative public-private partnership: a diagonal approach to combating women’s cancers in Africa. Bulletin of the World Health Organization 91: 691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z. et al. 2016. Rayyan-a web and mobile app for systematic reviews. Systematic Reviews 5: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DN, Tracy JK, Levitz L. et al. 2013. A cross-sectional study to assess HPV knowledge and HPV vaccine acceptability in Mali. PLoS One 8: e56402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ports KA, Reddy DM, Rameshbabu A.. 2013. Barriers and facilitators to HPV vaccination: perspectives from Malawian women. Women & Health 53: 630–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin W, Terris-Prestholt F, Changalucha J. et al. 2012. Costs of delivering human papillomavirus vaccination to schoolgirls in Mwanza Region, Tanzania. BMC Medicine 10: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remes P, Selestine V, Changalucha J. et al. 2012. A qualitative study of HPV vaccine acceptability among health workers, teachers, parents, female pupils, and religious leaders in northwest Tanzania. Vaccine 30: 5363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BL, Shepard A, Kahn JA.. 2018. US health care clinicians’ knowledge, attitudes, and practices regarding human papillomavirus vaccination: a qualitative systematic review. Academic Pediatrics 18: S53–S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGE. 2016. Midterm review of the global vaccine action plan. http://www.who.int/immunization/global_vaccine_action_plan/SAGE_GVAP_Assessment_Report_2016_EN.pdf, accessed 12 March 2020.
- Sankaranarayanan R, Anorlu R, Sangwa-Lugoma G. et al. 2012. Infrastructure requirements for human papillomavirus vaccination and cervical cancer screening in sub-Saharan Africa. Vaccine 31: F52. [DOI] [PubMed] [Google Scholar]
- Shen AK, Fields R, McQuestion M.. 2014. The future of routine immunization in the developing world: challenges and opportunities. Global Health: Science and Practice 2: 381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounga BK, Jaquet A, Coffie PA. et al. 2014. Cervical cancer prevention in reproductive health services: knowledge, attitudes and practices of midwives in Côte d’Ivoire, West Africa. BMC Health Services Research 14: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rueda S, Rulisa S, Burchett HED. et al. 2016. HPV vaccine introduction in Rwanda: impacts on the broader health system. Sexual & Reproductive Healthcare 7: 46–51. [DOI] [PubMed] [Google Scholar]
- Travis P, Bennett S, Haines A. et al. 2004. Overcoming health-systems constraints to achieve the millennium development goals. The Lancet 364: 900–6. [DOI] [PubMed] [Google Scholar]
- Turiho AK, Okello ES, Muhwezi WW.. et al. 2014. Effect of school-based human papillomavirus (hpv) vaccination on adolescent girls' knowledge and acceptability of the HPV vaccine in Ibanda District in Uganda. African Journal of Reproductive Health 18: 45–53. [PMC free article] [PubMed] [Google Scholar]
- Turiho AK, Okello ES, Muhwezi WW. et al. 2017. Perceptions of human papillomavirus vaccination of adolescent schoolgirls in western Uganda and their implications for acceptability of HPV vaccination: a qualitative study. BMC Research Notes 10: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwu EO, Obi SN, Ezechukwu PC. et al. 2013. Acceptability of human papillomavirus vaccine and cervical cancer screening among female health-care workers in Enugu, Southeast Nigeria. Nigerian Journal of Clinical Practice 16: 249–52. [DOI] [PubMed] [Google Scholar]
- Umeh IB, Nduka SO, Ekwunife OI.. 2016. Mothers’ willingness to pay for HPV vaccines in Anambra state, Nigeria: a cross sectional contingent valuation study. Cost Effectiveness and Resource Allocation 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasa M, Darj E.. 2011. Knowledge of cervical cancer and screening practices of nurses at a regional hospital in Tanzania. African Health Sciences 11: 48–57. [PMC free article] [PubMed] [Google Scholar]
- Venturas C, Umeh K.. 2017. Health professional feedback on HPV vaccination roll-out in a developing country. Vaccine 35: 1886–91. [DOI] [PubMed] [Google Scholar]
- Vermandere H, Naanyu V, Degomme O. et al. 2015. Implementation of an HPV vaccination program in Eldoret, Kenya: results from a qualitative assessment by key stakeholders. BMC Public Health 15: 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermandere H, Naanyu V, Mabeya H. et al. 2014. Determinants of acceptance and subsequent uptake of the HPV vaccine in a cohort in Eldoret, Kenya. PLoS One 9: e109353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter FM, Emery J, Braithwaite D. et al. 2004. Lay understanding of familial risk of common chronic diseases: a systematic review and synthesis of qualitative research. The Annals of Family Medicine 2: 583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamai RG, Ayissi CA, Oduwo GO. et al. 2013. Awareness, knowledge and beliefs about HPV, cervical cancer and HPV vaccines among nurses in Cameroon: an exploratory study. International Journal of Nursing Studies 50: 1399–406. [DOI] [PubMed] [Google Scholar]
- Wang SA, Hyde TB, Mounier-Jack S. et al. 2013. New vaccine introductions: assessing the impact and the opportunities for immunization and health systems strengthening. Vaccine 31: B122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson-Jones D, Mugo N, Lees S. et al. 2015. Access and attitudes to HPV vaccination amongst hard-to-reach populations in Kenya. PLoS One 10: e0123701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson-Jones D, Tomlin K, Remes P. et al. 2012. Reasons for receiving or not receiving HPV vaccination in primary schoolgirls in Tanzania: a case control study. PLoS One 7: e45231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt N, Sigfrid L, Legido-Quigley H. et al. 2017. Health systems facilitators and barriers to the integration of HIV and chronic disease services: a systematic review. Health Policy and Planning 32: iv13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2007. Everybody’s business—strengthening health systems to improve health outcomes: WHO’s framework for action. http://www.who.int/iris/handle/10665/43918, accessed 13 March 2020.
- WHO. 2011. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. http://www.who.int/healthinfo/systems/WHO_MBHSS_2010_full_web.pdf, accessed 13 March 2020.
- WHO. 2013a. The expanded programme on immunization. http://www.who.int/immunization/programmes_systems/supply_chain/benefits_of_immunization/en/, accessed 13 March 2020.
- WHO. 2013b. Global Vaccine Action Plan 2011-2020. https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/, accessed 13 March 2020.
- WHO. 2015. WHO’s mission and vision in immunization and vaccines 2015-2030. http://www.who.int/immunization/documents/general/WHO_Mission_VIsion_Immunization_Vaccines_2015_2030.pdf?ua=1, accessed 13 March 2020.
- WHO. 2017a. Human papillomavirus vaccines: WHO position paper, May 2017. Weekly Epidemiological Record 92: 241–68.28530369 [Google Scholar]
- WHO. 2017b. Human papillomavirus vaccines: WHO position paper, May 2017—recommendations. Vaccine 35: 5753–5. [DOI] [PubMed] [Google Scholar]
- WHO. 2019. WHO vaccine-preventable diseases: monitoring system. 2019 Global summary.http://apps.who.int/immunization_monitoring/globalsummary/schedules, accessed 13 March 2020.
- WHO. 2018. WHO regional office for Africa. https://www.afro.who.int/, accessed 13 march 2020.
- Wigle J, Coast E, Watson-Jones D.. 2013. Human papillomavirus (HPV) vaccine implementation in low and middle-income countries (LMICs): Health system experiences and prospects. Vaccine 31: 3811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Maximizing Positive Synergies Collaborative Group, Samb B, Evans T. et al. 2009. An assessment of interactions between global health initiatives and country health systems. Lancet (London, England) 373: 2137–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.