Abstract
Background
Despite the mainly reassuring outcomes for pregnant women with coronavirus disease 2019 reported by previous case series with small sample sizes, some recent reports of severe maternal morbidity requiring intubation and of maternal deaths show the need for additional data about the impact of coronavirus disease 2019 on pregnancy outcomes.
Objective
This study aimed to report the maternal characteristics and clinical outcomes of pregnant women with coronavirus disease 2019.
Study Design
This retrospective, single-center study includes all consecutive pregnant women with confirmed (laboratory-confirmed) or suspected (according to the Chinese management guideline [version 7.0]) coronavirus disease 2019, regardless of gestational age at diagnosis, admitted to the Strasbourg University Hospital (France) from March 1, 2020, to April 3, 2020. Maternal characteristics, laboratory and imaging findings, and maternal and neonatal outcomes were extracted from medical records.
Results
The study includes 54 pregnant women with confirmed (n=38) and suspected (n=16) coronavirus disease 2019. Of these, 32 had an ongoing pregnancy, 1 had a miscarriage, and 21 had live births: 12 vaginal and 9 cesarean deliveries. Among the women who gave birth, preterm deliveries were medically indicated for their coronavirus disease 2019–related condition for 5 of 21 women (23.8%): 3 (14.3%) before 32 weeks’ gestation and 2 (9.5%) before 28 weeks’ gestation. Oxygen support was required for 13 of 54 women (24.1%), including high-flow oxygen (n=2), noninvasive (n=1) and invasive (n=3) mechanical ventilation, and extracorporeal membrane oxygenation (n=1). Of these, 3, aged 35 years or older with positive test result for severe acute respiratory syndrome coronavirus 2 using reverse transcription polymerase chain reaction, had respiratory failure requiring indicated delivery before 29 weeks’ gestation. All 3 women were overweight or obese, and 2 had an additional comorbidity.
Conclusion
Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Its association with other well-known risk factors for severe maternal morbidity in pregnant women with no infection, including maternal age above 35 years, overweight, and obesity, suggests further studies are required to determine whether these risk factors are also associated with poorer maternal outcome in these women.
Key words: COVID-19 pneumonia, extracorporeal membrane oxygenation, intubation, maternal and neonatal outcomes, maternal morbidity, pregnancy, preterm birth, respiratory failure
Click Supplemental Materials under article title in Contents at ajog.org
Introduction
Since the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or coronavirus disease 2019 (COVID-19) outbreak, it has been argued that pregnant women are at increased risk of severe infection.1 , 2 Various experts and authorities rapidly published articles of advice, guidance, and information to aid professionals in caring for pregnant women with COVID-19.3, 4, 5, 6 Most of the early reports, mainly from the epicenter of the pandemic in China,7, 8, 9, 10, 11, 12, 13, 14, 15, 16 have suggested that pregnant women are not more severely affected than the general population.1 , 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Case reports and limited case series of pregnant women from the United States,22 Iran,2 , 3 and South America24 have contradicted these reassuring preliminary findings, however. They have instead described women with COVID-19 as patients who require invasive mechanical ventilation including extracorporeal membrane oxygenation (ECMO) and who are at risk of maternal death. These contradictory findings and the potential variation in disease severity between countries mean that additional information is urgently needed, especially from areas besides China, to determine whether or not pregnant women with COVID-19 are likely to experience severe cases with pneumonia.
We aim to present the clinical features and course of consecutive pregnant women with COVID-19 managed at a single French referral center, located in one of the main pandemic epicenters in France.
AJOG at a Glance.
Why was this study conducted?
Although the available data about maternal outcomes for pregnant women with coronavirus disease 2019 (COVID-19) are reassuring, some limited case series report adverse maternal outcomes justifying further investigation. This study was conducted to report the maternal characteristics and clinical outcomes of these women.
Key findings
Among the pregnant women with COVID-19, 13 of 54 (24.1%) received oxygen support, with 3 of 54 (5.5%) requiring invasive mechanical ventilation. Among the 21 women who gave birth, 5 of 21 (23.8%) had deliveries—3 of 21 (14.3%) very preterm—indicated for severe maternal conditions related to their COVID-19 condition.
What does this add to what is known?
In contrast to most existing data, this study shows that COVID-19 in pregnancy is associated with maternal morbidity and preterm birth.
Methods
Study design and patients
This single-center, retrospective study includes all consecutive pregnant women with COVID-19, regardless of gestational age at diagnosis, admitted to Strasbourg University Hospital (Strasbourg, France), a tertiary referral hospital, from March 1, 2020, to April 3, 2020. During the study period, only symptomatic women were tested using quantitative reverse transcription polymerase chain reaction (qRT-PCR) for SARS-CoV-2. Women with an ongoing pregnancy and a time from symptom onset to April 3 shorter than 14 days25 , 26 were excluded. COVID-19 was diagnosed according to the Chinese management guidelines (version 7.0),26 which distinguishes confirmed cases, defined as all cases with respiratory tract samples that test positive for SARS-CoV-2 using qRT-PCR, from suspected cases, which include an epidemiologic history (Strasbourg is a community with numerous COVID-19 cases) and at least 2 of the following clinical manifestations: (1) fever and/or respiratory syndrome, (2) imaging features of COVID-19 pneumonia, and (3) a decreased or normal total number of leukocytes and lymphocyte counts in the early stage of the disease.26 The imaging features of COVID-19 pneumonia used were typical radiographic features on chest computed tomography (CT), owing to their high diagnostic sensitivity and accuracy for this diagnosis.26, 27, 28
The Ethics Committee of Strasbourg University Hospital approved this study under a waiver of informed consent (reference number identification 2020: Hospital University of Strasbourg N7794).
Data collection
Maternal characteristics, laboratory findings, CT scans, management, and maternal and fetal outcomes were obtained from patients’ medical records.
All laboratory tests were performed at the Strasbourg University Hospital, including the laboratory confirmation of COVID-19 using qRT-PCR to tests for SARS-CoV-2 nucleic acid on nasopharyngeal swabs.29 Primer and probe sequences target 2 regions on the RdRp gene and are specific to SARS-CoV-2. Assay sensitivity is around 10 copies per reaction. For women with multiple RT-PCR assays, the diagnosis of COVID-19 was confirmed when any 1 nucleic acid test result was positive. Typical radiographic features of COVID-19 on chest CT were consolidation, ground-glass opacity, or bilateral pulmonary infiltration.27 Neonatal throat and rectal swab samples were also collected to test for SARS-CoV-2 using RT-PCR assays at birth or on day 1 of life, on day 3 of life in term newborns, and on days 7 and 14 of life in preterm neonates. Skin-to-skin contact between mothers and neonates and breastfeeding were both permitted at birth.
Definitions
Fever was defined as an axillary temperature of at least 37.8°C (100°F). The severity of COVID-19 was defined according to the Chinese management guidelines (version 7.0) as mild (mild clinical symptoms and no sign of pneumonia), moderate (patients have fever and respiratory symptoms; radiologic assessments found signs of pneumonia), severe (adults meeting any of the following criteria: shortness of breath, respiratory rate ≥30 breaths/min, oxygen saturation ≤93% at rest, or alveolar oxygen partial pressure (PaO2)/fraction of inspiration oxygen (FIO2) ≤300 mm Hg), and critical (≥1 of any mechanical ventilation, shock, or organ failure that needs intensive care unit [ICU] monitoring and treatment).26 Acute respiratory distress syndrome (ARDS) was diagnosed according to the Berlin definition in 3 mutually exclusive categories based on degree of hypoxemia: mild (200 mm Hg<PaO2/FIO2≤300 mm Hg), moderate (100 mm Hg<PaO2/FIO2≤200 mm Hg), and severe (PaO2/FIO2≤100 mm Hg).30
Statistical analysis
Descriptive characteristics were calculated for the variables of interest. Statistical analysis included determination of rates with their 95% confidence intervals, performed with R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
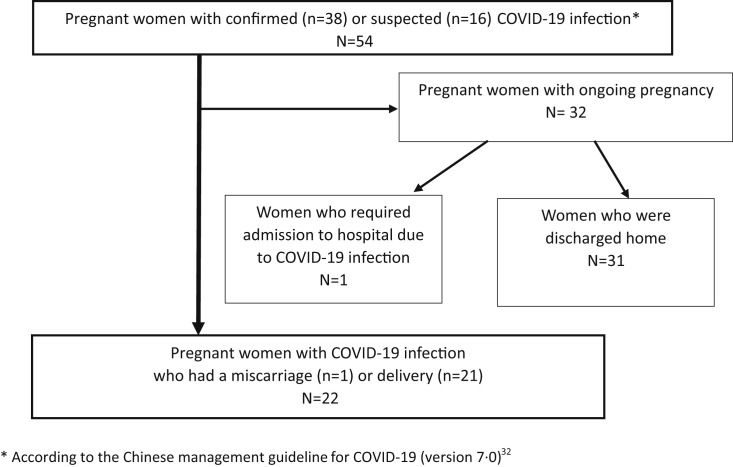
The study included 54 symptomatic pregnant women with COVID-19 during the study period; 38 had positive RT-PCR results for SARS-CoV-2, whereas 16 had negative RT-PCR results for SARS-CoV-2 but met the Chinese management guideline criteria for suspected COVID-19 (version 7.0).31 Among these 54 women, 32 had an ongoing pregnancy more than 14 days after symptom onset, 1 had a miscarriage, and 21 had live births by vaginal or cesarean delivery (Figure ).
Figure.
Flowchart of the study population
The severity of COVID-19 was defined according to the Chinese management guideline for COVID-19 (version 7.0). Adapted from China National Health Commision.26
COVID-19, coronavirus disease 2019.
Sentilhes et al. Coronavirus disease 2019 in pregnancy can cause severe maternal morbidity. Am J Obstet Gynecol 2020.
Maternal clinical characteristics
Table 1 summarizes maternal clinical characteristics. Their ages ranged from 19 to 42 years. Ten women (18.5%) had at least 1 comorbidity before pregnancy: 4 had asthma and 1 preexisting chronic hypertension, 4 were obese, and 4 had another comorbidity. No woman had a multiple pregnancy, and only 15 (27.3%) were nulliparous. The mean gestational age at diagnosis of COVID-19 was 30.4±9.0 weeks, whereas the mean time from symptom onset to hospital admission was 3.5±3.2 days. Fourteen (25.9%) had fever, 36 (66.7%) cough, 22 (40.7%) shortness of breath, 9 (16.7%) digestive disorders, and 20 (37.0%) anosmia or ageusia. Chest CT was performed on 12 women (22.2%) and showed typical features of COVID-19 for 10 (83.3%).
Table 1.
Women’s characteristics at admission
| Characteristics | All women (N=54) | Pregnant women with confirmed COVID-19 (n=38) | Pregnant women with suspected COVID-19 (n=16) |
|---|---|---|---|
| Age, y | 30.6±6.2 | 31.1±6.4 | 29.6±5.9 |
| Age >35 y, n (%) | 15 (27.8) | 12 (31.6) | 3 (18.8) |
| Geographic origin, n (%) | |||
| Europe | 34 (63.0) | 22 (57.9) | 12 (75.0) |
| Sub-Saharan Africa | 10 (18.5) | 10 (26.3) | 0 |
| North Africa | 10 (18.5) | 6 (15.8) | 4 (25.0) |
| Asia | 0 | 0 | 0 |
| Non-French nationality, n (%) | 21 (38.9) | 18 (47.4) | 3 (18.8) |
| BMI before pregnancy | 25.3±4.7 | 25.6±5.1 | 24.2±3.3 |
| BMI >25 kg/m2, n (%) | 20 (37.0) | 16 (42.1) | 4 (25) |
| BMI >30 kg/m2, n (%) | 4 (7.4) | 4 (10.5) | 0 |
| Preexisting chronic hypertension, n (%) | 1 (1.9) | 0 | 1 (6.25) |
| Asthma, n (%) | 5 (9.3) | 1 (2.6) | 4 (25.0) |
| Preexisting diabetes mellitus, n (%) | 0 | 0 | 0 |
| Other preexisting chronic disease, n (%) | 4 (7.4) | 3 (7.9) | 1 (6.25) |
| Nulliparous, n (%) | 15 (27.3) | 9 (23.7) | 6 (37.5) |
| Singleton pregnancy, n (%) | 54 (100) | 38 (100) | 16 (100) |
| Previous cesarean delivery, n (%) | 9 (16.7) | 6 (15.8) | 3 (18.8) |
| History of postpartum hemorrhage, n (%) | 2 (3.7) | 2 (5.3) | 0 |
| History of preterm delivery | 0 | 0 | 0 |
| Tobacco use during pregnancy, n (%) | 1 (1.9) | 0 | 1 (6.3) |
| Gestational diabetes, n (%) | 4 (7.4) | 4 (10.5) | 0 |
| Gestational hypertensive disorders, n (%) | 2 (3.7) | 1 (2.6) | 1 (6.3) |
| Gestational age at the diagnosis of COVID-19, wk | 30.4±9.0 | 29.3±8.5 | 31.8±6.3 |
| Contact history in epidemic area, n (%) | 54 (100) | 38 (100) | 16 (100) |
| Other family members affected, n (%) | 20 (37.0) | 16 (42.1) | 4 (25.0) |
| Clinical manifestations | |||
| Fever, n (%) | 14 (25.9) | 10 (26.3) | 4 (25.0) |
| Cough, n (%) | 36 (66.7) | 25 (65.8) | 11 (68.8) |
| Shortness of breath, n (%) | 22 (40.7) | 13 (34.2) | 9 (56.3) |
| Diarrhea, n (%) | 9 (16.7) | 7 (18.4) | 2 (12.5) |
| Fatigue, n (%) | 54 (100) | 38 (100) | 16 (100) |
| Sore throat, n (%) | 23 (42.6) | 16 (42.1) | 7 (43.8) |
| Anosmia or ageusia, n (%) | 20 (37.0) | 18 (47.4) | 2 (12.5) |
| Acute respiratory distress syndrome, n (%) | 1 (1.9) | 1 (2.6) | 0 |
| Disease severity statusa | |||
| Mild or moderate, n (%) | 37 (68.5) | 27 (71.1) | 10 (62.5) |
| Severe, n (%) | 11 (20.4) | 8 (21.1) | 3 (18.8) |
| Critical, n (%) | 6 (11.1) | 3 (7.9) | 3 (18.8) |
| Time from symptom onset to hospital admission, d | 3.5±3.2 | 3.6±2.8 | 4.0±4.2 |
| Chest computed tomography, n (%) | 12 (22.2) | 7 (18.4) | 5 (21.3) |
| Typical signs of viral infection, n/N (%) | 10/12 (83.3) | 7 (18.4) | 3 (18.8) |
| Consolidation, n/N (%) | 7/12 (58.3) | 5 (13.2) | 2 (12.5) |
| Ground-glass opacities, n/N (%) | 5/12 (41.6) | 4 (10.5) | 1 (6.3) |
| Bilateral pulmonary infiltration, n/N (%) | 1/12 (8.3) | 1 (2.6) | 0 |
Data are presented as mean±SD, unless otherwise indicated. Body mass index is weight in kilograms divided by the square of the height in meters. Other preexisting chronic disease means disease other than body mass index >25 kg/m2 or preexisting chronic hypertension, diabetes mellitus, and asthma. The data include 1 woman with sickle cell anemia, 1 with chronic alcohol abuse, 1 with chronic hepatitis B, and 1 with nephropathy. Contact history of epidemic area means exposure to the relevant environment (Alsace region) or contact with a person with infection.
BMI, body mass index; COVID-19, coronavirus disease 2019; SD, standard deviation.
Sentilhes et al. Coronavirus disease 2019 in pregnancy can cause severe maternal morbidity. Am J Obstet Gynecol 2020.
The severity of COVID-19 was defined according to the Chinese management guideline for COVID-19 (version 7.0).26
Laboratory tests
Data from laboratory tests showed that 24 women (44.4%) had lymphopenia; C-reactive protein was elevated in 22 (40.7%) and aspartate and/or alanine aminotransferases in 11 (20.4%). None had thrombocytopenia (Table 2 ).
Table 2.
Clinical laboratory results
| Laboratory characteristics | Reference range | All women (N=54) | Pregnant women with confirmed COVID-19 (n=38)a | Pregnant women with suspected COVID-19 (n=16)a |
|---|---|---|---|---|
| White blood cell count (×109 cells per L) | 4.02–11.42 | 8.9±3.7 | 8.4±3.7 | 10.2±3.7 |
| Lymphocyte count (×10⁹ cells per L) | 1.24–3.56 | 1.6±0.7 | 1.5±0.7 | 1.7±0.7 |
| Lymphopenia (<1.5×10⁹ cells per L), n (%) | N/A | 24 (44.4) | 17 (44.7) | 7 (43.8) |
| C-reactive protein concentration (mg/L) | <5 | 25.7±36.9 | 26.4±37.6 | 7.5±36.9 |
| Elevated C-reactive protein (>10 mg/L), n (%) | N/A | 22 (40.7) | 15 (41.7) | 6 (37.5) |
| Elevated ALT (>45 U/L) or AST (>35 U/L), n (%) | N/A | 11 (20.4) | 10 (27.0) | 1 (6.3) |
| ALT (U/L) | 6–55 | 26.6±17.1 | 29.1±19.0 | 19.9±8.4 |
| AST (U/L) | 5–34 | 25.7±20.4 | 27.4±19.7 | 21.5±22.3 |
| Hemoglobin (g/dL) | 11.5–14.9 | 11.2±1.3 | 11.1±1.3 | 11.2±1.4 |
| Hematocrit (%) | 34.4–43.9 | 36.4±10.8 | 34.3±4.0 | 41.2±18.2 |
| Thrombocytopenia <100,000/dL, n (%) | N/A | 0 | 0 | 0 |
| Blood urea nitrogen (mmol/L) | 2.5–6.7 | 2.7±1.0 | 2.5±1.0 | 3.0±1.1 |
| Creatinine (μmol/L) | 49–90 | 48.4±10.2 | 47.3±8.3 | 50.9±13.7 |
| Prothrombin ratio <80%, n (%) | N/A | 2 (3.7) | 1 (2.7) | 1 (6.3) |
| Prolonged aPTT, n (%) | >1.19 | 6 (11.1) | 5 (13.2) | 1 (6.3) |
Data are presented as mean±SD, unless otherwise indicated.
AST, aspartate aminotransferase; ALT, alanine aminotransferase; aPTT, activated partial thromboplastin time; COVID-19, coronavirus disease 2019; N/A, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Sentilhes et al. Coronavirus disease 2019 in pregnancy can cause severe maternal morbidity. Am J Obstet Gynecol 2020.
COVID-19 was diagnosed according to the Chinese management guidelines (version 7.0),26 which distinguishes confirmed cases, defined as all cases with respiratory tract samples that tested positive for SARS-CoV-2 using quantitative reverse transcription polymerase chain reaction (qRT-PCR), from suspected cases, which include an epidemiologic history (Strasbourg is a community with numerous COVID-19 cases) and at least 2 of the following clinical manifestations: (1) fever and/or respiratory syndrome, (2) imaging features of COVID-19 pneumonia, and (3) a decreased or normal total number of leukocytes and lymphocyte counts in the early stage of the disease.26
Clinical course of pregnancy
Thirteen women (24.1%) received oxygen support. Two of 13 (15.4%) required high-flow oxygen, one after delivery and the other before delivery. The latter subsequently required noninvasive mechanical ventilation and has not yet given birth. In addition, 3 of the 13 women (23.1%) who received oxygen subsequently had invasive mechanical ventilation after delivery. One woman (1.9%) later required ECMO.
Three women (5.6%) received antiviral therapy (lopinavir or ritonavir 400 mg/100 mg tablet twice a day for 5 days) (Table 3 ).
Table 3.
Management of COVID-19 pneumonia
| All women (N=54) | Pregnant women with confirmed COVID-19 (n=38) | Pregnant women with suspected COVID-19 (n=16) | |
|---|---|---|---|
| Management of COVID-19 pneumonia (before and after delivery) | |||
| Oxygen support (nasal cannula), n (%) | 13 (24.1) | 10 (26.3) | 3 (18.8) |
| High-flow oxygen, n (%) | 2 (3.7) | 1 (2.6) | 1 (6.3) |
| Noninvasive mechanical ventilation, n (%) | 1 (1.9) | 1 (2.6) | 0 |
| Invasive mechanical ventilation, n (%) | 3 (5.6) | 1 (2.6) | 2 (12.5) |
| Extracorporeal membrane oxygenation, n (%) | 1 (2.6) | 1 (2.6) | 0 |
| Antiviral therapy, n (%) | 3 (5.6) | 3 (7.9) | 0 |
| Antibiotic therapy, n (%) | 4 (7.4) | 4 (10.5) | 0 |
| Corticosteroid, n (%) | 0 | 0 | 0 |
| Hydroxychloroquine or chloroquine, n (%) | 0 | 0 | 0 |
| Management of COVID-19 pneumonia before delivery | |||
| Oxygen support (nasal cannula), n (%) | 13 (24.1) | 10 (13.2) | 3 (18.8) |
| High-flow oxygen, n (%) | 1 (1.9) | 0 | 1 (6.3) |
| Noninvasive mechanical ventilation, n (%) | 1 (1.9) | 0 | 1 (6.3) |
| Invasive mechanical ventilation, n (%) | 0 | 0 | 0 |
| Extracorporeal membrane oxygenation, n (%) | 0 | 0 | 0 |
| Antiviral therapy, n (%) | 0 | 0 | 0 |
| Antibiotic therapy, n (%) | 2 (3.7) | 2 (5.3) | 0 |
| Corticosteroid, n (%) | 0 | 0 | 0 |
| Hydroxychloroquine or chloroquine, n (%) | 0 | 0 | 0 |
| Management of COVID-19 pneumonia after delivery | |||
| Oxygen support (nasal cannula), n (%) | 0 | 0 | 0 |
| High-flow oxygen, n (%) | 1 (1.9) | 1 (6.3) | 0 |
| Noninvasive mechanical ventilation, n (%) | 0 | 0 | 0 |
| Invasive mechanical ventilation, n (%) | 3 (5.6) | 1 (2.6) | 2 (12.5) |
| Extracorporeal membrane oxygenation, n (%) | 1 (2.6) | 1 (2.6) | 0 |
| Antiviral therapy, n (%) | 3 (5.6) | 3 (7.9) | 0 |
| Antibiotic therapy, n (%) | 2 (3.7) | 2 (5.3) | 0 |
| Corticosteroid, n (%) | 0 | 0 | 0 |
| Hydroxychloroquine or chloroquine, n (%) | 0 | 0 | 0 |
Five women had more than 1 oxygen support procedure: 1 (woman 2; Supplemental Table) had invasive mechanical ventilation and then extracorporeal membrane oxygenation; 2 (women 3 and 4; Supplemental Table) had invasive mechanical ventilation; 1 (woman 7; Supplemental Table) received high-flow oxygen. The last one (woman 13; Supplemental Table) received high-flow oxygen and then required noninvasive mechanical ventilation.
COVID-19, coronavirus disease 2019.
Sentilhes et al. Coronavirus disease 2019 in pregnancy can cause severe maternal morbidity. Am J Obstet Gynecol 2020.
Among the 21 women who gave birth by April 17, 2020, 9 (42.9%) had cesarean deliveries, and 12 (57.1%) had vaginal deliveries. The mean gestational age at delivery was 37.4±4.7 weeks, but 5 women (23.8%) gave birth <37 weeks’ gestation, 3 (14.3%) <32 weeks’ gestation, and 2 (9.5%) <28 weeks’ gestation. These 5 premature births were medically indicated for severe maternal conditions related to COVID-19, and 2 women were transferred to the ICU after delivery. Five women (9.3%) had postpartum hemorrhages, 4 (7.4%) required a blood transfusion, and 1 (1.9%) had arterial embolization. Five women were (9.3%) admitted to the ICU, all owing to COVID-19 respiratory symptoms. No thromboembolic event nor any other significant maternal morbidity occurred (Table 4 ).
Table 4.
Maternal and neonatal outcomes of the pregnant women with confirmed or suspected COVID-19
| Characteristics | All women (N=54) | Pregnant women with confirmed COVID-19 (n=38) | Pregnant women with suspected COVID-19 (n=16) |
|---|---|---|---|
| Miscarriage, n (%) | 1 (1.9) | 1 (2.6) | 0 |
| Abortion, n (%) | 0 | 0 | 0 |
| Termination of pregnancy, n (%) | 0 | 0 | 0 |
| Ongoing pregnancy, n (%) | 32 (59.3) | 20 (52.6) | 12 (75.0) |
| Preeclampsia, n (%) | 3 (5.6) | 2 (5.3) | 1 (6.3) |
| Fetal growth restriction, n (%) | 1 (1.9) | 1 (2.6) | 0 |
| Preterm premature rupture of membranes, n (%) | 0 | 0 | 0 |
| Stillbirth, n (%) | 0 | 0 | 0 |
| Gestational age at delivery (n=21) | 37.4±4.7 | 36.9±5.2 | 39.0±0.8 |
| Delivery before 37 weeks’ gestation, n/N (%) | 5/21 (23.8) | 5/17 (29.4) | 0 |
| Delivery before 32 weeks’ gestation, n/N (%) | 3/21 (14.3) | 3/17 (17.6) | 0 |
| Delivery before 28 weeks’ gestation, n/N (%) | 2/21 (9.5) | 2/17 (11.8) | 0 |
| Cesarean delivery, n/N (%) | 9/21 (42.9) | 7/17 (41.1) | 2/4 (50) |
| Cesarean delivery related to COVID-19, n/N (%) | 7/21 (33.3) | 6/17 (35.3) | 1/4 (25) |
| Prelabor cesarean delivery, n/N (%) | 8/21 (38.1) | 6/17 (35.3) | 2/4 (50) |
| Cesarean delivery during labor, n/N (%) | 1/21 (4.8) | 1/17 (5.9) | 0 |
| Vaginal delivery, n/N (%) | 12/21 (57.1) | 10/17 (58.8) | 2/4 (50) |
| Operative vaginal delivery, n/N (%) | 2/21 (9.5) | 1/17 (5.9) | 1/4 (25) |
| Spontaneous vaginal delivery, n/N (%) | 10/21 (47.6) | 9/17 (52.9) | 1/4 (25) |
| Third- or fourth-degree perineal lacerations, n/N (%) | 1/21 (4.8) | 0 | 1/4 (25) |
| PPH, defined by blood loss ≥500 mL, measured with a graduated collector bag, n/N (%) | 5/21 (23.8) | 4/17 (23.5) | 1/4 (25) |
| Estimated total blood loss (n=21) | 1060±182 | 1100±183 | 900 |
| Additional uterotonics for excessive bleeding, n/N (%) | 4/21 (19.0) | 4/17 (23.5) | 0 |
| Blood transfusion, n/N (%) | 4/21 (19.0) | 4/17 (23.5) | 0 |
| Arterial embolization or surgery for PPH, n/N (%) | 1/21 (4.8) | 1/17 (5.9) | 0 |
| Acute respiratory distress syndrome, n/N (%)a | 1/21 (4.8) | 0/17 (0) | 0 |
| Thromboembolic events, n (%) | 0 | 0 | 0 |
| Kidney failure, n (%) | 0 | 0 | 0 |
| Admission to ICU, n (%) | 5 (9.3) | 3 (7.9) | 2 (12.5) |
| ICU length of stay (d), median (IQR) (n=5) | 9.2 (1.0–6.5) | 13.0 (1.5–13.0) | 4.0 (1.0–4.5) |
| Total hospital length of stay (d), median (IQR) | 4.0 (3–5.5) (n=30) | 3.0 (3.0–6.0) (n=19) | 4.0 (4.0–4.8) (n=11) |
Data are presented as mean±SD, unless otherwise indicated. Denominator is the number of pregnancies ended beyond the first trimester (n=21). In all preterm births, medically indicated premature delivery was decided because of severe maternal respiratory symptoms related to COVID-19.
COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range; PPH, postpartum hemorrhage; SD, standard deviation.
Sentilhes et al. Coronavirus disease 2019 in pregnancy can cause severe maternal morbidity. Am J Obstet Gynecol 2020.
Acute respiratory distress syndrome was diagnosed according to the Berlin definition (Acute Respiratory Distress Syndrome: The Berlin Definition).30
Neither neonatal acidosis nor fetal or neonatal death was observed. Three neonates, 2 born at 27 weeks’ gestation and 1 at 28 weeks’ gestation, underwent endotracheal intubation in the labor ward and were admitted to the neonatal ICU for problems related to their extremely or very preterm birth. All 21 neonates were tested for SARS-CoV-2 using RT-PCR of throat and rectal swab specimens, and SARS-CoV-2 was never detected (Table 5 ).
Table 5.
Neonatal outcomes of the pregnant women with confirmed or suspected COVID-19
| Characteristics | All women (N=54) | Pregnant women with confirmed COVID-19 (n=38) | Pregnant women with suspected COVID-19 (n=16) |
|---|---|---|---|
| Birthweight (n=21) | 2800±899 | 2810±982 | 2913±364 |
| Small for gestational age, n/N (%) | 2/21 (9.5) | 1/17 (5.9) | 1/4 |
| 5-min Apgar score <7, n/N (%) | 0 | 0 | 0 |
| Endotracheal intubation in the labor ward, n/N (%) | 3/21 (14.3) | 3/17 (17.6) | 0 |
| Admission to the neonatal ICU, n/N (%) | 3/21 (14.3) | 3/17 (17.6) | 0 |
| Attempted CPAP in the first 24 h of life, n/N (%) | 3/21 (14.3) | 3/17 (17.6) | 0 |
| Transfusion of blood products, n/N (%) | 1/21 (4.8) | 1/17 (5.9) | 0 |
| Phototherapy, n/N (%) | 1/21 (4.8) | 1/17 (5.9) | 0 |
| Neonatal death, n/N (%) | 0 | 0 | 0 |
| Neonatal samples positive for SARS-CoV-2 | 0 | 0 | 0 |
Data are presented as mean±SD, unless otherwise indicated. Denominator was the number of pregnancies ended beyond the first trimester (n=21). Small for gestational age was defined as birthweight less than the 10th percentile for gestational age and sex based on the French intrauterine growth curve.
COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Sentilhes et al. Coronavirus disease 2019 in pregnancy can cause severe maternal morbidity. Am J Obstet Gynecol 2020.
By the end of the follow-up (April 17, 2020), 1 woman (woman 2; Supplemental Table) remained in the ICU, 27 days after her transfer there. She had a secondary infection by Klebsiella pneumoniae and has required ECMO since April 9, 2020. Another woman (woman 13; Supplemental Table) remained hospitalized with an ongoing pregnancy but was transferred back to conventional care after 6 days in the ICU. She required high-flow oxygen therapy and transient noninvasive mechanical ventilation. All the other women have been discharged from the hospital. Eighteen neonates were also discharged, healthy and with no respiratory symptoms or fever. The remaining 3 newborns were still hospitalized for care related to their prematurity.
Clinical outcomes of the 13 women who required oxygen support
The characteristics and clinical course of the 13 pregnant women (24.1%) with COVID-19 who required oxygen support are described in the Supplemental Table. All but 3 had lymphopenia (<1.0×109 cells per L) or elevated concentrations of C-reactive protein (>10 mg/L). Four of them were still pregnant, whereas 9 had cesarean deliveries. Among the latter, 3 women aged ≥35 years with positive RT-PCR results for SARS-CoV-2 had medically indicated deliveries before 29 weeks’ gestation owing to severe maternal respiratory symptoms. Before delivery, all 3 women (women 1, 2, and 9) had respiratory failure even though they were receiving 5 liters of oxygen per minute. Preterm delivery was decided because physicians thought that maternal respiratory status would improve and oxygenation would be facilitated after birth.
One of these women (woman 2) needed invasive mechanical ventilation and then ECMO after delivery. All had at least 1 comorbidity: class III obesity (woman 1), overweight and gestational hypertension (woman 2), and overweight and gestational diabetes mellitus (woman 9).
Comment
Principal findings of the study
This is a descriptive study of the clinical characteristics and outcomes of 54 pregnant women with either laboratory-confirmed (n=38) or suspected (n=16) COVID-19; during our study, 32 pregnancies are ongoing, and 22 were completed. Oxygen support was required for 13 of 54 pregnant women (24.1%) with COVID-19 including 1 of 54 (1.9%) who required ECMO, and 3 of 21 births (14.3%) were very preterm deliveries indicated for severe COVID-19–related maternal conditions. The latter 3 women were aged 35 years or older, and all had at least 1 comorbidity.
Results of the study in the context of what is known
Most of the data about the maternal, fetal, and neonatal outcomes of pregnant women with COVID-19 have been globally reassuring.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Despite the unavailability of numerous details about maternal characteristics, including body mass index (BMI) and preexisting disease, or about the third stage of labor,7 , 11 , 14 the authors considered that the women’s outcomes were “good”13 or “satisfactory”11 or that there is “no evidence to suggest that COVID-19 pneumonia causes severe maternal and neonatal complications.”14
Zaigham and Andersson20 summarized these reports in a systematic review up to April 1, 2020, which included 108 pregnant women with COVID-19 from 18 case reports or series, mostly from China. They underlined that 3 of 108 women (3%) were admitted to ICUs and noted that severe maternal morbidity could therefore not be ruled out in COVID-19 during pregnancy,20 whereas Della Gatta et al21 pointed out in a systematic review of 6 Chinese studies totaling 51 pregnant women7 , 9 , 10 that preterm—mainly indicated—deliveries (31%) were frequent in women with COVID-19.
Finally, on April 17, 2020, Chen et al15 published a case series of 118 pregnant women with COVID-19 from 50 hospitals in Wuhan, China. It reported 3 spontaneous abortions, 2 ectopic pregnancies, 4 induced abortions, 41 ongoing pregnancies, and 68 deliveries; 63 of them cesarean deliveries (93%) because of the concern about the effect of COVID-19 on the pregnancy. No neonatal asphyxia or death occurred. Among the 14 preterm deliveries (21%), half (10.5%) were related to COVID-19. Overall, 109 women (92%) had mild disease, and 9 (8%) had severe disease (hypoxemia); only 1 (0.8%) received noninvasive mechanical ventilation. The only maternal characteristics described were age, parity, and singleton or multiple pregnancy, with no details about comorbidities, in particular BMI, preexisting conditions, or pregnancy-related disease. The authors concluded that their results “do not suggest an increased risk of severe disease among pregnant women.”15
Nevertheless, other authors have previously reported severe maternal complications related to COVID-19 in pregnant women, consistent with our findings.10 , 18 , 19 Liu et al10 retrospectively reported the clinical outcome of 13 pregnant women with COVID-19, including 3 ongoing pregnancies. Details were scarce, but the authors stated that 12 pregnant women were discharged without obvious complications. The remaining woman was admitted to the ICU with multiple organ dysfunction syndrome including ARDS, necessitating an emergency cesarean delivery. The neonate died. The woman required intubation, mechanical ventilation, and finally ECMO, but her final outcome was not reported.10
In a retrospective case series of 43 pregnant women with PCR-confirmed COVID-19 from 2 American centers, most of the women were obese, and 18 had an additional comorbidity.19 Among these 43 women, 18 gave birth, 10 by uncomplicated vaginal deliveries and 8 by cesarean deliveries for obstetrical reasons unrelated to COVID-19. Nonetheless, 2 women (4.7%) were admitted to the ICU.18 , 19 The first was a 38-year-old woman with a BMI of 38 kg/m2, diabetes mellitus, and intrahepatic cholestasis of pregnancy. Severe postpartum hemorrhage related to uterine atony occurred during cesarean delivery that followed failed induction of labor. Because she had fever and respiratory symptoms during delivery, a chest CT was performed and revealed ground-glass opacities in the lungs. RT-PCR was subsequently positive for SARS-CoV-2. She spent only 8 hours in the ICU and was discharged 4 days later.18 The second woman admitted to the ICU was 33 years old, with a BMI of 47 kg/m2, chronic hypertension, type 2 diabetes mellitus, and mild intermittent asthma. Twenty-five hours after cesarean delivery for failed induction of labor, she had coughing, respiratory distress, high fever, and reduced oxygen saturation. Although her respiratory status improved, she was admitted to the ICU after developing severe hypertension (blood pressure reaching 200/90 mm Hg). RT-PCR results returned positive for SARS-CoV-2. After 5 days, when the report concluded, she remained hospitalized, had acute kidney injury, and still needed oxygen supplementation.18 In both cases, COVID-19 seems to have been the—or at least the only—reason for ICU admission, as the first case involved severe postpartum hemorrhage and the second case involved severe hypertension.
Moreover, Yan et al16 reported a retrospective series expanded from 4 previous small case series,7 , 9 , 10 totaling 116 pregnant women with COVID-19 pneumonia from 25 hospitals in China. Preterm delivery before 34 and 37 weeks’ gestation occurred in 2% and 21.2% of cases: 8 of 116 women (6.9%) were admitted to the ICU, 6 of 116 (5.2%) required noninvasive and 2 of 116 (1.7%) invasive ventilation, and 1 of 116 (0.9%) required ECMO. Although these results were consistent with ours, the authors surprisingly concluded that “there is no evidence that pregnant women with COVID-19 are more prone to develop severe pneumonia, in comparison to nonpregnant patients.”16
Interestingly, since April 22, 2020, 3 different teams from distinct parts of the world have also alerted healthcare professionals about the possible detrimental effect of COVID-19 among pregnant women. Hirshberg et al22 described the clinical course of 5 American women, all requiring mechanical ventilation, including ECMO for one. All had at least 1 of the following comorbidities: overweight or obesity, chronic kidney disease, hypertension, insulin-dependent diabetes, obstructive sleep apnea, and mild asthma.22 Finally, maternal deaths have been reported in pregnant women with COVID-19.23 , 24 Amorim et al24 reported the cases of 9 women; the details were unfortunately limited, as the sources were mainly local media or the Brazilian or Mexican ministries of health. An Iranian case series reported the clinical outcomes of 9 pregnant women with severe COVID-19. Seven of them died, 3 aged 35 years or older.23 Nevertheless, no details were provided about any comorbidities related to these maternal deaths, including BMI.23 Thus, because of the considerable methodological limitations of the latter 2 reports, their principal information is that maternal deaths do occur in pregnant women with COVID-19.23 , 24
Clinical and research implications
Our case series confirms that COVID-19 during pregnancy may be responsible for severe maternal morbidity that may require very or extremely preterm elective delivery. In our series, 3 of 54 women (5.5%) had such medically indicated premature births; 13 of 54 (24.1%) required oxygen support, including invasive mechanical ventilation or ECMO for 3 of 54 women (5.5%); and 5 of 54 (9.2%) were admitted to the ICU. Interestingly, except for 1 of 2 women who required ECMO and for whom very few details were available,11 the most severe cases reported came from countries outside China, that is, from the United States,18 France (our series), South America,24 and Iran,23 where maternal characteristics and the prevalence of preexisting conditions may differ from those in China. Most of the French and American women had ≥1 maternal characteristics that are known to be associated with severe maternal morbidity in pregnant women, including an age of ≥35 years, overweight or obesity, gestational hypertension or diabetes, and preexisting asthma.32 These new findings should alert physicians and help them to manage and follow up pregnant women with COVID-19.
Another interesting finding of our study is the high rate of postpartum hemorrhage (9.3%) and blood transfusions (7.4%) observed in pregnant women with COVID-19, much higher than that reported in the general population of pregnant women.31 The literature thus far unfortunately fails to detail these women’s third stage of labor.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 Despite the overall low numbers and the single limited experience they come from, our findings warrant further investigation to examine whether COVID-19 might increase the risk of blood loss after delivery. Hemostasis changes may occur in these women, especially as clinically significant coagulopathy with antiphospholipid antibodies has been reported in nonpregnant patients with COVID-19 pneumonia.33
Finally, all the neonates we tested were negative for SARS-CoV-2, consistent with previous reports7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21; however, no conclusion can be drawn from our results about the possibility of intrauterine vertical transmission.
Strengths and limitations of the study
Our retrospective study is one of the largest series of pregnant women with confirmed and suspected cases of COVID-19, and it provides the most detailed look at maternal characteristics and maternal outcomes. It is one of the first to suggest that COVID-19 may be responsible for severe maternal morbidity that can require medically indicated extremely preterm delivery. Nevertheless, several limitations of our study must be underlined. The first is its retrospective design, common to all the studies that have thus far assessed maternal outcome in pregnant women with COVID-19. All the flaws of retrospective analyses apply. Moreover, the reported rates should be interpreted cautiously; an overestimation of severe cases is possible as Strasbourg University Hospital is the referral center for the management of pregnant women with COVID-19 in the Alsace region. Moreover, generalizability is limited to regions with COVID-19 prevalence similar to that in the region where our hospital is located, which is one of the principal pandemic hot spots in France (along with the Paris metropolitan region). Moreover, similar to previous authors,14 , 15 we have included suspected cases with typical chest CT findings but with RT-PCR testing negative for SARS-CoV-2, in accordance with the Chinese management guideline (version 7.0).26 Given the frequency of false negatives for COVID-19 cases, owing to low virus titers, inappropriate swabbing sites, and sampling at late disease stages,34 as well as the limited testing capacity in France during our study period (which also explains why only symptomatic women were tested during the study period), limiting inclusion to laboratory-confirmed cases would likely result in missing some COVID-19 cases.14 Nonetheless, all 5 women with premature births, including the 3 with the most severe illness in our study, those who required indicated extremely or very preterm cesarean delivery, had positive RT-PCR findings for SARS-CoV-2. Finally, even though our series is one of the largest so far reported, the small sample size should be taken into account when interpreting the results.
Conclusions
COVID-19 may be associated with severe maternal outcomes with hypoxemic respiratory failure despite oxygen support and can require an indicated—and sometimes very or extremely preterm—delivery. Healthcare providers should be aware that many of the standard risk factors associated with severe maternal morbidity without COVID-19, such as maternal age above 35 years, overweight or obesity, and preexisting and/or gestational hypertension or diabetes, may also increase the risk of severe maternal morbidity of pregnant women with COVID-19.
Acknowledgments
The authors are grateful to the participating women and their neonates. The authors thank Jo Ann Cahn for her help in editing this manuscript.
Footnotes
L.S. carried out consultancy work and was a lecturer for Ferring Laboratories in the previous 3 years. The other authors did not report any potential conflict of interest.
This study was funded by the Strasbourg University Hospital.
Cite this article as: Sentilhes L, De Marcillac F, Jouffrieau C, et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol 2020;223:914.e1-15.
Appendix
Supplemental Table.
Clinical characteristics and outcomes of the 13 women with confirmed or suspected COVID-19 who required oxygen support
| Characteristics | Woman 1 |
Woman 2 |
Woman 3 |
Woman 4 |
Woman 5 |
Woman 6 |
Woman 7 |
Woman 8 |
Woman 9 |
Woman 10 |
Woman 11 |
Woman 12 |
Woman 13 |
n/N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women who had vaginal or cesarean delivery | Ongoing pregnancies | |||||||||||||
| Baseline characteristics | ||||||||||||||
| Date of admission | March 16, 2020 | March 16, 2020 | March 20, 2020 | March 20, 2020 | March 20, 2020 | March 25, 2020 | March 26, 2020 | March 27, 2020 | March 28, 2020 | March 19, 2020 | March 26, 2020 | March 29, 2020 | April 1, 2020 | |
| Age, y | 36 | 42 | 32 | 33 | 41 | 24 | 42 | 36 | 36 | 34 | 36 | 32 | 21 | 34.2±6.3 |
| Gestational age at admission, wk+d | 27+2 | 26+5 | 37+2 | 38+1 | 40+3 | 39+2 | 35+0 | 33+5 | 28+2 | 34+0 | 34+5 | 36+0 | 25+0 | 32.5±5.1 |
| Non-French nationality | No | Yes | No | No | Yes | No | No | No | Yes | Yes | No | No | Yes | 5/13 (38.5) |
| Body mass index before pregnancy | 42 | 25.7 | 26.2 | 24.2 | 29.4 | 19.0 | 25.0 | 31.1 | 26.2 | 24.2 | 27.5 | 24.2 | 27.0 | 27.1±5.3 |
| Preexisting chronic basic disease | No | No | No | Asthma | No | No | No | No | No | Chronic hepatitis B | No | No | No | 2/13 (15.4) |
| Primiparous | No | Yes | No | No | Yes | No | No | No | No | No | Yes | No | No | 3/13 (23.1) |
| Gestational complication before admission | No | PIH | No | No | No | Preeclampsia | No | No | GDM | FGR | No | No | No | 4/13 (30.8) |
| Other family members affected | Yes | No | No | No | No | No | No | No | No | Yes | No | No | No | 2/10 (20) |
| Time from symptom onset to hospital admission, d | 5 | 0 | 8 | 0 | 0 | 0 | 3 | 6 | 5 | 1 | 7 | 5 | 5 | 3.5±2.9 |
| Clinical manifestations | ||||||||||||||
| Fever | Yes | Yes | Yes | No | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 9/13 (69) |
| Cough | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 12/13 (92) |
| Shortness of breath | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 11/13 (85) |
| Diarrhea | No | No | No | No | No | No | Yes | No | No | No | No | Yes | Yes | 3/13 (23) |
| Fatigue | Yes | No | Yes | No | No | No | Yes | No | Yes | No | No | Yes | Yes | 6/13 (46) |
| Sore throat | No | Yes | No | No | No | No | No | No | No | No | No | Yes | Yes | 3/13 (23) |
| Anosmia or ageusia | No | No | No | No | No | No | No | Yes | No | Yes | Yes | Yes | No | 4/13 (31) |
| Acute respiratory distress syndrome | No | Yes | No | No | No | No | No | No | No | No | No | No | No | 1/13 (7.7) |
| Chest CT | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | 10/13 (77) |
| Typical signs of viral infection on CTd | Yes | Yes | Yes | N/A | No | No | Yes | Yes | Yes | N/A | N/A | Yes | Yes | 8/10 (80) |
| Laboratory characteristics | ||||||||||||||
| RT-PCR positive for SARS-CoV-2 | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | No | 9/13 (69) |
| White blood cell count (×109 cells per L) | 5.68 | 7.33 | 3.32 | 9.6 | 19.1 | 10.3 | 10.9 | 5.1 | 5.3 | 7.8 | 11.4 | 9.1 | 4.37 | 8.4±4.1 |
| Lymphocyte count (×10⁹ cells per L) | 0.8 | 0.9 | 0.8 | 0.9 | 0.95 | 0.6 | 0.70 | 1.3 | 0.8 | NP | 1.8 | 1.7 | 0.8 | 1.0±0.4 |
| C-reactive protein concentration (mg/L) | 48.5 | 13.5 | 72 | 13.2 | 27.9 | 61.8 | 7.4 | 4.3 | 93.6 | 7.3 | 24 | 17.7 | 65.7 | 35.1±29.6 |
| ALT (U/L) | 55 | 48 | 22 | 14 | 13 | 15 | 36 | 76 | 26 | 12 | 75 | 12 | 12 | 32.0±24.0 |
| AST (U/L) | 65 | 49 | 45 | 23 | 29 | 30 | 76 | 71 | 36 | 15 | 64 | 18 | 13 | 42.1±22.2 |
| Hemoglobin (g/dL) | 12.4 | 10.1 | 11.6 | 8.8 | 13.10 | 10.7 | 9.4 | 11.7 | 11.6 | 10.9 | 12.9 | 10.4 | 9.6 | 11.0±1.3 |
| Creatinine (μmol/L) | 38.2 | 48.7 | 49.8 | 39.6 | 47.4 | 84.8 | 44.6 | 46.3 | 39.2 | 39.8 | NP | 59.9 | 35.2 | 47.8±13.4 |
| Treatment before delivery | ||||||||||||||
| Oxygen support (nasal cannula) | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 12/13 (92) |
| Oxygen (L/min) | 5 | 5 | 2 | 4 | 15b | N/A | 4 | 4 | 5 | 1 | 1 | 1 | 4 | — |
| High-flow oxygen therapy | No | No | No | No | No | No | No | No | No | No | No | No | Yes | 1 (8) |
| Noninvasive mechanical ventilation | No | No | No | No | No | No | No | No | No | No | No | No | Yes | 0 |
| Invasive mechanical ventilation | No | No | No | No | No | No | No | No | No | No | No | No | No | 0 |
| Admission to the ICU | No | No | No | No | No | No | No | No | No | No | No | No | Yes | 1 (8) |
| Antenatal steroids for fetal lung maturity | Yes | Yes | No | No | No | No | No | No | Yes | No | No | No | Yes | 4 (31) |
| Delivery | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | No | 9 (69) |
| Gestational age at delivery, wk+d | 27+5c | 27+2c | 37+2 | 38+1 | 40+3 | 39+2 | 35+0c | 34+0c | 28+4c | N/A | N/A | N/A | N/A | 35±4.8 |
| Spinal or epidural anesthesia | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | N/A | 9/9 (100) |
| Indicated cesarean delivery because of respiratory symptoms related to COVID-19 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | N/A | N/A | N/A | N/A | 7/9 (78) |
| Indicated cesarean delivery for other reasons | No | No | No | No | No | Yesa | No | No | No | N/A | N/A | N/A | N/A | 1/9 (11) |
| Cesarean delivery during labor | No | No | No | No | No | Yes | No | No | No | N/A | N/A | N/A | N/A | 1/9 (11) |
| Vaginal delivery | No | No | No | No | Yes | No | No | No | No | N/A | N/A | N/A | N/A | 1/9 (11) |
| PPH, defined by blood loss ≥500 mL, measured with a graduated collector bag | No | No | No | No | No | No | Yes | No | No | N/A | N/A | N/A | N/A | 1/9 (11) |
| Blood transfusion | No | No | No | No | No | No | Yes | No | No | N/A | N/A | N/A | N/A | 1/9 (11) |
| Treatment after delivery | ||||||||||||||
| Oxygen support (nasal cannula) | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | N/A | N/A | N/A | 8/9 (89) |
| Oxygen (L/min) | 5 | N/A | 4 | 1 | 1 | 4 | 4 | 2 | 3 | N/A | N/A | N/A | N/A | — |
| High-flow oxygen therapy | No | No | No | No | No | No | Yes | No | No | N/A | N/A | N/A | N/A | 1 (8) |
| Noninvasive mechanical ventilation | No | No | No | No | No | No | No | No | No | N/A | N/A | N/A | N/A | 0 |
| Invasive mechanical ventilation | No | Yes | No | Yes | Yes | No | No | No | No | N/A | N/A | N/A | N/A | 3/9 (33) |
| Extracorporeal membrane oxygenation | No | Yes | No | No | No | No | No | No | No | N/A | N/A | N/A | N/A | 1/9 (11) |
| Admission in ICU | Yes | Yes | No | Yes | Yes | No | No | No | No | N/A | N/A | N/A | N/A | 4/9 (44) |
| Antiviral therapy | Yes | Yes | Yes | No | No | No | No | No | No | N/A | N/A | N/A | N/A | 3/9 (33) |
| Antibiotic therapy | Yes | Yes | Yes | No | No | No | Yes | No | Yes | N/A | N/A | N/A | N/A | 4/9 (44) |
| Discharge from hospital at the end of follow-up | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 11/13 (85) |
| Neonatal outcome | ||||||||||||||
| Sex | Male | Male | Female | Female | Female | Female | Male | Male | Male | N/A | N/A | N/A | N/A | N/A |
| Birthweight (g) | 1080 | 1085 | 2220 | 3370 | 2840 | 2520 | 2590 | 2320 | 1340 | N/A | N/A | N/A | N/A | 2152±811 |
| Small for gestational age | No | No | No | No | No | No | No | No | No | N/A | N/A | N/A | N/A | 0 |
| 5-min Apgar score <7 | No | No | No | No | No | No | No | No | No | N/A | N/A | N/A | N/A | 1/9 (11) |
| Admission to neonatal ICU | Yes | Yes | No | Yes | No | No | No | Yes | Yes | N/A | N/A | N/A | N/A | 5/9 (55) |
| Attempted CPAP in the first 24 h of life | No | No | No | No | No | No | No | No | No | N/A | N/A | N/A | N/A | 0 |
| Breastfeeding | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No | N/A | N/A | N/A | N/A | 6/9 (67) |
| Discharge from hospital at the end of follow-up | No | No | Yes | Yes | Yes | Yes | Yes | No | No | N/A | N/A | N/A | N/A | 5/9 (55) |
Data are presented as mean±SD, unless otherwise indicated. The body mass index is the weight in kilograms divided by the square of the height in meters. Reference ranges of the different laboratory results are provided in Table 2. Small for gestational age was defined as birthweight less than the 10th percentile for gestational age and sex based on French intrauterine growth curves.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; CT, computed tomography; FGR, fetal growth restriction; GDM, gestational diabetes mellitus; ICU, intensive care unit; N/A, not applicable as the woman has not given birth; NP, not performed; PIH, pregnancy-induced hypertension; PPH, postpartum hemorrhage; SD, standard deviation.
Sentilhes et al.Coronavirus disease 2019 in pregnancy can cause severe maternal morbidity. Am J Obstet Gynecol 2020.
Cesarean delivery for preeclampsia
High concentration oxygen mask
Preterm birth was decided because of severe maternal respiratory symptoms related to COVID-19
Typical radiographic features of COVID-19 on chest CT ground-glass opacity, or bilateral pulmonary infiltration.27
Supplementary Data
References
- 1.Rasmussen S.A., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation. Obstet Gynecol. 2020;135:999–1002. doi: 10.1097/AOG.0000000000003873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Favre G., Pomar L., Musso D., Baud D. 2019-nCoV epidemic: what about pregnancies? Lancet. 2020;395:e40. doi: 10.1016/S0140-6736(20)30311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists Practice advisory: novel coronavirus 2019 (COVID-19) 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019 Available at:
- 4.Poon L.C., Yang H., Lee J.C.S. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020;55:700–708. doi: 10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dashraath P., Wong J.L.J., Lim M.X.K. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222:415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa200. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H., Wang L., Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu D., Li L., Wu X. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.23072. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25789. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu N., Li W., Kang Q. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N., Han L., Peng M. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L., Li Q., Zheng D. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020 doi: 10.1056/NEJMc2009226. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J., Guo J., Fan C. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal S.N., Overcash R., Mokhtari N. An uncomplicated delivery in a patient with Covid-19 in the United States. N Engl J Med. 2020;382:e34. doi: 10.1056/NEJMc2007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breslin N., Baptiste C., Miller R. Coronavirus disease 2019 in pregnancy: early lessons. Am J Obstet Gynecol MFM. 2020;2:100111. doi: 10.1016/j.ajogmf.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslin N., Baptiste C., Gyamfi-Bannerman C. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13867. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Della Gatta A.N., Rizzo R., Pilu G., Simonazzi G. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirshberg A., Kern-Goldberger A.R., Levine L.D. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amorim M.M.R., Soligo Takemoto M.L., Fonseca E.B.D. Maternal deaths with coronavirus disease 2019: a different outcome from low- to middle-resource countries? Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.023. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.China National Health Commision Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment (7th edition) 2020. http://kjfy.meetingchina.org/msite/news/show/cn/3337.html Available at:
- 27.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K., Kang S., Tian R., Zhang X., Zhang X., Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID-19) in the Xiaogan area. Clin Radiol. 2020;75:341–347. doi: 10.1016/j.crad.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization Protocol: real-time RT-PCR assays for the detection of SARS-CoV-2 Institut Pasteur, Paris. https://www.who.int/docs/default-source/coronaviruse/real-time-rt-pcr-assays-for-the-detection-of-sars-cov-2-institut-pasteur-paris.pdf?sfvrsn=3662fcb6_2 Available at: Accessed April 18, 2020.
- 30.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 31.Sentilhes L., Merlot B., Madar H., Sztark F., Brun S., Deneux-Tharaux C. Postpartum haemorrhage: prevention and treatment. Expert Rev Hematol. 2016;9:1043–1061. doi: 10.1080/17474086.2016.1245135. [DOI] [PubMed] [Google Scholar]
- 32.Freese K.E., Bodnar L.M., Brooks M.M., McTigue K., Himes K.P. Population-attributable fraction of risk factors for severe maternal morbidity. Am J Obstet Gynecol MFM. 2020;1:100066. doi: 10.1016/j.ajogmf.2019.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y., Yang M., Shen C. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. 2020. medRxiv. https://www.medrxiv.org/content/10.1101/2020.02.11.20021493v2 Available at: Accessed April 18, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.